Abstract
Purpose
Building and validating a clinical prediction model for novel coronavirus (COVID-19) re-positive cases in malnourished older adults.
Patients and Methods
Malnourished older adults from January to May 2023 were retrospectively collected from the Department of Geriatrics of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine. They were divided into a “non-re-positive” group and a “re-positive” group based on the number of COVID-19 infections, and into a training set and a validation set at a 7:3 ratio. The least absolute shrinkage and selection operator (LASSO) regression analysis was used to identify predictive factors for COVID-19 re-positivity in malnourished older adults, and a nomogram was constructed. Independent influencing factors were screened by multivariate logistic regression. The model’s goodness-of-fit, discrimination, calibration, and clinical impact were assessed by Hosmer-Lemeshow test, area under the curve (AUC), calibration curve, decision curve analysis (DCA), and clinical impact curve analysis (CIC), respectively.
Results
We included 347 cases, 243 in the training set, and 104 in the validation set. We screened 10 variables as factors influencing the outcome. By multivariate logistic regression analysis, preliminary identified protective factors, risk factors, and independent influencing factors that affect the re-positive outcome. We constructed a clinical prediction model for COVID-19 re-positivity in malnourished older adults. The Hosmer-Lemeshow test yielded χ2 =5.916, P =0.657; the AUC was 0.881; when the threshold probability was >8%, using this model to predict whether malnourished older adults were re-positive for COVID-19 was more beneficial than implementing intervention programs for all patients; when the threshold was >80%, the positive estimated value was closer to the actual number of cases.
Conclusion
This model can help identify the risk of COVID-19 re-positivity in malnourished older adults early, facilitate early clinical decision-making and intervention, and have important implications for improving patient outcomes. We also expect more large-scale, multicenter studies to further validate, refine, and update this model.
Keywords: malnutrition, COVID-19, re-positive, clinical prediction model
Introduction
COVID-19 infection has the characteristics of high mutation rate and a high transmissibility, 1 as of July 9, 2023, more than 767 million confirmed cases and more than 6.9 million deaths have been reported worldwide.2 Currently, China has entered a period of deep aging, with a large base and fast speed of aging population, 3,4 older adults are vulnerable groups during COVID-19 epidemic due to complex underlying diseases, atypical clinical symptoms, and multiple complications.5,6 Studies have found that COVID-19 infection is a high-risk group for nutritional risk and malnutrition, with rates of nutritional risk and malnutrition as high as 77% and 50%, respectively.7–10 A study in Wuhan, China, showed that among patients infected with COVID-19 aged over 65 years, the rates of nutritional risk and malnutrition were 27.5% and 52.7%, respectively.11 In addition, malnutrition can activate related inflammatory pathways, thereby damaging the human immune system. For older adults and critically ill patients with multiple underlying diseases, early initiation of enteral and parenteral nutrition support can effectively protect the intestinal mucosal barrier and immune function, reduce the incidence and mortality of infectious complications.12–14 Therefore, timely and standardized nutritional support can effectively improve the nutritional status, immune function, and clinical outcomes of COVID-19 infection, 15 promote recovery and reduce the risk of recurrence, 16,17 improve patient survival and quality of life.Therefore, how to quickly and accurately foresee the risk factors of COVID-19 “re-positive” in malnourished older adults is a clinical problem that needs to be solved urgently.
In recent years, clinical prediction models based on machine learning have helped to further understand the important determinants of disease risk, more accurately assess the condition and prognosis, and also facilitate communication and cooperation between doctors and patients by screening high-risk factors of disease as predictors and building relevant models to predict the probability of outcome occurrence.18–21 This has an important role in the three-level prevention system of disease. This study retrospectively analyzed the clinical data of 347 malnourished older adults, included relevant influencing factors affecting COVID-19 re-positive in malnourished older adults and constructed a clinical prediction model, aiming to predict the possible risk factors affecting COVID-19 re-positive, so as to intervene early, assist clinical decision-making, optimize clinical management, and improve patient prognosis.
Materials and Methods
Study Subjects and Inclusion and Exclusion Criteria
Older adults with malnutrition in the geriatric department of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine were retrospectively collected from January to May 2023. According to the number of COVID-19 infections, they were divided into “non-re-positive” and “re-positive” groups, and divided into training and testing sets at a ratio of 7:3.
Inclusion Criteria
(1) age ≥ 65 years; (2) meeting the diagnostic criteria for malnutrition; (3) “non-re-positive” patients who had not been infected, had been infected once, or were currently infected with COVID-19 for the first time; (4) “re-positive” patients who met the diagnostic criteria for COVID-19 “re-positive”; (5) complete relevant clinical data and informed consent.
Exclusion Criteria
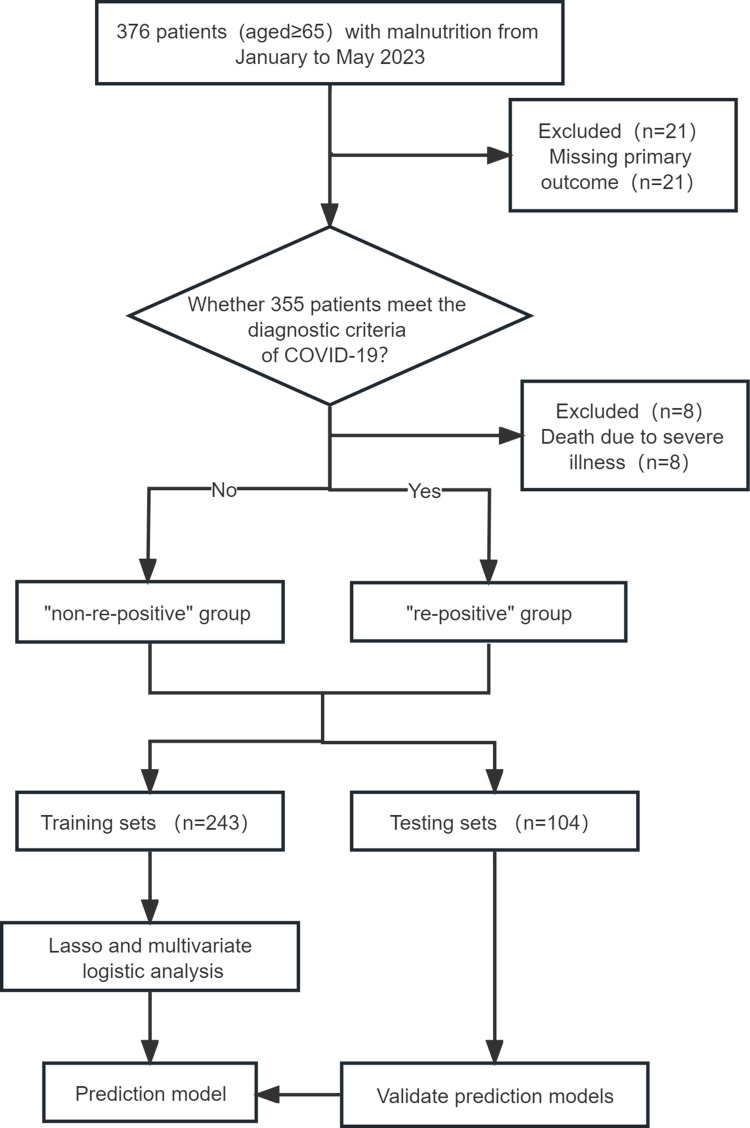
(1) incomplete relevant clinical data; (2) patients with dementia, mental illness, or who are unable to cooperate with data collection (Figure 1).
Figure 1.
Patient Flow Diagram.
Diagnostic Criteria
Older Adults Malnutrition Diagnostic Criteria
The Geriatric Nutritional Risk Index (GNRI) proposed by the American Society for Clinical Nutrition22 was used: GNRI=1.489*serum ALB(g/dL)+41.7*(actual weight/ideal weight). Ideal weight was calculated by the Lorentz formula: ideal weight=22*height(m)*height(m), If the actual weight > ideal weight, then the actual weight/ideal weight was taken as 1; if the actual weight < ideal weight, then the actual ratio was used for calculation. Scoring criteria: According to the value of GNRI, 92≤GNRI<98, 82≤GNRI<92, and GNRI<82 corresponded to mild malnutrition, moderate malnutrition, and severe malnutrition, respectively.
COVID-19 Diagnostic Criteria
Coronavirus Disease 2019 (COVID-19) Treatment Guidelines23 can be referred to. COVID-19 “re-positive” diagnostic criteria: (1) patient had previously been infected with COVID-19 and tested positive for nucleic acid, and then tested negative for nucleic acid after improvement, and then tested positive for nucleic acid again; (2) false positives caused by specimen collection and testing were excluded.
Procedure
The general clinical data and laboratory indicators of the patients in this study were obtained by reviewing the electronic record system of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine and by asking the patients themselves or their families, and the specific items included age, body mass index (BMI), gender, GNRI, cerebral infarction, renal failure, tumor, pneumonia, chronic obstructive pulmonary disease, type 2 diabetes, coronary disease, hypertension, skin ulcer, history of drinking, history of smoking, vaccination, white blood cell (WBC), neutrophil granulocyte, lymphocyte, c-reactive protein (CRP), d-dimer, hemoglobin (Hb), serum creatinine (Scr), albumin(ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST);The measurement of the patients’ nutritional status assessment was mainly referred to as part of the new version of the Mini Nutritional Assessment(MNAR)24 and made into a questionnaire form, which was measured, evaluated, and filled in by the staff who had received standardized training on the patients.The items included mid-arm circumference, gastrocnemius circumference, protein intake, intake of vegetables and fruits, diet status in recent 3 months, activity situation, and neurological and psychological problems.
Statistical Analysis
Data on gender, age, height, weight, BMI, underlying diseases, vaccination status, diet, and laboratory results were collected for all study subjects. According to the COVID-19 infection status of the patients, they were divided into “non-re-positive” and “re-positive” groups. Statistical analysis was performed using R-4.2.2 and SPSS 26.0 software. If the measurement data followed a normal distribution, they were expressed as mean±standard deviation (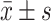 ) and independent samples t-test was used; if they followed a non-normal distribution, they were expressed as median (quartile) (M(Qn)) and Mann–Whitney U-test was used; count data were expressed as number (percentage) (n(%)) and χ2 test was used. LASSO regression was used to reduce the dimensionality of the included variables and screen out the influencing factors of COVID-19 “re-positive” in malnourished older adults; further, a clinical prediction model was established using multivariate logistic regression and a nomogram was drawn;Hosmer-Lemeshow test was used to evaluate the model fit; and χ2 statistic and P value were calculated; a calibration curve was drawn; an AUC was used to judge the discrimination of the model; and DCA and CIC were used to evaluate the validity and clinical impact of the model. P<0.05 indicated that the difference was statistically significant.
) and independent samples t-test was used; if they followed a non-normal distribution, they were expressed as median (quartile) (M(Qn)) and Mann–Whitney U-test was used; count data were expressed as number (percentage) (n(%)) and χ2 test was used. LASSO regression was used to reduce the dimensionality of the included variables and screen out the influencing factors of COVID-19 “re-positive” in malnourished older adults; further, a clinical prediction model was established using multivariate logistic regression and a nomogram was drawn;Hosmer-Lemeshow test was used to evaluate the model fit; and χ2 statistic and P value were calculated; a calibration curve was drawn; an AUC was used to judge the discrimination of the model; and DCA and CIC were used to evaluate the validity and clinical impact of the model. P<0.05 indicated that the difference was statistically significant.
Results
Comparison of General Data Between Training and Testing Sets
A total of 347 malnourished older adults were finally included, and divided into training and testing sets at a ratio of 7:3, with 243 and 104 cases, respectively. There were no statistically significant differences between the training and testing sets in age, gender, BMI, mid-arm circumference, calf circumference, smoking history, drinking history, vaccination status, activity status, psychological status, skin condition, dietary status in the past 3 months, vegetable and fruit intake, protein intake, hypertension, type 2 diabetes mellitus, coronary heart disease, cerebral infarction, chronic obstructive pulmonary disease, pneumonia, renal failure, tumor, WBC, neutrophil granulocyte, lymphocyte, CRP, Hb, d-dimer, ALB, ALT, AST, Scr (P>0.05), (Table 1).
Table 1.
Comparison of Clinical Information Between Training Set and Validation Set((n(%)) or Cases/%)
| Variables | Training Set (n=243) | Validation Set(n=104) | Z/χ2 | P value |
|---|---|---|---|---|
| Age (years) | 80.000(74.00, 86.00) | 78.000(74.00, 84.00) | −1.304 | 0.192 |
| BMI (kg/m2) | 19.835(18.80, 21.10) | 19.678(19.00, 21.60) | −0.505 | 0.613 |
| WBC (10^9/L) | 5.450(4.00, 7.20) | 5.850(4.30, 7.70) | −1.157 | 0.247 |
| Neutrophil granulocyte (10^9/L) | 3.760(2.80, 5.30) | 3.960(3.00, 5.70) | −0.932 | 0.352 |
| Lymphocyte (10^9/L) | 1.020(0.70, 1.40) | 1.075(0.70, 1.50) | −0.822 | 0.411 |
| CRP (mg/L) | 4.390(1.20, 24.10) | 8.780(1.90, 24.70) | −1.642 | 0.101 |
| D-dimer (ug/L) | 0.560(0.30, 1.30) | 0.645(0.30, 1.30) | −0.624 | 0.533 |
| Hb (g/L) | 116.000(102.00, 124.00) | 116.000(104.50, 123.80) | −0.314 | 0.753 |
| Scr (umol/L) | 77.900(62.30, 97.80) | 70.650(56.40, 91.70) | −1.701 | 0.089 |
| ALB (g/L) | 34.900(32.90, 36.10) | 34.950(33.50, 36.00) | −0.037 | 0.970 |
| AST (%) | ||||
| ≤35 | 158(65.02) | 69(66.35) | 1.755 | 0.625 |
| 35<AST≤105 | 77(31.69) | 31(29.81) | ||
| 105<AST≤175 | 3(1.23) | 3(2.88) | ||
| >175 | 5(2.06) | 1(0.96) | ||
| ALT (%) | ||||
| ≤40 | 167(68.72) | 76(73.08) | 2.255 | 0.521 |
| 40<AST≤120 | 68(27.98) | 27(25.96) | ||
| 120<AST≤200 | 4(1.65) | 1(0.96) | ||
| >200 | 4(1.65) | 0(0.00) | ||
| Gender (%) | ||||
| Male | 133(54.73) | 54(51.92) | 0.231 | 0.631 |
| Female | 110(45.27) | 50(48.08) | ||
| GNRI (%) | ||||
| <82 | 26(10.70) | 7(6.73) | 1.607 | 0.448 |
| 82≤GNRI<92 | 148(60.91) | 69(66.35) | ||
| 92≤GNRI<98 | 69(28.40) | 28(26.92) | ||
| Mid-arm circumference (%) | ||||
| <21 | 21(8.64) | 8(7.69) | 1.107 | 0.575 |
| 21≤Mid-arm circumference<22 | 92(37.86) | 34(32.69) | ||
| ≥22 | 130(53.50) | 62(59.62) | ||
| Gastrocnemius circumference (%) | ||||
| <31 | 72(29.63) | 24(23.08) | 1.563 | 0.211 |
| ≥31 | 171(70.37) | 80(76.92) | ||
| Cerebral infarction (%) | ||||
| No | 143(58.85) | 68(65.38) | 1.306 | 0.253 |
| Yes | 100(41.15) | 36(34.62) | ||
| Renal failure (%) | ||||
| No | 218(89.71) | 94(90.38) | 0.036 | 0.849 |
| Yes | 25(10.29) | 10(9.62) | ||
| Tumour (%) | ||||
| No | 215(88.48) | 95(91.35) | 0.629 | 0.428 |
| Yes | 28(11.52) | 9(8.65) | ||
| Pneumonia (%) | ||||
| No | 195(80.25) | 76(73.08) | 2.189 | 0.139 |
| Yes | 48(19.75) | 28(26.92) | ||
| Chronic obstructive pulmonary disease (%) | ||||
| No | 181(74.49) | 79(75.96) | 0.084 | 0.771 |
| Yes | 62(25.51) | 25(24.04) | ||
| Type 2 diabetes (%) | ||||
| No | 167(68.72) | 70(67.31) | 0.068 | 0.795 |
| Yes | 76(31.28) | 34(32.69) | ||
| Coronary disease (%) | ||||
| No | 166(68.31) | 77(74.04) | 1.138 | 0.286 |
| Yes | 77(31.69) | 27(25.96) | ||
| Hypertension (%) | ||||
| No | 105(43.21) | 39(37.50) | 0.978 | 0.323 |
| Yes | 138(56.79) | 65(62.50) | ||
| Skin ulcer (%) | ||||
| Yes | 78(32.10) | 26(25.00) | 1.749 | 0.186 |
| No | 165(67.90) | 78(75.00) | ||
| Protein intake (%) | ||||
| Less | 49(20.16) | 17(16.35) | 1.157 | 0.763 |
| Commonly | 92(37.90) | 42(40.38) | ||
| More | 102(41.94) | 45(43.27) | ||
| History of drinking (%) | ||||
| No | 127(52.26) | 56(53.85) | 0.073 | 0.787 |
| Yes | 116(47.74) | 48(46.15) | ||
| Intake of vegetables and fruits (%) | ||||
| Less than twice a day | 121(49.79) | 51(49.04) | 0.017 | 0.897 |
| Twice or more a day | 122(50.21) | 53(50.96) | ||
| Diet status in recent 3 months (%) | ||||
| Severe loss of appetite | 17(7.00) | 7(6.73) | 6.204 | 0.102 |
| Moderate loss of appetite | 77(31.69) | 21(20.19) | ||
| Mild loss of appetite | 73(30.04) | 43(41.35) | ||
| No | 76(31.28) | 33(31.73) | ||
| Activity situation (%) | ||||
| Bed-ridden or long-term seated person | 63(25.93) | 22(21.15) | 1.231 | 0.540 |
| Can leave the bed or table and chair, but can not go out | 103(42.39) | 50(48.08) | ||
| Can go out on your own | 77(31.69) | 32(30.77) | ||
| Nervous and psychological problems (%) | ||||
| Severe dementia or depression | 26(10.70) | 10(9.62) | 0.220 | 0.896 |
| Mild dementia | 62(25.51) | 25(24.04) | ||
| No psychological problems | 155(63.79) | 69(66.35) | ||
| History of smoking (%) | ||||
| No | 152(62.55) | 64(61.54) | 0.032 | 0.858 |
| Yes | 91(37.45) | 40(38.46) | ||
| Vaccination (%) | ||||
| No | 68(27.98) | 19(18.27) | 6.056 | 0.109 |
| Potion | 124(51.03) | 58(55.77) | ||
| Two doses | 36(14.81) | 23(22.12) | ||
| Three doses | 15(6.17) | 4(3.85) |
Abbreviations: BMI, body mass index; WBC, white blood cell count; CRP, c-reactive protein; Hb, hemoglobin; Scr, serum creatinine; ALB, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GNRI, geriatric nutritional risk index.
LASSO Regression Screening of Predictive Factors for COVID-19
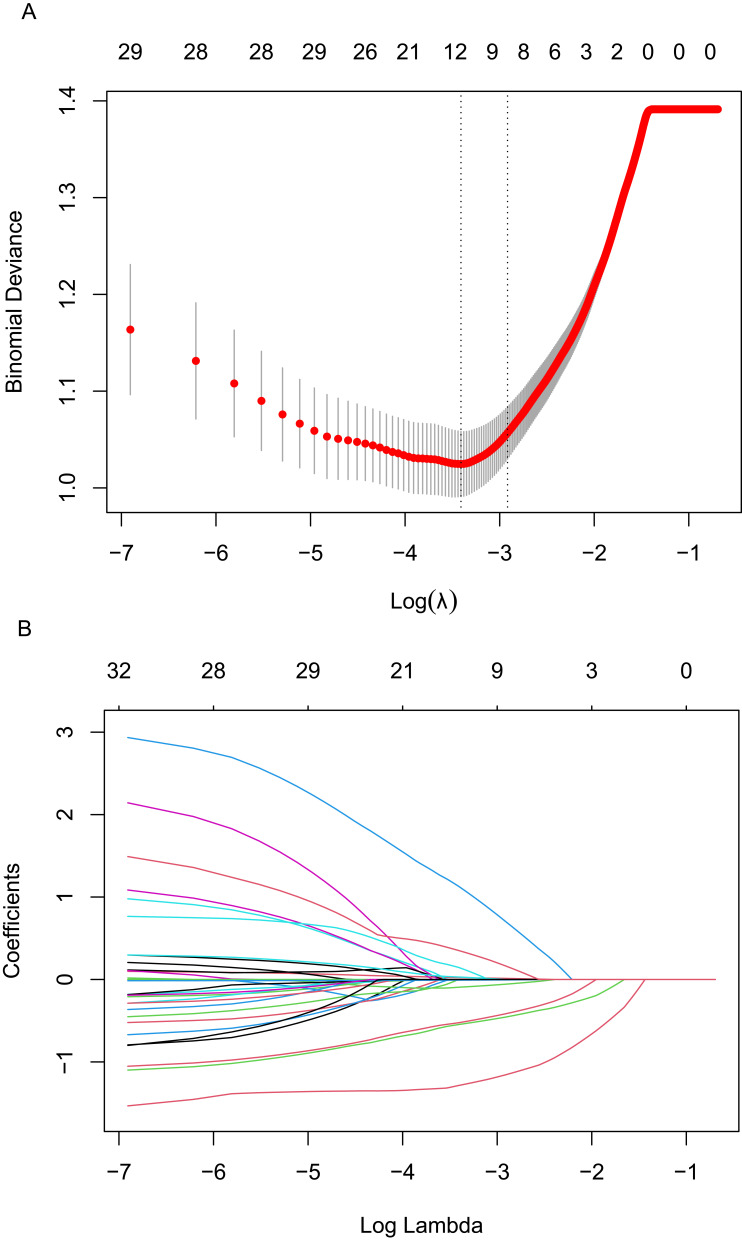
“re-positive” in malnourished older adults LASSO regression was used to reduce the dimensionality of the included variables. A total of 10-fold cross-validation was performed to analyze the correlation among the variables. The LASSO coefficient curve of the included variables was obtained as shown in Figure 2A. When the distance from the mean square error was one standard error (λ1se), that is, λ1se=0.054 and log(λ)=−2.917, the model was optimal. A total of 9 non-zero coefficient predictive variables were screened out as influencing factors for COVID-19 “re-positive”, as shown in Figure 2B. They were as follows: age, vaccination status, vegetable and fruit intake status, protein intake amount, renal failure, lymphocyte, Hb, d-dimer and AST.
Figure 2.
Plots for LASSO regression model. (A) 10-fold cross-validation plot for the penalty term. (B) A LASSO coefficient profiles plot of the 33 texture features was produced against the log (lambda) sequence.
Logistic Regression Analysis of Predictive Factors for COVID-19 “Re-Positive” in Malnourished Older Adults
Whether COVID-19 infection was “re-positive” or not was used as the dependent variable. The 9 predictive variables screened by LASSO regression analysis and GNRI which was closely related to this study were used as independent variables.22,25 Multivariate logistic regression analysis was performed to calculate the odds ratio (OR) and 95% confidence interval (CI). The size of the confidence interval depends on the sample size and the standard deviation of the study group.26 If the sample size is large, this leads to “greater confidence” and narrower confidence intervals; if the confidence interval is wide, this may mean that the sample is small; and if the dispersion is high, there is less certainty in the conclusions and wider confidence intervals.27 A positive correlation between exposure and outcome implies an OR > 1.0, and a negative correlation implies an OR < 1.0.28 Multifactorial logistic regression analyses in this study showed that age (OR 1.041; 95% CI 0.991–1.093), GNRI (OR 0.738; 95% CI 0.381–1.430), vaccination (OR 0.816; 95% CI 0.491–1.355), Intake of vegetables and fruits (OR 0.564; 95% CI 0.309 −1.028), protein intake (OR 0.588; 95% CI 0.353–0.982), renal failure (OR 9.299; 95% CI 2.580–33.516), lymphocyte (OR 0.121; 95% CI 0.060–0.242), Hb (OR 0.990; 95% CI 0.973–1.008); d-dimer (OR 1.236; 95% CI 0.901–1.696); AST (OR 1.016; 95% CI 1.005–1.028), where the CI for Renal failure was significantly larger, which may be related to the limited sample size and the inability to fully correct for bias and confounding.In addition, in this study, when the regression coefficients in the regression analysis were positive and the OR value was >1, the factors were determined to be risk factors affecting the outcome; conversely, they were protective factors, ie, high GRNI, higher number of doses of vaccination, high intake of vegetables and fruits, high intake of proteins, high lymphocytes, and high Hb were protective factors, and high age, renal failure, high d-dimer, and high AST were risk factors, as shown in Table 2. In addition, vegetable and fruit intake, protein intake, lymphocytes, renal failure, and AST were independent influences (P<0.05). Meanwhile, the Hosmer-Lemeshow test yielded χ2 = 5.916, P= 0.657 (P>0.05), indicating that the predictive model fit was good.
Table 2.
Multifactorial Logistic Regression of Predictive Factors for “Re-Positive” COVID-19 Infection in Malnourished Older Adults
| Index | β | SE | Wald | df | P value | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| Age | 0.040 | 0.025 | 2.584 | 1 | 0.108 | 1.041 | 0.991, 1.093 |
| GNRI | −0.303 | 0.337 | 0.809 | 1 | 0.368 | 0.738 | 0.381, 1.430 |
| Vaccination | −0.204 | 0.259 | 0.619 | 1 | 0.431 | 0.816 | 0.491, 1.355 |
| Intake of vegetables and fruits | −0.574 | 0.307 | 3.496 | 1 | 0.062 | 0.564 | 0.309, 1.028 |
| Protein intake | −0.530 | 0.261 | 4.113 | 1 | 0.043 | 0.588 | 0.353, 0.982 |
| Renal failure | 2.230 | 0.654 | 11.621 | 1 | 0.001 | 9.299 | 2.580, 33.516 |
| Lymphocyte | −2.115 | 0.355 | 35.398 | 1 | 0.000 | 0.121 | 0.060, 0.242 |
| Hb | −0.010 | 0.009 | 1.172 | 1 | 0.279 | 0.990 | 0.973, 1.008 |
| D-dimer | 0.212 | 0.161 | 1.727 | 1 | 0.189 | 1.236 | 0.901, 1.696 |
| AST | 0.016 | 0.006 | 7.544 | 1 | 0.006 | 1.016 | 1.005, 1.028 |
| Constant | 0.706 | 2.408 | 0.086 | 1 | 0.769 | 2.026 |
Abbreviations: GNRI, geriatric nutritional risk index; Hb, hemoglobin.
Establishment of a Clinical Prediction Model
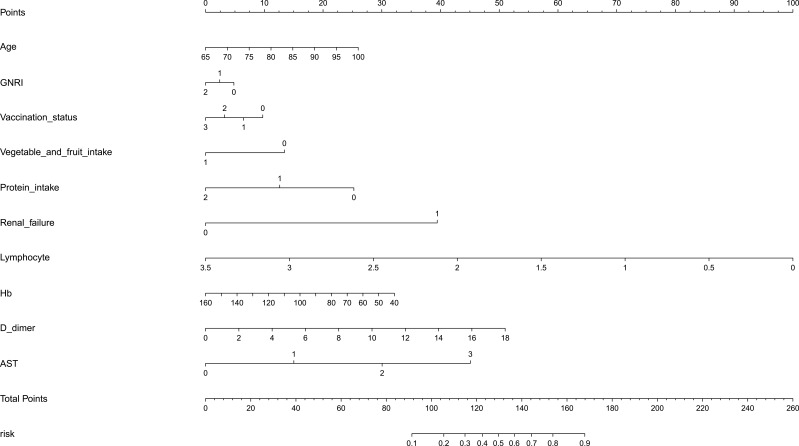
A clinical prediction model nomogram was constructed using the 10 variables included in the logistic regression model as predictive factors and whether COVID-19 infection was “re-positive” or not in malnourished older adults as clinical outcome. The length of each variable segment in the nomogram was positively correlated with its influence on the clinical outcome. The total score was the sum of the scores of each variable. The scale value corresponding to the total score was the risk probability of COVID-19 infection “re-positive” in malnourished older adults; see Figure 3.
Figure 3.
The nomogram based on the multivariable regression model.
Abbreviations: GNRI, Geriatric Nutritional Risk Index;Hb, hemoglobin; AST, aspartate aminotransferase.
Validation of a Clinical Prediction Model
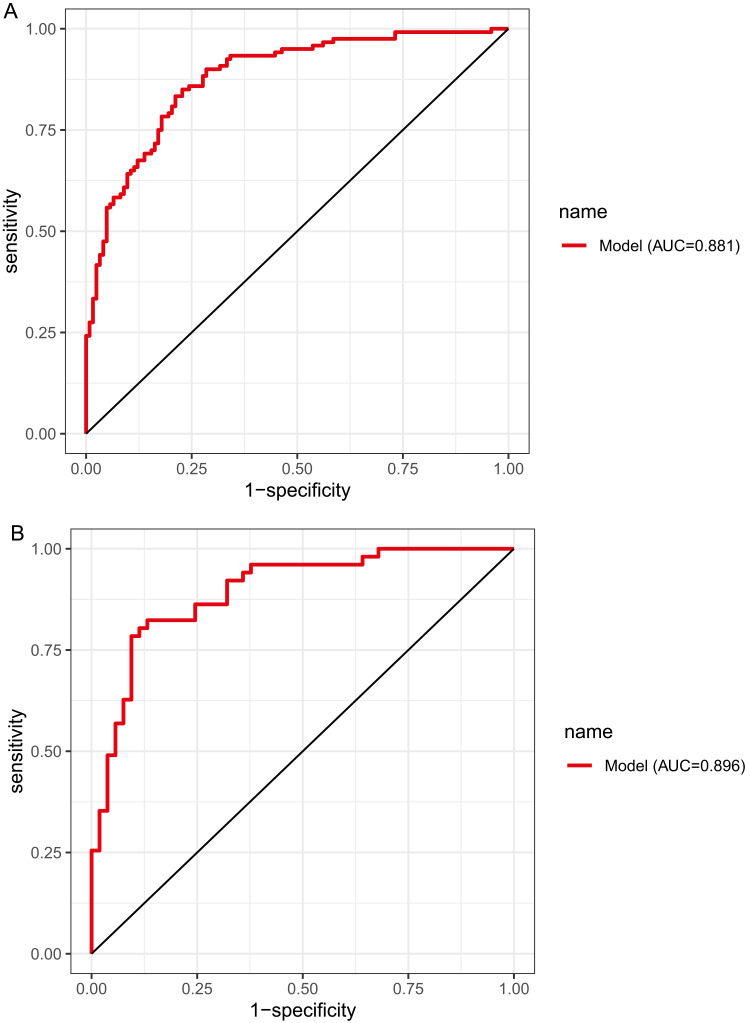
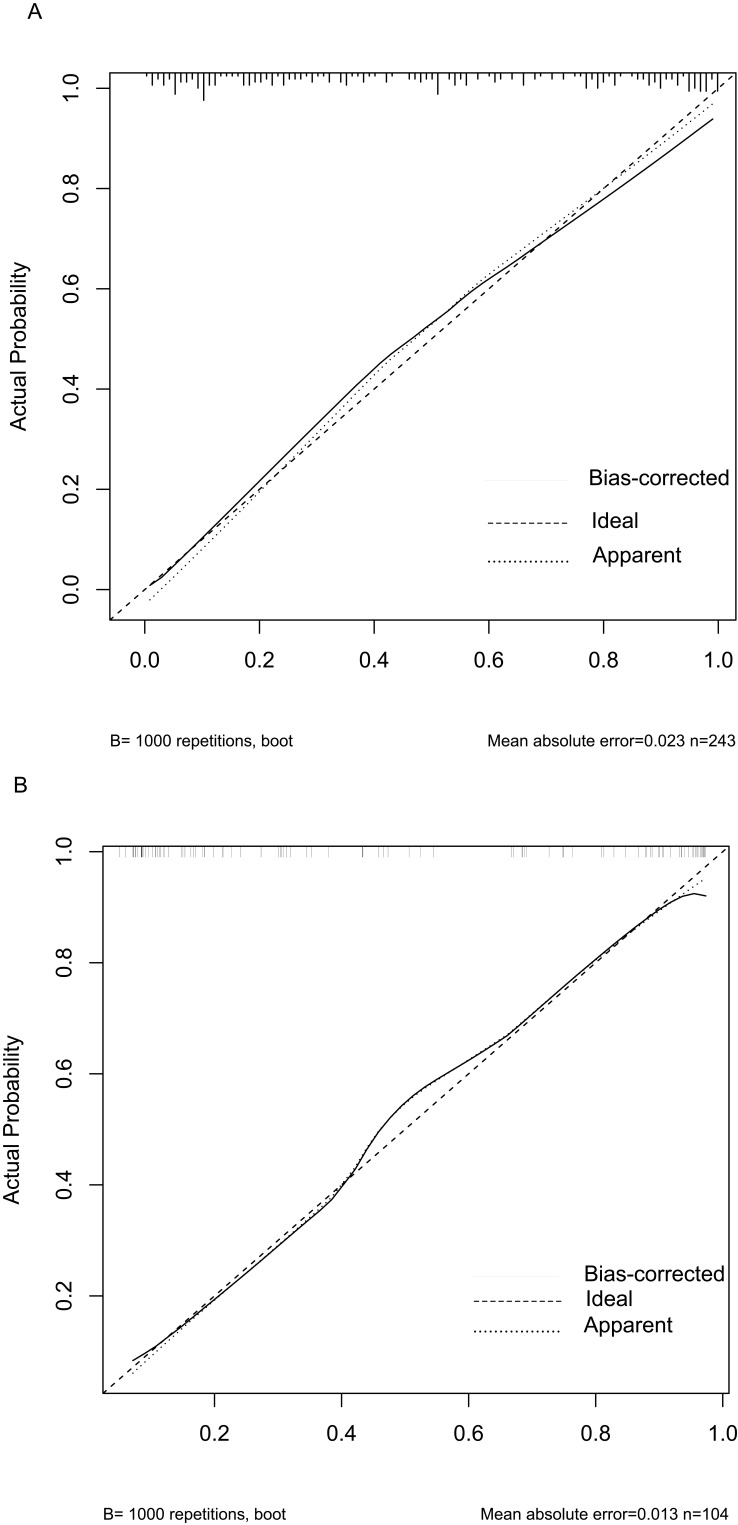
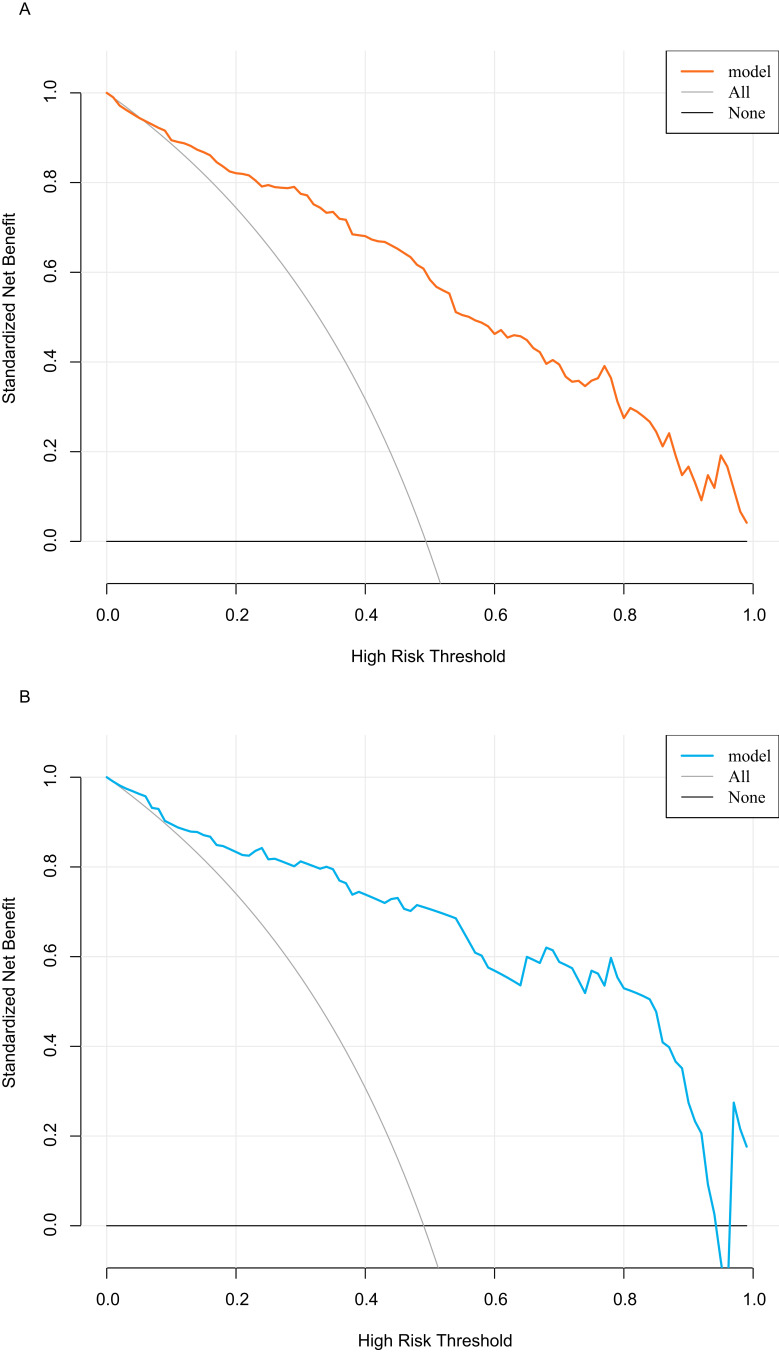
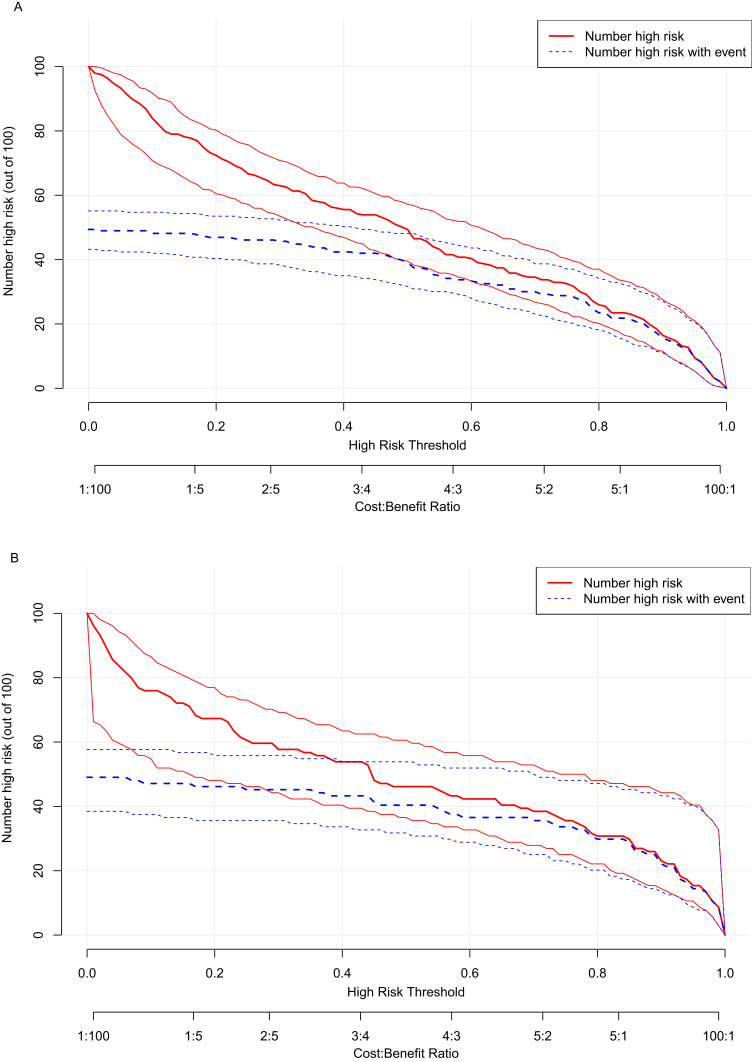
The above model was validated using data from 104 cases in the testing set. The predictive performance of the prediction model was evaluated from three aspects: discrimination, calibration and clinical impact. The AUC of the training set was 0.881 (95% CI: 0.839–0.923), see Figure 4A, and that of the validation set was 0.896 (95% CI: 0.835–0.956), see Figure 4B, indicating that the prediction model had high discrimination and good discrimination ability. At the same time, the calibration curve suggested that when the predicted probability was 15%-70%, the model prediction curve was close to the actual observation curve, indicating that the prediction model had good calibration ability; see Figure 5A and B. According to DCA analysis, when the threshold probability >8%, using this prediction model to predict whether COVID-19 infection is a “re-positive” risk in malnourished older adults is more beneficial than implementing an intervention plan for all patients. The net benefit of the prediction model was significantly higher than that of all or no intervention; see Figure 6A and B. Based on DCA, CIC was further drawn to evaluate the clinical impact of the model, showing the estimated number of positive cases and the actual number of cases at different risk thresholds. The results suggested that when the risk threshold was >80%, the positive estimate value was close to the actual number of cases. See Figure 7A and B.
Figure 4.
ROC curves. (A) Training set. (B) Validation set.
Figure 5.
Calibration curves. (A)Training set. (B) Validation set.
Figure 6.
Decision curve analysis. (A)Training set. (B) Validation set.
Figure 7.
Clinical impact curve analysis.(A)Training set. (B) Validation set.
Discussion
Since January 8, 2023, China has implemented “Class B management” for COVID-19 infection, and the focus of prevention and control has shifted to “maintaining health and preventing severe cases.” The incubation period of COVID-19 variants has shortened, and the pathogenicity has shown a significant downward trend, but related studies have shown that the risk of developing severe or fatal cases is still high for older adults, 29 another study found that malnourished older adults are more likely to develop severe cases, 30,31 leading to an increase in severe and mortality rates.32,33 In view of the prominent role of nutritional support in preventing and slowing down the development of infection, it is of great guiding significance for how to carry out the next step of nutritional support and predict the disease progression and prognosis of malnourished older adults to foresee the risk factors of COVID-19 “re-positive” in malnourished older adults as early as possible. At present, there are still few studies on COVID-19 “re-positive” in malnourished older adults at home and abroad. This study retrospectively analyzed the influencing factors of COVID-19 “re-positive” in malnourished older adults, screened risk factors by dimensionality reduction, constructed and validated models, aiming to provide a basis for accurately identifying high-risk population patients and provide reference for early intervention and medical decision-making.
A total of 347 valid cases were included in this study, with 243 cases in the training set and 104 cases in the testing set. The 9 predictive variables screened by LASSO regression were age, vaccination status, vegetable and fruit intake status, protein intake amount, renal failure, lymphocyte, Hb, d-dimer, AST and GNRI which were closely related to this study as factors affecting the outcome. Through multivariate logistic regression analysis, it was preliminary judged that high GNRI, more doses of vaccination, high vegetable and fruit intake amount, high protein intake amount, high lymphocyte and high Hb were protective factors; age, renal failure, high d-dimer and high AST were risk factors. Among them, protein intake amount, lymphocyte, renal failure and AST were independent influencing factors. A clinical prediction model for COVID-19 “re-positive” in malnourished older adults was constructed. Hosmer-Lemeshow test yielded χ2 =5.916, P=0.657, indicating that the prediction model had a good fit. Both the training set and the testing set showed that the prediction diagnosis was consistent with the actual diagnosis. The AUC of the prediction model in the training set was 0.881 and that in the testing set was 0.896, indicating that the prediction model had good accuracy. At the same time, DCA and CIC also suggested that the prediction model had high clinical validity and impact. The clinical prediction model for COVID-19 “re-positive” in older adults malnourished patients showed that controlling underlying diseases and balancing diet can help reduce the risk of COVID-19 “re-positive” in older adults.
Protein intake amount, renal failure, lymphocyte and AST were independent influencing factors for COVID-19 “re-positive” in malnourished older adults. Previous studies have shown that energy consumption and protein breakdown are accelerated in COVID-19 infection patients, 34 resulting in a reduction of available protein in the body, leading to a decrease in the number of functional active immunoglobulins and intestinal-associated lymphoid tissue, hindering the role of intestinal mucosal defense against infection;35,36 another study37 found that patients with comorbidities were more likely to have re-positive after discharge, compared with those with normal liver or kidney function, re-positive patients with liver or kidney function impairment had a 2.32-fold higher risk of developing severe cases, 38–40 and the tendency of severity and mortality risk increased significantly. Therefore, adequate intake of high-quality protein is essential for antibody production, 41 while for patients with liver or kidney dysfunction, protein intake should be strictly and standardized controlled.42 Lymphocyte count can predict disease severity, hospitalization time and prognosis of COVID-19 patients, 43–45 and several studies have shown46,47 that lymphocyte count can be used as a risk factor for re-positive rate of COVID-19 patients, and has certain clinical value for predicting recurrence, which is consistent with this study’s lymphocyte as an independent influencing factor and protective factor for “re-positive”. Age, renal failure, high d-dimer and high AST were risk factors for “re-positive”. Some studies48,49 have shown that elevated levels of ALT, AST, erythrocyte sedimentation rate, d-dimer and decreased neutrophil count are risk factors for re-positive. Previous studies have found that whether COVID-19 infection is “re-positive” or not is related to the age of the patient, 46 and due to the poor health status and low immunity of older adults, the virus clearance rate and tolerance in the body are relatively weak, which makes it easier to cause re-positive.50 High GNRI, more doses of vaccination, high vegetable and fruit intake amount, high protein intake amount, high lymphocyte and high Hb were protective factors for “re-positive”. GNRI is the geriatric nutritional risk index, 22 which integrates three indicators of serum albumin level, actual weight and ideal weight, and GNRI score is proportional to the nutritional status of the patient, and can more fully reflect the nutritional status of the patient’s body.51,52 Related studies have shown53 that GNRI assessment may provide valuable information for predicting the prognosis of COVID-19 pneumonia in older adults. Several previous studies have attempted to compare the GNRI with other standardized indices such as the MNA and the GNRI, and have found the GNRI to be valid for use in older adults, but mostly in combination with other indices.54 In terms of ongoing exploratory studies on the validity of the GNRI in Cairo and Egypt, it was found that the GNRI is more suitable for the classification of nutritional status and the identification of nutrition-related complications in hospitalized older adults than the MNA.55 In terms of using the GNRI to assess the nutritional status of older adults with specific diseases it was found that the GNRI predicts mortality in hemodialysis patients, that it is strongly related to exercise tolerance, that it can be used as a nutritional assessment scale for older patients with chronic obstructive pulmonary disease, that it predicts survival time in older patients with squamous cell carcinoma of the esophagus, and that the GNRI provides a reliable assessment of patients with a potential need for nutritional support, . Especially for older adults with dementia and aphasia.55–58 In addition, the GNRI is time-consuming, easy to use, and requires minimal involvement to help clinical staff (especially dietitians) diagnose malnourished individuals, which is one of the reasons why the GNRI has been accepted as a tool for assessing the nutritional status of hospitalized older adults.Vaccines are the “ultimate weapon” to end infectious diseases, and related studies have shown that vaccinating older adults can effectively reduce the risk of developing severe or critical cases or even death after COVID-19 infection, 59,60 and enhancing immunity can help further improve the protective effect.61 A series of studies in Malaysia found62 that the vaccine efficacy in intensive care admissions for older adults was significantly reduced. If this study is confirmed in other studies, increasing supplemental protection for severe disease and death (especially for older adults) before the expected wave of infection, strengthening vaccination is still a key tool to reduce the burden and mortality of COVID-19 on the health care system.63 Many studies have shown that eating more fruits, vegetables and whole grain foods can reduce the risk and incidence of COVID-19 pneumonia.64–66
Compared with previous studies, we have the following advantages. First, we based our study on a malnourished older adult population and then explored the risk factors associated with the COVID-19 re-positive, which has rarely been reported in previous studies. Relevant studies have shown that advanced age and malnutrition are all risk factors for susceptibility to COVID-19, 29 so exploring the risk factors associated with COVID-19 re-positive in malnourished older adults could help minimize the occurrence of adverse prognostic events. Second, we may be the first to develop a model to predict COVID-19 re-positive based on malnourished older adults, which is important for early diagnosis and prevention of the disease. Of course, our study has some limitations. First, as a cross-sectional study, our sample size was limited, which to some extent also affected the interpretation of the results of the confidence intervals of the associated factors. Second, the risk prediction model was only validated with an internal dataset, whereas validation with an external dataset is necessary. Therefore, our later research will expand the sample size, and we will also collaborate with several centers to obtain external data to validate the model.
Conclusion
In summary, this study exploratorily constructed a clinical prediction model for COVID-19 “re-positive” in malnourished older adults, and intuitively showed the risk probability of COVID-19 “re-positive” by drawing a nomogram. The analysis results showed that the model had good predictive performance, which could help clinical workers accurately assess patient prognosis. At the same time, the model suggested that controlling underlying diseases and balancing diet could help reduce the risk of COVID-19 “re-positive”, which could provide a basis for clinical decision-making and improve patient prognosis; in addition, accurate prevention of COVID-19 “re-positive” could enable patients to achieve health and economic benefits. However, as time changes, the risk factors of disease, unmeasured risk factors, treatment measures and so on are changing dynamically, which may lead to a decline in the standardization and performance of the model; in addition, there are many tools to evaluate the nutritional status of older adults, this study is a preliminary exploratory study, and the research methods are relatively single. Therefore, in future studies, we can include as many relevant influencing factors as possible, expand the sample size, conduct multi-center, multi-tool, multi-dimensional comprehensive research, to reduce information bias, and constantly improve and update the model.
Acknowledgments
The authors wish to express gratitude to all respondents and their families for their enthusiastic participation in this study.
Abbreviations
COVID-19, novel coronavirus;GNRI, Geriatric Nutritional Risk Index; LASSO, least absolute shrinkage and selection operator; AUC, area under the curve; DCA, decision curve analysis; CIC, clinical impact curve analysis; BMI, body mass index; WBC, white blood cell; CRP, c-reactive protein; Hb, hemoglobin; ALB, albumin; ALT, alanine aminotransferase, AST, aspartate aminotransferase; Scr, serum creatinine.
Data Sharing Statement
The dataset generated and analyzed during the current study is available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was conducted at the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine and has been approved by the Ethics Committee of our hospital in accordance with the STROBE criteria and the Declaration of Helsinki. As all included data were anonymized and retrospective, informed consent from patients was not required.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Novel Coronavirus(2019-Ncov) Situation Reports; 2020. [Google Scholar]
- 3.Cao H, Chen S, Xi X. Aging, migration, and structural transformation in China. Econ Modell. 2023;126:106430. doi: 10.1016/j.econmod.2023.106430 [DOI] [Google Scholar]
- 4.Xiao Y, Li J. Retraction Note: a conversational analysis of aging in China from a cross-section of the labour market: a corpus-based study. Humanit Soc Sci Commun. 2023;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu N. The Theme Evolution and Strategic Decision-Making of the Public’s Attention to the Theme of Aging in China Based on Big Data Analysis. Mathematical Problems in Engineering. 2022;2022:4455022. doi: 10.1155/2022/4455022 [DOI] [Google Scholar]
- 6.Jiang Q. Editorial: aging and health in China. Front Public Health. 2022;10:998769. doi: 10.3389/fpubh.2022.998769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Yan X, Sun K, et al. Estimation of disease burden and clinical severity of COVID-19 caused by Omicron BA.2 in Shanghai, February-June 2022. Emerg Microbes Infect. 2022;11(1):2800–2807. doi: 10.1080/22221751.2022.2128435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Horby PW, Hayden FG, Gao GF. Ping An Asset Management: china’s Aging Society Sparks Pension Interest, Becomes the Next Opportunity for Asset Management Industry. M2 Presswire. 2023. [Google Scholar]
- 9.Sadatnia M, Jalali A, Tapak L, Shamsaei F. The Relationship between Mental Health and Loneliness in the Elderly during the COVID-19 Pandemic. J Ageing Longevity. 2023;3(3). [Google Scholar]
- 10.Pironi L, Sasdelli AS, Ravaioli F, Baracco B, Musio A. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr. 2020;40(3):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Zhang Y, Gong C, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74(6):871–875. doi: 10.1038/s41430-020-0642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiertsema SP, van Bergenhenegouwen J, Garssen J, Knippels LMJ. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients. 2021;13(3):886. doi: 10.3390/nu13030886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverio R, Gonçalves DC, Andrade MF, Seelaender M. Coronavirus Disease 2019 (COVID-19) and Nutritional Status: the Missing Link? Adv Nutr. 2021;12(3):682–692. doi: 10.1093/advances/nmaa125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Deng J, Li J, et al. Changes of gut microbiota under different nutritional methods in elderly patients with severe COVID-19 and their relationship with prognosis. Front Immunol. 2023;14:1260112. doi: 10.3389/fimmu.2023.1260112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganmaa D, Chinbayar T, Khudaykov P, et al. Latent TB Infection, Vitamin D Status and COVID-19 Severity in Mongolian Patients. Nutrients. 2023;15(18):3979. doi: 10.3390/nu15183979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Chen Y, Zhang X, et al. Nutritional risk and a high NRS2002 score are closely related to disease progression and poor prognosis in patients with COVID-19. Front Nutr. 2023;10:1089972. doi: 10.3389/fnut.2023.1089972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng X, Liu Z, He X, et al. Risk of Malnutrition in Hospitalized COVID-19 Patients: a Systematic Review and Meta-Analysis. Nutrients. 2022;14(24):5267. doi: 10.3390/nu14245267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abedi V, Avula V, Chaudhary D, et al. Prediction of Long-Term Stroke Recurrence Using Machine Learning Models. J Clin Med. 2021;10(6):1286. doi: 10.3390/jcm10061286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daidone M, Ferrantelli S, Tuttolomondo A. Machine learning applications in stroke medicine: advancements, challenges, and future prospectives. Neural Regen Res. 2024;19(4):769–773. doi: 10.4103/1673-5374.382228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Song W, Patil N, Sainlaire M, Jasuja R, Dykes PC. Predicting COVID-19 severity: challenges in reproducibility and deployment of machine learning methods. Int J Med Inform. 2023;179:105210. doi: 10.1016/j.ijmedinf.2023.105210 [DOI] [PubMed] [Google Scholar]
- 21.Hassan N, Slight R, Morgan G, et al. Road map for clinicians to develop and evaluate AI predictive models to inform clinical decision-making. BMJ Health Care Inform. 2023;30(1):784. doi: 10.1136/bmjhci-2023-100784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi: 10.1093/ajcn/82.4.777 [DOI] [PubMed] [Google Scholar]
- 23.Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. 2022. https://www.covid19treatmentguidelines.nih.gov/. Accessed December 12, 2022. [PubMed] [Google Scholar]
- 24.Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature--What does it tell us? J Nutr Health Aging. 2006;10(6):466–485. [PubMed] [Google Scholar]
- 25.Zhao Y, Lin T, Hou L, et al. Association Between Geriatric Nutritional Risk Index and Frailty in Older Hospitalized Patients. Clin Interv Aging. 2021;16:1241–1249. doi: 10.2147/cia.S313827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien SF, Yi QL. How do I interpret a confidence interval? Transfusion. 2016;56(7):1680–1683. doi: 10.1111/trf.13635 [DOI] [PubMed] [Google Scholar]
- 27.du Prel JB, Hommel G, Röhrig B, Blettner M. Confidence interval or p-value?: part 4 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009;106(19):335–339. doi: 10.3238/arztebl.2009.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furcada JM, Patino CM, Ferreira JC. Estimating risk in clinical studies: odds ratio and risk ratio. J Bras Pneumol. 2020;46(2):e20200137. doi: 10.36416/1806-3756/e20200137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. medRxiv. 2021. doi: 10.1101/2021.12.14.21267755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tosato M, Calvani R, Ciciarello F, et al. Malnutrition in COVID-19 survivors: prevalence and risk factors. Aging Clin Exp Res. 2023;35(10):2257–2265. doi: 10.1007/s40520-023-02526-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths J, Seesen M, Sirikul W, Siviroj P. Malnutrition, Depression, Poor Sleep Quality, and Difficulty Falling Asleep at Night Are Associated with a Higher Risk of Cognitive Frailty in Older Adults during the COVID-19 Restrictions. Nutrients. 2023;15(13):2849. doi: 10.3390/nu15132849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its Impact on Patients with COVID-19. SN Compr Clin Med. 2020;2(8):1069–1076. doi: 10.1007/s42399-020-00363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Böhm M, Frey N, Giannitsis E, Sliwa K, Zeiher AM. Coronavirus Disease 2019 (COVID-19) and its implications for cardiovascular care: expert document from the German Cardiac Society and the World Heart Federation. Clin Res Cardiol. 2020;109(12):1446–1459. doi: 10.1007/s00392-020-01656-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu L, Abd Ghani MK, Aghemo A, et al. SARS-CoV-2 Infection, Inflammation, Immunonutrition, and Pathogenesis of COVID-19. Curr Med Chem. 2023;30(39):4390–4408. doi: 10.2174/0929867330666230330092725 [DOI] [PubMed] [Google Scholar]
- 35.Austic RE, Dietert RR, Sung YJ. Amino acids in immune function. Proceedings of the Cornell Nutrition Conference; 1991. [Google Scholar]
- 36.Al-Shami I, Hourani HMA, Alkhatib B. The use of prognostic nutritional index (PNI) and selected inflammatory indicators for predicting malnutrition in COVID-19 patients: a retrospective study. J Infect Public Health. 2023;16(2):280–285. doi: 10.1016/j.jiph.2022.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye H, Zhao C, Yang L, et al. Twelve out of 117 recovered COVID-19 patients retest positive in a single-center study of China. EClinicalMedicine. 2020;26:100492. doi: 10.1016/j.eclinm.2020.100492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohammedsaeed W, Ahmedseedi I, Alahmadey Z. Liver and Renal Impairments in COVID-19 Patients of Madinah City of Saudi Arabia: a Cross-Sectional Study (2020). Cureus. 2023;15(5):e39409. doi: 10.7759/cureus.39409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mottaghi A, Alipour F, Alibeik N, et al. Serum cystatin C and inflammatory factors related to COVID-19 consequences. BMC Infect Dis. 2023;23(1):339. doi: 10.1186/s12879-023-08258-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motavalli R, Abdelbasset WK, Rahman HS, et al. The lethal internal face of the coronaviruses: kidney tropism of the SARS, MERS, and COVID19 viruses. IUBMB Life. 2021;73(8):1005–1015. doi: 10.1002/iub.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51(3):249–255. doi: 10.1016/j.jacc.2007.10.016 [DOI] [PubMed] [Google Scholar]
- 42.Lu PH, Yu MC, Wei MJ, Kuo KL. The Therapeutic Strategies for Uremic Toxins Control in Chronic Kidney Disease. Toxins. 2021;13(8):573. doi: 10.3390/toxins13080573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Yang T, Wang S, et al. The value of lymphocyte count in determining the severity of COVID-19 and estimating the time for nucleic acid test results to turn negative. Bosn J Basic Med Sci. 2021;21(2):235–241. doi: 10.17305/bjbms.2020.4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henry BM. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8(4):e24. doi: 10.1016/s2213-2600(20)30119-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong Y, Huang H, Yang J, Yang L. Retrospective analysis on the clinical characteristics of patients who were reinfected with the Corona Virus in 2019. Am J Transl Res. 2021;13(5):5505–5511. [PMC free article] [PubMed] [Google Scholar]
- 47.Hoang T. Systematic review and meta-analysis of factors associated with re-positive viral RNA after recovery from COVID-19. J Med Virol. 2021;93(4):2234–2242. doi: 10.1002/jmv.26648 [DOI] [PubMed] [Google Scholar]
- 48.Zhou J, Zhang J, Zhou J, et al. Clinical characteristics of re-positive COVID-19 patients in Huangshi, China: a retrospective cohort study. PLoS One. 2020;15(11):e0241896. doi: 10.1371/journal.pone.0241896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Xu X, Hu J, et al. Clinical course and risk factors for recurrence of positive SARS-CoV-2 RNA: a retrospective cohort study from Wuhan, China. Aging (Albany NY). 2020;12(17):16675–16689. doi: 10.18632/aging.103795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren X, Ren X, Lou J, et al. A systematic review and meta-analysis of discharged COVID-19 patients retesting positive for RT-PCR. EClinicalMedicine. 2021;34:100839. doi: 10.1016/j.eclinm.2021.100839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durán Alert P, Milà Villarroel R, Formiga F, Virgili Casas N, Vilarasau Farré C. Assessing risk screening methods of malnutrition in geriatric patients: mini Nutritional Assessment (MNA) versus Geriatric Nutritional Risk Index (GNRI). Nutr Hosp. 2012;27(2):590–598. doi: 10.1590/s0212-16112012000200036 [DOI] [PubMed] [Google Scholar]
- 52.Fujioka H, Koike T, Imamura T, et al. Impact of Geriatric Nutritional Risk Index and Modified Creatinine Index Combination on Mortality in Hemodialysis Patients. Nutrients. 2022;14(4):801. doi: 10.3390/nu14040801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan Y, Mao J, Ou X, Huang L, Tu Q, Wang N. Geriatric Nutritional Risk Index assessment in elderly patients during the COVID-19 outbreak. Health Sci Rep. 2022;5(3):e560. doi: 10.1002/hsr2.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López-Gómez JJ, Calleja-Fernández A, Ballesteros-Pomar MD, et al. Valoración del riesgo nutricional en pacientes ancianos hospitalizados mediante diferentes herramientas. Endocrinología y Nutrición. 2011;58(3):104–111. doi: 10.1016/j.endonu.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 55.Abd-El-Gawad WM, Abou-Hashem RM, El Maraghy MO, Amin GE. The validity of Geriatric Nutrition Risk Index: simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with Mini Nutritional Assessment. Clin Nutr. 2014;33(6):1108–1116. doi: 10.1016/j.clnu.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 56.Jung YS, You G, Shin HS, Rim H. Relationship between Geriatric Nutritional Risk Index and total lymphocyte count and mortality of hemodialysis patients. Hemodial Int. 2014;18(1):104–112. doi: 10.1111/hdi.12077 [DOI] [PubMed] [Google Scholar]
- 57.Matsumura T, Mitani Y, Oki Y, et al. Comparison of Geriatric Nutritional Risk Index scores on physical performance among elderly patients with chronic obstructive pulmonary disease. Heart Lung. 2015;44(6):534–538. doi: 10.1016/j.hrtlng.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 58.Bo Y, Wang K, Liu Y, et al. The Geriatric Nutritional Risk Index Predicts Survival in Elderly Esophageal Squamous Cell Carcinoma Patients with Radiotherapy. PLoS One. 2016;11(5):e0155903. doi: 10.1371/journal.pone.0155903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50(2):279–283. doi: 10.1093/ageing/afaa274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z, Liu S, Li F, et al. Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: a systematic review and meta-analysis. Front Immunol. 2022;13:965971. doi: 10.3389/fimmu.2022.965971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta RK, Topol EJ. COVID-19 vaccine breakthrough infections. Science. 2021;374(6575):1561–1562. doi: 10.1126/science.abl8487 [DOI] [PubMed] [Google Scholar]
- 62.Suah JL, Husin M, Tok PSK, et al. Waning COVID-19 Vaccine Effectiveness for BNT162b2 and CoronaVac in Malaysia: an Observational Study. Int J Infect Dis. 2022;119:69–76. doi: 10.1016/j.ijid.2022.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lau JJ, Cheng SMS, Leung K, et al. Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nat Med. 2023;29(2):348–357. doi: 10.1038/s41591-023-02219-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laviano A, Koverech A, Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19). Nutrition. 2020;74:110834. doi: 10.1016/j.nut.2020.110834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muscogiuri G, Barrea L, Savastano S, Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. 2020;74(6):850–851. doi: 10.1038/s41430-020-0635-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated and analyzed during the current study is available from the corresponding author upon reasonable request.