Abstract
Background
No treatment other than platelet administration is known to protect against spontaneous hemorrhage in thrombocytopenic dogs.
Objectives
Primary: determine if treatment with ε‐aminocaproic acid (EACA) decreases the requirement for blood transfusions and improves outcome in dogs with severe thrombocytopenia. Secondary: find evidence of hyperfibrinolysis and determine the effect EACA administration on rapid (rTEG) and tissue plasminogen activator‐spiked (tPA‐rTEG) thromboelastography parameters.
Animals
Twenty‐seven dogs with severe thrombocytopenia were treated with EACA, and data from an additional 33 were obtained from the hospital database as historical control (HC) cohort.
Methods
Single arm clinical trial with HCs. The EACA group dogs received EACA (100 mg/kg IV followed by a constant‐rate infusion [CRI] of 400 mg/kg/24 hours). Thromboelastography before and during EACA infusion, hospitalization days, number of transfusions, and mortality were compared.
Results
No difference was found in number of transfusions per dog (median, interquartile range; 1, 0‐2.5 vs 0.9, 0‐2; P = .5) and hospitalization days (4, 4‐6 vs 4.5, 3.75‐6; P = .83) between HC and EACA groups, respectively, and no difference in survival was identified by log‐rank analysis (P = .15). Maximum amplitude on both rTEG and tPA‐rTEG increased after EACA administration (rTEG baseline: 23.6, 9.6‐38.9; post‐EACA: 27.3, 19.8‐43.2; P < .001; tPA‐rTEG baseline: 23, 10.9‐37.2; post‐EACA: 24.7, 16.7‐44.8; P < .002).
Conclusions and Clinical Importance
Although EACA increased clot strength, there was no effect on outcome. Treatment with EACA at this dosage cannot be recommended as a routine treatment but may be considered for dogs with severe ongoing hemorrhage.
Keywords: antifibrinolytics, hyperfibrinolysis, platelets, thrombocytopenia
Abbreviations
- aPTT
activated partial thromboplastin coagulation time
- BSA
bovine serum albumin
- CRI
constant‐rate infusion
- DOGiBAT
daily canine bleeding assessment tool
- EACA
ε‐aminocaproic acid
- FWB
fresh whole blood
- HC
historical control
- IQR
interquartile range
- ITP
immune‐mediated thrombocytopenia
- IVIG
intravenous immunoglobulin
- MA
maximum amplitude
- PAI‐1
plasminogen activator inhibitor 1
- PRBC
packed red blood cells
- PT
prothrombin coagulation time
- rTEG
rapid TEG
1. INTRODUCTION
Thrombocytopenia is a common finding in dogs presented to veterinary referral hospitals. In a previous report, >5% of admitted dogs had platelet counts lower than the reference range, and 11% of those dogs had platelet counts <50 000/μL. 1 Until the primary disease process can be controlled, blood products are administered to treat clinically relevant bleeding, adding substantial cost to hospital care. In a previous study, 81% of dogs with immune thrombocytopenia (ITP) had spontaneous hemorrhage at the time of admission and 41% required blood transfusions during hospitalization, with some developing severe complications of acute hemorrhage. 2
Epsilon‐aminocaproic acid (EACA) and tranexamic acid (TXA) are antifibrinolytic agents. They are lysine analogues that reversibly bind to lysine receptor sites on plasminogen, blocking the binding of plasmin to fibrin and therefore decreasing lysis of newly formed clots. Indications for the use of antifibrinolytics in humans include the treatment of hemorrhage associated with hyperfibrinolysis (eg, acute traumatic hemorrhage), postpartum bleeding, and intraoperative bleeding. 3 , 4 , 5 In veterinary medicine, studies in Greyhounds, a breed at risk of excessive fibrinolysis, suggest decreased postoperative bleeding in response to EACA. 6 Increased clot strength on viscoelastic testing was documented after the administration of EACA in another study. 7
The use of antifibrinolytics as adjunctive treatment of bleeding of any origin, including hemorrhage secondary to thrombocytopenia, has become increasingly common in veterinary medicine. 8 , 9 Reasons for this approach may include low cost, low adverse effect profile, and scarcity of point‐of‐care testing to screen for hyperfibrinolysis. These drugs also have been evaluated as a treatment to decrease spontaneous bleeding in severely thrombocytopenic humans, 10 based on speculation that antifibrinolytics may stabilize fragile blood clots in thrombocytopenic patients and therefore decrease further hemorrhage. 11
Some studies in humans with ITP and thrombocytopenia caused by hematologic malignancy suggest that antifibrinolytics may decrease clinical evidence of bleeding and the need for platelet transfusions, but the evidence is limited. 10 , 11 , 12 A report describing the use of TXA in 4 dogs with ITP found no clinical benefit when compared to a contemporary control cohort of 6 dogs. 13
A sensitive ex vivo method of identifying a tendency toward systemic hyperfibrinolysis is the addition of tissue plasminogen activator (tPA) to tissue factor or a kaolin and tissue factor‐activated thromboelastography. This technique of spiking the rTEG assay (tPA‐rTEG) may detect decreases in fibrinolysis inhibitor activity that are not sufficient to cause overt hyperfibrinolysis but place patients at increased risk for decompensation to a hyperfibrinolytic state. 14 It appears to provide rapid assessment of the endogenous fibrinolytic potential of whole blood in people 15 and also appears to be useful in dogs. 16 , 17 To our knowledge, the fibrinolytic potential of dogs with thrombocytopenia has not been characterized.
We hypothesized that treatment with EACA will decrease spontaneous bleeding and transfusion requirements in dogs with severe thrombocytopenia. Thus, our primary goals were to determine if prophylactic treatment with EACA decreases the frequency and severity of spontaneous hemorrhage, decreases the requirement for blood transfusions, and improves survival to discharge in dogs with severe thrombocytopenia. A secondary goal was to determine if thrombocytopenic dogs have thromboelastographic evidence of hyperfibrinolysis and to determine the effect of treatment with EACA on tPA‐rTEG parameters.
2. MATERIALS AND METHODS
2.1. Study design
Ours was a prospective single arm study using historical controls. The prospective arm was approved by the institutional animal care and use committee, and informed owner consent was obtained before enrollment of each dog (EACA group). Eligible dogs hospitalized for severe thrombocytopenia between 2018 and 2020 were identified by manual platelet count within the preceding 12 hours. Severe thrombocytopenia was defined as a platelet count <30 000/μL, or <50 000/μL if accompanied by evidence of bleeding consistent with thrombocytopenia (eg, petechiae, ecchymoses, epistaxis, melena). 18 Dogs weighing <2 kg (precluding safe blood collection for the study) and dogs suspected of having a disorder causing secondary hemostasis were excluded.
For each dog enrolled in the EACA group, a kaolin and tissue factor‐activated TEG (rapid TEG, rTEG) and a tPA‐modified rapid TEG (tPA stressed‐rTEG or tPA‐rTEG) were performed. Both rTEG and tPA‐rTEGs were performed before administration of EACA (T1), as described below. All assays were performed by the same 2 trained operators (JW or LR). After obtaining the assay samples, 100 mg/kg EACA (aminocaproic acid injection USP, 5 g/20 mL; Hospira Inc, Lake Forest, Illinois) was administered IV over 15 minutes, immediately followed by a constant‐rate infusion (CRI) of 400 mg/kg/24 hours. The rTEG and tPA‐rTEG assays then were repeated on blood samples collected before the end of the infusion, sometime during hours 18 to 24 as determined by operator availability (T2). If the dog was eating and judged to tolerate PO medication after T2 it was transitioned to PO administration of the IV EACA formulation at a dosage of 100 mg/kg q6h. 19 Dogs that were hyporexic or that had evidence of nausea or vomiting were continued on the IV CRI and monitored for adverse effects. Aminocaproic acid treatment was continued in each dog until it was discharged, its platelet count exceeded 50 000 μL/mL with no evidence of active bleeding, or it died or was euthanized. The number of days until the endpoints were reached was recorded. Day 1 was defined as the day of admission, day 2 (and all subsequent days) began at 11 the next morning. Manual platelets counts were obtained once daily, and packed cell volume (PCV) and total protein (TP) concentration were monitored q12h or more frequently if clinically indicated. Bleeding events, defined as observed hemorrhage including epistaxis, melena, or hematuria, or a percentage decrease in PCV or TP or both of >10%, were recorded. The more objective daily canine bleeding assessment tool (DOGiBAT) scoring system was not used in this study because the relevant information was not recorded for the control group. 20 All blood product transfusions including packed red blood cells (pRBC) and fresh whole blood (FWB) were documented. Approximately 10 mL/kg pRBC or 20 mL/kg FWB were administered per transfusion to dogs in both groups. Thus, depending on the size of the dog, a single transfusion may have used >1 unit of product. All additional diagnostic tests and treatments were at the discretion of the primary clinician.
For the HC group, medical records between 2013 and 2017 were screened for dogs with severe thrombocytopenia of any origin using the same inclusion and exclusion criteria as for the EACA group. Three records from dogs that received EACA during the course of their treatment were found and were not included in the HC group. Underlying etiology and additional treatments for both groups were recorded to ensure both groups had comparable underlying causes and received similar treatment.
2.2. TEG analysis
For each dog enrolled in the EACA group, paired rTEG and tPA‐rTEG were performed at T1 and T2. Both channels of a dedicated TEG machine (TEG 5000, Haemoscope, Skokie, Ill) were used and for each subject the channel assignment to each assay (rTEG and tPA‐rTEG) was randomly assigned in blocks of 10. Blood samples from each dog were collected from either a central venous catheter (Mila Small Animal Guidewire Catheter Kit, Mila International, Florence, KY, USA) freshly placed in the lateral saphenous vein or via direct cephalic or lateral saphenous venipuncture with a winged needle catheter during initial blood sampling at T1. All samples were collected from the central venous catheter at T2. For either technique, a purge of 1.2 mL (winged needle) or 3 mL (central venous catheter) blood was collected before diagnostic sample collection. The diagnostic sample of 1.4 mL of blood was collected directly into vacuum citrate tubes and the tubes then were mixed 5 times by inversion and allowed to rest for 30 minutes at room temperature. While the samples rested, a prediluted frozen tPA vial containing 25 IU of tPA in 10 μL of solution was retrieved and thawed at room temperature. The tPA solution was made in advance by serial dilutions of stock product (Cathflo Activase [Alteplase], Genentech, South San Francisco, CA; 2 mg/mL) in bovine serum albumin (BSA) buffer solution to a final concentration of 25 IU/10 μL. The tPA solution was made fresh every 6 months and stored at −80°F before use. The tubes were numbered to ensure a standardized sequence of use and each tube was assigned to either the left or right channel of the TEG analyzer to ensure an equal distribution of channel assignment for the stressed rTEG assays. The rTEG vials containing tissue factor activator and kaolin (RapidTEG, Haemonetics, Skokie, Ill) were reconstituted with 20 μL distilled water and gently swirled, and then allowed to rest. Immediately before assay, 10 μL of rTEG mixture and 20 μL of calcium chloride solution were added to each TEG cup. For the rTEG assays, the citrated tube was gently inverted 5 times and 340 μL of blood was pipetted directly into the test cup and gently mixed by pipetting the reagent‐blood mixture in and out of the cup 3 times. The analyzer carriage was raised to start the test. For the tPA‐rTEG assay, a 490 μL aliquot of citrated blood was pipetted into the tPA vial and was also gently mixed, yielding a final concentration of tPA in blood of 50 IU/mL. Then, 340 μL of the blood/tPA mixture were added to the test cup assigned to the tPA‐rTEG. This mixture also was pipetted out of and into the cup 3 times to mix all components before beginning the assay. The following values were recorded at T1 and T2: R (reaction time), K (clot formation time), α‐angle (a measure of the rapidity of clot formation), MA (maximum amplitude), and LY30 (clot lysis at 30 minutes).
2.3. Statistical analysis
Feasibility of the study was determined by a power analysis assuming a fixed number of thrombocytopenia cases would be enrolled in the 18 months that were available for recruitment. We used decreases in PCV to define bleeding events in HC group dogs, and based on contemporary hospital admissions we estimated that we would be able to enroll at least 16 dogs. Setting a threshold definition for bleeding as a decrease in PCV by 10%, the HC group bleeding event rate was 0.44 ± 0.08 episodes/day. Based on this HC group data, 16 EACA group dogs would allow us to detect a decrease to 0.37 episodes/day at an alpha error rate of 5% and a beta error level of 20%. Data including study days, mortality, and number of transfusions were compared between the HC and the EACA groups using the Mann Whitney U test for continuous data and the Pearson chi squared test for categorical data. Kaplan‐Meier survival curve and log rank analysis were performed to compare fatality rates. The analysis then was repeated including dogs with ITP only. In the EACA group, PCV, the rTEG, and tPA‐rTEG variables R, K, MA, and LY30% at T1 and T2 were compared using the Wilcoxon signed‐rank test. The correlation between the platelet count on admission and platelet count on day 2, between rTEG MA T1 and rTEG MA T2 and between tPA‐TEG MA T1 and tPA‐TEG MA T2 were determined by Pearson correlation. A P‐value of <.05 was used as the criterion for significance. Analyzing the influence of different adjunctive drugs was not attempted because of the high variability of different treatment protocols. Data analysis was conducted using commercial software (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp).
3. RESULTS
Records from 33 dogs were abstracted for the HC group, and 28 dogs were enrolled in the EACA group. Our initial enrollment criteria excluded dogs with prothrombin clotting time (PT) or activated partial thromboplastin clotting time (aPTT) results that exceeded the laboratory reference interval. After excluding several dogs with slight increases in their aPTT but no other clinical evidence of causes of secondary coagulation disorders, we modified the criteria to include dogs with results that were within 10% of the upper limit for either assay. The value of 10% was chosen because many dogs with no physical evidence of coagulopathy at the time of the assay fell within this range, and this figure is consistent with the magnitude of interassay variations between point‐of‐care device and laboratory assay results observed by us and others. 21 None of the dogs had any other evidence of disorders of secondary hemostasis, and all had normal reaction times on thromboelastography.
Six dogs did not complete the study. In 1 of these, the IV catheter was lost after day 1 and could not be replaced, and therefore serial data collection was not possible. Two dogs developed urethral obstructions secondary to blood clot formation in their urinary bladders and EACA administration therefore was discontinued. A fourth dog was discharged 24 hours after admission at the owner's request. The EACA was discontinued on day 5 in the fifth dog in response to persistent regurgitation. Where paired data was available, these dogs were included in the TEG analysis but not in the outcome analysis. A sixth dog was completely removed from the study after developing hemolysis and coagulation abnormalities consistent with disseminated intravascular coagulation the day after enrollment. Data for 1 rTEG T1 were unavailable because of an analyzer malfunction, and another dog died before T2. Thus, clinical outcome including transfusion data was evaluated for 22 EACA group dogs, paired rTEG data was available from 23, and paired tPA‐rTEG data was available from 24 dogs.
The majority of thrombocytopenic dogs in both the HC (30/33, 91%,) and the EACA group (19/22, 86.4%) were diagnosed with primary ITP. A summary of all diseases represented can be found in Table 1. Treatment of the underlying cause of the thrombocytopenia was variable, and several combinations of immunosuppressive and adjunctive treatments were used (Table 2). Nine dogs in the control group received IV immunoglobulin (IVIG) versus none in the EACA group. One dog, which was pancytopenic secondary to a suspected infectious cause, did not receive any immunosuppressants. To assess for an effect of IVIG treatment on study days and transfusion dependency, statistics were repeated excluding the dogs in the HC that received IVIG, and no differences in results were found. The influence of treatments other than EACA and IVIG on study endpoints was not analyzed because of the large number of treatment combinations used for dogs in both groups. Similarly, a repeated analysis was performed including only dogs with ITP, and no difference in results was found.
TABLE 1.
Underlying causes of thrombocytopenia in the control and EACA groups.
| Cause of thrombocytopenia | HC group | EACA group |
|---|---|---|
| N (%) | N (%) | |
| Primary ITP | 30 (91) | 19 (86.4) |
| Pancytopenia | 1 (3) | 1 (4.5) |
| Tick‐borne (suspected) | 0 | 1 (4.5) |
| Lymphoma (suspected) | 0 | 1 (4.5) |
| Acute leukemia | 1 (3) | 0 |
| ITP secondary to neoplasia | 1 (3) | 0 |
TABLE 2.
Overview of adjunctive treatment for thrombocytopenia for the HC and EACA groups.
| Immunosuppressants | HC group, N (%) | EACA group, N (%) |
|---|---|---|
| GC* only | 6 (18.2) | 8 (36.4) |
| GC + azathioprine | 10 (30.3) | 4 (18.2) |
| GC + cyclosporine | 5 (15.2) | 4 (18.2) |
| GC + mycophenolate | 8 (24.2) | 5 (22.7) |
| GC + 2 second agents | 3 (9.1) | 1 (4.5) |
| No immunosuppressants | 1 (3) | 0 |
| IVIG or vincristine | HC group, N (%) | EACA group, N (%) |
|---|---|---|
| Vincristine | 20 (60.6) | 15 (68.2 |
| IVIG | 3 (9.1) | 0 |
| IVIG + vincristine | 6 (18.2) | 0 |
| No IVIG or vincristine | 4 (12.1) | 7 (31.8) |
Abbreviation: GC*, glucocorticoid.
3.1. PCV and transfusions
Anemia was defined as PCV below the lower limit of the reference range. The median PCV and frequency of anemia at the time of admission was 37% (interquartile range [IQR], 21‐43) and 14 of 33 dogs (42%), respectively, in the HC group, and 39% (IQR, 24‐45) and 7 of 22 (32%) dogs in the EACA group. These results were not significantly different between groups (P = .31 and .48, respectively). Similarly, no difference between median platelet count upon admission was found between the HC (2000/μL; IQR, 0‐18 000) and the EACA groups (1725/μL; IQR, 0‐7500; P = .72).
Dogs in the HC group received a median of 1.33 transfusion/per dog of either pRBCs (24 transfusions) or FWB (20 transfusions). The EACA group dogs received a median of 1.32 transfusions/per dog of either pRBCs (19 transfusions) or FWB (10 transfusions). Additional results related to transfusions administered are shown in Figure 1. No significant group difference was found in the number of transfusions (pRBC or FWB) per dog (P = .5) or in the proportion of dogs receiving a FWB transfusion (P = .57). Although the proportion of dogs in the EACA group that did not need any blood products (59.1%) was larger than in the HC group (45.5%), the difference was not significant (P = .32). Some EACA dogs that required transfusions to treat hypovolemic hemorrhage did not have decreases in PCV of >10% and therefore did not fulfill that diagnostic criterion for a bleeding event, despite having clinically relevant bleeding. No difference was found in the frequency of bleeding events, defined as acute decreases in PCV or TP or both between groups (P = .31). Because observed episodes of hemorrhage were not reliably recorded in the EACA group, we assumed that the same problem existed in the HC group and consequently omitted observed bleeding events from our primary goal.
FIGURE 1.
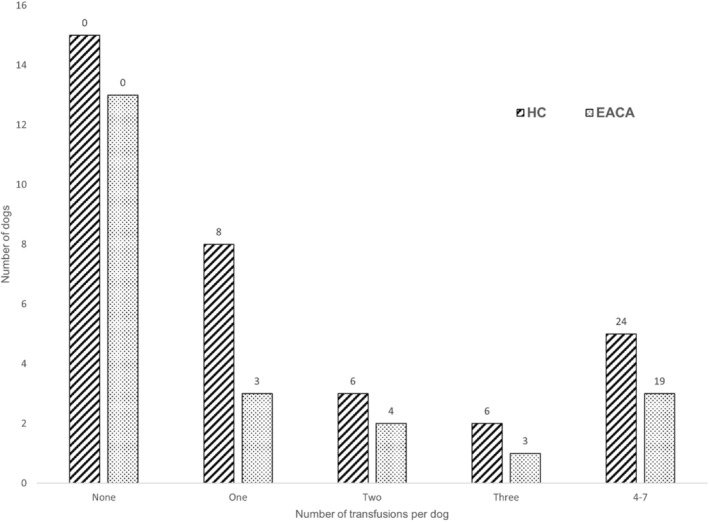
Bar chart showing the number of blood transfusions received per dog in both groups. Numbers at the top of each bar indicate the total number of transfusions administered to the control and EACA groups.
3.2. Outcome
The median time from admission to the study end point was 4 days (IQR, 4‐6) in the HC group and 4.5 days (IQR, 3.75‐6) in the EACA group (P = .83). In the HC group, 30 (91%) dogs survived to discharge. Two were euthanized, 1 failing to improve after 6 days of intensive treatment and the second after developing severe respiratory distress and neurological signs. One dog died of causes not related to thrombocytopenia. Seventeen (77%) dogs were discharged in the EACA group and 5 did not survive to discharge. Three patients died and 2 were euthanized (1 after 3 and 1 after 6 days of treatment) because of refractory thrombocytopenia. No significant difference was found in fatality rate (P = .08), and log‐rank analysis identified no significant difference (P = .15) in the survival time between the groups as demonstrated on a Kaplan‐Meier survival plot (Figure 2).
FIGURE 2.
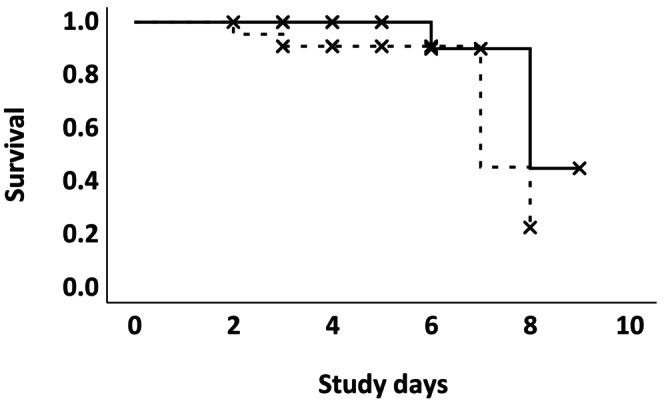
Kaplan‐Meier Curve comparing survival between the EACA (dotted line) and HC (solid line) groups. X indicate censored events. P‐value = .15.
3.3. TEG data
Only 1 dog was mildly hyperfibrinolytic on tPA‐TEG at T1 (LY30%, 23.4; reference range for our institution, <20%), and this finding resolved during administration of EACA. This dog was diagnosed with ITP, was not anemic on presentation, and did not require any blood products. A significant T1‐T2 difference in MA was found for both the rTEG (T1: 23.6; IQR, 9.6‐38.9; T2: 27.3; IQR, 19.8‐43.2, P < .001) and tPA‐rTEG (T1: 23; IQR, 10.9‐37.2, T2: 24.7; IQR, 16.7‐44.8, P < .002; Figure 3A). Five dogs had MA within reference range on both TEGs before EACA and 7 had MA within reference range post‐EACA. No other TEG parameters were different between T1 and T2. A summary of the TEG data including institutional reference ranges is presented in Table 3.
FIGURE 3.
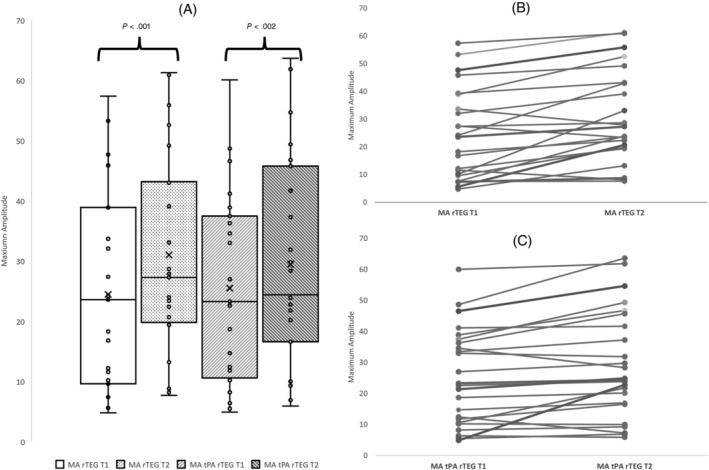
MA data. (A) rTEG and tPA rTEG bar‐and‐whisker plots at baseline (T1) and next day (T2). Bars represent the range between the 1st and 3rd quartiles, the horizontal lines represent median values, and the x marks represent average values. (B) Slope graph showing the changes in rTEG MA values for individual dogs between T1 and T2. (C) Slope graph showing the changes in tPA‐rTEG MA values for individual dogs between T1 and T2.
TABLE 3.
Summary of rapid and tPA stressed rapid TEG results including institutional reference ranges (in parentheses).
| Reference range rTEG | rTEG T1 | rTEG T2 | Reference range tPA‐rTEG | tPA‐rTEG T1 | tPA‐rTEG T2 | |
|---|---|---|---|---|---|---|
| R | 0‐1.26 | 0.2 (0.2‐0 .4) | 0.3 (0.2‐0.4) | 0‐1.4 | 0.3 (0.2‐0.3) | 0.3 (0.2‐0.4) |
| α‐angle | 50‐78 | 75.3 (58.6‐79.5) | 78.1 (65.6‐80) | 41‐83 | 77.2 (67‐80.4) | 77.4 (65‐79.9) |
| K | 0.4‐4.7 | 1.2 (0.8‐2.8) | 1.2 (0.8‐2.95) | 0.3‐5 | 0.85 (0.8‐3.8) | 1.2 (1.2‐4.2) |
| MA | 40‐67 | 23.6 (9.6‐38.9) | 27.3 (19.8‐43.2) | 38‐65 | 23 (10.9‐37.2) | 24.7 (16.7‐44.8) |
| LY30% | <3.8% | 0 | 0 | <20% | 0 (0‐0.35) | 0 |
A strong positive correlation was found between MA at T1 and T2 for both rapid (r = 0 .904, P < .001) and tPA‐TEGs (r = 0.942, P < .001), and the MA increased at T2 for 20/23 rTEG and 19/24 tPA‐rTEG assays (Figure 3B,C). Only a weak correlation was found for both TEG types between T1 platelet count and MA (rTEG r = 0.351, P = .07 and tPA‐TEG r = 0.36, P = .06) and T2 platelet count and MA (rTEG r = 0.27, P = .22 and tPA‐TEG r = 0.34, P = .11). The MA in 1 or both TEG assays increased from T1 to T2 in 10/12 dogs that experienced a concurrent decrease in platelet count. No significant difference was found between the PCV at both timepoints (T1: 39; IQR, 23‐45, T2: 32.5; IQR, 26.25‐37.25; P = .06).
4. DISCUSSION
Prophylactic treatment with EACA resulted in significantly increased clot strength (as measured by the MA) in severely thrombocytopenic dogs. However, this increase did not seem to be large enough to have a clinically relevant effect on variables such as hospitalization time, mortality, acute decreases of PCV or TP, and number of transfusions required.
Only a small number of published reports have investigated the efficacy of antifibrinolytics in dogs. To our knowledge, ours is the first prospective study investigating the use of EACA in thrombocytopenic dogs. A recent small study compared a group of dogs with ITP (n = 4) receiving TXA with a control group (n = 6) and did not find a clinical benefit of TXA. 13 In a retrospective study of the effect of TXA in dogs with acquired bleeding disorders, 15.7% of the study population was thrombocytopenic. Dogs that were treated with TXA in that study required fewer transfusions and had a lower mortality rate than dogs that did not receive TXA. Thrombocytopenic dogs however were not analyzed separately and therefore it is not possible to draw any conclusions regarding the efficacy of TXA specifically in thrombocytopenic dogs from that report. 8
The majority of dogs in our study were diagnosed with primary ITP. Most reported studies of antifibrinolytic treatment of humans with thrombocytopenia investigated their use in patients with hematologic malignancies rather than ITP. A 2016 Cochrane review concluded that there was a lack of high‐quality studies and insufficient evidence to justify the use of antifibrinolytics in thrombocytopenic patients secondary to hematological neoplasia. 11 Since then, a large randomized double‐blind placebo‐controlled trial did not identify significant decreases in transfusions, platelet administration, or bleeding events in patients with hematologic malignancies treated prophylactically with TXA. 22
We are aware of only 2 case series describing the efficacy of antifibrinolytics in human ITP patients with ongoing hemorrhage. In 12 patients that were treated with TXA as adjunctive treatment for active bleeding, high success rates for hemorrhage control were reported. 12 In a second series of 17 thrombocytopenic patients (15 with ITP) with uncontrolled bleeding, treatment with EACA appeared to facilitate discontinuation of platelet products in all 17. 23 We have not found any reports concerning the efficacy of prophylactic use of antifibrinolytic agents in ITP. Based on the lack of high‐quality data, current guidelines on treatment of ITP in human medicine recommend against the routine use of EACA or TXA for ITP. However, in patients with ongoing bleeding, these agents may be considered. 24 , 25
4.1. Adverse effects and complications
Aminocaproic acid appears to be well tolerated. In a retrospective study of 122 dogs with hemorrhage secondary to neoplastic and non‐neoplastic causes, only 3 dogs developed mild gastrointestinal signs possibly related to the EACA administration at dosages of 14‐24 mg/kg. 9 In our study, higher dosages of EACA (100 mg/kg PO q6h orally or as CRI) were administered based on recent evidence that increased doses are required to achieve more effective antifibrinolysis in dogs. 19 The high IV dose was chosen to maximize the potential to achieve constant therapeutic concentrations and identify any effect on thromboelastography at T2, because previously described characteristics of the drug in healthy dogs suggest that PO doses of 100 mg/kg are rapidly excreted and produce serum concentrations above the effective concentration of 25 μg/mL for only 3 hours. 19 We did not identify any clinically relevant adverse effects from this dose in the EACA group. One dog developed regurgitation during the study period and its PO EACA was stopped on day 5 as a precaution. It was not possible to determine if the regurgitation was more likely secondary to the dog's primary disease process or an adverse effect of EACA. However, because our study was not designed as a safety study, we cannot draw any conclusions with regard to safety of the EACA protocol used.
Two dogs with clinically relevant hematuria developed clots that caused urethral obstruction and EACA was discontinued in these dogs. Urinary tract obstruction secondary to blood clot formation has been reported previously in a dog with ITP, 26 and any contribution of EACA to this complication is unknown. However, considering that antifibrinolytics stabilize blood clots, the use of TXA or EACA in dogs and cats with severe hematuria should be considered carefully.
4.2. TEG data
Although the main reason for hemorrhage in dogs with thrombocytopenia is the lack of platelets, other mechanisms causing increased clot lysis might justify the use for antifibrinolytics in these dogs. One hypothesis regarding underlying mechanisms in thrombocytopenic patients is decreased availability of plasminogen activator inhibitor 1 (PAI‐1) released from granules on the platelet surface, increasing susceptibility to fibrinolysis. 27 , 28 Additionally, dogs with severe hemorrhage may develop hyperfibrinolysis similar to traumatic coagulopathy or hemoperitoneum. 29 To screen for evidence of systemic hyperfibrinolysis and to investigate the effect of EACA on TEG, we performed rTEGs and tPA‐rTEGs. Only 1 dog had mild hyperfibrinolysis on tPA‐rTEG, and it resolved after administration of EACA. This finding did not seem to have any clinical relevance because the dog did not show any signs of hemorrhage, had a normal PCV on presentation and did not require blood transfusions. Although it is possible that even tPA‐rTEG is not sensitive enough to detect endogenous hyperfibrinolysis, 30 the normal fibrinolytic profiles might explain the lack of response to prophylactic treatment with EACA in the dogs in our study. We found no other studies investigating hyperfibrinolysis in dogs with ITP. However, our findings are similar to data from thrombocytopenic human patients with hematologic malignancies. A previous study found that only 3 of 115 thrombocytopenic human patients had a hyperfibrinolytic profile, median plasma PAI‐I concentration was normal, and no differences in plasma PAI‐1 concentration were found between those who did or did not go on to develop clinically relevant hemorrhage. 28 Similarly, decreased plasma PAI‐I concentration was not identified in humans with ITP in another study. 30 Although localized hyperfibrinolysis cannot be excluded, our findings and data from humans suggest that systemic fibrinolytic activation does not play a major role in the pathophysiology of hemorrhage in thrombocytopenic patients.
We identified a significant increase in MA for both TEG types after EACA administration, a change that was not attributable to an increase in platelet count because no relationship between platelet count and MA could be shown. Unfortunately, fibrinogen concentrations were not consistently available for all patients at both T1 and T2, which is a limitation of our study. Another variable potentially increasing MA is decreased PCV, as suggested in previous publications. 31 In our study population, the PCV at T2 was slightly lower (32.5%) compared to T1 (39%), which represents a clinical change. However, the difference was not significant and the finding of previous studies that PCV influences MA could not be confirmed in a recent ex vivo study. 32 Although the increase in MA in the tPA‐rTEG assay in our study is consistent with a previous report showing an increase in MA using a tPA‐modified TEG after administration of a single dose of EACA to dogs, 19 the reason for the mild increase in rTEG MA is unclear. Because we have no TEG data on untreated control dogs, a possible explanation is that the MA increased with time in most dogs during the course of treatment with corticosteroids for their thrombocytopenia, independent of any effect of EACA or change in platelet numbers. 33 Another possible explanation could be that a low level of fibrinolytic activity is present in these dogs, decreasing the MA without causing measurable increases in LY30, and this fibrinolysis was inhibited by EACA. Regardless of the mechanism, this change was not associated with any identifiable clinical benefit in our dogs.
Additionally, 7 dogs in our study had MA within or just below reference range that normalized after EACA, which is an unexpected finding for thrombocytopenic dogs. Interestingly, only 1 of these dogs required a blood transfusion. Additional studies investigating the potential use of viscoelastic testing to predict clinical bleeding and correlation with bleeding scores in dogs with ITP are required.
4.3. Limitations
Our study had some limitations, including the small treatment group and variable individual treatment regimens. Although the treatment of primary ITP is immunosuppression, individual clinician preferences and variable need to escalate treatment make it difficult to control for variations in treatment for all dogs in a small study. Many different treatment protocols were used consisting of different combinations of immunosuppressants and adjunctive treatments, and there were group differences in the use of IVIG as presented in Table 2. Because of the small group sizes, marked variation in treatment and the timing of escalating drug treatment, it was not possible to determine if these treatments had a potential effect on study endpoints.
We used historical controls in an effort to maximize case numbers for our single site study, knowing that treatment for primary ITP at our institution has not changed substantially since 2013. The HC dogs were selected using the same inclusion and exclusion criteria, but selection bias may have occurred in the HC group from, for example, excluding dogs that were treated with EACA, which may have limited the HC group to dogs with a milder clinical course. More objective clinical severity scoring such as the DOGIBat system 26 was not possible because the necessary information was not recorded for the HC group. Bleeding events were not reliably documented and therefore could not be used for analysis. Baseline coagulation testing (PT and aPTT) for both the HC and EACA group dogs was done using different assays including a point‐of‐care device, commercial laboratories, and our hospital clinical pathology laboratory, and several dogs had slight increases of 1 or both values. Although no dogs had any other clinical evidence of a disorder of secondary hemostasis and their reaction times were normal on T1 thromboelastography, we cannot completely rule out subtle disorders of secondary hemostasis. However, the impact of any such disorder is likely small. 34
4.4. Conclusions
We found no evidence for hyperfibrinolysis in thrombocytopenic dogs or any survival benefit in dogs treated prophylactically with EACA, which is similar to results in studies of human patients. Based on our results, routine treatment with EACA cannot be recommended in dogs with ITP. Nevertheless, EACA administration might be considered for dogs with severe ongoing bleeding. A randomized controlled trial with a concurrent control group that differs only in the administration of EACA is necessary to further determine if antifibrinolytics are useful in dogs with ITP. However, our results suggest that such a study would require a very large number of subjects to demonstrate a treatment effect. Assays for hemostatic parameters, such as PAI‐1 concentration, might be of interest to investigate possible reasons of underlying local hyperfibrinolysis justifying the use of antifibrinolytic agents.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by North Carolina State University IACUC, number 18‐065.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
The authors thank Dr Alessio Vigani for providing funding for this study from his research fund.
Wolf J, Ruterbories LK, Handel I, Hansen B. The effect of ε‐aminocaproic acid on blood product requirement, outcome and thromboelastography parameters in severely thrombocytopenic dogs. J Vet Intern Med. 2024;38(2):1013‐1021. doi: 10.1111/jvim.16977
REFERENCES
- 1. Grindem CB, Breitschwerdt EB, Corbett WT, Jans HE. Epidemiologic survey of thrombocytopenia in dogs: a report on 987 cases. Vet Clin Pathol. 1991;20:38‐43. [DOI] [PubMed] [Google Scholar]
- 2. O'Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune‐mediated thrombocytopenia. J Am Vet Med Assoc. 2011;238:346‐352. [DOI] [PubMed] [Google Scholar]
- 3. Ker K, Roberts I, Shakur H, Coats TJ, Cochrane Injuries Group . Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2015;2015:CD004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry DA, Carless PA, Moxey AJ, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;2011:CD001886. [DOI] [PubMed] [Google Scholar]
- 5. Shakur HRI, Fawole B, Chaudhri R, El‐Sheikh M, Akintan A, Qureshi Z. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post‐partum haemorrhage (WOMAN): an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2017;389:2105‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marin LM, Iazbik MC, Zaldivar‐Lopez S, et al. Retrospective evaluation of the effectiveness of epsilon aminocaproic acid for the prevention of postamputation bleeding in retired racing greyhounds with appendicular bone tumors: 46 cases (2003‐2008). J Vet Emerg Crit Care. 2012;22:332‐340. [DOI] [PubMed] [Google Scholar]
- 7. Marin LM, Iazbik MC, Zaldivar‐Lopez S, et al. Epsilon aminocaproic acid for the prevention of delayed postoperative bleeding in retired racing greyhounds undergoing gonadectomy. Vet Surg. 2012;41:594‐603. [DOI] [PubMed] [Google Scholar]
- 8. Kelmer E, Marer K, Bruchim Y, Klainbart S, Aroch I, Segev G. Retrospective evaluation of the safety and efficacy of tranexamic acid (Hexakapron®) for the treatment of bleeding disorders in dogs. Israel J Vet Med. 2013;68:94‐100. [Google Scholar]
- 9. Davis M, Bracker K. Retrospective study of 122 dogs that were treated with the antifibrinolytic drug aminocaproic acid: 2010‐2012. J Am Anim Hosp Assoc. 2016;52:144‐148. [DOI] [PubMed] [Google Scholar]
- 10. Antun AG, Gleason S, Arellano M, et al. Epsilon aminocaproic acid prevents bleeding in severely thrombocytopenic patients with hematological malignancies. Cancer. 2013;119:3784‐3787. [DOI] [PubMed] [Google Scholar]
- 11. Estcourt LJ, Desborough M, Brunskill SJ, et al. Antifibrinolytics (lysine analogues) for the prevention of bleeding in people with haematological disorders. Cochrane Database Syst Rev. 2016;3:CD009733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayer B, Salama A. Successful treatment of bleeding with tranexamic acid in a series of 12 patients with immune thrombocytopenia. Vox Sang. 2017;112:767‐772. [DOI] [PubMed] [Google Scholar]
- 13. Olivares G, Sharman M, Miller R, Kisielewicz C, Seth M. Use of tranexamic acid in dogs with primary immune thrombocytopenia: a feasibility study. Front Vet Sci. 2023;10:946127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kupesiz A, Rajpurkar M, Warrier I, et al. Tissue plasminogen activator induced fibrinolysis: standardization of method using thromboelastography. Blood Coagul Fibrinolysis. 2010;21:320‐324. [DOI] [PubMed] [Google Scholar]
- 15. Moore HB, Moore EE, Chapman MP, et al. Viscoelastic tissue plasminogen activator challenge predicts massive transfusion in 15 minutes. J Am Coll Surg. 2017;225:138‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Osekavage KE, Brainard BM, Lane SL, Almoslem M, Arnold RD, Koenig A. Pharmacokinetics of tranexamic acid in healthy dogs and assessment of its antifibrinolytic properties in canine blood. Am J Vet Res. 2018;79:1057‐1063. [DOI] [PubMed] [Google Scholar]
- 17. Spodsberg EH, Wiinberg B, Jessen LR, Marschner CB, Kristensen AT. Endogenous fibrinolytic potential in tissue‐plasminogen activator‐modified thromboelastography analysis is significantly decreased in dogs suffering from diseases predisposing to thrombosis. Vet Clin Pathol. 2013;42:281‐290. [DOI] [PubMed] [Google Scholar]
- 18. Putsche JC, Kohn B. Primary immune‐mediated thrombocytopenia in 30 dogs (1997‐2003). J Am Anim Hosp Assoc. 2008;44:250‐257. [DOI] [PubMed] [Google Scholar]
- 19. Brown JC, Brainard BM, Fletcher DJ, Nie B, Arnold RD, Schmiedt CW. Effect of aminocaproic acid on clot strength and clot lysis of canine blood determined by use of an in vitro model of hyperfibrinolysis. Am J Vet Res. 2016;77:1258‐1265. [DOI] [PubMed] [Google Scholar]
- 20. Makielski KM, Brooks MB, Wang C, Cullen JN, O'Connor AM, LeVine DN. Development and implementation of a novel immune thrombocytopenia bleeding score for dogs. J Vet Intern Med. 2018;32:1041‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang W, Hosgood G, Luobikis K, Paul A. Agreement of point‐of‐care prothrombin and activated partial thromboplastin time in dogs with a reference laboratory. Aust Vet J. 2018;96:379‐384. [DOI] [PubMed] [Google Scholar]
- 22. Gernsheimer TB, Brown SP, Triulzi DJ, et al. Prophylactic tranexamic acid in patients with hematologic malignancy: a placebo‐controlled, randomized clinical trial. Blood. 2022;140:1254‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartholomew JR, Salgia R, Bell WR. Control of bleeding in patients with immune and nonimmune thrombocytopenia with aminocaproic acid. Arch Intern Med. 1989;149:1959‐1961. [PubMed] [Google Scholar]
- 24. Zitek T, Weber L, Pinzon D, Warren N. Assessment and management of immune thrombocytopenia (ITP) in the emergency department: current perspectives. Open Access Emerg Med. 2022;14:25‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3:3780‐3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hooi KS, Lemetayer JD. The use of intravesicular alteplase for thrombolysis in a dog with urinary bladder thrombi. J Vet Emerg Crit Care. 2017;27:590‐595. [DOI] [PubMed] [Google Scholar]
- 27. Heubel‐Moenen F, Henskens YMC, Verhezen PWM, et al. Fibrinolysis in patients with chemotherapy‐induced thrombocytopenia and the effect of platelet transfusion. J Thromb Haemost. 2019;17:1073‐1084. [DOI] [PubMed] [Google Scholar]
- 28. Ilich A, Gernsheimer TB, Triulzi DJ, et al. Absence of hyperfibrinolysis may explain lack of efficacy of tranexamic acid in hypoproliferative thrombocytopenia. Blood Adv. 2022;7:900‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fletcher DJ, Rozanski EA, Brainard BM, de Laforcade AM, Brooks MB. Assessment of the relationships among coagulopathy, hyperfibrinolysis, plasma lactate, and protein C in dogs with spontaneous hemoperitoneum. J Vet Emerg Crit Care (San Antonio). 2016;26:41‐51. [DOI] [PubMed] [Google Scholar]
- 30. Garabet L, Ghanima W, Monceyron Jonassen C, et al. Effect of thrombopoietin receptor agonists on markers of coagulation and P‐selectin in patients with immune thrombocytopenia. Platelets. 2019;30:206‐212. [DOI] [PubMed] [Google Scholar]
- 31. Smith SA, McMichael MA, Gilor S, et al. Correlation of hematocrit, platelet concentration, and plasma coagulation factors with results of thromboelastometry in canine whole blood samples. Am J Vet Res. 2012;73:789‐798. [DOI] [PubMed] [Google Scholar]
- 32. Lynch AM, Ruterbories L, Jack J, Motsinger‐Reif AA, Hanel R. The influence of packed cell volume versus plasma proteins on thromboelastographic variables in canine blood. J Vet Emerg Crit Care (San Antonio). 2020;30:418‐425. [DOI] [PubMed] [Google Scholar]
- 33. Rose LJ, Dunn ME, Allegret V, Bédard C. Effect of prednisone administration on coagulation variables in healthy beagle dogs. Vet Clin Pathol. 2011;40:426‐434. [DOI] [PubMed] [Google Scholar]
- 34. Poitout‐Belissent F. Nonclinical evaluation of compound‐related alterations in hemostasis. Brooks MB, Harr KE, Seelig DM, Wardrop KJ, Weiss DJ, (eds) In: Schalm's Veterinary Hematology. 7th ed. Hoboken, NJ: Wiley‐Blackwell, 2022:108‐115. [Google Scholar]


