Abstract
The Castleman triad has been described in a select few patients presenting with a retroperitoneal mass, mucocutaneous pemphigus vulgaris, and bronchiolitis obliterans. Here, we describe the Castleman triad in a 19-year-old male with unicentric hyaline vascular type Castleman disease (HV-CD). This patient presented with an array of positive antibodies, including anti-cyclic citrullinated peptide, anti-double-stranded DNA, and Sjogren's IgG. Interestingly, the patient's rheumatologic symptoms resolved after tumor resection, while his antibody profile remained relatively unchanged. HV-CD, with a triad presentation, was thought to be from a paraneoplastic syndrome secondary to an underlying lymphoproliferative disorder. The findings presented here identify multiple autoantibodies potentially contributing to this patient's presentation with HV-CD.
1. Introduction
Castleman disease (CD) is a rare, heterogeneous group of lymphoproliferative disorders with angiofollicular lymphatic hyperplasia, although, little is understood about its pathogenesis. CD may present with local (unicentric) or disseminated (multicentric) disease. The CD is further stratified microscopically as hyaline vascular, plasma cell, or mixed [1]. Due to the rare incidence of CD, few studies have systematically evaluated treatments and outcomes. There are few reports in the literature of CD presenting with rheumatologic findings, paraneoplastic pemphigus (PNP), and transient autoantibodies in the serum [2–4]. Treatment of unicentric CD (UCD) is surgical resection or rituximab if unresectable [5–7]. More recently, CD has been reported to be responsive to several immunotherapies (siltuximab, rituximab, tocilizumab) and antiviral drugs (ganciclovir, valganciclovir, and foscarnet for human herpes virus-8, HHV8, infected patients) [1, 8]. Yet, the immunopathophysiology and long-term outcomes of UCD remain understudied. We present a 19-year-old patient with the Castleman triad of retroperitoneal mass, mucocutaneous pemphigus vulgaris, bronchiolitis obliterans, and numerous persistently positive autoantibodies.
2. Case Presentation
A previously healthy 19-year-old male presented to the clinic with stomatitis in March 2018 (Figure 1(A)). His buccal biopsy revealed lichenoid interface dermatitis, which resolved without treatment. In December 2018, he presented again with buccal ulcers responsive to steroidal treatment. In January 2019, he returned with severe buccal ulcers, a papular rash along his trunk, painful genital ulcers, and blurry vision. Prednisone provided minimal relief. Three months later, he returned with worsening symptoms with new cuticle sanguineous crusting on his distal extremities, weight loss, pleuritic chest pain, arthralgias, and dyspnea with minimal exertion. The physical findings were consistent with mucocutaneous pemphigus vulgaris (Figure 1(B)–1(E)). Infectious workup for viruses, including HHV8, HIV, and other viremia and bacteremia, was unrevealing. Serologic testing for autoimmune antibodies was positive for several autoantibodies, including anti-nuclear antibodies, anti-cyclic citrullinated peptide, anti-double-stranded DNA, and Sjogren's IgG (Table 1). IgM and IgG against Mycoplasma pneumoniae and IgG against Chlamydia pneumoniae were positive, while the PCR detection of Mycoplasma pneumoniae was negative. CT of the chest, abdomen, and pelvis revealed ground glass opacities and bronchiectasis with signet rings (Figure 1(F)) and a 12 × 7.2 cm retroperitoneal mass with associated lymphadenopathy (Figures 1(G) and 1(H)).
Figure 1.

Clinical course and presentation of Castleman disease with retroperitoneal mass: (A) course of patient diagnosis and treatment. (B)–(E) physical findings from April 2019 showing (B) papular rash on the patient's trunk, (C) ulcerative stomatitis, (D) violaceous sanguineous cuticle encrustations, and (E) genital ulcers; (F) chest CT with red arrows indicating signet rings; (G, H) abdominal CT with red dotted circles indicating the retroperitoneal tumor.
Table 1.
Autoimmune and antibody testing.
| Antibody | Suggestive for | Before tumor removal | After tumor removal |
|---|---|---|---|
| Anti-nuclear antibodies | Nonspecific | Positive | Negative |
| Anti-cyclic citrullinated peptide | RA | Positive | Positive |
| Anti-double-stranded DNA | SLE | Positive | Positive |
| Anticentromere | Scleroderma, CREST syndrome | Negative | – |
| Anti-ribosome P | SLE | Negative | – |
| ss-A/RO | SS, SLE, RA | Negative | – |
| ss-B/la | SS, SLE, RA | Negative | – |
| Anti-smooth muscle | Autoimmune hepatitis | Negative | – |
| Anti-protinase-3 | Autoimmune vasculitis | Negative | – |
| Anti-myeloperoxidase | Autoimmune vasculitis | Negative | – |
| Rheumatoid factor | RA | Negative | – |
| Anti-ribonucleoprotein | SLE, MCTD | Negative | – |
| Anti-Scl70 | Scleroderma | Negative | – |
| Anti-Jo1 | Myositis | Negative | – |
| Sjogren's IgG | SS | Positive | Positive |
| Mycoplasma pneumoniae IgM | Acute infection | Positive | – |
| Mycoplasma pneumoniae IgG | Clearance/chronic infection | Positive | Positive |
| Chlamydia pneumoniae IgM | Acute infection | Negative | – |
| Chlamydia pneumoniae IgG | Clearance/chronic infection | Positive | Positive |
| HLA-B27 | B27-associated diseases | Negative | – |
| PNP panel | Bullous pemphigoid/pemphigus vulgaris | Positive | – |
RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjogren's syndrome; MCTD, mixed connective tissue disease; PNP, paraneoplastic pemphigus.
The tumor was surgically resected and revealed atretic follicles with paracortical vascular penetration and onion-skinning, consistent with unicentric hyaline vascular type CD (HV-CD) (Figure 2(a)–2(c)). Notably, these histologic findings did not reveal morphologic evidence of the tumor arising from an accessory spleen and instead strongly favored lymph node involvement. Mature B cells and IgG were identified using pan-IgG immunohistochemical staining. Immunophenotyping of the biopsy and resected tumor samples supported a B-cell predominant lymphocyte population in the HV-CD tumor (Figure 2(d)). Taken together, the patient was diagnosed with HV-CD with cutaneous and pulmonary involvement.
Figure 2.
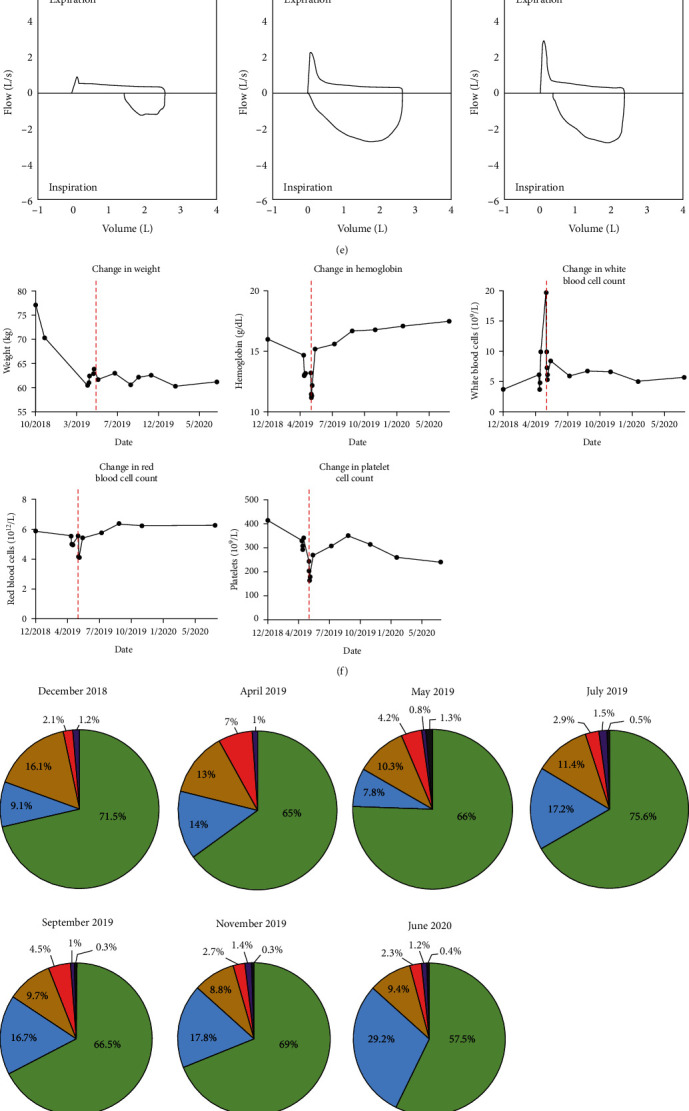
Diagnosis of unicentric hyaline vascular type Castleman disease: (a)–(d) immunohistochemical staining of the retroperitoneal mass showing (a) onionin-skinning and CD21 positive lymphocytes, (b) paracortical vascular penetration, (c) pan-IgG staining, and (d) flow cytometric immunophenotyping tumor biopsy and resection; (e) patient pulmonary function tests; (f) clinical data collected before and after surgery including weight, hemoglobin, white blood cell count, red blood cell count, and platelet count. Vertical dashed red line indicates the date of surgery; (g, h) white blood cell differential by (g) percent and (h) absolute number throughout the clinical course. Green designates to segmented neutrophils. Blue designates lymphocytes. Yellow designates monocytes. Red designates eosinophils. Purple designates basophils. Gray designates immature granulocytes.
Once the tumor was surgically removed, the patient appeared clinically improved but continued to struggle with dyspnea. Repeated pulmonary function tests revealed persistent severe obstruction consistent with bronchiolitis obliterans seen in the Castleman triad even after surgical resection of the tumor (Figure 2(e)). Over the disease course, the patient lost 16.8 kg, and prior to tumor removal, the patient had microcytic anemia and lymphopenia (Figure 2(f)–2(h)). In addition, he was at increased risk for bleeding with a prolonged prothrombin time (PT) of 14.6 s and high International Normalized Ratio (INR), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) (PT 14.6 s, ref 11.8–13.8 s; INR 1.2, ref 0.9–1.1; CRP 26.5 mg/L ref <10 mg/L; ESR 13 mm/hr, ref <10 mm/hr). After the removal of the tumor in May 2018, complete blood counts with differentials revealed lymphopenia had resolved. Notably, numerous serum autoantibodies were positive from this patient both prior to and after removing the tumor (Table 1). Interestingly, a number of these autoantibodies remained positive in his serum more than a year after tumor removal. Due to the slow clinical improvement, persistent breathlessness, and positive autoantibodies, we elected to treat the patient with four infusions of rituximab. The rituximab provided little clinical benefit and was discontinued after the patient developed bilateral lung nodules, which were biopsied and diagnosed as acute fibrinous organizing pneumonia with poorly formed nonnecrotizing granulomatous inflammation. Since 2020, the patient has continued to have severe airway obstruction and is under evaluation as a potential lung transplant.
3. Discussion
Initially, we postulated the patient's autoantibodies may be cross-reactive with (retroperitoneal) tumor-associated antigens and would resolve after tumor removal. However, upon tumor removal, the presence of autoantibodies did not resolve and remained positive more than 1-year postoperation despite resolution of other associated clinical pathologies. The remaining circulating autoantibodies are reactive with autoantigens (anti-cyclic citrullinated peptide, anti-double-stranded DNA, and Sjogren's IgG autoantibodies) characteristic of diverse autoimmune diseases, suggesting a general loss of immunological tolerance may accompany CD.
Notably, it is becoming increasingly evident that certain inflammatory settings that lead to the production of proinflammatory mediators (e.g., IL-6, IL-1, TNFα) are associated with breaches in peripheral tolerance. As examples, Zika and coronavirus infections are typically accompanied by severe inflammation and autoantibody production that promote autoimmune syndromes and pathology, respectively [9, 10]. While the precise mechanism by which these infections appear to impair peripheral tolerance is not yet understood, proinflammatory cytokines have been shown to breach tolerance to grafts in both animal models and humans.
Indeed, CD has not only been associated with IL-6 production [11] but is also treated with tocilizumab [12]. In this scenario, CD tumor burden and associated IL-6 production break peripheral tolerance, which precipitated the patient's rheumatologic symptoms. There are anecdotal reports in the literature of pathogen-driven or sterile inflammatory settings that result in transient autoantibody production [3, 13]. Preexisting, autoreactive B cells found in healthy individuals could be released from peripheral restrain and contribute to the autoimmune pathophysiology observed and in presented in this report.
Typically, autoimmune responses are seen in multicentric CD and can present with PNP, autoimmune hemolytic anemia, interstitial lung disease, cytokine storm, and even mimic systemic lupus erythematosus (SLE) [1, 14, 15]. Autoimmune symptoms have been previously reported in UCD, yet these symptoms typically resolve after tumor removal [16–21]. Notably, UCD is proposed to be derived from follicular dendritic cells (FDCs), which specialize in antigen capture through immune complex formation [1]. The key functions of FDCs are to trap antigens, activate B cells, and promote follicular microarchitecture and formation of germinal centers [22]. In addition to these functions, FDCs have a unique capability of retaining native antigens in long-lasting antigen depots [22, 23]. This may explain why CD responds to rituximab and why immunomodulators are currently promising exploratory treatment modalities of CD over chemotherapy [24].
Importantly, both the tumor biopsy and resection showed a lymphocyte-predominant population. It is possible these relatively long-lived autoantibodies may reflect production by long-lived antibody-secreting plasma cells. Emerging evidence may suggest that long-lived antibody-secreting plasma cells are refractory to rituximab treatment [25–27] and may explain why our patient received minimal benefit from anti-CD20 therapy. This is the first report of a UCD patient with long-lived antibodies persisting in the serum more than a year after tumor removal. Our case suggests that the long-term clinical consequences of enduring autoantibodies in UCD are associated with lasting lung damage, potentially requiring future lung transplants. Importantly, these potential mechanisms appear to be taking place in a neoplastic and reactive lymph node. Interestingly, reactive lymph node involvement is frequently observed in a rare CD subvariant of TAFRO (thrombocytopenia, ascites/anasarca, myelofibrosis/fever, renal dysfunction/reticulin fibrosis, and organomegaly) [28]. A few studies have found unicentric HV-CD arising from an accessory spleen or reported Castleman tumors arising from other uncommon locations [29–33]. However, our case strongly favors the tumor arising from a lymph node.
The triad of problems in this patient with UCD have been previously reported, yet no large series has been reported [2–4, 21]. Our patient presented with PNP as the primary clinical complaint, accompanied by respiratory symptoms before/after CD excision. After treatment with various combinations of immunosuppressive and anti-inflammatory agents, most patients have great or total improvement in mucosal erosions, while their pulmonary function does not improve despite further immunosuppressive therapy. As with our patient, this sequalae of events has led to consider lung transplantation in such patients. In the patients reported so far, disease onset to lung transplant were 1, 2, and 5 years. All antibodies were negative or were present at low titers before the lung transplant. Altogether, this report identifies a case of HV-CD and coinciding autoantibody production with precipitating pemphigus vulgaris and bronchiolitis obliterans, which should be clinically recognized as the Castleman triad. CD patients presenting with autoimmune symptoms and autoreactive antibodies could have consequential long-term lung damage. While postsurgical rituximab offered minimal clinical benefit, earlier immunomodulatory intervention could be more effective to deplete B cells. Thus, autoantibody testing should be performed in CD patients and could potentially serve as an earlier interventional target. In this study, we highlight important and unexplored potential mechanisms that could be exploited to understand the pathophysiology, clinical course, and treatment of patients with CD.
Acknowledgments
The authors would like to thank the University of Colorado Cancer Center. This study was funded by the Amy Davis Foundation, the Moore Family Foundation, and the Heidi Horner Foundation with funding provided to W.A.R. and J.A.T. was funded by the Hertz Graduate Fellowship.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper. Any additional laboratory values, clinical data, or follow-up information can be made available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Clinical data were extracted from the medical record by J.A.T., W.A.R., J.T.S., J.M.S., and B.J.S. The patient treatment and clinical care were coordinated by W.A.R., J.M.S, J.T.S, and B.J.S. Histology and immunohistochemical analysis was performed and determined by J.T.S. and B.J.S. Key expertise required for writing the manuscript was provided by H.L., A.K., and R.M.T. The manuscript was drafted by J.A.T. and reviewed and edited by all authors.
References
- 1.Dispenzieri A., Fajgenbaum D. C. Overview of Castleman disease. Blood . 2020;135(16):1353–1364. doi: 10.1182/blood.2019000931. [DOI] [PubMed] [Google Scholar]
- 2.Sun D.-P., Chen W.-M., Wang L., et al. Clinical characteristics and immunological abnormalities of Castleman disease complicated with autoimmune diseases. Journal of Cancer Research and Clinical Oncology . 2021;147(7):2107–2115. doi: 10.1007/s00432-020-03494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L., Bu D., Yang Y., Chen X., Zhu X. Castleman’s tumours and production of autoantibody in paraneoplastic pemphigus. The Lancet . 2004;363(9408):525–531. doi: 10.1016/S0140-6736(04)15539-6. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., Chen H., Shi J., et al. Castleman disease mimicking systemic lupus erythematosus: a case report. Medicine . 2018;97(38) doi: 10.1097/MD.0000000000012291.e12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek H. J., Kook H., Han D. K., Shin M.-G., Kim H. S., Hwang T. J. Unicentric Castleman disease relapsed after rituximab-CHOP chemotherapy or radiation therapy in an adolescent. Journal of Pediatric Hematology/Oncology . 2012;34(5):e206–e208. doi: 10.1097/MPH.0b013e3182352dc7. [DOI] [PubMed] [Google Scholar]
- 6.Seo H. Y., Kim E. B., Kim J. W., Shin B. K., Kim S. J., Kim B. S. Complete remission in a patient with human herpes virus-8 negative multicentric Castleman disease using CHOP chemotherapy. Cancer Research and Treatment . 2009;41(2):104–107. doi: 10.4143/crt.2009.41.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estephan F. F., Elghetany M. T., Berry M., Jones D. V., Jr. Complete remission with anti-CD20 therapy for unicentric, non-HIV-associated, hyaline-vascular type, Castleman’s disease. Cancer Investigation . 2005;23(2):191–191. doi: 10.1081/CNV-50484. [DOI] [PubMed] [Google Scholar]
- 8.Fajgenbaum D. C., Kurzrock R. Siltuximab: a targeted therapy for idiopathic multicentric Castleman disease. Immunotherapy . 2016;8(1):17–26. doi: 10.2217/imt.15.95. [DOI] [PubMed] [Google Scholar]
- 9.Burnett D. L., Reed J. H., Christ D., Goodnow C. C. Clonal redemption and clonal anergy as mechanisms to balance B cell tolerance and immunity. Immunological Reviews . 2019;292(1):61–75. doi: 10.1111/imr.12808. [DOI] [PubMed] [Google Scholar]
- 10.Reed J. H., Jackson J., Christ D., Goodnow C. C. Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. Journal of Experimental Medicine . 2016;213(7):1255–1265. doi: 10.1084/jem.20151978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leger-Ravet M. B., Peuchmaur M., Devergne O., et al. Interleukin-6 gene expression in Castleman’s disease. Blood . 1991;78(11):2923–2930. doi: 10.1182/blood.V78.11.2923.2923. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T., Narazaki M., Ogata A., Kishimoto T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Seminars in Immunology . 2014;26(1):88–96. doi: 10.1016/j.smim.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Agazio A., Cimons J., Shotts K. M., et al. Histone H2A-reactive B cells are functionally anergic in healthy mice with potential to provide humoral protection against HIV-1. Frontiers in Immunology . 2020;11 doi: 10.3389/fimmu.2020.01565.1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S.-H., Ruan Z., Huang H.-L., Song K.-S. A rare case of Castleman disease presenting as pulmonary mass mimicking central pulmonary malignancy. Chinese Medical Journal . 2009;122(8):990–991. [PubMed] [Google Scholar]
- 15.Fajgenbaum D. C., Shilling D. Castleman disease pathogenesis. Hematology/Oncology Clinics of North America . 2018;32(1):11–21. doi: 10.1016/j.hoc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Chorzelski T., Hashimoto T. P., Amagai M., et al. Paraneoplastic pemphigus with cutaneous and serological features of pemphigus foliaceus. British Journal of Dermatology . 1999;141(2):357–359. doi: 10.1046/j.1365-2133.1999.02999.x. [DOI] [PubMed] [Google Scholar]
- 17.Kop E. N., MacKenzie M. A. Castleman disease and paraneoplastic pemphigus. Canadian Medical Association Journal . 2010;182(1) doi: 10.1503/cmaj.081504.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzano A. V., Vezzoli P., Mariotti F., Boneschi V., Caputo R., Berti E. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma and Castleman disease. British Journal of Dermatology . 2005;153(1):214–215. doi: 10.1111/j.1365-2133.2005.06695.x. [DOI] [PubMed] [Google Scholar]
- 19.Singh M., Saroha V., Gupta P., Gupta P., Khurana N., Singh T. Hyaline vascular Castleman disease relapsing as T cell rich B cell lymphoma with paraneoplastic pemphigus. Journal of Clinical Pathology . 2011;64(1):93–94. doi: 10.1136/jcp.2010.076554. [DOI] [PubMed] [Google Scholar]
- 20.Sun H., Wang R., Bin H., et al. Localized Castleman disease with paraneoplastic pemphigus and pulmonary involvement: clinical features and histopathology. Zhonghua Yi Xue Za Zhi . 2002;82(8):530–533. [PubMed] [Google Scholar]
- 21.Wang J., Zhu X., Li R., et al. Paraneoplastic pemphigus associated with Castleman tumor: a commonly reported subtype of paraneoplastic pemphigus in China. Archives of Dermatology . 2005;141(10):1285–1293. doi: 10.1001/archderm.141.10.1285. [DOI] [PubMed] [Google Scholar]
- 22.Kranich J., Krautler N. J. How follicular dendritic cells shape the B-cell antigenome. Frontiers in Immunology . 2016;7 doi: 10.3389/fimmu.2016.00225.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heesters B. A., Chatterjee P., Kim Y.-A., et al. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity . 2013;38(6):1164–1175. doi: 10.1016/j.immuni.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Rhee F., Voorhees P., Dispenzieri A., et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood . 2018;132(20):2115–2124. doi: 10.1182/blood-2018-07-862334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuwana M., Iki S., Urabe A. The role of autoantibody-producing plasma cells in immune thrombocytopenic purpura refractory to rituximab. American Journal of Hematology . 2007;82(9):846–848. doi: 10.1002/ajh.20951. [DOI] [PubMed] [Google Scholar]
- 26.Mahévas M., Michel M., Weill J.-C., Reynaud C.-A. Long-lived plasma cells in autoimmunity: lessons from B-cell depleting therapy. Frontiers in Immunology . 2013;4 doi: 10.3389/fimmu.2013.00494.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahévas M., Patin P., Huetz F., et al. B cell depletion in immune thrombocytopenia reveals splenic long-lived plasma cells. Journal of Clinical Investigation . 2013;123(1):432–442. doi: 10.1172/JCI65689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D., Lim M. S., Jaffe E. S. Pathology of Castleman disease. Hematology/Oncology Clinics of North America . 2018;32(1):37–52. doi: 10.1016/j.hoc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Mantas D., Damaskos C., Dailiani P., Samarkos M., Korkolopoulou P. Castleman’s disease of the spleen. Acta Chirurgica Belgica . 2017;117(3):203–208. doi: 10.1080/00015458.2016.1246273. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi C., Hama Y., Kadota Y., Sugiura Y., Aida S., Kosuda S. Castleman’s disease arising from the accessory spleen: ultrasonography, computed tomography, and magnetic resonance imaging findings. Clinical Imaging . 2005;29(5):352–355. doi: 10.1016/j.clinimag.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Sbrana F., Zhou D., Zamfirova I., Leonardi N. Castleman’s disease: a rare presentation in a retroperitoneal accessory spleen, treated with a minimally invasive robotic approach. Journal of Surgical Case Reports . 2017;2017(10) doi: 10.1093/jscr/rjx195.rjx195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tunru-Dinh V. W., Ghani A., Tom Y. D. Rare case of Castleman disease involving the pancreas. The American Surgeon . 2007;73(12):1284–1287. doi: 10.1177/000313480707301220. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y.-X., Ji Y., Wu S. A CARE-compliant article: unicentric Castleman disease presenting as a retroperitoneal mass of the upper edge of the pancreas: a case report. Medicine . 2020;99(11) doi: 10.1097/MD.0000000000019515.e19515 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper. Any additional laboratory values, clinical data, or follow-up information can be made available from the corresponding author upon request.


