Abstract
Introduction
Vitamin D3 plays a vital role in bone health, with low levels of vitamin D3 being related to skeletal fragility, fractures, and metabolic disorders such as diabetes. Metformin is known as an antihyperglycemic agent for regulating blood sugar. A correlation between diabetes mellitus and osteoporosis is attracting considerable interest, and research to find the prevention and treatment is gradually being studied. In this study, we investigated the effect of metformin and vitamin D3 on osteogenic differentiation of human adipose tissue-derived mesenchymal stem cells (AT-MSCs) under high d-glucose concentrations and optimized by combining vitamin D3 and metformin in the process.
Methods
ROS production of AT-MSCs under high d-glucose conditions was measured by DCFH-DA assay. The differentiated AT-MSCs were analyzed by Alizarin Red S staining and optical density measurement. The investigation involved the examination of osteogenic master genes' expressions using quantitative reverse transcription polymerase chain reaction (qRT-PCR) techniques.
Results
Interestingly, the results have shown that human AT-MSCs will exhibit high ROS accumulation and low osteogenic differentiation capabilities, indicated by low calcium deposition, as well as low expression of indicative genes such as ALP, Runx-2 under high d-glucose conditions. The combination of vitamin D3 and metformin remarkedly accelerated the osteogenic differentiation of AT-MSCs under high d-glucose concentrations more effectively than the administration of either agent.
Conclusions
This study partially explains an aspect of an in vitro model for pre-clinical drug screening for osteoporosis-related diabetic pathological mechanisms, which can be applied for further research on the prevention or treatment of osteoporosis in diabetic patients.
Keywords: Diabetes mellitus, Osteoporosis, Vitamin D, Metformin, Mesenchymal stem cells, AT-MSCs, Osteogenic differentiation
1. Introduction
Diabetes mellitus (DM) is distinguished by hyperglycemia, elevated levels of blood sugar, stemming from the dysfunction of insulin production, insulin action, or both. Patients with DM are associated with higher risks of lowered bone density due to the various complications caused by the disease [1,2], leading to increased risk of bone fracture. A common condition of DM is increased reactive oxygen species (ROS) formation elevated receptor activator of nuclear factor kappa beta (NF-κB) ligand and receptor for advanced glycation endproducts expression, which is expressed in osteoclasts and stimulates osteoclast productions, adding to the reduction of bone mass [[3], [4], [5]]. Previous reports demonstrated that ROS production also increases in mesenchymal stem cells-derived from diabetic donors [3,4].
Metformin is a first-line medication for the treatment of type 2 diabetes and has been shown to have benefactor potential for bone health of diabetic patients. The mechanism action of metformin on bone is not yet fully understood. However, an in vivo study hinted at the ability of metformin to improve glucose metabolism, insulin sensitivity, and to suppress inflammation via the regulation of AMPK, NF-κB, p65, and Hmgb1 gene expression [6]. In addition, metformin can diminish the negative effects of hyperglycemia on bone health [7]. It is worth noting that conflicting studies exist on the effect of metformin on bone health. A recently published meta-analysis indicated that there is not strong correlation between decreased fracture risk and metformin administration. However, the study was conducted with limited data samples [8]. Further investigations about metformin in bone health are necessary to provide a concrete conclusion. On the other hand, metformin induces osteoblastic differentiation of human dental pulp stem cells and induced pluripotent stem cell-derived mesenchymal stem cells [9,10].
On the other hand, vitamin D3 (1,25-dihydroxyvitamin D3 (1α,25(OH)2D3), an active form of vitamin D, is one of the primary factors regulating the osteoblast differentiation [11], which mainly works by attaching to its nuclear vitamin D receptor (VDR) [12,13]. According to previous reports, vitamin D3 and VDR are crucial for the control of osteogenic differentiation of stem/progenitor cells [[14], [15], [16], [17]]. Studies have revealed that vitamin D3 stimulates the calcification of human osteoblasts and induces the osteogenesis of human MSCs [18,19]. During the process of bone development, Runx-2 and ALP are the master transcription factor that is necessary for determining the cell lineage of osteoblasts [20].
Vitamin D3 and metformin both have been shown to have positive effects on skeletal disorders and insulin resistance in previous studies [21,22]. Vitamin D3 has been studied nearly entirely in terms of its function in the calcium homeostasis [23]. At the same time, metformin is a commonly used anti-hyperglycemic medication that reduces hepatic insulin resistance by acting as an insulin sensitizer [24]. Many studies have shown that metformin induced bone formation in diabetic patients [25] and diabetic mice models [26]. Moreover, the present results also demonstrated that osteogenic differentiation ofAT-MSCs was improved by the addition of vitamin D3, but there were few studies about the effect of vitamin D under high d-glucose levels on the osteogenesis of MSCs. On the other hand, the effect of high d-glucose levels, vitamin D3, metformin, and the combination of both vitamin D3 and metformin on the properties of AT-MSCs, including proliferation, differentiation, migration, and toxicity should be examined to complete the stimulation of osteoporosis-related diabetes stem cell model, which is further employed in pre-clinical drug screening and osteoporosis-related diabetes researches, such as treatment and prevention.
In addition, our previous studies showed that insulin resistance-related gene induction was significantly elevated while the osteogenic ability in human AT-MSCs was impaired under 25 mM, 50 mM, and 100 mM d-glucose treatment [27,28]. Hence, we hypothesize that AT-MSCs may be a good candidate for examining the effect of vitamin D3 and metformin on the osteogenic differentiation under high d-glucose conditions. Therefore, we aim to investigate the optimal dosages for the combination of metformin and vitamin D3 treatment in human AT-MSCs under high d-glucose conditions.
2. Materials and methods
2.1. Stem cell culture
Human adipose tissue-derived mesenchymal stem cells (AT-MSCs) were provided by the Laboratory of Stem Cell Biology and Regenerative Medicine, University of Tsukuba, Japan. The ethical declaration were mentioned in our previous reports [29,30]. AT-MSCs were cultured in complex Iscove's modified Dulbecco's medium (IMDM, Gibco, USA), with 10 % fetal bovine serum (FBS, Gibco, USA), 1 % Pen-strep (Gibco, Sigma), and 5 ng/ml human bFGF (Sigma, USA) at 37 °C, 5 % CO2 and 98 % humidity. The fresh medium was replaced every three days. Cells were stocked in solution including 90 % FBS and 10 % dimethylsulfoxide (DMSO, Sigma, USA) at −80 °C or liquid nitrogen. The AT-MSCs that were used for all experiments were in passages 9–12.
2.2. The supplement of high d-glucose concentrations
The d-glucose solutions at different concentrations (25, 50, and 100 mM) (Sigma, USA) were supplemented to the osteogenic induced-medium before adding to the culture plates used for investigating ROS release and osteogenic differentiation process.
2.3. DCFH-DA assay
AT-MSCs were seeded in 24-well-plate and incubated at 37 °C, 5 % CO2 overnight. Hydrogen peroxide (H2O2) (2 mM) diluting in serum-free medium was used as the positive control sample. High d-glucose (25, 50 and 100 mM) was supplemented to the plate and cultured for three days. DCFH-DA (40 μM) was added and incubated for 30 min to interact with the ROS inside the cells. Images were taken using a fluorescence microscope (Nikon, Japan) at magnification of 10×. The fluorescent signal was measured using microplate reader (Thermo, USA) at 495 nm and 535 nm for excitation and emission, respectively.
2.4. In vitro osteogenic differentiation
The osteogenic induction in AT-MSCs was referenced from a previous study [29]. AT-MSCs were seeded in 48-well-plate with the density of 3 × 104 cells/well and cultured at 37 °C, 5 % CO2, and 98 % humidity for 24 h. The osteogenic differentiation medium was made of IMDM, 1 % FBS, 1 % antibiotics, supplemented with dexamethasone (Sigma, USA), human epidermal growth factor (hEGF, Sigma, USA), vitamin C (Sigma, USA), and β-glycerol-2-phosphate (β-GDP, Sigma, USA). The complex medium was added to the cells the day after seeding. The medium was freshly renewed every 3 days in total 25 days. The change in cell morphology was captured by using NIS-Elements BR software at day 0 (before differentiation) and day 25 in order to express changing of AT-MSCs to differentiated cells.
2.5. Alizarin Red S staining for osteogenic differentiation recognition
Calcium formation of AT-MSCs was qualified by the Alizarin Red staining method [29] at day 25. Differentiated cells were fixed with formalin 10 % for 10 min and rinsed with distilled water. Alizarin Red S (Sigma, USA) was added to the cells and incubated for 5 min. Images were captured under an inverted microscope (Nikon, Japan) at magnification 10×. Then cell lysis buffer was added to measure optical density at 480 nm by using microplate reader (Thermo, USA).
2.6. Total mRNA isolation
AT-MSCs were seeded in a 24-well-plate with the density of 5 × 104 cells/well and underwent osteogenic differentiation process. mRNA was isolated using Sepasol-RNA I Super G (Nacalai tesque, Japan) on day 7 of the process.
2.7. Reverse transcriptase polymerase chain reaction
Total RNA was firstly reverse-transcribed by High-Capacity cDNA Reverse Transcription Kit (Thermo, USA). Complementary DNA (cDNA) was used to examine the expression of Runx-2, and ALP by real-time PCR (q-PCR) using Maxima SYBR Green/ROX qPCR Master Mix (2X) Kit (Thermo, USA) and reacted in Qiagen Rotor-Gene Q. Expression of genes in interest were then calculated by the formula. The internal control utilized in the study was β-actin. Table 1 displays the primers employed in the PCR experiments [28,29].
Table 1.
The primers employed for quantitative polymerase chain reaction.
| Function | Gene | Primer | Sequence |
|---|---|---|---|
| Internal control | β-actin | 5′-primer | GTGCGTGACATTAAGGAGAAGCTGTGC |
| 3′-primer | GTACTTGCGCTCAGGAGGAGCAATGAT | ||
| Osteogenic markers | Runx-2 | 3′-primer | CAGATGGGACTGTGGTTACTGTCATGG |
| 5′-primer | CCTAAATCACTGAGGCGGTCAGAGAAC | ||
| ALP | 3′-primer | ACGTGGCTAAGAATGTCATC | |
| 5′-primer | CTGGTAGGCGATGTCCTTA |
2.8. Statistical analysis
One-way analysis of variance (ANOVA) (Tukey post-hoc test; SPSS 20 software, IBM Corp.) was used to identify significant differences between groups. The experiments conducted in the study were replicated by a minimum of 3 times, with a significance level of p < 0.05 being accepted as statistically significant. The data were presented as the mean standard deviation (SD).
3. Results
3.1. High d-glucose concentrations induced ROS generation
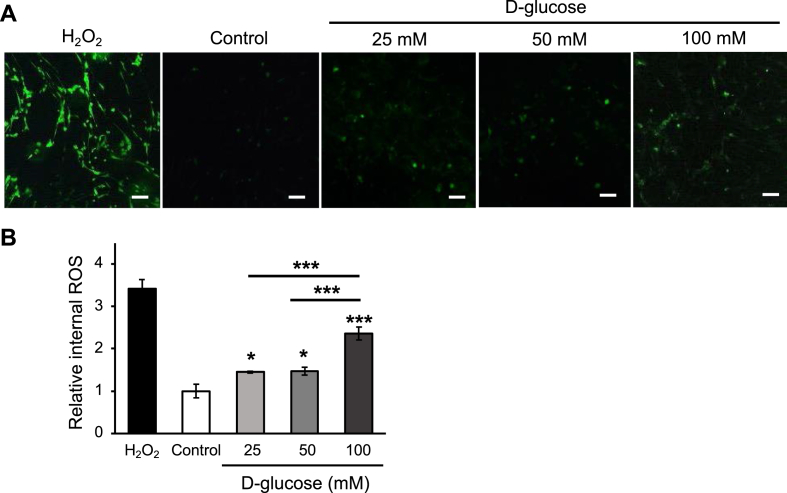
The internal ROS generation of AT-MSCs under different conditions, including normal and high d-glucose concentrations (25, 50, and 100 mM), was investigated by DCFH-DA assay. The result showed that high d-glucose at 25, 50, and 100 mM had increased the internal ROS release in AT-MSCs (Fig. 1A). Furthermore, the intensity of ROS in 25 mM and 50 mM-treated groups was not significantly different. Remarkably, the ROS production under 100 mM d-glucose has increased 2.35-fold (p < 0.005, n = 3) and 1.61-fold (p < 0.005, n = 3) higher than that in the control group and 25 mM d-glucose-treated group, respectively (Fig. 1B). The results indicated that the characteristic of AT-MSCs, such as osteogenic differentiation under high d-glucose conditions, may be altered due to the over-expression of ROS.
Fig. 1.
The ROS generation of AT-MSCs under 25, 50, and 100 mM d-glucose conditions were recognized by DCFH-DA assay. (A) The fluorescent-stained cells were captured under a fluorescent microscope. Control: non-treated AT-MSCs. The scale bar is at 100 μm; (B) The histogram indicated ROS release of AT-MSCs under different conditions. The data are expressed as the means ± SD, ∗∗∗p < 0.001, ∗p < 0.05, n = 3.
3.2. The effect of metformin concentrations on the osteogenic differentiation ability of AT-MSCs under high d-glucose conditions
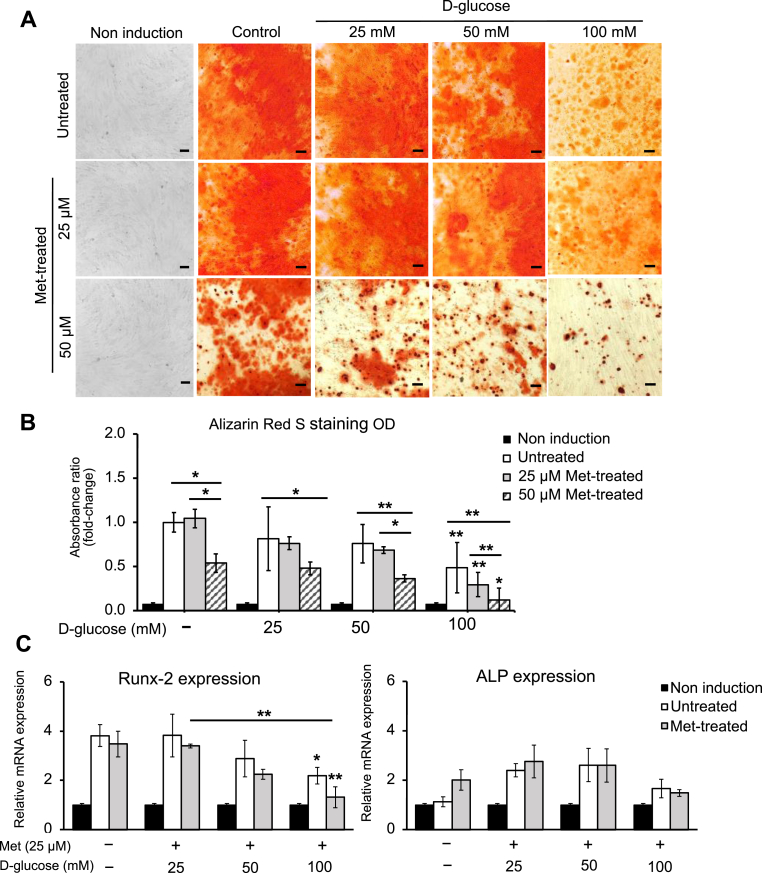
In this study, two concentrations of metformin (25 and 50 μM) were added to the osteogenic differentiation-induced medium. Under high d-glucose concentration (100 mM), the osteogenic differentiation ability of AT-MSCs was inhibited (Fig. 2A). The optical density (OD) measurement of 100-mM d-glucose treated group was significantly lower than the untreated group (2.05-fold lower, p < 0.05, n = 3) (Fig. 2B). Moreover, it was also indicated by the reduction in the expression of osteogenic-specific gene Runx-2. The expression of Runx-2 in the 100-mM treated group was 1.75-fold (p < 0.05, n = 3) lower than the untreated group (Fig. 2C). Overall, treating with metformin (25 and 50 μM) has not shown any supportive effect on osteogenic differentiation of AT-MSCs under high d-glucose conditions. Under high d-glucose conditions, the calcium deposition in 50-μM treated groups was significantly lower than those in the untreated groups (Fig. 2B). However, the osteogenic differentiation under 25 μM metformin had no significant difference compared to the untreated group (Fig. 2B). Moreover, the expression of Runx-2 and ALP under metformin (25 μM) treated groups was similar to those in the untreated (Fig. 2C). This indicated that 50 μM metformin was inappropriate to support osteogenic differentiation of AT-MSCs under high d-glucose conditions. Hence, we suggested using 25 μM metformin for further experiments.
Fig. 2.
The effect of different concentrations of metformin (25 and 50 μM) on osteogenic differentiation of AT-MSCs under high d-glucose concentrations (25, 50, and 100 mM). (A) The matrix mineralization of AT-MSCs was recognized by Alizarin Red S (stained in orange red) at day 25 of osteogenic differentiation process. The scale bar is at 100 μm. (B) The quantitative result of calcification of AT-MSCs was measure at wavelength 480 nm. (C) The effect of metformin (25 μM) on the expression of osteocyte-specific genes Runx-2 and ALP under high d-glucose conditions. The level of mRNA expression was measured by qRT-PCR on day 7 of osteogenic induction and normalized to β-actin. The data demonstrated the average values of three independent experiments (mean ± SD); n = 3. ∗p < 0.05, ∗∗p < 0.01. Non-induction: the undifferentiated group; Untreated: the differentiated group without being treated with either metformin or d-glucose.
3.3. Evaluation of vitamin D3 concentrations on osteogenic differentiation
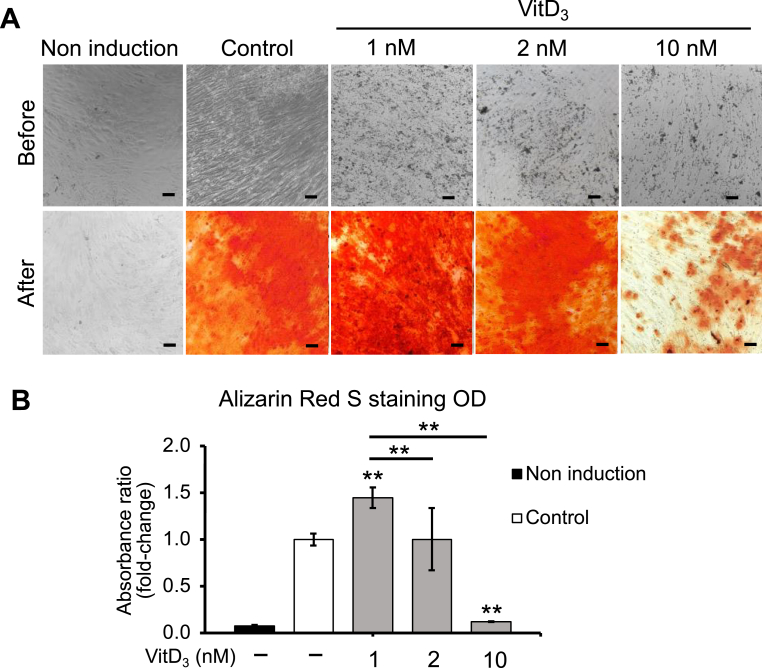
We evaluated the different concentrations of vitamin D3 (1, 2, and 10 nM) on osteogenic differentiation. The staining result demonstrated that vitamin D3 at 1 nM has increased the calcium deposition significantly compared to the other groups (Fig. 3A). The OD measurement supported that the calcium accumulation in 1 nM treated group was increased 1.45-fold higher (p < 0.01, n = 3) than that in the untreated groups (Fig. 3B). Moreover, when increasing the concentration of vitamin D3 to 2 nM, we observed that the ability to differentiate to osteoblasts of the untreated group and 2 nM treated group were similar (Fig. 3B). Surprisingly, the pre-stained result of 10 nM vitamin D3 showed detachment of cells during the differentiation process (Fig. 3A), as well as the inhabitation in osteogenic differentiation (Fig. 3B). The Alizarin Red S (ARS) staining of 10 nM treated group was 8.3-fold decreased (p < 0.01, n = 3) compared to the untreated group, indicating that this concentration of vitamin D3 was not applicable for this study. Thus, we decided to use 1 nM and 2 nM of vitamin D3 for further investigation.
Fig. 3.
The effect of vitamin D3 on osteogenic differentiation ability of AT-MSCs. (A) Qualitative result of differentiated cells under different concentrations of vitamin D3 (1, 2, and 10 nM). The scale bar is at 100 μm. (B) The quantification result of AT-MSCs supplemented with vitamin D3 (1, 2, and 10 nM). The data demonstrated the average values of three independent experiments (mean ± SD); n = 3. ∗∗p < 0.01.
3.4. Vitamin D3 improved osteogenic differentiation under high d-glucose conditions
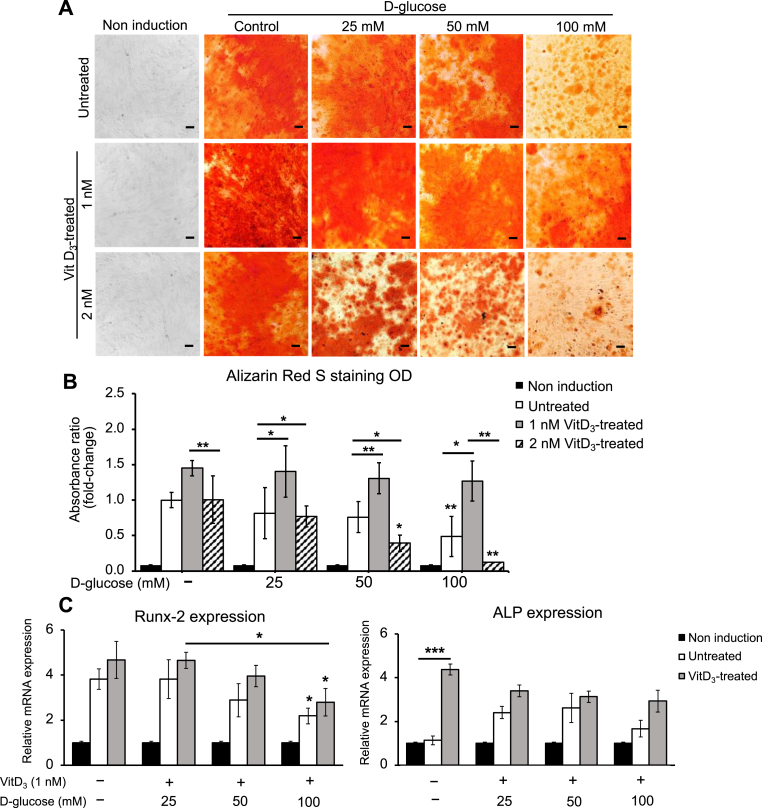
The effect of vitamin D3 at those levels (1 nM and 2 nM) on osteogenic differentiation in the presence of varying amounts of high concentrations of d-glucose was investigated. Overall, when comparing the effect of two concentrations of vitamin D3 (1 nM and 2 nM) on the enhancement of osteogenic differentiation under various high d-glucose conditions, 1 nM of vitamin D3 enhanced the mineralization of AT-MSCs more effectively (Fig. 4A). The quantification results showed that osteoblasts differentiated from AT-MSCs in 1 nM treated group was increased 1.87 (p < 0.05, n = 3), 1.72 (p < 0.01, n = 3), and 2.59 (p < 0.05, n = 3), compared to those in untreated groups under 25 mM, 50 mM, and 100 mM d-glucose, respectively (Fig. 4B). On the other hand, 2 nM of vitamin D3 has inhibited the osteogenic differentiation under high d-glucose conditions instead of improving it (Fig. 4A). In correlation with staining results, the expression of Runx-2 and ALP has been increased under the treatment of 1 nM vitamin D3 (Fig. 4C). Although the presented data was not significantly comparable, the results showed the trend in upregulating osteogenic-specific genes using vitamin D3 at 1 nM.
Fig. 4.
The effect of vitamin D3 (1 and 2 nM) on osteogenic differentiation ability of AT-MSCs under different concentrations of high d-glucose (25, 50, and 100 mM). (A) Qualitative result of differentiated cells under different high d-glucose concentrations supplemented with vitamin D3 (1 nM and 2 nM). The scale bar is at 100 μm. (B) The quantification of calcium deposition of AT-MSCs. (C) The effect of vitamin D3 (1 nM) on the expression of osteocyte-specific gene Runx-2 and ALP. The data demonstrated the average values of three independent experiments (mean ± SD); n = 3. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005.
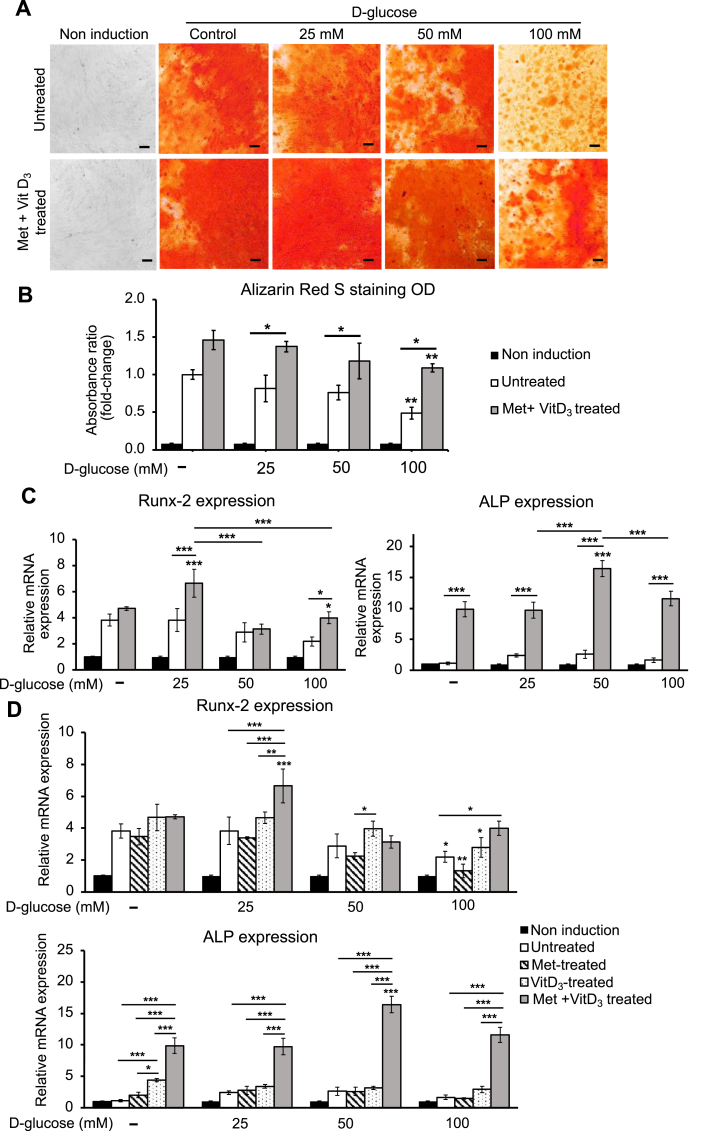
3.5. The investigation of synergistic effect of metformin and vitamin D3 on osteogenic differentiation of AT-MSCs under different high d-glucose conditions
According to the previous results, metformin (25 μM) and vitamin D3 (1 nM) have been selected to supplement examine for the synergistic effect on osteogenic differentiation under high d-glucose conditions. The staining result showed that the osteogenic differentiation of AT-MSCs in the combined group (metformin+vitamin D3-treated) was significantly increased compared to the untreated groups (Fig. 5A). The quantitative result of the combined group was 1.67-fold, 1.55-fold, and 2.22-fold (p < 0.05) higher than those of the untreated groups under 25 mM, 50 mM, and 100 mM d-glucose, respectively (Fig. 5B). Interestingly, osteogenic differentiation was progressively inhibited under 100 mM d-glucose condition, while the combined treatment of metformin and vitamin D3 has shown their recovery effect. It was supported by the expression of Runx-2 and ALP that was significantly enhanced in the combined group under high d-glucose condition (100 mM). Runx-2 and ALP in the combined group have increased 1.82 (p < 0.05) and 6.94 (p < 0.005) times than the untreated groups (Fig. 5C). Moreover, we observed that combined groups showed better effects than the single treatment with metformin or vitamin D3. Although under 100 mM d-glucose condition, the increase in Runx-2 expression of the combined group was not significant, the expression of ALP was 3.95-fold (p < 0.005) higher, compared to that in the vitamin D3 treated group (Fig. 5D). The results indicated that the supplement of metformin has promoted the effect of vitamin D3 in osteogenic differentiation under high d-glucose conditions, especially 100 mM.
Fig. 5.
The combined effect of metformin and vitamin D3 on osteogenic differentiation under high d-glucose conditions. (A) The qualitative results of differentiated cells in untreated and combined groups under high d-glucose conditions (25, 50, and 100 mM). The scale bar is at 100 μm. (B) The quantitative result of AT-MSCs supplemented with metformin (25 μM) and VitD3 (1 nM), compared to the untreated groups. (C) The mRNA expression level of Runx-2 and ALP in the combined groups. (D) The comparison in Runx-2 and ALP expression among single treatment with Met, VitD3, and combined treatment with Met and VitD3. The data demonstrated the average values of three independent experiments (mean ± SD); n = 3. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
4. Discussion
Although many studies have focused on bone marrow which is one of the main sources of mesenchymal osteoprogenitor cells, the success of bone-tissue regeneration depends on the osteogenic differentiation potential and secretomes. Both BM-MSCs and AT-MSCs have high osteogenic potential, osteoinductive ability, and immunomodulation properties. The secretomes of AT-MSCs has beneficial effects on recruiting other progenitor cells and can induce osteogenic differentiation in vitro and in vivo [31]. Osteoinduction is initiated by several transcription factors that govern AT-MSCs to an osteoblastic lineage. The key transcription factor commencing osteogenic differentiation of AT-MSCs is Runx2. After differentiation into preosteoblasts, Runx2, osterix, and β-catenin are in control of the cells to become immature osteoblasts. Then, ALP is a membrane-bound enzyme expressed as a marker in early differentiation and plays a key role in enhancing mineralization and calcification in these osteoblasts [32,33]. Notably, AT-MSCs has been proven to perform higher calcification and higher ability to regenerate new bone than BM-MSCs and dental pulp-derived MSCs in mouse bone fracture model. Besides, the advantage of AT-MSCs cells over BM-MSCs is the possibility to harvest them in a less invasive manner [31,34]. However, type 2 diabetic patients suffer from insulin resistance and chronic hyperglycemia, from which AT-MSCs’ functions were impaired in osteogenic differentiation potential and wound healing ability [27,29,35]. In previous report, we also demonstrated that the expressions of insulin resistance-related genes PTEN, EGR-1, GGPS-1 in healthy human AT-MSCs-treated with high d-glucose concentrations at 25 mM, 50 mM, and 100 mM were accelerated in comparison with those of human diabetic-derived AT-MSCs [27]. Additionally, according to our previous report, the expression of ALP and Runx-2 were remarkably decreased in non-diabetic-derived AT-MSCs under high d-glucose conditions and in diabetic-derived AT-MSCs [28]. Hankamolsiri et al. (2016) also demonstrated high d-glucose concentration (25 mM) has downregulated the osteogenic genes in MSCs derived from other sources including the bone marrow, umbilical cord, placenta and chorion [36]. Therefore, in the scope of this study, we focused on a reverse effect of both metformin and vitamin D3 on the osteogenic differentiation under high d-glucose concentrations at 25 mM, 50 mM, and 100 mM, which had obvious recovered not only the expression of Runx2 and ALP which indicate the early osteogenic differentiation at day 7 of the process but also confirmed by Alizarin red S staining for localization and quantification of increasing calcium deposits at day 25 (Fig. 5). For futher investigation, we will investigate the molecular mechanisms of metformin and vitamin D3 underlying the synergistic effects of the combination treatment on osteogenesis.
Here, we also provided more evidence about the effect of high d-glucose concentrations at 25 mM, 50 mM, and 100 mM on increasing intracellular ROS accumulation and inhibiting osteogenic differentiation in AT-MSCs, especially AT-MSCs-treated with 100 mM d-glucose (Fig. 1, Fig. 2). Besides, we reported that metformin significantly reduced ROS release in AT-MSCs-treated with 100 mM d-glucose [37]. Our results are consistent with the report of Zhou R et al. (2020) and Marycz K et al. (2016) on mouse BM-MSCs and mouse AT-MSCs, respectively [38,39]. Besides, metformin at a concentration of 50 μM significantly enhanced the proliferation, migration, and osteogenic differentiation of periodontal ligament stem cells [40]. However, our result showed that metformin supplements at both 50 and 25 μM expressed little effect on improving suppressed osteogenic differentiation of AT-MSCs under high d-glucose concentrations (Fig. 2), suggesting that metformin alone may not able to rescue the osteogenic differentiation potential under severe high glucose-induced oxidative stress.
Although we have not examined the effect of vitamin D3 on ROS reduction in our research, vitamin D3 has been proven to significantly reduce the intracellular ROS production in human AT-MSCs-treated with H2O2 [41]. Previous research has shown that vitamin D3 at 10 and 50 nM has promoted osteogenic differentiation of iPS-derived osteoprogenitor cells [42]. However, our results indicated that 10 nM vitamin D3 has not supported AT-MSCs osteogenic differentiation and caused cell detachment leading to a decrease in mineralization (Fig. 3A). This was consistent with the result of Mostafa et al. [43], vitamin D3 (10 nM) combined with Dex in osteogenic induced-medium caused a reduction in cell proliferation and mineralization in hBM-MSCs. On the other hand, we investigated the smaller concentrations of vitamin D3 (1 and 2 nM), and the results demonstrated that 1 nM of vitamin D3 has increased osteogenesis under high d-glucose conditions (Fig. 4). It was similar to the previous study, vitamin D3 (1 nM) has shown its effect in promoting dexamethasone (Dex), resulting in increasing ALP activity and matrix mineral formation [15]. Although the staining results of vitamin D3 treated groups showed a significant increase in matrix mineralization (Fig. 4B), the expression of Runx-2 and ALP was only significantly enhanced under 100 mM d-glucose condition (Fig. 4C). This can be explained by the stage of differentiation that vitamin D3 affected as it could enhanced not only early but also late-stage markers of osteogenic differentiation [15].
Additionally, previous research demonstrated that metformin had neither deleterious impact on the successful treatment of vitamin D deficiency in T2DM patients nor on the subgroup with osteoporosis [44]. Metformin and vitamin D3 may have a synergistic effect on the heterogeneous structure of skeletal muscle, according to previous report [22]. Notably, we demonstrated that the inhibition of osteogenic differentiation in AT-MSCs according to the increase of high d-glucose conditions as well as the significant recovery in osteogenic differentiation of AT-MSCs treated with metformin (25 μM) and vitamin D3 (1 nM), especially at 100 mM d-glucose (Fig. 5). Although the molecular mechanisms underlying the synergistic effects of the combination treatment of metformin and vitamin D3 on osteogenesis in human AT-MSCs has not been elucidated, metformin regulates osteogenesis by the AMPK-Runx2 signal pathway which increased ALP activity, collagen synthesis, osteocalcin production, and extracellular calcium deposition in AT-MSCs and BM-MSCs in vitro [38,39]. In addition, metformin directly enhances not only AMPK activation but also SIRT1 enzymatic activity to reduce oxidative stress. SIRT1/AMPK pathway also enforce the osteogenic differentiation potential of human MSCs under oxidative stress condition [45,46]. On the other hand, vitamin D3 contributes to enhanced osteogenic differentiation of MSCs under oxidative stress condition via activating the endogenous antioxidant system [41]. Vitamin D3 activates SIRT1-FOXO3 signaling pathways enforced the osteogenic differentiation potential of BM-MSC [47]. Furthermore, pre-administration of vitamin D3 (10 nM or 20 nM) attenuates the ROS-induced damage and MSC dysfunction by activating the endogenous antioxidant system by SIRT1 signaling in bone remodeling regulation [47]. Taken together, the results of the present study suggest that metformin and vitamin D3 exerts a protective role on osteogenic differentiation potential of AT-MSCs from relativelyhigh glucose-induced oxidative stress. For futher investigation, the regulatory mechanism of metformin and vitamin D3 on the ROS-SIRT1-FOXO3 axis under high d-glucose conditions, and the effect of metformin and vitamin D3 on bone formation in diabetic in vivo model should be elucidated, which will contribute in pre-clinical drug screening and osteoporosis-related diabetes applications.
5. Conclusions
This finding has demonstrated that the effect of a single treatment with metformin, vitamin D3, and the combined treatment. The results indicated that high d-glucose conditions have negatively affected the osteogenic differentiation of AT-MSCs, especially at the concentration of 100 mM d-glucose. Metformin (25 μM) has not shown significant effect on promoting osteogenic differentiation under high d-glucose conditions. On the other hand, despite the fact that vitamin D3 at 1 nM recover the ability to differentiate into osteoblasts of AT-MSCs under high d-glucose conditions, the changes at the molecular level were not highly evaluated. Interestingly, the combined metformin and vitamin D3 treated groups have shown outstanding effects in recovering osteogenic differentiation of AT-MSCs under high d-glucose conditions, especially at 100 mM d-glucose. Our observations pave the way for future investigations to better explain the impact of vitamin D3 and meformin on bone development under high glucose-stress conditions, such as investigating the timing of gene and protein expression during the process. The findings obtained from this study have the potential to enhance comprehension of the association between DM and osteoporosis, as well as advance the management of DM and its associated complications.
Author contributions
Conceptualization and methodology, N-T.T.; validation, investigation and data analysis, N.N-Y.H., H.T.K.T., P.N.U.P. N-T.T.; writing original draft preparation, N.N-Y.H., L.B.V., N-T.T.; supervision, L.B.V., N-T.T.; project administration, T-H.N., L.B.V., N-T.T.; funding acquisition, N-T.T. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors have no competing financial interests to declare.
Acknowledgments
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number C2021-28-02. We appreciate the support of Professor Osamu Ohneda for providing stem cells and Biotechnology Center of Ho Chi Minh city for supporting some research materials and equipment.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Long Binh Vong, Email: vblong@hcmiu.edu.vn.
Nhu-Thuy Trinh, Email: tnthuy@hcmiu.edu.vn.
References
- 1.Zhang Y.-S., Zheng Y.-D., Yuan Y., Chen S.-C., Xie B.-C. Effects of anti-diabetic drugs on fracture risk: a systematic review and network meta-analysis. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.735824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 3.Tiganis T. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol Sci. 2011;32(2):82–89. doi: 10.1016/j.tips.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Styskal J., Van Remmen H., Richardson A., Salmon A.B. Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med. 2012;52(1):46–58. doi: 10.1016/j.freeradbiomed.2011.10.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercer N., Ahmed H., Etcheverry S.B., Vasta G.R., Cortizo A.M. Regulation of advanced glycation end product (AGE) receptors and apoptosis by AGEs in osteoblast-like cells. Mol Cell Biochem. 2007;306:87–94. doi: 10.1007/s11010-007-9557-8. [DOI] [PubMed] [Google Scholar]
- 6.Araújo A.A., Pereira A., Medeiros C., Brito G. A.C., Leitão R.F.C., Araújo L.S., et al. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0183506. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecka-Czernik B. Diabetes, bone and glucose-lowering agents: basic biology. Diabetologia. Jul 2017;60(7):1163–1169. doi: 10.1007/s00125-017-4269-4. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Yu L., Ye Z., Lin R., Sun A.R., Liu L., et al. Association of metformin use with fracture risk in type 2 diabetes: a systematic review and meta-analysis of observational studies. Front Endocrinol. 2022;13:1038603. doi: 10.3389/fendo.2022.1038603. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin W., Gao X., Ma T., Weir M.D., Zou J., Song B., et al. Metformin enhances the differentiation of dental pulp cells into odontoblasts by activating AMPK signaling. J Endod. 2018;44(4):576–584. doi: 10.1016/j.joen.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P., Ma T., Guo D., Hu K., Shu Y., Xu H.H., et al. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2018;12(2):437–446. doi: 10.1002/term.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haussler M., Haussler C., Jurutka P., Thompson P., Hsieh J., Remus L., et al. The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J Endocrinol. 1997;154(3_Suppl):S57–S73. [PubMed] [Google Scholar]
- 12.Amling M., Priemel M., Holzmann T., Chapin K., Rueger J.M., Baron R., et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. Nov 1999;140(11):4982–4987. doi: 10.1210/endo.140.11.7110. (in eng) [DOI] [PubMed] [Google Scholar]
- 13.Gurlek A., Pittelkow M.R., Kumar R. Modulation of growth factor/cytokine synthesis and signaling by 1alpha,25-dihydroxyvitamin D(3): implications in cell growth and differentiation. Endocr Rev. Dec 2002;23(6):763–786. doi: 10.1210/er.2001-0044. (in eng) [DOI] [PubMed] [Google Scholar]
- 14.Prince M., Banerjee C., Javed A., Green J., Lian J.B., Stein G.S., et al. Expression and regulation of Runx2/Cbfa1 and osteoblast phenotypic markers during the growth and differentiation of human osteoblasts. J Cell Biochem. 2001;80(3):424–440. doi: 10.1002/1097-4644(20010301)80:3<424::aid-jcb160>3.0.co;2-6. (in eng) [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen N.R., Henriksen Z., Sørensen O.H., Civitelli R. Dexamethasone, BMP-2, and 1,25-dihydroxyvitamin D enhance a more differentiated osteoblast phenotype: validation of an in vitro model for human bone marrow-derived primary osteoblasts. Steroids. Apr 2004;69(4):219–226. doi: 10.1016/j.steroids.2003.12.005. (in eng) [DOI] [PubMed] [Google Scholar]
- 16.van Driel M., Koedam M., Buurman C., Hewison M., Chiba H., Uitterlinden A., et al. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. Faseb J. Nov 2006;20(13):2417–2419. doi: 10.1096/fj.06-6374fje. (in eng) [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y.S., Liu Y.S., Tan J.G. Is 1, 25-dihydroxyvitamin D3 an ideal substitute for dexamethasone for inducing osteogenic differentiation of human adipose tissue-derived stromal cells in vitro? Chin Med J (Engl) Aug 5 2006;119(15):1278–1286. (in eng) [PubMed] [Google Scholar]
- 18.Nebel D., Svensson D., Arosenius K., Larsson E., Jönsson D., Nilsson B.O. 1α,25-dihydroxyvitamin D3 promotes osteogenic activity and downregulates proinflammatory cytokine expression in human periodontal ligament cells. J Periodontal Res. Oct 2015;50(5):666–673. doi: 10.1111/jre.12249. (in eng) [DOI] [PubMed] [Google Scholar]
- 19.Uchiyama M., Nakamichi Y., Nakamura M., Kinugawa S., Yamada H., Udagawa N., et al. Dental pulp and periodontal ligament cells support osteoclastic differentiation. J Dent Res. 2009;88(7):609–614. doi: 10.1177/0022034509340008. 2009/07/01. [DOI] [PubMed] [Google Scholar]
- 20.Wysokinski D., Pawlowska E., Blasiak J. RUNX2: a master bone growth regulator that may Be involved in the DNA damage response. DNA Cell Biol. May 2015;34(5):305–315. doi: 10.1089/dna.2014.2688. (in eng) [DOI] [PubMed] [Google Scholar]
- 21.Montagnani A., Gonnelli S., Alessandri M., Nuti R. Osteoporosis and risk of fracture in patients with diabetes: an update. Aging Clin Exp Res. Apr 2011;23(2):84–90. doi: 10.1007/bf03351073. (in eng) [DOI] [PubMed] [Google Scholar]
- 22.Amin S.N., Hussein U.K., Yassa H.D., Hassan S.S., Rashed L.A. Synergistic actions of vitamin D and metformin on skeletal muscles and insulin resistance of type 2 diabetic rats. J Cell Physiol. Aug 2018;233(8):5768–5779. doi: 10.1002/jcp.26300. (in eng) [DOI] [PubMed] [Google Scholar]
- 23.Christakos S., Hewison M., Gardner D.G., Wagner C.L., Sergeev I.N., Rutten E., et al. Vitamin D: beyond bone. Ann N Y Acad Sci. 2013;1287(1):45–58. doi: 10.1111/nyas.12129. 2013/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M., Li X., Zhang H., Lu Y. Molecular mechanisms of metformin for diabetes and cancer treatment. Front Physiol. 2018;9:1039. doi: 10.3389/fphys.2018.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegazy S.K. Evaluation of the anti-osteoporotic effects of metformin and sitagliptin in postmenopausal diabetic women. J Bone Miner Metabol. 2015/03/01 2015;33(2):207–212. doi: 10.1007/s00774-014-0581-y. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Q., Guan Z., Liu S., Xuan Y., Han G., Chen H., et al. The effects of metformin and alendronate in attenuating bone loss and improving glucose metabolism in diabetes mellitus mice. Aging (Albany NY) Jan 14 2022;14(1):272–285. doi: 10.18632/aging.203729. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mai H.-P., Trinh N.-T., Long V.B., Binh N.T., Nguyen D.-Q., Duong H.-X. International conference on the development of biomedical engineering in Vietnam. Springer; 2020. The influence of high D-glucose concentrations on increasing the expression of EGR-1, PTEN and GGPS-1 involved in insulin resistance of AT-MSCs; pp. 489–499. [Google Scholar]
- 28.Nguyen Y.-N.H., Vong L.B., Pham H.A., Nguyen D.Q., Phuong P.N., Mai H.P., et al. The impairment of osteogenic differentiation of human adipose tissue-derived mesenchymal stem cells under high d-glucose concentrations. Vietnam J Sci Technol Eng. 2022;64(1):72–77. [Google Scholar]
- 29.Trinh N.-T., Yamashita T., Ohneda K., Kimura K., Salazar G.T.a., Sato F., et al. Increased expression of EGR-1 in diabetic human adipose tissue-derived mesenchymal stem cells reduces their wound healing capacity. Stem Cell Dev. 2016;25(10):760–773. doi: 10.1089/scd.2015.0335. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4870610/pdf/scd.2015.0335.pdf [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinh N.T., Yamashita T., Tu T.C., Kato T., Ohneda K., Sato F., et al. Microvesicles enhance the mobility of human diabetic adipose tissue-derived mesenchymal stem cells in vitro and improve wound healing in vivo. Biochem Biophys Res Commun. 2016;473(4):1111–1118. doi: 10.1016/j.bbrc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 31.Mende W., Götzl R., Kubo Y., Pufe T., Ruhl T., Beier J.P. The role of adipose stem cells in bone regeneration and bone tissue engineering. Cells. Apr 21 2021;10(5) doi: 10.3390/cells10050975. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojas A., Aguilar R., Henriquez B., Lian J.B., Stein J.L., Stein G.S., et al. Epigenetic control of the bone-master Runx2 gene during osteoblast-lineage commitment by the histone demethylase JARID1B/KDM5B. J Biol Chem. Nov 20 2015;290(47):28329–28342. doi: 10.1074/jbc.M115.657825. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dvorakova J., Wiesnerova L., Chocholata P., Kulda V., Landsmann L., Cedikova M., et al. Human cells with osteogenic potential in bone tissue research. Biomed Eng Online. 2023/04/03 2023;22(1):33. doi: 10.1186/s12938-023-01096-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura K., Nagano M., Salazar G., Yamashita T., Tsuboi I., Mishima H., et al. The role of CCL5 in the ability of adipose tissue-derived mesenchymal stem cells to support repair of ischemic regions. Stem Cell Dev. 2014;23(5):488–501. doi: 10.1089/scd.2013.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cramer C., Freisinger E., Jones R.K., Slakey D.P., Dupin C.L., Newsome E.R., et al. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cell Dev. 2010;19(12):1875–1884. doi: 10.1089/scd.2010.0009. [DOI] [PubMed] [Google Scholar]
- 36.Hankamolsiri W., Manochantr S., Tantrawatpan C., Tantikanlayaporn D., Tapanadechopone P., Kheolamai P. The effects of high glucose on adipogenic and osteogenic differentiation of gestational tissue-derived MSCs. Stem Cell Int. 2016;2016 doi: 10.1155/2016/9674614. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha N.N.-y., Vong L.B., Trinh T.N. International conference on the development of biomedical engineering in Vietnam. Springer; 2022. Evaluation of reactive oxygen species production in human adipose tissue-derived mesenchymal stem cells under high D-glucose condition; pp. 231–240. [Google Scholar]
- 38.Zhou R., Ma Y., Qiu S., Gong Z., Zhou X. Metformin promotes cell proliferation and osteogenesis under high glucose condition by regulating the ROS-AKT-mTOR axis. Mol Med Rep. Oct 2020;22(4):3387–3395. doi: 10.3892/mmr.2020.11391. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marycz K., Tomaszewski K.A., Kornicka K., Henry B.M., Wroński S., Tarasiuk J., et al. Metformin decreases reactive oxygen species, enhances osteogenic properties of adipose-derived multipotent mesenchymal stem cells in vitro, and increases bone density in vivo. Oxid Med Cell Longev. 2016;2016:9785890. doi: 10.1155/2016/9785890. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang R., Liang Q., Kang W., Ge S. Metformin facilitates the proliferation, migration, and osteogenic differentiation of periodontal ligament stem cells in vitro. Cell Biol Int. 2020;44(1):70–79. doi: 10.1002/cbin.11202. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J., Wang F., Ma Y., Wei F. Vitamin D3 contributes to enhanced osteogenic differentiation of MSCs under oxidative stress condition via activating the endogenous antioxidant system. Osteoporos Int. Aug 2018;29(8):1917–1926. doi: 10.1007/s00198-018-4547-0. (in eng) [DOI] [PubMed] [Google Scholar]
- 42.Kato H., Ochiai-Shino H., Onodera S., Saito A., Shibahara T., Azuma T. Promoting effect of 1, 25 (OH) 2 vitamin D3 in osteogenic differentiation from induced pluripotent stem cells to osteocyte-like cells. Open Biol. 2015;5(2) doi: 10.1098/rsob.140201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mostafa N.Z., Fitzsimmons R., Major P.W., Adesida A., Jomha N., Jiang H., et al. Osteogenic differentiation of human mesenchymal stem cells cultured with dexamethasone, vitamin D3, basic fibroblast growth factor, and bone morphogenetic protein-2. Connect Tissue Res. 2012;53(2):117–131. doi: 10.3109/03008207.2011.611601. [DOI] [PubMed] [Google Scholar]
- 44.Kos E., Liszek M.J., Emanuele M.A., Durazo-Arvizu R., Camacho P. Effect of metformin therapy on vitamin D and vitamin B12 levels in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(2):179–184. doi: 10.4158/EP11009.OR. [DOI] [PubMed] [Google Scholar]
- 45.Chen H., Liu X., Chen H., Cao J., Zhang L., Hu X., et al. Role of SIRT1 and AMPK in mesenchymal stem cells differentiation. Ageing Res Rev. Jan 2014;13:55–64. doi: 10.1016/j.arr.2013.12.002. (in eng) [DOI] [PubMed] [Google Scholar]
- 46.Goel S., Singh R., Singh V., Singh H., Kumari P., Chopra H., et al. Metformin: activation of 5′ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front Genet. 2022-October-31;13 doi: 10.3389/fgene.2022.1022739. (in English) 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borojević A., Jauković A., Kukolj T., Mojsilović S., Obradović H., Trivanović D., et al. Vitamin D3 stimulates proliferation capacity, expression of pluripotency markers, and osteogenesis of human bone marrow mesenchymal stromal/stem cells, partly through SIRT1 signaling. Biomolecules. 2022;12(2):323. doi: 10.3390/biom12020323. https://www.mdpi.com/2218-273X/12/2/323 [Online]. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]