Abstract
We report results of a large multisite double-blind randomized trial investigating the short and long-term efficacy of repetitive transcranial magnetic stimulation (rTMS) applied to patients with Alzheimer's disease (AD) at mild to moderate stages, in doses of either 2 or 4 weeks of treatment (5 days/week), whilst compared with 4 weeks of sham rTMS. Randomization to treatment group was stratified based on age and severity. The objectives of this study were to: 1) investigate the efficacy of active rTMS versus sham, 2) investigate the effect of dose of treatment (2 or 4 weeks), and 3) investigate the length of benefits from treatment. The rTMS pulses (20 Hz, 30 pulses/train, 25 trains, 10-s intertrain interval) were applied serially to the left and right dorsolateral prefrontal cortex using neuro-navigation. We compared the primary outcome measure's (ADAS-Cog) score changes from pre- to post-treatment, with assessments at baseline and 4 more times up to 6 months post-treatment. Data of 135 patients were analyzed. The mean total ADAS-Cog score at baseline did not differ between the active and sham treatment groups, nor across the three study sites. The overall results show significant cognitive improvement after treatment up to two months post-treatment with either sham or active coils. The results show both short and long-term benefits of active rTMS treatment but also show similar benefits for sham coil treatment of mild/moderate AD. We discuss this finding in the context of the existing literature on rTMS therapy for AD, as well as evidence of the sham coil's potential to induce a low-level current in the brain.
Trial Registration
Keywords: Alzheimer's disease, rTMS efficacy, Dorsolateral prefrontal cortex, Double-blind, Randomized clinical trial
Introduction
The use of repetitive transcranial magnetic stimulation (rTMS) treatment for improving or stabilizing cognition in patients with mild to moderate Alzheimer's disease (AD) has been on the rise in recent years due to some encouraging results from pilot studies. Most of the studies applying rTMS as a treatment for AD have applied the pulses with a high-frequency (10–20 Hz); some have chosen the dorsolateral prefrontal cortex (DLPFC) either only the left side [[1], [2], [3]] or bilaterally [[4], [5], [6]] as the brain area(s) for stimulation, while some applied stimulation to additional sites such as the Broca and Wernicke areas [5], and more recently some chose the precuneus as the area of stimulation [7]. However, the majority of studies were conducted in small sample sizes mainly due to the demanding protocols of rTMS treatment. The maximum sample size reported to date was 109 (out of 130 enrolled), which has been the phase III randomized sham-controlled multisite study of the neuroAD™ system in patients with mild to moderate AD [5].
Although a majority of rTMS studies have reported some positive outcomes on the cognitive status of people with AD, yet there is reason to be cautious in concluding that rTMS represents a breakthrough in the treatment of AD. Forty years of rTMS research has shown that its behavioural effects are influenced by a wide range of factors, including the targeted brain region as well as the frequency, duration and number of treatment sessions. Systematic reviews of a decade of rTMS studies in the context of AD have not yielded consistent conclusions about the optimal stimulation parameters for enhancing cognition. For example, the frequency of the applied pulses is well established as a key parameter influencing physiological and behavioural effects. High-frequency pulses (10–20 Hz) increase cortical excitability and synaptic plasticity through long-term potentiation mechanisms [8]; however, a systematic review paper on the studies on rTMS efficacy for AD treatment did not find any statistically significant differences in outcomes whether using pulse frequencies <10 Hz or >10 Hz [9].
The target stimulation area of the brain is another important variable whose effects are not very well established. Based on knowledge of functional neuroanatomy and the brain regions most affected by AD, previous studies have targeted the precuneus, Broca area, Wernicke's area, the DLPFC, or even combinations of these regions [5,7,[10], [11], [12], [13]]; all of these areas of the brain are known to be impacted in AD. The DLPFC has been the most common site of stimulation due to its important role in executive function of the brain such as decision-making, involvement in coordinating activities of the rest of the brain including storage and retrieval of information and therefore having a role in working memory. Nevertheless, it seems that neither the brain region targeted for stimulation, nor whether the rTMS pulses were delivered with cognitive training, played a significant role in rTMS treatment efficacy [9].
Another important parameter is the duration of the treatment. Most commonly, researchers have used 2–4 weeks of everyday (5 days/week) treatment, while some have applied maintenance treatments (either 1 or 2 sessions/week) up to 6 months [5,14], and another study in a limited sample performed maintenance treatment every 3–7 months with two weeks treatment up to two years after the baseline [4]. Other than the latter study in a very limited sample size (n = 10) [4], no other previous study has followed up with the patients to investigate the duration of rTMS effect. On the other hand, the review paper [9] found a significant correlation between the total number of pulses delivered per protocol and the study effect size. The total number of the pulses obviously is correlated with the duration of the study; however, it is not known whether there is a difference in delivering the same number of pulses over a short period of time or over a longer period of time. A review of most common rTMS parameters, including the number of delivered pulses and their frequency is provided in Ref. [15]. Most studies applied between 1500 and 2000 pulses at either 10 or 20 Hz, and literature suggests little difference in effect.
In the last 5 years, we have been running a large, multisite (Winnipeg and Montreal in Canada and Melbourne in Australia), randomized, placebo-controlled, dose finding double-blind clinical trial investigating the effect of rTMS treatment for improving or stabilizing cognition in patients with mild to moderate AD. The detailed protocol of this current study can be found in Ref. [15]. In this paper, we present the results of the study using data including 156 participants. To the best of our knowledge, this is the largest clinical trial to date using rTMS treatment in AD and the first exploring duration of treatment (dose - 2-weeks vs 4-weeks) efficacy as a variable of interest. In addition, this is the only large study that has followed up measuring the long-term effect of rTMS treatment up to 6 months post-intervention and that can be compared to the expected average changes in cognitive functioning of patients with AD over a period of 6 months previously reported longitudinally in large cohorts [16].
Materials and Methods
Study design and participants
This was a multisite (Winnipeg and Montreal in Canada and Melbourne in Australia), randomized, placebo-controlled, double-blind randomized clinical trial (NCT02908815) for investigating the effect of rTMS treatment for improving or stabilizing cognition in patients in the mild to moderate stage of AD. The details of the protocol are described in Ref. [15]. In brief, the study randomized the participants into three groups to receive one of the two doses of active treatment (either 2 or 4 weeks of 5 days/week) or 4 weeks (5 days/week) with a sham coil wherein 1500 pulses at 20 Hz were delivered in 1.5-s trains with 10-s intertrain intervals each session (each day); the pulses were applied to DLPFC bilaterally left then right sides. The DLPFC was chosen for best comparative purposes as most studies used this location for stimulation, and also for its important role in executive functioning, a cognitive domain that is typically impaired in patients with AD have impairment; for a detailed rationale on the choice of DLPFC, please see Ref. [15].
The sham stimulation was applied with a protocol exactly the same as the active stimulation but with a Magstim Sham coil. The sham coil was also identical to the active coil in terms of produced sound and sensation over the scalp during the application. Inclusion criteria were to have: age >55 years; Montreal Cognitive Assessment (MoCA) score between 7 and 25; a Clinical Dementia Rating (CDR) score of 1–2; a Cornell Scale for Depression in Dementia (CSDD) score of 18 or less to rule out moderate to severe depression; a diagnosis of probable mild or moderate AD as confirmed by the treating neurologist or psychiatrist, and be on a stable dose (or no dose) of an acetylcholinesterase inhibitor for at least 3 months prior to study entry with no plans to change medication for the duration of the study. The diagnosis of AD was made by a neuropsychiatrist or neurologist and involved MRI and/or FDG-PET scans. Additional AD biomarkers (e.g., cerebrospinal fluid (CSF) measures, amyloid or tau PET) were not available. We excluded patients with vascular dementia as their main diagnosis. However, since cerebrovascular symptomology is commonly mixed with AD, especially at older ages, we have calculated the modified Hachinski Ischemic Score (HIS) [17] from the diagnostic reports of the participant and their MRI scans (Table 1). Differential diagnosis of AD and AD with cerebrovascular disease (AD-CVD) is challenging due to overlapping symptomologies. Despite several attempts at AD and AD-CVD differential diagnosis [[18], [19], [20], [21]], still brain autopsy is the only way to confirm the diagnosis. HIS is a common clinical method to identify AD from vascular dementia (VaD) and those with mixed AD and cerebrovascular conditions (AD-CVD). Modified HIS that uses the imaging results too, is a simple clinical tool for differential diagnosis of AD and AD-CVD [20]. A HIS is considered as VaD, while a 4 < HIS < 7 is considered as AD-CVD [22].
Table 1.
Demographic and clinical characteristics (frequencies or means±SDs) of participants in each group and site.
| Site | Group | N, #Males, # Females | Age (years) | CDR | MoCA | Baseline ADAS-Cog | CSDD | HIS (modified version) |
|---|---|---|---|---|---|---|---|---|
| Winnipeg | R2 | 25 (14 M, 11 F) | 72.9 ± 7.6 | 1.08 ± 0.37 | 13.8 ± 5.2 | 25.8 ± 7.1 | 3.5 ± 2.9 | 2.6 ± 1.8 |
| R4 | 24 (15 M, 9 F) | 72.4 ± 7.4 | 1.12 ± 0.34 | 17.0 ± 5.4 | 21.7 ± 8.0 | 3.0 ± 2.7 | 2 ± 2.1 | |
| S4 | 23 (15 M, 8 F) | 73.5 ± 9.7 | 1.09 ± 0.29 | 17.3 ± 4.5 | 21.2 ± 8.0 | 4.2 ± 3.8 | 2.6 ± 1.3 | |
| Montreal | R2 | 15 (7 M, 8 F) | 72.8 ± 11.5 | 1.13 ± 0.35 | 16.1 ± 4.5 | 24.9 ± 10.3 | 5.2 ± 4.0 | 1.1 ± 0.9 |
| R4 | 16 (7 M, 9 F) | 72.4 ± 6.7 | 1.19 ± 0.40 | 13.4 ± 5.4 | 29.9 ± 11.2 | 5.6 ± 2.7 | 1.2 ± 1.5 | |
| S4 | 16 (6 M, 10 F) | 72.9 ± 7.4 | 1.19 ± 0.40 | 16.1 ± 4.4 | 25.1 ± 8.5 | 5.4 ± 3.4 | 1.6 ± 1.3 | |
| Melbourne | R2 | 12 (11 M, 1 F) | 76.9 ± 3.9 | 1.17 ± 0.39 | 15.8 ± 4.1 | 21.4 ± 5.0 | 3.7 ± 2.2 | 2.8 ± 1.6 |
| R4 | 13 (5 M, 8 F) | 76.1 ± 5.9 | 1.23 ± 0.44 | 15.8 ± 5.2 | 24.3 ± 13.0 | 4.4 ± 4.7 | 1.2 ± 1.5 | |
| S4 | 12 (5 M, 7 F) | 80.7 ± 8.2 | 1.17 ± 0.39 | 12.6 ± 3.9 | 24.1 ± 8.5 | 3.4 ± 2.9 | 1.6 ± 1.3 | |
| All sites | R2 | 52 (32 M, 20 F) | 73.8 ± 8.3 | 1.12 ± 0.37 | 14.9 ± 4.8 | 24.4 ± 7.9 | 4.0 ± 3.2 | 2.2 ± 1.7 |
| R4 | 53 (27 M, 26 F) | 73.3 ± 6.9 | 1.17 ± 0.38 | 15.6 ± 5.5 | 24.9 ± 10.8 | 4.1 ± 3.4 | 1.9 ± 1.9 | |
| S4 | 51 (26 M, 25 F) | 75.0 ± 9.1 | 1.14 ± 0.35 | 15.8 ± 4.6 | 23.1 ± 8.3 | 4.4 ± 3.5 | 2.3 ± 1.7 | |
| Total | All | 156 (85 M, 71 F) | 74.0 ± 8.1 | 1.14 ± 0.36 | 15.4 ± 5.0 | 24.2 ± 9.1 | 4.2 ± 3.3 | 2.2 ± 1.8 |
The study was approved by the Ethics board of each site of the study, and all participants and their primary caregiver signed an informed consent form prior to enrollment according to Declaration of Helsinki. Participants were randomized based on their age (less or greater than 70 years) and severity (measured by CDR as 1 or 2) to be in three groups of active 2-weeks (R2), active 4-weeks (R4) or sham 4-weeks (S4) treatment. Participants were not informed that there was no 2-weeks sham group and we assessed all participants at Week 3 (after two weeks of treatment) in order to mask the group assignment. Participants, their caregivers, assessors and those who analyzed data were all blind to treatment group assignment.
Trial procedures
Using the Super Rapid-2 Magstim system (including one air-cooled figure-8 active and one sham coil), the rTMS pulses were administered at 90–100 % intensity of the resting motor threshold (RMT) of each participant as described in Ref. [15]. The DLPFC location was determined using the Brainsight Neuronavigation system software and each patient's MRI scan using Talairach coordinates (x, y, z) = (Left: −50, 30, 36; Right: 50, 30, 36) with the coil held at approximately 45° relative to the horizontal axis [23]. The same targeting procedures were used whether using the active or the sham coil. If a participant missed a treatment session due to any reason (e.g. illness or public holiday), they received double treatment sessions on the following day. Missing more than 10 % of the total treatment sessions was an automatic withdrawal from the study.
Outcome measures
The primary outcome measure was the change in the Alzheimer Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) score from pre- to post-treatment at different times up to 6 months after the baseline. There were several secondary outcome measures as described in Ref. [15]; however, in this paper we present the results of only the main primary (ADAS-Cog) and secondary outcome measures (changes in neuropsychiatric symptoms measured by Neuropsychiatric Inventory–Questionnaire (NPI-Q) and changes in activities of daily living measured by Alzheimer Disease Co-operative Study-Activities of Daily Living Inventory (ADCS-ADL)). We assessed participants within one week prior to the start of intervention (baseline) and at 3, 5, 12, 20, and 28 weeks after the start of the intervention. The assessments on Weeks 3 and 5 were performed within 3–5 days post-treatment, while later follow-up visits (Week 12, 20, 28) were completed within a week of their target date. If it was not possible to schedule an assessment within a week of the ideal date (due to participant's illness or travel), the assessment was skipped and was considered as missing data in the analysis. Also, to reduce practice effect, we used four different versions of ADAS-Cog, which were different in the word recall section, in each of the four sequential assessments. The initial sample size, to have a minimum of 80 % statistical power and a significance level of 0.05 at Week 5 assessment, had been estimated as 208 considering 10 % dropout [15].
Objectives of the study
The objectives of this study were to investigate 1) whether patients in either of the active treatment groups (R2, R4) respond better (shown by a decrease of ≥3 points of ADAS-Cog with respect to baseline or the way it is defined in the “Response Analysis” section) than those in sham group (S4), 2) whether there is a difference between the effects of R2 and R4 treatments, and 3) to assess the duration of treatment effects, if any.
Statistical analysis
The detailed statistical analysis procedures have been described and discussed in Ref. [24]. The required sample size was initially determined to satisfy 5 % level of significance (corrected using the Bonferroni adjustment for three treatment groups) and 80 % power to detect an expected difference of 3 points on the ADAS-Cog score scale (based on a published studies [25,26]) and a standard deviation of 4.9 points (based on our pilot study [4]). Each participating patient was randomized to either the sham or active treatment group, using stratified block randomization with a block size of 3. The chi-square test was used to test the homogeneity of proportions over all enrolled patients, between the proportion of females and males between the sites, or between the treatment groups.
We tested the primary outcome measure (ADAS-Cog) for any significant differences between the three study sites at baseline, with the intention of combining their data, if appropriate, when comparing the treatment groups using both descriptive analysis and formal statistical tests. Baseline comparisons were done using parametric analysis of variance (ANOVA) or its non-parametric equivalent in case assumptions of normality and homogeneity of variances were not satisfied. Post-hoc analysis was also conducted after the ANOVA test. All important assumptions such as normality and equal variance were statistically tested, and remedial measures were taken in case of assumption violations. All tests are based on 5 % level of significance.
Then, we performed a complete analysis of the ADAS-Cog data. Logarithmic transformation of the scores was used to adjust for outliers and any departure from normality assumptions. A mixed effects model for longitudinal data was used to test for the effects of Group (R2, R4, Sham), Time and the interaction of Group and Time using the changes in ADAS-Cog scores from baseline to post-treatment assessments.
We conducted a repeated measure ANOVA to compare the changes of log(ADAS-Cog) scores (the effect of treatment) over assessment weeks. Then, we used a multivariate ANOVA (MANOVA) to compare the three groups within each study site. Finally, the three sites were compared based on the ADAS-Cog scores obtained in all assessments using a MANOVA approach. If there were any missing assessments, they were imputed from the preceding week.
Response rate analysis
While the ADAS-Cog score change with respect to baseline is the primary outcome measure of this study, we also considered a term called “Response Rate” using the following criteria that were derived based on similar literature monitoring improvement/decline in AD patients undergoing pharmaceutical treatments using similar outcome measures [25,[27], [28], [29], [30]]. We defined a “Response Rate” based on ADAS-Cog score along with caregiver scores on the patient's symptoms and severity and functionality [24].
The “Response Rate” of the participants was defined as the following: a “Marked Positive Response” if their ADAS-Cog score has ≥3 point improvement compared to baseline at either Week5 or Week8 assessment sessions. A “Moderate Positive Response” if the ADAS-Cog score has a non-significant improvement (<3 points) in ADAS-Cog AND improvement or no change in ADCS-ADL OR NPI-Q at either Week5 or Week8. If the AND part does not hold, then it is considered as a “Small/Stabilized Response”. Likewise, if there is a non-substantial decline in ADAS-Cog (<3 points) AND there is an improvement in both NPI-Q and ADCS-ADL at either Week 5 or Week8, it is considered a “Small/Stabilized Response”; otherwise if the AND part does not hold OR the ADAS-Cog score shows a substantial increase (i.e., worsening performance), it is considered as “Non-Responsive”. All the AND/OR are logical notions. It worth noting that ADAS-Cog change with respect to baseline still plays the dominant role in the definition of the Response Rate. The main difference of this response rate definition compared to simply using ≥3 points reduction of ADAS-cog score (respect to baseline) as a response rate is for patients with a marginal ADAS-Cog change with respect to baseline.
Next, we investigated the effects of treatment groups (R2, R4 and Sham) given the site of the study and time of assessment on responders (the combined groups of Marked, Moderate and Small Responders) versus non-responders as defined above. We also analyzed the effect of Time on the response to treatment (the difference between assessments).
Data availability
The de-identified performance data along with the corresponding information file about the data, will be made available to the public within one year after the study is fully finished. The data will be shared with researchers upon a signed data access agreement on the FTP server of the study PI.
Results
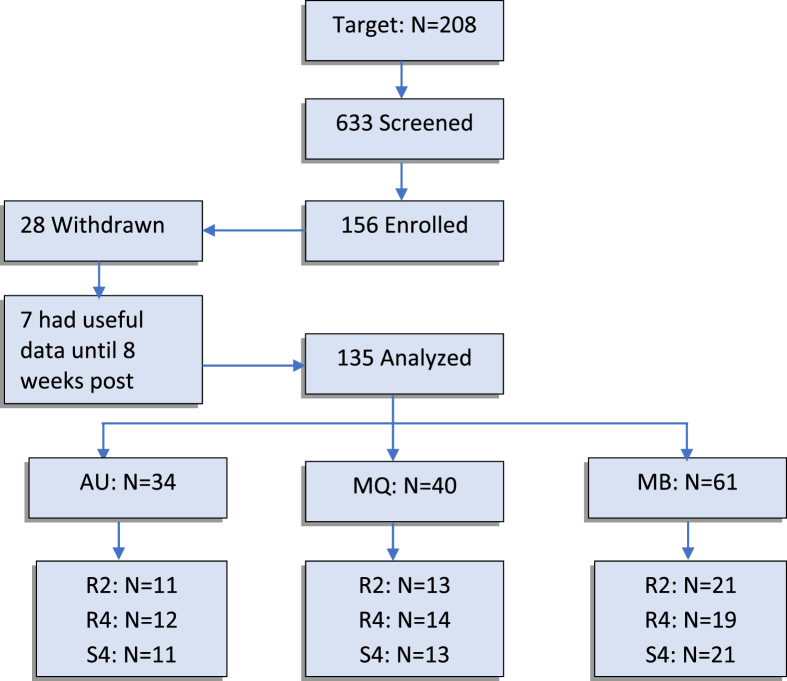
With a target number of 208 participants based on our initial sample size estimation [15], 633 individuals were screened, of whom 156 were enrolled over a period of 5 years, of which two years included the COVID19 pandemic. The study was ceased at that point following the conduct of the analysis presented in this paper. Twenty-eight participants withdrew; however, seven of them had data up to 8 weeks post-treatment. Thus, a total of 135 (156 − 28 + 7) participants’ data were analyzed in this study (Fig. 1). Table 1 shows the demographics of the participants in different groups and sites for all enrolled patients.
Fig. 1.
Flowchart of the study groups and number in each group and site.
Overall, the rate of withdrawal/drop-out from the study (18 %) was higher than the 10 % expected rate; however, a number of participants withdrew due to COVID-19 pandemic and lab closure in the Australia site. The rTMS treatment, overall, was found tolerable without any serious adverse effects although two participants were withdrawn by the PI for safety concerns of a health issue that could be related to rTMS treatment (one participant had felt numbness in left arm and left side of their face for a couple of minutes at night after the 3rd treatment and the other felt chest pain after rTMS that continued for a few days; thus, the site PI withdrew the participant although later was found the heart was fine and the issue was probably not related to rTMS application). Table 2 shows the participants’ withdrawal reasons. As can be seen in Table 2, only two participants withdrew because they found the rTMS pulses too painful. The rest of the participants found the treatment sessions well tolerated. We asked our participants before and after each rTMS treatment session about any adverse effect (either related or even non-related). All those adverse effects that were all considered as minor (except in two participants that the PI withdrew from the study). A quantitative analysis of those minor adverse effects is being submitted as a separate manuscript. Overall, a general pain including headache, jaw pain, toothache and twitching were the most common ones which were diminished as soon as the treatment session was over; a few patients reported a mild headache up to a couple of hours after the sessions. Dizziness feeling was the second most common reported adverse effect. Overall, 82 % of the participants in active treatment and 79 % of the participants in sham treatment experienced at least one minor adverse effect. There was no significant difference between the frequency and severity of the adverse effects between the active and sham groups. Furthermore, and interestingly, there was no significant correlation between the stimulation intensity (RMT) and the normalized frequency of a participant experiencing an adverse effect in active treatment group, while there was a positive significant correlation of those in sham treatment group.
Table 2.
Participants’ withdrawal reasons and their distribution among the sites and the treatment groups; AU, MQ and MB refer to Australia (Melbourne), Quebec (Montreal) and Manitoba (Winnipeg) sites; R2, R4 and S4 refer to 2 and 4 weeks active and 4 weeks sham treatment groups.
| Withdrawal reasons | Site/Group, # |
||
|---|---|---|---|
| AU | MQ | MB | |
| Finding treatment painful and/or exhausting | 1 R2 | 1 R4 | |
| Other unrelated illnesses | 1 R2, 1 S4 | 1 R2 | |
| Missing several treatments and/or assessments due to weather, commuting issues, etc. | 1 S4 | 1 R2, 1 R4 | |
| Caregiver felt treatment is unproductive | 1 S4 | 1 R4, 1 S4 | |
| Caregiver sickness | 1 S4 | ||
| Patient being non-compliant (too much movement under the treatment); PI decision to withdraw | 1 R2 | ||
| Patient felt too anxious before starting the treatment | 1 R4 | 2 R4 | |
| Patient changed medication during the treatment | 1 R4 | 1 R2 | |
| Plausible side effect, PI decision to withdraw for safety (MQ patient felt brain fog and chest pain after one session of treatment; MB patient felt numbness in the left side of face and left arm for a few minutes after the second treatment at night) | 1 R4 | 1 R2 | |
| Due to pandemic and labs' closure | 1 R2, 2 R4, 1 S4 | ||
| Patient and carer became unresponsive to contact | 1 S4 | 1 R4, 1 S4 | |
| Patient became ineligible by postponing the starting date | 1 R2 | ||
| Total (count, %) | 9 (24 %) | 8 (17 %) | 11 (15 %) |
The majority (86.7 %) of participants received the rTMS pulses at 100 % of their respective RMT in their treatment sessions. For some patients who found the 100 % intensity painful, we started the first few trains (1–5 trains) of pulses of the two first treatment sessions at 90 % intensity and gradually increased the intensity to 100 % when they got used to it. Only 13.3 % (n = 18) of participants received the pulses at 90 % in majority of their treatment sessions.
Overall, six participants out of 135 missed 10 treatment sessions in total. Three participants in Manitoba and two in Australia sites missed their last 1–3 treatment sessions that were not compensated for the next day because due to COVID illness; as it happened during the last days of treatment block, it could not be rescheduled due to the isolation requirements for COVID. Their immediate post-treatment assessments were also performed online; overall 59 assessments of 25 participants were performed online (we analyzed all in-person and online assessments together). The other participant missed only one treatment session that was compensated on the following day.
Using chi-square test of homogeneity of proportions over all enrolled patients, there was no significant difference between the proportion of females and males between the sites ( or between the treatment groups ). Also, there was no significant difference between the proportion of females and males between the treatment groups in Montreal and Winnipeg sites and , respectively). However, there was a significant difference in Melbourne site : the proportion in group R2 of Melbourne site was different from other groups. Note that sex was not a parameter for stratified randomization.
To compare the ages of participants between the sites and treatment groups, we used the ANOVA test. The mean ages of participants between the groups (R2, R4, and S4) were not significantly different as age was a randomization parameter for group assignment. The sites and different treatment groups were found not significantly different in terms of baseline ADAS-Cog scores, which means we could combine data of the different sites for comparison between the treatment groups.
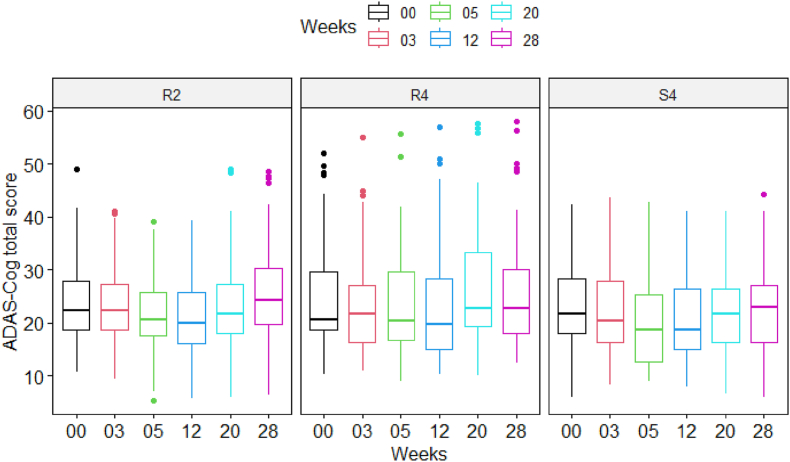
Fig. 2 shows the boxplot of the ADAS-Cog total scores averaged amongst participants (N = 135 total analyzed) of each group at different assessment times. As can be seen, each group's participants on average had an improvement in ADAS-Cog total scores (i.e., lower scores) after the treatment. This improvement lasted for two months post-treatment and then gradually returned to baseline values after 6 months. However, there were no differences in this trend between active and sham groups or the 2 dose groups.
Fig. 2.
Boxplot of the ADAS-Cog total score of participants at baseline and a given time point in different participant treatment groups.
Analysis of mixed effect models for comparing treatment and time interval effects
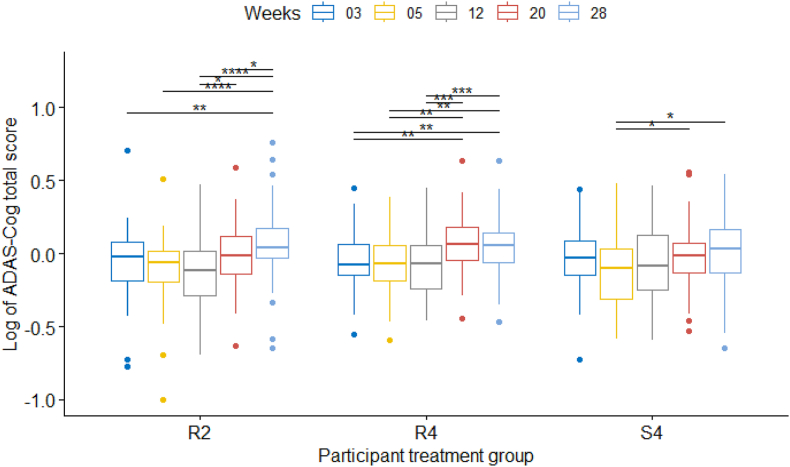
A mixed effect model for repeated measures was used to investigate the effect of treatment (the R2, R4, S4 groups), time intervals (weeks) and the interaction of treatment and time on the difference between log(ADAS-Cog) at baseline and a given time point in all sites together and each site separately. The results of the mixed effects model on all participants and all sites are shown in Table 3. The results showed that the ADAS-Cog scores of different weeks were significantly different but not different for different treatment groups, congruent with the results mentioned above. Fig. 3 shows post-hoc paired comparison tests of the difference in scores of different time periods (weeks) in each site using the pairwise t-test. As can be seen, again the change of log(ADAS-Cog) score at any point of assessment time compared to baseline is not significantly different between the three treatment groups. There were some significant improvements with respect to baseline in some weeks post-treatment in all 3 groups (decreases and increases in mean scores) but no clear pattern of difference between the 3 groups (Fig. 3).
Table 3.
Results of comparing the time (weeks) and treatment groups in all sites.
| # of DF | Den. DF | F-value | P-value | |
|---|---|---|---|---|
| Weeks | 4 | 528 | 21.40 | <0.00001 |
| Groups (R2, R4, S4) | 2 | 132 | 0.63 | 0.53 |
| Weeks∗Groups | 8 | 528 | 1.59 | 0.13 |
Fig. 3.
Boxplot of the difference between the log(ADAS-Cog) of participants at baseline and a given time point in different participant treatment groups with their statistical comparisons.
Comparisons based on the response rate
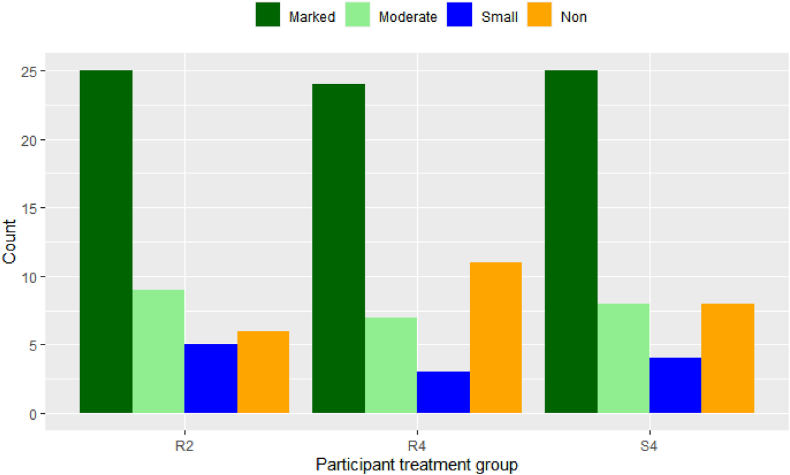
Table 4 shows the distribution of different responses across the sites and in total, averaged for the two active groups combined versus sham group. Using Chi-square test on the equality of different response proportions in different treatment groups, we observed that the Marked, Moderate, Small and Non-responders were not equal in all treatment groups, nor in any individual group, but the proportions were homogeneous between the sites. In all groups, “Marked Responses” were more frequent than moderate and small responses (Fig. 4). All the statistical analyses done on ADAS-Cog (and its log) changes as the primary outcome generated similar results when considering the “Response Rate” as defined in the Statistical Analysis section instead of the ADAS-Cog score changes with respect to baseline. This was expected since ADAS-Cog score plays a major role in the definition of the Response Rate as well and since the “Marked” Response (of which the ADAS-Cog score is the main determinant) was more frequent than the other types of responses.
Table 4.
Distribution of the response rates (n = 135) across the sites for the active and sham groups; active group includes both R2 and R4 groups.
| Marked/Moderate | Mild/Stabilized | Non-responsive/declined | ||
|---|---|---|---|---|
| Active rTMS | Winnipeg (n = 40) | 72.5 % | 15.0 % | 12.5 % |
| Montreal (n = 27) | 77.8 % | 0.0 % | 22.2 % | |
| Melbourne (n = 23) | 65.2 % | 8.7 % | 26.1 % | |
| All sites (n = 90) | 72.2 % | 8.9 % | 18.9 % | |
| Sham rTMS | Winnipeg (n = 21) | 81.0 % | 9.5 % | 9.5 % |
| Montreal (n = 13) | 61.5 % | 7.7 % | 30.8 % | |
| Melbourne (n = 11) | 72.7 % | 9.1 % | 18.2 % | |
| All sites (n = 45) | 73.3 % | 8.9 % | 17.8 % | |
Bolded rows show the distribution across all sites.
Fig. 4.
Frequency of the different response types in each group of R2, R4 and S4 across all sites.
Next, we partitioned the responders into two groups of Positive- and Non-Responders, and repeated the analyses. The results showed the proportion of the Positive Responders was similar across the three Groups (R2, R4 and S4) and much higher than the non-responders. It shows there were improvements across repeated assessments, but in all active and sham groups similarly; that can easily be observed by the distribution of the responses shown in Table 4.
Discussion
In this paper, we present the results of a randomized double-blind placebo-controlled rTMS treatment study applied to AD patients with two doses of active treatments versus sham; participants were followed for a period of 6 months post-baseline. To the best of our knowledge, this study is the largest of its kind to date, with a sample size of 156 participants and the longest follow-up duration (6 months) post-treatment investigating the short and long-term effect of rTMS as a treatment for AD. Overall, our outcomes indicate that high frequency rTMS treatment applied to bilateral DLPFC does not yield greater cognitive improvement than the “sham” group either immediately post-intervention or in the longer term. On average, the two active treatment groups and the sham group show similar cognitive outcomes across the assessment times up to 6 months follow-up. In addition, and interestingly, on average all groups showed no decline compared to baseline at follow-up assessment. We briefly compare these results to those reported for other clinical trials of rTMS in AD, then move on to the present evidence in support of a potential explanation for the improvement in cognitive status seen in sham-treated participants.
Our results are contrary to some recently reported findings on the effect of active rTMS treatment on AD. A recent study with a relatively high number of participants (a total of 109 in a randomized grouping) [5] reports statistically significant benefit of rTMS treatment applied to DLPFC and to Broca and Wernicke areas when paired with simultaneous cognitive exercises for those who received active treatment versus sham (cognitive exercises were also given during sham). The benefit of active treatment was not seen immediately after the intervention but at 5 weeks post-treatment. In a separate study [14], 25 AD patients randomized to receive active rTMS over the precuneus area showed better cognitive performance after treatment than the 25 participants in sham intervention. In both studies, cognitive performance of the patients in the active group remained stable during the period of the intervention, while cognitive performance in the sham group declined, and the difference between the two groups at post-intervention was statistically significant. Our study on 156 (135 analyzed) participants randomized into active and sham groups yielded very different results, i.e., improvements were seen in the sham and the active treatment groups, not only immediately post-intervention but also at the 6 months follow-up assessment.
We attempted to control for practice effect by using alternate versions of the ADAS-Cog in which different word lists were presented for memory testing; however, the remaining test items are consistent across assessments and it is conceivable that growing familiarity with the assessment procedures in general, in particular from baseline through Week 5, may have led to better test outcomes, even in patients with a limited capacity to remember from one session to the next. Some studies have claimed the degree of practice effect of standard cognitive tests, in particular episodic memory tests, can be used as a biomarker for predicting Alzheimer's development amongst older adults with mild cognitive impairment [31]. However, the research on ADAS-Cog's practice effect amongst patients with Alzheimer's without any intervention with large enough samples is limited, and is a topic in need of further research. It should also be noted that ADAS-Cog score and almost all other neuropsychological tests can be affected by the mood of the patient at the time of assessment. There are several confounding variables, such as any incident that the patient might have on the day of assessment, the quality of sleep in the night before, any illness, etc. that can affect the assessment's score. To overcome these confounding variables, there is a need for a very large sample size of patients to be observed over a period of time with frequent assessments across fixed intervals and also not having any intervention including placebo over the period of observation.
In examining the literature on rTMS treatment efficacy in AD, we note that many research publications do not specify exactly when the assessments were done. The timing of post-intervention assessment has been reported as “after the last rTMS session” [6], “immediately after” [32], the week of assessment [5] or simply “post-treatment” [7]. Distinguishing between “immediately after” as in 5 min later on the same day, and three days later (as we did in this study), is crucial to separating out the potential acute effects of rTMS from the cumulative effects of several weeks of intervention. Future clinical trials should specify clearly the time elapsed between the last rTMS pulse and the assessment of cognitive function.
Notwithstanding important differences in the brain regions targeted with rTMS in our studies and those that yielded more positive outcomes, the fact that our sham participants on average did not decline even at 6 months post-intervention is intriguing. This led us to consider the possibility of a potentiating effect of the sham coil, which was intended to be an inactive control condition. The present study used the Magstim sham coil, as have the majority of trials of rTMS in Alzheimer's disease. Experiments have shown [20,21] that significant perpendicular magnetic fields are produced by this coil, a finding that we have now replicated in our own laboratory (see Supplementary Materials). As suggested by Ref. [33], the induced significant electric field can produce low-field effects that affect neuromodulation. The study in Ref. [34] showed the weak electric fields from the sham rTMS coil can modify the cortical excitability of the neurons. Therefore, it is possible that the induced weak electrical fields of the sham coil perhaps have an effect similar to that of transcranial alternating electrical stimulation (TACS), even though TACS is given in a continuous mode and rTMS fields are induced within the duration of the given train of pulses. Our protocol had 10-s intertrain intervals between each train of rTMS pulses given at 20 Hz. It is feasible that the induced electric currents in the brain may have had a similar effect to TACS at 20 Hz.
Another support for a possible sham coil effect, as discussed above, is from the analysis of the adverse effects collected in two active and sham arms of the study. As mentioned in results section, there was no significant difference in the type and frequency of the reported adverse effects between and sham and active groups. Interestingly, the correlation between the adverse effects and the rTMS pulses’ intensity was significant in the sham group but not in active group. This intriguing outcome indirectly supports a potential benefit from the fact that the sham coil also generates a magnetic field and it spreads over the scalp; the higher the intensity, the more the feeling of the pulses over the scalp and hence plausible adverse effect such as headache.
To test the hypothesis of a neuromodulatory effect of the sham coil, a future study would need to include: 1) a control condition with no direct neuromodulatory effect that was equivalent to a sham coil condition in terms of time commitment and attention/care provided to participants; 2) a control condition involving a control stimulation site (i.e., stimulation of a brain area that is theoretically unlikely to affect brain systems involved in AD); or 3) a way of quantifying the physiological effect of active treatment and sham condition on brain activity in the DLPFC.
Earlier studies such as [1,4] used a different technique for sham stimulation; they used a 2-cm thick wooden piece with the shape of the same coil placed under the active coil. The wooden spacer beneath the active coil effectively reduces the penetration of the TMS pulses into the brain significantly while not changing the sound of the pulses. However, the wooden piece under the active coil significantly reduces the scalp sensation of the pulses and that can potentially unblind the participant. On the other hand, the authors of the study in Ref. [4] argued that, since the participants had memory impairment, and there were wash-out periods between the two blocks of active and sham treatment, none of the 10 participants reported feeling any difference. The results of the study in Ref. [4] showed the sham group's cognitive status stayed the same with respect to baseline, while the active group showed improvement.
While our results indicate that the active groups’ positive responses (improvements) were similar to that of the sham group, it does not necessarily mean that active rTMS treatment should not be used as a treatment for any AD patients. One plausible explanation for our results could be that the improvement is all due to improvement in depression. However, this explanation is unlikely because the majority of our participants were not depressed at baseline (see CSDD scores on Table 1). Out of the 135 patients, only 13 had a CSDD score of 10 or higher that may suggest a mild level of depression (see Table 5), and participants with higher levels of depression were excluded by design. The majority of participants with mild depressive symptoms (58.3 %) were from the Montreal site, which showed better results for the Active group versus Sham for R2 group. Nevertheless, these numbers are small and represent less than 10 % of the entire subset of patients who showed improvement in the course of this study. Further, in the absence of a post-intervention measure of depression we cannot test the hypothesis that a reduction in depressive symptoms underlies the observed cognitive improvement.
Table 5.
Distribution of the 13 patients with major depression (CSDD ≥ 10) across the sites and groups, their response rate as Marked, Moderate, Small or Non-responsive.
| Site | Group | # (Response rate) |
|---|---|---|
| Winnipeg | R2 | 2 (1 marked, 1 moderate) |
| R4 | 0 | |
| S4 | 2 (1 marked, 1 small) | |
| Montreal | R2 | 4 (3 marked, 1 non) |
| R4 | 1 (moderate) | |
| S4 | 2 (1 marked, 1 moderate) | |
| Melbourne | R2 | 0 |
| R4 | 1 (marked) | |
| S4 | 1 (marked) |
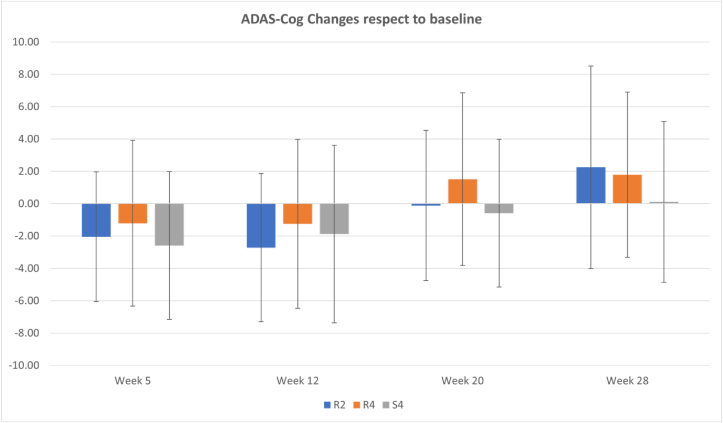
One may attribute the improvement in both active and sham groups to a “care effect” of the interaction due to attending the treatment (either active or sham) but that is unlikely as such a care effect in our experience (pilot studies) usually does not last more than a month after the interaction (treatment) time is over. We see the improvement lasted more than 2 months in the majority (about 68 %) of patients of active and sham groups and some even up to 6 months post-treatment (see Fig. 5).
Fig. 5.
ADAS-Cog changes (μ ± σ) with respect to baseline at assessment Weeks of 5, 12, 20 and 28 after the baseline for each group of the study across all sites. Negative and positive values imply improvement and decline, respectively (the lower ADAS-Cog, the better cognitive status).
Our data show about 10 % of patients either in active or sham groups cognitively declined after rTMS treatment; although the amount of decline measured by ADAS-Cog score changes with respect to baseline is higher in the active group compared to sham group (average values of 2.6 and 1.8 for R2 and R4, respectively, compared to −0.11 for S4), the numbers are too small to draw any meaningful conclusion.
A recent study on a subset of our data who also went through an exploratory assessment, called Electrovestibulography (EVestG), showed a high (76 %) accuracy in predicting at baseline whether a participant improves, declines or has no change after an active rTMS treatment [35]. The feature that was most different between responders and non-responders was a lower frequency (efferent) modulation of the vestibular afferent firing pattern for responders, and this was hypothesized to be related to GABAergic changes [35]. Unfortunately, the number of EVestG study participants who received sham rTMS treatment was too small to draw any conclusion on sham rTMS’ efficacy at baseline.
The results of our randomized clinical trial demonstrate that active rTMS is not superior to sham rTMS coil (Magstim coil and Magstim Sham coil used in this study) when following the stimulation protocol used herein. Our protocol was designed to match those most commonly used for studies in AD at the outset of this trial. The results do not preclude that rTMS may be found effective in treating AD when using different stimulation parameters or targeting other brain regions or using a sham coil that does not induce any neuromodulation (if it exists). Moreover, given the importance to society of finding novel interventions for AD, further studies should seek to identify patient characteristics that best predict response to interventions using brain stimulation.
Limitations of the study
This study despite, being carefully designed, is not free of limitations. One limitation is that the assessor personnel changed over the 5 years of the study. With training videos, regular video calls to discuss the assessment procedures, and regular site visits, we made every effort to ensure consistency in administration of interventions and assessments; however, turnover of personnel could possibly affect the scoring. Second, our experiments on the magnetic fields induced by the sham coil raise concern that the use of this coil may have reduced the ability to detect differences between the control and active treatment conditions. Third, due to the COVID19 pandemic, we had to run some assessments online. While we developed a procedure for doing so as close as possible to in-person assessments, it remains plausible that online assessment might have had an effect on the outcomes for those participants. We will study if that indeed had an impact on the outcomes in the near future. Finally, important differences between our study and recent work by others demonstrating efficacy of rTMS in AD include the target site and the absence of a cognitive training intervention. The current study was designed to provide a definitive estimate of the efficacy of rTMS in a protocol that has been used most commonly in the literature, i.e., stand-alone treatment with rTMS over the DLPFC. It is important to recognize these methodological differences, as they preclude generalization of our null results to the wider range of rTMS protocols that have yet to be fully explored for treating AD.
This study for the first time examined the efficacy of rTMS for treatment of cognitive impairment in AD in a randomized double-blind placebo-controlled study up to 6 months post-treatment. In summary, the results of our analysis of 135 participants in three groups, active treatment in two doses of 2 and 4 weeks versus sham, indicate 1) an overall significant improvement for all groups after treatment, and 2) no difference between the sham and active groups even at any point up to 6 months after the baseline. Since this result lasted till 6 months post-intervention, it is unlikely to be entirely due to placebo effects. We hypothesize that the sham TMS coil may have had a therapeutic effect, akin to a modified tACS. While active coil has shown positive therapeutic effect for the majority (>68 %) of patients, there was continued decline or no effect for other patients. We believe the future of rTMS application as a treatment for AD should be further explored with “true” sham coils.
Ethics approval and consent to participate
Each site of the study received the Ethics approval from their respective university's Ethics Board prior to starting the study. All studies were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki, and all participants gave written consent prior to the experiments. All participants (and their caregiver in cases where the caregiver was a legal representative acting on behalf of the participant) read and signed an informed consent form prior to participate in the study.
Consent for publication
All participants (and their caregiver in cases where the caregiver was a legal representative acting on behalf of the participant) read and signed an informed consent form to publish the outcomes of the study.
Availability of data and materials
The de-identified performance data along with the corresponding information file about the data, will be made available to the public within one year after the study is fully finished. The data will be shared with researchers upon a signed data access agreement on the FTP server of the study PI.
Authors contributions
Conceptualization: all authors
Methodology: ZM, PF, BL, CM, XW, BM, LF, LK
Drafting the original and revised version of manuscript: ZM
Contributing to the writing of manuscript: ZM, BL, MU, CM, XW, BM, PF, LK
Commenting for the discussion: BL, CM, XK, BM, CO, LF, PF, LK
Data Analysis: GR, CS and MU
Supplementary Analysis: MU and GR
Funding and Ethics Acquisition: ZM, PF, and LK (ZM was the PI and PF and LK were the main site-PI in Melbourne and Montreal, respectively.)
All authors approved the submitted revision.
Funding
This study was funded by Weston Brain Institute. PBF co-author is supported by a National Health and Medical Research Council of Australia Investigator grant (1193596).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Zahra Moussavi reports financial support was provided by Weston Brain Institute. Paul B Fitzgerald reports a relationship with National Health and Medical Research Council of Australia Investigator grant (1193596) that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The PI wishes to acknowledge the generous donation of the Puchniak family to help with the travel expense of out-of-town participants in Manitoba site.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurot.2024.e00331.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cotelli M., Manenti R., Cappa S.F., Zanetti O., Miniussi C. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry. 2010;82:794–797. doi: 10.1136/jnnp.2009.197848. [DOI] [PubMed] [Google Scholar]
- 2.Li X., Qi G., Yu C., Lia G., Zheng H., Wu S., et al. Cortical plasticity is correlated with cognitive improvement in Alzheimer’s disease patients after rTMS treatment. Brain Stimul. 2021;14(3):503–510. doi: 10.1016/j.brs.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhang F., Qin Y., Xie L., Zheng C., Huang X., Zhang M. High-frequency repetitive transcranial magnetic stimulation combined with cognitive training improves cognitive function and cortical metabolic ratios in Alzheimer’s disease. J Neural Transm. 2019;126(8):1081–1094. doi: 10.1007/s00702-019-02022-y. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford G., Lithgow B., Moussavi Z. Short and long-term effects of rTMS treatment on Alzheimer's disease at different stages: a pilot study. J Exp Neurosci. 2015;9:43–51. doi: 10.4137/JEN.S24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabbagh M., Sadowsky C., Tousi B., Agronin M.E., Alva G., Armon C., et al. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimer’s Dementia. 2019;16(4):641–650. doi: 10.1016/j.jalz.2019.08.197. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed M.A., Darwish E.S., Khedr E.M., El Serogy Y.M., Ali A.M. Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. J Neurol. 2012;259(1):83–92. doi: 10.1007/s00415-011-6128-4. [DOI] [PubMed] [Google Scholar]
- 7.Koch G., Casula E.P., Bonnì S., Borghi I., Assogna M., Minei M., et al. Precuneus magnetic stimulation for Alzheimer’s disease: a randomized, sham-controlled trial. Brain. 2022;145(11):3776–3786. doi: 10.1093/brain/awac285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406(6792):147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 9.Menardi A., Dotti L., Ambrosini E., Vallesi A. Transcranial magnetic stimulation treatment in Alzheimer’s disease: a meta-analysis of its efficacy as a function of protocol characteristics and degree of personalization. J Neurol. 2022;269(10):5283–5301. doi: 10.1007/s00415-022-11236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliasova I., Anderkova L., Marecek R., Rektorova I. Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer’s disease: a pilot study. J Neurol Sci. 2014;346(1–2):318–322. doi: 10.1016/j.jns.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Lee J., Choi B.H., Oh E., Sohn E.H., Lee A.Y. Treatment of Alzheimer’s disease with repetitive transcranial magnetic stimulation combined with cognitive training: a prospective, randomized, double-blind, placebo-controlled study. J Clin Neurol. 2016;12:57–64. doi: 10.3988/jcn.2016.12.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen J.P., Suarez A., Kemoun G., Meignier M., Le Saout E., Damier P., et al. Repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer’s disease. Neurophysiol Clin. 2017;47(47–53) doi: 10.1016/j.neucli.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Rabey J.M., Dobronevsky E., Aichenbaum S., Gonen O., Marton R.G., Khaigrekht M. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. J Neural Transm. 2013;120:813–819. doi: 10.1007/s00702-012-0902-z. [DOI] [PubMed] [Google Scholar]
- 14.Koch G., Bonni S., Pellicciari M.C., Casula E.P., Mancini M., Esposito R., et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. NeuroImage. 2018;169:302–311. doi: 10.1016/j.neuroimage.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 15.Moussavi Z., Rutherford G., Lithgow B., Millikin C., Modirrousta M., Mansouri B., et al. Repeated transcranial magnetic stimulation for improving cognition in patients with Alzheimer disease: protocol for a randomized, double-blind, placebo-controlled trial. JMIR Res Protoc. 2021;10(1) doi: 10.2196/25144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podhorna J., Krahnke T., Michael S., Harrison J.E. Alzheimer’s disease assessment scale–cognitive subscale variants in mild cognitive impairment and mild Alzheimer’s disease: change over time and the effect of enrichment strategies. Alzheimer’s Res Ther. 2016;8(1):8. doi: 10.1186/s13195-016-0170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hachinski V.C., Iliff L.D., Zilhka E., Du Boulay G.H., McAllister V.L., Marshall J., et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32(9):632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 18.Di Lazzaro V., Bella R., Benussi A., Bologna M., Borroni B., Capone F., et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin Neurophysiol. 2021;132(10):2568–2607. doi: 10.1016/j.clinph.2021.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Cantone M., Di Pino G., Capone F., Piombo M., Chiarello D., Cheeran B., et al. The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin Neurophysiol. 2014;125(8):1509–1532. doi: 10.1016/j.clinph.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Lithgow B.J., Dastgheib Z., Anssari N., Mansouri B., Blakley B., Ashiri M., et al. Physiological separation of Alzheimer’s disease and Alzheimer’s disease with significant levels of cerebrovascular symptomology and healthy controls. Med Biol Eng Comput. 2021;59(7):1597–1610. doi: 10.1007/s11517-021-02409-8. [DOI] [PubMed] [Google Scholar]
- 21.Dastgheib Z.A., Lithgow B.J., Moussavi Z.K. An unbiased algorithm for objective separation of Alzheimer's, Alzheimer's mixed with cerebrovascular symptomology, and healthy controls from one another using electrovestibulography (EVestG) Med Biol Eng Comput. 2022;60(3):797–810. doi: 10.1007/s11517-022-02507-1. [DOI] [PubMed] [Google Scholar]
- 22.Moroney J.T., Bagiella E., Desmond D.W., Hachinski V.C., Mölsä P.K., Gustafson L., et al. Meta-analysis of the Hachinski Ischemic score in pathologically verified dementias. Neurology. 1997;49(4):1096–1105. doi: 10.1212/wnl.49.4.1096. [DOI] [PubMed] [Google Scholar]
- 23.Rusjan P.M., Barr M.S., Farzan F., Arenovich T., Maller J.J., Fitzgerald P.B., et al. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp. 2010;31(11):1643–1652. doi: 10.1002/hbm.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moussavi Z., Koski L., Fitzgerald P.B., Millikin C., Lithgow B., Jafari-Jozani M., et al. Repeated transcranial magnetic stimulation for improving cognition in Alzheimer disease: protocol for an interim analysis of a randomized controlled trial. JMIR Res Protoc. 2021;10(8) doi: 10.2196/31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrag A., Schott J.M. What is the clinically relevant change on the ADAS-Cog? J Neurol Neurosurg Psychiatry. 2012;83(2):171–173. doi: 10.1136/jnnp-2011-300881. [DOI] [PubMed] [Google Scholar]
- 26.Lansdall C.J., McDougall F., Butler L.M., Delmar P., Pross N., Qin S., et al. Establishing clinically meaningful change on outcome assessments frequently used in trials of mild cognitive impairment due to Alzheimer’s disease. J Prev Alzheimers Dis. 2023;10(1):9–18. doi: 10.14283/jpad.2022.102. [DOI] [PubMed] [Google Scholar]
- 27.Burns A., Yeates A., Akintade L., Del Valle M., Zhang R.Y., Schwam E.M., et al. Defining treatment response to Donepezil in Alzheimer’s disease. Drugs Aging. 2008;25(8):707–714. doi: 10.2165/00002512-200825080-00007. [DOI] [PubMed] [Google Scholar]
- 28.Lanctot K.L., Herrmann N., Yau K.K., Khan L.R., Liu B.A., LouLou M.M., et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. Can Med Assoc J. 2003;169(6):557–564. [PMC free article] [PubMed] [Google Scholar]
- 29.Cook R.J., Sackett D.L. The number needed to treat: a clinical useful measure of treatment effect. BMJ. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posch M., Bauer P. Interim analysis and sample size reassessment. Biometrics. 2000;56(4):1170–1176. doi: 10.1111/j.0006-341x.2000.01170.x. [DOI] [PubMed] [Google Scholar]
- 31.Hassenstab J., Ruvolo D., Jasielec M., Xiong C.X., Grant E., Morris J.C. Absence of practice effects in preclinical Alzheimer’s disease. Neuropsychology. 2015;29(6):940–948. doi: 10.1037/neu0000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saitoh Y., Hosomi K., Mano T., Takeya Y., Tagami S., Mori N., et al. Randomized, sham-controlled, clinical trial of repetitive transcranial magnetic stimulation for patients with Alzheimer’s dementia in Japan. Front Aging Neurosci. 2022:14. doi: 10.3389/fnagi.2022.993306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith J.E., Peterchev A.V. Electric field measurement of two commercial active/sham coils for transcranial magnetic stimulation. J Neural Eng. 2018;15(5) doi: 10.1088/1741-2552/aace89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opitz A., Legon W., Mueller J., Barbour A., Paulus W., Tyler W.J. Is sham cTBS real cTBS? The effect on EEG dynamics. Front Hum Neurosci. 2014;8:1043. doi: 10.3389/fnhum.2014.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lithgow B.J., Dastgheib Z., Moussavi Z. Baseline Prediction of rTMS efficacy in Alzheimer patients. Psychiatr Res. 2022;308 doi: 10.1016/j.psychres.2021.114348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The de-identified performance data along with the corresponding information file about the data, will be made available to the public within one year after the study is fully finished. The data will be shared with researchers upon a signed data access agreement on the FTP server of the study PI.
The de-identified performance data along with the corresponding information file about the data, will be made available to the public within one year after the study is fully finished. The data will be shared with researchers upon a signed data access agreement on the FTP server of the study PI.