Abstract
The combination of systemic amyloid A (AA) amyloidosis and xanthogranulomatous pyelonephritis (XGP) resulting from a chronic urinary tract infection is extremely rare. We herein report a case of systemic AA amyloidosis secondary to XGP for which clinical remission developed after nephrectomy. To our knowledge, this is the first case report describing the clinical improvement of systemic AA amyloidosis secondary to XGP after nephrectomy in Japan. Clinicians should be aware of this uncommon combination and search for amyloid depositions in cases of XGP.
Keywords: systemic AA amyloidosis, xanthogranulomatous pyelonephritis, serum amyloid A
Introduction
Amyloidosis is characterized by the deposition of insoluble amyloid fibrils formed by disease-specific precursor proteins in the extracellular interstitium of various organs throughout the body, resulting in organ damage. The stable β-sheet structure of amyloid deposits exhibits a unique green birefringence by polarized light microscopy when stained with Congo red dye. Once amyloid is found, the protein type is determined by immunohistochemistry. Forty-two types of amyloidosis have been identified and classified based on the type of individual precursor protein (1). Amyloid A (AA) amyloidosis occurs in the setting of chronic inflammatory or infectious diseases.
Xanthogranulomatous pyelonephritis (XGP) is a relatively rare form of chronic pyelonephritis characterized by the formation of inflammatory renal masses rich in macrophages loaded with lipids (2). The association of XGP and systemic AA amyloidosis is infrequent. To our knowledge, however, few reports have described the clinical course of AA amyloidosis after treating XGP.
We herein report a rare case of systemic AA amyloidosis caused by XGP with refractory anorexia and diarrhea in which the gastrointestinal symptoms dramatically improved after nephrectomy.
Case Report
A 67-year-old Japanese woman was referred to our hospital for anorexia and watery diarrhea that had occurred several times daily in the past 2 months. She had a history of myasthenia gravis, a left renal tumor, paroxysmal atrial fibrillation, osteoporosis, and chronic kidney disease stage G4A3 of unknown cause. Her recent history was significant for admission twice to our hospital one year prior because of left ureteral stones with pyelonephritis. Her medications included prednisolone 6 mg, tacrolimus hydrate 3 mg, famotidine 10 mg, bisoprolol fumarate 5 mg, azosemide 30 mg, apixaban 5 mg, alendronate sodium hydrate 5 mg, alfacalcidol 0.25 μg, and rupatadine fumarate 10 mg.
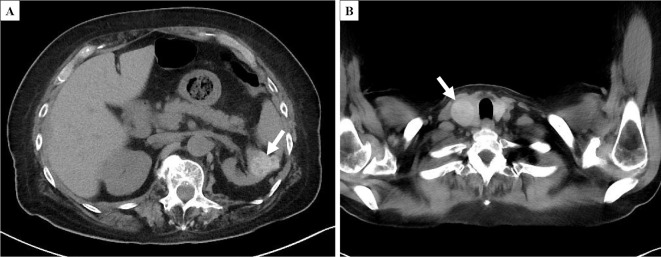
At the current admission, the patient was 152.8 cm tall and weighed 67.4 kg, approximately 10 kg less than 2 months earlier. Her temperature was 36.3°C, pulse 61 beats per minute, blood pressure 90/67 mmHg, and oxygen saturation 100% on ambient air. She appeared mildly unwell, and physical examination showed dry mouth and bilateral pitting edema; other physical parameters, including joint swelling and tenderness, were within normal limits. Laboratory studies demonstrated anemia, renal dysfunction, hypokalemia, hypoalbuminemia, proteinuria, hematuria, and an elevated C-reactive protein concentration. Protein fractionation did not include M protein, and serologic markers of collagen disease, except for anti-nuclear antibody, were negative. Although the levels of matrix-metalloproteinase 3 and serum amyloid A (SAA) were elevated, the anti-cyclic citrullinated peptide antibody was negative. Other clinical laboratory data are summarized in Table. The urine culture grew Escherichia coli with a colony count greater than 105/mm3; the blood culture was sterile. Computed tomography (CT) revealed a left kidney tumor and a right thyroid tumor, with no marked changes in size compared to six months prior (Fig. 1). Echocardiography revealed left ventricular hypertrophy with a normal systolic function and diastolic dysfunction, represented by a reduced early transmitral flow.
Table.
Laboratory Data on Admission to Our Hospital.
| Blood count | Serum chemistry | ||||
| WBC | 8,300 | /µL | TP | 5.5 | g/dL |
| Neu | 86.5 | % | Alb | 2.1 | g/dL |
| Ba | 0.1 | % | Protein fractionation | ||
| Eo | 0.2 | % | Alb% | 39.5 | % |
| Ly | 9.8 | % | α1-G% | 6.2 | % |
| Mo | 3.4 | % | α2-G% | 18.8 | % |
| RBC | 363 | ×104/µL | β1-G% | 4.6 | % |
| Hb | 9.8 | g/dL | β2-G% | 6.2 | % |
| Ht | 29.3 | % | γ-G% | 24.7 | % |
| Plt | 38.6 | ×104/µL | M Protein | (-) | |
| Urinalysis | BUN | 54 | mg/dL | ||
| Protein | (2+) | Cr | 4.7 | mg/dL | |
| Occult blood | (3+) | UA | 15.9 | mg/dL | |
| Urinary sediment | Na | 138 | mEq/L | ||
| RBC | 20-29 | /HPF | K | 2.5 | mEq/L |
| WBC | 100↑ | /HPF | Cl | 110 | mEq/L |
| Pro/Cr | 1.74 | g/gCr | Ca | 7.3 | mg/dL |
| Na(u) | 29 | mEq/L | P | 7.6 | mg/dL |
| Cr(u) | 145.6 | mg/dL | AST | 20 | U/L |
| FENa | 0.7 | % | ALT | 27 | U/L |
| LDH | 552 | U/L | |||
| ALP | 116 | U/L | |||
| Immunological findings | |||||
| CRP | 3.59 | mg/dL | FT4 | 0.74 | ng/dL |
| FT3 | 1.55 | pg/mL | TSH | 2.74 | μIU/mL |
| SS-A | Negative | IgG | 1,510 | mg/dL | |
| SS-B | Negative | IgA | 153 | mg/dL | |
| Scl-70 | <1.0 | U/mL | IgM | 80 | mg/dL |
| CENPB | 7.3 | U/mL | IgG4 | 181 | mg/dL |
| Sm | Negative | CH50 | 63.8 | U/mL | |
| Jo-1 | <1.0 | U/mL | ANA | 1,280 | index |
| RF | 7 | IU/mL | MMP-3 | 419.4 | ng/mL |
| ACPA | 1.4 | U/mL | MPO-ANCA | 11.1 | U/mL |
| dsDNA (RIA) | <2.0 | IU/mL | PR3-ANCA | <1.0 | U/mL |
| UI-RNP | Negative | RNAP3 | Negative | ||
| ARS | Negative | Amyloid A | 248 | μg/mL | |
Figure 1.
CT (A: left kidney tumor; B: right thyroid tumor). A left kidney tumor with a well-defined border and a mixture of high- and low-density areas (A, arrow) and a right thyroid tumor (B, arrow) with no marked changes in size since follow-up CT six months prior.
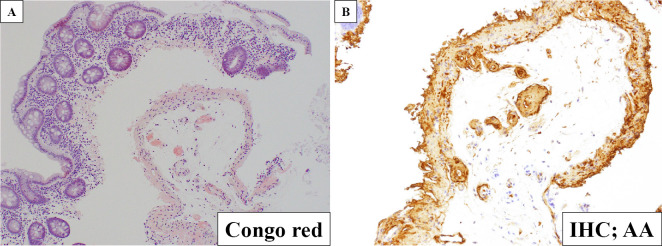
Despite two weeks of conservative management, the patient's symptoms persisted, and her nutritional condition worsened. Lower gastrointestinal endoscopy was performed to search for the cause of her symptoms. In the specimen obtained from the lower gastrointestinal tract, AA deposits were identified by Congo red staining and immunohistochemical staining with anti-AA antibody, indicating intestinal AA amyloidosis (Fig. 2).
Figure 2.
Histological findings in the lower gastrointestinal tract (A: Congo red staining; B: immunostaining with anti-AA antibody). Amyloid was deposited in the specimen obtained by the lower gastrointestinal tract and showed positive immunostaining with anti-AA antibody, indicating AA amyloidosis. Magnification: ×10. IHC: immunohistochemistry
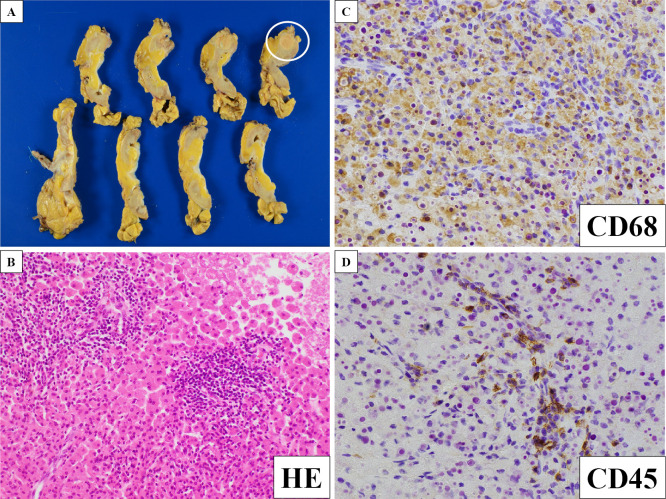
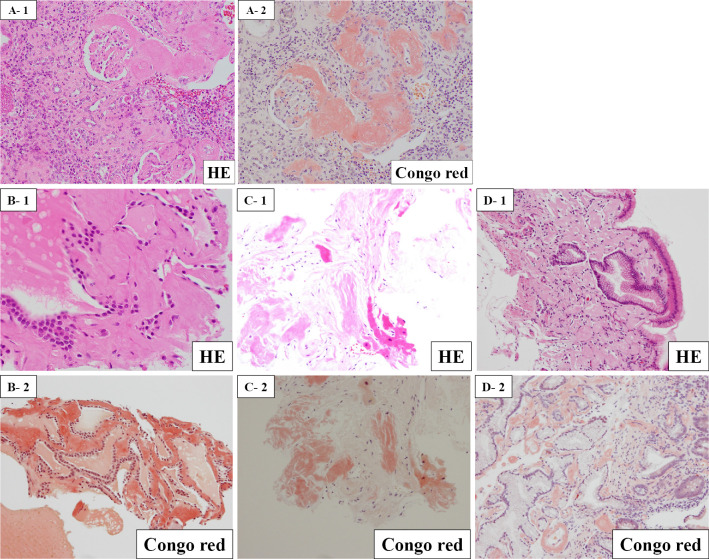
A consultation with collagen specialists did not lead to a diagnosis of rheumatoid arthritis or other collagen diseases. Because the renal tumor was suspicious for the underlying illness of AA amyloidosis, the patient underwent left nephrectomy three months after admission. A yellowish nodule measuring 1.8×1.8×2.0 cm was apparent on the cut surface. A histopathologic examination revealed xanthogranulomatous kidney inflammation with abundant lipid-laden foamy macrophages and lymphocytes (Fig. 3). The residual renal parenchyma showed extensive amyloid depositions in the mesangium and vascular walls (Fig. 4). Furthermore, amyloid deposition was revealed in the thyroid, myocardium, and stomach tissues by biopsies (Fig. 4). These histopathologic findings confirmed the diagnosis of systemic AA amyloidosis.
Figure 3.
Histological findings at left nephrectomy (A: macroscopic findings; B: Hematoxylin and Eosin staining; C: immunostaining with anti-CD68 antibody; D: immunostaining with anti-CD45 antibody). A: A yellowish nodule measuring 1.8×1.8×2.0 cm was present on the cut surface. B-D: This examination revealed xanthogranulomatous kidney inflammation with abundant lipid-laden foamy macrophages and lymphocytes. CD68 is a marker of macrophages, and CD45 is a marker of lymphocytes. Magnification: ×10.
Figure 4.
Histological findings after the biopsy (A: the residual renal parenchyma; B: thyroid; C: myocardium; D: stomach). A: Amyloid deposited in the mesangium and vascular walls of the residual renal parenchyma. B-D: Amyloid deposited in the thyroid, myocardium, and stomach tissues.
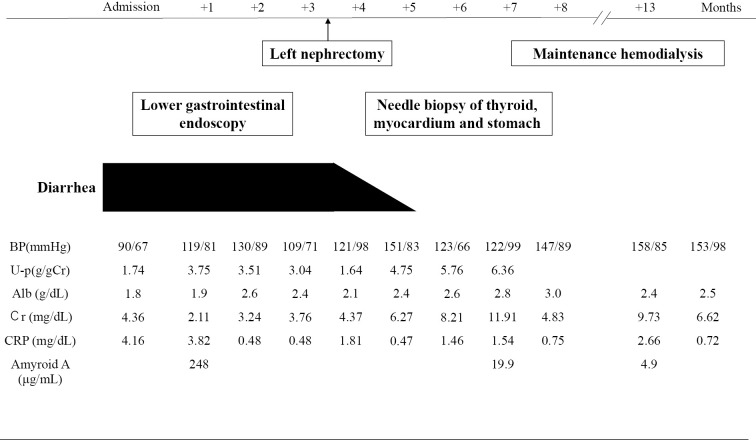
The patient's diarrhea condition dramatically improved with the decreased concentrations of SAA, although her renal function deteriorated progressively due to amyloid deposition in the residual kidney, and maintenance hemodialysis was initiated at four months postoperatively (Fig. 5).
Figure 5.
Clinical course of the patient after the onset of diarrhea.
Discussion
We herein report a rare case of systemic AA amyloidosis secondary to XGP. XGP is an uncommon, severe, chronic suppurative process characterized by the destruction and replacement of the renal parenchyma with granulomatous tissue that contains lipid-laden macrophages intermixed with lymphocytes, giant cells, and plasma cells on a microscopic examination (2). The primary factors involved in the pathogenesis of this process are nephrolithiasis, obstruction, previous urologic procedures, and chronic urinary tract infection (3). In a New Zealand study of 35 patients assessed from 2001-2013, where 91% of the patients were women, staghorn calculi occurred in 51.4%, obstructing ureteric calculi were present in 22.9%, and the most common organisms isolated were E. coli (46%) and Proteus mirabilis (20%) (4). Other related factors include abnormal lipid metabolism, arterial circulatory failure, venous obstruction, steroid use, and an abnormal immune response associated with underlying diseases, such as diabetes mellitus (5-7).
The most useful imaging modality in evaluating patients with XGP is CT, which successfully identifies the abnormality in 74-90% of cases; however, the diagnosis of segmental XGP without calculi is difficult on CT (8,9). The differential diagnosis of XGP includes malignancy and tuberculosis. The usual management is nephrectomy, whereas antimicrobial therapy has only a secondary role (10,11). In this case report, because making a definitive diagnosis by CT was difficult, nephrectomy was performed to confirm the presence of macrophage-charged lipids in inflamed areas.
AA amyloidosis is caused by the deposition of amyloid fibrils, which are composed of an 8-kDa, 76-amino-acid N-terminal portion of a 12-kDa precursor protein, SAA. These macromolecular deposits result in organ dysfunction by the cellular uptake of the oligomeric amyloid precursors, thus inducing toxicity in the target cells. The diagnosis of AA amyloidosis is based on clinical organ involvement and histological evidence of amyloid A deposits by polarized light microscopy stained with Congo red dye and immunohistochemistry. The involved organs can be biopsied in systemic amyloidosis, but amyloid deposits may be found in any body tissue. Furthermore, AA amyloidosis can be associated with almost any chronic inflammatory disease, neoplasm, or chronic infection.
A review of the literature uncovered a number of characteristics of AA amyloidosis. Lane described both a decreasing incidence of AA amyloidosis and better outcomes over the past 25 years, reflecting advances in the treatment and overall management of the complex chronic disease (12). Okuda et al. reported that underlying diseases of AA amyloidosis in Japan are rheumatoid arthritis (60.3%), uncharacterized inflammatory disorders (11.1%), neoplasms (7.0%), other rheumatic diseases (6.5%), inflammatory bowel diseases (4.5%), chronic infection (4.5%), Castleman disease (4.0%), and autoinflammatory diseases (2.0%) (13). Regarding the underlying chronic infections in AA amyloidosis, various respiratory infections have been reported, including tuberculosis, nontuberculous mycobacteria, and pulmonary aspergillosis, as well as other infections, including osteomyelitis and complicated urinary tract infection (14-17). Lachmann et al. reported that the median duration of symptomatic inflammatory disease before the diagnosis of amyloidosis was 17 years, and there were no significant differences in latency among the various underlying disorders (18).
The primary therapy in AA amyloidosis involves treating the underlying infectious disease. Tanaka et al. reported that gastrointestinal AA amyloidosis secondary to chronic pyelonephritis presenting with refractory diarrhea was dramatically improved by ureteral drainage and antibiotics (19). In the present case, the symptoms were dramatically improved after nephrectomy. Identifying the underlying disease of AA amyloidosis secondary to chronic infection is thus essential, as treating the infectious disease can cure AA amyloidosis.
The association of XGP and amyloidosis is infrequent. Bilbao Garay et al. estimates that amyloidosis complicates XGP in less than 1% of all XGP cases (20). Our search of PubMed articles published between 1986 and 2023 found only 12 patients with amyloidosis secondary to XGP. Of the 12 cases, only 2 patients presented with postnephrectomy courses of systemic AA amyloidosis secondary to XGP. Lauzurica et al. reported the case of a 38-year-old woman who presented with weight loss, anemia, and generalized edema. She had a history of renal colic without stone elimination one year previously. CT in that patient revealed multiple low-density areas in the left kidney with a pelvic stone. Left nephrectomy was performed, and the histopathologic diagnosis was XGP and AA renal amyloidosis. The general condition improved spectacularly and was stable with good general health for three years after the nephrectomy. In another case in the same report, a 67-year-old woman was admitted for paroxysmal dyspnea and general edema. A laboratory examination showed renal dysfunction and proteinuria, and CT revealed a large hydronephrotic kidney with calculi. Left XGP was suspected, and nephrectomy was performed. A histopathologic study showed XGP and renal AA amyloidosis. A rectal biopsy and fine-needle biopsy of the abdominal subcutaneous fat were also positive for AA. Her renal function stabilized, and proteinuria became negative after nephrectomy. At that time, a fine-needle biopsy of the abdominal fat and a rectal biopsy were repeated, and Congo red staining was negative. These reports are rare cases of type AA amyloidosis caused by XGP, in which removal of the renal tumor resulted in dramatic improvement of symptoms (21). Based on the course of the case presented herein, treatment of the underlying disease can inhibit the progression of amyloidosis.
No reports to date have shown an association between systemic AA amyloidosis secondary to XGP and the SAA level. Deposition of amyloid fibrils derived from circulating SAA causes systemic AA amyloidosis. SAA is an acute-phase apoprotein synthesized in the liver and transported by the high-density lipoprotein HDL3 in the plasma. Several years of an underlying inflammatory disease causing chronic elevation of SAA usually precede fibril formation. The concentration of SAA is strongly associated with the outcome in patients with AA amyloidosis. Lachmann et al. reported that amyloid deposits regressed in 60% of patients with a median SAA concentration of <10 mg/L, and the survival among these patients was superior to that among patients in whom amyloid deposits did not regress. Treatment that suppresses or eliminates inflammation or infection decreases the SAA concentration (18). In the present case, nephrectomy caused not only the improvement of diarrhea but also the normalization of the SAA concentration.
In conclusion, we reported a rare case of systemic AA amyloidosis secondary to XGP in which the gastrointestinal symptoms improved after nephrectomy. Although the association of amyloidosis and XGP is rare, clinicians should maintain an awareness of this uncommon combination and search for amyloid depositions in cases of XGP.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Buxbaum JN, Dispenzieri A, Eisenberg DS, et al. Amyloid nomenclature 2022: update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 29: 213-219, 2022. [DOI] [PubMed] [Google Scholar]
- 2.LE N. Urinary Tract Infection in Adults. Elsevier 1244, 2020. [Google Scholar]
- 3.Gregg CR, Rogers TE, Munford RS. Xanthogranulomatous pyelonephritis. Curr Clin Top Infect Dis 19: 287-304, 1999. [PubMed] [Google Scholar]
- 4.Addison B, Zargar H, Lilic N, Merrilees D, Rice M. Analysis of 35 cases of Xanthogranulomatous pyelonephritis. ANZ J Surg 85: 150-153, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Malek RS, Elder JS. Xanthogranulomatous pyelonephritis: a critical analysis of 26 cases and of the literature. J Urol 119: 589-593, 1978. [DOI] [PubMed] [Google Scholar]
- 6.Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine (Baltimore) 58: 171-181, 1979. [PubMed] [Google Scholar]
- 7.Lin HH, Chien CC, Fang JT, Lai RH, Huang CC. Unusual clinical presentation of Klebsiella pneumoniae induced endogenous endophthalmitis and xanthogranulomatous pyelonephritis in a non-nephrolithiasis and non-obstructive urinary tract. Ren Fail 24: 659-665, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Demertzis J, Menias CO. State of the art: imaging of renal infections. Emerg Radiol 14: 13-22, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Kim JC. US and CT findings of xanthogranulomatous pyelonephritis. Clin Imaging 25: 118-121, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Elkhammas EA, Mutabagani KH, Sedmak DD, Tesi RJ, Henry ML, Ferguson RM. Xanthogranulomatous pyelonephritis in renal allografts: report of 2 cases. J Urol 151: 127-128, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Gravestock P, Moore L, Harding C, Veeratterapillay R. Xanthogranulomatous pyelonephritis: a review and meta-analysis with a focus on management. Int Urol Nephrol 54: 2445-2456, 2022. [DOI] [PubMed] [Google Scholar]
- 12.Lane T, Pinney JH, Gilbertson JA, et al. Changing epidemiology of AA amyloidosis: clinical observations over 25 years at a single national referral centre. Amyloid 24: 162-166, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Okuda Y, Yamada T, Ueda M, Ando Y. First nationwide survey of 199 patients with amyloid A amyloidosis in Japan. Intern Med 57: 3351-3355, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deshayes S, Aouba A, Grateau G, Georgin-Lavialle S. Infections and AA amyloidosis: an overview. Int J Clin Pract 75: e13966, 2021. [DOI] [PubMed] [Google Scholar]
- 15.Torii R, Noguchi S, Shimabukuro I, et al. Pulmonary Mycobacterium abscessus infection with reactive AA amyloidosis: a case report and brief review of the literature. Intern Med 58: 557-561, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura S, Matsumae T, Murakami Y, et al. Chronic renal failure due to amyloid nephropathy caused by chronic infection after total hip replacement. CEN Case Rep 3: 217-222, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya Y, Ubara Y, Suwabe T, et al. AA-amyloidosis in autosomal dominant polycystic kidney disease caused by chronic cyst infections lasting for 30 years. Intern Med 52: 791-794, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med 356: 2361-2371, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka T, Naito T, Midori Y, et al. Gastrointestinal AA amyloidosis secondary to chronic pyelonephritis presenting with refractory diarrhea and severe hypoalbuminemia. Clin J Gastroenterol 14: 1642-1648, 2021. [DOI] [PubMed] [Google Scholar]
- 20.Bilbao Garay J, Zapatero Gaviria A, Domínguez Frajo P, Llorente Abarca C, Fernández Juárez G. [Amyloidosis secondary to xanthogranulomatous pyelonephritis: a case report and review of the literature]. Rev Clin Esp 206: 43-47, 2006. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 21.Lauzurica R, Felip A, Serra A, et al. Xanthogranulomatous pyelonephritis and systemic amyloidosis: report of 2 new cases and the natural history of this association. J Urol 146: 1603-1606, 1991. [DOI] [PubMed] [Google Scholar]