Abstract
Objectives
The objective of the present study was to investigate whether NOD-like receptor family pyrin domain-containing 3 (NLRP3) and absent in melanoma 2 (AIM2) inflammasomes pathways were involved in an experimental model of fibroblast activation named nemosis, which was used to mimic circumstances without bacteria stimulation.
Methods
Nemosis of human dental pulp fibroblast (DPFs) was induced by three-dimensional culture in U-shaped 96-well plates and investigated by scanning electron microscopy (SEM). DPFs monolayers were used as control. Annexin V-FITC/7-AAD apoptosis assay was performed on the DPFs spheroids by flowcytometry. Caspase-1 activity detection assay was conducted on the DPFs spheroids. Quantitative real-time polymerase chain reaction (qRT-PCR), cytokine measurements, Western blot and the effect of COX-2 inhibitor on spheroids was studied.
Results
SEM study observed human dental pulp fibroblast clusters and cell membranes damage on the surface of DPFs spheroids. The percentages of necrotic cells from DPFs spheroids gradually increased as the incubation time increased. A statistically significant increase in caspase-1 activity was observed after DPFs spheroids formation. DPFs spheroids displayed significant amounts of NLRP3, AIM2 mRNA and protein expression, caspase-1 mRNA expression and cleaved Caspase-1 protein expression and high IL-1β concentrations (P < 0.05) than DPFs monolayers. Specific COX-2 inhibitor (NS-398) decreased NLRP3 mRNA and protein expression, cleaved Caspase-1 protein expression, Caspase-1 activity and IL-1β mRNA expression and IL-1β concentrations (P < 0.05). However, Specific COX-2 inhibitor had no impact on AIM2 mRNA and protein expression, caspase-1 mRNA expression and pro-Caspase-1 protein expression.
Conclusions
In conclusion, clustering human DPFs spontaneously activated NLRP3 and AIM2 inflammasomes and induced IL-1β secretion which could be partially attenuated by COX-2 inhibitor. Thus, nemosis could become a powerful model for studying mechanisms underlying aseptic pulpitis.
Keywords: Dental pulp fibroblast, Nemosis, Inflammasome, Pyrin domain-containing 3, Absent in melanoma 2
1. Introduction
Pulpitis is an inflammatory disease caused by the progression of dental caries [1]. It is often reversible, however, if the pulp is exposed to the cavity, the inflammation becomes irreversible [2]. In such conditions, even the removal of the infected tissues does not provide predictable treatment options [3]. For effective clinical therapy, the patient should be treated before the infection invades the dental pulp. Thus, it is important to study the mechanism of aseptic pulp inflammation for the rational treatment of pulpitis.
Dental pulp fibroblasts (DPFs) are the major cells in dental pulp tissue involved in the synthesis of extracellular matrix and play important role in immune defense functions. Nemosis is a process of cell activation involving the death of human fibroblasts and the destruction of the dental pulp. Bizik et al. showed that normal fibroblasts are induced to form clusters, called spheroids, which undergo a cell activation pathway called “nemosis” [4]. Nemosis can be induced in the 3-dimensional culture of fibroblasts by seeding 10,000 cells per well (U-bottomed 96-well plate). The fibroblasts in these spheroids are activated to a proinflammatory, proteolytic and growth factor response at approximately two days after initiation, and synchronously begin to decompose in a process characterized as programmed necrosis-like death. Nemotic fibroblasts release large amounts of proinflammatory mediators, such as prostaglandins, and growth factors, such as hepatocyte growth factor/scatter factor in neonatal foreskin, fetal skin, and fetal and adult lung fibroblasts [[4], [5], [6]]. Clustering of DPFs could release interleukin-8 and CC chemokine ligand 20 (CCL-20) which are associated with dental pulp pathophysiological processes [7]. In our pervious study, we proved LDH release from the DPFs spheroids and demonstrated that nemosis provides a model to understand the relationship between DPFs and other cells in the dental pulp, which is useful for characterizing the role of fibroblasts in pulpal inflammation [8]. The nemosis model also appears to be suitable for studying aseptic pulpitis, as clustering of fibroblasts cells spontaneously releases inflammatory factors in absence of external microbes.
DPFs can express various proinflammatory mediators and cytokines (on stimulation by bacteria and other virulence factors) such as interleukin1 (IL-1) [[9], [10], [11], [12]]. IL-1 activity in the symptomatic dental pulp (caries) is significantly higher compared to healthy dental pulp. IL-1 can further be divided into interleukin 1α (IL-1α) and interleukin 1β (IL-1β). The high level of IL-1β in irreversible pulpitis samples compared to normal healthily pulp indicates that IL-1β shows immunostimulatory activity in pulpitis [13]. To date, the spontaneous release of IL-1β due to the clustering of DPFs is not clear. The synthesis and secretion of IL-1β are divided into two steps. Briefly, in the first step, the nuclear factor kB (NF-kB) activation promotes inactive pro-IL-1β accumulation in the cytoplasm. In the second step, inflammasomes activate intracellular caspase-1 to cleave inactive pro-IL-1β into bioactive IL-1β [14].
Inflammasomes are the pivotal signaling platforms that detect causative microbe and sterile stressors. At present, the most thoroughly studied inflammasome is NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome. It takes adaptor protein apoptosis-associated speck-like CARD (ASC) and pro-caspase-1 to make NLRP3 inflammasome. The two steps are involved in the activation of NLRP3 inflammasome. In the first step, NF-κB induces expression of NLRP3 and pro-IL-1β. The DAMPs (damage-associated molecular patterns) like ATP and reactive oxygen species (ROS) activate NLRP3 inflammasome. The mature NLRP3 inflammasome cause pro-caspase-1 clustering which induces autocleavage and formation of active caspase-1 p10/p20 tetramer, followed by pro-IL-1β into mature form [12]. On other hand, the absence in melanoma 2 (AIM2) inflammasome recognize double-stranded DNA due to its DNA-binding ability [15]. The AIM2 inflammasome is activated by the pathogen or cytosolic DNA (self-derived) as a danger single in primed myeloid and epithelial cells. AIM2 can initiate an immune response mechanism like the NLRP3 inflammatory pathway. Both NLRP3 and AIM2 inflammasomes have been reported to be overexpressed in dental pulp and tissue cells [16,17].
Our former study demonstrated that AIM2 mediates IL-1β secretion during pulpitis [18]. Also, NLRP3/caspase-1 pathway in DPFs showed a biological role in the dental pulp innate immune response [12,19]. It is worth mentioning that in the previous research, DPFs cells were stimulated by microbial virulence. Alternatively, data come from caries-derived pulpitis in infectious conditions. Thus, the inflammasome pathway plays an important role in infectious pulpitis. Although most pulpitis cases are induced by microbial infection, certain physical, chemical, physiological, and iatrogenic factors can also cause pulp inflammation, which is aseptic. However, whether the NLRP3 and AIM2 inflammasomes in DPFs are functional during aseptic pulpitis has not yet been revealed.
Clustering fibroblasts are sufficient to stimulate NF-kB activity and promote leukocyte migration [20]. Since NF-kB activation is the first stage in which pro-IL-1β is produced. Clustering fibroblasts might likely stimulate inflammasome pathway and induce IL-1β release. However, to date, there is no research on whether clustering fibroblasts motivate inflammasomes. Therefore, the objective of this paper was to investigate, whether activation of inflammasomes induces IL-1β secretion in human DPFs. The three-dimensional culture of human DPFs (nemosis induce) was studied which mimics aseptic inflammation and determined the NLRP3 and AIM2 inflammasome expression levels and the IL-1β release level in normal and clustering human DPFs. This study enriches understanding of the characteristics of nemosis in dental pulp fibroblasts and provides a model for further investigation of inflammasome function in the dental pulp immune response.
2. Material and methods
2.1. Ethical approval
The study was approved (protocol number: 2020XFM01) by the ethical committee of Xi'an Medical University (Shaanxi Province, China). Human DPFs were obtained from six healthy individuals (aged 20 to 30 years) who underwent third molar extraction at the Department of Stomatology in the Third Affiliated Hospital of Xi'an Medical University (Shaanxi Province, China). Signed written informed consent were obtained from all study participants.
2.2. Cell culture
As previously described [8], the human DPFs cells were cultured in α-minimum essential media (HyClone, UT, USA) supplemented with fetal bovine serum (10%, FBS, HyClone), penicillin-G (100 U/ml) and streptomycin (100 mg/mL) at 37 °C in a humidified atmosphere (5% CO2). Human DPFs between passages 4 and 6 were used in all experiments.
2.3. Initiation and formation of dental pulp fibroblast spheroids
Dental pulp fibroblast spheroids were produced as described in the previous reports [8]. Briefly, a 96-well plate (U-bottomed, Costar, MA, USA) was treated with agarose (0.8% w/v in sterile water) to form a thin non-adhesive layer. The human DPFs cells were seeded (10,000 cells/well) to form one spheroid in α-MEM (150 μl) media supplemented with FBS (10% w/v) at 37 °C in a humidified incubator (5% CO2). Reader should note that FBS concentration in the ELISA assay was 5% w/v.
2.4. Scanning electron microscopy (SEM)
Four U-bottomed, 96-well plates containing 10,000 cells per well were centrifuged for 5 min at 8000 rpm (maintained at 5 °C). The supernatant was discarded and the cell samples were processed as described in the previous report [21]. The spheroids were washed twice gently using phosphate buffer saline (PBS, 0.01 mol/L, pH 7.4). After removal of the supernatant by centrifugation for 5 min at 6000 rpm, all the deposits were immersed in osmium tetroxide fixative (1% w/v) for 2 h. The depositions were again washed (twice) using sterile water and dehydrated using a series of acetonitrile solutions at different concentrations (50%, 70%, 80%, 90% and 100% each) for 20 min (twice). The samples were dried and sputter-coated with gold. The morphology of spheroids was observed using field emission scanning electron microscopy (S-4800, Hitachi, Tokyo, Japan).
2.5. AnnexinV-FITC/7 -AAD apoptosis assay
Annexin V-FITC/7-AAD apoptosis assay was performed on the DPFs spheroids according to the manufacturer's protocol (Annexin V-FITC/7-AAD Apoptosis Detection Kit, Pricella, Wuhan, China). Briefly, the cells were washed twice with PBS and then resuspended in Binding Buffer at a concentration of 1.0 × 106 cells/mL. The cells were then gently mixed and 100 μL of the cell suspension was transferred to a 5 mL test tube, followed by the addition of 2.5 μL ofAnnexin V-FITC, and 2.5 μL of 7-AAD staining solution to the tube. The cells were gently vortexed and incubated for 15 min at room temperature in the dark. Then, 400 μL of Binding Buffer were added to each tube, following which the samples were analyzed by flowcytometry (BD FACSCalibur, USA).
2.6. Caspase-1 activity detection assay
Caspase-1 activity detection was performed on the monolayer DPFs and DPFs spheroids according to the manufacturer's protocol (Caspase-1 activity Detection Kit, beyotime, Shanghai, China). Briefly, the cells were washed with PBS and then resuspended in Binding Cells were lysed with the lysis buffer at a concentration of 2 × 107 cells/mL on ice for 15 min. After removal of the supernatant by centrifugation for 10 min at 20,000 g, the supernatant was transferred to a pre-cooled centrifuge tube. 40 μL of Detection Buffer was transferred to a 5 mL test tube, followed by the addition of 50 μL of the supernatant, and 10 μL of Ac-YVAD-pNA (2 mM) to the tube. Then, the samples were incubated for 1 h at 37 °C, following which the absorbance was measured at 405 nm using an automatic microplate reader (BioTek, Winooski, VT, USA). The experiments were performed in triplicate and repeated twice.
2.7. RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
From the human DPFs cells, RNA was extracted using RNA plus and treated with RNase-free DNase I (Promega, USA) at different time intervals (0, 24, 48, 72 and 96 h) after cell seeding. The extraction of RNA and qRT-PCR were performed as described in the previous report [19]. Primers sequences were NLRP3, forward 5′-GATCTTCGCTGCGATC AACA-3′ and reverse 5′-GGGATTCGAAACACGTGCATTA-3’; AIM2,forward5′-TCAAGCTGAAATGAGTCCTGC-3′ and reverse 5′-CTTGGGTCTC.
AAACGTGAAGG-3’; caspase-1, forward 5′-GCCTGTTCCTGTGATGTGGAG-3′ and reverse 5′-TGCCCACAGACATTCATACAGTTTC-3’; IL-1β, forward 5′-CCAGGGACAG.
GATATGGAGCA-3′ and reverse 5′-TTCAACACGCAGGACAGGTACAG-3’; GAPDH, forward 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-GCACCGTCAAGGCT.
GAGAAC-3’. One microgram of total RNA (as a template) was used to prepare first-strand complementary DNA via oligo-dT priming with in Omni script RTkit (Qiagen, Valencia, California, USA). ABI Prism 7500 PCR system was used to perform RT-PCR analysis with SYBR Green PCR master mix reagent in a 40 cycles PCR program. In PCR cycle, the denaturing, annealing and extension conditions were 95 °C for 30 s, 95 °C for 5 s, 60 °C for 34 s, and 95 °C for 15 s respectively. The relative amount (fold change) of target gene expression was normalized to the level of d-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and to control the monolayer of human DPFs at 0 h. The data for targeted expression were analyzed using 2−ΔΔCt method.
2.8. Western blot
Western blot analysis was performed as previously described [22]. The total protein was extracted from the cells by using lysis buffer containing protease inhibitors (SigmaAldrich). The protein concentration was measured by using a BCA-200 protein assay kit (Pierce). Equal amounts of a sample protein (50 μg) were loaded onto an 12% SDS-PAGE gel. After electrophoresis, the samples were transferred onto a nitrocellulose membrane. Then, the membrane was blocked for 2 h at room temperature and incubated overnight at 4 °C with the following primary antibodies: anti-AIM2 (cat. no. ab93015; 1:500; Abcam, Cambridge, MA, USA), anti-NLRP3 (cat. no. 15101; 1:500; CST, Danvers, MA, USA), anti-caspase-1 (cat. no. 3866; 1:400; CST, Danvers, MA, USA), and anti-GAPDH (cat. no. sc-47724; 1:1000, Santa Cruz, Dallas, TX, USA). The activation of caspase-1 was assessed by measuring caspase-1 p20. Appropriate secondary antibodies including anti-mouse IgG antibody (cat. no. 7076; 1:2000; CST, Danvers, MA, USA) or anti-rabbit IgG antibody (cat. no. 7074; 1:2000; CST, Danvers, MA, USA) were selected to incubate with the membrane for 2 h at room temperature. Then, blot bands were visualized with an ECL reagent (Amersham, Little Chalfont UK). Non-saturated bands were selected to perform densitometry quantification using Image J software (Image J 1.51, NIH, USA). Three independent experiments were performed, and each time the target protein was compared to the internal reference protein for quantitative analysis.
2.9. Cytokine measurements
Human DPFs supernatants were collected at regular time intervals (0, 24, 48, 72, and 96 h) after cell seeding. The supernatant was harvested at each time point and then stored at −20 °C until analysis. The levels of IL-1β were determined in duplicate using a commercial ELISA kit (enzyme-linked immunosorbent assay) in accordance with the supplier protocol (eBioscience, San Diego, California, USA) [23]. The samples were quantified in duplicate.
2.10. Effects of COX-2 inhibitor on spheroids
A selective COX-2 inhibitor (NS-398) was procured from MedChemExpress Corp. (Shanghai, China). NS-398 was dissolved in DMS (dimethyl sulfoxide) and diluted with culture media to produce a final concentration of 10 μM. The human pulp cells are noncytotoxic at this solvent concentration [24,25]. NS-398 was added to human DPFs at the time of seeding and spheroids were cultured for 72 h. The RNA extraction, PCR methods,Caspase-1 activity and Western blot methods were the same as described above. Human DPFs spheroids treated only with solvent served as the control group.
2.11. Data analysis
All experiments were repeated three times. The analysis was performed using SPSS software (version 24.0; SPSS Inc., IBM, NY, USA). Data are reported as mean ± standard deviation. The significance of differences was determined using two-way mixed model ANOVA analysis. Wilcoxon test and Bonferroni's correction were used. The statistical significance of the differences between the groups was analyzed using a two-tailed Student's t-test. All the data passed the normally distributed test. Statistical significance was set at p < 0.05.
3. Results
3.1. Observation of ultra-structural of DPFs monolayer and spheroids
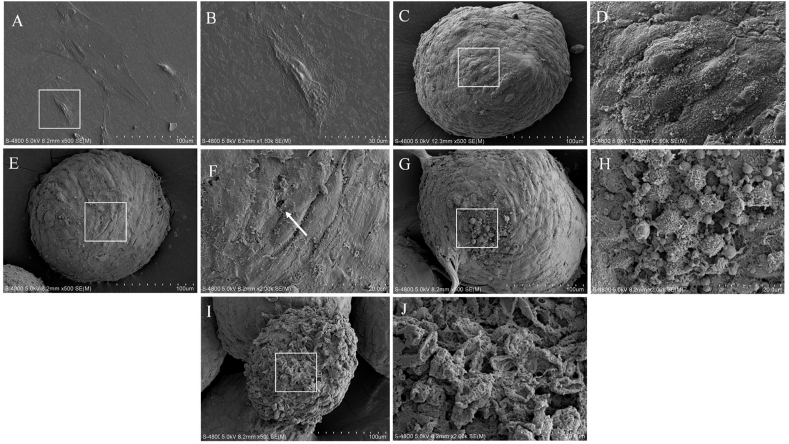
The scanning electron microscopy investigation of DPFs monolayer (ultra-structure) is shown in Fig. 1. The morphological images exhibited normal structure of DPFs cells showing long fusiform shape with crisscrossed fibers arranged on the surface of the cell. The adherent DPFs were flat with intact and uniform cell membrane. The cell bodies and antennae of human DPFs were clear (Fig. 1A and B). After 24 h of cell seeding the DPFs cells aggregated into a single independent spherical shape. The cell bodies and the antennae in the DPFs spheroids could be roughly distinguished with gap between the cells (Fig. 1C and D). As the culture time extended, the distance between the cells decreased and the cell spheres became denser. Some cell membranes appeared perforated and ruptured, and even the membrane structure disappeared (Fig. 1E and F). After 72 h, some DPFs lost their fusiform antennae and the surface area of the DPFs spheroids shrank (Fig. 1G and H). Partial DPFs spheroids were severely damaged and the cell membrane structure disappeared after 96 h (Fig. 1I and J).
Fig. 1.
Scanning electron microscopy images of normal monolayer of human dental pulp fibroblasts (A) and (B) and human dental pulp fibroblasts in spheroids at 24 h (C) and (D), 48 h (E) and (F), 72 h (G) and (H) and 96 h (I) and (J) after spheroid formation. (B, D, F, H and J are local magnifications of A, C, E, G and I respectively). The arrow in (F) indicates perforation of the cell membrane surface.
3.2. Annexin V-FITC/7-AAD flowcytometry of DPFs spheroids
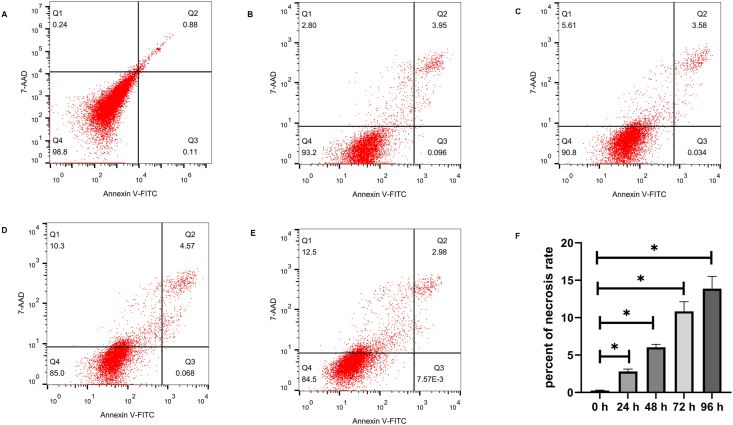
To evaluate whether the spheroid cells were undergoing apoptosis or necrosis, Annexin V-FITC/7-AAD apoptosis assay was performed on the DPFs spheroids by flowcytometry. We observed the percentage of necrotic cells were gradually increased with culture time in DPFs spheroids. The percentages of necrotic cells were 0.25 ± 0.7%, 2.81 ± 0.31%, 6.04 ± 0.38%, 10.84 ± 1.28%, and 13.88 ± 1.63% in 0 h (Fig. 2A), 24 h (Figs. 2B), 48 h (Fig. 2C), 72 h (Figs. 2D), and 96 h (Fig. 2E), respectively. These results revealed that DPFs spheroids underwent necrosis. The percent of necrosis rate in DPFs spheroids were significantly increased at 24 h, 48 h, 72 h, and 96 h compared with 0 h (P < 0.05) (Fig. 2F).
Fig. 2.
Annexin V-FITC/7-AAD flowcytometry images of human dental pulp fibroblast (DPFs) spheroids at 0 h (A), 24 h (B), 48 h (C), 72 h (D) and 96 h (E). The necrotic cells cells are AnnexinV− 7-AAD+(Q1). The late apoptotic cells are AnnexinV+ 7-AAD+(Q2). The early apoptotic cells are AnnexinV+ 7-AAD-(Q3). The viable cells are AnnexinV− 7-AAD-(Q4). (F) indicated the necrosis rate of DPFs spheroids at 0 h, 24 h, 48 h, 72 h and 96 h ∗p < 0.05 24 h, 48 h, 72 h and 96 h vs. 0 h.
3.3. Caspase-1 activity in DPFs spheroids
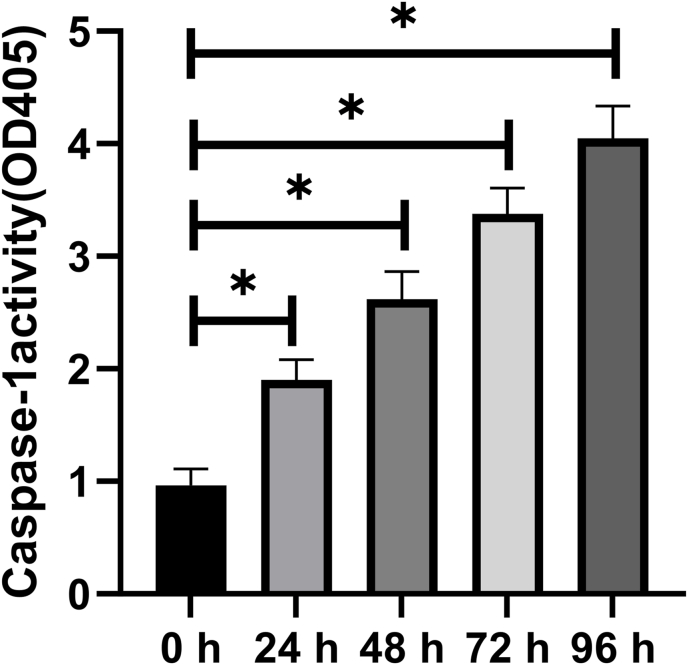
This Caspase 1 activity detection kit is based on the fact that caspase 1 can catalyze substrate Ac-YVAD-pNA (acetyl-Tyr-Val-Ala-Asp p-nitroanilide) to produce yellow pNA (p-nitroaniline). Thus, the activity of caspase 1 can be detected by measuring absorbance at 405 nm. At each time point from 24 h to 96 h, OD (optical density) value of DPFs spheroids was more than that of DPFs spheroids at 0 h (Fig. 3) (P < 0.05).
Fig. 3.
Caspase-1 enzymatic activity was measured by caspase-1 activity assays. ∗p < 0.05 vs. 0 h.
3.4. Gene expression of NLRP3, AIM2, caspase-1 and IL-1β in monolayer DPFs and DPFs spheroids
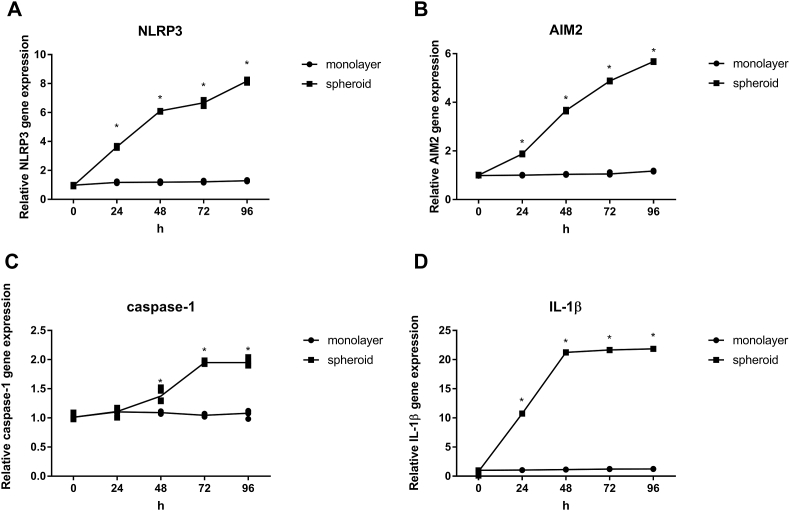
To further investigate the role inflammasomes play in DPFs spheroids. RNA extraction was performed to analyze the amounts of NLRP3, AIM2, caspase-1 and IL-1β mRNA in monolayer DPFs and DPFs spheroids. As the cultivation time increased, the mRNA expression of NLRP3 (Fig. 4A), AIM2 (Fig. 4B) and IL-1β (Fig. 4D) increased in a time-dependent manner after DPFs spheroid formation from 24 h to 96 h. Meanwhile, the mRNA expression level of caspase-1(Fig. 4C) in clustering DPFs showed no significant changes until 24 h, a slight increase at 48 h, reached a maximum at 72 h, and showed little decrease from 72 h to 96 h. Moreover, at each time point from 24 h to 96 h, NLRP3 (Fig. 4A), AIM2 (Fig. 4B) and IL-1β (Fig. 4D) mRNA expression levels were significantly increased in spheroid compared with monolayer (P < 0.05). At the same time, caspase-1 (Fig. 4C) mRNA expression levels were also significantly increased in spheroid compared with monolayer from 48 h to 96 h (P < 0.05).
Fig. 4.
mRNA expression at different time points (0, 24, 48, 72 and 96 h) in human dental pulp fibroblast (DPFs) monolayers and DPFs spheroids. (A) NLRP3, (B) AIM2, (C) caspase-1, (D) IL-1β. ∗p < 0.05 spheroid vs. monolayer.
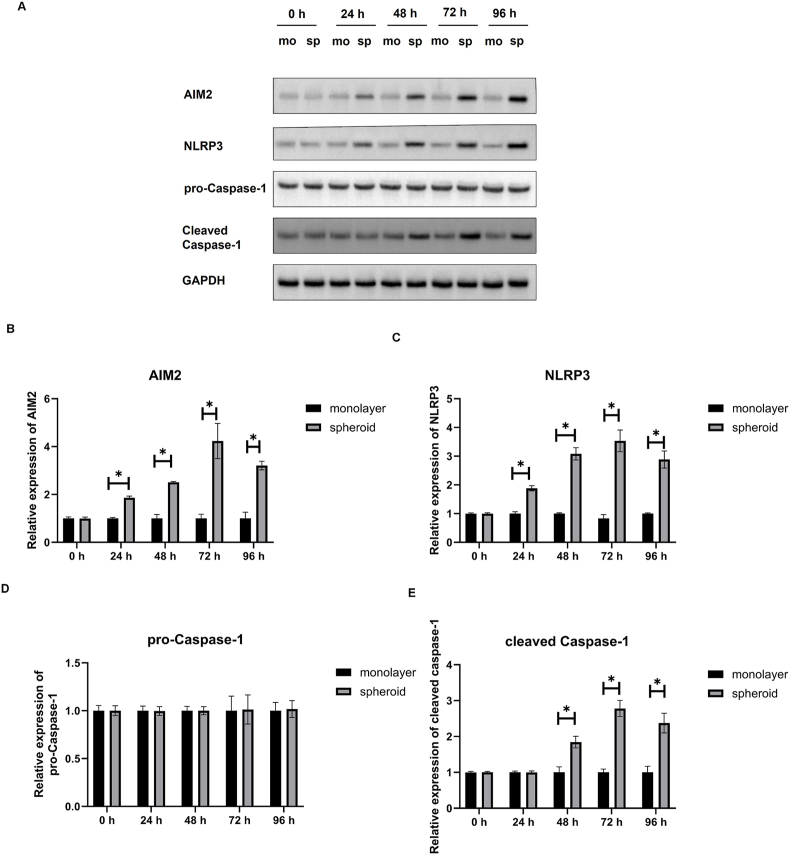
3.5. Protein expression of NLRP3, AIM2, pro-caspase-1 and cleaved Caspase-1 in monolayer DPFs and DPFs spheroids
Western blot was performed to analyze the amounts of inflammasomes related proteins NLRP3, AIM2, pro-Caspase-1 and cleaved Caspase-1 in DPFs spheroids (Fig. 5A). Caspase-1 activation was assessed by the appearance of cleaved caspase-1. The quantitative results showed that protein levels of AIM2 (Fig. 5B) and NLRP3(Fig. 5C) in the DPFs spheroids were higher than monolayer DPFs at 24 h, 48 h, 72 h and 96 h (P < 0.05). No significant difference was existed in the protein expression of pro-Caspase-1 between the monolayer DPFs and DPFs spheroids at each time point (Fig. 5D). Protein levels of cleaved Caspase-1 (Fig. 5E) in the DPFs spheroids were higher than monolayer DPFs at 48 h, 72 h and 96 h (P < 0.05). No significant difference was existed in the protein expression of cleaved Caspase-1(Fig. 5E) between the monolayer DPFs and DPFs spheroids at 24 h.
Fig. 5.
Protein expression at different time points (0, 24, 48, 72 and 96 h) in human dental pulp fibroblast (DPFs) monolayers and DPFs spheroids. (A) Western blotting analysis of Protein of AIM2, NLRP3, pro-Caspase-1, and cleaved Caspase-1. (B), (C), (D), and (E) indicated relative protein expressions of AIM2, NLRP3, pro-Caspase-1, and cleaved Caspase-1 in the monolayer DPFs and DPFs spheroids, respectively. ∗p < 0.05 spheroid vs. monolayer.
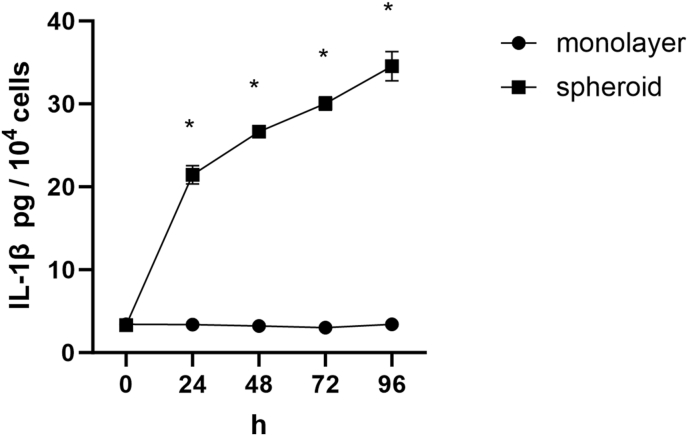
3.6. Release of IL-1β in monolayer DPFs and DPFs spheroids
In DPFs monolayer group, the IL-1β was released at basal levels without an observed increase throughout the incubation process. While, in DPFs spheroid group, the IL-1β release was gradually increased with culture time, with sharp burst from 24 h to 48 h, followed by a slower increase from 72 h to 96 h. At each time point from 24 h to 96 h, clustering DPFs secreted more IL-1β than monolayer DPFs (Fig. 6) (P < 0.05).
Fig. 6.
Concentrations of IL-1β secreted by human dental pulp fibroblasts cultured in monolayer and spheroid. ∗p < 0.05 spheroid vs. monolayer.
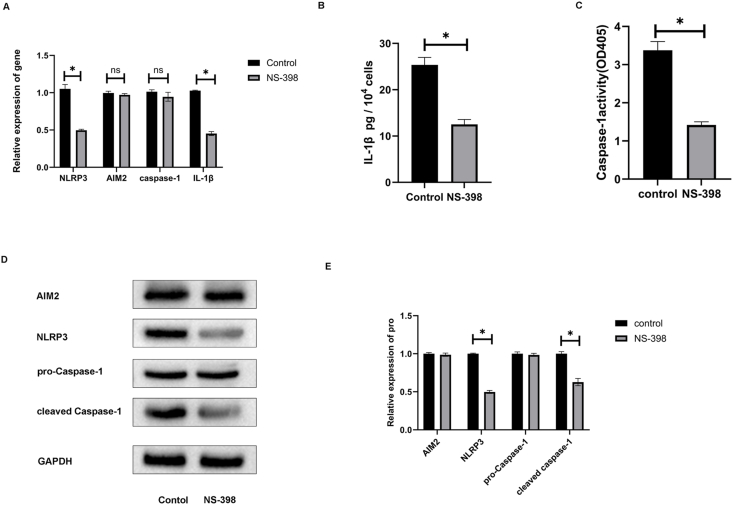
3.7. Selective COX-2 inhibitor effect on NLRP3 and AIM2 inflammasomes gene and protein expression and IL-1β secretion
In spheroids, the effect of NS-398 (selective COX-2 inhibitor) on expression of NLRP3, AIMs and IL-1β were evaluated after 72 h of a noncytotoxic dose (NS-398 = 10 μM) which reduced NLRP3 and IL-1β mRNA expression (P < 0.05) but had no impact on AIM2 and caspase-1 mRNA expression (Fig. 7A). Meanwhile, NS-398 decreased the secretion of IL-1β (Fig. 7B) and the activity of Caspase-1(Fig. 7C) in human DPFs spheroids. Western blot was performed to analyze the amounts of inflammasomes related proteins NLRP3, AIM2, pro-Caspase-1 and cleaved Caspase-1 in DPFs spheroids (Fig. 7D). The effect of NS-398 reduced NLRP3 and cleaved Caspase-1 protein expression (P < 0.05) but had no impact on AIM2 and pro-caspase-1 protein expression (Fig. 7E).
Fig. 7.
Selective COX-2 inhibitor effect on NLRP3 and AIM2 inflammasomes gene and protein expression, Caspase-1 activity and IL-1β secretion. The mRNA expressions and protein expressions of NLRP3 and AIM2 inflammasomes and secretion of IL-1β in human dental pulp fibroblast spheroids after 72 h without or with NS-398 treatment. (A) represents NLRP3, AIM2, caspase-1, and IL-1β mRNA expression. (B) represents IL-1β secretion amount. (C) represents Caspase-1 enzymatic activity. (D) and (E) represent AIM2, NLRP3, pro-caspase-1, and cleaved caspase-1 protein expression. ∗p < 0.05 with NS-398 (NS-398) vs. without NS-398 (control). NS, not significant. mon, monolayer. sph, spheroid.
4. Discussion
Fibroblasts are main body cells in the dental pulp tissue with immunomodulatory function [26]. Understanding the regulatory immune characteristics of the pulp fibroblasts can help to prevent and treat pulpitis. Interesting to note that, in addition to the microbial infections, chemical stimuli (such as phenols) and physical stimuli (such as high temperature) can also cause pulpitis. Under these conditions, pulp inflammation is aseptic [3]. However, molecular signaling in the aseptic pulp inflammation is still not clear. Thus, for better illustrating the regulation mechanism, it is valuable to find an in vitro research model that simulates aseptic pulpitis. The tissue microenvironment can be recapitulate using three-dimensional culture model to provide good platform for investigation of pulpitis pathogenesis [27]. Nemosis is a newly discovered way of fibroblast activation. Many investigations have reported that this type of dental fibroblast activation (nemosis) may be involved in pathophysiological conditions in dental pulp. Our experimental SEM results showed that without external stimulation, only cell–cell contact of human DPFs could induce cell membrane damage. This processes of nemosis provide a sterile environment without pathogenic bacterial stimulation. Thus, underlying molecular mechanisms in clustering human DPFs help to understand their biology function.
Clustering DPFs could express the angiogenic promoting factor vascular endothelial-derived growth factor (VEGF), growth factor hepatocyte growth factor/scatter factor (HGF/SF) and chemokines (CCL20 and CXCL8) [7]. The implication of soluble factors secreted by clustering fibroblasts is significant. Prostaglandin E2 (PGE2) is the most abundantly detected prostaglandin (PG) in clustering fibroblasts. It is important in modulating the inflammatory and healing processes in pulp inflammation [28]. PGE2 is biosynthesized from arachidonic acid by cyclooxygenases (COXs) as the rate-limiting enzymes. At present, there are three isozymes of COX, namely, COX-1, COX-2 and COX-3. COX-1 is mainly involved in normal physiological functions, and its expression level remains unchanged during inflammation. COX-3 is a splice variant of COX-1. COX-2 is an inducible enzyme and is rarely expressed under physiological conditions. Under inflammation stimulation, COX-2 expression can increase rapidly. Normal pulp tissues express little COX-2 protein, whereas COX-2 protein can be detected in pulpal fibroblasts and macrophages in inflamed pulp tissues [29]. Our previous study results showed that COX-2 was induced in clustering human DPFs [8]. IL-1β stimulates COX-2 expression in pulp cells and affects inflammatory and healing processes in dental pulp [30]. It is reasonable to speculate that IL-1β is expressed in cell spheroids. As we showed in DPFs spheroids, significantly higher IL-1β mRNA and protein levels were induced compared with monolayer DPFs. However, basal levels of IL-1β in the monolayer group were detected throughout the incubation time, without a significant change throughout the culture period. This is consistent with fibroblasts lacking external stimuli and thus expressing very low physiological levels of IL-1 [31].
In pulpitis, IL-1β is one of the important inflammatory mediators. The production of IL-1β is regulated by a two-step signaling process. The first step is activation of NF-kB which leads to production of pro-IL-1β. Maturation and secretion of IL-1β requires the formation of inflammasomes and caspase-1 activation [19]. Previous research has demonstrated that NF-κB is active in nemosis [20,32]. The pan-caspase inhibitor Z-VAD-FMK inhibited cell death by 80% [33]. One possibility is that the proinflammatory caspases in “inflammasomes” might be involved in nemosis [34]. Because an increased amount of IL-1β was induced in clustering human DPFs, we wanted to explore the underlying molecular mechanism, especially in the inflammasome activation step.
In this study, we used nemosis model to explore NLRP3 and AIM2 inflammasomes pathway in the aseptic inflammation. Our results suggest that NLRP3 and AIM2 inflammasome-dependent sterile inflammation can be induced in clustering human DPFs. Inflammasomes can be activated without bacteria stimulation in three-dimensional cultured human DPFs spheroids. This is a new idea different from the traditional relationship in which inflammasome activation can be induced by microbial infection in DPFs [18,19]. Similarly, NLRP3 inflammasome-dependent sterile inflammation was also found in dead odontoblasts [35]. Overall, inflammasome-dependent sterile inflammation might play a role in the dental pulp immune defense system. Although this study was limited to in vitro analysis, the findings provide important insights into inflammasome activation in aseptic pulpitis.
Several studies have suggested mutual regulation between COX-2 and IL-1β. Th dual COX-2 and soluble epoxide hydrolase (sEH) inhibitor PTUPB inhibit NLRP3 inflammasome activation to exert anti-inflammatory effects in acute lung injury [36]. NLRP3 inflammasome derived IL-1β secretion in macrophages was reduced by genetic knockdown of COX-2 [37]. Thus, COX-2 targeted treatment can be beneficial for diseases involving NLRP3 inflammasome. However, whether COX-2 regulates the NLRP3 inflammasome and IL-1β production in dental pulp fibroblasts is still unknown.
Pharmacological inhibition of COX-2 has been widely implemented in experimental studies [38,39]. Since both COX-2 and NLRP3 inflammasome were found to be activated in clustering dental pulp fibroblasts, we applied NS-398, a selective inhibitor of COX-2, to study whether COX-2 can regulate the NLRP3 inflammasome and IL-1β expression in clustering dental pulp fibroblasts. Our present study found that pharmacological inhibition of COX-2 attenuated activation of the NLRP3 inflammasome and IL-1β expression. This result provides a new understanding that differs from the former traditional relationship in which COX-2 can be stimulated by the cytokine IL-1β in dental pulp cells, gingival fibroblasts, and periodontal ligament fibroblasts [28,[40], [41], [42], [43]]. Notably, these clustering dental pulp fibroblasts can induce an inflammatory response without the addition of external cytokines or microbial components [7]. Thus, when dental pulp fibroblasts are stimulated by pathogenic bacteria, whether COX-2 regulates NLRP3 inflammasome-derived IL-1β production is still unknown. Further experiments on COX-2-targeted therapy might be beneficial for regulating cellular biological activity involving the NLRP3 inflammasome during pulpitis. To date, nemosis has been found to exist in neonatal foreskin, fetal skin, fetal and adult lung fibroblasts, etc [4,6]. It is unclear whether inflammasome activity and IL-1β secretion can be induced in fibroblasts other than DPFs. Further research to evaluate whether inflammasome activity and IL-1β secretion can be induced in other type of fibroblasts is needed, which could ultimately be used more extensively in potential biomedical application.
5. Conclusions
To our knowledge, it is the first report to investigate the role of NLRP3 and AIM2 inflammasomes in fibroblasts activation (nemosis). The work indicates that the human DPFs can spontaneously express NLRP3 and AIM2 and secrete IL-1β without the addition of external cytokines or microbial components. In addition, pharmacological inhibition of COX-2, attenuated activation of the NLRP3 inflammasome and IL-1β expression in clustering human DPFs. Thus, nemosis can become a powerful model for studying the mechanisms of the sterile inflammation that occurs during pulpitis. Hopefully, the results of this study will aid in the discovery of new anti-inflammatory treatments for pulpitis.
Credit author statement
Shafei Zhai: Conceptualization, Project administration, Methodology and Writing-original draft. Changkui Liu: Funding acquisition. Lihui Zhang: Formal analysis, Data Curation. Xue Li: Resources. Qi Yu: Writing - Review & Editing.
Ethics approval
The study was accordance with the Helsinki Declaration and approved by the Ethics Committee of Xi'an Medical University (Approval number: 2020ZXFM01).
Funding
This study was supported by the Shaanxi Education Youth Innovation Team Project (21JP109).
Declaration of competing interest
Authors declare no competing interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Zero D.T., Zandona A.F., Vail M.M., Spolnik K.J. Dental caries and pulpal disease. Dental Clinics. 2011;55(1):29–46. doi: 10.1016/j.cden.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Bjørndal L., Simon S., Tomson P., Duncan H. Management of deep caries and the exposed pulp. Int Endod J. 2019;52(7):949–973. doi: 10.1111/iej.13128. [DOI] [PubMed] [Google Scholar]
- 3.Zheng J., Wu Z., Niu K., Xie Y., Hu X., Fu J., et al. Microbiome of deep dentinal caries from reversible pulpitis to irreversible pulpitis. J Endod. 2019;45(3):302–309 e1. doi: 10.1016/j.joen.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Vaheri A., Enzerink A., Räsänen K., Salmenperä P. Nemosis, a novel way of fibroblast activation, in inflammation and cancer. Exp Cell Res. 2009;315(10):1633–1638. doi: 10.1016/j.yexcr.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhai S., Wang Y., Jiang W., Jia Q., Li J., Wang W., et al. Nemotic human dental pulp fibroblasts promote human dental pulp stem cells migration. Exp Cell Res. 2013;319(10):1544–1552. doi: 10.1016/j.yexcr.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Enzerink A., Salmenperä P., Kankuri E., Vaheri A. Clustering of fibroblasts induces proinflammatory chemokine secretion promoting leukocyte migration. Mol Immunol. 2009;46(8–9):1787–1795. doi: 10.1016/j.molimm.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Le Clerc J., Tricot-Doleux S., Pellen-Mussi P., Perard M., Jeanne S., Perez F. Expression of factors involved in dental pulp physiopathological processes by nemotic human pulpal fibroblasts. Int Endod J. 2018;51(Suppl 2):e94–e106. doi: 10.1111/iej.12762. [DOI] [PubMed] [Google Scholar]
- 8.Zhai S., Wang Y., Jiang W., Jia Q., Li J., Wang W., et al. Nemotic human dental pulp fibroblasts promote human dental pulp stem cells migration. Exp Cell Res. 2013;319(10):1544–1552. doi: 10.1016/j.yexcr.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Hirao K., Yumoto H., Takahashi K., Mukai K., Nakanishi T., Matsuo T. Roles of TLR2, TLR4, NOD2, and NOD1 in pulp fibroblasts. J Dent Res. 2009;88(8):762–767. doi: 10.1177/0022034509341779. [DOI] [PubMed] [Google Scholar]
- 10.Nagaoka S., Tokuda M., Sakuta T., Taketoshi Y., Tamura M., Takada H., et al. Interleukin-8 gene expression by human dental pulp fibroblast in cultures stimulated with Prevotella intermedia lipopolysaccharide. J Endod. 1996;22(1):9–12. doi: 10.1016/S0099-2399(96)80228-7. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi M., Kojima T., Kanekawa M., Aihara N., Nogimura A., Kasai K. Neuropeptides stimulate production of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha in human dental pulp cells. Inflamm Res. 2004;53(5):199–204. doi: 10.1007/s00011-003-1243-z. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A., Wang P., Ma X., Yin X., Li J., Wang H., et al. Mechanisms that lead to the regulation of NLRP3 inflammasome expression and activation in human dental pulp fibroblasts. Mol Immunol. 2015;66(2):253–262. doi: 10.1016/j.molimm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch V., Wolgin M., Mitronin A.V., Kielbassa A.M. Inflammatory cytokines in normal and irreversibly inflamed pulps: a systematic review. Arch Oral Biol. 2017;82:38–46. doi: 10.1016/j.archoralbio.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 14.van de Veerdonk F.L., Netea M.G., Dinarello C.A., Joosten L.A. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32(3):110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Sun L., Byrd K.M., Ko C.C., Zhao Z., Fang J. AIM2 inflammasome's first decade of discovery: focus on oral diseases. Front Immunol. 2020;11:1487. doi: 10.3389/fimmu.2020.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S., Song Z., Jiang L., Chen L., Wang R., Qin W., et al. Absent in melanoma 2 (AIM2) expressed in human dental pulp mediates IL-1beta secretion in response to cytoplasmic DNA. Inflammation. 2015;38(2):566–575. doi: 10.1007/s10753-014-9963-5. [DOI] [PubMed] [Google Scholar]
- 17.Song Z., Lin Z., He F., Jiang L., Qin W., Tian Y., et al. NLRP3 is expressed in human dental pulp cells and tissues. J Endod. 2012;38(12):1592–1597. doi: 10.1016/j.joen.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Zhai S., Wang H., Jia Q., Jiang W., Zhang X., et al. Absent in melanoma 2 (AIM2) in rat dental pulp mediates the inflammatory response during pulpitis. J Endod. 2013;39(11):1390–1394. doi: 10.1016/j.joen.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Jiang W., Lv H., Wang H., Wang D., Sun S., Jia Q., et al. Activation of the NLRP3/caspase-1 inflammasome in human dental pulp tissue and human dental pulp fibroblasts. Cell Tissue Res. 2015;361(2):541–555. doi: 10.1007/s00441-015-2118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enzerink A., Salmenpera P., Kankuri E., Vaheri A. Clustering of fibroblasts induces proinflammatory chemokine secretion promoting leukocyte migration. Mol Immunol. 2009;46(8–9):1787–1795. doi: 10.1016/j.molimm.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Tong Z., Tao R., Jiang W., Li J., Zhou L., Tian Y., et al. In vitro study of the properties of Streptococcus mutans in starvation conditions. Arch Oral Biol. 2011;56(11):1306–1311. doi: 10.1016/j.archoralbio.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W., Sun S., Wang D., Qiu J., Song Y., Zhang Q., et al. MicroRNA-22 suppresses NLRP3/CASP1 inflammasome pathway-mediated proinflammatory cytokine production by targeting the HIF-1α and NLRP3 in human dental pulp fibroblasts. Int Endod J. 2022;55(11):1225–1240. doi: 10.1111/iej.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miwa K., Shibayama N., Moriguchi T., Goto J., Yanagisawa M., Yamazaki Y., et al. A rapid enzyme-linked immunosorbent assay with two modes of detection for measuring cytokine concentration. J Clin Lab Anal. 2009;23(1):40–44. doi: 10.1002/jcla.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang F.M., Tsai C.H., Ding S.J., Chang Y.C. Induction of cyclooxygenase-2 expression in human pulp cells stimulated by dentin bonding agents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(4):501–506. doi: 10.1016/j.tripleo.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 25.Huang F.M., Yang S.F., Hsieh Y.S., Liu C.M., Yang L.C., Chang Y.C. Examination of the signal transduction pathways involved in matrix metalloproteinases-2 in human pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(3):398–403. doi: 10.1016/j.tripleo.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Ma L., Makino Y., Yamaza H., Akiyama K., Hoshino Y., Song G., et al. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swaminathan S., Cranston A.N., Clyne A.M. A three-dimensional in vitro coculture model to quantify breast epithelial cell adhesion to endothelial cells. Tissue Eng C Methods. 2019;25(10):609–618. doi: 10.1089/ten.tec.2019.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang M.C., Chen Y.J., Tai T.F., Tai M.R., Li M.Y., Tsai Y.L., et al. Cytokine-induced prostaglandin E2 production and cyclooxygenase-2 expression in dental pulp cells: downstream calcium signalling via activation of prostaglandin EP receptor. Int Endod J. 2006;39(10):819–826. doi: 10.1111/j.1365-2591.2006.01156.x. [DOI] [PubMed] [Google Scholar]
- 29.Guven G., Altun C., Gunhan O., Gurbuz T., Basak F., Akbulut E., et al. Co-expression of cyclooxygenase-2 and vascular endothelial growth factor in inflamed human pulp: an immunohistochemical study. J Endod. 2007;33(1):18–20. doi: 10.1016/j.joen.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Chang M.C., Hung H.P., Lin L.D., Shyu Y.C., Wang T.M., Lin H.J., et al. Effect of interleukin-1beta on ICAM-1 expression of dental pulp cells: role of PI3K/Akt, MEK/ERK, and cyclooxygenase. Clin Oral Invest. 2015;19(1):117–126. doi: 10.1007/s00784-014-1227-0. [DOI] [PubMed] [Google Scholar]
- 31.Heino J., Heinonen T. Interleukin-1 beta prevents the stimulatory effect of transforming growth factor-beta on collagen gene expression in human skin fibroblasts. Biochem J. 1990;271(3):827–830. doi: 10.1042/bj2710827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kankuri E., Babusikova O., Hlubinova K., Salmenpera P., Boccaccio C., Lubitz W., et al. Fibroblast nemosis arrests growth and induces differentiation of human leukemia cells. Int J Cancer. 2008;122(6):1243–1252. doi: 10.1002/ijc.23179. [DOI] [PubMed] [Google Scholar]
- 33.Bizik J., Kankuri E., Ristimaki A., Taieb A., Vapaatalo H., Lubitz W., et al. Cell-cell contacts trigger programmed necrosis and induce cyclooxygenase-2 expression. Cell Death Differ. 2004;11(2):183–195. doi: 10.1038/sj.cdd.4401317. [DOI] [PubMed] [Google Scholar]
- 34.Vaheri A., Enzerink A., Rasanen K., Salmenpera P. Nemosis, a novel way of fibroblast activation, in inflammation and cancer. Exp Cell Res. 2009;315(10):1633–1638. doi: 10.1016/j.yexcr.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Al Natour B., Lundy F.T., Moynah P.N., About I., Jeanneau C., Irwin C.R., et al. Odontoblast cell death induces NLRP3 inflammasome-dependent sterile inflammation and regulates dental pulp cell migration, proliferation and differentiation. Int Endod J. 2021;54(6):941–950. doi: 10.1111/iej.13483. [DOI] [PubMed] [Google Scholar]
- 36.Yang H.H., Duan J.X., Liu S.K., Xiong J.B., Guan X.X., Zhong W.J., et al. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Theranostics. 2020;10(11):4749–4761. doi: 10.7150/thno.43108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hua K.F., Chou J.C., Ka S.M., Tasi Y.L., Chen A., Wu S.H., et al. Cyclooxygenase-2 regulates NLRP3 inflammasome-derived IL-1beta production. J Cell Physiol. 2015;230(4):863–874. doi: 10.1002/jcp.24815. [DOI] [PubMed] [Google Scholar]
- 38.Chi Y.C., Shi C.L., Zhou M., Liu Y., Zhang G., Hou S.A. Selective cyclooxygenase-2 inhibitor NS-398 attenuates myocardial fibrosis in mice after myocardial infarction via Snail signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21(24):5805–5812. doi: 10.26355/eurrev_201712_14028. [DOI] [PubMed] [Google Scholar]
- 39.Liu H., Xu X.F., Zhao Y., Tang M.C., Zhou Y.Q., Gao F.H. NS-398 promotes pancreatic cancer cell invasion by CD147 and MMP-2 via the activation of P38. Mol Med Rep. 2016;13(3):2208–2214. doi: 10.3892/mmr.2016.4783. [DOI] [PubMed] [Google Scholar]
- 40.Ahn S.H., Lee J.K., Kim N.D., Kim S.H., Lee S., Jung S., et al. DPIE [2-(1,2-diphenyl-1H-indol-3-yl)ethanamine] augments pro-inflammatory cytokine production in IL-1beta-stimulated primary human oral cells. Int J Mol Sci. 2018;19(7) doi: 10.3390/ijms19071835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rausch-Fan X., Ulm C., Jensen-Jarolim E., Schedle A., Boltz-Nitulescu G., Rausch W.D., et al. Interleukin-1beta-induced prostaglandin E2 production by human gingival fibroblasts is upregulated by glycine. J Periodontol. 2005;76(7):1182–1188. doi: 10.1902/jop.2005.76.7.1182. [DOI] [PubMed] [Google Scholar]
- 42.Tipton D.A., Flynn J.C., Stein S.H., Dabbous M. Cyclooxygenase-2 inhibitors decrease interleukin-1beta-stimulated prostaglandin E2 and IL-6 production by human gingival fibroblasts. J Periodontol. 2003;74(12):1754–1763. doi: 10.1902/jop.2003.74.12.1754. [DOI] [PubMed] [Google Scholar]
- 43.Noguchi K., Shitashige M., Watanabe H., Murota S., Ishikawa I. Interleukin-4 and interferon-gamma inhibit prostaglandin production by interleukin-1beta-stimulated human periodontal ligament fibroblasts. Inflammation. 1999;23(1):1–13. doi: 10.1023/a:1020231331932. [DOI] [PubMed] [Google Scholar]