Abstract
Background
In the absence of prognostic biomarkers, most patients with early-stage triple-negative breast cancer (eTNBC) are treated with combination chemotherapy. The identification of biomarkers to select patients for whom treatment de-escalation or escalation could be considered remains an unmet need. We evaluated the prognostic value of histopathologic traits in a unique cohort of young, (neo)adjuvant chemotherapy-naïve patients with early-stage (stage I or II), node-negative TNBC and long-term follow-up, in relation to stromal tumor-infiltrating lymphocytes (sTILs) for which the prognostic value was recently reported.
Materials and methods
We studied all 485 patients with node-negative eTNBC from the population-based PARADIGM cohort which selected women aged <40 years diagnosed between 1989 and 2000. None of the patients had received (neo)adjuvant chemotherapy according to standard practice at the time. Associations between histopathologic traits and breast cancer-specific survival (BCSS) were analyzed with Cox proportional hazard models.
Results
With a median follow-up of 20.0 years, an independent prognostic value for BCSS was observed for lymphovascular invasion (LVI) [adjusted (adj.) hazard ratio (HR) 2.35, 95% confidence interval (CI) 1.49-3.69], fibrotic focus (adj. HR 1.61, 95% CI 1.09-2.37) and sTILs (per 10% increment adj. HR 0.75, 95% CI 0.69-0.82). In the sTILs <30% subgroup, the presence of LVI resulted in a higher cumulative incidence of breast cancer death (at 20 years, 58%; 95% CI 41% to 72%) compared with when LVI was absent (at 20 years, 32%; 95% CI 26% to 39%). In the ≥75% sTILs subgroup, the presence of LVI might be associated with poor survival (HR 11.45, 95% CI 0.71-182.36, two deaths). We confirm the lack of prognostic value of androgen receptor expression and human epidermal growth factor receptor 2 -low status.
Conclusions
sTILs, LVI and fibrotic focus provide independent prognostic information in young women with node-negative eTNBC. Our results are of importance for the selection of patients for de-escalation and escalation trials.
Key words: triple-negative breast cancer, prognostic biomarkers, lymphovascular invasion, fibrotic focus, stromal tumor-infiltrating lymphocytes
Highlights
-
•
Oncologists treat most eTNBC patients with chemotherapy due to a lack of implemented prognostic and predictive biomarkers.
-
•
We studied a cohort of systemic treatment-naïve young women with node-negative eTNBC with 20 years of median follow-up.
-
•
LVI (HR 2.35), fibrotic focus (HR 1.61) and sTILs (HR 0.75 per 10% increment) had independent prognostic value for BCSS.
-
•
The presence of LVI and sTILs <30% identified patients with an ultra-high risk of recurrence or death.
-
•
De-escalation trials should consider the exclusion of patients when LVI is present.
Introduction
Triple-negative breast cancer (TNBC) represents about 15% of breast cancers (BCs) and is more prevalent in younger women.1,2 TNBCs are known for their aggressive behavior, with frequent early relapses and poor survival once metastasized.3,4 Currently, almost all patients with early-stage TNBC (eTNBC) are treated with three or four chemotherapeutic agents with or without anti-programmed cell death protein 1 therapy due to a lack of implemented prognostic and predictive biomarkers. The only exception to forego chemotherapy applies to low-risk TNBC, which includes T1a-b node-negative TNBCs and special histological subtypes such as adenoid cystic, secretory carcinoma or low-grade metaplastic carcinoma.5, 6, 7 Extensive efforts have been undertaken to evaluate other prognostic biomarkers8, 9, 10, 11; however, many findings were confounded by (neo)adjuvant chemotherapy or lacked long-term survival outcomes, and so far, only stromal tumor-infiltrating lymphocytes (sTILs) show promise to change clinical practice.
sTILs have emerged as a robust and important prognostic biomarker in TNBC.12, 13, 14 In addition, sTILs show potential to identify patients with an excellent prognosis without chemotherapy,15,16 which has opened the door for clinical trials evaluating treatment de-escalation strategies based on sTILs. It is unknown, however, what the contribution of other histopathologic tumor characteristics is, on top of sTILs, in the risk stratification of young patients with eTNBC. Moreover, the majority (52%-71%) of node-negative eTNBC have low (<30%) sTILs and represents a heterogeneous group of tumors considering the wide range of associated overall survival (OS) (at 10 years, OS estimates of 60%-85%).14, 15, 16, 17, 18 Especially in this subgroup, there is an urgent need for additional clinically valuable biomarkers to more accurately predict the risk of recurrence or death.
Recently, the treatment landscape of eTNBC has changed dramatically with the Food and Drug Administration and European Medicines Agency approval of neoadjuvant pembrolizumab added to chemotherapy for high-risk eTNBC19 and the poly (ADP-ribose) polymerase inhibitor olaparib for BRCA1/2-mutated TNBC.20,21 Given the impressive efficacy of antibody–drug conjugates (ADCs) such as sacituzumab govitecan and trastuzumab deruxtecan for metastatic TNBC,22,23 the incorporation of these agents in the early setting is expected. With the advent of clinical trials evaluating promising treatment options in eTNBC, there is a growing need to stratify patients based on baseline risk, which underlines the importance to identify prognostic biomarkers in addition to standard clinicopathological factors.
In the present study, we evaluated the prognostic value of histopathologic tumor characteristics independent of sTILs in a unique cohort of (neo)adjuvant systemic treatment-naïve, stage I or II TNBC patients younger than 40 years with 20 years of follow-up from the nationwide and population-based PARADIGM cohort,24 in which previously sTILs showed strong prognostic value for survival.15 In addition, we explored whether the prognostic impacts of these tumor characteristics are similar in a cohort of older chemotherapy-naïve patients with TNBC. Our findings may aid in the identification of patients with an ultra-high risk of recurrence or death, thus most in need of systemic treatment. Moreover, our data are of importance for future clinical trials that evaluate (neo)adjuvant treatment (de-)escalation strategies in eTNBC.
Materials and methods
Patient selection
For the current study, we selected all patients with eTNBC (stage I or II) (hereafter referred to as the young cohort) from the PARADIGM cohort.15 The retrospective PARADIGM cohort study included all women younger than 40 years diagnosed with a node-negative primary invasive BC in the Netherlands between 1989 and 2000 and prospectively registered in the Netherlands Cancer Registry. Women had no prior malignancies and tumor tissue was available.24 All patients were (neo)adjuvant systemic treatment-naïve. Clinicopathologic information and long-term follow-up data were collected from individual hospital records and through linkage with the municipality population register. Detailed information about the cohort, data collection and follow-up has been published previously.15,24
In addition, for exploratory analyses, we studied older patients with node-negative TNBC (hereafter referred to as the old cohort) that did not receive (neo)adjuvant chemotherapy and with long-term follow-up. Patients were derived from the prospective multicenter Dutch IKA trial (1982-1994), diagnosed with a T1-4N0-3M0 primary BC between 50 and 76 years of age,25 for which sufficient formalin-fixed paraffin-embedded (FFPE) tumor tissue could be recollected.26,27 The central ethics committee of the Netherlands Cancer Institute approved this trial and all patients gave informed consent. The trial was carried out in accordance with the Declaration of Helsinki.
The Institutional Review Board of the Netherlands Cancer Institute approved the use of archival tissue from included patients for the current retrospective translational study. This study complied with reporting recommendations for tumor-marker prognostic studies (REMARK) criteria.28
Histopathology and sTILs evaluation
For both cohorts, dedicated breast pathologists revised tumor characteristics on hematoxylin and eosin (H&E)-stained whole slides of surgical resection specimens according to international guidelines and blinded to clinical data, as described previously.15,24 In brief, TILs were reported for the stromal compartment as a percentage of TILs between 0% and 100% (in bins of 10%), and grouped into low (<30%), intermediate (30% to <75%) and high (≥75%) level.29 Lymphovascular invasion (LVI) was categorized as present when tumor cells were seen within a definite endothelial-lined space (either lymphatic or vascular) within the peritumoral tissue.30 No immunohistochemical (IHC) stainings for endothelial markers were used. Presence of a fibrotic focus, a scar-like area in the center of a carcinoma consisting of fibroblasts and collagen with few tumor cells, was assessed according to the criteria from Hasebe et al.31 The size in mm of the largest fibrotic focus present was assessed, with a minimum size of 0 mm. The presence of pushing borders was scored as <25%, 25%-75% or >75% of the tumor perimeter.32 Central necrosis was scored as a percentage between 0% and 100% (in bins of 10%), and considered present when >0%. Mitotic count (≤7, 8-12 or ≥13 mitoses per 2 mm2), glandular/tubular differentiation (<10%, 10%-75% or >75% of tumor area) and nuclear pleomorphism (minimal, moderate or marked variation in size and shape of the nuclei) were scored separately, after which tumor grade was derived according to the modified Bloom and Richardson scoring system.33
Immunohistochemistry
Tissue microarrays (TMAs) were constructed using three 0.6 mm cores/patient obtained from FFPE tumor blocks. TMA slides were IHC stained and scored for estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2).15,27,34 We considered tumors as triple-negative when the ER and PR receptor stained positive in <10% of tumor cells and HER2 was not overexpressed [IHC 0/1+ or IHC 2+ and in situ hybridization (ISH) not amplified]. Tumors with an ER expression of 1%-9% were considered ER low-positive, while tumors with HER2 IHC1+ or IHC2+ and negative ISH were considered HER2-low.35 In addition, tumors were evaluated by IHC for androgen receptor (AR) expression. A more detailed description of all IHC stainings and scoring methods is provided in the Supplementary Methods, available at https://doi.org/10.1016/j.esmoop.2024.102923.
Tumor BRCA1 mutation status
For the young cohort, tumor BRCA1 status was previously obtained.15 In brief, tumor DNA was extracted from FFPE tumor tissue and sequenced using an Illumina NextSeq (Illumina, San Diego, CA). Samples were considered tumor BRCA1-mutated (harboring a class 4/class 5 pathogenic variant, tBRCA1m) or tumor BRCA1 wild-type (all other samples, tBRCA1wt).
Statistical analysis
Patient and tumor characteristics were summarized using descriptive statistics and compared between groups using Fisher’s exact, chi-square or linear-by-linear tests for categorical variables, and Mann–Whitney U tests for continuous variables. In addition, associations between a tumor characteristic and age group were assessed using logistic regressions, unadjusted and adjusted for T-stage, grade and histology.
Survival endpoints were breast cancer-specific survival (BCSS), recurrence-free interval (RFI) and OS defined according to STEEP2.0 criteria.36 Follow-up started at TNBC diagnosis and ended at the occurrence of an event, at 20 years after TNBC diagnosis, or at the date of a second primary tumor, whichever occurred first. Since cause of death was unknown for the patients from the young cohort, death was considered to be related to the primary TNBC when a patient without a second primary tumor died within 5 years after diagnosis or following a distant recurrence. Median follow-up was calculated using the reverse Kaplan–Meier method. For every tumor characteristic of interest, univariable and multivariable Cox proportional hazards models with follow-up as the time scale were used to determine its effect on the endpoints, i.e. their prognostic value. Separate multivariable models were built for each covariate and tumor characteristic of interest. All models were adjusted for covariates that had P < 0.05 in univariable models. Additionally, other covariates were added if adjustment for it resulted in >10% change in the hazard ratio (HR) for the variable of interest. The prognostic value of each covariate and tumor characteristic of interest was estimated separately for both cohorts but also for a pooled cohort. In addition, the prognostic value of each trait was compared between the cohorts using an interaction term between the covariate of interest and the age status. The proportional hazards assumption was checked and fulfilled using Schoenfeld residuals and by testing interactions between variables and time. Furthermore, a BCSS tree was built to evaluate whether a combination of different traits aids in the risk stratification of young patients. More information about the methodology can be found in the Supplementary Methods, available at https://doi.org/10.1016/j.esmoop.2024.102923.
Sensitivity analyses for BCSS were carried out using death not related to BC as a competing event. Subdistribution HRs of patient and tumor characteristics were estimated with the Fine and Gray model. Cumulative incidences of death from any cause, death due to BC and causes other than BC were estimated separately by age group using a non-parametric approach.37
Statistical analyses were carried out based on a predefined statistical analyses plan and using SPSS version 27, Stata version 16 and R software version 4.1.1 with R package ‘rpart’.38 All P values were two-sided and considered statistically significant if they were <0.05. No adjustments for multiple testing were made.
Results
Patient and tumor characteristics
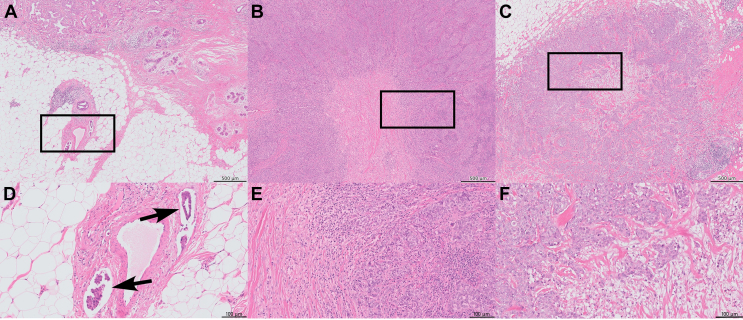
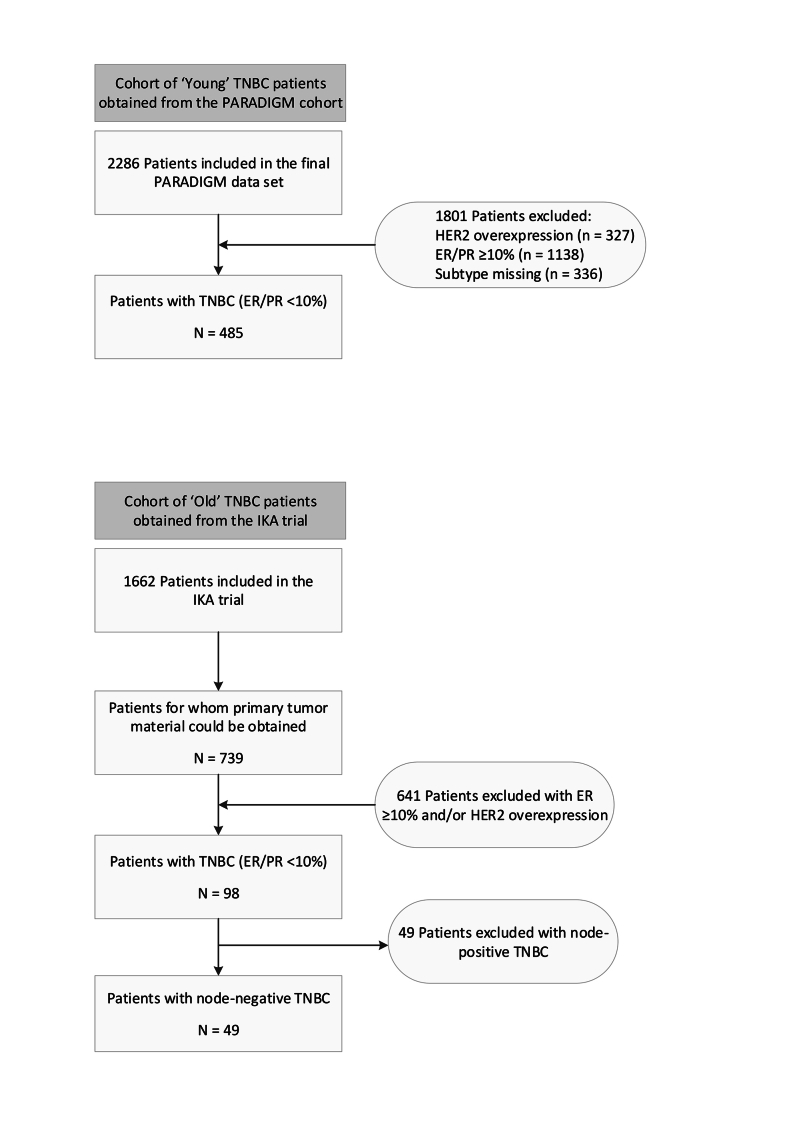
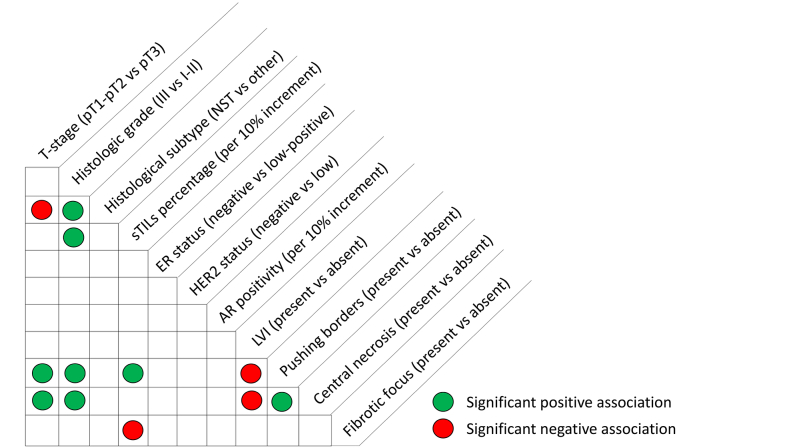
The final PARADIGM cohort contained 2286 patients younger than 40 years with node-negative BC, of which 327 had HER2 overexpression, 1138 had ER/PR ≥10%, 485 were considered triple-negative and for 336 BCs the subtype was missing, as has been described before.15 For the current study, we included all 485 patients with eTNBC from this PARADIGM cohort (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.102923). Patient and tumor characteristics of the young cohort with TNBC are summarized in Table 1. Most tumors were pT1-pT2 (58.8% and 38.8%, respectively), of histologic grade 3 (85.6%) and classified as carcinoma of no special type (NST, 91.8%). ER low-positive, HER2-low and AR-positive tumors represented 4.9%, 9.1% and 16.5% of tumors, respectively, and 23.5% were tBRCA1m. LVI and fibrotic focus were present in 11.5% and 28.7% of tumors, respectively. The associations between the evaluated histopathologic tumor characteristics are summarized in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.102923. The status of LVI was not significantly different among the sTILs categories (<30%, 30%-75%, ≥75%) (P = 0.12), whereas tumors with fibrotic focus had significantly lower levels of sTILs (P < 0.01). The presence of pushing borders was associated with significantly higher levels of sTILs (P < 0.01). Representative images of LVI, fibrotic focus and sTILs are shown in Figure 1.
Table 1.
Patient and tumor characteristics of young patients with node-negative triple-negative breast cancer
| Characteristic | All patients |
|---|---|
| N (%) | |
| Total | 485 (100) |
| Age at diagnosis, median years (IQR) | 35 (32-38) |
| T-stage, n (%) | |
| pT1 | 285 (58.8) |
| pT2 | 188 (38.8) |
| pT3 | 10 (2.1) |
| Unknown | 2 (0.4) |
| Histologic grade, n (%) | |
| 1-2 | 70 (14.4) |
| 3 | 415 (85.6) |
| Histological subtype, n (%) | |
| Carcinoma NST | 445 (91.8) |
| Metaplastic carcinoma | 27 (5.6) |
| Othera | 13 (2.7) |
| sTILs percentage, median (IQR) | 25 (5-70) |
| sTILs percentage | |
| <30% | 247 (50.9) |
| 30%-75% | 127 (26.2) |
| ≥75% | 107 (22.1) |
| Unknown | 4 (0.8) |
| ER status | |
| ER-negative (0%) | 461 (95.1) |
| ER low-positive (1%-9%) | 24 (4.9) |
| HER2 statusb | |
| HER2-negative | 441 (90.9) |
| HER2-low | 44 (9.1) |
| AR status | |
| Negative (0%) | 367 (75.7) |
| Positive (≥1%) | 80 (16.5) |
| Unknown | 38 (7.8) |
| LVI | |
| Absent | 429 (88.5) |
| Present | 56 (11.5) |
| Pushing borders | |
| Absent | 186 (38.4) |
| <25%c | 56 (11.5) |
| 25%-75%c | 78 (16.1) |
| ≥75%c | 152 (31.3) |
| Unknown | 13 (2.7) |
| Central necrosis | |
| Absent | 234 (48.2) |
| Present | 251 (51.8) |
| Fibrotic focus | |
| Absent | 346 (71.3) |
| Present | 139 (28.7) |
| Tumor BRCA1 status | |
| Wild-type | 303 (62.5) |
| Mutated | 114 (23.5) |
| Unknown | 68 (14.0) |
| Local treatment, n (%) | |
| Lumpectomy | 324 (66.8) |
| Mastectomy | 152 (31.3) |
| Surgery not specified | 9 (1.9) |
| Radiotherapy | |
| No | 141 (29.1) |
| Yes | 344 (70.9) |
AR, androgen receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IQR, interquartile range; LVI, lymphovascular invasion; NST, no special type; sTILs, stromal tumor-infiltrating lymphocytes.
Includes: adenoid cystic carcinoma, apocrine carcinoma, invasive cribriform carcinoma, ductolobular carcinoma, invasive papillary carcinoma, invasive lobular carcinoma and invasive micropapillary carcinoma.
HER2-low is defined as HER2 IHC1+ or IHC2+ with negative in situ hybridization.
Of tumor perimeter.
Figure 1.
LVI, fibrotic focus and sTILs in triple-negative breast cancer. Whole slide images of H&E-stained triple-negative breast cancer representative for (A) the presence of LVI, (B) fibrotic focus with >75% sTILs and (C) fibrotic focus with <30% sTILs. (D) A detailed region of panel A with LVI, (E) of panel B with a sTILs score of >75% and (F) of panel C with a sTILs score of <30%. Scale bar A, B and C: 500 μm. Scale bar D, E and F: 100 μm. H&E, hematoxylin and eosin; LVI, lymphovascular invasion; sTILs, stromal tumor-infiltrating lymphocytes.
Association of histopathological traits with breast cancer-specific survival
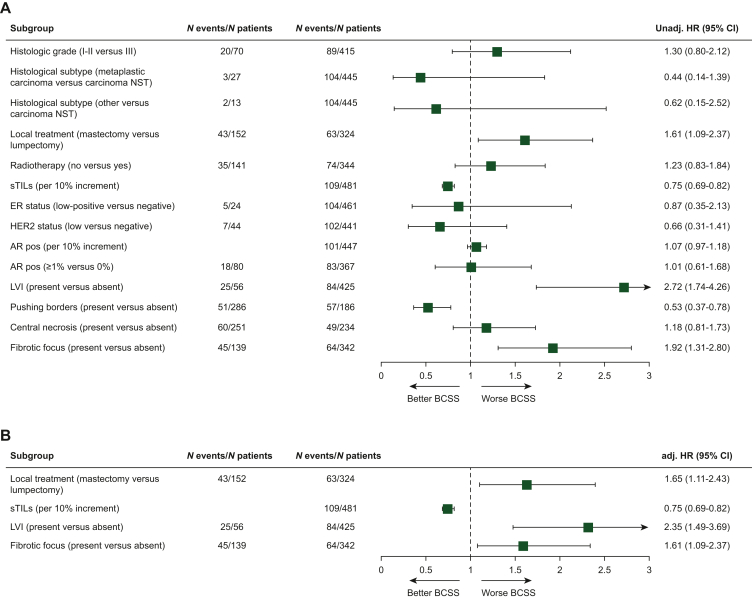
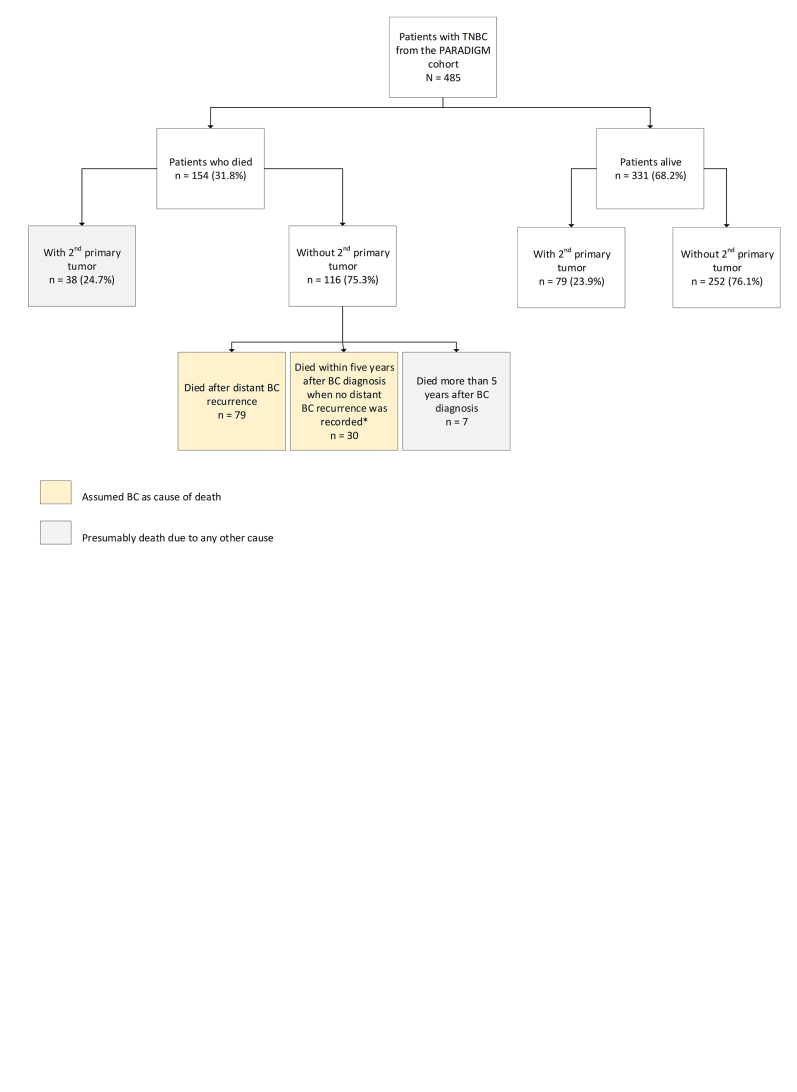
During a median follow-up of 20.0 years, 154/485 (31.8%) patients died. Seventy-one percent of deaths were considered BC related (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.102923). Local treatment, sTILs and LVI showed significant unadjusted effects on BCSS and were used for adjustment of effects of all tumor characteristics of interest, whereas histologic grade and T-stage were not significantly associated with BCSS (Figure 2, Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.102923). sTILs had a strong prognostic impact in this cohort, as previously shown.15 Interestingly, here we show that LVI and fibrotic focus were significantly associated with BCSS, independently of sTILs. The presence of LVI [adjusted hazard ratio (adj. HR) 2.35, 95% confidence interval (CI) 1.49-3.69] and fibrotic focus (adj. HR 1.61, 95% CI 1.09-2.37) resulted in a significantly increased risk of death due to BC compared to tumors without these characteristics, respectively (Figure 2, Supplementary Table S1 and S2, available at https://doi.org/10.1016/j.esmoop.2024.102923). In patients with TNBC considered tBRCA1m (n = 114), a more pronounced effect was observed for LVI (adj. HR 3.73, 95% CI 1.64-8.51) (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.102923). In contrast, no significant association with BCSS was observed for AR, pushing borders and central necrosis. Importantly, ER low-positive, HER2-low and histologic grade also lacked prognostic value for BCSS. Similar findings were reported in sensitivity analyses using multivariable competing risk analyses for BCSS with non-BC-related death as a competing event, and when using RFI and OS as survival endpoint (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.102923).
Figure 2.
Unadjusted and adjusted HRs for BCSS among young patients with node-negative triple-negative breast cancer by tumor characteristics. (A) Plot of unadjusted hazard ratios (unadj. HR) and (B) adjusted hazard ratios (adj. HR) for BCSS comparing young (<40 years) patients with a specific tumor characteristic (depicted in the figure) versus those without that specific characteristic. For every tumor characteristic separately a (A) univariable and (B) multivariable Cox proportional hazards model was built. In the multivariable models, HRs were adjusted for local treatment (lumpectomy, mastectomy, surgery not specified), sTILs (per 10% increment) and LVI (absent, present), unless otherwise specified. Models for sTILs and LVI as the covariate of interest were adjusted for local treatment (lumpectomy, mastectomy, surgery not specified) and LVI (absent, present) or sTILs (per 10% increment), respectively. T-stage (1/2, 3) and grade (1/2, 3) were not detected as prognostic or confounding variables and therefore not included in the multivariable models. The group with the highest number of events was used as the reference group. sTILs and AR positive were used as continuous variables with HR showing change in risk per 10% increment. Patients with unknown values were omitted. The square shows unadj./ adj. HR and its size reflects the number of patients included, while whiskers represent the 95% CI. AR, androgen receptor; BCSS, breast cancer-specific survival; CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; NST, carcinoma of no special type; sTILs, stromal tumor-infiltrating lymphocytes.
Patient risk stratification
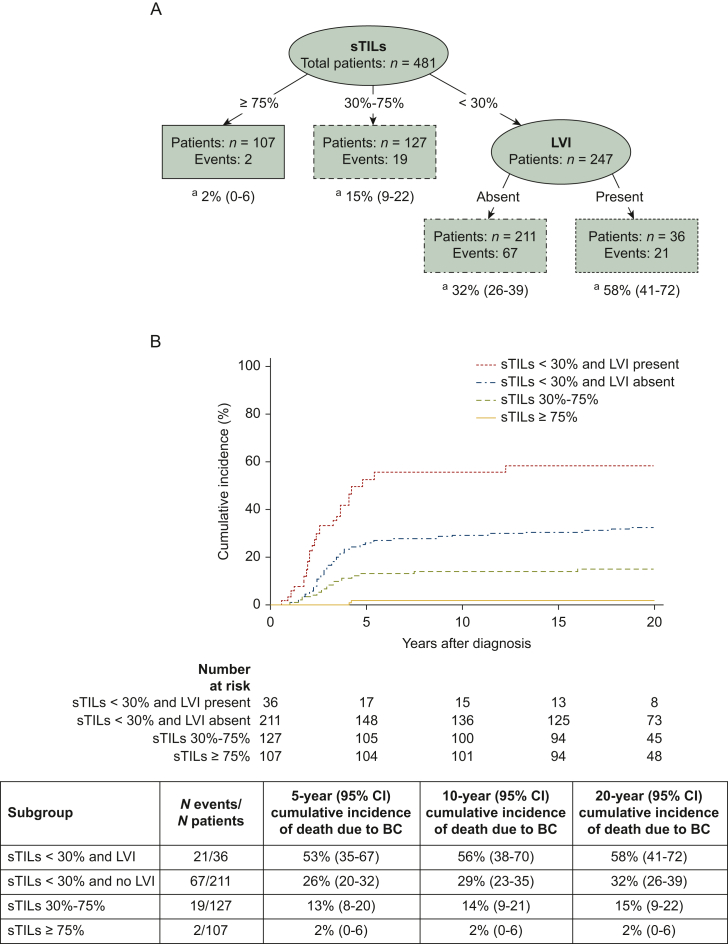
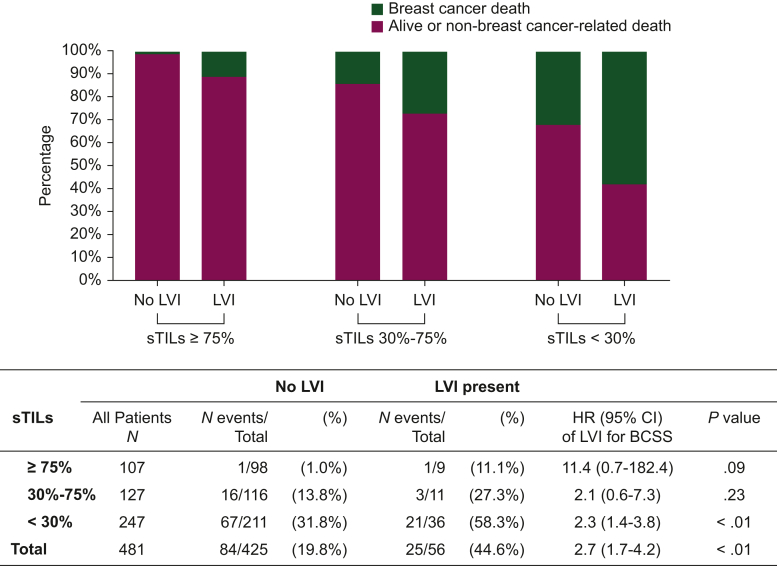
Survival tree analysis identified sTILs (≥75%, 30%-75%, <30%) and LVI (absent, present) as the most important predictors for BCSS, with an associated Brier score of 0.14, i.e. a survival tree with a good accuracy (Figure 3). It appeared that LVI has additional value in the risk stratification of patients with low sTIL tumors (<30%); the presence of LVI resulted in a 58% 20-year cumulative incidence of death due to BC versus 32% when LVI was absent. Note that the presence of LVI appeared also associated with poor survival in the subgroups with 30%-75% sTILs (HR 2.12, 95% CI 0.62-7.29, 19 deaths) and ≥75% sTILs (HR 11.45, 95% CI 0.71-182.36, 2 deaths), although the CIs were wide and associations were not significant due to the small number of deaths in these groups (Figure 4).
Figure 3.
Risk stratification of young patients with node-negative triple-negative BC for death due to BC by survival tree analysis. Survival tree model partitioning of 481 patients with node-negative triple-negative BC (for four patients, no sTILs score was available). (A) The resulting tree with four terminal groups was split by sTILs and LVI. a20-year cumulative incidences for death due to BC. (B) Corresponding cumulative incidence functions of death due to BC for selected groups based on survival tree analysis. BC, breast cancer; CI, confidence interval; LVI, lymphovascular invasion; sTILs, stromal tumor-infiltrating lymphocytes.
Figure 4.
Breastcancer deaths among patients stratified by LVI and sTILs. The effects of LVI (presence versus absence of LVI) on breast cancer-specific death in the different sTILs categories were estimated with a Cox proportional hazard model that included an interaction term between LVI and sTILs. The total effect of LVI was estimated with a univariable Cox proportional hazard model. Patients with missing information on sTILs (n = 4) were excluded from analyses. BCSS, breast cancer-specific survival; CI, confidence interval; HR, hazard ratio; LVI, lymphovascular invasion; sTILs, stromal tumor-infiltrating lymphocytes.
Exploratory: prognostic value within old patients
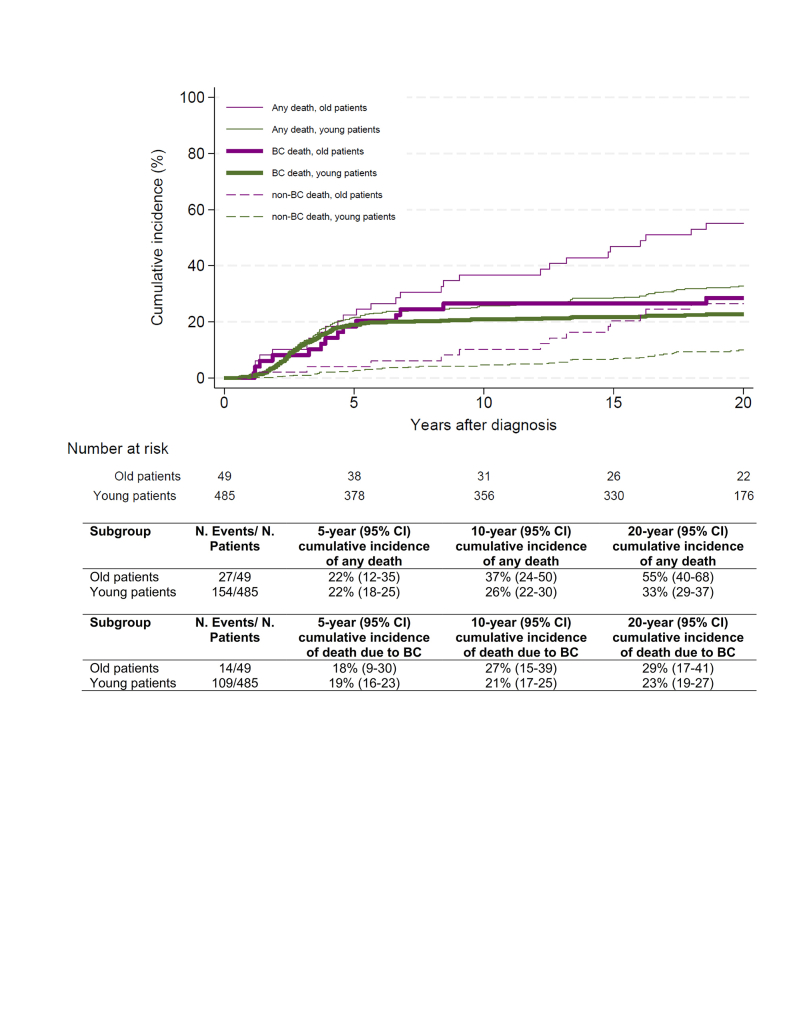
We identified 49 old patients, aged 51-74 years, with node-negative TNBC from a prospective multicenter trial (Supplementary Figure S1 and Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.102923). Old patients were more likely to have HER2-low tumors [adjusted odds ratio (adj. OR) 4.22, P < 0.01] or to have tumors with pushing borders (adj. OR 0.18, P < 0.01) (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2024.102923). Only one patient had a tumor with LVI. With a median follow-up of 20.0 years, 27/49 (55.1%) patients died (from any cause). During follow-up, the risk of death due to BC was comparable in young and old patients with a 10-year cumulative incidence of 21% (95% CI 17% to 25%) versus 27% (95% CI 15% to 39%), respectively (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2024.102923). Although in the old patients no significant independent histopathological prognostic factors for BCSS could be identified (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.102923), the direction and magnitude of the associations with BCSS appeared similar to that in young patients (P for interaction tests between age status and the tumor trait of interest >0.05). Considering the small sample size of the old cohort, these analyses should be considered exploratory.
Discussion
Our cohort of (neo)adjuvant chemotherapy-naïve young women with node-negative eTNBC and long-term follow-up provided the unique opportunity to evaluate the prognostic impact of putative biomarkers on BCSS, not biased by systemic treatment. As also shown by others,12, 13, 14, 15 sTIL is a very strong prognostic factor and the key question was whether other histopathological features have independent effect. Here we showed that LVI and fibrotic focus are independent prognostic biomarkers for BCSS in young patients with node-negative eTNBC. Moreover, LVI appeared to have additional value in the risk stratification of patients with low levels of sTILs, suggesting that the presence of LVI may have important clinical implications in identifying patients with an ultra-high risk of recurrence, thus most in need of systemic treatment. Our observations, if confirmed in independent series, may help to optimize the allocation and escalation of (neo)adjuvant treatment strategies for individual patients with eTNBC.
Our observations of a poor prognostic effect of the presence of LVI in eTNBC is in line with numerous prior studies in (triple-negative) BC, especially in women with node-negative disease.30,39,40 However, LVI is not yet systematically incorporated in international guideline algorithms for adjuvant systemic therapy decisions,5,6,41, 42, 43 even though LVI has been reported in pathology reports for decades. This is likely explained by the poor reproducibility of LVI.44, 45, 46, 47 However, the strong prognostic value of LVI merits international efforts to improve reproducibility and clinical adoption, as has been done with sTILs by The International Immune-Oncology Biomarker Working Group, also called TILs-WG,29 and with Ki67 by the International Ki67 in Breast Cancer working group.48 Possible solutions are the training of pathologists and adherence to assessment criteria, and the use of digital pathology and machine-learning algorithms for the objective assessment of LVI.49,50
Treatment de-escalation in patients with TNBC is an emerging area of investigation and sTILs showed the potential to identify patients with an excellent survival in the absence of adjuvant chemotherapy.15,16 In the current study, we observed that patients with the presence of LVI show a higher risk of a breast cancer-specific death in comparison to when LVI is absent, which is in line with other studies.30,39,40 Therefore, it is of vital importance to consider the exclusion of patients with LVI from de-escalation trials.
Fibrotic focus is an easily assessable, well-reproducible feature in routine histological tissue sections.51 Fibrotic focus has been associated with tumor progression, possibly explained by its relation with hypoxia, (lymph)angiogenesis and an immunosuppressive tumor microenvironment,52, 53, 54 and many prior studies reported the independent prognostic relevance of fibrotic focus in BC in general.31,51,52,55,56 However, limited data exist about its value in TNBC. The present study contributes herein, and strengthens the promising potential of fibrotic focus as an independent and unbiased predictor for worse outcome within eTNBC.
In contrast, we did not find significant prognostic value for the other evaluated histopathological tumor traits. The lack of prognostic significance of HER2-low in eTNBC has been described before.9,57, 58, 59 We, however, are the first to have studied HER2-low in systemic treatment-naïve patients and thereby provide more reliable information on its relation with the natural history of disease. In the studied cohort, grade 3 was not associated with a worse survival outcome, which is consistent with a previous report on eTNBC.14 In addition, we showed that ER-low tumors had similar long-term prognosis as ER-negative tumors in the absence of systemic treatment. This is in line with accumulating evidence showing that ER-low/HER2-negative BC share similar molecular and immunological features and clinical behavior with ER-negative (0%) BCs.60, 61, 62, 63 Given that current eTNBC clinical trials investigating the use of new targeted agents such as immunotherapy are often limited to tumors with ER <1%, the inclusion of the ER low-positive subgroup in these trials is encouraged.62
The main cohort in the present study consisted of patients with eTNBC <40 years of age. Although eTNBC is associated with younger age, there is still a substantial number of eTNBCs diagnosed within patients of older age.18,64,65 The modifying effect of age on the association of a tumor characteristic with outcome is poorly understood. Therefore, our results cannot be extrapolated to patients of all ages, even though there are indications that TNBC in young versus old patients represent similar molecular diseases in the context of underlying genomic phenotypes.18 To explore whether the associations of tumor traits with BCSS in young patients are similar within old patients, we additionally studied a small cohort of older node-negative chemotherapy-naïve patients with eTNBC from a prospective trial with long-term follow-up. The associations of histopathological tumor traits with outcome appeared largely similar in the two age groups, although these analyses are merely exploratory and should be interpreted with caution. Follow-up studies in larger cohorts of older systemically untreated patients with eTNBC, such as the Stockholm Tamoxifen Trial (STO-3),66 or PARADIGM initiatives for patients older than 40 years are recommended to validate our findings.
In the present study, young and old patients with eTNBC appeared to have similar BCSS. Prior studies assessing the association of age with survival in patients with eTNBC reported conflicting results,18,65,67,68 which may be explained by the use of small cohorts, different case mixes and (partly) treated patients with different chemotherapy regimens. Thus, confounding by indication and bias by treatment selection may have affected prior studies.69,70 To the best of our knowledge, the present study is the first to compare BCSS of young and old patients with eTNBC who did not receive (neo)adjuvant chemotherapy, with long follow-up.
It is important to consider that our cohort consisted mainly of women with node-negative, pT1-pT2 tumors. Secondly, since the cause of death was unknown for women below 40 years of age, we had to approximate the cause of death. The risk of mistakenly reporting a death as BC related is very low given that most women did not develop a second primary tumor (75.3%) and their young age makes a non-cancer-related death unlikely. Thirdly, our findings may be less applicable for germline BRCA1 mutation carriers since these women are more likely to develop a second primary tumor. Of note, we observed that LVI was prognostic for BCSS in women with a tBRCA1 mutation, suggesting that LVI may also be of value in the prognostication of gBRCA1m carriers. Lastly, the histopathological tumor characteristics were assessed on whole slides of surgical resection specimens. Ideally, risk stratification and treatment choice take place at diagnosis, based on pretreatment diagnostic breast biopsies. As it stands now, LVI, especially its absence, cannot reliably be determined on core needle biopsies,45 in contrast to sTILs.71,72 Further work is therefore needed to evaluate whether the assessment of LVI in biopsies accurately reflects surgical resection specimens, and whether the biopsy procedure (one versus multiple biopsies; core needle versus vacuum assisted) contributes herein.
Despite the limitations, our study is unique in providing information on the prognostic impact of several common histopathological tumor characteristics in a cohort of systemically untreated patients. Our studied patients were diagnosed with node-negative BC in an era when, according to the Dutch guidelines at the time, node negativity was considered a favorable prognostic factor without indication for adjuvant chemotherapy. Evaluating treatment-naïve patients is the only way to study the true prognostic value of biomarkers. Additional strengths include the long-term follow-up, the homogenous patient selection limited to pN0 TNBC patients and the evaluation of biomarkers that could be assessed on FFPE tumor material with techniques widely available and affordable in most pathology clinics, which will contribute to their chances of being routinely integrated for prognostication in clinical practice. Future research is needed to confirm our observation that tumors with low sTILs and LVI represents a subgroup with an ultra-high risk of recurrence, and to investigate whether this subgroup derives increased benefit of adjuvant chemotherapy.
In conclusion, here we report the independent prognostic value of LVI and fibrotic focus for BCSS in young patients with node-negative eTNBC (stage I or II), not influenced by (neo)adjuvant chemotherapy and independent from sTILs. Based on our results, it should be considered to exclude patients from de-escalation trials when tumor LVI is present. Moreover, the presence of LVI and low levels of sTILs identified patients with eTNBC with an ultra-high risk of recurrence or death. Our findings may help, if confirmed in independent series, to select patients most in need of systemic treatment. In addition, it may be of importance to stratify for LVI and fibrotic focus in future clinical trials to take the biological and clinical heterogeneity of patients with TNBC into account.
Acknowledgements
We are grateful to all patients who participated in these studies. We would like to acknowledge the NKI-AVL Core Facility Molecular Pathology & Biobanking (CFMPB) for supplying NKI-AVL Biobank material and lab support. We thank Vincent van der Noort for his contribution to this project. We are also grateful to all general practitioners and research nurses for their help with data collection.
Funding
This work was supported by institutional research grants from the Dutch Cancer Society [grant number NKI 2018-11655], The Netherlands Organization for Health Research and Development [grant number 836021019], A Sister’s Hope, De Vrienden van UMC Utrecht, and [Z]aan de Wandel to the Netherlands Cancer Institute (Marleen Kok, Marjanka K. Schmidt and Sabine C. Linn). The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
PJvD has advisory relationships with Paige, Pantarei and Samantree, paid to the institution, and research grants paid to the institute from Pfizer. NS received institutional research funding from Pfizer. ZV has a consulting role for Roche. CHMvD received institutional research funding from AstraZeneca/Daiichi Sankyo. SMW has a consulting role for Roche, and received institutional research funding from Roche, Pfizer, Bayer, MSD, AstraZeneca/Merck and Amgen. SMW has a consulting role for IDDI, Sensorion, Biophytis, Servier, Yuhan, Amaris Consulting and Roche. JW received institutional research funding from Cancer Research UK and KWF Dutch Cancer Society. AR has a consulting role for MSD Oncology, Amgen, Roche, AstraZeneca/Daiichi Sankyo and Bristol Myers Squibb/Pfizer. SCL reports grants from ZonMw and A Sister’s Hope during the conduct of the study; has been an advisory board member for AstraZeneca, Cergentis, IBM, Novartis, Pfizer, Sanofi and Roche; and received institutional research grants from Agendia, AstraZeneca, Eurocept-pharmaceuticals and Merck and Pfizer. In addition, SCL received institutional research grants and institutional non-financial support from Agendia, Genentech, Novartis, Roche, Tesaro and Immunomedics and other institutional support from AstraZeneca, Pfizer, Cergentis, Daiichi Sankyo, IBM and Bayer outside the submitted work. MK is an advisory board member and/or received speakers’ fee for/from Alderaan, Bristol Myers Squibb (BMS), Domain Therapeutics, Gilead, Roche, Medscape, MSD and AZ/Daiichi and received institutional research support from AstraZeneca/Daiichi, BMS and Roche outside the submitted work. RS reports non-financial support from Merck and BMS, research support from Merck, Puma Biotechnology and Roche, and personal fees from Roche, BMS and Exact Sciences for advisory boards. IRK received research grants from Novartis and Gilead. RHTK is an advisory board member for Amgen, AstraZeneca, Bayer, BMS, MSD, Novartis, Pfizer, Pierre Fabre Sante, Sanofi and Servier. All other authors have declared no conflicts of interest.
Data Sharing
The datasets used and analyzed during the present study are not publicly available due to privacy-protection restrictions, but are available from the corresponding author upon reasonable request and with permission of the Netherlands Cancer Registry, hosted by the Netherlands Comprehensive Cancer Center (IKNL).
Supplementary data
Supplementary Figure S1.
Supplementary Figure S2.
Supplementary Figure S3.
Supplementary Figure S4.
References
- 1.Kim N.H., Bang H.W., Eom Y.H., Choi S.H. The different prognostic impact of age according to individual molecular subtypes in breast cancer. Ann Surg Treat Res. 2022;103(3):129–144. doi: 10.4174/astr.2022.103.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge A.H., Hughes M.E., Warner E.T., et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34(27):3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 3.Keegan T.H., DeRouen M.C., Press D.J., Kurian A.W., Clarke C.A. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14(2):R55. doi: 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent R., Trudeau M., Pritchard K.I., et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F., Kyriakides S., Ohno S., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 6.Burstein H.J., Curigliano G., Thürlimann B., et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216–1235. doi: 10.1016/j.annonc.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cserni G., Quinn C.M., Foschini M.P., et al. Triple-negative breast cancer histological subtypes with a favourable prognosis. Cancers. 2021;13(22):5694. doi: 10.3390/cancers13225694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H.J., Seo A.N., Park S.Y., et al. Low prognostic implication of fibroblast growth factor family activation in triple-negative breast cancer subsets. Ann Surg Oncol. 2014;21(5):1561–1568. doi: 10.1245/s10434-013-3456-x. [DOI] [PubMed] [Google Scholar]
- 9.Denkert C., Seither F., Schneeweiss A., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 10.Conte B., Brasó-Maristany F., Pascual T., et al. 3MO Association of the research-based HER2DX signatures with survival in early-stage triple-negative breast cancer (eTNBC) Ann Oncol. 2022;33:S124–S125. [Google Scholar]
- 11.Denkert C., Liedtke C., Tutt A., von Minckwitz G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet. 2017;389(10087):2430–2442. doi: 10.1016/S0140-6736(16)32454-0. [DOI] [PubMed] [Google Scholar]
- 12.Adams S., Gray R.J., Demaria S., et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denkert C., von Minckwitz G., Darb-Esfahani S., et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 14.Loi S., Drubay D., Adams S., et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–569. doi: 10.1200/JCO.18.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong V.M.T., Wang Y., Ter Hoeve N.D., et al. Prognostic value of stromal tumor-infiltrating lymphocytes in young, node-negative, triple-negative breast cancer patients who did not receive (neo)adjuvant systemic therapy. J Clin Oncol. 2022;40:2361–2374. doi: 10.1200/JCO.21.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J.H., Jonas S.F., Bataillon G., et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol. 2019;30(12):1941–1949. doi: 10.1093/annonc/mdz395. [DOI] [PubMed] [Google Scholar]
- 17.Loi S., Michiels S., Salgado R., et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 18.Aine M., Boyaci C., Hartman J., et al. Molecular analyses of triple-negative breast cancer in the young and elderly. Breast Cancer Res. 2021;23(1):20. doi: 10.1186/s13058-021-01392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid P., Cortes J., Dent R., et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–567. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 20.Tutt A.N.J., Garber J.E., Kaufman B., et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geyer C.E., Sikov W.M., Huober J., et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol. 2022;33(4):384–394. doi: 10.1016/j.annonc.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Modi S., Jacot W., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bardia A., Hurvitz S.A., Tolaney S.M., et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 24.Dackus G.M., ter Hoeve N.D., Opdam M., et al. Long-term prognosis of young breast cancer patients (≤40 years) who did not receive adjuvant systemic treatment: protocol for the PARADIGM initiative cohort study. BMJ Open. 2017;7(11) doi: 10.1136/bmjopen-2017-017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermorken J., Burgers J., Taat C., et al. 21st Annual San Antonio Breast Cancer Symposium - December 12-15, 1998; Adjuvant tamoxifen in breast cancer: interim results of a comprehensive cancer center Amsterdam trial. Breast Cancer Res Treat. 1998;50(3):203–335. [Google Scholar]
- 26.Kruger D.T., Opdam M., Sanders J., van der Noort V., Boven E., Linn S.C. Hierarchical clustering of PI3K and MAPK pathway proteins in breast cancer intrinsic subtypes. APMIS. 2020;128:298–307. doi: 10.1111/apm.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beelen K., Opdam M., Severson T.M., et al. Phosphorylated p-70S6K predicts tamoxifen resistance in postmenopausal breast cancer patients randomized between adjuvant tamoxifen versus no systemic treatment. Breast Cancer Res. 2014;16(1):R6. doi: 10.1186/bcr3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97(16):1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 29.Salgado R., Denkert C., Demaria S., et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakha E.A., Martin S., Lee A.H.S., et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118(15):3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 31.Hasebe T., Sasaki S., Imoto S., Mukai K., Yokose T., Ochiai A. Prognostic significance of fibrotic focus in invasive ductal carcinoma of the breast: a prospective observational study. Mod Pathol. 2002;15(5):502–516. doi: 10.1038/modpathol.3880555. [DOI] [PubMed] [Google Scholar]
- 32.Lakhani S.R., Jacquemier J., Sloane J.P., et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;90(15):1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- 33.Elston C.W., Ellis I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 34.Kruger D.T., Beelen K.J., Opdam M., et al. Hierarchical clustering of activated proteins in the PI3K and MAPK pathways in ER-positive, HER2-negative breast cancer with potential therapeutic consequences. Br J Cancer. 2018;119(7):832–839. doi: 10.1038/s41416-018-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarantino P., Hamilton E., Tolaney S.M., et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38(17):1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 36.Tolaney S.M., Garrett-Mayer E., White J., et al. Updated Standardized Definitions for Efficacy End Points (STEEP) in adjuvant breast cancer clinical trials: STEEP version 2.0. J Clin Oncol. 2021;39(24):2720–2731. doi: 10.1200/JCO.20.03613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coviello V., Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4(2):103–112. [Google Scholar]
- 38.Therneau T.M., Atkinson E.J. Mayo Foundation; 2022. An introduction to recursive partitioning using the RPART routines.https://cran.r-project.org/web/packages/rpart/vignettes/longintro.pdf Available at. [Google Scholar]
- 39.Steenbruggen T.G., van Werkhoven E., van Ramshorst M.S., et al. Adjuvant chemotherapy in small node-negative triple-negative breast cancer. Eur J Cancer. 2020;135:66–74. doi: 10.1016/j.ejca.2020.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Houvenaeghel G., Cohen M., Classe J.M., et al. Lymphovascular invasion has a significant prognostic impact in patients with early breast cancer, results from a large, national, multicenter, retrospective cohort study. ESMO Open. 2021;6(6) doi: 10.1016/j.esmoop.2021.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wishart G.C., Bajdik C.D., Azzato E.M., et al. A population-based validation of the prognostic model PREDICT for early breast cancer. Eur J Surg Oncol. 2011;37(5):411–417. doi: 10.1016/j.ejso.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Korde L.A., Somerfield M.R., Carey L.A., et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39(13):1485–1505. doi: 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goetz M.P., Gradishar W.J., Anderson B.O., et al. NCCN Guidelines Insights: breast cancer, Version 3.2018: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2019;17(2):118–126. doi: 10.6004/jnccn.2019.0009. [DOI] [PubMed] [Google Scholar]
- 44.Gilchrist K.W., Gould V.E., Hirschl S., et al. Interobserver variation in the identification of breast carcinoma in intramammary lymphatics. Hum Pathol. 1982;13(2):170–172. doi: 10.1016/s0046-8177(82)80121-4. [DOI] [PubMed] [Google Scholar]
- 45.Hoda S.A., Hoda R.S., Merlin S., Shamonki J., Rivera M. Issues relating to lymphovascular invasion in breast carcinoma. Adv Anat Pathol. 2006;13(6):308–315. doi: 10.1097/01.pap.0000213048.69564.26. [DOI] [PubMed] [Google Scholar]
- 46.de Mascarel I., Bonichon F., Durand M., et al. Obvious peritumoral emboli: an elusive prognostic factor reappraised. Multivariate analysis of 1320 node-negative breast cancers. Eur J Cancer. 1998;34(1):58–65. doi: 10.1016/s0959-8049(97)00344-4. [DOI] [PubMed] [Google Scholar]
- 47.Rakha E.A., Abbas A., Pinto Ahumada P., et al. Diagnostic concordance of reporting lymphovascular invasion in breast cancer. J Clin Pathol. 2018;71(9):802–805. doi: 10.1136/jclinpath-2017-204981. [DOI] [PubMed] [Google Scholar]
- 48.Dowsett M., Nielsen T.O., A'Hern R., et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bera K., Schalper K.A., Rimm D.L., Velcheti V., Madabhushi A. Artificial intelligence in digital pathology - new tools for diagnosis and precision oncology. Nat Rev Clin Oncol. 2019;16(11):703–715. doi: 10.1038/s41571-019-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Echle A., Rindtorff N.T., Brinker T.J., Luedde T., Pearson A.T., Kather J.N. Deep learning in cancer pathology: a new generation of clinical biomarkers. Br J Cancer. 2021;124(4):686–696. doi: 10.1038/s41416-020-01122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baak J.P.A., Colpaert C.G.A., van Diest P.J., et al. Multivariate prognostic evaluation of the mitotic activity index and fibrotic focus in node-negative invasive breast cancers. Eur J Cancer. 2005;41(14):2093–2101. doi: 10.1016/j.ejca.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 52.Van den Eynden G.G., Colpaert C.G., Couvelard A., et al. A fibrotic focus is a prognostic factor and a surrogate marker for hypoxia and (lymph)angiogenesis in breast cancer: review of the literature and proposal on the criteria of evaluation. Histopathology. 2007;51(4):440–451. doi: 10.1111/j.1365-2559.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 53.Van den Eynden G.G., Smid M., Van Laere S.J., et al. Gene expression profiles associated with the presence of a fibrotic focus and the growth pattern in lymph node-negative breast cancer. Clin Cancer Res. 2008;14(10):2944–2952. doi: 10.1158/1078-0432.CCR-07-4397. [DOI] [PubMed] [Google Scholar]
- 54.Ding J.-H., Xiao Y., Zhao S., et al. Integrated analysis reveals the molecular features of fibrosis in triple-negative breast cancer. Mol Ther Oncol. 2022;24:624–635. doi: 10.1016/j.omto.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasebe T., Tsuda H., Hirohashi S., et al. Fibrotic focus in infiltrating ductal carcinoma of the breast: a significant histopathological prognostic parameter for predicting the long-term survival of the patients. Breast Cancer Res Treat. 1998;49(3):195–208. doi: 10.1023/a:1006067513634. [DOI] [PubMed] [Google Scholar]
- 56.Colpaert C., Vermeulen P., van Beest P., et al. Intratumoral hypoxia resulting in the presence of a fibrotic focus is an independent predictor of early distant relapse in lymph node-negative breast cancer patients. Histopathology. 2001;39(4):416–425. doi: 10.1046/j.1365-2559.2001.01238.x. [DOI] [PubMed] [Google Scholar]
- 57.de Moura Leite L., Cesca M.G., Tavares M.C., et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat. 2021;190(1):155–163. doi: 10.1007/s10549-021-06365-7. [DOI] [PubMed] [Google Scholar]
- 58.Tarantino P., Gandini S., Nicolò E., et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer. 2022;163:35–43. doi: 10.1016/j.ejca.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 59.Horisawa N., Adachi Y., Takatsuka D., et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer. 2022;29(2):234–241. doi: 10.1007/s12282-021-01303-3. [DOI] [PubMed] [Google Scholar]
- 60.Fujii T., Kogawa T., Dong W., et al. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol. 2017;28(10):2420–2428. doi: 10.1093/annonc/mdx397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villegas S.L., Nekljudova V., Pfarr N., et al. Therapy response and prognosis of patients with early breast cancer with low positivity for hormone receptors - an analysis of 2765 patients from neoadjuvant clinical trials. Eur J Cancer. 2021;148:159–170. doi: 10.1016/j.ejca.2021.02.020. [DOI] [PubMed] [Google Scholar]
- 62.Voorwerk L., Sanders J., Keusters M.S., et al. Immune landscape of breast tumors with low and intermediate estrogen receptor expression. NPJ Breast Cancer. 2023;9(1):39. doi: 10.1038/s41523-023-00543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massa D., Vernieri C., Nicolè L., et al. 1MO Tumor immune microenvironment in ER-negative vs ER-low, HER2-neg breast cancer. ESMO Open. 2023;8(1 suppl 4) [Google Scholar]
- 64.Ma D., Jiang Y.Z., Xiao Y., et al. Integrated molecular profiling of young and elderly patients with triple-negative breast cancer indicates different biological bases and clinical management strategies. Cancer. 2020;126(14):3209–3218. doi: 10.1002/cncr.32922. [DOI] [PubMed] [Google Scholar]
- 65.Tzikas A.K., Nemes S., Linderholm B.K. A comparison between young and old patients with triple-negative breast cancer: biology, survival and metastatic patterns. Breast Cancer Res Treat. 2020;182(3):643–654. doi: 10.1007/s10549-020-05727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutqvist L.E., Johansson H. Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol (Stockholm, Sweden) 2007;46(2):133–145. doi: 10.1080/02841860601034834. [DOI] [PubMed] [Google Scholar]
- 67.Freedman R.A., Keating N.L., Lin N.U., et al. Breast cancer-specific survival by age: Worse outcomes for the oldest patients. Cancer. 2018;124(10):2184–2191. doi: 10.1002/cncr.31308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liedtke C., Hess K.R., Karn T., et al. The prognostic impact of age in patients with triple-negative breast cancer. Breast Cancer Res Treat. 2013;138(2):591–599. doi: 10.1007/s10549-013-2461-x. [DOI] [PubMed] [Google Scholar]
- 69.Grimes D.A., Schulz K.F. Bias and causal associations in observational research. Lancet. 2002;359(9302):248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 70.Giordano S.H., Kuo Y.-F., Duan Z., Hortobagyi G.N., Freeman J., Goodwin J.S. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112(11):2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cha Y.J., Ahn S.G., Bae S.J., et al. Comparison of tumor-infiltrating lymphocytes of breast cancer in core needle biopsies and resected specimens: a retrospective analysis. Breast Cancer Res Treat. 2018;171(2):295–302. doi: 10.1007/s10549-018-4842-7. [DOI] [PubMed] [Google Scholar]
- 72.Buisseret L., Desmedt C., Garaud S., et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol. 2017;30(9):1204–1212. doi: 10.1038/modpathol.2017.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.