Abstract
Intrathecal chemotherapy is often administered for prophylaxis and treatment of central nervous system involvement in hematological malignancies. However, it may rarely cause neurotoxicity as a side effect.
We herein report a 74-year-old woman with diffuse large B-cell lymphoma including a spinal lesion. She received systemic and intrathecal chemotherapy. After five doses of intrathecal chemotherapy, she developed intrathecal chemotherapy-induced myelopathy. Intrathecal treatment was discontinued, and she was administered vitamin B12 and folic acid, along with steroid pulses. However, her symptoms did not improve.
Intrathecal chemotherapy-induced myelopathy is rare, but may be irreversible; therefore, clinicians should be aware of this potential complication.
Keywords: intrathecal chemotherapy, myelopathy, diffuse large B-cell lymphoma
Introduction
Intrathecal chemotherapy is often administered for prophylaxis and treatment of central nervous system (CNS) involvement in hematological malignancies. CNS relapse is a devastating and often fatal complication of malignant lymphoma. Patients with involvement of the nasal sinuses, testes, orbits, or bone marrow are considered to be at high risk for CNS relapse (1).
The CNS International Prognostic Index (CNS-IPI) is a robust, highly reproducible scoring system that can be used to estimate the risk of CNS relapse in patients with diffuse large B-cell lymphoma (DLBCL) (2). The risk of CNS relapse in the CNS-IPI high-risk group has been reported to be 10.2%, therefore, prophylactic intervention to prevent CNS involvement is considered in these patients (2).
The prophylactic strategy for CNS relapse remains controversial; however, intrathecal chemotherapy is a widely used method (3). The three most commonly used drugs in intrathecal chemotherapy regimens are methotrexate (MTX), cytosine arabinoside (Ara-C), and corticosteroids (3). Neurotoxicity attributed to intrathecal chemotherapy, including myelopathy, seizures, and encephalopathy, has been reported (4). Of these complications, myelopathy is of paramount importance, as it results in irreversible functional impairment.
We herein report a 74-year-old woman with DLBCL who developed intrathecal chemotherapy-induced myelopathy. In addition, pathophysiology and treatment strategies for this complication are also discussed.
Case Report
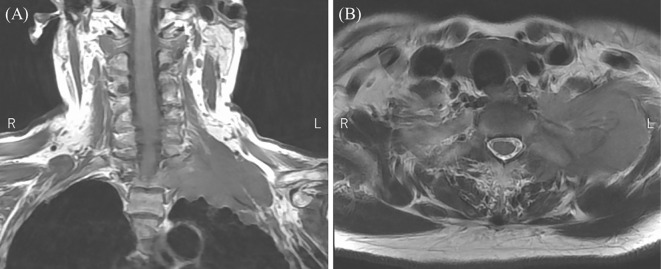
A 74-year-old woman visited another hospital with chief complaints of decreased grip strength and numbness in the left arm. Cervical spine magnetic resonance imaging (MRI) revealed a mass lesion extending from the left lung apex to the neck (Fig. 1). The patient was therefore referred to our hospital for further investigation.
Figure 1.
T2-weighted cervical spine MRI revealed a mass lesion from the left lung apex to the neck. (A) Coronal and (B) axial images.
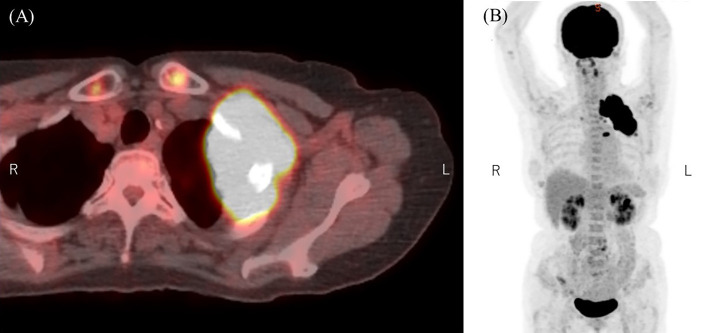
She was diagnosed with DLBCL based on the findings of a computed tomography (CT)-guided biopsy of the mass in the left lung apex. The lymphoma lesions extended to the left chest wall, ribs, and cervical spine. Positron emission tomography (PET)-CT revealed an increased 18F-fluorodeoxyglucose uptake by the bulky mass. However, no other lesions were observed (Fig. 2). The Ann Arbor Classification was stage IV, and the revised IPI score was 4 with elevated lactate dehydrogenase (LDH) levels. In addition to numbness and decreased grip strength in the left arm, she also exhibited left Horner syndrome, including left blepharophimosis, left miosis, and decreased sweating on the left side of the face, likely caused by brachial plexus involvement of the lung apex mass.
Figure 2.
PET-CT revealed an increased 18F-fluorodeoxyglucose uptake in the bulky mass (A) but no other lesions (B).
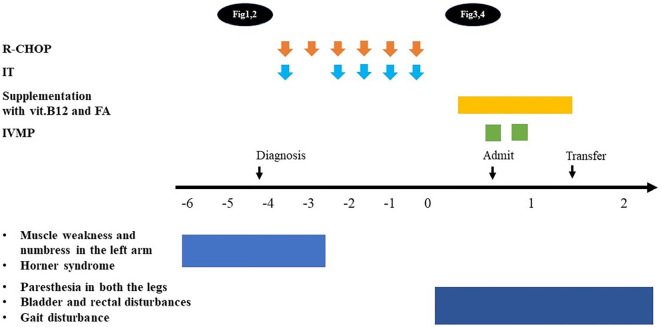
The patient was administered six cycles of R-CHOP (rituximab, doxorubicin, vincristine, cyclophosphamide, prednisolone) for the treatment of DLBCL. Cytology of the pretreatment cerebrospinal fluid (CSF) was negative, but she was considered to be at high risk for CNS invasion since the lesion extended to the spine. Therefore, she was treated with a combination of intrathecal chemotherapies (MTX, Ara-C, and dexamethasone) to prevent CNS relapse. After the commencement of chemotherapy, her symptoms in the left arm and Horner syndrome improved. Furthermore, a marked reduction in the mass size was evident on imaging.
However, four days after the fifth intrathecal injection, she developed urinary incontinence and paresthesia in both legs. She also reported loss of sensation during urination and defecation, muscle weakness, and decreased pain and temperature sensation in both the lower limbs. Six days later, she exhibited decreased deep sensation and loss of bilateral Achilles tendon reflexes. The patient was then referred to a neurologist for a thorough examination.
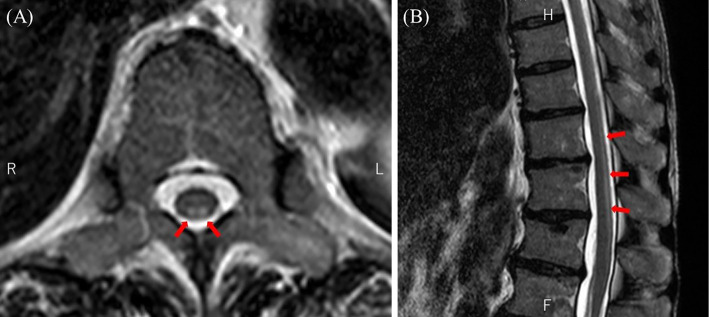
Thoracolumbar MRI showed a high-intensity signal in the inferior thoracic posterior cord on T2-weighted imaging (Fig. 3). Considering the possibility of subacute combined degeneration of the spinal cord (SCD), oral supplementation with vitamin B12 and folic acid was initiated. However, a blood examination prior to supplement initiation revealed only mildly low levels of folic acid (3.5 ng/mL) and no vitamin B12 deficiency (6,720 pg/mL). Serum copper levels and blood concentrations of MTX were normal (124 μg/dL and <0.04 μM, respectively), but serum homocysteine levels were elevated (26.9 nmol/mL). Even after the commencement of replacement therapy, the patient experienced worsening difficulty in walking; therefore, she was admitted to our hospital for a further investigation 21 days after the last R-CHOP and intrathecal chemotherapy session.
Figure 3.
Thoracolumbar MRI showed a high signal in the inferior thoracic posterior cord on T2-weighted imaging (arrows). (A) Axial and (B) sagittal images.
At the time of admission, she had already completed six cycles of R-CHOP and was in complete remission based on a CT evaluation (Fig. 4). Her consciousness was clear, and there were no remarkable abnormalities on a physical examination, other than the neurological findings. A neurological examination revealed abnormal sensation of the bilateral lower limbs with distal dominance. Tendon reflexes were decreased in the lower limbs, and the bilateral Babinski sign was positive. She could not walk without assistance, and her gait was wide-based. In the Manual Muscle Test, there was no pathological muscle weakness in either lower limb, and the cause of gait disturbance was thought to be sensory ataxia. A blood examination revealed no organ dysfunction or electrolyte abnormalities, except for a slight increase in the LDH value. In addition, her serum homocysteine levels had normalized. Antinuclear antibody, anti-Ro/SSA and La/SSB antibody, myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA), proteinase-3 anti-neutrophil cytoplasmic antibody (PR3-ANCA), and angiotensin converting enzyme were all negative. Human T-lymphotropic virus type I antibody and human immunodeficiency virus antibody were also negative. A CSF analysis revealed no elevated cells (2 /μL) or protein (43 mg/dL) and normal glucose levels (56 mg/dL). However, the level of myelin basic protein (MBP), a marker of CNS inflammation (7), was increased to 1,700 pg/mL. The concentration of MTX in CSF was <0.04 μM. A cytological examination of the CSF revealed no malignant cells. Spinal contrast-enhanced MRI showed a high-intensity signal involving the bilateral posterior cord of Th11-12 and the spinal cone on T2-weighted imaging, with no contrast effect.
Figure 4.
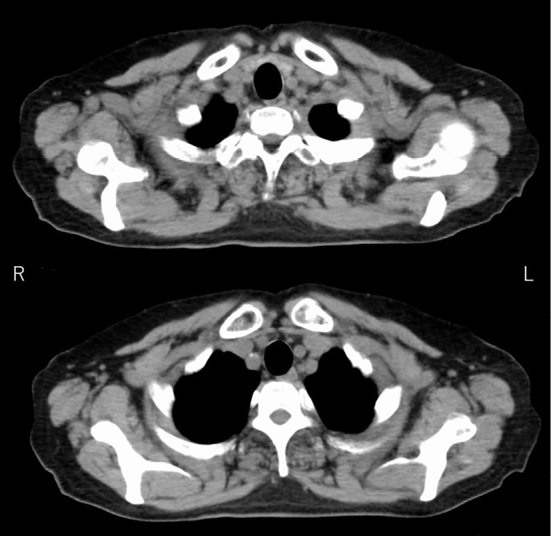
After six courses of R-CHOP, thoracoabdominal CT showed that the mass in the left pulmonary apex had disappeared.
Her neurological symptoms did not improve even after vitamin B12 and folic acid supplementation. Based on her clinical course, blood tests, CSF analysis, and MRI findings, we considered myelopathy associated with systemic inflammatory disease, autoimmune disease, or infection to be negative and diagnosed her with intrathecal chemotherapy-induced myelopathy. Steroid pulse (methylprednisolone 1 g/day for 3 days) was administered due to concerns of irreversible neurological damage. However, no obvious improvement in her gait or other neurological findings was observed, so the patient was transferred to another hospital for rehabilitation three weeks after admission (Fig. 5).
Figure 5.
The neurological symptoms caused by lymphoma resolved after the start of chemotherapy, but symptoms due to intrathecal chemotherapy-induced myelopathy developed later and did not improve with treatment.
The patient has been followed for three years since the completion of R-CHOP therapy. Her neurological symptoms have not improved significantly, but rehabilitation enabled her to walk with the help of a cane. Fortunately, there were no signs of DLBCL recurrence, and the patient remained in remission.
Discussion
Intrathecal chemotherapy is often performed for prophylaxis and treatment of CNS involvement in hematological malignancies. Myelopathy associated with intrathecal chemotherapy is a rare but serious adverse event, with a reported incidence rate of approximately 3% (5).
Frequent symptoms of this entity include bladder and rectal disturbances, and paraplegia. In addition, saddle anesthesia and sensory disturbances have also been commonly reported (6).
Myelopathy associated with intrathecal chemotherapy often involves the dorsal column of the spinal cord (7). MRI of the spine is useful for the diagnosis. The characteristic findings include a high-intensity signal involving the dorsal column on T2-weighted images (8). The clinical presentation and MRI findings of intrathecal chemotherapy-induced myelopathy are similar to those of SCD caused by severe vitamin B12 or folate deficiency. However, in intrathecal chemotherapy-induced myelopathy, the levels of these vitamins are usually normal or only mildly decreased, and replacement therapy rarely results in symptomatic improvement.
The pathophysiology of intrathecal chemotherapy-induced myelopathy is not clear, but local folate depletion due to MTX may result in deficiency at the spinal cord level (8).
MTX prevents the conversion of dihydrofolate to tetrahydrofolate (THF) and decreases intrathecal 5-methyl-THF production (9). 5-Methyl-THF is required for the conversion of cobalamin to methylcobalamin, which is essential for the conversion of homocysteine to methionine. S-adenosylmethionine (SAM) is synthesized from methionine and is essential for the maintenance of the myelin sheath. Intrathecal injection of MTX results in SAM deficiency and induces demyelination of the spinal cord, resulting in myelopathy symptoms (9).
Pinnix et al. examined the clinical characteristics of 13 patients who developed intrathecal MTX-induced myelopathy. They reported that serum homocysteine levels were measured in four patients, and all of them showed elevated values (7). A previous study reported that homocysteine levels in CSF were predominantly increased in pediatric patients treated with MTX (10). Another case report found elevated levels of homocysteine in the CSF of a patient with intrathecal MTX-induced myelopathy (9). Elevated homocysteine levels in CSF reflect impaired methionine synthesis in the CNS, which can result in spinal cord demyelination. Measuring homocysteine levels in the serum or CSF may therefore help recognize this complication.
In addition, elevated protein and MBP levels in the CSF may also suggest spinal cord toxicity due to MTX. In the abovementioned report by Pinnix et al, MBP in CSF was elevated in all four patients assessed for this marker (7). However, the cut-off values of these markers to be used as diagnostic tests have yet to be determined. Furthermore, whether or not these markers can predict the onset of myelopathy in advance is unclear. Further research is needed to explore the diagnostic potential of these markers in order to prevent the development of symptomatic myelopathy.
There is no established treatment strategy for intrathecal chemotherapy-induced myopathy, but the usual recommendation is to immediately discontinue intrathecal chemotherapy. Although there have been some reports of mild improvement in the symptoms of MTX-induced neurotoxicity with dextromethorphan, most studies have failed to show any improvement with it (4,6). In addition, MRI findings are similar to those of SCD, but vitamin B12 or folic acid supplementation seldom results in improvement of the symptoms (6). The symptoms of myelopathy are often irreversible; therefore, prompt discontinuation of intrathecal chemotherapy should be considered when myelopathy is suspected.
When neurological symptoms occur during chemotherapy, the possibility of intrathecal chemotherapy-induced myelopathy should be considered, as well as CNS neoplastic infiltration or neuropathy associated with systemic chemotherapy (e.g., vincristine).
Conclusion
We encountered a case of irreversible myelopathy associated with intrathecal chemotherapy. Clinicians administering intrathecal chemotherapy should be aware of this potential complication. If a patient develops neurological abnormalities, it is necessary to consider discontinuing intrathecal therapy after an appropriate evaluation. T2-weighted MRI (axial and sagittal) and CSF evaluations, including protein, MBP, and homocysteine measurements, may aid in the diagnosis. Since there is no established method for diagnosing or treating this entity, further research is warranted.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hill QA, Owen RG. CNS prophylaxis in lymphoma: who to target and what therapy to use. Blood Rev 20: 319-332, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated With R-CHOP. J Clin Oncol 34: 3150-3156, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Kwong YL, Yeung DY, Chan JC. Intrathecal chemotherapy for hematologic malignancies: drugs and toxicities. Ann Hematol 88: 193-201, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues PGB, Lima TT, Duarte FB, Nóbrega PR. Myelopathy associated with intrathecal methotrexate. Pract Neurol 22: 141-144, 2022. [DOI] [PubMed] [Google Scholar]
- 5.Teh HS, Fadilah SA, Leong CF. Transverse myelopathy following intrathecal administration of chemotherapy. Singapore Med J 48: e46-e49, 2007. [PubMed] [Google Scholar]
- 6.Pinnix CC, Chi L, Jabbour EJ, et al. Dorsal column myelopathy after intrathecal chemotherapy for leukemia. Am J Hematol 92: 155-160, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cachia D, Kamiya-Matsuoka C, Pinnix CC, et al. Myelopathy following intrathecal chemotherapy in adults: a single institution experience. J Neurooncol 122: 391-398, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murata KY, Maeba A, Yamanegi M, Nakanishi I, Ito H. Methotrexate myelopathy after intrathecal chemotherapy: a case report. J Med Case Rep 9: 135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn CT, Griener JC, Bottiglieri T, Hyland K, Farrow A, Kamen BA. Elevation of homocysteine and excitatory amino acid neurotransmitters in the CSF of children who receive methotrexate for the treatment of cancer. J Clin Oncol 15: 2800-2806, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Bidikian AH, Bazarbachi A, Hourani R, El-Cheikh J, Abou Dalle I. Intrathecal methotrexate induced myelopathy, rare yet serious complication: a case report and review of the literature. Curr Res Transl Med 69: 103296, 2021. [DOI] [PubMed] [Google Scholar]