Summary
Background
The US overdose crisis is driven by fentanyl, heroin, and prescription opioids. One evidence-based policy response has been to broaden naloxone distribution, but how much naloxone a community would need to reduce the incidence of fatal overdose is unclear. We aimed to estimate state-level US naloxone need in 2017 across three main naloxone access points (community-based programmes, provider prescription, and pharmacy-initiated distribution) and by dominant opioid epidemic type (fentanyl, heroin, and prescription opioid).
Methods
In this modelling study, we developed, parameterised, and applied a mechanistic model of risk of opioid overdose and used it to estimate the expected reduction in opioid overdose mortality after deployment of a given number of two-dose naloxone kits. We performed a literature review and used a modified-Delphi panel to inform parameter definitions. We refined an established model of the population at risk of overdose by incorporating changes in the toxicity of the illicit drug supply and in the naloxone access point, then calibrated the model to 2017 using data obtained from proprietary data sources, state health departments, and national surveys for 12 US states that were representative of each epidemic type. We used counterfactual modelling to project the effect of increased naloxone distribution on the estimated number of opioid overdose deaths averted with naloxone and the number of naloxone kits needed to be available for at least 80% of witnessed opioid overdoses, by US state and access point.
Findings
Need for naloxone differed by epidemic type, with fentanyl epidemics having the consistently highest probability of naloxone use during witnessed overdose events (range 58–76% across the three modelled states in this category) and prescription opioid-dominated epidemics having the lowest (range 0–20%). Overall, in 2017, community-based and pharmacy-initiated naloxone access points had higher probability of naloxone use in witnessed overdose and higher numbers of deaths averted per 100 000 people in state-specific results with these two access points than with provider-prescribed access only. To achieve a target of naloxone use in 80% of witnessed overdoses, need varied from no additional kits (estimated as sufficient) to 1270 kits needed per 100 000 population across the 12 modelled states annually. In 2017, only Arizona had sufficient kits to meet this target.
Interpretation
Opioid epidemic type and how naloxone is accessed have large effects on the number of naloxone kits that need to be distributed, the probability of naloxone use, and the number of deaths due to overdose averted. The extent of naloxone distribution, especially through community-based programmes and pharmacy-initiated access points, warrants substantial expansion in nearly every US state.
Funding
National Institute of Health, National Institute on Drug Abuse.
Introduction
In the USA, overdose is the leading cause of adult death due to injury,1 associated with substantial reductions in life expectancy, and annual estimated costs of US$20·4 billion.2,3 The opioid crisis seems to be rapidly transforming into multiple and, in some geographical areas, overlapping public health disasters involving prescription opioids, heroin, and illicitly manufactured fentanyl and fentanyl analogues.3–6
The evolving nature of the crisis requires approaches that reduce mortality and morbidity across all three epidemic types. One such approach is overdose education and naloxone distribution to people at risk of experiencing or witnessing an opioid overdose. Different from naloxone administered by police or medical professionals, peers often witness opioid use and might be the first to respond to an overdose. Greater layperson access to naloxone has gained widespread acceptance as an evidence-based strategy to reduce deaths due to opioid overdose.7
Historically, individuals thought to be at risk of having an opioid overdose included patients prescribed high doses of opioid analgesics, people using illicit opioids or with opioid use disorders, and people who misuse opioid medications. However, with the advent of illicitly manufactured fentanyl entering the illicit drug supply, anyone using unregulated drugs in powder or pill form might be exposed to drugs contaminated with illicitly manufactured fentanyl, leading to an increased burden of fatal opioid overdoses in the community. The US Department of Health and Human Services and the US Food and Drug Administration have called for expanded naloxone access through physicians and other prescribers,8–10 which might reduce opioid-related emergency visits11 and geographical disparities in naloxone availability.12 Other experts and behavioural health leaders encourage broader provision of naloxone to people in drug treatment, codified in the Substance Abuse and Mental Health Services Administration’s Opioid overdose prevention toolkit.13 In 2018, the US Surgeon General issued a rare special advisory, calling on all Americans to prevent overdose, get naloxone, and ready themselves to use it.14 Although these actions convey consistent support for naloxone access, just how much naloxone is needed to substantially reduce opioid-involved overdose deaths in the USA is unclear.
In the USA, naloxone access is permitted through state legal permissions for community-based programmes, by prescription from a health-care provider, and from pharmacist-initiated models defined by state laws and regulations (eg, collaborative pharmacy practice agree ments, prescriptive authority, prescriptive protocol, and pharmacy standing orders).8,12,15 These legal permissions allow community-based programmes and laypeople to distribute no-cost naloxone in venues such as jails, drug treatment programmes, or syringe service programmes. Provider-initiated and pharmacy-initiated provision models might include a cost and require interaction with either the provider for a prescription or with a community pharmacist who can provide naloxone directly without having to first see the provider.16,17
We aimed to construct a mathematical model that considered the type of naloxone access point (ie, community programme, provider prescribed, or pharmacy initiated); the dynamics of the prescription opioid, heroin, or fentanyl epidemic in each state; and how choice of access point and the type of epidemic affects estimates of naloxone need. Mathematical models are becoming established as important tools for assessment of naloxone-based public health policy.18,19 Our approach was to extend an established population model of overdose to incorporate key differences in subpopulations and naloxone distribution types in different US states for data collected in 2017.20,21 We aimed to generate actionable estimates to reduce fatal opioid overdoses over a 1-year time horizon.
Methods
Study design
In this modelling study, to capture the influence of epidemic type on naloxone need, first we defined the different epidemic types in each US state in 2017, and then selected representative states for which complete data were available. Next, we constructed a stochastic Markov chain model to estimate state-wide naloxone needs for the representative states.22 Non-representative states were those that did not have data on overdose-related outcomes and naloxone distribution available for full model inclusion, and so we projected naloxone need on the basis of how well the model fitted to representative states of the same epidemic type using key demographic population size estimates. We also investigated counterfactual scenarios, in which we considered alternative combinations of access points and their effect on outcomes.
Modified Delphi process
Because data to inform several model parameters were unknown and the epidemic type for each state was inferred, we undertook a standardised process for gathering expert opinion to streamline model building. We carried out a modified Delphi process involving a panel of ten nationally recognised experts in public health policy, harm reduction, naloxone, illicit drug markets, and law enforcement who were identified from the literature, public speaking events, and community acknowledged or academically recognised expertise. Experts were asked to: provide realistic starting estimates and consider assumptions for prescription opioid-dominated, heroin-dominated, and fentanyl-dominated epidemics (eg, base rate of witnessing overdoses by epidemic type); classify US states into epidemic type; and generate and rank counterfactual scenarios for subsequent modelling. Experts provided input via anonymous online surveys (available on request from the corresponding author) in the first two of three total waves. In the third wave, an in-person meeting was held (three members attended virtually), with consensus reached at 75% agreement. Experts were offered a $200 gift card for participating. We used 2017 National Vital Statistics System and US state-based data on deaths due to overdose by opioid to supplement categorisation of each state’s epidemic type.
Data sources
The Delphi panel identified three opioid epidemic types plus fentanyl-mixed epidemic types (eg, fentanyl plus prescription opioid or fentanyl plus heroin), which were combined for ease of interpretation (hereafter referred to as fentanyl-mixed epidemics). However, in 2017, no heroin-dominated epidemic types were observed, although several states were evolving from prescription opioid to more illicit drug involvement. In these instances, the epidemic type was described by the Delphi panel as such and labelled as a heroin-prescription opioid epidemic. The panel also provided estimates for which of the four identified epidemic types (ie, prescription opioid-dominated, heroin-prescription opioid, fentanyl-dominated, fentanyl-mixed epidemics) each state was experiencing in 2017, and three representative states of each epidemic type were selected on the basis of availability of public data.
Data were obtained from proprietary data sources, state health departments, and national surveys; via database review of published (ie, PubMed, MEDLINE, and PsycInfo databases) and grey literature (search engines included Google and Bing) using the search terms “naloxone” and “overdose”; and state and national harm reduction community programme inquiries for the period Jan 1 to Dec 31, 2017. State health department data included the following key model inputs: the aggregate annual number of patients dispensed prescriptions of over 90 morphine mg equivalents daily; the number of patients dispensed an opioid analgesic and benzodiazepine prescription within 30 days of each other; the number of emergency medical services callouts for suspected opioid overdoses; and the annual number of opioid-involved overdose deaths, and by prescription opioid, heroin, and fentanyl involvement.
Naloxone kits (containing two doses) were defined as being community based, provider prescribed, and pharmacy initiated. Several states’ community-based naloxone programmes do not routinely collect use data, so we only used distribution data to inform our model. We catalogued provider-prescribed and pharmacy-initiated naloxone data using the SymphonyHealth database. We defined provider-prescribed naloxone kits as a naloxone prescription first initiated by a provider and pharmacy-initiated kits as a naloxone prescription first initiated by a pharmacist through an authorised mechanism or provided to individuals requesting a kit at a pharmacy.12,15 We estimated pharmacy-initiated prescription rates per state on the basis of previous research.12
Several data queries of state health departments were unavailable because of staffing constraints, ongoing litigation, or statutes preventing data release, so we imputed point estimates on the basis of previous year data or similar states with available data.
Model input parameters
We derived model input parameters from published literature, the modified Delphi process, and epidemiological and interventional studies that are ongoing or have not yet been published. Parameters included the initial populations with a 10% SD to allow for a general uncertainty of data and literature estimates. Each population had an associated risk of overdose that was derived from literature estimates. The model prior’s sources and initial values are in the appendix (pp 9–15). A conceptual framework for parameters is shown in figure 1.
Figure 1: Simplified model overview of fatal and non-fatal opioid overdose with naloxone use by US state.
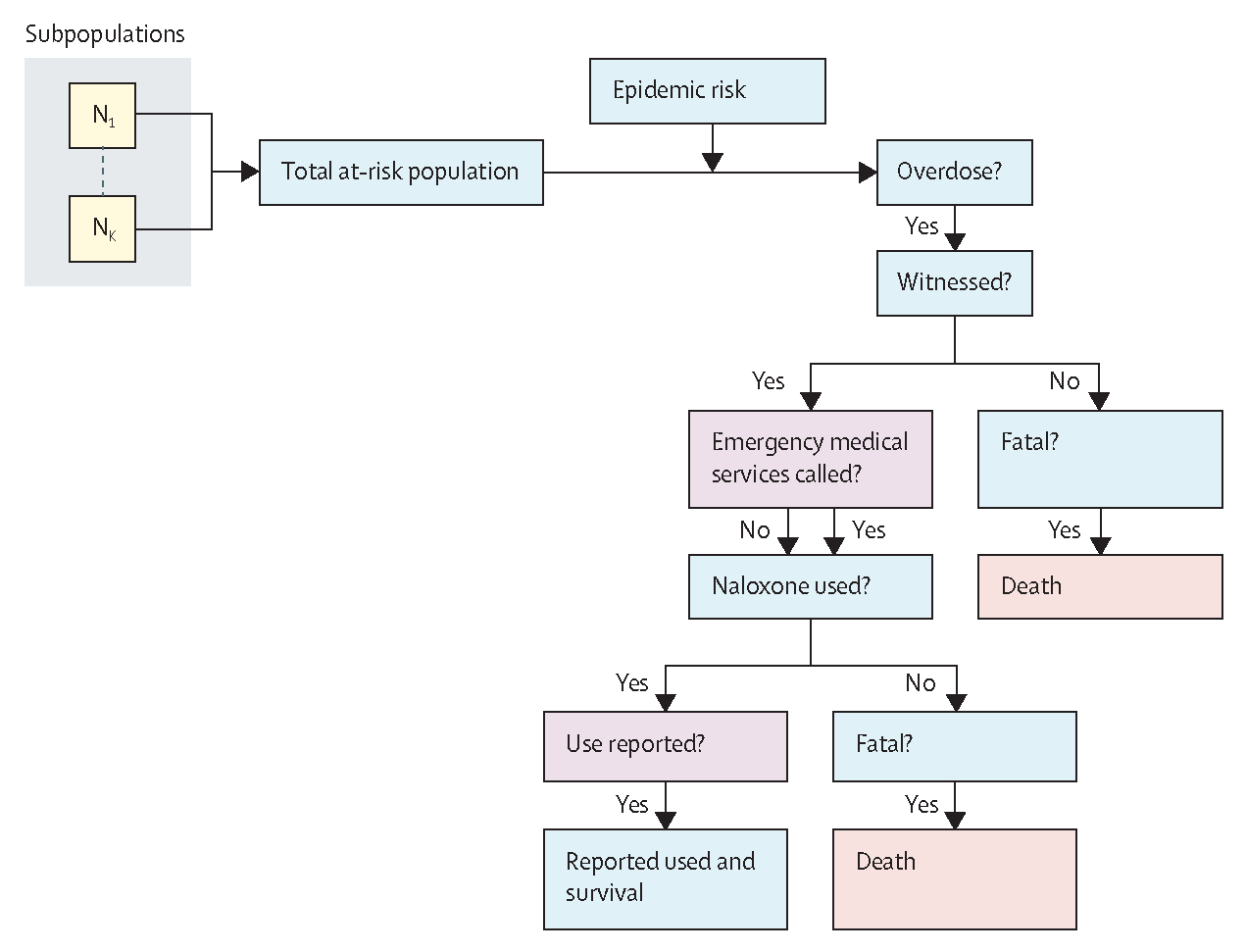
Diagram indicates where data are captured in the model (non-blue boxes) with other latent branches suppressed. The at-risk population is stratified by risk type (N1 to Nk), which are combined together to produce an effective population size of individuals at risk of an opioid overdose. A per-month rate of opioid overdose is dependent on epidemic type and underlying circulation of fentanyl and its derivatives (indicted by the epidemic risk box). Each estimated opioid overdose is then entered into a decision tree to determine the probability of the opioid overdose resulting in death, whether there is a reported use of naloxone, or neither. Whether an opioid overdose is fatal is dependent on whether it is witnessed, whether emergency medical services were called, whether naloxone is used, and the underlying risk of death for an intervened overdose. The probability of naloxone use is dependent on whether an overdose is witnessed, naloxone is available and used by a witness, and whether its use is reported.
The primary endpoint of interest was the reduction in opioid-involved overdose mortality. Therefore, we considered patients taking prescribed opioids medically (at high dose or in combination with a benzodiazepine) and those using opioids illicitly to be at-risk of overdose on the basis of established literature and guidelines prioritising these subgroups.8,23 We also considered special circumstances that increase the risk of a fatal overdose (eg, when people with opioid use disorders leave incarceration), with adjustment for possible overlap.24 On the basis of emerging evidence of the possibility for fentanyl-contaminated cocaine and subsequent increased risk of fentanyl-involved overdose,25,26 we included estimates of the number of people discharged for treatment of cocaine use disorder as a proxy for the size of this at-risk population. Model prior distribution uncertainty was incorporated to account for potential biases in these populations (appendix pp 9–13).
Although the model’s framework broadly considers reduction in mortality due to overdose, we calculated the range and saturation point of naloxone needed to: (1) reduce the number of deaths due to opioid overdose per 100 000 people and (2) increase the probability of naloxone being available at a witnessed overdose. The modified Delphi process informed both the choice of outcomes and selection of 80% as the target saturation point for the probability of naloxone being available at a witnessed overdose.
Model design
The mathematical model was based on previous work estimating the effect of deaths averted due to naloxone distributed in British Columbia, Canada.20,21 The scheme is established from a Bayesian evidence synthesis method, in which multiple disparate data sources are combined together in a mechanistic fashion to estimate latent (ie, unobserved) processes, such as the proportion of fentanyl in the illicit opioid supply. In broad terms, a monthly estimated rate of opioid overdoses is established on the basis of population risk factors. This rate of opioid overdoses is then used to inform the probability of death after an opioid overdose depending on whether a bystander intervenes or no intervention occurs. Using this estimate of the probability of death after an opioid overdose, we could then estimate the number of deaths due to opioid overdose averted due to a given amount of naloxone distributed through various methods within a given year. Here we will briefly outline the model, and a more in-depth description is in the appendix (pp 2–5).
Because the time horizon of the model was 1 year, the population was considered to be static. The population had a number of underlying risk factors including opioid use disorders, coprescriptions of benzodiazepines and opioid analgesics, stimulant use, recent (ie, past month) release from incarceration, and return to use after a period of treatment. Each risk factor was assigned a corresponding weight representing the estimated relative risk of opioid overdose. The proportion of contact with fentanyl in a given month was used as an additional risk factor that was directly fit to data and varied over time with dependency on the previous month (this model feature is absent in heroin-prescription opioid and prescription opioid-only epidemics). This proportion of contact with fentanyl, which is a latent variable that incorporates the proportion of deaths due to fentanyl-related overdose, was combined with the population risk structure to produce total population rates of fentanyl and non-fentanyl opioid overdoses. Additionally, the probability of an opioid overdose being witnessed was combined with the probability of the emergency medical services being called to a witnessed overdose to give the rate of overdoses to which there was an emergency medical services response.
The probability of naloxone use for a witnessed opioid overdose was modelled as a non-linear function of the total number of naloxone kits distributed annually. Non-linearity was incorporated to account for potential saturation because large numbers of naloxone kits were distributed, which would reduce the probability that a particular naloxone kit was used.
As a simplification, we assumed that naloxone use during a witnessed overdose would result in survival. Estimates of opioid overdose deaths averted were dependent both on the estimated risk of an unwitnessed opioid overdose and total rate of opioid overdoses within the population, in addition to the number of naloxone kits distributed and used. Systematic under-reporting of opioid overdoses could lead to a biased increase in the risk of death after an opioid overdose that could overestimate the impact of naloxone. Conversely, if use of naloxone kits is systematically under-reported then this could lead to underestimation of the impact of naloxone to reduce death due to overdose. To mitigate this effect, we used an expert-derived prior probability of under-reporting from the modified Delphi panel. The probability of death for overdoses that were attended by emergency medical services was assumed to be negligible compared with overdoses that were not attended by emergency medical services. The probability of naloxone use was assumed to saturate at 100% of all witnessed opioid overdoses, and we used a simple saturating function to describe this association. Finally, the rate of death due to fentanyl-related and non-fentanyl-related overdose was calculated as a composition of the population rate of opioid overdose, the probability that the opioid overdose was unwitnessed, the probability that naloxone was not used, and the probability that an unwitnessed opioid overdose resulted in death. Rates of deaths due to prescription-based opioid overdose were calculated in a similar fashion; however, without the incorporation of fentanyl involvement and incorporating prescription opioid-based overdose deaths.
Calibration and validation of the model
Model fitting was done within a Bayesian framework. This method allowed the incorporation of disparate data sources and expert-driven and literature-based estimates in the form of informed priors (listed in the appendix [pp 9–16]). We constructed the likelihood for each model state from observed deaths due to fentanyl-related and non-fentanyl-related overdose, the number of opioid overdoses that were attended by emergency medical services, the number of naloxone kits used, and the number of deaths due to fentanyl, non-fentanyl, and prescription opioid overdose. We assumed each observed outcome was independently Poisson distributed according to the population rates. We sampled the model using a No U-Turn Sampling algorithm,27 assessed convergence via visual inspection of the chains, and internally validated the model fit by graphically assessing the posterior distribution to all outcomes and comparing main outcomes to different choices of prior distributions (appendix p 5). We built all models using the Python package PyMC3 modelling library.28
Counterfactual scenarios
After we completed state-level calibrations of the model, we used these state-specific parameters to develop several counterfactual scenarios. We explored two sets of scenarios: the number of deaths due to opioid overdose averted at varying naloxone distribution levels, and the number of naloxone kits needed to be available for at least 80% of witnessed opioid overdoses. Based on the large volume of naloxone distributed by community-based naloxone programmes,17 we contrasted the effect of epidemic type using community-based access as the reference.
We calculated counterfactual simulations by chan ging a posterior parameter (eg, probability of fentanyl involvement) or altering the input data (eg, amount of naloxone distributed), or both. All counterfactual scenarios drew a sample set of parameters from the posterior for the given model fit. By changing the parameter posterior or input data, or both, we modelled the effect of additional kit distribution or the absence of its distribution. For each scenario, we repeated these calculations 1000 times and took the median and 95 percentiles to produce the estimated effect with credible intervals (CrIs). We summed the outcomes from the counterfactuals over a quarter of the year to establish the yearly effect of varying kit distribution levels. We produced counterfactual scenarios for every model state and extended these to non-representative states where applicable.
Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Opioid epidemic types and selected representative states in the USA are shown in figure 2. In 2017, fentanyl overwhelmingly dominated the east coast. Mixed epidemics of prescription opioids and fentanyl and of heroin and fentanyl predominated in the midwest. Non-contiguous states in the midwestern and western areas were primarily characterised by prescription opioid epidemics, except for the southwest and Pacific Northwest, which had a mixture of prescription opioid and heroin epidemics. Alaska and Hawaii had prescription opioid-dominated epidemics.
Figure 2: Map of opioid epidemic types and representative states selected for modelling across the USA, 2017.
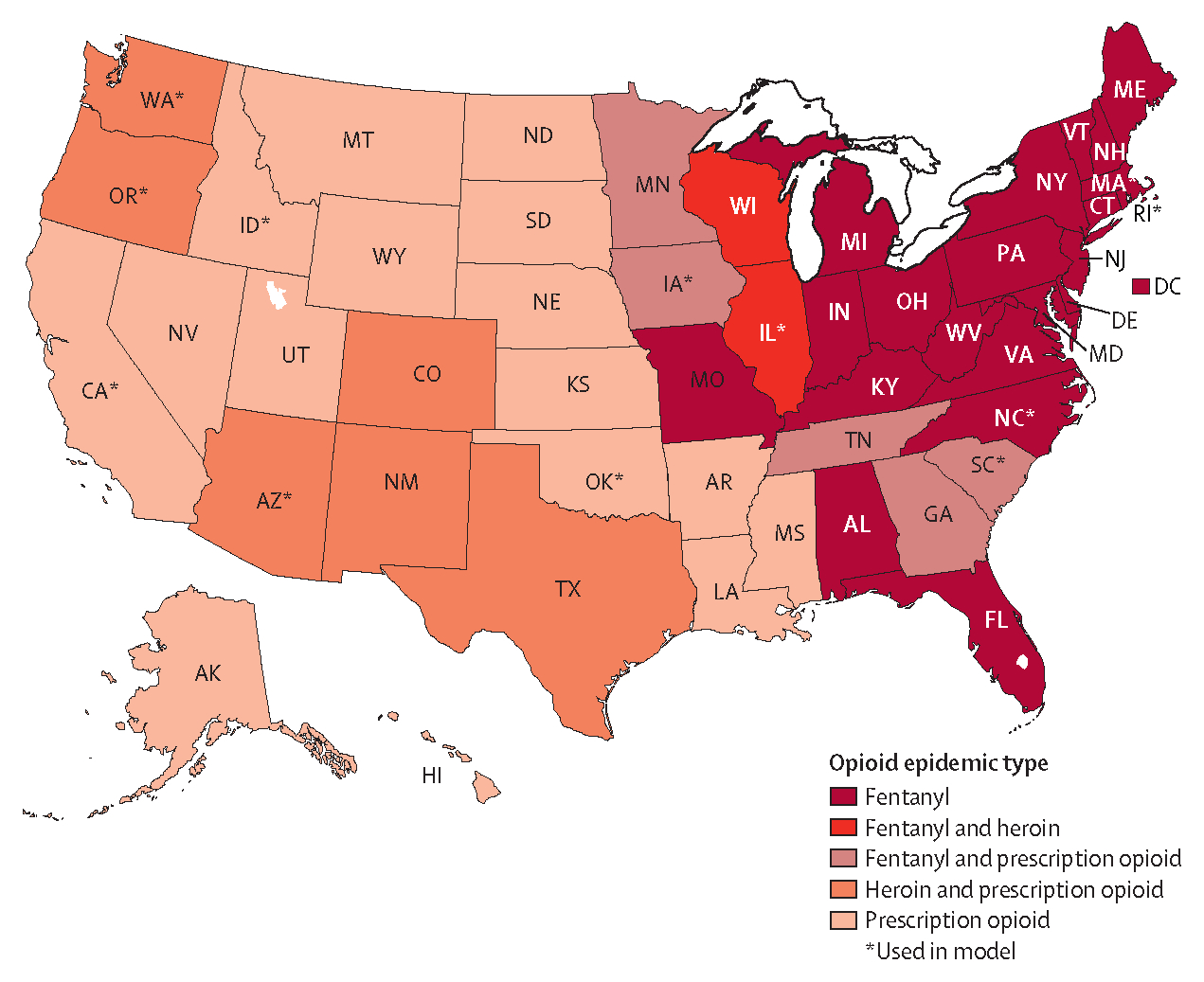
State abbreviations are shown in place of full names.
Across a range of community-based kit distribution volumes, our models indicated that the probability of naloxone use in witnessed overdoses and the number of deaths averted varied greatly by opioid epidemic type (figure 3). Generally, fentanyl-dominated epidemics had the highest estimated number of deaths due to opioid overdose averted (eg, Rhode Island had an estimated 12 deaths averted [95% Crl 6–18] per 100 000 people in 2017 with naloxone distribution compared with no community-based naloxone distribution). Given the observed 2017 kit distribution, the highest estimated probability of naloxone use in witnessed overdoses was found in states with a fentanyl epidemic—ranging between 58–76% in our modelled states. By contrast, prescription opioid-dominated epidemics had lower probabilities of naloxone use in witnessed overdoses (ranging 0–20%; eg, Oklahoma had 12% naloxone use [95% Crl 5–23] and <1 death averted [95% Crl 0–1] per 100 000) than did the other epidemic types. The pattern was more varied in the fentanyl-mixed and heroin-prescription opioid epidemic types, for which both high (Arizona 93% [95% Crl 40–99]) and low (South Carolina 0% [0–0]) probabilities of naloxone use, at 2017 kit distribution levels, in witnessed opioid overdoses were estimated. Saturation was not directly observed for high amounts of naloxone kits distributed in any state.
Figure 3: Model-derived expected probability of naloxone use in the event of a witnessed opioid overdose and deaths averted for 0 to 1000 distributed naloxone two-dose kits (through community-based access points) per 100 000 total population per year for three modelled states within each of the four dominant opioid epidemic types.
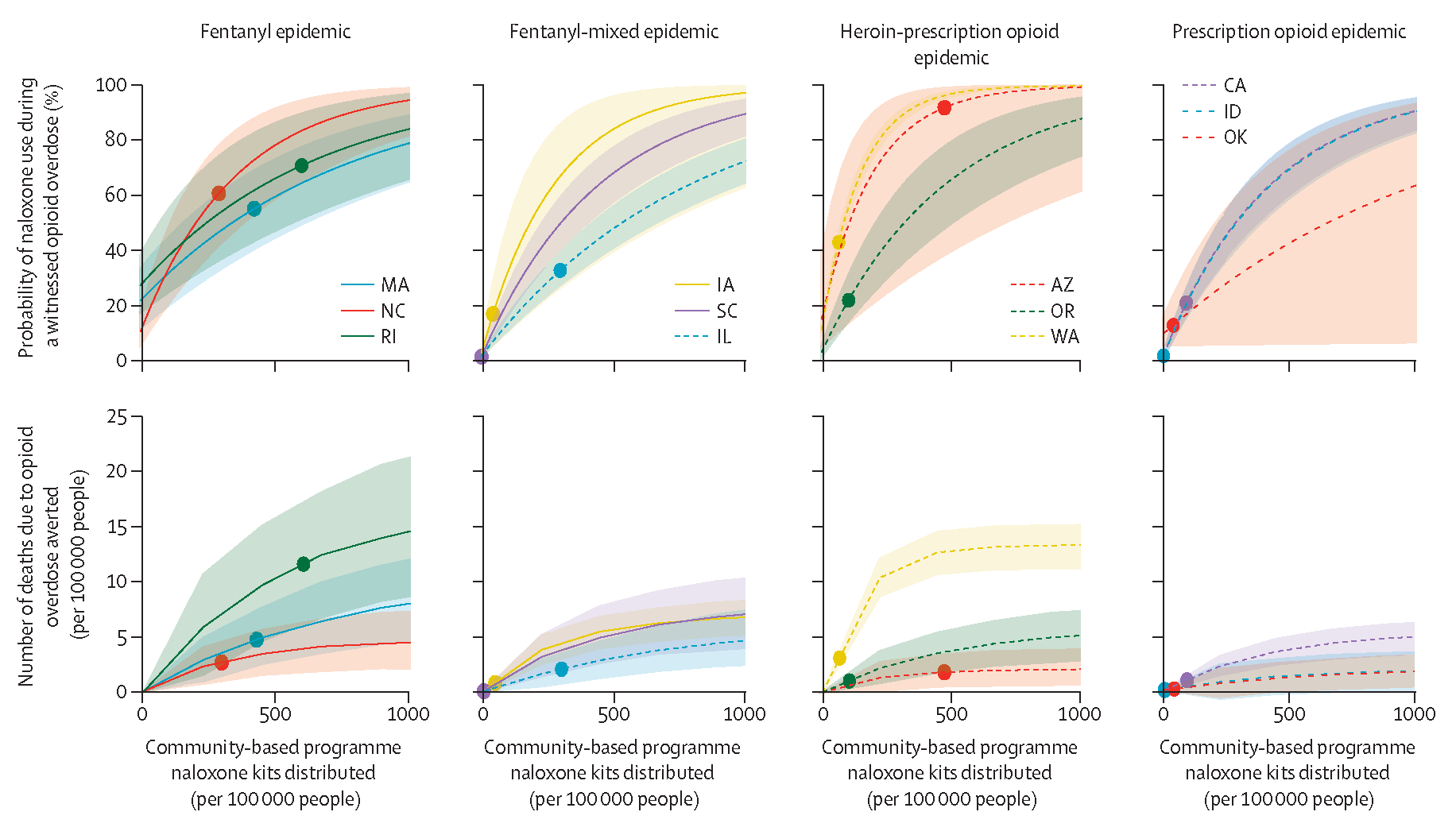
Datapoints are observed volumes of distributions of naloxone kits in 2017, with lines showing estimated probabilities of naloxone use and deaths due to opioid overdose averted. Shaded areas show 95% credible intervals. State abbreviations are shown in place of full names.
We did counterfactual modelling by shifting the kits distributed through each access point (ie, community-based programme, provider-prescribed, pharmacy-initiated kits) among representative states and estimating the total probability of naloxone use during a witnessed opioid overdose (figure 4). For example, in Arizona we estimated that increasing the number of provider-prescribed or pharmacy-initiated kits relative to 2017 distribution levels would not substantially affect the probability of naloxone use during a witnessed overdose; however, if community-based naloxone distribution decreased to zero, the probability of naloxone use in a witnessed overdose was estimated to be just 10%. The lowest probability of naloxone use for South Carolina occurred with no pharmacy-initiated kits distributed (2% [95% CrI 2–3]) because there were no community-based programmes distributing naloxone in 2017; however, this probability increased rapidly when other naloxone kit distribution points were considered. To reach a target of 60% probability of naloxone use, community-based programme distribution in South Carolina would need to distribute 390 kits per 100 000 people per year or the pharmacy-initiated programme would need to provide 800 kits per 100 000 people per year.
Figure 4: Model-derived expected probability of naloxone use in the event of a witnessed opioid overdose with respect to number of naloxone kits distributed, by naloxone source, per year, for 12 US states used in modelling.
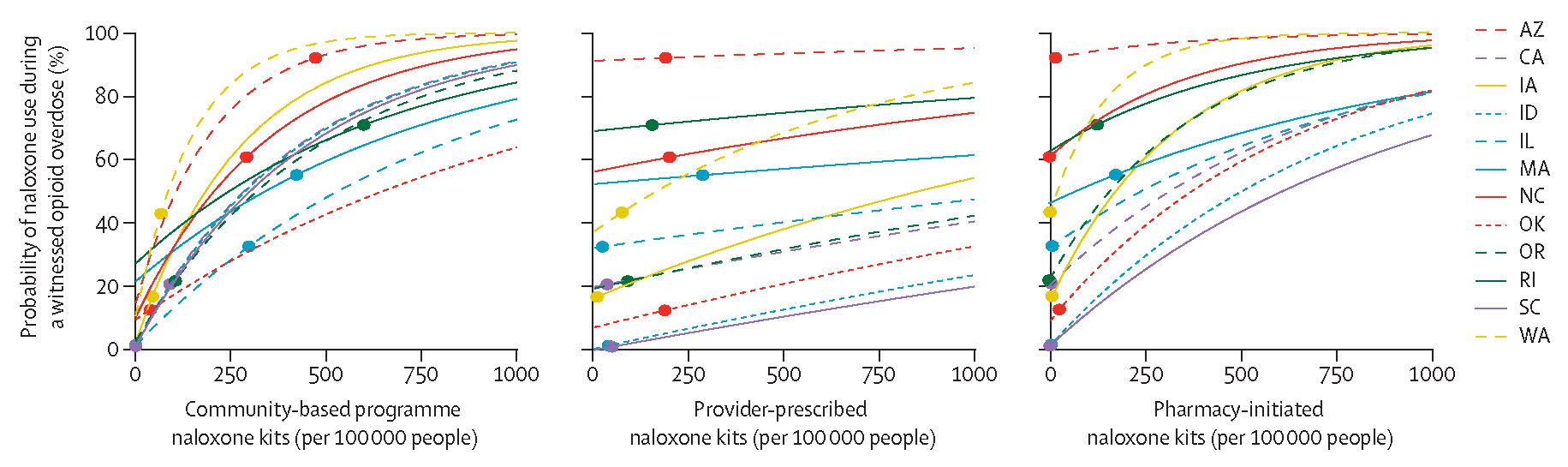
Datapoints are observed volumes of naloxone kits distributed in 2017, with lines showing estimated probability of naloxone use. 95% credible intervals are not shown here for clarity.
Other states with at least medium levels of distribution showed a similar distinction between effectiveness by distribution type. For example, Washington had an estimated probability of naloxone use of 42% (95% CrI 40–45) when no pharmacy-initiated kits were distributed, but this probability increased to 67% (64–70) after 82 pharmacy-initiated naloxone kits per 100 000 people were distributed. Washington also had an estimated 11% (10–12) naloxone use when no community-based kits were distributed, increasing to 49% (46–52) after distributing 80 community-based kits per 100 000 people per year. Increasing distribution of provider-prescribed kits had less of an effect on the probability of naloxone use, with an increase in probability of use from 37% (95% CrI 35–40) with no distribution to 44% (41–47) if 80 provider-prescribed kits per 100 000 people were distributed per year.
Because each model state had different levels of naloxone distribution in 2017, we estimated the total number of community-based kits required to avert deaths in 80% of all witnessed overdoses. For instance, in Massachusetts, an estimated 740 kits need to be distributed per 100 000 people to achieve this goal. In states where kits were already distributed widely, fewer additional kits were needed to achieve the intervention goal and in one state, Arizona, no additional kits were needed because the target of 80% was met. Among the 12 representative states, Illinois had the highest number of kits needed to achieve the 80% intervention goal, with 1270 total kits needed per 100 000 people per year (figure 3 and Naloxone Needed to Save website).
Estimated deaths averted and probability of naloxone use for 100, 500, and 1000 kits distributed per 100 000 people by distribution pathway for each non-model US state and for the District of Columbia are shown in the appendix (pp 16–23). For 100 kits per 100 000 people there is considerable range in the probability of naloxone use if distributed by community-based programmes (range 20–51%), provider-prescribed (4–14%), and pharmacy-initiated pathways (20–51%). Ranges in use narrowed when naloxone kits distributed by community-based (range 89–100%) and pharmacy-initiated pathways (89–100%) were modelled at thresholds of 1000 kits per 100 000 people, but remained variable for prescriber-based pathways (35–76%; data are available on Naloxone Needed To Save website and in the appendix [pp 16–23]). Across state-specific results, naloxone kits provided through community-based and pharmacy-initiated access points resulted in higher probability of naloxone use in witnessed overdose and a higher number of deaths averted per 100 000 population than did provider-prescribed access points (data are available on Naloxone Needed to Save website and in the appendix [pp 16–23]).
Discussion
To our knowledge, this is a first-of-its-kind assessment of the current and projected effect of naloxone distribution across the USA. We found that almost all US states have underdeveloped naloxone distribution efforts and that few are able to avert 80% of witnessed deaths due to opioid overdose with naloxone. Our models indicate that community-based and pharmacy-initiated naloxone distribution pathways have a larger public health effect in terms of deaths averted and potential for naloxone intervention than does an approach reliant only on prescriber-based naloxone access. Naloxone saturation, although not observed here, is possible. Our study adds to the increasing evidence that expanding naloxone access, in combination with other prevention and harm reduction initiatives, can have a substantial impact on the overdose epidemic. A recent modelling study found that a coordinated effort to increase initiation of medications for opioid use disorders, as well as retention and naloxone distribution, was required to reduce overdose deaths.29 To facilitate use of these findings, we developed an interactive website, Naloxone Needed to Save, that displays model outputs for each state by naloxone access pathway.
Using our model, we estimated the current and potential future impact of naloxone distribution; however, care should be taken when interpreting the explored counterfactual scenarios. The probability of naloxone use and the number of deaths due to overdose averted were found to vary by opioid epidemic type. The probability of naloxone use is a product of naloxone availability at the time of overdose and whether the overdose is witnessed, whereas the number of deaths due to overdose averted further includes the rate of overdose and the survivability of an unattended overdose. If only the number of total deaths due to overdose averted is examined, states with a fentanyl-dominated drug supply might appear to have a highly effective intervention simply because of the relatively higher probability of death after fentanyl-involved overdose. Therefore, we examined the probability of naloxone use and deaths due to overdose averted together when determining intervention effect.
Our analysis indicated varying effectiveness of naloxone by distribution pathway; however, when total distribution of naloxone is low, distribution through any mechanism can have a large effect on the probability of naloxone use during a witnessed overdose. Our model further indicated that when naloxone distribution is high, it is more effective to increase community-based and pharmacy-initiated distribution approaches than provider-initiated distribution approaches. Our findings on effects of high-volume community-based pathways that prioritise those at greatest risk of overdose are corroborated by data from Arizona.30 Known but addressable barriers to expanding these pathways include funding constraints, overly restrictive laws around naloxone access through pharmacies, and social stigma around obtaining naloxone.31–34 Inherent to the more effective community-based naloxone pathway is heavy reliance on peer social networks of people who use drugs, who are often undervalued in this context as being first responders. Communities could consider provision of mental health and crisis support for these responders and reducing or removing barriers to access to community naloxone to convey the value of services provided.
Although we calculated substantial potential public health effects of expanded naloxone distribution, because there is no effect of naloxone in an unwitnessed overdose, and using opioids alone is a risk factor for fatal overdose, interventions aimed at increasing the probability of witnessing an overdose are needed. Services such as Never Use Alone, supervised consumption sites, or harm reduction practices (eg, taking turns or partnering with others during use episodes) might increase the probability that an overdose is witnessed and potentially intervened on with naloxone.35,36 Finally, although naloxone access is fundamental to addressing immediate needs to reduce the harm of drug use in a community, underlying structural factors and social determinants deserving of investment exist that give rise to and perpetuate overdose risk, such as excessive incarceration, unjust drug laws, and structural racism. Increasing distribution of naloxone cannot be at the expense of re-examining these roadblocks; efforts need to be synergistic. For example, when a community is determining its naloxone strategy, the proportion and community burden of deaths due to overdose in specific subgroups, such as among Black people and minority ethnic groups, should be incorporated.37
Our model had several limitations. We assumed a saturation in naloxone distribution, which might have led to differences in the estimation of its effect when considering different levels of distribution. Some key assumptions were necessary considering the survivability of specific events and we sought expert input from a modified Delphi panel to inform these decisions. Finally, data incorporated were from 2017, and rates of overdose have now increased in many locations and epidemic types have evolved.38
Our model relied on data from community-based naloxone programmes, estimated overdose events, and estimated deaths due to overdose. Because of the paucity of these data, we drew on expert opinion and incorporated a large amount of uncertainty into our final estimates within the parameter prior probabilities. Furthermore, we note that some jurisdictions under-report deaths due to overdose; therefore, state-specific calculations might underestimate the need for naloxone. With more accurate and timely reporting of these data, we could reduce uncertainty in our estimates and uncover and address potential biases that might affect our model estimates. A consistent national framework for reporting opioid overdoses and cataloguing community-based and pharmacy-initiated naloxone distribution would enable better comparison between states and further support allocation of resources. Finally, we encourage other countries to employ mathematical modelling to help inform policy-relevant questions about naloxone need. Although our model structure itself is fairly general, the specific model priors and data that inform such models need to be gathered at geographically-specific and epidemic-specific level. The context-dependent nature of these models makes them inherently complex and are best informed by close alignment and involvement of harm reduction organisations and people who use drugs to promote valid model structure and interpretation.
Supplementary Material
Research in context.
Evidence before this study
Naloxone has been a highly effective evidence-based tool to reduce opioid overdose-related mortality and morbidity. In 2017, the US Department of Health and Human Services declared the opioid crisis in the USA to be a public health emergency. The advent of illicitly manufactured fentanyl entering the illicit drug market, in both powder-based and pill-based drugs, led to an increase in opioid overdoses. We searched PubMed on April 22, 2021, for publications in English between Jan 1, 2015, and April 22, 2021, using the search terms “naloxone” AND “fentanyl” AND “overdose”, with no restrictions on geographical location. Of the 186 publications identified, none aimed to estimate the amount of naloxone needed to reduce overdoses across fentanyl, heroin, and prescription opioid epidemics within the USA.
Added value of this study
To our knowledge, this is the first study to estimate the naloxone need for the entire USA. Estimates for each state include the estimated number of deaths averted by each of the three main access points for naloxone, and the number of kits that need to be distributed to avert a targeted 80% of witnessed opioid overdose deaths. Additionally, the data collection and parameterisation method provide programme-based and expert-driven estimates of the current extent and impact of naloxone access mechanisms.
Implications of all the available evidence
Our results highlight that current naloxone access pathways in most US states are underdeveloped. Additionally, we estimate that community-based programmes and pharmacy-initiation models provide the greatest effect per kit distributed compared with prescription-based programmes. We provide state-based targets of distribution to guide policy makers in their future investment in naloxone distribution efforts.
Acknowledgments
We acknowledge support from the National Institutes of Health, National Institute on Drug Abuse (grant number R01DAQ45745-01S1).
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Michael A Irvine, British Columbia Centre for Disease Control, Vancouver, BC, Canada; Health Sciences, Simon Fraser University, Burnaby, BC, Canada.
Declan Oller, Health Sciences, Simon Fraser University, Burnaby, BC, Canada, Providence, RI, USA.
Jesse Boggis, Heller School for Social Policy and Management, Brandeis University, Waltham, MA, USA.
Brian Bishop, University of Rhode Island College of Pharmacy, Kingston, RI, USA.
Daniel Coombs, Department of Mathematics, University of British Columbia, Vancouver, BC, Canada.
Eliza Wheeler, Department of Mathematics, University of British Columbia, Vancouver, BC, Canada, San Francisco, CA, USA.
Maya Doe-Simkins, Department of Mathematics, University of British Columbia, Vancouver, BC, Canada, Chicago, IL, USA.
Alexander Y Walley, Boston Medical Center, Crosstown Center, Boston, MA, USA.
Brandon D L Marshall, Brown University School of Public Health, Providence, RI, USA.
Jeffrey Bratberg, University of Rhode Island College of Pharmacy, Kingston, RI, USA.
Traci C Green, Brown University School of Public Health, Providence, RI, USA; Heller School for Social Policy and Management, Brandeis University, Waltham, MA, USA.
Data sharing
The datasets analysed during the current study are not publicly available. To request access, contact the corresponding author. The data dictionary and de-identified non-proprietary data will be made available as applicable with a signed data access agreement and a protocol approved by an institutional review board.
References
- 1.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2016; 65: 1445–52. [DOI] [PubMed] [Google Scholar]
- 2.Arias E, Tejada-Vera B, Ahmad F, Kochanek KD. Provisional life expectancy estimates for 2020. In: Vital statistics rapid release; report no 15. Hyattsville, MD: National Center for Health Statistics, July, 2021. https://www.cdc.gov/nchs/data/vsrr/vsrr015-508.pdf (accessed June 3, 2021). [Google Scholar]
- 3.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2016; 64: 1378–82. [DOI] [PubMed] [Google Scholar]
- 4.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths – 27 states, 2013–2014. MMWR Morb Mortal Wkly Rep 2016; 65: 837–43. [DOI] [PubMed] [Google Scholar]
- 5.Carroll JJ, Marshall BDL, Rich JD, Green TC. Exposure to fentanyl-contaminated heroin and overdose risk among illicit opioid users in Rhode Island: a mixed methods study. Int J Drug Policy 2017; 46: 136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson AB, Gladden RM, Delcher C, et al. Increases in fentanyl-related overdose deaths - Florida and Ohio, 2013–2015. MMWR Morb Mortal Wkly Rep 2016; 65: 844–49. [DOI] [PubMed] [Google Scholar]
- 7.Compton WM, Volkow ND, Throckmorton DC, Lurie P. Expanded access to opioid overdose intervention: research, practice, and policy needs. Ann Intern Med 2013; 158: 65–66. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services. Naloxone: the opioid reversal drug that saves lives: how healthcare providers and patients can better utilize this life-saving drug. US Department of Health and Human Services. https://www.hhs.gov/opioids/sites/default/files/2018-12/naloxone-coprescribing-guidance.pdf (accessed June 3, 2021). [Google Scholar]
- 9.US Food and Drug Administration. FDA requiring labeling changes for opioid pain medicines, opioid use disorder medicines regarding naloxone July, 23, 2020. https://www.fda.gov/news-events/press-announcements/fda-requiring-labeling-changes-opioid-painmedicines-opioid-use-disorder-medicines-regarding#:~:text=The%20U.S.%20Food%20and%20Drug,availability%20of%20naloxone%20with%20patients (accessed June 3, 2021).
- 10.Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002–2013. MMWR Morb Mortal Wkly Rep 2015; 64: 719–25. [PMC free article] [PubMed] [Google Scholar]
- 11.Baca CT, Grant KJ. Take-home naloxone to reduce heroin death. Addiction 2005; 100: 1823–31. [DOI] [PubMed] [Google Scholar]
- 12.Green TC, Davis C, Xuan Z, Walley AY, Bratberg J. Laws mandating coprescription of naloxone and their impact on naloxone prescription in five US states, 2014–2018. Am J Public Health 2020; 110: 881–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Substance Abuse and Mental Health Services Administration. Opioid overdose prevention toolkit. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. [Google Scholar]
- 14.Office of the Surgeon General. U.S. Surgeon General’s advisory on naloxone and opioid overdose. US Department of Health & Human Services, 2018. https://www.hhs.gov/surgeongeneral/priorities/opioids-and-addiction/naloxone-advisory/index.html (accessed June 3, 2021). [Google Scholar]
- 15.Adams AJ, Weaver KK. The continuum of pharmacist prescriptive authority. Ann Pharmacother 2016; 50: 778–84. [DOI] [PubMed] [Google Scholar]
- 16.Wenger LD, Showalter D, Lambdin B, et al. Overdose education and naloxone distribution in the San Francisco county jail. J Correct Health Care 2019; 25: 394–404. [DOI] [PubMed] [Google Scholar]
- 17.Lambdin BH, Bluthenthal RN, Wenger LD, et al. Overdose education and naloxone distribution within syringe service programs - United States, 2019. MMWR Morb Mortal Wkly Rep 2020; 69: 1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irvine MA, McGowan R, Hammond K, Davison C, Coombs D, Gilbert M. The role of mathematical modelling in aiding public health policy decision-making: a case study of the BC opioid overdose emergency. Int J Drug Policy 2021; 88: 102603. [DOI] [PubMed] [Google Scholar]
- 19.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med 2013; 158: 1–9. [DOI] [PubMed] [Google Scholar]
- 20.Irvine MA, Buxton JA, Otterstatter M, et al. Distribution of take-home opioid antagonist kits during a synthetic opioid epidemic in British Columbia, Canada: a modelling study. Lancet Public Health 2018; 3: e218–25. [DOI] [PubMed] [Google Scholar]
- 21.Irvine MA, Kuo M, Buxton JA, et al. Modelling the combined impact of interventions in averting deaths during a synthetic-opioid overdose epidemic. Addiction 2019; 114: 1602–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carta A, Conversano C. On the use of Markov models in pharmacoeconomics: pros and cons and implications for policy makers. Front Public Health 2020; 8: 569500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Recomm Rep 2016; 65: 1–49. [DOI] [PubMed] [Google Scholar]
- 24.Mital S, Wolff J, Carroll JJ. The relationship between incarceration history and overdose in North America: a scoping review of the evidence. Drug Alcohol Depend 2020; 213: 108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiSalvo P, Cooper G, Tsao J, et al. Fentanyl-contaminated cocaine outbreak with laboratory confirmation in New York City in 2019. Am J Emerg Med 2021; 40: 103–05. [DOI] [PubMed] [Google Scholar]
- 26.Hughto JMW, Gordon LK, Stopka TJ, et al. Understanding opioid overdose risk and response preparedness among people who use cocaine and other drugs: mixed-methods findings from a large, multi-city study. Subst Abus 2021; published online July 6. 10.1080/08897077.2021.1946893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman MD, Gelman A. The No-U-Turn sampler: adaptively setting path lengths in Hamiltonian Monte Carlo. J Mach Learn Res 2014; 15: 1593–623. [Google Scholar]
- 28.Salvatier J, Wiecki TV, Fonnesbeck C. Probabilistic programming in Python using PyMC3. PeerJ Comput Sci 2016; 2: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linas BP, Savinkina A, Madushani RWMA, et al. Projected estimates of opioid mortality after community-level interventions. JAMA Netw Open 2021; 4: e2037259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyerson BE, Moehling TJ, Agley JD, Coles HB, Phillips J. Insufficient access: naloxone availability to laypeople in Arizona and Indiana, 2018. J Health Care Poor Underserved 2021; 32: 819–29. [DOI] [PubMed] [Google Scholar]
- 31.Guadamuz JS, Alexander GC, Chaudhri T, Trotzky-Sirr R, Qato DM. Availability and cost of naloxone nasal spray at pharmacies in Philadelphia, Pennsylvania, 2017. JAMA Netw Open 2019; 2: e195388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoniou T, McCormack D, Campbell T, et al. Geographic variation in the provision of naloxone by pharmacies in Ontario, Canada: a population-based small area variation analysis. Drug Alcohol Depend 2020; 216: 108238. [DOI] [PubMed] [Google Scholar]
- 33.Cone DC, Bogucki S, Burns K, et al. Naloxone use by emergency medical services during the COVID-19 pandemic: a national survey. J Addict Med 2020; 14: e369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunlop A, Lokuge B, Masters D, et al. Challenges in maintaining treatment services for people who use drugs during the COVID-19 pandemic. Harm Reduct J 2020; 17: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauly B, Wallace B, Pagan F, et al. Impact of overdose prevention sites during a public health emergency in Victoria, Canada. PLoS One 2020; 15: e0229208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris MD, Bates A, Andrew E, Hahn J, Page K, Maher L. More than just someone to inject drugs with: injecting within primary injection partnerships. Drug Alcohol Depend 2015; 156: 275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolen S, Zang X, Chatterjee A, et al. Community-based naloxone coverage equity for the prevention of opioid overdose fatalities in racial/ethnic minority communities in Massachusetts and Rhode Island. Addiction 2021; published online Nov 25. 10.1111/add.15759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciccarone D The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr Opin Psychiatry 2021; 34: 344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are not publicly available. To request access, contact the corresponding author. The data dictionary and de-identified non-proprietary data will be made available as applicable with a signed data access agreement and a protocol approved by an institutional review board.


