Summary
The expression of growth/differentiation factor (GDF) 15 increases in the ganglionic eminence (GE) late in neural development, especially in neural stem cells (NSCs). However, GDF15 function in this region remains unknown. We report that GDF15 receptor is expressed apically in the GE and that GDF15 ablation promotes proliferation and cell division in the embryonic GE and in the adult ventricular-subventricular zone (V-SVZ). This causes a transient generation of additional neuronal progenitors, compensated by cell death, and a lasting increase in the number of ependymal cells and apical NSCs. Finally, both GDF15 receptor and the epidermal growth factor receptor (EGFR) were expressed in progenitors and mutation of GDF15 affected EGFR signaling. However, only exposure to exogenous GDF15, but not to EGF, normalized proliferation and the number of apical progenitors. Thus, GDF15 regulates proliferation of apical progenitors in the GE, thereby affecting the number of ependymal cells and NSCs.
Keywords: GDF15, GFRAL, EGFR, CXCR4, radial glia, subapical progenitors, neural stem cells, ganglionic eminence
Highlights
-
•
GDF15 receptor GFRAL is expressed in ganglionic eminence progenitors
-
•
GDF15 controls Cell cycle progression and reentry in neural stem cells
-
•
GDF15 controls the number of ependymal and apical neural stem cells
-
•
GDF15 controls proliferation independent of EGFR
In this report, Baur and colleagues report that GDF15 and its receptor GFRAL control proliferation in the germinal niche lining the lateral ventricle from late development onward. This determines the number of adult NSCs and ependymal cells in the adult ventricular-subventricular zone. These findings describe a functional role for GDF15 in this region for the first time.
Introduction
All neural cells derive directly or indirectly, through the generation of intermediate progenitors, from radial glia (RG) (Anthony et al., 2004; Bandler et al., 2017; Turrero García and Harwell, 2017). In the ventral telencephalon, intermediate progenitors are represented by short neural progenitors (SNPs), similar to those in the dorsal pallium (Tyler and Haydar, 2013), and subapical progenitors (SAPs) (Pilz et al., 2013). Despite displaying an apical membrane, SAPs divide at a basal position in the ventricular zone (VZ) unlike RG and SNPs, which divide at the apical border and will be collectively referred to here as apical progenitors (APs). During development, neurogenesis occurs increasingly from progenitors located at the basal side of the niche, forming the secondary germinal epithelium called the subventricular zone (SVZ) (Haubensak et al., 2004; Kosodo and Huttner, 2009; Noctor et al., 2004; Petros et al., 2015; Pilz et al., 2013). The ganglionic eminence (GE) largely contributes to the pool of neural stem cells (NSCs) in the ventricular-subventricular zone (V-SVZ) (Borrett et al., 2020; Young et al., 2007), which represents the largest neurogenic niche in the adult murine brain. In the V-SVZ, two distinct types of NSCs coexist, which are referred to as apical and basal NSCs because they display the morphological characteristics of apical and basal RG, respectively (Baur et al., 2022). Whereas the origin of basal NSCs is still unclear, apical NSCs share a common apical RG progenitor with ependymal cells (Epen) (Ortiz-Alvarez et al., 2019).
Several niche factors affect the proliferation and differentiation of neural progenitors (Ferent et al., 2020). From mid-development onward, the expression of the epidermal growth factor receptor (EGFR) and its multiple ligands increases in the periventricular tissue (Zhang et al., 2023). In the developing GE, NSCs acquire EGF responsiveness around embryonic day 18 (E18) (Ciccolini, 2001) and EGF is the main mitogen for postnatal NSCs (Robson et al., 2018). Despite being associated with proliferation, EGFR activation in neural progenitors controls pleiotropic effects, including survival differentiation and migration (Abdi et al., 2018; Ciccolini et al., 2005; Scalabrino, 2022). This likely reflects different levels of EGFR activation (Burrows et al., 1997) and differential recruitment of downstream pathways (Cochard et al., 2021). Instead, ablation of EGFR in developing neural precursors affects especially gliogenesis and astrocytic function (Robson et al., 2018; Zhang et al., 2023).
Growth/differentiation factor (GDF)15, a member of the transforming growth factor β superfamily, is also expressed in the apical V-SVZ from late development onward; however, its function in this region is still unknown. In the adult brain, the choroid plexus represents the site of strongest and almost exclusive GDF15 expression in the adult CNS (Bottner et al., 1999; Schober et al., 2001). The neonatal brain displays GDF15 immunoreactivity not only in Epen but also in the subependymal area (Schober et al., 2001), and GDF15 regulates the proliferation and EGFR expression of hippocampal NSCs (Carrillo-Garcia et al., 2014). This suggests that GDF15 may affect the development of APs in the V-SVZ. To investigate this possibility, here, we have analyzed progenitors in the embryonic GE and in the V-SVZ of Gdf15-deficient mice. Our observations provide the first evidence that GDF15 regulates the proliferation of apically and subapically dividing progenitors in the embryonic VZ and in the postnatal counterpart, thereby controlling the total number of adult Epen and NSCs.
Results
Expression of GDF15 receptor glial cell line-derived neurotrophic factor family receptor α-like (GFRAL) in embryonic and adult APs
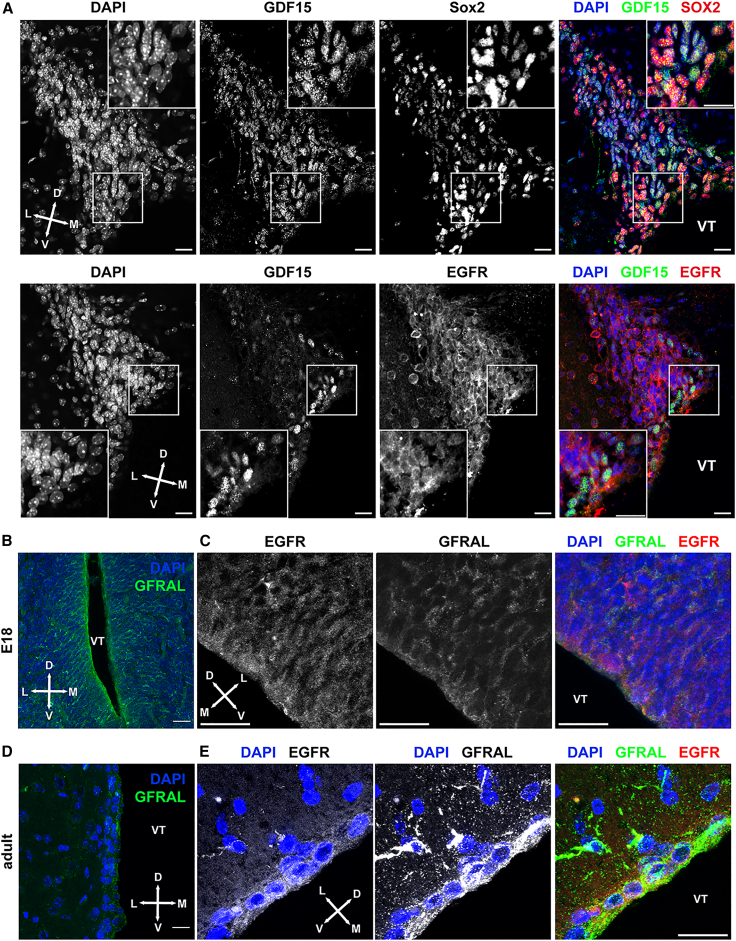
Previous studies have reported the highest levels of expression of Gdf15 mRNA and protein in the brain in the region lining the lateral ventricle and in the choroid plexus (Bottner et al., 1999; Schober et al., 2001). Consistent with these observations, we have previously shown that Gdf15 transcripts increase at late developmental age in the GE and that they remain high in the adult V-SVZ (Carrillo-Garcia et al., 2014). We have also found GDF15 expression in neural progenitors endowed with NSC potential in both the hippocampus and in the GE of E18 embryos. In the latter region, these include cells displaying high levels of the EGFR (EGFRh) (Carrillo-Garcia et al., 2014), suggesting that GDF15 may affect the development of NSCs in the V-SVZ. Supporting this hypothesis, Gdf15 transcripts were observed in neurosphere progenitors obtained from the E18 GE (Figure S1A) and GDF15 immunoreactivity was present in cells expressing SOX2, a marker for NSCs (Ellis et al., 2004), and EGFR, which is upregulated in activated NSCs and cycling progenitors in the adult V-SVZ (Figure 1A). Transcripts of Gfral, which was recently identified as the GDF15 receptor (Li et al., 2017; Mullican et al., 2017; Yang et al., 2017), were also similarly expressed in the GE of wild-type (WT) and Gdf15−/− E18 embryos (Figures S1B–S1D). Finally, GFRAL immunoreactivity was often colocalized with EGFR immunoreactivity and stronger at the apical portion of the GE (Figures 1B and 1C) and adult V-SVZ (Figures 1D and 1E). Thus, like its ligand, GFRAL is expressed in the embryonic and adult germinal niche lining the lateral ventricle.
Figure 1.
Analysis of GDF15 and GFRAL expression
Representative confocal images illustrating immunofluorescent staining of the adult V-SVZ (A, D, and E) and E18 GE (B and C) and DAPI nuclear counterstaining as indicated. See also Figure S1.
Scale bars, 20 μm. D, dorsal; L, lateral; M, medial; V, ventral; VT, ventricle.
GDF15 affects the proliferation of apically and subapically dividing progenitors in the embryonic GE
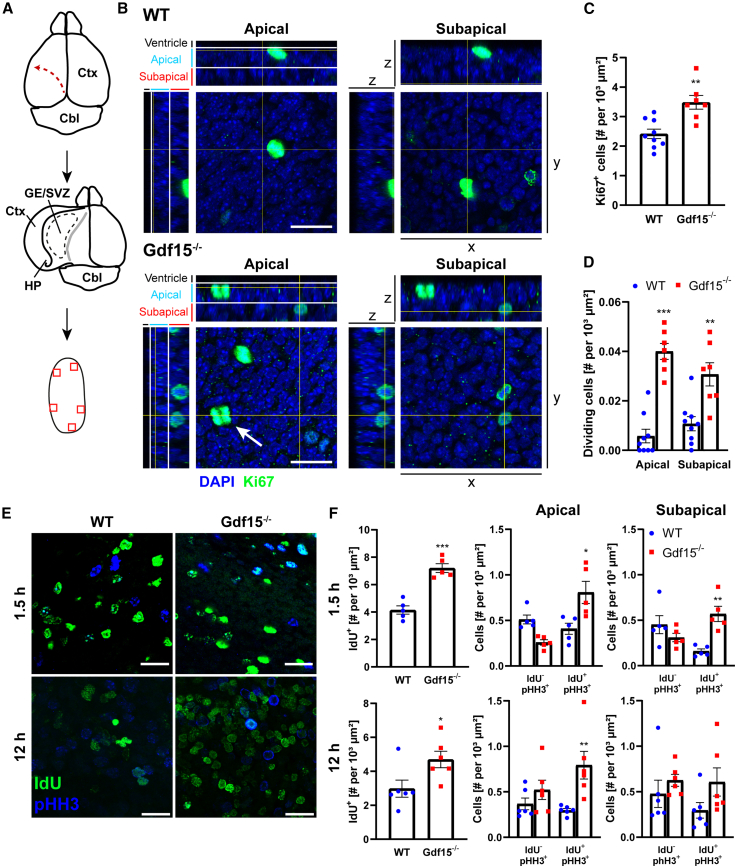
Having observed that both GDF15 and its receptor are expressed in the GE and in the V-SVZ, we next investigated whether in these regions, as in the hippocampus (Carrillo-Garcia et al., 2014), GDF15 also increases progenitor proliferation. We first immunostained whole-mount samples with antibodies to Ki67 (Figures 2A and 2B), which is present in the nuclei of all cycling cells. In these preparations, we also identified dividing progenitors based on the morphological appearance of nuclei in anaphase and telophase (Figure 2B, white arrow; see also supplemental experimental procedures). As illustrated in Figure 2B, the distance of the dividing nuclei from the apical surface of the GE allowed us to identify dividing APs (i.e., RG and SNPs), whose nuclei localized within the 10-μm-thick apical border, and SAPs, dividing at a basal position away from the apical border. We found that GDF15 ablation significantly affects proliferation also in the E18 GE. However, unlike in the hippocampus, GDF15 expression in this region was associated with a decrease in the number of cycling progenitors and of dividing APs and SAPs (Figures 2C and 2D). A similar effect of GDF15 was also observed in the V-SVZ of adult mice (Figures S2A–S2C). To better understand the effect of GDF15 ablation on proliferation in the E18 GE, we took advantage of whole-tissue explants. Progenitors duplicating their DNA were labeled by culturing the explants for 1.5 h in medium containing 5-Iodo-2'-deoxyuridine (IdU). The tissue was then fixed either immediately or after a further 12 h of culturing without IdU. Fixed explants were then processed for double immunostaining to score IdU incorporation and mitosis by means of phospho-histone H3 (pHH3) immunoreactivity (Figures 2E and 2F). At both time points, more IdU+ and mitotic pHH3+/IdU+ progenitors were counted in the mutant tissue (Figure 2F). In contrast, the genotype did not affect the number of pHH3+/IdU− cells. The fact that only the number of pHH3+/IdU+ cells increases in the Gdf15−/− GE could indicate a shortening of the time required for the transition between DNA replication and cell division in mutant progenitors. Supporting this conclusion, the effect of the genotype on pHH3+ cells, which include all cells in M-phase, was smaller than the effect on Ki67+ dividing cells, which include only cells in advanced stages of mitosis (Figure 2, compare panels D and F). Moreover, in the mutant tissue, a consistent portion of the additional IdU+ cells were non cycling, even at the earliest time point analyzed (Figures S2D–S2F), indicating that in the absence of GDF15, more cells divided and exited the cell cycle 1.5 h after DNA replication. Notably, at the earlier time point, regardless of the genotype, IdU+ cells displayed similar fluorescence levels (Figure 2E, upper panels). In contrast, cells displaying lighter IdU immunoreactivity were additionally present at the later time point (Figure 2D, lower panels). Some of these cells could represent second-generation SAPs, which have a significantly shorter cell cycle (∼12 h) than other progenitors in the embryonic GE (Pilz et al., 2013). Although they were not quantified, these cells also appeared increased in the mutant tissue, suggesting that in the absence of GDF15, the additional dividing cells include secondary progenitors. In explants fixed after a further 12 h, only the number of apical pHH3+/IdU+ cells in the mutant tissue was still increased, whereas pHH3+/IdU+ SAPs displayed only a trend increase that was not significant (Figure 2F). Furthermore, all of the additional IdU+ cells in the mutant GE had exited the cell cycle at this time point (Figure S2F), indicating that they are not continuously cycling.
Figure 2.
Increased proliferation in the E18 GE in the absence of GDF15
(A) Schematic illustration of whole-mount dissection and of the imaged regions of interest (red squares) at the apical side.
(B) Representative confocal images of the apical GE illustrating Ki67 (green) and DAPI (blue) staining viewed as z planes (x-y axis; large images) and orthogonal (yz and xz axes, small images) projections. The apical and subapical regions are shown on the orthogonal projections. Arrow indicates a dividing cell. X, y, z, 3-dimensional axes. Scale bar, 20 μm.
(C and D) Quantification of total Ki67+ cells (C) and apically and subapically dividing cells (D).
(E) Representative confocal micrographs of the apical surface of tissue explants illustrating pHH3 (blue) and IdU (green) immunostaining detected after 1.5 h of IdU pulse or after a further 12 h of incubation without IdU.
(F) Quantification of total IdU+ and apical and subapical pHH3+ cells. See also Figure S2.
Bars represent mean ± SEM; N number is represented by single data points, each showing the average data for 1 individual animal; asterisks indicate significance to respective WT: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, determined by 2-tailed Student’s t test (C and F, left column) or by 2-way ANOVA with Sidak’s multiple comparisons test (D and F, center and right columns). Scale bars, 20 μm.
Next, we examined the cell division angles of dividing APs relative to an axis orthogonal to the ventricular surface in the embryonic GE and in the adult V-SVZ to determine whether the absence of GDF15 also alters the mode of cell division (i.e., symmetric vs. asymmetric). At both ages, most AP divisions were symmetrical, with a 0° angle in both genotypes (Figures S3A–S3C). The proportion of APs dividing asymmetrically at a 45° angle was ∼40% in the embryonic tissue (Figure S3D), which is consistent with previous observations (Falk et al., 2017). In the adult V-SVZ, this was reduced to 20% (Figure S3E), indicating a decrease in neurogenic divisions (Chenn and McConnell, 1995; Zhang et al., 2004). Thus, at either age, the division mode is not changed by the absence of GDF15.
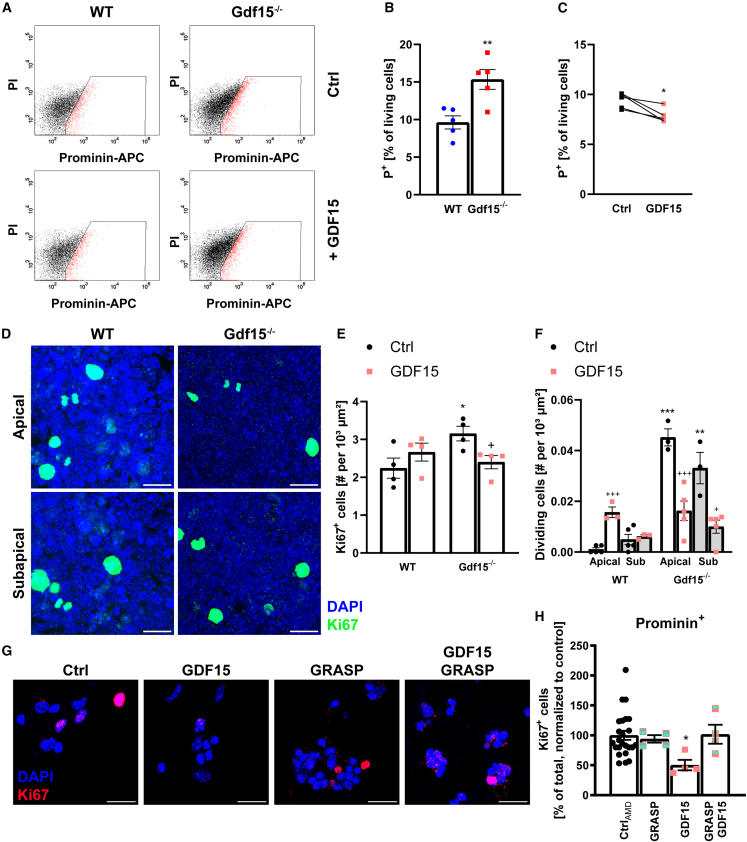
Our data indicate that in the GE, endogenous GDF15 decreases proliferation, in contrast to the age-matched hippocampus, where it promotes progenitor proliferation (Carrillo-Garcia et al., 2014). Consistent with this conclusion, compared to the WT counterpart, the number of apical prominin-1-expressing (P+) progenitors was increased in the mutant E18 GE (Figures 3A and 3B) and decreased by treatment with exogenous GDF15 (Figure 3C). This treatment also normalized the number of cycling progenitors to WT levels in whole-mount preparations (Figures 3D and 3E), and it reduced the number of dividing APs and SAPs in mutant explants (Figure 3F). In contrast, when the WT E18 GE was exposed to exogenous GDF15, only the number of dividing APs was significantly increased (Figure 3F). Remarkably, the number of dividing APs was virtually identical in the two groups of GDF15-treated explants, suggesting that the seemingly opposite effect GDF15 exerts on WT and mutant progenitors is likely due to the initial difference in the number of dividing APs between the two genotypes.
Figure 3.
Application of exogenous GDF15 to the mutant E18 GE
(A) FACS plots showing cells positive for P+ (red) in the WT and Gdf15−/− E18 GE, with GDF15 application and without (Ctrl).
(B) Quantification of the FACS analysis in (A).
(C) Change in the number of total P+ cells upon GDF15 treatment as percentage of total P+ cells in untreated cells from the same animal.
(D) Representative confocal images of apical and subapical planes of explants treated with GDF15 overnight upon Ki67 (green) and DAPI (blue) staining.
(E and F) Quantification of total (E) or dividing (F) Ki67+ cells from (D).
(G) Dissociated, P+-sorted E18 GE cells after treatment as indicated and Ki67 (red) and DAPI (blue) staining. Scale bars, 20 μm.
(H) Quantification of Ki67+ cells from (G), as percentage of total cells, normalized to Ctrl.
Bars represent mean ± SEM; N number is represented by single data points, each showing the average data for 1 individual animal; ∗/+ indicate significance: ∗/+p < 0.05, ∗∗p < 0.01, ∗∗∗/+++p < 0.001; ∗ indicates significance to respective WT control, + indicates significance to respective untreated control. p values were determined by 2-tailed Student’s t test (B unpaired, C paired as indicated), 2-way ANOVA with Sidak’s multiple comparisons test (E and F), or one-way ANOVA against Ctrl (H).
Since GFRAL is expressed in APs, our data indicate that GDF15 directly affects the proliferation of VZ progenitors in the developing GE. Supporting this conclusion, we found that exposure to exogenous GDF15 in the presence of the GRASP antagonist peptide, which prevents GFRAL and RET co-receptor interaction (Borner et al., 2023), also prevented the ability of exogenous GDF15 to affect the proliferation of sorted APs (Figures 3G and 3H). Thus, GDF15 decreases the proliferation of progenitors in the embryonic GE.
GDF15 regulates the proliferation of intermediate neuronal progenitors in the developing GE
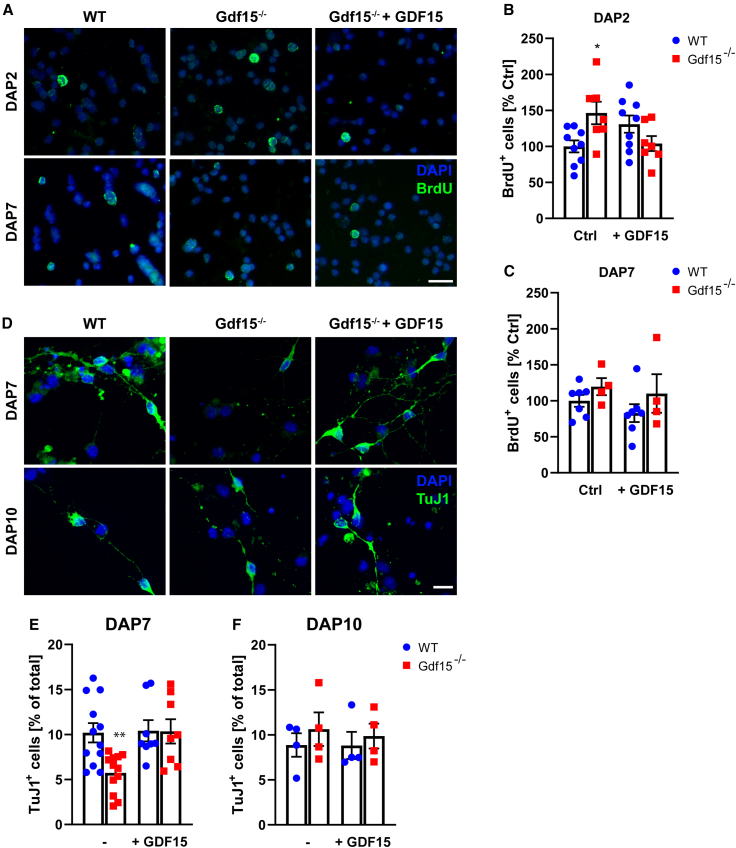
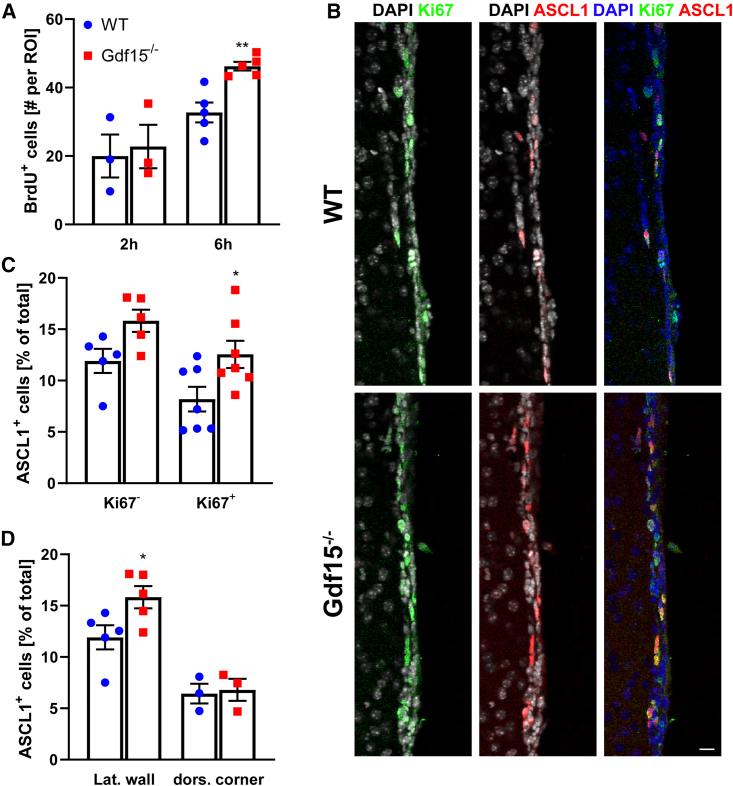
Our analysis of dividing cells indicates that the number of fast proliferating secondary SAPs is increased upon GDF15 mutation. Cell-cycle regulation affects neuronal output in the developing GE, and in differentiating neurosphere cultures, most immature neurons are generated from dividing progenitors (Ostenfeld and Svendsen, 2004; Suh et al., 2009). Therefore, we used differentiating neurosphere cultures to begin to characterize whether GDF15 affects the generation of neuronal progenitors. Consistent with our observations in situ, Gdf15−/− neurospheres contained significantly more cells undergoing DNA replication (Figures 4A and 4B) and mitosis (Figures S3F and S3G) than their WT counterpart when examined 2 days after plating (DAP) in differentiation conditions. This also resulted in a faster increase in Gdf15−/− cell culture confluence within the first 24 h of plating (Figure S3H). The difference in proliferation was no longer observed in neurospheres that differentiated in the presence of GDF15, or when cultures were examined at DAP7 (Figures 4B and 4C). At DAP2, the number of pycnotic nuclei was unchanged in the two groups of cultures (Table S2), showing that the change in cell proliferation was not due to a selective effect of GDF15 on cell viability. However, at DAP4, the mutant cultures that were not treated with exogenous GDF15 had more pycnotic nuclei than their treated and WT counterparts (Table S2). At DAP7, the genotype also affected neuronal (Figures 4D and 4E) but not oligodendrocyte (Table S2) differentiation. The differences in the percentage of TuJ1+ neurons were no longer observed in cultures that differentiated in the presence of exogenous GDF15 or for 3 additional days (Figure 4F). Taken together, these data indicate that GDF15 decreases the proliferation of neuronal progenitors while promoting their differentiation and survival. We next investigated changes in proliferation and neurogenesis in vivo in deeper areas of the GE, containing differentiated neurons by means of intraperitoneal bromodeoxyuridine (BrdU) injection of the pregnant dams. The number of dividing cells was only significantly increased in the tissue analyzed 6, but not 2 h after the injection (Figure 5A). This indicates that the additional proliferating cells in the mutant GE are slower proliferating neuronal precursors. Consistent with this, the number of cells expressing achaete-scute homolog 1 (ASCL1), which affects both proliferation and neuronal specification in the developing GE (Casarosa et al., 1999; Torii et al., 1999), was higher in cells dissociated from the mutant E18 GE than in the WT counterpart (% immunopositive cells: WT = 22.84 ± 3.4; Gdf15−/− = 30.51 ± 2.2; p < 0.05). A similar change was observed in the postnatal V-SVZ, where ASCL1+ progenitors largely represent rapidly proliferating transit amplifying progenitors and preneuroblasts (Cesetti et al., 2009; Parras et al., 2004). The number of ASCL1+ progenitors was also higher in the V-SVZ of adult Gdf15−/− mice than the WT counterpart (Figures 5B and 5C). This increase was limited to the fraction of ASCL1+/Ki67+ proliferating progenitors (Figure 5C) and was not observed at the level of the dorsolateral corner (Figure 5D). A subset of ASCL1+ cells, but not preneuroblasts, also expresses oligodendrocyte transcription factor 2 (OLIG2), a helix-loop helix transcription factor associated to oligodendrocyte differentiation (Cesetti et al., 2009; Lu et al., 2002; Zhou and Anderson, 2002). Therefore, we next analyzed the expression of OLIG2 by immunofluorescence in the mediolateral V-SVZ of WT and Gdf15−/− mice. Independent of ASCL1 expression, the number of OLIG2+ cells (Figures S4A and S4B), and total or proliferating doublecortin-positive (DCX+)/Ki67+ neuroblasts (Figures S4C and S4E) was not affected by the genotype. Next, we investigated whether GDF15 affects cell viability also in vivo. Flow cytometric analysis showed a higher percentage of propidium iodide (PI)+ dying cells in both mutant EGFRh (fold increase 2.00 ± 0.3382; p = 0.0369) and EGFRl (fold increase 1.380 ± 0.1094; p = 0.0511) cells than in WT counterparts. Likewise, more annexin V+ cells were observed in mutant than WT brain slices (Figures S4F and S4G). Taken together, our data indicate that both in vitro and in vivo, the increase in the proliferation of mutant neuronal progenitors does not cause increased neurogenesis, likely due to compensatory cell death.
Figure 4.
Effect of GDF15 on differentiation of neurospheres derived from the E18 GE
(A) BrdU (green) and DAPI (blue) staining of WT and Gdf15−/− neurosphere cultures fixed DAP2 or -7 in differentiation medium and treated with GDf15 as indicated. BrdU was added to the culture medium 24 h before fixation.
(B and C) Quantifications of BrdU+ cells from (A).
(D) TuJ1 (green) and DAPI (blue) staining of WT and Gdf15−/− neurosphere cultures fixed DAP7 or -10 and treated with GDF15 as indicated.
(E and F) Quantifications of TuJ1+ cells from (D).
Scale bars, 20 μm. Bars represent mean ± SEM; N number is represented by single data points, each showing the average data for 1 individual animal; ∗ indicates significance: ∗p < 0.05, determined by 2-way ANOVA with Tukey’s multiple comparisons test.
Figure 5.
Increased ASCL1+ cells in the lateral-ventral V-SVZ
(A) Quantitative analysis of the nuclei displaying BrdU immunoreactivity per ROI within the deep GE of WT and Gdf15−/− E18 embryos 2 and 6 h after BrdU injection.
(B) Coronal sections of the V-SVZ of adult WT and Gdf15−/− mice after Ki67 (green), ASCL1 (red), and DAPI (blue) staining. Scale bar, 20 μm.
(C and D) Quantifications of ASCL1+/Ki67− and ASCL1+/Ki67 (C) and total ASCL1+ (D) cells from (B).
See also Figure S4. Lat. wall = lateral wall, dors. corner = dorsolateral corner. Bars represent mean ± SEM; N number is represented by single data points, each showing the average data for 1 individual animal; ∗ indicates significance: ∗p < 0.05, ∗∗p < 0.01, determined by 2-way ANOVA with Sidak’s multiple comparisons test.
GDF15 regulates the number of NSCs and Epen
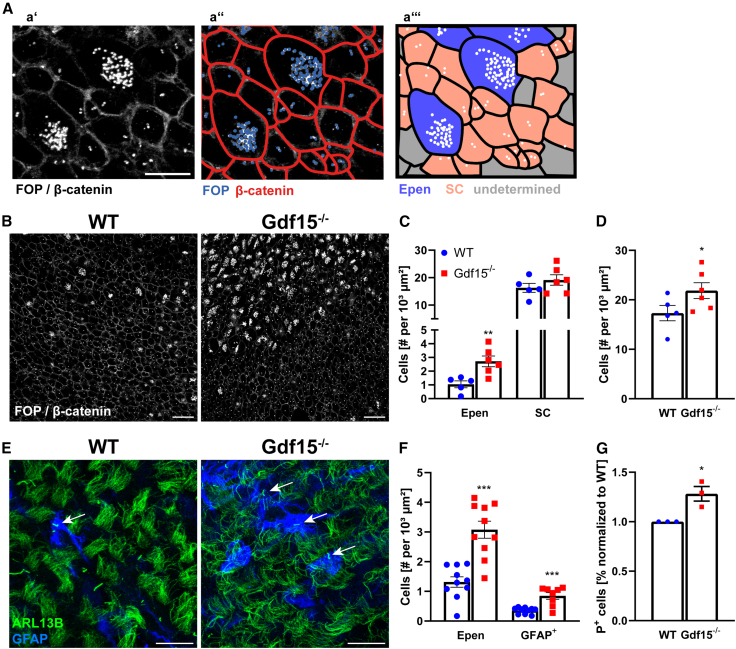
Dividing APs in the developing GE also gives rise to Epen and adult apical NSCs, which are largely derived from a common progenitor whose proliferation at mid-development (Ortiz-Alvarez et al., 2019) is regulated by a mechanism that involves bone morphogenetic protein-mediated control of the cell cycle (Omiya et al., 2021). Therefore, we next investigated whether the increase in the proliferation in the mutant GE also affects the number of multiciliated Epen and progenitors displaying only one primary cilium at the apical surface of newborn mice at postnatal day 2 (P2). We here used β-catenin to label cell-to-cell contacts and fibroblast growth factor receptor 1 oncogene partner (FOP), a centrosomal protein, thereby visualizing cell boundaries and the basal body of the cilia, respectively. Because both β-catenin and FOP antibodies were derived from the same host species, the antigens were labeled in a single fluorescent channel and differentiated based on label localization and intensity (Figure 6A). Cells with a single centrosome or centrosome pair (one to two FOP+ dots) were counted as single ciliated (SC), whereas cells with more than two centrosomes (i.e., multiciliated cells) were counted as Epen (Figure 6A). This analysis revealed a significant increase in multiciliated Epen as well as a trend increase in SC cells in the mutant tissue compared to the WT counterpart and a significant increase in the number of total cells (Figures 6B–6D). Moreover, in the adult V-SVZ, where multiciliated Epen are fully differentiated, the number of both Epen and apical NSCs, identified based on the elongated cell morphology, glial fibrillary acidic protein (GFAP) expression, and absence of motile cilia, were increased (Figures 6E and 6F). Both apical NSCs and Epen continue to express P+ in the postnatal V-SVZ, but only the first are capable of undergoing clone formation (Baur et al., 2022; Carrillo-Garcia et al., 2010; Khatri et al., 2014). Therefore, we determined the number of P+ cells and their clonogenic ability. These analyses revealed that their number was increased in the mutant V-SVZ (Figure 6G) but the incidence of P+ clonogenic cells was not affected (Table 1), indicating an increase in the number of both Epen and apical NSCs.
Figure 6.
Lack of GDF15 leads to increased number of Epen and apical NSCs
(A) Schematic showing Epen and SC cells using FOP and β-catenin immunoreactivity. (a′) Closeup of WT image in (B), showing FOP and β-catenin, indicating ciliary basal bodies/centrosomes and cell-to-cell contacts, respectively. (a″) The latter are highlighted in red. (a‴) Cells containing 1/2 or multiple centrosomes were considered SC (red) and Epen (blue) cells, respectively. Scale bar, 10 μm.
(B) Whole mounts of the E18 lateral GE immunofluorescently labeled with FOP and β-catenin. Scale bars, 20 μm.
(C and D) Quantification of SC and Epen (C) and total cells (D).
(E) Arl13b (green) and GFAP (blue) staining on whole mounts of the adult V-SVZ. Arrows indicate SC NSCs. Scale bars, 20 μm.
(F) Quantification of multiciliated (Epen) and GFAP immunopositive cells.
(G) FACS analysis of dissociated P8 V-SVZ cells labeled with antibodies against P+.
Bars represent mean ± SEM; N number is represented by single data points, each showing the average data for 1 individual animal; ∗ indicates significance to respective WT control: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, determined by 2-tailed Student’s t test.
Table 1.
Number of clonogenic cells (as percentage of plated) within the indicated population sorted from the dissociated V-SVZ of adult WT and Gdf15−/− mice
| Clones DIV 0 | WT | Gdf15−/− |
|---|---|---|
| P−Eh cells | 14.37 ± 7.4 | 12.71 ± 3.9 |
| P+Eh cells | 21.3 ± 1.79 | 38.35 ± 9.51 |
Data are displayed as mean ± SEM. N = 2 separate experiments with sorted cells pooled from 4 to 6 animals each.
Lack of GDF15 affects EGFR expression and signaling dynamics in neural progenitors
The acquisition of EGF responsiveness (Ciccolini, 2001) and the increase in GDF15 expression in the telencephalon both occur at late developmental ages and in the developing hippocampus, where GDF15 affects EGFR expression (Carrillo-Garcia et al., 2014). Therefore, we next investigated EGFR expression and its activation in the developing WT and mutant GE. Independent of the genotype, EGFR immunoreactivity was stronger at the basal side than the apical side of the VZ, with EGFR+ cells showing a more pronounced radial orientation in WT than in mutants in coronal sections (Figure S5A, white arrows). At higher magnification, EGFR immunoreactivity had mainly a punctuate appearance in the mutant embryonic GE (Figure S5B) and especially adult V-SVZ (Figure S5C), unlike in the WT counterpart, where it was present also at the cell borders. Compared to age-matched WT tissue, the EGFR fluorescence levels were significantly reduced in the adult but not in the embryonic tissue (Figures S5D and S5E). On the other side, endogenous EGFR activation, measured by immunostaining with antibodies recognizing phosphorylated EGFR (pTyr1092; pEGFR), revealed at both ages no significant effect of the genotype (Figures S5F and S5G). Notably, there was not a clear overlap between pEGFR and EGFR immunoreactivities, with the first being mostly expressed in weakly EGFR+ cells in the embryonic GE and especially in the adult V-SVZ (Figures S5Band S5C). The lack of correlation between EGFR expression and phosphorylation is known, since the EGFR protein is degraded in the presence of strong EGFR activation (Tomas et al., 2014). Analysis of EGFR by flow cytometry also showed a significant reduction in the number of EGFRh cells both in apical mutant P+ and P− cells, which was rescued by a short exposure to exogenous GDF15 (Figures S5H–S5J). Notably, the decrease in the number of EGFRh cells in the mutant GE was detected only when EGFR was measured using EGF bound to the less sensitive Alexa 488 but not Alexa 647 (Figures S5K–S5M), which unlike the first allows the detection of EGFR-expressing cells displaying also moderate levels of surface EGFR. Taken together, these data indicate that, rather than a general downregulation of EGFR, lack of GDF15 leads to a decrease in the levels of EGFR expressed at the cell membrane. Similar amounts of Egfr transcripts were expressed in the mutant and WT GE (Figure S5N).
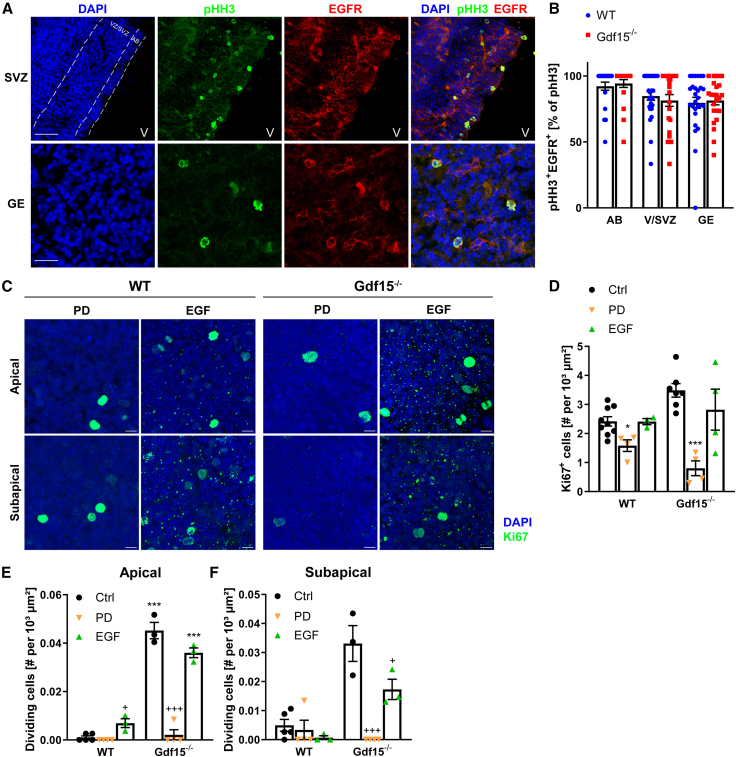
We next investigated the dynamics of EGFR activation in WT and mutant progenitors. Independent of the genotype, a short exposure to exogenous EGF induced similar levels of ERK phosphorylate in acutely dissociated E18 GE (Figure S6A). Moreover, a short application of GDF15 was also able to induce the phosphorylation of ERK, consistent with the fact that it triggers GFRAL-dependent signaling. However, levels of pERK were significantly downregulated already 1 h after EGF application in mutant but not WT cells, and a lower concentration of EGF was necessary to evoke a significant response in WT than in mutant cells (Figures S6B and S6C). Thus, although both WT and mutant progenitors are capable of responding to EGF, the dynamics of EGFR activation differ between the two groups of progenitors, which also suggests that in mutant cells the receptor is more rapidly degraded upon ligand activation. Underscoring the mild nature of these differences, pHH3 and EGFR immunoreactivities were strongly colocalized both in the WT and in the mutant GE (Figures 7A and 7B). Moreover, independent of the genotype, pharmacological blockade of EGFR with PD158780 (PD) reduced the number of cycling progenitors (Figures 7C and 7D) and dividing cells (Figures 7E and 7F). However, the effect of the treatment was significant only in the mutant GE, which probably reflects the problematic quantification of mitotic cells in the WT GE, due to their low number. On the contrary, independent of the genotype, exposing the GE to exogenous EGF for 24 h did not greatly affect the number of cycling progenitors, indicating saturation of endogenous EGFR activation. Nevertheless, the treatment caused a small but significant increase in the number of dividing APs in the WT tissue and a decrease in the number of dividing APs and SAPs in the mutant GE, which was significant only in the latter group (Figures 7E and 7F). Thus, unlike exposure to exogenous GDF15, manipulation of EGFR activation does not rescue the proliferation of mutant progenitors. Nevertheless, our analysis indicates that in the mutant GE, proliferation is more reliant on endogenous EGFR activation than in the WT counterpart.
Figure 7.
Effect of EGFR activation on proliferation in the E18 GE
(A) Coronal sections of the GE germinal region of E18 WT and Gdf15−/− mice illustrating pHH3 (green), EGFR (red), and DAPI (blue) staining. AB, apical border; GE, deep ganglionic eminence; V, lateral ventricle. Scale bar, 37.5 μm.
(B) Quantification of (A).
(C) Representative confocal pictures showing the apical surface of explants upon Ki67 (green) and DAPI (blue) staining after treatment with PD or EGF as indicated. Scale bars, 10 μm.
(D–F) Quantifications of total (D) or dividing Ki67+ (E and F) cells.
Bars represent mean ± SEM; N number is represented by single data points, each showing the average data for 1 individual animal; ∗/+ indicate significance: ∗/+p < 0.05, ∗∗/++p < 0.01, ∗∗∗/+++p < 0.001; ∗ indicates significance to respective WT control, + indicates significance to respective untreated control, determined by 2-way ANOVA with Tukey’s multiple comparisons test.
A similar effect of GDF15 on EGFR expression was previously observed also in the developing hippocampus, where we found that GDF15 requires active C-X-C chemokine receptor type 4 (CXCR4) signaling to increase the number of EGFRh hippocampal progenitors (Carrillo-Garcia et al., 2014). Consistently, also in the embryonic GE, blockade of CXCR4 signaling by medium application of AMD3100 (AMD) prevented the increase in the number of EGFRh progenitors induced by GDF15 exposure (Figure S7A), and the addition of AMD to the culture medium affected specifically the ability of EGFRh progenitors to undergo clone formation (Figure S7B). Since AMD blockade interferes specifically with this class of progenitors, we applied it in situ to investigate how many of the proliferating EGFRh cells are present in the WT and mutant GE. Treatment with AMD overnight led to a significant decrease both in terms of total cycling cells and dividing APs in the WT and mutant tissue (Figures S7C–S7F). Independent of the location, no dividing cells could be observed in AMD-treated WT tissue, whereas in the mutant tissue CXCR4 blockade significantly decreased only the number of dividing APs (Figures S7E and S7F). Taken together, these data indicate that some of the dividing APs and SAPs represent EGFRh cells and that these cells, which are more reliant on active CXCR4 to undergo proliferation, are more abundant in the WT GE than in the mutant counterpart.
Discussion
The neonatal periventricular zone and the choroid plexus are the main sources of GDF15 within the brain (Bottner et al., 1999; Schober et al., 2001). Our data provide for the first time a function for GDF15 in this region, showing that it regulates growth factor responsiveness, cell-cycle kinetics, and cell survival, ultimately affecting the number of adult NSCs and Epen.
Our analysis focuses here on the effect of GDF15 on apical NSCs, a minor pool of NSCs in the adult V-SVZ characterized by P+ expression, an apical membrane and a primary cilium (Baur et al., 2022). Most Epen are born during mid- to late development, although they differentiate only after birth (Redmond et al., 2019). Around the same time, NSCs begin a transition into quiescence, which continues into the early postnatal weeks (Borrett et al., 2020; Fuentealba et al., 2015; Furutachi et al., 2015). The generation of Epen and NSCs is linked because they share a common progenitor (Ortiz-Alvarez et al., 2019). The increase of GDF15 expression at the late stages of development is temporally correlated with changes in cell-cycle dynamics and in EGFR expression in neural progenitors, suggesting a developmental role for the growth factor in the regulation of these processes. Supporting this hypothesis, we found that short exposure to exogenous GDF15 reduces progenitor proliferation and promotes EGFR expression, showing that the phenotype observed in the mutant V-SVZ is not the result of a selective process.
At a cellular level, GDF15 decreases the pool of cycling cells and apical cell division and it slows cell-cycle progression. However, GDF15 is not required for progenitors to permanently exit the cell cycle and differentiate. Instead, our data in vitro and in vivo support the notion that the extra progenitor cells generated in the mutant germinal niche will proceed to differentiate in a normal manner, leading to the generation of supernumerary Epen and NSCs and to neuronal progenitors that will undergo compensatory cell death. Compensatory cell death was also observed upon the increase in neural progenitor proliferation following the loss of mCD24 expression (Belvindrah et al., 2002), suggesting that it may represent a common route of regulation of neurogenesis in the postnatal niche. However, in the mutant embryonic GE, the increase in the number of ASCL1+ cells was not associated with extra cell death. Since many of the cells generated at E18 in the GE migrate away and undergo terminal differentiation in the dorsal telencephalon, it is possible that the supernumerary cells generated in the Gdf15−/− GE eventually undergo compensatory cell death in other regions of the telencephalon and therefore escaped our analysis. It is interesting to note that the increase in GDF15 expression temporally coincides with apical RG progenitors starting to withdraw from the cell cycle and transitioning to the state of quiescent adult NSCs. Gene signature analysis has revealed that this transition involves shutting down several biological processes occurring between E17 and P6 (Borrett et al., 2020), and that upon reactivation, adult NSCs reacquire characteristics of RG. Indeed, we here found that proliferating adult NSCs display the same increase in cell-cycle kinetics as observed in embryonic progenitors.
Our data also indicate that at the molecular level, GDF15 may affect at least in part the proliferation of APs by modulating EGFR expression. A brief exposure to exogenous GDF15 increased EGFR expression, and modulation of EGFR signaling affected the proliferation and number of APs. From late development onward, EGFR expression is also upregulated in NSCs, and EGF is the main mitogen for NSCs (Cesetti et al., 2009; Ciccolini, 2001). Extending these previous observations, we show here a close association between EGFR expression and proliferation also in vivo. Mutant progenitors appear more dependent on endogenous EGFR signaling to undergo fast proliferation, whereas the activation of EGFR had opposite effects in mutant and WT GE progenitors, suggesting that levels of EGFR expression affect the response to EGF. That EGFR signaling is an important component of the regulation of APs is consistent with the observation that in rodents, EGFR ligands are a constant component of the cerebrospinal fluid (Van Setten et al., 1999). Consistent with the regional expression of EGFR ligands, in the postnatal brain EGFR activation is crucial for the regulation of the differentiation of Epen cells from RG progenitors (Abdi et al., 2019). Embryonic human NSCs also overexpress GDF15 (Wang et al., 2010), and EGF is present in the human cerebrospinal fluid (Shnaper et al., 2009). Thus, our observation may have potential relevance for the regulation of proliferation in human NSCs.
Despite the effect of GDF15 on EGFR transduction, modulation of EGFR signaling did not rescue the Gdf15−/− phenotype in a manner similar to GDF15 application. This indicates that GDF15 also works through other, as yet unknown, mechanisms to regulate proliferation of apical NSCs.
Experimental procedures
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author, Francesca Ciccolini (ciccolini@nbio.uni-heidelberg.de).
Materials availability
No unique reagents were generated in this study.
Data and code availability
Large datasets and original code were not generated in this study.
Animals
Details are provided in the supplemental information. All of the animal experiments were approved by the Regierungspräsidium in Karlsruhe, Germany.
BrdU labeling in vivo
Time-mated (E18) pregnant Gdf15+/− females from heterozygous matings were injected intraperitoneally with BrdU (10 μg/g body weight) once and sacrificed after 2 h, or twice every 2 h and killed 6 h after the first injection.
Tissue and neurosphere cultures
Whole-mount preparations, brain tissue of 8-week-old animals, or E18 embryos were dissected as described before (Khatri et al., 2014). Details are provided in the supplemental information. Neurosphere cultures were established as previously described (Gakhar-Koppole et al., 2008).
Fluorescence-activated cell sorting (FACS)
Sorting was performed using a FACSVantage and a FACSAriaIII sorter (Becton Dickinson) as previously described (Carrillo-Garcia et al., 2010; Cesetti et al., 2009; Ciccolini et al., 2005). Details are provided in the supplemental information.
Western blot
Western blot was performed as previously described (Gakhar-Koppole et al., 2008). Details are provided in the supplemental information.
Immunofluorescence
Immunofluorescence was performed as previously described (Baur et al., 2022). Details are provided in the supplemental information.
Image acquisition and imaging
Capture and analysis of images were performed as previously described (Baur et al., 2022). Details are provided in the supplemental information.
Real-time PCR
RNA isolation and real-time PCR were performed as previously described (Baur et al., 2022). Details are provided in the supplemental information.
Acknowledgments
We thank Dr. Robert P. Doyle (Syracuse University, New York) for providing the GRASP peptide. K.B. was supported by the Interdisciplinary Center for Neuroscience (IZN) and the Landesgraduiertenförderung (LGF) of the Heidelberg University Graduate Academy. C.C.-G. was supported by contract research from the program “Adult Neural Stem Cell” of the Baden-Württemberg Stiftung. J.S. acknowledges the Deutsche Forschungsgemeinschaft (UN 34/23-1).
Author contributions
Conceptualization, project administration, and funding acquisition: F.C. Methodology: F.C. and J.S. Supervision: K.B. and F.C. Investigation: K.B., C.C.-G., Ş.Ş., M.v.H., G.H.-W., and C.M. Formal analysis: K.B. and C.C.-G. Visualization: K.B. and C.C.-G. Writing – original draft: K.B. and F.C. Writing – review & editing: K.B., C.C.-G., Ş.Ş., M.v.H., and F.C.
Declaration of interests
The authors declare no competing interests.
Published: February 15, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2024.01.008.
Supplemental information
References
- Abdi K., Lai C.H., Paez-Gonzalez P., Lay M., Pyun J., Kuo C.T. Uncovering inherent cellular plasticity of multiciliated ependyma leading to ventricular wall transformation and hydrocephalus. Nat. Commun. 2018;9:1655. doi: 10.1038/s41467-018-03812-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdi K., Neves G., Pyun J., Kiziltug E., Ahrens A., Kuo C.T. EGFR Signaling Termination via Numb Trafficking in Ependymal Progenitors Controls Postnatal Neurogenic Niche Differentiation. Cell Rep. 2019;28:2012–2022.e4. doi: 10.1016/j.celrep.2019.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony T.E., Klein C., Fishell G., Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Bandler R.C., Mayer C., Fishell G. Cortical interneuron specification: the juncture of genes, time and geometry. Curr. Opin. Neurobiol. 2017;42:17–24. doi: 10.1016/j.conb.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur K., Abdullah Y., Mandl C., Hölzl-Wenig G., Shi Y., Edelkraut U., Khatri P., Hagenston A.M., Irmler M., Beckers J., Ciccolini F. A novel stem cell type at the basal side of the subventricular zone maintains adult neurogenesis. EMBO Rep. 2022;23 doi: 10.15252/embr.202154078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R., Rougon G., Chazal G. Increased neurogenesis in adult mCD24-deficient mice. J. Neurosci. 2002;22:3594–3607. doi: 10.1523/JNEUROSCI.22-09-03594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner T., Tinsley I.C., Milliken B.T., Doebley S.A., Najjar N.R., Kerwood D.J., De Jonghe B.C., Hayes M.R., Doyle R.P. Creation of a Peptide Antagonist of the GFRAL-RET Receptor Complex for the Treatment of GDF15-Induced Malaise. J. Med. Chem. 2023;66:11237–11249. doi: 10.1021/acs.jmedchem.3c00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrett M.J., Innes B.T., Jeong D., Tahmasian N., Storer M.A., Bader G.D., Kaplan D.R., Miller F.D. Single-Cell Profiling Shows Murine Forebrain Neural Stem Cells Reacquire a Developmental State when Activated for Adult Neurogenesis. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108022. [DOI] [PubMed] [Google Scholar]
- Böttner M., Suter-Crazzolara C., Schober A., Unsicker K. Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res. 1999;297:103–110. doi: 10.1007/s004410051337. [DOI] [PubMed] [Google Scholar]
- Burrows R.C., Wancio D., Levitt P., Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Carrillo-García C., Prochnow S., Simeonova I.K., Strelau J., Hölzl-Wenig G., Mandl C., Unsicker K., von Bohlen Und Halbach O., Ciccolini F. Growth/differentiation factor 15 promotes EGFR signalling, and regulates proliferation and migration in the hippocampus of neonatal and young adult mice. Development. 2014;141:773–783. doi: 10.1242/dev.096131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-García C., Suh Y., Obernier K., Hölzl-Wenig G., Mandl C., Ciccolini F. Multipotent precursors in the anterior and hippocampal subventricular zone display similar transcription factor signatures but their proliferation and maintenance are differentially regulated. Mol. Cell. Neurosci. 2010;44:318–329. doi: 10.1016/j.mcn.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Casarosa S., Fode C., Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- Cesetti T., Obernier K., Bengtson C.P., Fila T., Mandl C., Hölzl-Wenig G., Wörner K., Eckstein V., Ciccolini F. Analysis of stem cell lineage progression in the neonatal subventricular zone identifies EGFR+/NG2- cells as transit-amplifying precursors. Stem Cell. 2009;27:1443–1454. doi: 10.1002/stem.74. [DOI] [PubMed] [Google Scholar]
- Chenn A., McConnell S.K. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Ciccolini F. Identification of two distinct types of multipotent neural precursors that appear sequentially during CNS development. Mol. Cell. Neurosci. 2001;17:895–907. doi: 10.1006/mcne.2001.0980. [DOI] [PubMed] [Google Scholar]
- Ciccolini F., Mandl C., Hölzl-Wenig G., Kehlenbach A., Hellwig A. Prospective isolation of late development multipotent precursors whose migration is promoted by EGFR. Dev. Biol. 2005;284:112–125. doi: 10.1016/j.ydbio.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Cochard L.M., Levros L.C., Jr., Joppé S.E., Pratesi F., Aumont A., Fernandes K.J.L. Manipulation of EGFR-Induced Signaling for the Recruitment of Quiescent Neural Stem Cells in the Adult Mouse Forebrain. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.621076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P., Fagan B.M., Magness S.T., Hutton S., Taranova O., Hayashi S., McMahon A., Rao M., Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev. Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Falk S., Bugeon S., Ninkovic J., Pilz G.A., Postiglione M.P., Cremer H., Knoblich J.A., Götz M. Time-Specific Effects of Spindle Positioning on Embryonic Progenitor Pool Composition and Adult Neural Stem Cell Seeding. Neuron. 2017;93:777–791.e3. doi: 10.1016/j.neuron.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferent J., Zaidi D., Francis F. Extracellular Control of Radial Glia Proliferation and Scaffolding During Cortical Development and Pathology. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.578341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba L.C., Rompani S.B., Parraguez J.I., Obernier K., Romero R., Cepko C.L., Alvarez-Buylla A. Embryonic Origin of Postnatal Neural Stem Cells. Cell. 2015;161:1644–1655. doi: 10.1016/j.cell.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutachi S., Miya H., Watanabe T., Kawai H., Yamasaki N., Harada Y., Imayoshi I., Nelson M., Nakayama K.I., Hirabayashi Y., Gotoh Y. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat. Neurosci. 2015;18:657–665. doi: 10.1038/nn.3989. [DOI] [PubMed] [Google Scholar]
- Gakhar-Koppole N., Hundeshagen P., Mandl C., Weyer S.W., Allinquant B., Müller U., Ciccolini F. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur. J. Neurosci. 2008;28:871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- Haubensak W., Attardo A., Denk W., Huttner W.B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P., Obernier K., Simeonova I.K., Hellwig A., Hölzl-Wenig G., Mandl C., Scholl C., Wölfl S., Winkler J., Gaspar J.A., et al. Proliferation and cilia dynamics in neural stem cells prospectively isolated from the SEZ. Sci. Rep. 2014;4:3803. doi: 10.1038/srep03803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y., Huttner W.B. Basal process and cell divisions of neural progenitors in the developing brain. Dev. Growth Differ. 2009;51:251–261. doi: 10.1111/j.1440-169X.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- Li F., Ruan X., Min L. Targeting both sides of the GDF15-GFRAL-RET receptor complex: A new approach to achieve body weight homeostasis. Genes Dis. 2017;4:183–184. doi: 10.1016/j.gendis.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q.R., Sun T., Zhu Z., Ma N., Garcia M., Stiles C.D., Rowitch D.H. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Mullican S.E., Lin-Schmidt X., Chin C.N., Chavez J.A., Furman J.L., Armstrong A.A., Beck S.C., South V.J., Dinh T.Q., Cash-Mason T.D., et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017;23:1150–1157. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- Noctor S.C., Martínez-Cerdeño V., Ivic L., Kriegstein A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Omiya H., Yamaguchi S., Watanabe T., Kuniya T., Harada Y., Kawaguchi D., Gotoh Y. BMP signaling suppresses Gemc1 expression and ependymal differentiation of mouse telencephalic progenitors. Sci. Rep. 2021;11:613. doi: 10.1038/s41598-020-79610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Álvarez G., Daclin M., Shihavuddin A., Lansade P., Fortoul A., Faucourt M., Clavreul S., Lalioti M.E., Taraviras S., Hippenmeyer S., et al. Adult Neural Stem Cells and Multiciliated Ependymal Cells Share a Common Lineage Regulated by the Geminin Family Members. Neuron. 2019;102:159–172.e7. doi: 10.1016/j.neuron.2019.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld T., Svendsen C.N. Requirement for neurogenesis to proceed through the division of neuronal progenitors following differentiation of epidermal growth factor and fibroblast growth factor-2-responsive human neural stem cells. Stem Cell. 2004;22:798–811. doi: 10.1634/stemcells.22-5-798. [DOI] [PubMed] [Google Scholar]
- Parras C.M., Galli R., Britz O., Soares S., Galichet C., Battiste J., Johnson J.E., Nakafuku M., Vescovi A., Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros T.J., Bultje R.S., Ross M.E., Fishell G., Anderson S.A. Apical versus Basal Neurogenesis Directs Cortical Interneuron Subclass Fate. Cell Rep. 2015;13:1090–1095. doi: 10.1016/j.celrep.2015.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz G.A., Shitamukai A., Reillo I., Pacary E., Schwausch J., Stahl R., Ninkovic J., Snippert H.J., Clevers H., Godinho L., et al. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat. Commun. 2013;4:2125. doi: 10.1038/ncomms3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond S.A., Figueres-Oñate M., Obernier K., Nascimento M.A., Parraguez J.I., López-Mascaraque L., Fuentealba L.C., Alvarez-Buylla A. Development of Ependymal and Postnatal Neural Stem Cells and Their Origin from a Common Embryonic Progenitor. Cell Rep. 2019;27:429–441.e3. doi: 10.1016/j.celrep.2019.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson J.P., Wagner B., Glitzner E., Heppner F.L., Steinkellner T., Khan D., Petritsch C., Pollak D.D., Sitte H.H., Sibilia M. Impaired neural stem cell expansion and hypersensitivity to epileptic seizures in mice lacking the EGFR in the brain. FEBS J. 2018;285:3175–3196. doi: 10.1111/febs.14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalabrino G. Epidermal Growth Factor in the CNS: A Beguiling Journey from Integrated Cell Biology to Multiple Sclerosis. An Extensive Translational Overview. Cell. Mol. Neurobiol. 2022;42:891–916. doi: 10.1007/s10571-020-00989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A., Böttner M., Strelau J., Kinscherf R., Bonaterra G.A., Barth M., Schilling L., Fairlie W.D., Breit S.N., Unsicker K. Expression of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in the perinatal, adult, and injured rat brain. J. Comp. Neurol. 2001;439:32–45. doi: 10.1002/cne.1333. [DOI] [PubMed] [Google Scholar]
- Shnaper S., Desbaillets I., Brown D.A., Murat A., Migliavacca E., Schluep M., Ostermann S., Hamou M.F., Stupp R., Breit S.N., et al. Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. Int. J. Cancer. 2009;125:2624–2630. doi: 10.1002/ijc.24639. [DOI] [PubMed] [Google Scholar]
- Suh Y., Obernier K., Hölzl-Wenig G., Mandl C., Herrmann A., Wörner K., Eckstein V., Ciccolini F. Interaction between DLX2 and EGFR regulates proliferation and neurogenesis of SVZ precursors. Mol. Cell. Neurosci. 2009;42:308–314. doi: 10.1016/j.mcn.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Tomas A., Futter C.E., Eden E.R. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24:26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M.a., Matsuzaki F., Osumi N., Kaibuchi K., Nakamura S., Casarosa S., Guillemot F., Nakafuku M. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development. 1999;126:443–456. doi: 10.1242/dev.126.3.443. [DOI] [PubMed] [Google Scholar]
- Turrero García M., Harwell C.C. Radial glia in the ventral telencephalon. FEBS Lett. 2017;591:3942–3959. doi: 10.1002/1873-3468.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler W.A., Haydar T.F. Multiplex genetic fate mapping reveals a novel route of neocortical neurogenesis, which is altered in the Ts65Dn mouse model of Down syndrome. J. Neurosci. 2013;33:5106–5119. doi: 10.1523/JNEUROSCI.5380-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Setten G.B., Edström L., Stibler H., Rasmussen S., Schultz G. Levels of transforming growth factor alpha (TGF-alpha) in human cerebrospinal fluid. Int. J. Dev. Neurosci. 1999;17:131–134. doi: 10.1016/s0736-5748(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Wang F., Guo Y., Yu H., Zheng L., Mi L., Gao W. Growth differentiation factor 15 in different stages of heart failure: potential screening implications. Biomarkers. 2010;15:671–676. doi: 10.3109/1354750X.2010.510580. [DOI] [PubMed] [Google Scholar]
- Yang L., Chang C.C., Sun Z., Madsen D., Zhu H., Padkjær S.B., Wu X., Huang T., Hultman K., Paulsen S.J., et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017;23:1158–1166. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- Young K.M., Fogarty M., Kessaris N., Richardson W.D. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J. Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Zhang Z., Zhang C., Zhang L., Robin A., Wang Y., Lu M., Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J. Neurosci. 2004;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xiao G., Johnson C., Cai Y., Horowitz Z.K., Mennicke C., Coffey R., Haider M., Threadgill D., Eliscu R., et al. Bulk and mosaic deletions of Egfr reveal regionally defined gliogenesis in the developing mouse forebrain. iScience. 2023;26 doi: 10.1016/j.isci.2023.106242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Anderson D.J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Large datasets and original code were not generated in this study.