Abstract
Cervical ectopic pregnancies account for <1% of ectopic pregnancies. Early diagnosis may reduce the morbidity and mortality associated with treatment.
A 43-year-old woman, gravida 4 para 2, presented at 5 + 6 weeks of gestation of pregnancy via in vitro fertilisation with painless vaginal bleeding. Her initial serum β-hCG level was 51,495 mIU/mL. Ultrasound showed a live ectopic pregnancy within the upper cervical canal with no sliding sign. Surgery was avoided initially due to risk of haemorrhage. Multi-dose systemic intramuscular methotrexate was used in an alternate-day regimen with rescue folic acid to arrest further pregnancy development. Repeat ultrasound seven days later showed absent cardiac activity. Serum β-hCG remained high at 91,764 mIU/mL. A suction dilatation and curettage was performed to remove the pregnancy from the cervix, with an estimated blood loss of 50 mL. The patient was discharged and her serum β-hCG declined to an undetectable level over three months of follow-up.
This case adds to the small body of evidence in the management of live cervical ectopic pregnancy. Neo-adjuvant multi-dose methotrexate was successfully used to reduce the risk of haemorrhage associated with surgical management.
Keywords: Ectopic, Methotrexate, Cervical ectopic, Early pregnancy, Case report
Highlights
-
•
Cervical ectopic pregnancies are rare presentations.
-
•
Management should be individualised to the specific patient.
-
•
Methotrexate can reduce the risk of haemorrhage during surgical management.
1. Introduction
Cervical ectopic pregnancies (CEPs) implant within the endocervical canal and account for <1% of ectopic pregnancies [1]. Cervical ectopic pregnancies have been associated with a high risk of life-threatening haemorrhage. Early diagnosis may reduce the morbidity and mortality associated with treatment [2]. Risk factors for their development include previous caesarean section, assisted reproductive techniques and previous ectopic pregnancy [3].
Current management strategies aim to preserve fertility as previously this presentation has been associated with hysterectomy. Medical management predominantly involves systemic or local methotrexate in single- or multi-dose regimens [[4], [5], [6], [7]]. Surgical management strategies involve dilatation and curettage or hysteroscopic resection with subsequent steps to control haemorrhage [[8], [9], [10]]. Uterine artery embolisation has also been demonstrated to be a successful interventional approach.
Factors which alter success rates of medical management include serum β-hCG level, gestational age, fetal cardiac activity and crown-rump length [11].
This report describes the use of a multi-dose regimen of methotrexate to reduce the risk of haemorrhage during dilatation and curettage for a live CEP.
2. Case Presentation
A 43-year-old woman, gravida 4 para 2, with two previous caesarean sections, presented to the emergency department at 5 + 6 weeks of gestation of pregnancy via in vitro fertilisation (IVF) with painless vaginal bleeding. She reported regular menses without menorrhagia or dysmenorrhoea. Her cervical screening had always been normal. She denied a history of sexually transmitted infections. Regular medication included fluvoxamine and multivitamins. The patient was a non-smoker.
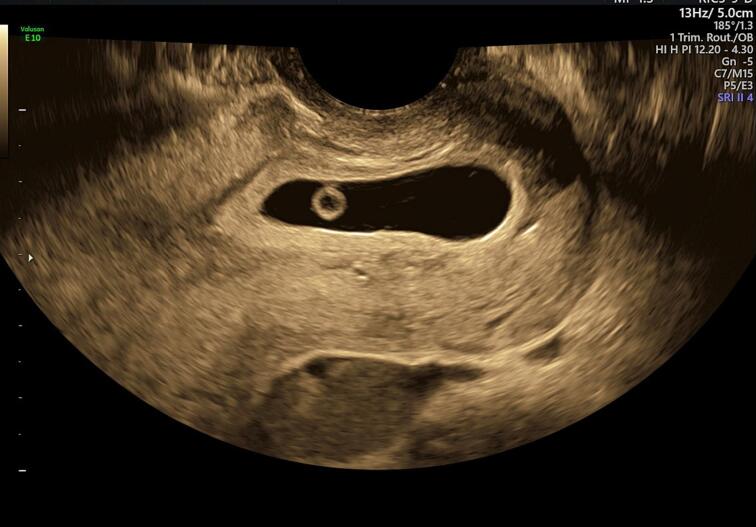
A physical examination elicited mild suprapubic tenderness with no guarding or signs of peritonism. Observations were all within normal limits. Laboratory investigations included Hb 127, WBC 6.9, neutrophils 4.35, CRP 0.5 and serum β-hCG 51,495 mIU/mL. The patient was discharged from the emergency department with an impression of threatened miscarriage. An outpatient ultrasound four days later showed a gestational sac (crown-rump length 6.1 mm, yolk sac 4.3 mm) within the upper cervical canal with fetal cardiac activity (Fig. 1). The sliding sign present in sonographic assessment of cervical stage of miscarriage (the gestational sac should slide against the endocervical canal with probe pressure) was absent [12]. Imaging was reviewed to rule out caesarean scar pregnancy and a diagnosis of CEP was made.
Fig. 1.
Transvaginal ultrasound at 6 + 3 weeks of gestation. A gestational sac is seen within the upper cervical canal (crown rump length 6.1 mm, yolk sac 3.6 mm) with fetal cardiac activity. Sliding sign was negative.
Presentation to the emergency department was recommended post ultrasound. The patient described ongoing vaginal spotting with nausea and pelvic pressure. Serum beta β-hCG had now risen to 72,954 mIU/mL (Fig. 2). Inpatient admission was facilitated and management options were explored. Conservative management was not offered given the diagnosis of CEP. Medical management with systemic or intra-sac methotrexate was discussed, including the risk of bone marrow suppression. Surgical management alone or with adjuncts of uterine artery embolization or intracervical balloon tamponade was also considered.
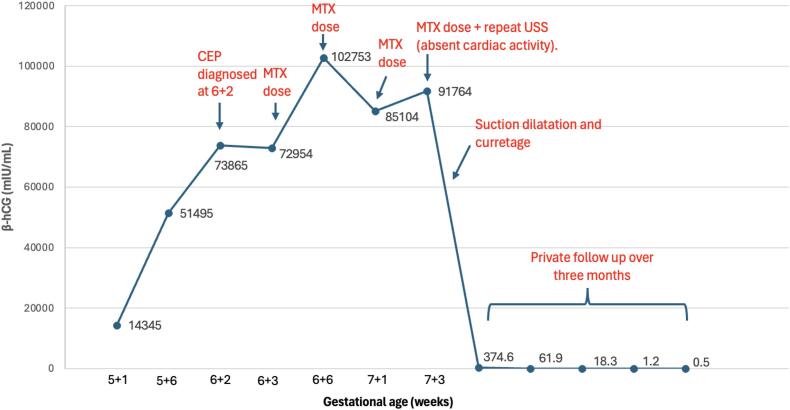
Fig. 2.
Timeline depicting serum β-hCG level and interventions in the management of cervical ectopic pregnancy.
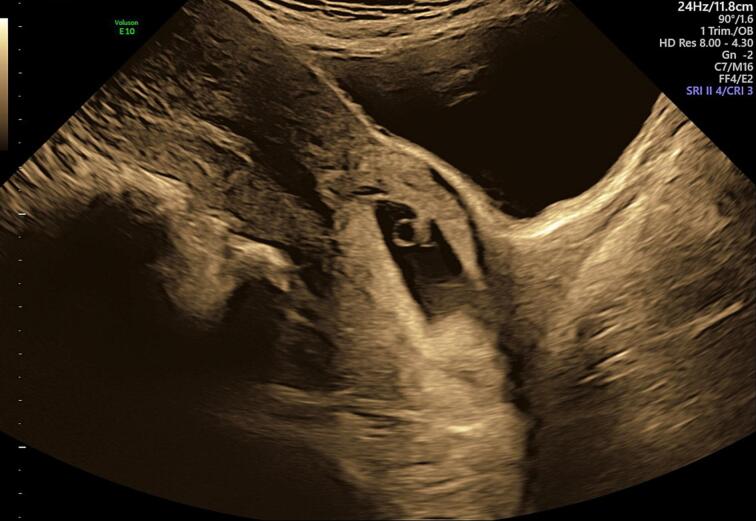
Surgery was avoided initially, due to the high risk of haemorrhage with a live ectopic pregnancy with serum β-hCG of 72,954 mIU/mL. Four doses of systemic intramuscular methotrexate (50 mg/m2) were used in an alternate-day regimen with rescue folic acid to arrest further pregnancy development. Serum β-hCG initially rose to 102,753 mIU/mL before falling to 85,104 mIU/mL on day 4. On day 6, serum β-hCG increased to 91,764 mIU/mL and a repeat ultrasound (Fig. 3) showed a gestational sac within the cervical canal (crown-rump length 6.3 mm, yolk sac 5.2 mm) but with absent fetal cardiac activity. Surgical management was agreed upon by the treating team and patient.
Fig. 3.
Transabdominal ultrasound at 7 + 2 weeks of gestation. A gestational sac (crown-rump length 6.3 mm, yolk sac 5.2 mm) is visualised within the cervical canal with absent fetal cardiac activity. There is distension of the cervix and lower part of the uterus.
A suction dilatation and curettage was performed under general anaesthetic. The cervix was open on initial inspection and the pregnancy was easily removed from the cervix with sponge forceps without dilatation. Suction aspiration was performed with a size 8 Carmen catheter. 1 g intravenous tranexamic acid and 600 μg per-rectal misoprostol aided haemostasis with an estimated blood loss of only 50 mL. The methotrexate was well tolerated with no reported side-effects. The patient was discharged the following day.
The patient chose to have follow-up through her private IVF provider with serial β-hCG blood tests. Initial serum β-hCG ten days post procedure was 374.6 mIU/mL. An appropriate decline of serum β-hCG to an undetectable level was seen over three months. Histopathology sections showed first-trimester-type chorionic villi with prominent nucleated red blood cells plus small fragments of decidua. There was no evidence of gestational trophoblastic disease.
3. Discussion
This article reports a case in which combined medical and surgical management was required to successfully manage a live CEP presenting at 5 + 6 weeks of gestation. CEP is a rare presentation and much of the literature remains low quality. Management options range from conservative, medical, interventional to surgical but clear algorithms are yet to be agreed.
Hysterectomy was previously common due to severe or sudden haemorrhage with CEP. Risk factors for haemorrhage in this case included a high β-hCG level (72,954 mIU/mL) and fetal cardiac activity. As many as 5 out of 12 cases required hysterectomy due to uncontrolled bleeding during curettage or ruptured CEP in published case series [13]. A similar case of live CEP with a serum β-hCG of 97,388 mIU/mL required hysterectomy and uterine artery embolisation despite a more advanced gestation [14]. Minimally invasive dilatation and curettage or hysteroscopy has largely replaced hysterectomy but may still require balloon catheter insertion to tamponade haemorrhage [8,15,16]. In a case series of 10 patients, small-calibre hysteroscopy has been suggested to be superior to dilatation and curettage with shorter hospital stay and less blood loss but notably median β-hCG was <10,000 mIU/mL [8]. Hence, surgery was avoided as primary management to reduce potential morbidity.
Uterine artery embolisation has been demonstrated to control bleeding and treat CEP [17]. As the primary desire for the patient was to preserve fertility, less invasive approaches were first considered given the potential long-term implications of uterine artery embolization, including amenorrhoea [18].
Medical management with a combination of systemic or local methotrexate is now common in the initial management of CEP. The success rate of multi-dose regimens of methotrexate have been reported as high as 92.7% [19]. An inadequate response to methotrexate has been related to a gestational age ≥ 9 weeks, initial β-HCG ≥ 10,000 mIU/mL and fetal cardiac activity. The goal of primary methotrexate therapy was to optimise these factors before a likely concomitant therapy rather than isolated treatment.
The risk of amniotic sac rupture and subsequent haemorrhage with intra-sac methotrexate administration directed us toward systemic therapy only [11]. A multi-dose regimen of methotrexate was initiated given evidence of its greater success than a single-dose regimen in unruptured ectopic pregnancy [20]. A high methotrexate dose of 50 mg/m2 was chosen in an alternate-day regimen in the absence of definitive literature.
Serum β-hCG level remained stagnant with the multi-dose regimen of systemic methotrexate. Repeat ultrasound indicated devascularisation of the pregnancy with a crown rump length of 6.3 mm and no fetal cardiac activity. A β-hCG plateau is a significant prognostic indication for additional therapy and therefore suction dilatation and curettage was arranged [11]. Continuing with methotrexate therapy prompted concerns about side-effect development. The procedure was successful, with only 50 mL blood loss and an appropriate postoperative decline of β-hCG to <5 mIU/mL.
The main limitation of this case report is that only one case is brought to the literature. As CEP remains a rare pathology, the clinical reasoning behind the approach in this article still adds value. Methotrexate has revolutionised management of CEP but requires three months of contraception due to risk of fetal anomaly. When minimally invasive surgical options such as hysteroscopic resection are available, this is a limitation of medical management. Lastly, surgical management often necessitates a general anaesthetic, exposing the patient to additional risk. This could have been avoided had further doses or an alternative route of methotrexate been attempted.
4. Conclusion
Neo-adjuvant methotrexate is a viable management strategy in CEP before dilatation and curettage. This case report strengthens the body of evidence that concomitant medical and surgical management may be required for a live CEP with high initial β-hCG. More evidence is required to help substantiate risk factors and management algorithms to guide the care of these women.
Acknowledgments
Contributors
Matteo Di Carlofelice contributed to conception of the case report, acquiring and interpreting the data, undertaking the literature review, drafting the manuscript and revising it critically for important intellectual content.
Danica Vress contributed to patient care, conception of the case report and revising it critically for important intellectual content.
Both authors approved the final submitted manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Written informed consent was obtained from the patient for publication of the case report and accompanying images.
Provenance and peer review
This article was not commissioned and was peer reviewed.
Acknowledgments
Conflict of interest statement
The authors declare that they have no conflict of interest regarding publication of this case report.
Contributor Information
Matteo Di Carlofelice, Email: matteo.dicarlofelice@health.nsw.gov.au.
Danica Vress, Email: danica.vress@act.gov.au.
References
- 1.Hosni M.M., Herath R.P., Rashid M. Diagnostic and therapeutic dilemmas of cervical ectopic pregnancy. Obstet. Gynecol. Surv. 2014;69(5):261–276. doi: 10.1097/OGX.0000000000000062. https://pubmed.ncbi.nlm.nih.gov/25101692/ Available from: [DOI] [PubMed] [Google Scholar]
- 2.Gnlkglihjwk Cunningham F.G. Williams Obstetrics. 21st ed. McGraw-Hill; New York: 2001. Ectopic pregnancy, chapter 24; pp. 884–910. [Google Scholar]
- 3.Hoyos L.R., Tamakuwala S., Rambhatla A., Brar H., Vilchez G., Allsworth J., et al. Risk factors for cervical ectopic pregnancy. J. Gynecol. Obstet. Hum. Reprod. 2020;49(10) doi: 10.1016/j.jogoh.2019.101665. [DOI] [PubMed] [Google Scholar]
- 4.Surampudi K. A case of cervical ectopic pregnancy: successful therapy with methotrexate. J. Obstet. Gynaecol. India. 2012;62(Suppl. 1):1. doi: 10.1007/s13224-013-0351-0. (Available from: /pmc/articles/PMC3632676/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerveira I., Costa C., Santos F., Santos L., Cabral F. Cervical ectopic pregnancy successfully treated with local methotrexate injection. Fertil. Steril. 2008;90(5):2005.e7–2005.e10. doi: 10.1016/j.fertnstert.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Dilday E., Douglas C., Brennan K. Case Rep Womens Health; 2021. Single-dose intramuscular methotrexate for treatment of cervical ectopic pregnancy: a case report; p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouda A., Enayat A., Ahmed W.E. Conservative management of a viable cervical ectopic pregnancy with systemic and multiple local methotrexate injections. A case report. Eur. J. Contracept. Reprod. Health Care. 2022;27(3):265–268. doi: 10.1080/13625187.2022.2026325. [DOI] [PubMed] [Google Scholar]
- 8.Maglic R., Rakic A., Nikolic B., Maglic D., Jokanovic P., Mihajlovic S. Management of cervical ectopic pregnancy with small-caliber hysteroscopy. JSLS. 2021;25(2) doi: 10.4293/JSLS.2021.00016. (Available from: /pmc/articles/PMC8249221/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aryad R., Molakatalla S. Successful surgical management of a cervical ectopic pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 2019;59(S1):11–82. https://wslhd.intersearch.com.au/wslhdjspui/handle/1/3044 Available from: [Google Scholar]
- 10.Ghoubara A.S.M., Elsheikh J.S.A., Abdulwahab H.R., Taha A.A.A. Intra-amniotic and systemic administration of methotrexate with concomitant surgical evacuation of 11 + 5 weeks cervical ectopic pregnancy: a case report. BMC Pregn. Childbirth. 2023;23(1):1–5. doi: 10.1186/s12884-023-05794-0. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung T.H., Shau W.Y., Hsieh T.T., Hsu J.J., Soong Y.K., Jeng C.J. Prognostic factors for an unsatisfactory primary methotrexate treatment of cervical pregnancy: a quantitative review. Hum. Reprod. 1998;13(9):2636–2642. doi: 10.1093/humrep/13.9.2636. Available from: [DOI] [PubMed] [Google Scholar]
- 12.Jurkovic D., Hacket E., Campbell S. Diagnosis and treatment of early cervical pregnancy: a review and a report of two cases treated conservatively. Ultrasound Obstet. Gynecol. 1996;8(6):373–380. doi: 10.1046/j.1469-0705.1997.08060373.x. (PMID: 9014275) [DOI] [PubMed] [Google Scholar]
- 13.Vela G., Tulandi T. Cervical pregnancy: the importance of early diagnosis and treatment. J. Minim. Invasive Gynecol. 2007;14(4):481–484. doi: 10.1016/j.jmig.2006.11.012. https://pubmed.ncbi.nlm.nih.gov/17630167/ Available from: [DOI] [PubMed] [Google Scholar]
- 14.Stabile G., Zinicola G., Romano F., Buonomo F., Mangino F.P., Ricci G. Management of non-tubal ectopic pregnancies: a single center experience. Diagnostics (Basel) 2020;10(9) doi: 10.3390/diagnostics10090652. https://pubmed.ncbi.nlm.nih.gov/32878097/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitale S.G., Rapisarda A.M.C., Laganà A.S. Cervical ectopic pregnancy: the role of hysteroscopy. Hysteroscopy. 2018:171–179. doi: 10.1007/978-3-319-57559-9_18. Available from: [DOI] [Google Scholar]
- 16.Timor-Tritsch I.E., Monteagudo A., Bennett T.A., Foley C., Ramos J., Kaelin Agten A. A new minimally invasive treatment for cesarean scar pregnancy and cervical pregnancy. Am. J. Obstet. Gynecol. 2016;215(3) doi: 10.1016/j.ajog.2016.03.010. https://pubmed.ncbi.nlm.nih.gov/26979630/ 351.e1–351.e8. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Xu B., Wang Y.K., Zhang Y.H., Wang S., Yang L., Dai S.Z. Angiographic uterine artery embolization followed by immediate curettage: an efficient treatment for controlling heavy bleeding and avoiding recurrent bleeding in cervical pregnancy. J. Obstet. Gynaecol. Res. 2007;33(2):190–194. doi: 10.1111/j.1447-0756.2007.00512.x. https://pubmed.ncbi.nlm.nih.gov/17441894/ Available from: [DOI] [PubMed] [Google Scholar]
- 18.Martin J., Bhanot K., Athreya S. Complications and reinterventions in uterine artery embolization for symptomatic uterine fibroids: a literature review and meta analysis. Cardiovasc. Intervent. Radiol. 2013;36(2):395–402. doi: 10.1007/s00270-012-0505-y. https://pubmed.ncbi.nlm.nih.gov/23152035/ Available from: [DOI] [PubMed] [Google Scholar]
- 19.Halimeh R., Sleiman R., Geahchan A., Massoud D., Abdallah A., Feghali J., et al. Cervical ectopic pregnancy, a case report and literature review. J. Gynecol. Obstet. 2020;8(4):85–90. http://www.sciencepublishinggroup.com/j/jgo Available from: [Google Scholar]
- 20.Talwar P., Sandeep K., Naredi N., Duggal B.S., Jose T. Systemic methotrexate: an effective alternative to surgery for management of unruptured ectopic pregnancy. Med. J. Armed Forces India. 2013;69(2):130. doi: 10.1016/j.mjafi.2012.08.032. (Available from: /pmc/articles/PMC3862614/) [DOI] [PMC free article] [PubMed] [Google Scholar]