Abstract
The characteristics of antibody-dependent cellular cytotoxicity (ADCC) directed by a panel of human and chimpanzee antienvelope (anti-Env) monoclonal antibodies (MAbs) of different epitope specificities were studied; this was accomplished by using target cells expressing human immunodeficiency virus type 1 (HIV-1) Envs of either primary or laboratory-adapted strains. Human MAbs of similar apparent affinities (1 × 109 to 2 × 109 liters/mol) against either a “cluster II”-overlapping epitope of gp41 or against the CD4 binding site, V3 loop, or C5 domain of gp120 directed substantial and comparable levels of specific lysis against targets infected with laboratory-adapted strains of HIV-1. As expected, those MAbs specific for relatively conserved regions of Env generally exhibited ADCC activity against a broader range of HIV-1 strains than those directed against variable epitopes. Significant ADCC activities of selected MAbs against primary isolate Env-expressing cells were demonstrated. In addition, a new ADCC epitope in the V2 domain of gp120 was defined. CD56+ cells were demonstrated to be the effector cells in these studies by fluorescence-activated cell sorting followed by ADCC assays. Notably, all anti-Env MAbs tested in this study, including MAbs directed against each of the known neutralization epitope clusters in gp120, directed significant levels of ADCC against targets expressing Env of one or more HIV-1 strains. These results imply that many, if not most, HIV-1-neutralizing human Abs of high affinity (≥3 × 108 liters/mol in these studies) and of the immunoglobulin G1 (IgG1) subclass (i.e., the predominate IgG subclass) are capable of directing ADCC. Since neutralizing Abs have been associated with long-term survival following HIV-1 infection, this suggests that ADCC activity may be beneficial in vivo.
The in vivo role(s) of antibodies (Abs) that can direct antibody-dependent cellular cytotoxicity (ADCC) against human immunodeficiency virus type 1 (HIV-1) Env-expressing cells in vitro remains unclear. In ADCC, anti-Env Abs direct effector cells to kill target cells bearing HIV-1 envelope on their surfaces; this is accomplished via specific binding of the Abs’ antigen-binding sites to Envs and their Fc regions to Fc receptors on the effector cells. Broadly strain reactive, ADCC-directing Abs arise early in the immune response to HIV-1 infection in vivo (14) and may be partially responsible for the initial clearance of viremia.
Earlier in the HIV-1 epidemic, concerns were raised that shed soluble gp120 in HIV-1-infected individuals might bind to CD4+ cells, including uninfected ones, and could target these cells for “innocent bystander” killing by ADCC (6). However, effector cells armed with serum Abs able to direct ADCC in vitro against either innocent bystanders or HIV-1-infected cells were found at highest frequency in asymptomatic, seropositive individuals; patients with AIDS-related complex and AIDS showed progressively diminished reactivities (20). Furthermore, in a recent study (1), the ability of monoclonal Abs (MAbs) against three distinct gp120 epitopes to direct ADCC against uninfected CD4+ cells to which rgp120SF2 had been adsorbed (i.e., innocent bystanders) was demonstrated to be less efficient by at least an order of magnitude than their ability to direct ADCC against HIV-1-infected cells.
The existing data from in vivo studies (reviewed in reference 1) supports the efficacy, rather than the pathogenicity, of ADCC-directing Abs against HIV-1. Consistent with this data is our recent characterization of two MAbs, 42F and 43F, isolated from a long-term survivor of HIV-1 infection (1); these MAbs directed significant levels of ADCC and defined a new, conserved ADCC epitope in the C5 domain of HIV-1 gp120. Preliminary evidence indicated that concentrations of 42F- and 43F-like Abs in the serum of the donor were in the range required to direct high levels of ADCC, and these MAbs were shown to bind both oligomeric primary-isolate and laboratory-adapted Env efficiently (1).
Because of the potential importance of ADCC-directing Abs against HIV-1, in this study we have evaluated ADCC directed against cells expressing HIV-1 Envs of primary or laboratory-adapted strains by a panel of human and chimpanzee anti-Env MAbs of different epitope specificities. Significant ADCC activities of selected MAbs against primary-isolate Env-expressing cells were demonstrated, and a new ADCC epitope in the V2 domain of gp120 was defined. Finally, a MAb’s ability to direct ADCC against a specific target cell type was shown to be dependent on additional factors beyond its ability to efficiently bind antigen on the target cell and its possession of an Fc region of the appropriate isotype to engage FcγR on effector cells.
MATERIALS AND METHODS
Human and chimpanzee MAbs and fragments.
Human MAbs utilized in this study were anti-CD4 binding site (bs) MAbs 1125H (10, 11) and 5145A (7), anti-V3 loop MAbs 4117C (12) and 41148D (8), anti-C5 MAb 42F (1), and anti-gp41 MAb 31710B (11). Chimpanzee MAbs utilized were anti-V2 MAb C108G (17, 19, 21) and anti-V3 loop MAb C311E (18). An F(ab′)2 fragment of MAb 1125H was produced by pepsin digestion as described previously (8). All MAbs are of the immunoglobulin G1 (IgG1) isotype.
HIV-1 strains, HIV-1-infected cells, and recombinant HIV-1 envelope-expressing cells.
HIV-1 strains used in these studies and the derivation of CEM.NKR cells chronically infected with these strains have been described previously (1). Recombinant Env-expressing vaccinia viruses and their use in similar studies have also been described previously (1).
Purification and quantitation of MAbs.
Human and chimpanzee MAbs were purified, concentrated, and quantitated as described previously (7, 11).
Flow cytometric analyses.
Unless indicated otherwise (see Table 1), these analyses were carried out as previously described (1).
TABLE 1.
Percentages of HIV-1-infected cells staining above background by flow cytometry analysis following incubation with ant-Env Abs and MAbs under different conditionsa
| Conditions of incubation (°C/min) | Ab or MAb used | % SF-2-infected cells staining, gate 2 | % RF-infected cells staining, gate 2 |
|---|---|---|---|
| 4/30 | None | 1 | <1 |
| Seropositive sera | 100 | 100 | |
| 4117C | 96 | 96 | |
| 41148D | 80 | 98 | |
| 37/30 | None | <1 | <1 |
| Seropositive sera | 100 | 100 | |
| 4117C | 93 | 92 | |
| 41148D | 67 | 86 | |
| 4/30, then 37/30 | None | <1 | <1 |
| Seropositive sera | 100 | 100 | |
| 4117C | 97 | 97 | |
| 41148D | 71 | 96 | |
| 4/30, then 37/60 | None | <1 | <1 |
| Seropositive sera | 100 | 100 | |
| 4117C | 96 | 92 | |
| 41148D | 72 | 85 | |
| 4/30, then 37/240 | None | <1 | <1 |
| Seropositive sera | 100 | 100 | |
| 4117C | 97 | 93 | |
| 41148D | 74 | 88 |
All incubations were done under the conditions given in the table using either no Ab or MAb, pooled seropositive sera at 10−3 dilution, or 4117C or 41148D at 20 μg/ml in a buffer containing 10 mM NaN3 as described previously (1). An aliquot of washed cells from each of these incubations was then counted for viability, while the remainder were reacted with fluorescein isothiocyanate-conjugated goat anti-human IgG at 4°C prior to measurement of fluorescence intensity by flow cytometry as described previously (1). Cell viability after the 4-h incubation at 37°C (final rows of table) was ≥75%, while for all other conditions, it was ≥95%.
Preparation of specific human effector cell populations.
For effector cell identification studies, aliquots of approximately 107 peripheral blood mononuclear cells (PBMC) were incubated at 4°C for 30 min with cell type-specific, fluorescently labeled MAbs in 1 ml of phosphate-buffered saline (PBS) containing 2% heat-inactivated fetal calf serum (FCS). The MAbs were obtained from Coulter Corp. and were used at a concentration of 60 μl of MAb per ml; this concentration was determined in preliminary experiments to give optimal staining of the appropriate cell populations. For specific staining of NK cells, B cells, and monocytes, MAbs NKH-1-RD1 (anti-CD56), B4-FITC (anti-CD19), and Mo2-FITC (anti-CD14), respectively, were used. Following incubation with these MAbs, cells were washed in PBS containing 2% FCS; then, positively and negatively staining fractions of cells were sorted in an EPICS Elite cell sorter (Coulter Corp.). Diagnostic flow cytometry analyses of the sorted cells confirmed that the positively selected cells were 85 to 90% pure and that the PBMC which were depleted of specific cell types, i.e., negatively selected cells, retained ≤3% of the positively stained cells. Control PBMC for these experiments were incubated and washed under the same conditions as the sorted cells but were not treated with MAbs or sorted. When sorting was complete, all cells were pelleted and resuspended in media for use in ADCC assays as described previously (1).
Anti-HIV-1 ADCC assay.
This assay was carried out essentially as described previously (1). In some assays, normal chimpanzee PBMC rather than human PBMC were used as the source of effector cells. Fresh blood from a young, healthy chimpanzee was obtained for these experiments, and PBMC were isolated and prepared as described previously (1).
Epitope mapping.
Synthetic peptides were used to coat polyvinyl chloride enzyme-linked immunosorbent assay (ELISA) plates at 500 ng per well in Na2CO3-NaHCO3 buffer, pH 9.8, overnight at 4°C. Following a blocking step described previously (11), cell supernatants containing approximately 1 μg of MAb per ml or purified MAb at 20 μg/ml were incubated with each peptide. Diluted sera from seropositive individuals were also separately incubated with the peptides on plates and served as positive controls to ensure that each peptide had attached to the plate in reactive form. A standard ELISA protocol (11) was used to detect bound human and chimpanzee IgG Abs.
RESULTS
Binding and ADCC directed by MAbs against targets chronically infected with various HIV-1 strains.
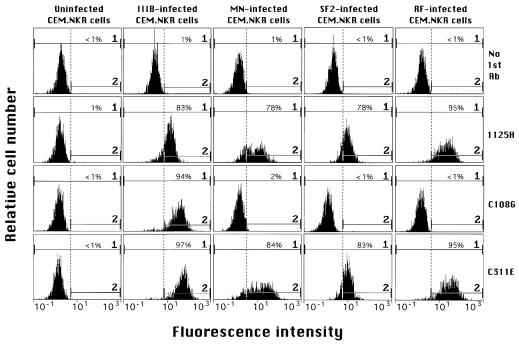
The abilities of a panel of six human and two chimpanzee anti-Env MAbs of different epitope specificities to bind and/or direct ADCC against target cells infected with various HIV-1 strains were assessed in this study. Figure 1 shows the results of representative flow cytometry analyses of selected mAbs’ binding to CEM.NKR cells chronically infected with the IIIB, MN, SF-2, or RF strain. MAbs 1125H (anti-CD4 bs) and C311E (anti-V3 loop) efficiently stained cells that were chronically infected with each of the four HIV-1 strains, while C108G (anti-V2) specifically stained only the cells chronically infected with the IIIB strain. These results correlated with the strain specificities of these three MAbs previously observed in neutralization assays (11, 18, 18a, 19).
FIG. 1.
Results of flow cytometry analyses of uninfected or HIV-1-infected CEM.NKR cells. MAbs 1125H, C108G, and C311E were used at 20 μg/ml. In this experiment, gate 2 was set to exclude ≥99% of the background fluorescence observed with no first Ab added (only fluorescein isothiocyanate conjugate was added) for each type of infected or uninfected cells. The percentage of cells staining within gate 2 (above background) is shown in each panel.
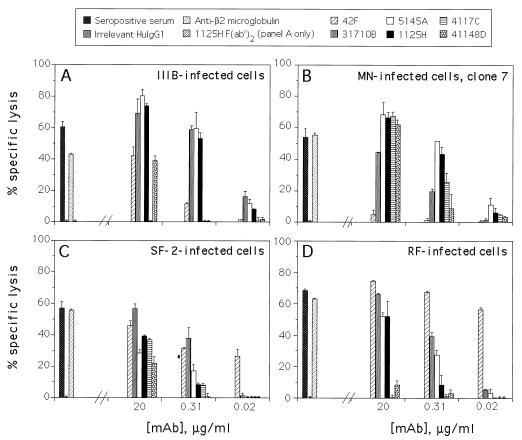
While MAbs against the CD4 bs and V3 loop of gp120 and “cluster II” of gp41 (reviewed in reference 13), as well as those against the C5 domain of gp120 (1), were previously observed to direct ADCC, the relative activities of MAbs specific for these different epitope clusters and their breadths of strain specificity in ADCC have not been compared prior to this study. Figure 2 shows that the anti-gp41 MAb, 31710B, and the two anti-CD4 bs MAbs, 5145A and 1125H, directed comparable levels of specific lysis against targets infected with each of the four HIV-1 strains tested. This is consistent with previous observations that 31710B and the anti-CD4 bs MAbs had similar apparent affinities for monomeric rgp160LAI, i.e., 1 × 109 to 2 × 109 liters/mol, and that 1125H and 5145A had comparable potencies, within experimental error, for neutralizing the strains involved in this experiment (7, 11). The anti-V3 MAb, 4117C, directed specific lysis comparable to that directed by 31710B, 5145A, and 1125H against the MN- and SF2-infected cells, but not against the IIIB- or RF-infected cells. This correlates with findings that 4117C had a potency comparable to that of 1125H in the neutralization of the MN and SF2 strains but did not neutralize the IIIB or RF strains at ≤20 μg of mAb per ml (12, 13a).
FIG. 2.
Representative ADCC assay results obtained with a panel of human anti-Env MAbs. CEM.NKR cells were chronically infected with the HIV-1 strains indicated. The left side of each panel shows results with the following controls: pooled seropositive serum at 10−3 dilution, irrelevant human (Hu) IgG1 (myeloma protein; ICN Immunobiological, Costa Mesa, Calif.) at 20 μg/ml, and rabbit antiserum against β2 microglobulin (Accurate Chemical, Westbury, N.Y.), a positive control against both uninfected and infected cells, at 33 μg/ml. Results with the latter control against uninfected cells were comparable to those seen against the four infected cell lines shown, while no specific lysis above background was seen against uninfected cells for any of the other Abs and MAbs in this experiment (data not shown). The right side of each panel shows results with various concentrations of each human MAb. The error bars represent standard deviations of duplicate points.
Surprisingly, anti-V3 MAb 41148D directed substantial lysis against the IIIB-, MN-, and SF2-infected cells (Fig. 2), though in previous neutralization assays this MAb was found to be less potent by at least an order of magnitude than MAb 4117C against these HIV-1 strains (13a). Thus, viral neutralization potency and ADCC activity may often be correlated, as seen when comparing the activities of 5145A, 1125H, and 4117C, but exceptions clearly exist, as seen upon comparing the results for 4117C and 41148D in Fig. 2B and C to those of previous neutralization assays.
As previously reported (1), anti-C5 MAb 42F directed significant lysis against the IIIB, SF-2, and RF strains, but not against clone 7 of the MN-infected cells used in this experiment (Fig. 2). However, in separate experiments, uncloned MN-infected cells were moderately lysed by 42F (1). Since the apparent affinity of 42F for rgp160LAI was similar to that of 1125H (1, 11), the level of specific lysis directed by 42F was slightly lower than expected against IIIB-infected cells. Nevertheless, the level of specific lysis directed by 42F against SF2- and RF-infected cells, especially at lower MAb concentrations, was notably high (Fig. 2).
In all cases in Fig. 2 where cell lysis was observed with a given MAb-target combination, the percent specific lysis was a function of the MAb concentration used in the assay. Furthermore, the lysis was dependent on intact Fc regions of the Abs and MAbs used, since the F(ab′)2 fragment of 1125H directed no specific lysis against IIIB-infected targets (Fig. 2A), though this fragment bound efficiently to antigen in ELISA assays (data not shown). These findings corroborate antibody-dependent cellular cytotoxicity as the mechanism responsible for the specific lysis observed.
In general, an excellent correlation was observed between the level of staining of a given infected target cell type by a MAb as seen by flow cytometry and the level of specific lysis directed against that target by the MAb (1) (e.g., results with 1125H in Fig. 1 and 2). Notable exceptions to this pattern were seen, however, in the interaction of anti-V3 MAbs 4117C and 41148D with RF-infected cells. That is, these MAbs were observed to bind the RF-infected cells under our standard conditions (4°C, 30 min) as well as or better than they bound SF2-infected cells, for example (Table 1), but to direct no significant ADCC against the RF-infected targets (Fig. 2D).
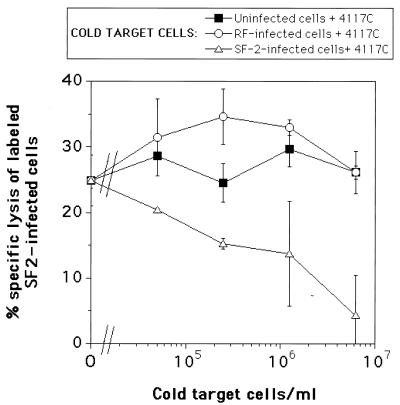
To address this inconsistency, we varied the primary binding step to include various periods of incubation at 37°C, reasoning that this would more closely mimic the conditions of the ADCC assay itself. The results, shown in Table 1, demonstrate that the binding of RF-infected cells by both 4117C and 41148D is, indeed, slightly reduced at 37°C compared to that at 4°C. Nevertheless, the binding of RF-infected cells by both MAbs is under all conditions greater than or equal to the binding of SF2-infected cells by the MAbs. However, the MAbs direct substantial ADCC against SF2-infected cells but not against RF-infected cells (Fig. 2C and D). We interpret this conundrum to indicate that binding of 4117C or 41148D to RF-infected targets results in the MAbs’ Fc regions becoming sterically or functionally inaccessible to effector cells for the multivalent engagement of Fc receptors. This interpretation is corroborated by results of the cold-target competition assays shown in Fig. 3. While SF-2-infected, unlabeled cells preincubated with 4117C could effectively inhibit the specific lysis of labeled, 4117C-coated targets (SF-2-infected cells) by effector cells, the RF-infected, unlabeled cells preincubated with 4117C were not recognized by the effector cells and did not inhibit the effectors’ lysis of labeled targets (Fig. 3). In contrast, RF-infected, unlabeled cells preincubated with MAb 1125H could effectively inhibit such lysis (data not shown).
FIG. 3.
ADCC assay results obtained by using Na251CrO4-labeled SF2-infected targets in the presence of various numbers of cold-target cells. Various numbers of unlabeled cells were preincubated with 26 μg of 4117C MAb per ml, while the standard number of labeled targets (104 cells) was separately preincubated with the same amount of 4117C MAb. Following this preincubation, the labeled target-MAb mixture was added to the unlabeled target-MAb (cold-target) mixture, attaining the cold-target cell-per-milliliter concentrations shown in the figure. The remainder of the ADCC assay was carried out as previously described (1). The error bars represent standard deviations of duplicate points.
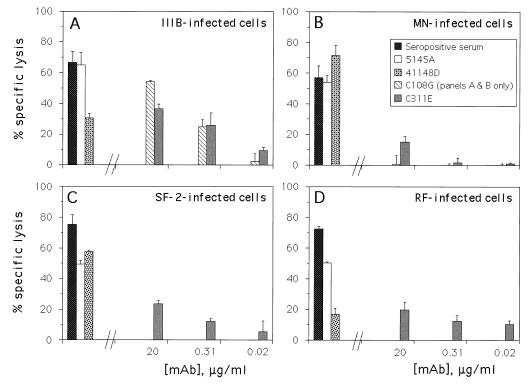
Figure 4 shows representative results of ADCC assays using the two chimpanzee MAbs, C108G and C311E, to direct lysis against targets infected with each of the four laboratory-adapted strains. As expected from the strain specificities exhibited by these MAbs in neutralization assays (18, 18a, 19) and in flow cytometry (Fig. 1), C108G directed ADCC only against the IIIB-infected targets, while C311E directed significant lysis against targets infected with all four HIV-1 strains. To our knowledge, this is the first observation of ADCC directed by a MAb (i.e., C108G) against an epitope in the V2 domain of gp120.
FIG. 4.
ADCC assay results obtained by using two chimpanzee anti-Env MAbs. CEM.NKR cells were chronically infected with the HIV-1 strains indicated. The left side of each panel shows results with the following controls: pooled seropositive serum at 10−3 dilution and human MAbs 5145A and 41148D, each at 20 μg/ml. The right side of each panel shows results with various concentrations of each chimpanzee MAb. No specific lysis above background was seen against uninfected cells with any of the Abs or MAbs in this experiment (data not shown). The error bars represent standard deviations of triplicate points.
The level of specific lysis directed by C108G against the IIIB-infected targets (Fig. 4) was somewhat lower than that expected based on its level of staining of these cells (Fig. 1) and its potency in neutralization of the IIIB strain (19). In addition, the specific lysis directed by C311E against IIIB-infected targets and targets infected by other strains was significantly less than that directed by C108G against IIIB-infected cells or by 5145A against each of the infected targets (Fig. 4), though the levels of staining of these cells by C311E were greater than or equal to those seen with 1125H (Fig. 1) or 5145A (data not shown) and the neutralization potency of C311E against IIIB was similar to that of C108G (18, 18a).
Since human PBMC were used as effector cells in these ADCC assays (Fig. 4), we questioned whether the interaction of the chimpanzee MAb Fc regions and human Fcγ receptors on effector cells was of significantly lower avidity than the homologous human Fc-human FcγR or chimpanzee Fc-chimpanzee FcγR interactions; if so, this might account for the reduced efficacies of the chimpanzee MAbs in these experiments. To address this question, we performed ADCC assays using either human or chimpanzee PBMC as effectors in combination with both human and chimpanzee anti-Env sera and MAbs. In each of two experiments (data not shown), chimpanzee PBMC were significantly (0.001 < P < 0.01) less effective than human PBMC in mediating ADCC; this was the case whether human or chimpanzee Abs or MAbs were directing the ADCC observed. Nevertheless, if one hypothesized that the chimpanzee Abs and MAbs were more effective than human Abs and mAbs when using chimpanzee PBMC as effectors, then the ratio of specific lysis directed by chimpanzee effectors to that directed by human effectors should be significantly higher for the chimpanzee Abs and MAbs than for the human Abs and MAbs. Calculation of these ratios from our data (data not shown) and performance of Student’s t test indicate that this hypothesis is improbable (P = 0.1 to 0.2). Thus, there was no detectable difference in these experiments between homologous versus heterologous Fc-FcγR interactions in ADCC.
ADCC directed by MAbs against primary-isolate Env-expressing targets.
Previous studies (1) demonstrated the binding of anti-C5 MAb 43F and anti-CD4 bs MAb IgG1b12 (2) to CD4− T cells infected with recombinant vaccinia viruses expressing laboratory-adapted and primary-isolate Envs from clades B and E. Further binding studies of this type were done with additional MAbs of various epitope specificities (data not shown), and the results of these confirmed our earlier impression that, of the three vaccinia virus recombinant primary-isolate Envs tested, the clade E Env of vCB53 was most efficiently recognized by seropositive serum and a panel of anti-Env MAbs. This appeared to be due to a higher level of Env expression from the vCB53 recombinant virus (1). Thus, we utilized cells infected with vCB53 as the prototypic targets for primary-isolate Env-specific ADCC in these studies.
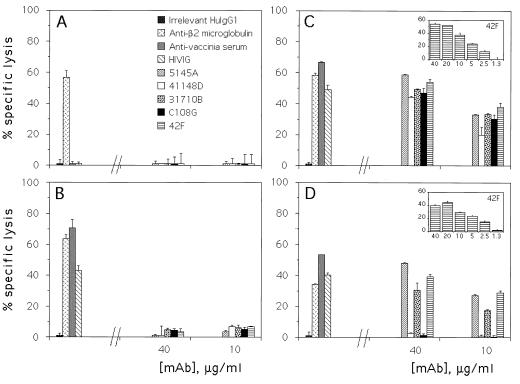
Figure 5D shows that three MAbs of different epitope specificities, 5145A (anti-CD4 bs), 31710B (anti-gp41), and 42F (anti-CD5), directed substantial ADCC against the vCB53-infected cells, while two MAbs specific for variable domains in gp120, 41148D (anti-V3) and C108G (anti-V2), did not direct ADCC against these targets. The latter was expected, since these two MAbs were raised against a clade B isolate(s) and variable-domain-specific MAbs are usually clade specific. MAbs 41148D and C108G do bind to cells infected with certain primary isolates of clade B (data not shown), and C108G neutralizes such isolates (18). Figure 5C shows that each of the MAbs tested is capable of directing substantial ADCC against vPE16-infected cells; this recombinant virus encodes Env of the BH8 clone of IIIB, a clade B laboratory-adapted virus. The positive controls, anti-vaccinia virus serum and HIV immuneglobulin (HIVIG), directed ADCC against cells infected with wild-type vaccinia virus (Fig. 5B) or recombinant vaccinia virus (Fig. 5C and D), while anti-β2 microglobulin directed ADCC against both uninfected (Fig. 5A) and vaccinia virus-infected (Fig. 5B to D) cells. Considering the apparently lower level of vaccinia virus protein (and, presumably, recombinant Env) expression by vCB53 versus that by vPE16, as evidenced by lower levels of ADCC directed by anti-vaccinia virus serum and HIVIG in Fig. 5D compared to those in Fig. 5C, the levels of ADCC directed by MAbs 5145A, 31710B, and 42F against primary-isolate versus laboratory-adapted Env-expressing cells are comparable in these experiments. The insets in Fig. 5C and D show that anti-C5 MAb 42F retains significant ADCC-directing activity against either the primary isolate or laboratory-adapted Env-expressing targets down to MAb concentrations as low as 2.5 μg/ml.
FIG. 5.
Results of ADCC assays using as targets A2.01 cells that were uninfected (A), infected with wild-type vaccinia virus (WR) (B), infected with vPE16, a vaccinia virus recombinant expressing Env of laboratory-adapted strain BH8 (C), or infected with vCB53, a vaccinia virus recombinant expressing a clade E primary isolate Env (D). The left side of each panel shows results with the following controls: irrelevant human (Hu) IgG1 (myeloma protein; ICN Immunobiological) at 40 μg/ml; rabbit antiserum against β2 microglobulin (Accurate Chemical), a positive control against both uninfected and infected cells, at 30 μg/ml; serum from a healthy individual recently immunized against vaccinia virus (anti-vaccinia serum) at 1/200; and HIVIG at 40 μg/ml. The right side of each panel shows results for two or more concentrations of each human or chimpanzee MAb tested. The error bars represent standard deviations of triplicate points.
CD56+ cells are the effector cells in these ADCC studies.
Previous studies (9, 15) addressing the effector cell type in PBMC responsible for mediating ADCC against HIV-1 Env-expressing or -bearing cells indicated that primarily NK cells are involved. However, the results of Posner et al. (9) suggested that additional populations of effector cells functioned in ADCC mediated by polyclonal sera as opposed to that mediated by anti-Env MAb. These earlier studies involved the blocking of FcγR (CD16) on NK cells with Ab (9, 15) and/or the elimination of NK cells by Ab and complement (15).
We revisited this issue via fluorescence-activated cell sorting of specific cell populations from whole PBMC, followed by ADCC assays utilizing the positively selected or specifically depleted populations as effector cells. Table 2 shows that the positively selected CD56+ cells, but not the CD14+ or CD19+ cells, were capable of mediating ADCC against infected cells in the presence of seropositive serum. The CD56+ cells were capable of mediating levels of specific lysis comparable to that mediated by total PBMC, but at lower effector-to-target (E/T) ratios than those for unseparated PBMC, as expected if one has enriched for the appropriate effector population. The depletion experiments (Table 3) indicate that the CD56+ cells are entirely responsible for the ADCC mediated by total PBMC, since PBMC depleted of these cells mediate no significant lysis, while those depleted of CD14+ or CD19+ cells show no significant reduction in specific lysis. Similar results were obtained when MAbs against HIV-1 Env were utilized to sensitize target cells (data not shown).
TABLE 2.
Percent specific lysis of IIIB-infected CEM.NKR cells by different positively selected human effector cell populations in the presence of pooled, human seropositive sera (10−3 dilution)
| E/T ratio | % Specific lysis for indicated effector cell populationa
|
|||
|---|---|---|---|---|
| Total PBMC | CD56+ | CD19+ | CD14+ | |
| 100 | 33 ± 2 | ND | ND | ND |
| 33 | 16 ± 4 | 30 ± 4 | 0 ± 1 | 0 ± 2 |
| 11 | 0 ± 4 | 13 ± 3 | 0 ± 2 | 0 ± 2 |
| 3 | ND | 6 ± 2 | 0 ± 1 | 1 ± 1 |
Effector cell populations were prepared as described in Materials and Methods. Errors represent the standard deviations of triplicate measurements. ND, not determined.
TABLE 3.
Percent specific lysis of IIIB-infected CEM.NKR cells by total PBMC and PBMC depleted of different cell types in the presence of pooled, human seropositive sera (10−3 dilution)
| Expt no. | E/T ratio | % Specific lysis for indicated effector cell populationa
|
|||
|---|---|---|---|---|---|
| Total PBMC | CD56+-depleted PBMC | CD19+-depleted PBMC | CD14+-depleted PBMC | ||
| 1 | 100 | 32 ± 1 | 5 ± 2 | 24 ± 4 | 25 ± 5 |
| 2 | 100 | 34 | 4 | 31 | 34 |
Effector cell populations were prepared as described in Materials and Methods. Errors represent the standard deviations of triplicate measurements.
Epitope of anti-gp41 MAb 31710B.
Having observed significant and broad ADCC directed by the anti-gp41 MAb, 31710B (Fig. 2 and 5), we sought to map its epitope as part of this study. Initially, this was attempted using an epitope scanning kit (Cambridge Research Biochemicals, Valley Stream, N.Y.) as previously described (12). The kit contained 12-mer peptides with 10-amino-acid overlaps that spanned the entire extracellular domain of gp41LAI. Despite the reactivities of control antibodies and peptides in these experiments, 31710B, at concentrations as high as 10 μg/ml, did not react with any of these gp41-derived peptides, though it reacted with rgp160LAI (data not shown). These results suggested that the 31710B epitope was conformational and/or required a peptide greater than 12 residues in length for its formation. Thus, truncation mutants of gp160 were utilized to localize the 31710B epitope by radioimmunoprecipitation and sodium dodecyl sulfate gel analysis essentially as described previously (5). Results of these experiments (data not shown) demonstrated that 31710B could precipitate the vPE17 (5) and vPE12B (4) truncation mutants but not the shorter vPE18 (5) truncation mutant, while the control seropositive serum could precipitate each of these mutants. Since vPE12B contains 43 amino acids just N-terminal to the transmembrane region of gp41 which are not contained in vPE18 (5), the 31710B epitope was localized to this region.
To map the 31710B epitope more precisely, we utilized an ELISA approach involving a series of 20-mer peptides with 10-amino-acid overlaps from the 43-amino-acid region of gp41 described above. Representative results from these assays are shown in Table 4. The 31710B MAb reacted with a single peptide (2030) from this region that contained amino acids 651 to 670 (Los Alamos numbering for the MN strain). The fact that the MAb did not react with peptides 2029 and 2031, which share 10 amino acids at the N terminus and C terminus, respectively, of peptide 2030 indicates that the epitope is centered near the middle of peptide 2030 and/or requires additional amino acids on either side of its core sequence for recognition by 31710B, as suggested by earlier experiments discussed above. The 31710B epitope overlaps, but does not coincide precisely with, the cluster II epitope of previously described ADCC-directing human MAb 120-16 (16).
TABLE 4.
Epitope mapping of 31710B to the extracellular domain of HIV-1 gp41 by peptide ELISA
| Viral strain and peptide (amino acid position) | Amino acid numbering or sequencea | Reactivity (OD450b) |
|---|---|---|
| HIV-1 MN | 641 660 670 680 | |
| 2029 (641–660) | SLIYSLLEKSQTQQEKNEQE | −0.31 |
| 2030 (651–670) | QTQQEKNEQELLELDKWASL | 1.22 |
| 2031 (661–680) | LLELDKWASLWNWFDITNWL | −0.24 |
Los Alamos amino acid numbering for the MN strain is shown.
OD450, optical density at 450 nm.
DISCUSSION
The specific activities and breadths of HIV-1 strain recognition in ADCC of MAbs against five different HIV-1 Env epitope clusters were compared in this study. The results demonstrated that human MAbs of similar apparent affinities (1 × 109 to 2 × 109 L/mol) against either a cluster II-overlapping epitope of gp41 or against the CD4 bs, V3 loop, or C5 domain of gp120 directed substantial and comparable levels of specific lysis against targets infected with laboratory-adapted strains of HIV-1. Thus, these four epitope clusters can elicit comparably potent, ADCC-directing Abs. A new ADCC epitope in the V2 domain of HIV-1 gp120 was identified via the observed specific lysis of IIIB-infected targets directed by anti-V2 chimpanzee MAb C108G (19).
As expected, the MAbs directed against relatively conserved regions of Env, i.e., the CD4 bs and C5 domain of gp120 and the cluster II-overlapping region of gp41, generally exhibited ADCC activity against a broader range of HIV-1 strains than those directed against variable epitopes, i.e., V3 loop and V2 domain. This extended to ADCC studies employing cells expressing Env of a primary, clade E isolate; these cells could be lysed only in the presence of the broadly clade-reactive MAbs. Nevertheless, the MAbs against variable epitopes were capable of binding to Envs of clade B primary isolates (1a, 18) and, as ADCC-directing MAbs, are likely to be able to direct ADCC against cells expressing Env of such strains. To our knowledge, these are the first observations of ADCC directed against primary-isolate Env-expressing targets by anti-Env MAbs.
There was a precise correlation between the strain specificities of neutralizing MAbs previously observed in neutralization assays and those seen here in ADCC assays. However, attempts to correlate MAb potencies seen in previous neutralization experiments with those observed for the direction of ADCC in this study yielded disparate results. Anti-V3 MAb 4117C and anti-CD4 bs MAbs 5145A and 1125H had comparable potencies in neutralization (7, 11, 12), and their specific ADCC activities were also similar. However, human anti-V3 MAb 41148D was surprisingly efficient at directing ADCC against IIIB-, MN-, and SF2-infected targets, considering its poor neutralizing activity against the same strains (13a). Conversely, chimpanzee anti-V3 MAb C311E was significantly less efficient at directing ADCC than it was at neutralizing the same strains of virus (18, 18a). These disparities between neutralizing and ADCC-directing activities may reflect differences in oligomeric Env structure as expressed on the surfaces of virions versus infected cells and/or the fact that ADCC activity, unlike neutralizing activity, depends on multivalent presentation of MAb Fc regions to FcγR on effector cells, in addition to the binding of MAb to viral antigens.
The lack of high-avidity, multivalent presentation of MAb Fc regions to FcγR on effector cells appears to be the explanation for our unusual observations that anti-V3 MAbs 4117C and 41148D were not able to direct ADCC against RF-infected targets, though they could bind to such cells efficiently. These findings cannot be explained by a lack of appropriate Fc regions in these MAbs, since the MAbs directed ADCC efficiently against targets infected with other HIV-1 strains, e.g., the MN strain. Furthermore, MAbs against other epitopes, including a MAb (C311E) against a distinct V3 epitope, could direct ADCC against the RF-infected cells, so these targets were not generally resistant to ADCC. Perhaps when the 4117C and 41148D MAbs engaged their epitopes on RF Env, but not on the Env of other strains, their Fc regions became sterically inaccessible to FcγR on effector cells. Another possibility is that, during the ADCC assay, the 4117C and 41148D MAbs were rapidly internalized upon binding to the RF Env (but not upon binding to Env of other strains) on target cells and, in this way, their Fc regions became inaccessible to FcγR. Our binding assays, which were done in the presence of azide, would not have detected such an internalization if it were energy dependent. Whatever the explanation, it is clear that, in this case, the binding of MAbs to target cells and the MAbs’ possession of Fc regions of the appropriate isotype did not result in their ability to direct ADCC against these targets. Further research will be required in order to understand the frequency with which such cases may occur and their potential biological significance.
Not all anti-Env human MAbs of the appropriate isotype mediated significant ADCC in previous studies (16), indicating that some of these MAbs had insufficient affinities and/or were not directed against epitopes that are accessible for ADCC. Given these observations, it is noteworthy that all anti-Env MAbs tested here, including MAbs directed against each of the known neutralization epitope clusters in gp120, directed significant levels of ADCC against targets infected separately with one or more HIV-1 strains. These results suggest that many, if not most, HIV-1-neutralizing Abs with high levels of affinity (≥3 × 108 liters/mol in these studies) and of the IgG1 subclass (i.e., the predominate subclass) in humans are capable of directing ADCC. This finding suggests that ADCC-directing Abs may be beneficial in vivo, given that neutralizing Abs (now seen to be ADCC-directing Abs as well) have been associated with long-term survival following HIV-1 infection (3).
ACKNOWLEDGMENTS
We thank Douglas Cohn, Laboratory for Experimental Medicine and Surgery in Primates, Tuxedo, N.Y., for normal chimpanzee blood samples; P. Earl, C. Broder, and B. Moss, NIH, for recombinant vaccinia viruses expressing truncation mutants of gp160 and complete primary-isolate Envs; Ruth Herz, BMCC/CUNY, New York, N.Y., for sharing preliminary results and technical expertise in ADCC assays; Carol Reiss, New York University, and Abraham Pinter, Public Health Research Institute, New York, N.Y., for critical reading of the manuscript; and M. Racho and R. Georgescu for valuable technical assistance. The A2.01 cells (contributed by T. Folks), HIVIG (contributed by A. Prince), WR vaccinia virus (contributed by B. Moss), vPE16 (contributed by P. Earl and B. Moss), and 20-mer gp41 peptides were obtained from the NIH AIDS Research and Reference Reagent Program.
This work was supported by Pediatric AIDS Foundation Summer Student Research Awards to O.A., by NIH grant AI26081 and a Lucille P. Markey Charitable Trust Award to S.A.T., and by NIH CFAR grant AI-72659.
REFERENCES
- 1.Alsmadi O, Herz R, Murphy E, Pinter A, Tilley S A. A novel antibody-dependent cellular cytotoxicity epitope in gp120 is identified by two monoclonal antibodies isolated from a long-term survivor of human immunodeficiency virus type 1 infection. J Virol. 1997;71:925–933. doi: 10.1128/jvi.71.2.925-933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Alsmadi, O., and S. A. Tilley. Unpublished data.
- 2.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. New Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 4.Earl P A, Broder C C, Long D, Lee S A, Peterson J, Chakrabarti S, Doms R W, Moss B. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J Virol. 1994;68:3015–3026. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earl P L, Koenig S, Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991;65:31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyerly H K, Matthews T J, Langlois A J, Bolognesi D P, Weinhold K J. Human T-cell lymphotropic virus IIIB glycoprotein (gp120) bound to CD4 determinants on normal lymphocytes and expressed by infected cells serves as target for immune attack. Proc Natl Acad Sci USA. 1987;84:4601–4605. doi: 10.1073/pnas.84.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinter A, Honnen W J, Racho M E, Tilley S A. A potent, neutralizing human monoclonal antibody against a unique epitope overlapping the CD4-binding site of HIV-1 gp120 that is broadly conserved across North American and African virus isolates. AIDS Res Hum Retroviruses. 1993;9:985–996. doi: 10.1089/aid.1993.9.985. [DOI] [PubMed] [Google Scholar]
- 8.Pinter A, Honnen W J, Tilley S A. Conformational changes affecting the V3 and CD4-binding domains of human immunodeficiency virus type 1 gp120 associated with env processing and with binding of ligands to these sites. J Virol. 1993;67:5692–5697. doi: 10.1128/jvi.67.9.5692-5697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posner M R, Elboim H S, Cannon T, Cavacini L, Hideshima T. Functional activity of an HIV-1 neutralizing IgG human monoclonal antibody: ADCC and complement-mediated lysis. AIDS Res Hum Retroviruses. 1992;8:553–558. doi: 10.1089/aid.1992.8.553. [DOI] [PubMed] [Google Scholar]
- 10.Thali M, Furman C, Ho D D, Robinson J, Tilley S, Pinter A, Sodroski J. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 1992;66:5635–5641. doi: 10.1128/jvi.66.9.5635-5641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilley S A, Honnen W J, Racho M, Hilgartner M, Pinter A. A human monoclonal antibody against the CD4 binding site of HIV-1 gp120 exhibits potent, broadly neutralizing activity. Res Virol. 1991;142:247–259. doi: 10.1016/0923-2516(91)90010-z. [DOI] [PubMed] [Google Scholar]
- 12.Tilley S A, Honnen W J, Racho M E, Chou T C, Pinter A. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4 binding site of gp120. AIDS Res Hum Retroviruses. 1992;8:461–467. doi: 10.1089/aid.1992.8.461. [DOI] [PubMed] [Google Scholar]
- 13.Tilley S A, Pinter A. Human and chimpanzee monoclonal antibodies with antiviral activity against HIV-1. In: Koff W C, Wong-Staal F, Kennedy R C, editors. AIDS research reviews. Vol. 3. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 255–287. [Google Scholar]
- 13a.Tilley, S. A., and A. Pinter. Unpublished data.
- 14.Tyler D S, Lyerly H K, Weinhold K J. Minireview: anti-HIV-1 ADCC. AIDS Res Hum Retroviruses. 1989;5:557–563. doi: 10.1089/aid.1989.5.557. [DOI] [PubMed] [Google Scholar]
- 15.Tyler D S, Nastala C L, Stanley S D, Matthews T J, Lyerly H K, Bolognesi D P, Weinhold K J. gp120 specific cellular cytotoxicity in HIV-1 seropositive individuals: evidence for circulating CD16+ effector cells armed in vivo with cytophilic antibody. J Immunol. 1989;142:1177–1182. [PubMed] [Google Scholar]
- 16.Tyler D S, Stanley S D, Zolla-Pazner S, Gorny M K, Shadduck P P, Langlois A J, Matthews T J, Bolognesi D P, Palker T J, Weinhold K J. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J Immunol. 1990;145:3276–3282. [PubMed] [Google Scholar]
- 17.Vijh-Warrier S, Murphy E, Yokoyama I, Tilley S A. Characterization of the variable regions of a chimpanzee monoclonal antibody with potent neutralizing activity against HIV-1. Mol Immunol. 1995;32:1081–1092. doi: 10.1016/0161-5890(95)00081-x. [DOI] [PubMed] [Google Scholar]
- 18.Vijh-Warrier S, Pinter A, Honnen W J, Tilley S A. Synergistic neutralization of human immunodeficiency virus type 1 by a chimpanzee monoclonal antibody against the V2 domain of gp120 in combination with monoclonal antibodies against the V3 loop and the CD4-binding site. J Virol. 1996;70:4466–4473. doi: 10.1128/jvi.70.7.4466-4473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Vijh-Warrier, S., and S. A. Tilley. Unpublished data.
- 19.Warrier S V, Pinter A, Honnen W J, Girard M, Muchmore E, Tilley S A. A novel, glycan-dependent epitope in the V2 domain of human immunodeficiency virus type 1 gp120 is recognized by a highly potent, neutralizing chimpanzee monoclonal antibody. J Virol. 1994;68:4636–4642. doi: 10.1128/jvi.68.7.4636-4642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinhold K J, Matthews T J, Ahearne P M, Langlois A J, Lyerly H K, Tyler D S, Stine K C, Durack D T, Bolognesi D P. Cellular anti-gp120 cytolytic reactivities in HIV-1 seropositive individuals. Lancet. 1988;i:902–905. doi: 10.1016/s0140-6736(88)91713-8. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, Kayman S C, Honnen W, Revesz K, Chen H, Vijh-Warrier S, Tilley S A, McKeating J, Shotton C, Pinter A. Characterization of neutralization epitopes in the V2 region of human immunodeficiency virus type 1 gp120: role of glycosylation in the correct folding of the V1/V2 domain. J Virol. 1995;69:2271–2278. doi: 10.1128/jvi.69.4.2271-2278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]