Abstract
Background
H101, an innovative oncolytic adenovirus, has shown potential in modifying the tumor microenvironment from immunologically ‘cold’ to ‘hot’. When combined with nivolumab, a programmed cell death protein 1 inhibitor, this synergy may offer substantial therapeutic benefits beyond the capabilities of each agent alone.
Patients and methods
In this pilot study, we assessed the efficacy and safety of combining H101 with nivolumab in advanced hepatocellular carcinoma (HCC) patients who failed prior systemic therapy. The participants received initial oncolytic virus (OV) pretreatment with intratumoral H101 injections (5.0 × 1011 vp/0.5 ml/vial, two vials per lesion) on days 1 and 3. Combination therapy started on day 8, with H101 administered every 2 or 4 weeks and nivolumab (240 mg) injections every 2 weeks. Treatment continued up to 12 months or until disease progression, intolerable toxicity, consent withdrawal, or study conclusion. The primary endpoint was the objective response rate (ORR).
Results
Between March 2020 and March 2022, 18 of 21 screened patients were assessable, showing an ORR of 11.1% [two cases of partial response (PR) and five cases of stable disease], with extrahepatic injections often leading to favorable outcomes. The disease control rate stood at 38.9%, with a 6-month survival rate of 88.9%. Median progression-free survival was 2.69 months, and overall survival (OS) was 15.04 months. Common adverse events included low-grade fever (100%) and pain related to centesis (33.3%), and no grade 3/4 events were reported. Significantly, local H101 injection showed potential in reversing immune checkpoint inhibitor resistance, evidenced by over 2.5 years of extended OS in PR cases with low α-fetoprotein. Additionally, decreasing neutrophil-to-lymphocyte ratio during OV pretreatment may predict positive outcomes.
Conclusions
This study demonstrates the potential efficacy of combining H101 with nivolumab in treating refractory advanced HCC, with well-tolerated toxicities.
Key words: advanced hepatocellular carcinoma, oncolytic adenovirus, nivolumab, viral immunotherapy
Highlights
-
•
First pilot trial of oncolytic virus and programmed cell death protein 1 inhibitors in refractory HCC.
-
•
H101 injections show promise in reversing immune checkpoint inhibitor resistance, extending OS in PR cases.
-
•
Dynamic neutrophil-to-lymphocyte ratio changes during OV pretreatment may predict outcomes.
Introduction
Hepatocellular carcinoma (HCC) is the primary malignancy of hepatocytes, ranking as the fourth most common cause of cancer-related death worldwide. The average 5-year survival rate of HCC patients is 18%, but this figure drops to as low as 2.5% for advanced, metastatic cases, making it one of the most lethal cancers.1 For over a decade, sorafenib, a multi-targeted kinase inhibitor, remained the only Food and Drug Administration-approved systemic therapy for advanced/metastatic HCC, offering a median survival of just 11.7 months and often being poorly tolerated.2 This highlights a significant unmet need for novel therapeutic strategies in advanced HCC.
Immunotherapy, particularly agents like nivolumab, an anti-programmed cell death protein 1 monoclonal antibody, has emerged as a new era in cancer treatment. Despite showing promise in studies like CheckMate 040, nivolumab’s first-line application did not significantly enhance overall survival (OS) compared to sorafenib, as per the CheckMate 459 trial.3 Nevertheless, it is listed as a first-line therapy option for certain advanced HCC patients by the National Comprehensive Cancer Network Clinical Practice Guidelines.4 Moreover, the combination of atezolizumab and bevacizumab, targeting programmed death-ligand 1 and vascular endothelial growth factor, respectively, has shown superior survival to sorafenib, becoming a new standard regimen for unresectable or metastatic HCC in May 2020.5
However, the tumor microenvironment of HCC can impact the response to immunotherapy. Oncolytic viruses (OVs), known for specifically lysing cancer cells and activating the immune system,6 thus converting ‘cold’ tumors into ‘hot’ ones,7, 8, 9 have shown potential in preclinical models. This makes OV an appealing therapeutic strategy in combination with immune checkpoint inhibitors (ICIs) for solid tumors, including melanoma and head and neck carcinoma.10,11 H101 (Oncorine) is a human recombinant type 5 adenovirus, engineered by removing the gene E1B, which is responsible for coding the anti-apoptotic E1B55K protein that deactivates p53.12,13 Additionally, to enhance safety, a portion of its E3 region has also been deleted.14 It has been shown to enhance transarterial chemoembolization (TACE) for HCC15, 16, 17 and conventional chemotherapy for other tumors.18, 19, 20 Approved in China for advanced nasopharyngeal carcinoma since 2005, H101 represents the first OV to gain regulatory approval. Currently, over 30 types of OVs, including reovirus, herpes simplex virus, and vaccinia virus, are under investigation for HCC treatment, but the efficacy of OVs combined with ICIs is still being evaluated in ongoing trials.14
Whether the combination of oncolytic virotherapy and ICIs can replicate the successful paradigms observed in other tumors remains unknown. Given this background, our pilot trial aims to evaluate the efficacy and safety of combining H101 with nivolumab in patients with advanced HCC who have failed prior systemic therapy.
Patients and methods
Study design and patients
This open-label, single-arm, pilot trial was conducted in Shanghai, China. The eligibility criteria were patients aged >18 years with histologically or radiologically confirmed unresectable advanced HCC [according to the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition)], who failed or were not tolerant from at least one or more prior systemic therapy. Additional inclusion criteria were: an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; at least one measurable untreated lesion as per RECIST v1.1, which can be evaluated as a target lesion for follow-up measurement; at least one measurable untreated lesion for local injection of H101, which can be evaluated as an injection lesion; sufficient hematological, hepatic, renal, and cardiac functions.
Key exclusion criteria included: previous oncolytic adenovirus and/or immune therapy; radiotherapy, major surgical procedure, open biopsy, or significant traumatic injury within 4 weeks, or those who receive minor surgical procedures within 1 week before enrollment; hepatic encephalopathy; clinically significant ascites; evidence of portal hypertension with bleeding esophageal or gastric varices within the past 6 months; prior liver transplant. Full details of the eligibility criteria are provided in the protocol (Supplementary Material S1, available at https://doi.org/10.1016/j.esmoop.2024.102239).
The trial protocol was approved by the institutional ethics review board of the participating center (ChiCTR1900025377). It was done according to the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines, with any applicable regulatory requirements. All patients provided written informed consent.
Procedures
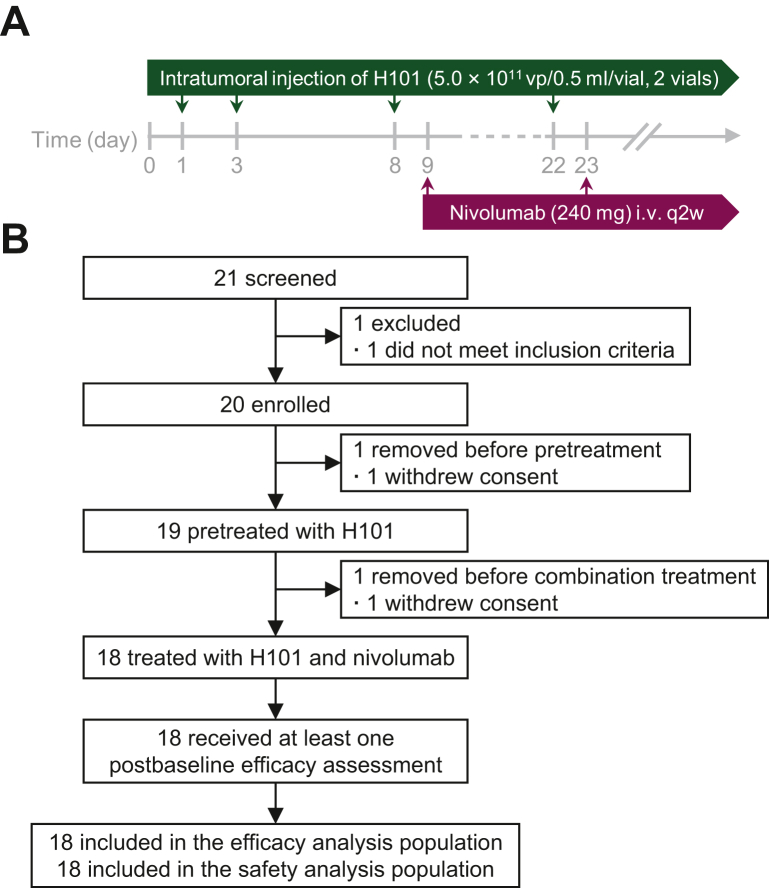
We established clear criteria for selecting lesions suitable for intratumoral injection of H101. Only one lesion was chosen for injection in each patient, ensuring a focused and targeted delivery. Eligible lesions were determined based on their accessibility under ultrasound guidance and their size, prioritizing intrahepatic lesions >2 cm in diameter. However, in cases where there were more accessible extrahepatic metastatic lesions, such as subcutaneous metastatic nodules, these were preferentially chosen for injection due to their ease of operation. In the procedure for OV pretreatment, patients received an intratumoral injection of H101 (5.0 × 1011 vp/0.5 ml/vial, two vials) for each selected lesion on days 1 and 3, to trigger seroconversion and a defense immune response to the viral vector. The operation method was meticulously executed as follows: under the guidance of ultrasound, the most accessible treatment lesion was identified. A fine needle aspiration was then carried out at the center of this lesion and we slowly injected H101, ensuring optimal distribution of the viral vector within the lesion. Following the completion of the injection, the fine needle was carefully withdrawn to prevent any damage to the surrounding tissues and to minimize patient discomfort. In the absence of any complications, this same tumor site would be selected for medication injection during the subsequent combined treatment period. The combination period started from day 8, which was recorded as the first day of the first cycle [i.e. day 8 was recorded as cycle 1 day 1 (C1D1)], and H101 (two vials, on C1D1) was injected every 2 or 4 weeks or suspended, depending on the patient’s tolerance. Nivolumab (240 mg) was administered intravenously every 2 weeks beginning on C1D2 (if the patient was well tolerated, it could also be administered on C1D1). Treatment continued until disease progression (as assessed in the RECIST v1.1 criterion), intolerable toxic effects, informed consent withdrawal, or the end of the study (Figure 1A). The maximum total combination treatment period for this study was 12 months (24 cycles). The investigators might use their judgment in non-protocol situations and make choices after weighing the risks and benefits to the patient.
Figure 1.
Study design and trial profile. (A) Pilot study design schema. (B) Consolidated standards of trial diagram.
Before the first administration, baseline assessments were done within 2 weeks. Patients underwent essential screening evaluations, which included a complete medical history and physical examination; ECOG PS; a standard 12-lead electrocardiogram; a magnetic resonance imaging (MRI) or computed tomography (CT) scan of the chest, abdomen, and pelvis; Child–Pugh score. Laboratory tests such as hematology, biochemistry, viral safety biomarkers [for hepatitis B virus (HBV)- and hepatitis C virus (HCV)-infected participants], HBV-DNA for surface antigen of HBV (HBsAg) (+), HCV-RNA for HCV patients, quantitative detection of immunocyte subtypes, coagulation function, urine analysis, and α-fetoprotein (AFP) were carried out as well.
Outcomes
The primary endpoint was the confirmed objective response rate (ORR), as evaluated by researchers according to RECIST v1.1. Secondary endpoints included the investigator-assessed 6-month OS%, progression-free survival (PFS), OS, disease control rate (DCR), duration of response (DOR), safety, and exploratory bioinformatics analysis. All these endpoints were calculated in the assessable population, defined as subjects who received at least four nivolumab treatments and underwent at least one cancer evaluation.
Statistical analyses
The ORR of nivolumab treatment for HCC was 14.3% in CheckMate 040 in 2014.3 Under the assumption of an ORR of 40% for the combined treatments, with α = 0.1 (one-tailed), 80% power could be achieved by a sample size of 16 cases. Allowing for a 20% dropout rate, we estimated that a total of 20 patients were required.
Tumor responses were evaluated by researchers based on RECIST v1.1, defined as complete response (CR), partial response (PR), and stable disease (SD), respectively. For this evaluation, target lesions were selected based on specific criteria: when multiple measurable lesions were present at baseline, up to five total lesions (maximum two per organ) were identified as target lesions for consistent recording and measurement. The selection was based on the longest diameter and representativeness of all involved organs. Special attention was given to lymph nodes; only nodes with a short axis of ≥15 mm on CT scan were considered as target lesions. Unless there were no other suitable target lesions available, lesions treated with intratumoral injections were generally not considered as target lesions for evaluation, to prevent the influence of injections on the appearance of the lesions. All validity analyses were based on a full analysis set. The two-sided 95% confidence intervals (CIs) were evaluated for the ORR and DCR using the Clopper–Pearson method. Time-to-event data (PFS, OS, DOR) were estimated based on the Kaplan–Meier (KM) method, with the median and the 95% CIs calculated using the Brookmeyer–Crowley method. The 6-month OS% was determined based on the KM curve.
Results
Participant characteristics
Between March 2020 and March 2022, 21 patients were screened, of whom 18 enrolled in this study were assessable (Figure 1B). The baseline characteristics are summarized in Table 1. Briefly, 18 participants were predominantly male (88.9%), with an average age of 52.6 years, most of whom were under 60 years (72.2%). The majority of the participants had an ECOG PS score of 1 (72.2%) and were classified as Child–Pugh score A (88.9%). A significant proportion (83.3%) were infected with HBV, and 77.8% were at Barcelona Clinic Liver Cancer stage C. Regarding AFP levels, the median was 95.9 ng/ml, with 38.9% of participants having levels of 400 ng/ml or higher. Microvascular invasion was present in 11.1% of the participants, while a notable 77.8% had extrahepatic metastases, primarily in the lungs (55.6%) and bones (33.3%). In terms of treatment history, a majority underwent TACE (83.3%), and half of the participants had previous surgical resection and ablation. Regarding systemic therapies, most participants (83.3%) had one prior treatment, with sorafenib (72.2%) and lenvatinib (27.8%) being the most common types used. The characteristics of specific locations of the injection sites and target lesions for observation for each of the 18 patients are detailed in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.102239.
Table 1.
Baseline characteristics, n = 18
| Number of cases (%) | |
|---|---|
| Age, years | |
| Mean ± SD | 52.6 ± 10.9 |
| Median [ITR] | 54 [42-60.5] |
| <60 | 13 (72.2) |
| ≥60 | 5 (27.8) |
| Gender | |
| Male | 16 (88.9) |
| Female | 2 (11.1) |
| ECOG PS | |
| 0 | 5 (27.8) |
| 1 | 13 (72.2) |
| Child–Pugh score | |
| A | 16 (88.9) |
| B | 2 (11.1) |
| Virus infection | |
| HBV | 15 (83.3) |
| None | 3 (16.7) |
| BCLC stage | |
| B | 4 (22.2) |
| C | 14 (77.8) |
| AFP, ng/ml | |
| Median [ITR] | 95.9 [6.8-3033] |
| ≤20 | 6 (33.3) |
| 20-400 | 5 (27.8) |
| ≥400 | 7 (38.9) |
| MVI | |
| Yes | 2 (11.1) |
| No | 16 (88.9) |
| EHS | |
| Yes | 14 (77.8) |
| No | 4 (22.2) |
| Metastatic organ involvement | |
| Liver only | 4 (22.2) |
| Lung | 10 (55.6) |
| Bone | 6 (33.3) |
| Adrenal gland | 2 (11.1) |
| Soft tissue | 5 (27.8) |
| Lymph nodes | 3 (16.7) |
| Previous local treatments | |
| Surgical resection | 9 (50.0) |
| TACE | 15 (83.3) |
| Ablation | 9 (50.0) |
| HIFU | 1 (5.6) |
| Number of prior systemic treatments | |
| 1 | 15 (83.3) |
| 2 | 3 (16.7) |
| Types of systemic therapy | |
| Sorafenib | 13 (72.2) |
| Lenvatinib | 5 (27.8) |
| Chemotherapy | 3 (16.7) |
| Other | 1 (5.6) |
The total percentages for metastatic organ involvement, previous local treatments, and types of systemic therapy exceeded 100%, respectively. This was due to some patients having metastases in more than one organ and receiving multiple previous interventions.
AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; EHS, extrahepatic metastases; HBV, hepatitis B virus; HIFU, high-intensity focused ultrasound; ITR, interquartile range; MVI, microvascular invasion; SD, standard deviation; TACE, transarterial chemoembolization.
Efficacy
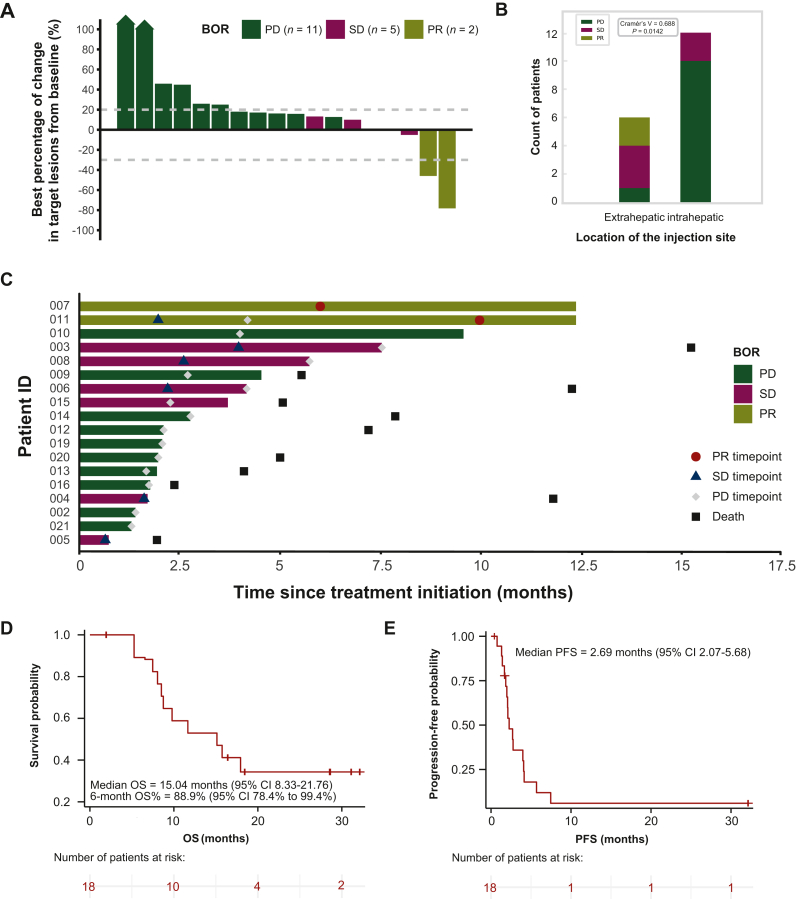
The database cut-off was 27 June 2023, and all 18 patients were included in the data analysis. Compared to baseline, five patients (27.8%) experienced a decrease in the change of target lesion size (Figure 2A). In the best overall response (BOR), there were no patients with a CR, 2 with a confirmed PR, 5 with SD, and 11 with progressive disease (PD). The ORR and DCR were 11.1% and 38.9%, respectively (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.102239). Upon further analysis of the correlation between the location of injection lesions and treatment response, we found that when the injection sites were located within the liver, there appeared to be a greater tendency toward PD (Figure 2B). Conversely, patients with extrahepatic injection sites, such as those in the lung and chest wall mass, were more likely to exhibit instances of PR and SD. The median time to initial response was 7.98 months (range 6-9.97 months) in the two patients with an objective response, and the median DOR was 18.79 months [95% CI not estimable (NE)-NE, Figure 2C]. In addition, 16 (89.9%) confirmed PD events occurred, with a median PFS of 2.69 months (95% CI 2.07-5.68 months, Figure 2E). The median follow-up was 11.89 months (range 1.94-32.13 months), and 12 (66.7%) events of death occurred. The median OS was 15.04 months (95% CI 8.33-21.76 months, Figure 2D), with a 6-month OS% of 88.9% (95% CI 78.4% to 99.4%). Post-study treatments were not recorded.
Figure 2.
Tumor response and survival data. (A) Best percentage change from baseline in the target lesion; the BOR is indicated by the color coding of the bar. (B) Correlation between injection lesion location and BOR; P = 0.0142, Cramér’s V = 0.688, analyzed using the chi-square test. (C) Records of responses during treatment; the bar length represents the treatment duration for each patient. (D) KM curve of OS. (E) KM curve of PFS. BOR, best overall response; CI, confidence interval; KM, Kaplan–Meier; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
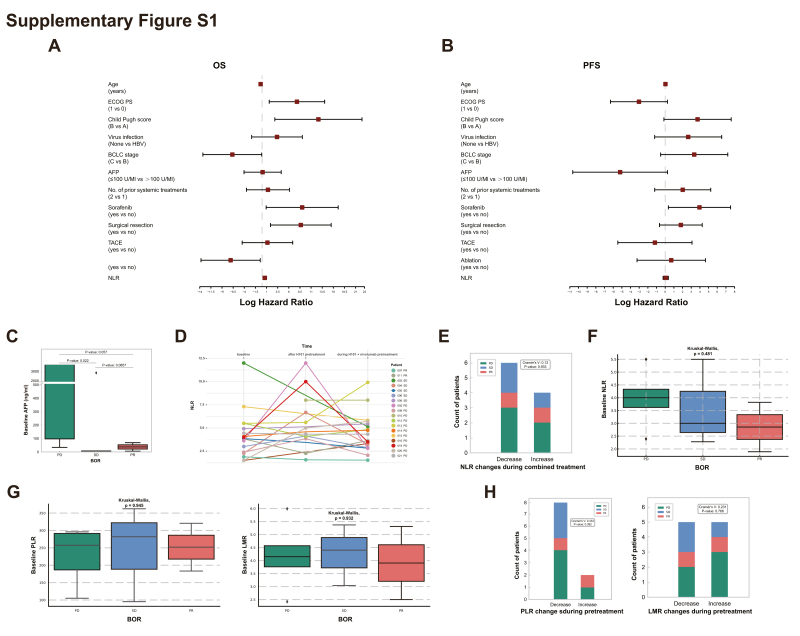
In our analysis of a non-predefined subgroup, we explored clinical prognostic variables that significantly influenced OS and PFS. For OS, an ECOG PS of 1, a Child–Pugh score of B, a history of treatment with sorafenib or surgical resection treatments, and a high neutrophil-to-lymphocyte ratio (NLR) were identified as risk factors in the hazard ratio analysis (Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2024.102239). Interestingly, a history of ablation treatment emerged as a protective factor. In terms of PFS, sorafenib remained a risk factor, but no other classical prognostic variables showed significant differences (Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2024.102239).
Safety
In the evaluation of the combined treatment’s safety profile, we observed various adverse events (AEs), aligning with the National Cancer Institute Common Terminology Criteria (version 5.0), as detailed in Table 2. Notably, all 18 cases reported low-grade (grade 1 or 2) fever related to H101, which typically occurred within 12 h post-H101 injection and mostly subsided spontaneously after 2-4 h, without necessitating special treatment. Additionally, we observed complications such as infection (5.6%) and liver hemorrhage (5.6%), potentially linked to frequent centesis. Injection site pain was reported in 33.3% of the cases, a foreseeable consequence of the intratumoral injection of H101.
Table 2.
Adverse events, n = 18
| AEs |
n (%) |
|
|---|---|---|
| Grade 1 or 2 | Grade 3 or 4 | |
| Systemic or general symptoms | ||
| Fever | 18 (100.0) | 0 (0.0) |
| Decreased appetite | 2 (11.1) | 0 (0.0) |
| Fatigue | 2 (11.1) | 0 (0.0) |
| Weight loss | 1 (5.6) | 0 (0.0) |
| Cancer pain | 3 (16.7) | 0 (0.0) |
| Local reactions | ||
| Injection site pain | 6 (33.3) | 0 (0.0) |
| Liver hemorrhage | 1 (5.6) | 0 (0.0) |
| Immune-related hepatitis | ||
| Increased bilirubin | 2 (11.1) | 0 (0.0) |
| Elevated ALT/AST | 2 (11.1) | 0 (0.0) |
| Immune-related endocrine events | ||
| Increased TSH | 2 (11.1) | 0 (0.0) |
| Infectious complications | ||
| Infection | 1 (5.6) | 0 (0.0) |
| Cough | 1 (5.6) | 0 (0.0) |
| Metabolic abnormalities | ||
| Hypophosphatemia | 2 (11.1) | 0 (0.0) |
| Hypomagnesemia | 1 (5.6) | 0 (0.0) |
| Hypoalbuminemia | 1 (5.6) | 0 (0.0) |
AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TSH, thyroid-stimulating hormone.
Regarding immune-related adverse events (irAEs), we noted increased bilirubin and elevated alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels were observed in 11.1% of patients, indicative of immune-related hepatitis. Additionally, 11.1% of patients experienced increased thyroid-stimulating hormone (TSH) levels, signaling potential immune-related endocrine events. These AEs were predominantly low grade (grade 1 or 2) in our study and were managed effectively upon symptomatic treatment or temporary discontinuation of the therapy, without leading to any grade 3 or 4 AEs. These findings aligned with the known safety profile of nivolumab and indicated that the combination of H101 with nivolumab was well tolerated in our patient cohort.
Reversal of resistance to ICIs by local H101 injection and long-lasting response
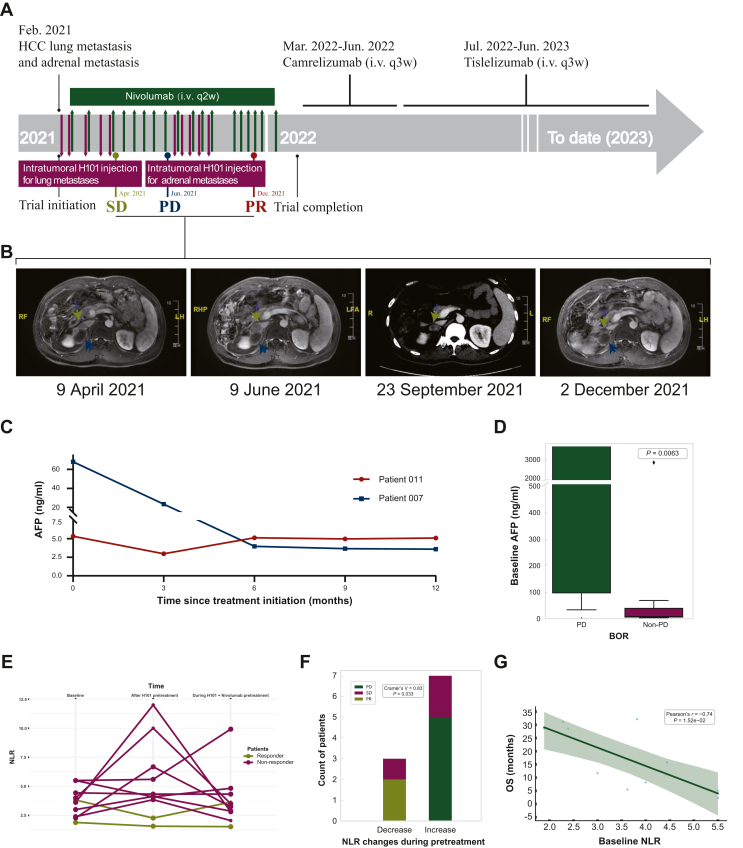
Patient 011 was enrolled in the clinical trial with a diagnosis of lung and adrenal metastases from HCC and had a history of liver tumor and gallbladder resection, multiple TACE sessions, sorafenib treatment, and microwave ablation for lung and adrenal tumors. In the treatment timeline (Figure 3A), H101 injection was initially administered to the lung metastases, while the adrenal metastases were monitored for diameter measurement. The treatment resulted in SD with tumor regression. However, after a 2-month discontinuation of virotherapy, the patient developed resistance to nivolumab monotherapy, as evidenced by increased adrenal lesion size on follow-up MRI scans, indicating PD. Subsequently, researchers administered H101 directly into the adrenal metastases, resulting in sustained tumor shrinkage and a PR outcome (Figure 3B). This suggests that OVs can activate the local immune environment and overcome resistance to ICIs.
Figure 3.
Multifaceted clinical data of the PR patients and non-responders. (A) Timeline of treatment and clinical events of patient 011. (B) Representative MRI images of the pelvis [the adrenal metastatic lesion (green) and lesion previously treated with microwave ablation (blue) are marked by triangle]. (C) AFP levels of PR cases during baseline and throughout the treatment. (D) Comparison of baseline AFP levels between non-PD and PD patient groups; P = 0.0063, analyzed using the Mann–Whitney U test. (E) NLR observed in the responders and non-responders at baseline, after pretreatment with H01, and during the course of combined treatment. (F) Correlation between NLR changes during H101 pretreatment and treatment efficacy; P = 0.033, Cramér’s V = 0.83, analyzed using the chi-square test. (G) Correlation between baseline NLR levels and OS; P = 1.52e−02, Pearson’s r = 0.83, analyzed using Pearson’s correlation coefficient.AFP, α-fetoprotein; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PD, progressive disease; PR, partial response; SD, stable disease.
Following the completion of the trial, the patient opted for self-administration of single-agent ICI therapy and achieved ongoing remission. The combination of OV therapy with nivolumab demonstrated enduring efficacy, with both PR cases experiencing an OS of >2.5 years. In our analysis, a notable trend emerged: patients whose AFP levels remained <100 ng/ml both at baseline and throughout the treatment appeared to respond more favorably (Figure 3C). Recognizing the limitations posed by our small sample size (Supplementary Figure S1C, available at https://doi.org/10.1016/j.esmoop.2024.102239), we grouped patients with PR and SD into a single ‘non-PD’ category for a more comprehensive comparison. This aggregated group demonstrated significantly lower baseline AFP levels compared to those in the PD group (P = 0.0063; Figure 3D), thereby highlighting a more distinct trend. These findings tentatively indicate that individuals with lower AFP levels might be better candidates for the combined OV and nivolumab treatment regimen. However, given the limited number of PR cases, this conclusion remains preliminary and calls for further validation through research in a larger, more diverse cohort.
Changes in the functional phenotype of circulating neutrophils and lymphocytes with combined therapy
The NLR was determined based on absolute neutrophil count and absolute lymphocyte count. We closely monitored the dynamic changes in NLR in peripheral blood during different phases of treatment (Supplementary Figure S1D, available at https://doi.org/10.1016/j.esmoop.2024.102239). In our analysis of 10 patients, after H101 pre-injection, NLR decreased in 2 responders and increased in 7 out of 8 non-responders (Figure 3E). After combining with nivolumab, the majority (7 out of 10) showed a further decrease in NLR. Notably, we found that NLR changes during the pretreatment phase were strongly correlated with treatment efficacy (P = 0.033; Figure 3F), suggesting its potential as an early indicator of response to oncolytic viral immunotherapy. In contrast, during the combined treatment phase with nivolumab, the predictive significance of these NLR changes appeared to diminish (Supplementary Figure S1E, available at https://doi.org/10.1016/j.esmoop.2024.102239).
Regarding baseline NLR, our analysis showed that there was no significant correlation with treatment efficacy (Supplementary Figure S1F, available at https://doi.org/10.1016/j.esmoop.2024.102239), possibly due to the limited sample size. Additionally, baseline NLR levels were negatively correlated with OS (Figure 3G), suggesting its potential as a prognostic marker, although this correlation was not observed in PFS (Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2024.102239). Additionally, we expanded our analysis to include other hematological markers such as platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR). However, we did not find a significant correlation between their baseline levels or changes during pretreatment and the response in our cohort (Supplementary Figure S1G and H, available at https://doi.org/10.1016/j.esmoop.2024.102239).
Discussion
Though recent research on OVs has garnered considerable attention, there remains a notable scarcity of published data specifically related to their use in HCC. Our study represents the first pilot trial reported in assessing the safety and effectiveness of the combined administration of H101 and nivolumab in HCC. The ORR of 11.1% and a median OS of 15.04 months provide preliminary evidence of positive outcomes, particularly considering that our cohort consisted of patients with refractory HCC.
One of our pivotal findings is the correlation between injection site location and treatment response. We discovered that extrahepatic injection sites, such as those in superficial areas like the lung and chest wall, often led to PR and SD. This aligns with other studies in melanoma and head and neck tumors, suggesting that such subcutaneous injection sites may allow for better viral spread,10,11 compared to dense and hypoxic environments like solid tumors.21,22 Conversely, liver injections, as well as a case involving the muscular layer of the buttock, were more likely associated with PD. The distinct immunosuppressive contexture of the liver and its role in drug metabolism may contribute to this variance in efficacy.23,24 This emerging pattern highlights the importance of strategic injection site selection in the application of oncolytic virotherapy for HCC patients. Additionally, our findings tentatively indicate that individuals with lower AFP levels might be better candidates for the combined OV and nivolumab treatment regimen. However, given the limited number of PR cases, this conclusion remains preliminary and calls for further validation through research in a larger, more diverse cohort.25,26
In our cohort, we also noted that some patients with SD achieved prolonged OS post-treatment (Figure 2C). This observation is in line with recent findings underscoring the long-tail effects of immunotherapy,27 suggesting that initial response patterns might not fully capture the potential benefits of such treatments and highlighting the importance of extended OS in efficacy evaluations.28, 29, 30 Furthermore, a critical bottleneck in current immunotherapy is the occurrence of acquired resistance, especially in cases like non-small-cell lung cancer where it can affect up to 46% of patients in remission.31 Approximately 56% of these cases are characterized as oligo-acquired resistance, where localized treatments can still be effective.32 Our study proposes that local OV administration, by potentially reactivating the immune system, in conjunction with continued immunotherapy, could reverse such resistance, thereby extending the period of disease control.
Of note, we observed an increase in circulating lymphocytes and a decrease in NLR among responders following H101 pretreatment, indicating systemic immune activation. Conversely, non-responders showed no significant change or an increase in NLR, suggesting a less activated immune system. Interestingly, despite a subsequent decrease in NLR after adding nivolumab, non-responders’ outcomes remained unfavorable. Moreover, these observations suggest a potential link between early immune responses, as indicated by NLR fluctuations, and the overall treatment efficacy. These NLR changes in the pretreatment phase may serve as convenient and safer predictors of outcomes compared to traditional tumor tissue biopsy. We also explored additional hematological biomarkers including PLR and LMR, but found no significant correlation with treatment response, possibly due to the relatively small size of our patient cohort. While baseline NLR holds predictive value,33,34 the variability of optimal NLR cut-offs across studies suggests that it is not competent to be considered as the sole marker of immunotherapy responses.35,36 A more robust approach would be to combine baseline NLR measurements with the monitoring of dynamic NLR changes during the OV pre-injection phase.
The safety profile of our treatment regimen, consistent with the known effects of nivolumab and those observed in other oncolytic viral immunotherapy trials,37 showed manageable low-grade irAEs including elevated bilirubin, increased ALT/AST levels, and changes in TSH levels.38 This indicates the combination regimen’s suitability for advanced HCC patients. Our trial also contributes to the ongoing discussion on the optimal dosing of OVs. In our study, the dosage of H101 used (5.0 × 1011 vp/0.5 ml/vial, two vials) was found to be safe and well tolerated. Additionally, in the treatment of malignant ascites with a reported 40% response rate, another study tailored the H101 dose based on the volume of ascites, ranging from 5.0 × 1011 vp for smaller volumes to 2.0 × 1012 vp for larger volumes.37 While in phase Ib trial of talimogene laherparepvec combined with pembrolizumab in melanoma, a total dose of up to 4 ml × 108 pfu/ml leads to an ORR of 61.9%.10 These examples illustrate that effective dosages of OVs can vary significantly depending on the specific condition and treatment context.39 However, whether the effectiveness and toxicity of H101 are dose-dependent remains an open question. Our findings suggest the possibility of a wider safe dose than two vials for H101, which warrants further exploration.
In selecting our study cohort, we uniquely focused on refractory HCC patients who have undergone systemic treatment, contrasting with many OV trials that typically involve early-stage, non-metastatic patients receiving first-line treatment. Applying OV therapies to advanced-stage and metastatic cancers is challenging, as shown by limited efficacy in recent studies. For example, a phase Ib study of talimogene laherparepvec combined with atezolizumab reported an ORR of just 10% in triple-negative breast cancer and 0% in colorectal cancer with liver metastases,22 and a phase III trial of virus-based immunotherapy in melanoma revealed low response rates in patients with visceral metastases.40 This context underscores the importance of our study in exploring the potential of H101 combined with nivolumab for hard-to-treat HCC cases. Other than H101, the burgeoning field of oncolytic virotherapy is developing novel viruses,41,42 and more sophisticated delivery systems,43,44 which may further improve treatment efficacy in the future.
This study has several limitations, including its single-center, single-arm design, and a relatively small sample size. Reflecting on our trial’s design, it was registered on 25 August 2019, a time when more potent immunotherapy combinations were not widely available. The trial’s duration and the 1-year nivolumab treatment plan were determined based on the knowledge and standards at that time. However, it is worth noting that the optimal duration of immunotherapy, particularly the timing of discontinuation, remains a topic of ongoing debate, and longer or differently structured treatment courses might yield different outcomes. At the start of our trial, significant developments such as the IMbrave 150 study’s findings,45 which highlighted the superior ORR of combining immunotherapy with anti-angiogenic therapy, had not been concluded.46 In response to evolving insights, we have launched an extended trial (NCT05303090) to investigate whether a triple combination of OVs, anti-angiogenic therapy, and immunotherapy can offer enhanced therapeutic effects in solid tumors compared to a dual combination. This new trial builds upon our current findings and incorporates higher doses of OVs, aiming to refine treatment efficacy. Future research needs to focus on establishing specific biomarkers predictive of efficacy to identify patients most likely to respond to oncolytic viral immunotherapies, thereby optimizing treatment duration and outcomes.
Conclusion
In summary, this pilot study is the first to demonstrate the potential efficacy and well-tolerated toxicities of combining H101 with nivolumab in patients with refractory advanced HCC who failed prior systemic therapy. Notably, local administration of H101 may represent a promising strategy for reversing ICI resistance, leading to extended OS of >2.5 years in responders, particularly in those with lower AFP levels. Furthermore, our findings suggest that a decrease in NLR during OV pretreatment could serve as an early predictive marker for favorable treatment outcomes. However, given the limited number of participants, these conclusions remain preliminary and call for further validation through research in a larger, more diverse cohort. Future research is warranted to optimize the therapeutic approach for refractory advanced HCC patients.
Acknowledgements
This study is an investigator-initiated trial sponsored by Bristol-Myers Squibb Company, which provides free medication to the participants. The project number is CA209-7CE (BMS). The authors would like to thank the patients, their families, and the study personnel involved in this trial.
Funding
This research is supported by the Xisike Clinical Oncology Research Foundation (Grant Number: Y-HR2018-104).
Disclosure
The authors have declared no conflicts of interest.
Contributor Information
J. Xie, Email: isable624@163.com.
Z. Meng, Email: mengshca@fudan.edu.cn.
Supplementary data
Supplementary Figure S1.
Comprehensive analysis of prognostic indicators. (A) HR analysis for OS. (B) HR analysis for PFS. (C) Comparison of baseline AFP levels between PD, SD and PR patient groups; analyzed using the Mann-Whitney U test. (D) Changes in NLR in peripheral blood during different phases of treatment of each patient. (E) Correlation between NLR changes during combined treatment and treatment efficacy; analyzed using the chi-square test. (F) Comparison of baseline NLR between PD, SD and PR patient groups; analyzed using the Mann-Whitney U test. (G) Comparison of baseline PLR (left) and LMR (right) levels between PD, SD and PR patient groups; analyzed using the Mann-Whitney U test. (H) Correlation between PLR (left) and LMR (right) changes during H101 pretreatment and treatment efficacy; analyzed using the chi-square test. HR, hazard ratio; OS, overall survival; PFS, progression-free survival; AFP, α-fetoprotein; PD, progressive disease; PR, partial response; SD, stable disease; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
References
- 1.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson A.B., D’Angelica M.I., Abbott D.E., et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 3.El-Khoueiry A.B., Sangro B., Yau T., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yau T., Park J.W., Finn R.S., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 5.Casak S.J., Donoghue M., Fashoyin-Aje L., et al. FDA approval summary: atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin Cancer Res. 2021;27:1836–1841. doi: 10.1158/1078-0432.CCR-20-3407. [DOI] [PubMed] [Google Scholar]
- 6.Li R., Zhang J., Gilbert S.M., Conejo-Garcia J., Mulé J.J. Using oncolytic viruses to ignite the tumour immune microenvironment in bladder cancer. Nat Rev Urol. 2021;18:543–555. doi: 10.1038/s41585-021-00483-z. [DOI] [PubMed] [Google Scholar]
- 7.Foerster F., Gairing S.J., Ilyas S.I., Galle P.R. Emerging immunotherapy for HCC: a guide for hepatologists. Hepatology. 2022;75:604–1626. doi: 10.1002/hep.32447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L., Zhao H., Shan M., et al. Oncolytic adenovirus H101 ameliorate the efficacy of anti-PD-1 monotherapy in colorectal cancer. Cancer Med. 2022;11:4575–4587. doi: 10.1002/cam4.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garofalo M., Bellato F., Magliocca S., et al. Polymer coated oncolytic adenovirus to selectively target hepatocellular carcinoma cells. Pharmaceutics. 2021;13:949. doi: 10.3390/pharmaceutics13070949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribas A., Dummer R., Puzanov I., et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119.e1110. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington K.J., Kong A., Mach N., et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153–5161. doi: 10.1158/1078-0432.CCR-20-1170. [DOI] [PubMed] [Google Scholar]
- 12.McCormick F. Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015. Semin Cancer Biol. 2000;10:453–459. doi: 10.1006/scbi.2000.0336. [DOI] [PubMed] [Google Scholar]
- 13.O’Shea C.C., Johnson L., Bagus B., et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Li X., Sun X., Wang B., Li Y., Tong J. Oncolytic virus-based hepatocellular carcinoma treatment: current status, intravenous delivery strategies, and emerging combination therapeutic solutions. Asian J Pharm Sci. 2023;18 doi: 10.1016/j.ajps.2022.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Q., Liu Y., Zou Q., Guan Y.S. Transarterial injection of H101 in combination with chemoembolization overcomes recurrent hepatocellular carcinoma. World J Gastroenterol. 2011;17:2353–2355. doi: 10.3748/wjg.v17.i18.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin X.J., Li Q.J., Lao X.M., Yang H., Li S.P. Transarterial injection of recombinant human type-5 adenovirus H101 in combination with transarterial chemoembolization (TACE) improves overall and progressive-free survival in unresectable hepatocellular carcinoma (HCC) BMC Cancer. 2015;15:707. doi: 10.1186/s12885-015-1715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao M., Cheng S., Zhai X., et al. Prognostic comparison between cTACE and H101-TACE in unresectable hepatocellular carcinoma (HCC): a propensity-score matching analysis. Appl Bionics Biomech. 2022;2022 doi: 10.1155/2022/9084852. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zhang R., Cui Y., Guan X., Jiang X. A recombinant human adenovirus type 5 (H101) combined with chemotherapy for advanced gastric carcinoma: a retrospective cohort study. Fron Oncol. 2021;11 doi: 10.3389/fonc.2021.752504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu W., Zheng S., Li X.F., Huang J.J., Zheng X., Li Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial. World J Gastroenterol. 2004;10:3634–3638. doi: 10.3748/wjg.v10.i24.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Z.J., Chang J.H., Zhang L., et al. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus] Ai Zheng. 2004;23:1666–1670. [PubMed] [Google Scholar]
- 21.Waddington S.N., McVey J.H., Bhella D., et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Hecht J.R., Raman S.S., Chan A., et al. Phase Ib study of talimogene laherparepvec in combination with atezolizumab in patients with triple negative breast cancer and colorectal cancer with liver metastases. ESMO Open. 2023;8 doi: 10.1016/j.esmoop.2023.100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng J., Liu S., Zhou Q., et al. Intratumoral PPT1-positive macrophages determine immunosuppressive contexture and immunotherapy response in hepatocellular carcinoma. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2022-006655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Xun Z., Ma K., et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. 2023;78:770–782. doi: 10.1016/j.jhep.2023.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhu A.X., Dayyani F., Yen C.J., et al. Alpha-fetoprotein as a potential surrogate biomarker for atezolizumab + bevacizumab treatment of hepatocellular carcinoma. Clin Cancer Res. 2022;28:3537–3545. doi: 10.1158/1078-0432.CCR-21-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L., Feng J., Kuang T., et al. Blood biomarkers predict outcomes in patients with hepatocellular carcinoma treated with immune checkpoint inhibitors: a pooled analysis of 44 retrospective sudies. Int Immunopharmacol. 2023;118 doi: 10.1016/j.intimp.2023.110019. [DOI] [PubMed] [Google Scholar]
- 27.Nassiri F., Patil V., Yefet L.S., et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial. Nat Med. 2023;29:1370–1378. doi: 10.1038/s41591-023-02347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbst R.S., Garon E.B., Kim D.W., et al. Five year survival update from KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol. 2021;16:1718–1732. doi: 10.1016/j.jtho.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 30.de Castro G., Jr., Kudaba I., Wu Y.L., et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥1% in the KEYNOTE-042 study. J Clin Oncol. 2023;41:1986–1991. doi: 10.1200/JCO.21.02885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenfeld A.J., Rizvi H.A., Memon D., et al. Systemic and oligo-acquired resistance to PD-(L)1 blockade in lung cancer. Clin Cancer Res. 2022;28:3797–3803. doi: 10.1158/1078-0432.CCR-22-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuo N., Liu C., Zhang Q., et al. Characteristics and prognosis of acquired resistance to immune checkpoint inhibitors in gastrointestinal cancer. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassidy M.R., Wolchok R.E., Zheng J., et al. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine. 2017;18:56–61. doi: 10.1016/j.ebiom.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valero C., Lee M., Hoen D., et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. 2021;12:729. doi: 10.1038/s41467-021-20935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motzer R.J., Ravaud A., Patard J.J., et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur Urol. 2018;73:62–68. doi: 10.1016/j.eururo.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin N., Li J., Yao X., et al. Prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer liver metastasis: a meta-analysis of results from multivariate analysis. Int J Surg. 2022;107 doi: 10.1016/j.ijsu.2022.106959. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y., Qian L., Chen K., et al. Intraperitoneal oncolytic virotherapy for patients with malignant ascites: characterization of clinical efficacy and antitumor immune response. Mol Ther Oncolytics. 2022;25:31–42. doi: 10.1016/j.omto.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zibelman M., MacFarlane A.W., 4th, Costello K., et al. A phase 1 study of nivolumab in combination with interferon-gamma for patients with advanced solid tumors. Nat Commun. 2023;14:4513. doi: 10.1038/s41467-023-40028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J., Zhang J., Zhang H., Liu Y. Adaptive virotherapy strategy for organism with constrained input using medicine dosage regulation mechanism. IEEE Trans Cybern. 2023 doi: 10.1109/tcyb.2023.3241344. [DOI] [PubMed] [Google Scholar]
- 40.Poh A. First oncolytic viral therapy for melanoma. Cancer Discov. 2016;6:6. doi: 10.1158/2159-8290.CD-NB2015-158. [DOI] [PubMed] [Google Scholar]
- 41.Hoang H.D., Said A., Vaidya N., et al. Adaptation of transgene mRNA translation boosts the anticancer efficacy of oncolytic HSV1. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2022-006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang A., Zhang Z., Chaurasiya S., et al. Development of the oncolytic virus, CF33, and its derivatives for peritoneal-directed treatment of gastric cancer peritoneal metastases. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2022-006280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Q., Huang H., Sun M., et al. Inhibition of tumor metastasis by liquid nitrogen-shocked tumor cells with oncolytic viruses infection. Adv Mater. 2023;35 doi: 10.1002/adma.202212210. [DOI] [PubMed] [Google Scholar]
- 44.Chen J., Gao P., Yuan S., et al. Oncolytic adenovirus complexes coated with lipids and calcium phosphate for cancer gene therapy. ACS Nano. 2016;10:11548–11560. doi: 10.1021/acsnano.6b06182. [DOI] [PubMed] [Google Scholar]
- 45.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 46.Cheng A.L., Qin S., Ikeda M., et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.