Abstract
Methamphetamine (MA) use disorder poses significant challenges to both the affected individuals and society. Current non-drug therapies like transcranial direct-current stimulation and transcranial magnetic stimulation have limitations due to their invasive nature and limited reach to deeper brain areas. Transcranial focused ultrasound (FUS) is gaining attention as a noninvasive option with precise spatial targeting, able to affect deeper areas of the brain. This research focused on assessing the effectiveness of FUS in influencing the infralimbic cortex (IL) to prevent the recurrence of MA-seeking behavior, using the conditioned place preference (CPP) method in rats. The study involved twenty male Sprague-Dawley rats. Neuronal activation by FUS was first examined via electromyography (EMG). Rats received alternately with MA or saline, and confined to one of two distinctive compartments in a three compartment apparatus over a 4-day period. After CPP test, extinction, the first reinstatement, and extinction again, FUS was applied to IL prior to the second MA priming-induced reinstatement. Safety assessments were conducted through locomotor and histological function examinations. EMG data confirmed the effectiveness of FUS in activating neurons. Significant attenuation of reinstatement of MA CPP was found, along with successful targeting of the IL region, confirmed through acoustic field scanning, c-Fos immunohistochemistry, and Evans blue dye staining. No damage to brain tissue or impaired locomotor activity was observed. The results of the study indicate that applying FUS to the IL markedly reduced the recurrence of MA seeking behavior, without harming brain tissue or impairing motor skills. This suggests that FUS could be a promising method for treating MA use disorder, with the infralimbic cortex being an effective target for FUS in preventing MA relapse.
Keywords: Focused ultrasound, Conditioned place preference, Methamphetamine, Methamphetamine relapse, Neuromodulation
Introduction
Methamphetamine (MA) use disorder is a growing global public health problem affecting both individuals and society. MA is a psychostimulant that can overexcite the monoamine neurotransmitter systems of the brain. MA users often initially experience intense euphoria, prolonged wakefulness, and enhanced energy [[1], [2], [3]]. The highly addictive nature of MA can lead to strong dependency even after a single use. Long-term use of MA can lead to various health issues, including heart disease, hypertension, skin problems, and dental damage [2,4]. Moreover, the impact of MA on mental health can be even more severe, triggering conditions such as anxiety, depression, paranoia, or even cognitive impairments [[4], [5], [6]].
Currently, the FDA has not sanctioned any drug treatments specifically for MA addiction. Nonetheless, neuromodulation methods are emerging as innovative, hopeful, and readily accessible strategies to address addiction issues. Research exploring the impact on MA relapse prevention has examined both invasive methods like deep brain stimulation (DBS) and noninvasive ones, including repetitive transcranial magnetic stimulation (rTMS) and transcranial direct-current stimulation (tDCS). These investigations utilized the conditioned place preference (CPP) reinstatement model in rats. This addiction model can imitate addiction in humans, which includes the three main phases of development, extinction, and reinstatement, and it is commonly employed to study the effects of neuromodulation of relapse-like behavior in animals.
It has been reported that high-frequency DBS targeting the substantia nigra pars reticulata or orbitofrontal cortex prevents the reinstatement of MA-seeking behaviors [7,8]. Moreover, chronic rTMS (over a 3-day period) significantly inhibited the reinstatement of MA-induced CPP [9]. However, DBS involves complex neurosurgical interventions for embedding electrodes deep in the brain, often leading to poor adherence from patients. In addition, rTMS and tDCS suffer from limitations in spatial precision and the depth of target area they can affect [10].
Emerging as a prominent method in neuromodulation is transcranial focused ultrasound stimulation (FUS). This noninvasive technique, unlike its counterparts, has the theoretical ability to target deep brain structures accurately. It achieves modulation of neural activity in these deeper regions with millimeter-level precision, leveraging the effective penetration of sound waves through the skull and soft tissues [11,12]. Experiments with transcranial FUS across a frequency spectrum of 0.25–1 MHz and spatial peak pulse average intensity (ISPPA) levels ranging from 1 to 100 W/cm2 have successfully elicited neural and behavioral responses in both animal and human subjects, without inducing adverse effects [[13], [14], [15]]. By fine-tuning the parameters, FUS can even decrease human motor cortical excitability [16]. The neural modulation properties of FUS, especially the inhibition of neuronal excitability, may help prevent MA relapse.
The infralimbic cortex (IL) in rodents, a lower subsection of the medial prefrontal cortex, corresponds to a portion of the human ventromedial prefrontal cortex and plays a role in inhibiting drug-seeking actions [17]. Experimentally manipulating IL activity, including by applying DBS, affects the relapse of drug-seeking behaviors [18]. Since the penetration of the skull and soft tissue, as well as the neuromodulatory effects, increase as the FUS frequency decreases [19,20], the present proof-of-concept animal study examined whether low-frequency FUS stimulation (at 0.5 MHz) of the IL region can reduce the reinstatement of MA-induced CPP. Furthermore, histological assays and locomotor activity tests were conducted to assess the safety of FUS treatment, and the extent of the FUS-stimulated region covering the IL region was confirmed using acoustic field scanning and Evans blue dye staining.
Methods
Animals
This study utilized 30 male Sprague-Dawley rats from BioLASCO (Taipei, Taiwan). The study utilized animals aged between 10 and 12 weeks, with a body weight ranging from 200 to 250 g, sourced from BioLASCO Taiwan Co. Ltd. Before initiating the experiments, a 3-day acclimation period was allowed for the rats in a controlled setting at 25 °C, with humidity levels between 40 % and 60 % and a 12-h light/dark cycle (lights active from 07:00 to 19:00). Throughout this acclimation phase, the rats had unrestricted access to food and water. The animal care procedures used in this research were sanctioned by the Institutional Animal Care and Use Committee of the National Health Research Institutes (NHRI-IACUC-111004-A).
Effectiveness of FUS-induced neuromodulation estimated using electromyography
To identify the acoustic pressure threshold for FUS stimulation to activate brain neurons, electromyography (EMG) was used to monitor the appearance of leg muscle contractions in a rat while applying FUS. A 0.5-MHz FUS transducer (V318, Olympus, Westborough, MA, USA) was focused at the primary motor cortex (bregma +2.0 mm, midline +1.0 mm, and depth −1.0 mm) and triggered by a pulse generator (Agilent 33220A, Agilent Technology, Palo Alto, CA, USA) and a power amplifier (325LA, Eni Technology, Rochester, NY, USA). The parameters were an acoustic pressure ranging from 0 to 400 kPa, 40,000 cycles, and a pulse repetition interval (PRI) of 5 s (sec). Long cycles in the parameters are used to accumulate enough energy in the targeted brain region for generating strong EMG signals. The experiment consists of collecting 1 min of background EMG data, administering 12 FUS stimulations with specified parameters, and repeating this sequence once for a total duration of 4 min (min). Experiments were conducted three times for each acoustic pressure, with a 10-min interval between trials to prevent parameter interference.
The experimental procedure began with anesthetizing the rat using 1 %–3 % isoflurane, after which EMG electrodes were attached to the rat's leg and the common ground was inserted into the rat's tail. The detailed anesthesia procedure was as follows: anesthesia was administered through a vaporizing system (MATRX VIP 3000, Midmark, Ohio, USA). Initially, rats were anesthetized for 10 min using the following anesthesia parameters: O2 flow rate set at 4.5 cc/min and isoflurane level adjusted to 3 %. Subsequently, for the measurement of EMG signals, the anesthesia parameters were modified to an O2 flow rate of 4.5 cc/min and Isoflurane level at 1 %, during which period the electrical signals were recorded. EMG signals were collected using a 16-channel neural signal processor (CerePlex Direct, Blackrock Microsystems, Salt Lake City, UT, USA) at a sampling frequency of 10 kHz before, during, and after FUS stimulation. To evaluate the neuron activity, the acquired EMG signals were identified and estimated the areas under the curves as described previously [21].
Hematoxylin and eosin staining
Whether the applied FUS had induced tissue damage was determined by performing hematoxylin and eosin (H&E) staining. The rats were divided into two groups: the control group (without treatment) and the FUS-treated group (0.5-MHz, 80 cycles, 328 kPa, and a PRI of 2 ms for 20 min). After finishing the experiments, each rat was perfused with saline, the brain was harvested, and the brain sample was sliced into 20 μm-thick coronal sections. The tissue sections were stained with H&E to detect erythrocyte extravasation and other potential damage to brain tissue using microscopy.
Establishment and validation of the MA-induced CPP extinction and reinstatement animal model
A three-compartment apparatus constructed as described previously was used to perform MA CPP [22]. A black-and-white striped compartment, a purple compartment, and an unpainted compartment protruding from the rear of the two painted compartments, connected by two entrances with removable wooden partitions. Black-and-white and purple compartments were alternately used for MA and saline control conditioning.
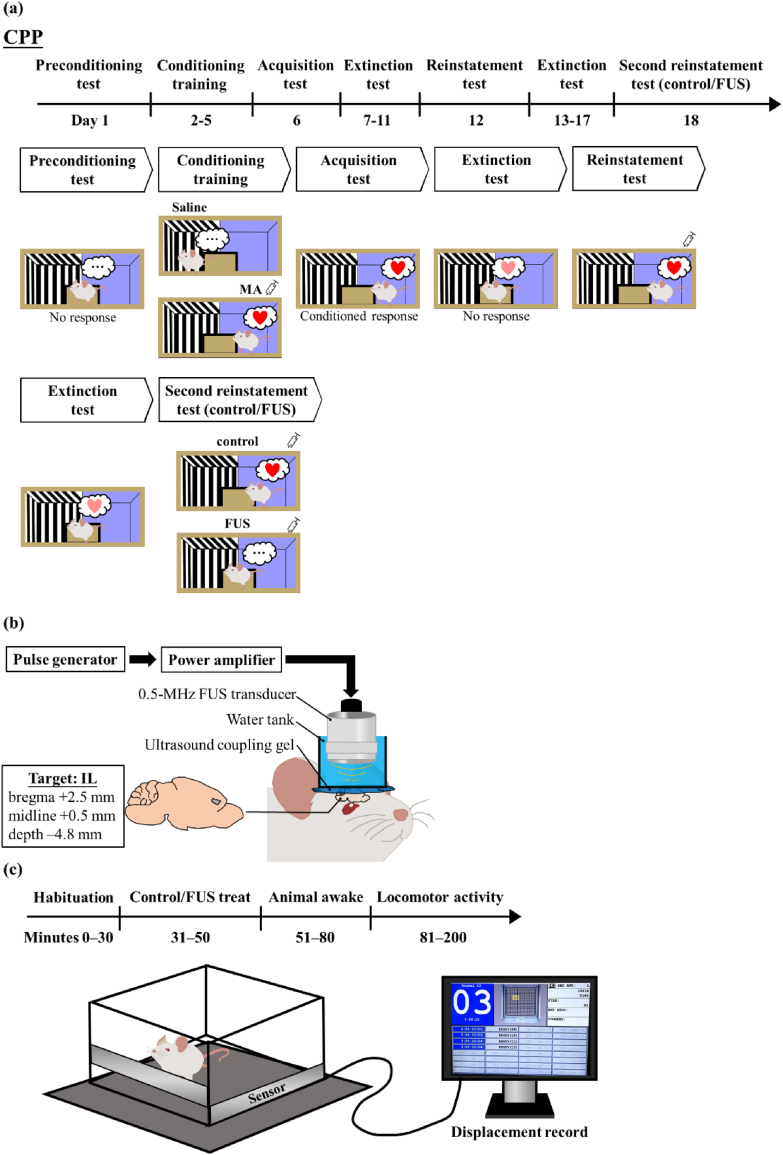
The experiment consisted of five stages: a preconditioning test (day 1), conditioning (days 2–5), acquisition test (day 6), extinction (days 7–11), and reinstatement test (day 12) (Fig. 1a). In the preconditioning test, each rat was placed in the rear compartment and allowed to explore freely for 15 min, with the partitions open. The time difference between the rats spent in MA-paired and control-paired compartment (CPP score, Equation (1)) was recorded as the unconditioned preference. The CPP experiment employed an unbiased design, with the MA-paired compartment being randomly assigned.
| (1) |
where the subscript indicates the condition under which the time was measured.
Fig. 1.
Timeline and setup of experiments on (a) CPP training, (b) FUS treatment and (c) locomotor activity.
In the subsequent conditioning training, rats received either MA (2 mg/kg, i.p.) or saline and were then placed in the MA-paired or saline-paired compartment for 20 min. On the following day, the rats received the opposite injection (MA or saline) and placed in the other compartment for 20 min. Four days after the paired training protocol, the CPP acquisition test was conducted, which followed the same procedure as the preconditioning test. The rats exhibited a preference for the drug-paired compartment during the test. Subsequently, they underwent nonconfined extinction training. This involved a 15-min session that used the same procedure as the preconditioning test and continued until the CPP score approximated that during the preconditioning test. Following the extinction sessions, the reinstatement test was conducted in which MA (1 mg/kg, i.p.) was administered and placed in the rear compartment for 15 min to record the CPP score [23,24].
FUS treatment procedure
To determine if the FUS affect the MA relapse, after the rats were performed 2nd extinction training (days 13–17), the rats were divided into two groups: control group (without treatment) and FUS-treated group. All rats received isoflurane anesthesia (1 %–3 %) for 20 min. Rats in the FUS-treated group received 0.5-MHz FUS stimulation (Fig. 1b) in the IL region (bregma +2.5 mm, midline +0.5 mm, and depth −4.8 mm) for 20 min (80 cycles, 328 kPa, and PRI = 2 ms). Once the rats recovered from the anesthesia and exhibited normal locomotor activity, they were performed the 2nd reinstatement test (Day 18). The control group underwent the same procedure as the FUS-treated group with the exclusion of FUS exposure. As the IL region is deeper than primary motor cortex (depth: 4.8 vs 1.0 mm) and the soft tissue attenuation coefficient of ultrasound is 0.75 dB/cm/MHz [25]. Therefore, an acoustic pressure of 328 kPa was selected for the treatment.
Locomotor activity
To verify whether FUS affected the MA-induced locomotor activity in rats, the distance traveled after FUS treatment was measured. A photocell-based activity monitor (Opto-Varimex-3, Columbus Instruments, Columbus, OH, USA) was utilized in combination with AutoTrack software (version 4.4, Columbus Instruments) to quantify the total distance, which served as an indicator of their locomotor activity. One hour prior to the test, rats were transferred to the measurement room and subsequently individually placed into the activity cage (43 × 43 × 17 cm3) for a 30-min recording of spontaneous activity (habituation) (Fig. 1c). The rats were then subjected to either FUS stimulation or the sham control. Once the rats recovered from the anesthesia and exhibited normal locomotor activity (between 51 and 80 min), the rats were injected with MA (1 mg/kg, i.p.) and immediately returned to the activity cage to record their locomotor activity (between 81 and 200 min).
Scanning the acoustic field induced by the FUS transducer
The specific range of acoustic pressure effects during FUS stimulation was elucidated by measuring the acoustic field. During the measurement, the 0.5-MHz FUS transducer (serving as a transmitter) and a hydrophone (HNC-0400, Onda Corporation, Sunnyvale, CA, USA) (acting as a receiver) were coaxially replaced at a focal distance of 20 mm inside a water tank. The measurement procedure entailed driving the FUS transducer with three cycles of a 0.5-MHz sinusoidal wave that was generated by the pulse generator. The hydrophone was mounted on a 3D customized motor scanning system (YO SO TECHNOLOGY, New Taipei, Taiwan) with a stepping interval of 100 μm along both the X and Y axes and of 500 μm along the Z axis, which was the direction of ultrasound propagation. There were two scanning regions: (1) at Z = 20 mm on the X–Y plane, with both length and width being 10 mm, and (2) at Y = 5 mm on the X-Z plane, with a length of 45 mm and a width of 10 mm. Signals received by the hydrophone were amplified by a preamplifier and then displayed on an oscilloscope (MSO46, Tektronix, Beaverton, OR, USA). The signals were processed and constructed the acoustic field of the FUS transducer using MATLAB (The MathWorks, Natick, MA, USA) software. The pressure at the acoustic focus was calibrated relative to the input voltage using the hydrophone.
Confirmation of FUS-treated region by Evans blue dye and microbubbles
To verify whether the focal site of FUS was precisely in the IL region, Evans blue dye staining and FUS-actuated microbubble cavitation were performed. Evans blue is a brain-vessel-impermeable dye that only appeared in FUS-treated brain tissue, which was due to the mechanical forces generated by combining FUS with microbubbles permeabilizing the local brain vessels [26,27]. During the experiment, the rats were anesthetized with isoflurane. A bolus of Evans blue dye (1.3 ml/kg) and microbubbles (1 ml diluted in saline) was IV administered 5 min before 0.5-MHz FUS was delivered to the IL region without opening the skull. The FUS was operated with 328 kPa, 5000 cycles, and PRI = 1 s for 60 s. One hour after the experiment, the rat was perfused by saline and the brain was then harvested. The brain sample was sliced into coronal sections to observe the dye distribution.
Immunohistochemical analysis for c-Fos expression
To ascertain FUS affect the neuronal activity in the IL region, the c-Fos expression was determined. The rats were segregated into two groups: a control group (without treatment) and a FUS group (0.5-MHz, 80 cycles, 328 kPa, and a PRI of 2 ms for 20 min). Sixty minutes post-experiment, each rat was sequentially perfused with saline and 4 % paraformaldehyde, followed by post-fixation of the brain in 4 % paraformaldehyde at 4 °C overnight. The brain tissues were then saturated in a 30 % sucrose solution until they sank and subsequently sectioned into 50 μm thick coronal slices using a cryostat.
c-Fos protein expression within brain section nuclei was analyzed using immunohistochemistry [28,29], which produced brown spots visible under a standard optical microscope. The section preparation involved the following steps: initially, the sections were soaked in a 1 % solution of hydrogen peroxide for 30 min to suppress endogenous peroxidase and reduce unspecific staining. This was succeeded by three washes of 10 min each in a PBS solution containing 0.4 % Triton X-100 and 2 % Bovine Serum Albumin (BSA). The sections were then treated with 10 % BSA for 1 h before an overnight incubation at 4 °C with a c-Fos polyclonal antibody (1:1000 dilution, Abcam, Cambridge, UK, catalog number: ab190289). After the primary antibody incubation, the sections underwent three PBS washes, followed by a 2-h room temperature incubation with biotinylated anti-rabbit IgG (1:200 dilution, Sigma Aldrich, St. Louis, USA), and then a 1-h treatment with 0.2 % avidin-biotinylated horseradish peroxidase complex (ABC solution, Vector Laboratories, Burlingame, CA). Staining with 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma Aldrich, St. Louis, USA) was conducted for 15 min. Following three more PBS washes, the sections were placed on gelatin-coated slides, left to dry in air overnight, and then coverslipped. Imaging was performed using a Nikon digital sight 10 camera (Nikon, Tokyo, Japan) on a Nikon Eclipse Ti2 microscope (Nikon, Tokyo, Japan) at 60x magnification. The c-Fos immunoreactive cells in a 0.24 mm2 area of the IL region were counted using Nikon's NIS-Elements AR v5.42.02 imaging software (Nikon Instruments, Melville, NY). Data were collected from four brain slices per rat, with sample sizes comprising four rats per group.
Statistical analyses
Data is presented as the standard error of the mean, derived from at least three separate experiments. Statistical evaluations encompassed a two-tailed Student's t-test and a one-way ANOVA for broad comparisons. Two-way mixed ANOVA was employed for comparing these groups in specific tests like the preconditioning test, acquisition test, reinstatement test, etc. A probability value of p < 0.05 was considered statistically significant. All computations were performed using the SPSS software package (SPSS, Chicago, IL, USA).
Results
Assessing FUS stimulation threshold with EMG and biosafety with tissue staining
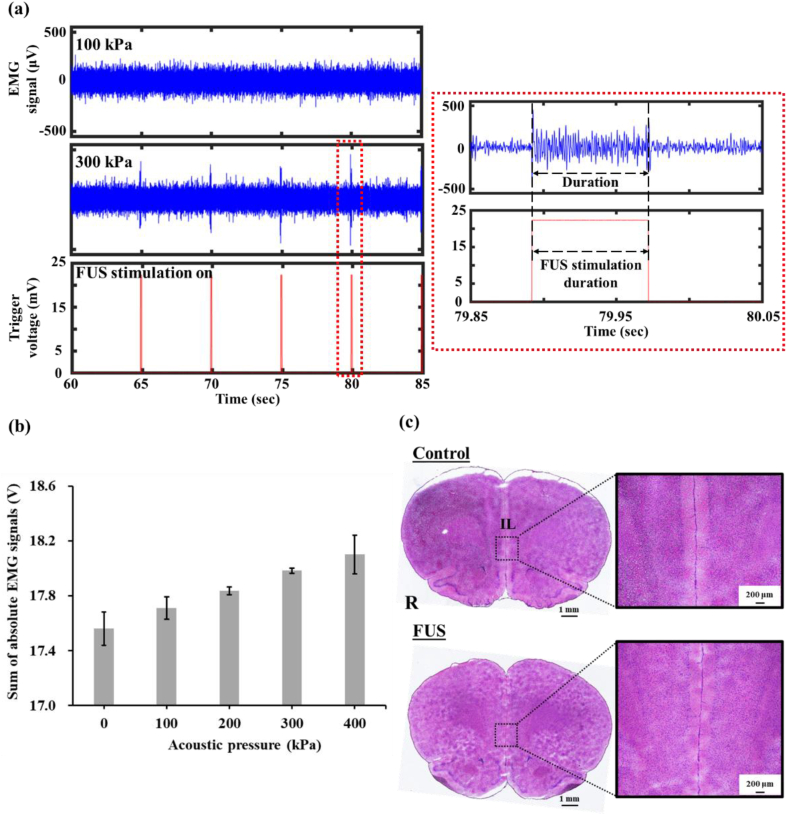
It has been reported that activating neurons in the primary motor cortex, a movement-related brain region, changes leg muscle electric signals [30]. In the present study we used EMG to identify the threshold of FUS pressure required to activate brain neurons. The results demonstrated that FUS at an acoustic pressure of 100 kPa did not affect the EMG signal (background signal = 78.9 ± 4.3 μV) (Fig. 2a). However, increasing the acoustic pressure to 300 kPa resulted in a peak EMG signal of 451 μV. This 5.7-fold increase in signal strength during FUS stimulation confirmed that stimulation at 300 kPa is effective at stimulating the rat brain for achieving neuromodulation. Additionally, the EMG signal duration consisted with the duration of FUS stimulation. Processing and quantifying the EMG signals for acoustic pressures from 0 to 400 kPa revealed a difference between 0 and 200 kPa (17.5 ± 0.1 vs 17.8 ± 0.0 V), indicating that a minimum acoustic pressure of 200 kPa is sufficient to stimulate brain neurons. Increasing the acoustic pressure to 300 kPa (17.9 ± 0.0 V) increased the stimulation effects. However, further increasing the pressure to 400 kPa did not induce any differences in stimulation effects compared with 300 kPa (18.1 ± 0.1 vs 17.9 ± 0.0 V, Fig. 2b). Note that the IL region is deeper than primary motor cortex (depth 4.8 vs 1.0 mm) and soft tissue attenuation coefficient of ultrasound is 0.75 dB/cm/MHz [25]. Therefore, an acoustic pressure of 328 kPa was selected to assess the effect of transcranial FUS on the reinstatement of MA seeking behavior.
Fig. 2.
(a) EMG raw signals elicited by FUS at 100 and 300 kPa. Red rectangular: magnification of single spike and FUS trigger signal. (b) Sum of absolute EMG signals postprocessed from three repeated trials on one animal for each acoustic pressure. N = 1 per group; (c) H&E staining in control and FUS samples.
To verify the biosafety of the specified acoustic parameters, we applied H&E staining to rats from the control group (surgery without FUS) and the FUS group (surgery with 328 kPa FUS) to detect signs of erythrocyte extravasation. The results for the FUS group confirmed that erythrocyte extravasation did not occur (Fig. 2c), affirming that the applied acoustic parameters did not induce brain tissue damage.
Effects of FUS on reinstatement of MA-induced CPP
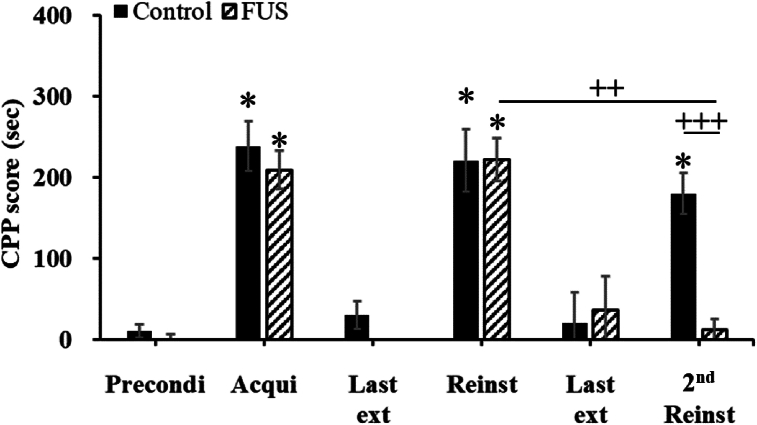
Following two pairs of condition training sessions, the CPP scores during the acquisition test increased significantly by >20-fold relative to those during the preconditioning test, demonstrated a markedly stronger preference for the MA-paired compartment. The rats were separated into control and FUS groups (Fig. 3). During the acquisition test, the CPP score increased from 10.4 to 238.4 s in the control group, and from −7.4 to 209.3 s in the FUS group.
Fig. 3.
Comparing the effects of FUS on MA-induced CPP between the control and FUS groups. The corresponding CPP periods on the x-axis are as follows: Precondi, preconditioning test; Acqui, acquisition test; Last ext, the final consecutive extinction test; Reinst, reinstatement test; 2nd Reinst, second reinstatement test; Control, underwent surgery before the second reinstatement test but without FUS; FUS, underwent surgery and received FUS before the second reinstatement. N = 8 per group. ∗p < 0.05: significantly different from the preconditioning test, ++ p < 0.01, +++ p < 0.001.
During the 5 sessions of extinction, the CPP score gradually decreased. Once the CPP score was confirmed to have returned to levels similar to the preconditioning test (Control 30.3 ± 17.1 s; FUS –25.0 ± 15.8 s), a reinstatement test was conducted. In the reinstatement test with MA injection, both the control and FUS groups exhibited a significant preference for the MA-paired compartment (Control 221.1 ± 38.7 s; FUS 222.6 ± 26.4 s, Fig. 3), reflecting the results observed in the acquisition test. This indicates that the rats retained memory of the MA injection and showed a preference for the MA-paired compartment. After undergoing 5 sessions of a second extinction, and confirming that the CPP score returned to levels similar to the preconditioning test (Control 20.2 ± 38.8 s; FUS 36.6 ± 41.6 s), a 2nd reinstatement test was conducted.
In the 2nd reinstatement test with MA injection, the control group showed a significant preference for the MA-paired compartment, akin to the initial reinstatement test. This supported the hypothesis that MA injection can revive the memory of MA use disorder in rats, leading to a preference for the MA-paired compartment. A paired t-test analysis comparing the CPP scores of the control group between the reinstatement tests showed no significant differences (180.3 ± 25.2 vs 221.1 ± 13.6 s, Fig. 3), indicating that the surgical procedure without FUS did not alter the rats’ preference.
Furthermore, the CPP experiment results were analyzed using a two-way mixed ANOVA. This involved within-subject factors, assessing whether rats exhibited induced reoffending responses across different CPP phases, and between-subject factors, comparing the CPP scores between control and FUS groups. The analysis revealed significant within-group differences in CPP phases: F(1, 14) = 29.274, p = 0.00001; and a notable interaction between CPP phases and groups: F(1, 14) = 5.815, p = 0.00899. Further analysis using the Student Newman-Keuls post hoc test compared CPP scores between the FUS and control groups during the 2nd reinstatement test following MA injection, revealing control vs FUS: p < 0.001. This indicates that FUS application significantly lowered CPP scores during the 2nd reinstatement phase, affirming its effectiveness in reducing MA-induced reoffending responses.
Assessing the effect of FUS on MA-induced locomotor activity
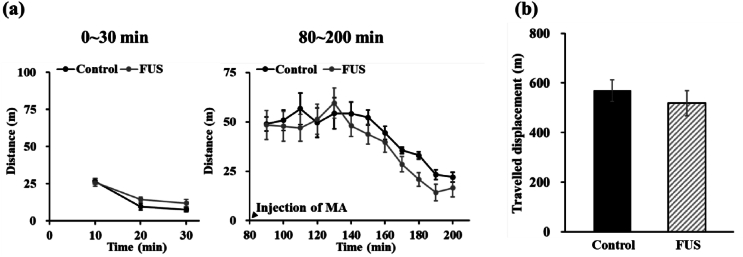
To assess whether the reduction of MA priming-induced reinstatement of CPP after FUS was associated with the changes in MA-induced locomotor hyperactivity, the locomotor activity test was performed. As shown in Fig. 4a, after habituation for 30 min, MA injection significantly increased the locomotor activity in both control and FUS groups. No significant difference was shown in the displacement across all time points after MA injection for both the control and FUS groups (F(1, 10) = 0.359, p = 0.56237, Fig. 4b). These findings demonstrate that MA-induced locomotor hyperactivity was not impacted by FUS. However, FUS did reduce the reinstatement of MA-induced CPP. These finding revealed that FUS did not impair the motor ability of rats, like in the control group, thereby indicating that the motor abilities of rats remain normal when they are exposed to FUS using the parameters employed in this study.
Fig. 4.
(a) Displacement over each 10-min period and (b) total displacement in the effects of FUS on locomotor activity in MA-pretreated rats. N = 6 per group.
FUS transducer acoustic fields and FUS-induced c-Fos expression in the targeted brain region
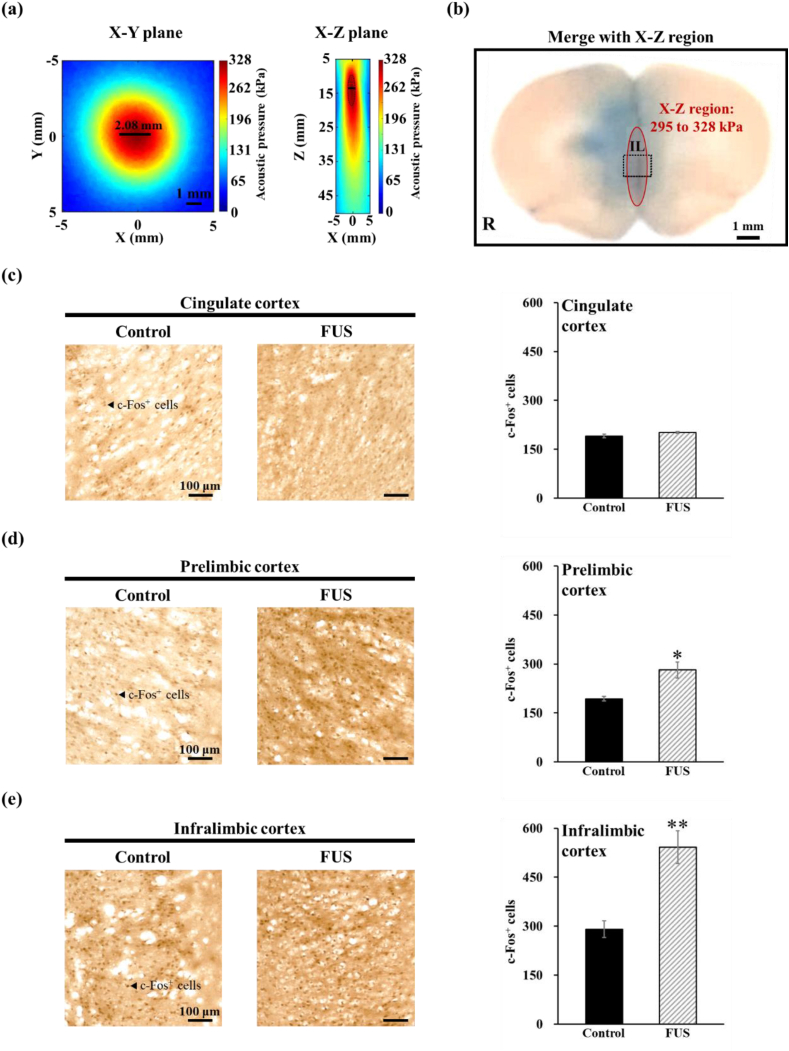
We measured the acoustic field to determine the effective range of acoustic stimulation provided by the FUS transducer on the FUS-treated regions. The results presented in Fig. 5a reveal that within the X–Y plane, a region of 295–328 kPa appears as an approximately circular area with a diameter of 2.08 mm. Conversely, on the X-Z plane, the coverage of the same normalized intensity range approximates an elliptical region with minor and major axes measuring 1.04 and 4.80 mm, respectively.
Fig. 5.
(a) Acoustic field of the FUS transducer. (b) Histological examination of the Evans blue dye distribution within the FUS-treated brain. Red ellipse: the acoustic field of the FUS transducer. Comparison of c-Fos-positive cells in the (c) cingulate cortex, (d) prelimbic cortex, and (e) infralimbic cortex regions between the control and FUS groups. An arrow points to a c-Fos-positive cell. N = 4 per group. ∗p < 0.05, ∗∗p < 0.01 compared with the untreated control groups.
We subsequently evaluated the focal site of the FUS directed at the IL region by observing the distribution of Evans blue dye within the brain tissue (Fig. 5b). The dye was continuously present in the IL region with no visually identifiable damage to brain tissue. Additionally, by integrating the data from the X-Z region in Fig. 5a with observations from brain tissue sections, it was revealed that the FUS-treated area extended beyond the IL. Importantly, the IL region corresponded to the location with the highest acoustic energy in the sonication field.
Moreover, our investigation extended to assess the impact of FUS on cellular activity, not only with a particular focus on c-Fos expression in the focal position of the IL region but also including analyses of the adjacent prelimbic and cingulate cortices. The c-Fos analysis results were compelling: after FUS treatment, the number of c-Fos positive cells in the IL region, located at the FUS focus, showed a significant increase, with a 1.8-fold rise compared to the control groups (291 ± 26 vs 542 ± 50 cells, F(1, 6) = 14.706, p = 0.00861), as depicted in Fig. 5e. These results substantiate our hypothesis that FUS stimulation notably enhances c-Fos expression in the targeted brain region.
Furthermore, our expanded analysis revealed differential effects of FUS in adjacent brain regions. In the cingulate cortex (Fig. 5c), farther from the focal point of FUS, there was no significant difference in the number of c-Fos positive cells between the control and FUS groups (191 ± 5 vs 201 ± 2 cells, F(1, 6) = 2.723, p = 0.15000). However, in the prelimbic cortex (Fig. 5d), closer to the focal point, a significant increase in c-Fos positive cells was observed (193 ± 7 vs 282 ± 24 cells, F(1, 6) = 9.138, p = 0.02331). These findings indicate that the biological effects of FUS, particularly in terms of c-Fos upregulation, are not confined to the IL but also affect neighboring regions within the medial prefrontal cortex. Although this suggests that FUS influences adjacent brain areas outside the focal point, the IL located at the focus still exhibits a higher level of c-Fos expression (IL: p < 0.01, prelimbic cortex: p < 0.05).
Discussion
The present study demonstrates that FUS applied to the IL cortex can significantly reduce the reinstatement of MA-seeking behaviors induced by MA priming in CPP tests. However, under the same FUS stimulation conditions, there was no alteration observed in MA-induced locomotor hyperactivity. In this context, it is noteworthy to mention the specific effects of FUS on the IL cortex, as evidenced by our acoustic field measurements and the resulting c-Fos expression. The acoustic field's impact, precisely delineated in the targeted IL region, and the adjacent prelimbic cortex, aligns with the significant increase in c-Fos positive cells observed, particularly in these regions after FUS treatment. This increase in c-Fos expression, a marker of neuronal activation, highlights the targeted and specific effects of FUS stimulation, distinct from its non-effect on locomotor activity.
However, this finding also underscores the broader influence of FUS, extending beyond the IL to neighboring regions within the medial prefrontal cortex, particularly the prelimbic cortex. This finding rules out the possibility that the reduction in MA-seeking behaviors is associated with a decrease in locomotor activity, indicating that FUS stimulation of the IL cortex can precisely modulate craving behavior in response to MA exposure. Consistent with our results, previous research has shown that DBS of the IL cortex attenuates cocaine priming-induced drug-seeking reinstatement [18]. This suggests the potential for noninvasive transcranial FUS to replace the invasive DBS procedure for the treatment of MA use disorder.
It has been reported that increasing neuronal activity in the IL cortex with the glutamate agonist AMPA could suppress the cocaine priming-induced reinstatement of drug-seeking by a cocaine injection in extinguished animals [17]. In our study, we hypothesized an increase in neuronal activity in the IL cortex when subjected to FUS treatment, utilizing a 0.5-MHz frequency, acoustic pressure of 328 kPa, a PRI of 2 ms, and a 20-min sonication duration.
It's crucial to recognize that the effects of ultrasound neuromodulation hinge on the employed stimulation parameters. Five key factors define a sonication protocol: fundamental frequency, pulse repetition frequency (PRF), duty cycle (DC), sonication duration (SD), and intensity [21,31,32]. To reduce risks of tissue heating or damage, pulsed ultrasound is typically employed for neuromodulation. The fundamental frequency, denoting the rate of oscillatory cycles per time unit and inversely related to wavelength, greatly influences the spatial precision of targeting brain areas. Higher frequencies lead to more focused stimulation (above 1 MHz, the focus can narrow down to a few millimeters). However, higher frequencies also mean increased attenuation and scattering when penetrating the skull; hence, frequencies between 200 and 650 kHz are commonly used in human and animal studies. PRF indicates the pulse delivery rate, while DC represents the fraction of each pulse comprising ultrasound cycles. The interplay of these parameters can affect either the inhibition or excitation of cortical neurons.
The studies explored the effects of FUS on drug addiction are limited. It has been reported that applying 2.4-MHz FUS to the nucleus accumbens region for 10 min before administering morphine for additional CPP training prevented the increase in morphine-induced place preference scores in rats that had already developed morphine CPP. However, FUS did not cause a significant reduction of morphine preference [33]. Our current study examined the effects of FUS on reinstatement of MA CPP, which mimics drug relapse, which is the first to reveal the beneficial potential of FUS stimulation in the treatment of drug use disorder.
While this study has demonstrated the feasibility of reducing MA-seeking behaviors using FUS, there are still some limitations to consider. Firstly, the focus on male subjects only is a limitation to the study. Research on sex differences in the effects of MA [34] reveals that female rats exhibit more intense locomotor and stereotypic behaviors after MA administration compared to males, and also show higher MA intake in self-administration models Despite differences in MA intake, male and female rats exhibited similar levels of MA priming and cue-induced reinstatement after extend access to MA intravenous self-administration [35]. Accordingly, male and female rats might also exhibit similar levels of reinstatement in MA CPP. On the other hand, the size of the FUS focal spot is larger than the IL region, which may reduce the precision of neuromodulation. For future efforts to enhance FUS precision, adopting a dual-crossed transducer system could be considered [36]. This system, designed for more accurate ultrasonic targeting, intersects two ultrasound beams to concentrate energy efficiently, potentially reducing stimulated area size and improving targeting precision. Additionally, integrating technologies such as sonogenetics, nanoscale piezoelectric materials, or liposomes could enhance the precision of neural stimulation [[37], [38], [39]]. This includes our team's development of proteins sensitive to 0.5 MHz ultrasound, which has demonstrated potential in precisely stimulating the dopamine neuron regions in mice [38]. Conducting a thorough examination of ultrasound parameters concerning MA-seeking behaviors can also yield advantages.
Our data indicated that the CPP scores in the FUS group had returned to levels similar to those observed in the reinstatement test one week following FUS stimulation, suggesting a transient therapeutic effect of FUS (data not shown). Despite its temporary efficacy, FUS shows promise as a novel, fast-acting option for the treatment of drug addiction in clinical settings. The long-term treatment effects and safety of FUS treatment need to be monitored over time. Finally, the underlying mechanism of how FUS reduces MA-seeking behaviors requires further exploration. It has been implicated that activation of voltage- and calcium-gated potassium channels in the brain potentially benefit the treatment of MA use disorder [32,33]. Given that voltage-gated ion channels are one of the targets for FUS-induced neuromodulation [34,35], it is of importance to explore whether FUS suppresses the reinstatement of MA seeking behavior by altering the characteristics of ion channels in the targeted brain regions.
This study illustrates the potential of FUS as a safe and effective neuromodulatory tool for managing MA use disorder. The parameters of FUS used did not cause significant tissue damage or alter the MA-induced locomotor activity. Significantly, they led to increased c-Fos expression in the IL cortex, reflecting the neuromodulatory capability of FUS. This was effective in reducing the reinstatement of MA-induced CPP. These observations shed the light that FUS might be a safe and effective neuromodulatory tool for addiction management. Further investigations are necessary to elucidate the specific neuromodulatory mechanisms of FUS and gain a comprehensive understanding of the duration and temporal dynamics of its effects.
Author contributions
Each author declares substantial contributions through the following:
(1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, Please indicate for each author the author contributions in the text field below. Signatures are not required.
Chia-Wei Lin: Software, Validation, Formal analysis, Investigation, Writing - Original Draft, Visualization, Project administration.
Min-Hsuan Cheng: Investigation, Software.
Ching-Hsiang Fan: Writing - Review & Editing, Visualization.
Hwei-Hsien Chen: Methodology, Supervision, Conceptualization, Resources, Writing - Review & Editing.
Chih-Kuang Yeh: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The research team extends their heartfelt thanks for the financial assistance provided by the National Science and Technology Council (NSTC) of Taiwan. The support was granted through the projects numbered 110-2221-E-007-019-MY3, 111-2221-E-007-019-MY3, 112-2321-B-002-021, and 112-2636-E-006-007. Further contributions came from National Tsing Hua University (Hsinchu, Taiwan), through grants no. 112Q2713E1 awarded to C.H.F. and C.K.Y. Additionally, the study received funding from National Health Research Institutes through internal grants NP-111-SP-05 and NP-112-SP-08, allocated to H.H.C. The team also wishes to express their appreciation to Mei-Yee Lee and Yung-Ting Hung from the National Health Research Institutes for their invaluable assistance in the immunohistochemical analysis for c-Fos expression.
Contributor Information
Hwei-Hsien Chen, Email: hwei@nhri.org.tw.
Chih-Kuang Yeh, Email: ckyeh@mx.nthu.edu.tw.
References
- 1.Freese T.E., Miotto K., Reback C.J. The effects and consequences of selected club drugs. J Subst Abuse Treat. 2002;23(2):151–156. doi: 10.1016/s0740-5472(02)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Klasser G.D., Epstein J. Methamphetamine and its impact on dental care. J Can Dent Assoc. 2005;71(10):759–762. [PubMed] [Google Scholar]
- 3.Looby A., Earleywine M. The impact of methamphetamine use on subjective well-being in an Internet survey: preliminary findings. Hum Psychopharmacol. 2007;22(3):167–172. doi: 10.1002/hup.831. [DOI] [PubMed] [Google Scholar]
- 4.Cruickshank C.C., Dyer K.R. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104(7):1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman W.F., Moore M., Templin R., McFarland B., Hitzemann R.J., Mitchell S.H. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188(2):162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 6.Kalayasiri R., Verachai V., Gelernter J., Mutirangura A., Malison R.T. Clinical features of methamphetamine-induced paranoia and preliminary genetic association with DBH-1021C-->T in a Thai treatment cohort. Addiction. 2014;109(6):965–976. doi: 10.1111/add.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fattahi M., Eskandari K., Riahi E., Khosrowabadi R., Haghparast A. Distinct suppressing effects of deep brain stimulation in the orbitofrontal cortex on the development, extinction, and reinstatement of methamphetamine-seeking behaviors. Life Sci. 2023;322:121613. doi: 10.1016/j.lfs.2023.121613. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Meng S., Chen W., Chen Y., Huang E., Zhang G., et al. High-frequency deep brain stimulation of the substantia nigra pars reticulata facilitates extinction and prevents reinstatement of methamphetamine-induced conditioned place preference. Front Pharmacol. 2021;12:705813. doi: 10.3389/fphar.2021.705813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X., Ju Y., Jiao D., Zhao M. The effect of repetitive transcranial magnetic stimulation on the reinstatement of methamphetamine-induced conditioned place preference in rats. Shanghai Arch Psychiatry. 2018;30(3):188–198. doi: 10.11919/j.issn.1002-0829.218007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahoney J.J., 3rd, Hanlon C.A., Marshalek P.J., Rezai A.R., Krinke L. Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: review of modalities and implications for treatment. J Neurol Sci. 2020;418:117149. doi: 10.1016/j.jns.2020.117149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowary P., Greenberg B.D. Noninvasive focused ultrasound for neuromodulation: a review. Psychiatr Clin North Am. 2018;41(3):505–514. doi: 10.1016/j.psc.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Rezayat E., Toostani I.G. A review on brain stimulation using low intensity focused ultrasound. Basic Clin Neurosci. 2016;7(3):187–194. doi: 10.15412/J.BCN.03070303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tufail Y., Matyushov A., Baldwin N., Tauchmann M.L., Georges J., Yoshihiro A., et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66(5):681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Folloni D., Verhagen L., Mars R.B., Fouragnan E., Constans C., Aubry J.F., et al. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron. 2019;101(6):1109–1116 e5. doi: 10.1016/j.neuron.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legon W., Sato T.F., Opitz A., Mueller J., Barbour A., Williams A., et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. 2014;17(2):322–329. doi: 10.1038/nn.3620. [DOI] [PubMed] [Google Scholar]
- 16.Legon W., Bansal P., Tyshynsky R., Ai L., Mueller J.K. Transcranial focused ultrasound neuromodulation of the human primary motor cortex. Sci Rep. 2018;8(1):10007. doi: 10.1038/s41598-018-28320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters J., LaLumiere R.T., Kalivas P.W. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guercio L.A., Wimmer M.E., Schmidt H.D., Swinford-Jackson S.E., Pierce R.C., Vassoler F.M. Deep brain stimulation of the infralimbic cortex attenuates cocaine priming-induced reinstatement of drug seeking. Brain Res. 2020;1746:147011. doi: 10.1016/j.brainres.2020.147011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darrow D.P. Focused ultrasound for neuromodulation. Neurotherapeutics. 2019;16(1):88–99. doi: 10.1007/s13311-018-00691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilham S.J., Kiani M. Towards high-resolution ultrasound neuromodulation with crossed-beam phased arrays. IEEE Trans Biomed Circuits Syst. 2023 doi: 10.1109/TBCAS.2023.3285724. [DOI] [PubMed] [Google Scholar]
- 21.King R.L., Brown J.R., Newsome W.T., Pauly K.B. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med Biol. 2013;39(2):312–331. doi: 10.1016/j.ultrasmedbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Kuo C.S., Chai S.C., Chen H.H. Mediodorsal nucleus of the thalamus is critical for the expression of memory of methamphetamine-produced conditioned place preference in rats. Neuroscience. 2011;178:138–146. doi: 10.1016/j.neuroscience.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y.L., Chen S.T., Chan T.Y., Hung T.W., Tao P.L., Liao R.M., et al. Delayed extinction and stronger drug-primed reinstatement of methamphetamine seeking in rats prenatally exposed to morphine. Neurobiol Learn Mem. 2016;128:56–64. doi: 10.1016/j.nlm.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Chao Y.L., Chen H.H., Chen C.H. Effects of repeated electroconvulsive shock on methamphetamine-induced behavioral abnormalities in mice. Brain Stimul. 2012;5(3):393–401. doi: 10.1016/j.brs.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Shankar H., Pagel P.S. Potential adverse ultrasound-related biological effects: a critical review. Anesthesiology. 2011;115(5):1109–1124. doi: 10.1097/ALN.0b013e31822fd1f1. [DOI] [PubMed] [Google Scholar]
- 26.Shen Y., Zhang A., Guo J., Dan G., Chen S., Yu H. Fluorescence imaging of Evans blue extravasation into mouse brain induced by low frequency ultrasound with microbubble. Bio Med Mater Eng. 2014;24(6):2831–2838. doi: 10.3233/BME-141101. [DOI] [PubMed] [Google Scholar]
- 27.Goldim M.P.S., Della Giustina A., Petronilho F. Using Evans blue dye to determine blood-brain barrier integrity in rodents. Curr Protoc Immunol. 2019;126(1):e83. doi: 10.1002/cpim.83. [DOI] [PubMed] [Google Scholar]
- 28.Chiu H.Y., Chan M.H., Lee M.Y., Chen S.T., Zhan Z.Y., Chen H.H. Long-lasting alterations in 5-HT2A receptor after a binge regimen of methamphetamine in mice. Int J Neuropsych. 2014;17(10):1647–1658. doi: 10.1017/S1461145714000455. [DOI] [PubMed] [Google Scholar]
- 29.Lee M.Y., Lin B.F., Chan M.H., Chen H.H. Increased behavioral and neuronal responses to a hallucinogenic drug after adolescent toluene exposure in mice: effects of antipsychotic treatment. Toxicology. 2020;445:152602. doi: 10.1016/j.tox.2020.152602. [DOI] [PubMed] [Google Scholar]
- 30.Legrand M., Galineau L., Novell A., Planchez B., Brizard B., Leman S., et al. 2019. (Efficacy of chronic ultrasound neurostimulation on behaviors and distributed brain metabolism in depressive-like mice). [DOI] [Google Scholar]
- 31.Younan Y., Deffieux T., Larrat B., Fink M., Tanter M., Aubry J.F. Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med Phys. 2013;40(8) doi: 10.1118/1.4812423. [DOI] [PubMed] [Google Scholar]
- 32.Yu K., Niu X., Krook-Magnuson E., He B. Intrinsic functional neuron-type selectivity of transcranial focused ultrasound neuromodulation. Nat Commun. 2021;12(1):2519. doi: 10.1038/s41467-021-22743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deveci E., Kilic A., Yilmaz O., Nabi A., Ergun A.S., Bozkurt A., et al. The effects of focused ultrasound pulsation of nucleus accumbens in opioid-dependent rats. Psych Clinic Psychopharmacol. 2019;29(4):748–759. doi: 10.1080/24750573.2019.1631942. [DOI] [Google Scholar]
- 34.Daiwile A.P., Jayanthi S., Cadet J.L. Sex differences in methamphetamine use disorder perused from pre-clinical and clinical studies: potential therapeutic impacts. Neurosci Biobehav Rev. 2022;137:104674. doi: 10.1016/j.neubiorev.2022.104674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Everett N.A., Baracz S.J., Cornish J.L. The effect of chronic oxytocin treatment during abstinence from methamphetamine self-administration on incubation of craving, reinstatement, and anxiety. Neuropsychopharmacology. 2020;45(4):597–605. doi: 10.1038/s41386-019-0566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S., Jo Y., Kook G., Pasquinelli C., Kim H., Kim K., et al. Transcranial focused ultrasound stimulation with high spatial resolution. Brain Stimul. 2021;14(2):290–300. doi: 10.1016/j.brs.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Qu F., Wang P., Zhang K., Shi Y., Li Y., Li C., et al. Manipulation of Mitophagy by "All-in-One" nanosensitizer augments sonodynamic glioma therapy. Autophagy. 2020;16(8):1413–1435. doi: 10.1080/15548627.2019.1687210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan C.H., Wei K.C., Chiu N.H., Liao E.C., Wang H.C., Wu R.Y., et al. Sonogenetic-based neuromodulation for the amelioration of Parkinson's disease. Nano Lett. 2021;21(14):5967–5976. doi: 10.1021/acs.nanolett.1c00886. [DOI] [PubMed] [Google Scholar]
- 39.Kim T., Kim H.J., Choi W., Lee Y.M., Pyo J.H., Lee J., et al. Deep brain stimulation by blood-brain-barrier-crossing piezoelectric nanoparticles generating current and nitric oxide under focused ultrasound. Nat Biomed Eng. 2023;7(2):149–163. doi: 10.1038/s41551-022-00965-4. [DOI] [PubMed] [Google Scholar]