Abstract
Nonalcoholic fatty liver disease (NAFLD) is a stress-induced liver injury related to heredity, environmental exposure and the gut microbiome metabolism. Short-chain fatty acids (SCFAs), the metabolites of gut microbiota (GM), participate in the regulation of hepatic steatosis and inflammation through the gut-liver axis, which play an important role in the alleviation of NAFLD. However, little progress has been made in systematically elucidating the mechanism of how SCFAs improve NAFLD, especially the epigenetic mechanisms and the potential therapeutic application as clinical treatment for NAFLD. Herein, we adopted PubMed and Medline to search relevant keywords such as ‘SCFAs’, ‘NAFLD’, ‘gut microbiota’, ‘Epigenetic’, ‘diet’, and ‘prebiotic effect’ to review the latest research on SCFAs in NAFLD up to November 2023. In this review, firstly, we specifically discussed the production and function of SCFAs, as well as their crosstalk coordination in the gut liver axis. Secondly, we provided an updated summary and intensive discussion of how SCFAs affect hepatic steatosis to alleviate NAFLD from the perspective of genetic and epigenetic. Thirdly, we paid attention to the pharmacological and physiological characteristics of SCFAs, and proposed a promising future direction to adopt SCFAs alone or in combination with prebiotics and related clinical drugs to prevent and treat NAFLD. Together, this review aimed to elucidate the function of SCFAs and provide new insights to the prospects of SCFAs as a therapeutic target for NAFLD.
Keywords: Nonalcoholic fatty liver disease, Gut microbiota, Short-chain fatty acids, Genetic mechanisms, Epigenetic mechanisms
1. Introduction
The prevalence of nonalcoholic fatty liver disease (NAFLD) is increasing worldwide and becoming the leading cause of chronic liver disease in developed countries. However, to date, the specific mechanisms leading to NAFLD remain elusive. With the deepening of NAFLD research, it became clear that the two-strike theory could not effectively explain the interactions between genetics and the environment, the effects of microorganisms in the body, and the interactions between different organ systems. Therefore, the multiple-strike theory gradually became widely accepted [1]. In the multiple hits theory, dysbiosis of the gut microbiota (GM) has been identified as one of the factors necessary to influence the development of NAFLD [2]. The liver and intestines are closely related anatomically and functionally, and both develop from the same germ layer in the embryo. The enterohepatic axis subtly links the liver to the intestines, and it is precisely the existence of this enterohepatic axis that allows, under certain pathological conditions, the migration of intestinal bacteria to the liver through the portal vein, leading to an abnormal activation of the immune system, resulting in an inflammatory response and injury [3,4].
Intriguingly, short-chain fatty acids (SCFAs) as the metabolites of bacterial fermentation of intestinal dietary fiber, are the key factors in inhibiting NAFLD progression through portal vein branches [5]. Numerous studies have shown that SCFAs exerted a wide spectrum of positive effects. Shimizu et al. revealed that SCFAs acted as signalling molecules that link gut conditions to physiological metabolism [6]. Leung et al. found that SCFAs maintained the homeostasis of gut barrier and the integrity of gut mucosa, preventing some toxic substances and inflammatory mediators from entering the liver [7]. Thus, SCFAs play a vital role in alleviating of NAFLD.
A growing number of reviews began to report the function about SCFAs in NAFLD. Zhang et al. mainly emphasized the crosstalk between SCFAs and host metabolism in relation to NAFLD pathophysiology [8]. Forlano et al. preferred to focus on SCFAs produced by GM as a potential direction for liver disease [9]. Amiri et al. specialized in potential effects of butyrate on NAFLD [3]. Park et al. tended to identify the dietary fiber and GM as essential carbon sources for hepatic fat synthesis during the development of NAFLD [10]. However, rare reviews systematically summarized the complex mechanisms of SCFAs in mitigation of NAFLD and their detail therapeutic application.
Herein, we perform this review to concluding the complex mechanism of SCFAs in hepatic metabolism at the genetic and epigenetic levels, and propose a promising future direction to adopt SCFAs alone or in combination with prebiotics and related clinical drugs to alleviate NAFLD, which provides a theoretical basis for the clinical intervention and treatment of NAFLD.
2. SCFAs-generation and function
SCFAs are produced in the colon, mainly including acetate, propionate, butyrate, and valerate. The proportions of acetate, propionate and butyrate are highest, with a ratio of 3:1:1, respectively (Table 1). SCFAs are derived from the conversion of pyruvate produced by the GM through glycolytic, acetyl-CoA, lactic acid or succinic acid pathways under the action of intestine [8]. SCFAs are rare in our daily diet, mainly derived from GM fermentation of dietary fibre. Different GM can produce different SCFAs [11,12]. For instance, Bifidobacterium and Streptococcus produce acetate, while Dalister succinatiphilus and Enteric bacteria generate propionate and butyrate [[13], [14], [15]]. Specifically, researchers have observed GM that produce SCFAs in different models of NAFLD (Table 2). In the mice model, Hong et al. found that Astragalus polysaccharides (APS) enriched D. vulgaris is effective on attenuating hepatic steatosis possibly through producing acetic acid, and modulation in hepatic lipids metabolism [16]. For rats model, Zhao et al. showed that rats treated with sodium alginate (SA) had significantly increased levels of fecal SCFAs, decreased serum lipopolysaccharide (LPS) levels, attenuated hepatic steatosis and the abundance of Colidextribacter and Oscillibacter was higher in rats of high fatty diet with 150 mg/kg/d sodium alginate group (HAS) compared to rats of high-fat diet group (HFD) [17]. In addition, in a randomized controlled trial, a total of 96 patients with NAFLD were selected as research subjects. And Clostridium butyricum capsules combined with rosuvastatin can effectively improve liver function damage in NAFLD patient [18]. This accumulating evidence further suggests that SCFAs show good therapeutic efficacy in NAFLD in both animal and clinical trials.
Table 1.
Classification, source, component content, main signal receptors, and signalling modes of SCFAs.
| Classification of SCFAs | The content of the individual parts in SCFAs | Related microbes | SCFAs receptors | Signalling mechanisms |
|---|---|---|---|---|
| Acetate | 60% | Enteric bacteria, Lactis GCL 2505, Human-derived Bifidobacterium breve UCC2003, Bifidobacterium longum NCIMB 8809 [12,19,20] |
|
Histone Deacetylases (HDACS) |
| Propionate | 20% | Firmicutes and Bacteroidetes [12] |
|
|
| Butyrate | 20% | Enteric bacteria, Eubacterium hallii, Ruminococus bromii [15,21] |
|
|
Abbreviations: SCFAs, short-chain fatty acids; GPR41, G protein-coupled receptor 41; GPR43, G protein-coupled receptor 43; GPR109A, G protein-coupled receptor 109A; HDACs, histone deacetylases; GPCRs, G protein-coupled receptors.
Table 2.
Beneficial SCFAs produced by bacteria in different NAFLD model.
Abbreviations: FASN, fatty acid synthase; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HFD, high-fat diet; ALT, alternative lengthening of telomeres; TNF-α, tumor necrosis factor alpha.
SCFAs are incredibly critical for regulating NAFLD progression, mainly through five pathways (Fig. 1). First of all, SCFAs are natural inhibitors of histone deacetylases (HDACs) in T cells, modulating inflammation by suppressing the immune response of T cells [22]. Moreover, SCFAs interact with G protein-coupled receptors (GPCRs, GPR41, GPR43, GPR109A), which are expressed in gut epithelium and immune cells [23]. For example, SCFAs activate GPR41 and GPR43 to act on the surface of intestinal endocrine L cells to promote glucagon-like peptide-1 (GLP-1) secretion, inhibit gastrointestinal peristalsis and gastric juice secretion, thereby controlling appetite and intake to affect lipid oxidation in the liver [[24], [25], [26]]. Meanwhile, SCFAs activate brown adipose tissue, affect energy expenditure and anti-obesity [27]. SCFAs also regulate liver mitochondrial function, inhibit fat accumulation and reverse insulin resistance [28]. It is also essential for SCFAs to maintain homeostasis of energy cycle in the body and promote metabolic homeostasis of the liver [29].
Fig. 1.
Generation and function of SCFAs.
GM converts dietary fibre into pyruvate by glycolysis, then converts pyruvate into acetate, propionate, and butyrate through acetyl CoA, lactic acid and succinate pathways, respectively. Firstly, SCFAs as inhibitors of HDACs regulate inflammatory response of NAFLD. Secondly, SCFAs bind to GPR41 and GPR43 to promote intestinal endocrine L cells secretion of GLP-1 to restrain hepatic steatosis. Thirdly, SCFAs stimulate brown adipose tissue to improve NAFLD. Fourthly, SCFAs regulate liver mitochondrial function to improve NAFLD. Fifthly, SCFAs maintain energy circulation of body and promote hepatic metabolic balance. GM, gut microbiota; SCFAs, short-chain fatty acids; NAFLD, Nonalcoholic fatty liver disease; HDAC, histone deacetylase; GPR41, G protein-coupled receptor 41; GPR43, G protein-coupled receptor 43; GLP-1, glucagon-like peptide-1.
Together, SCFAs play inimitable roles in preventing the development of NAFLD through various pathways, which is of significance for providing new strategies for early diagnosis and target treatment of NAFLD.
3. SCFAs coordinate crosstalk in the gut and liver
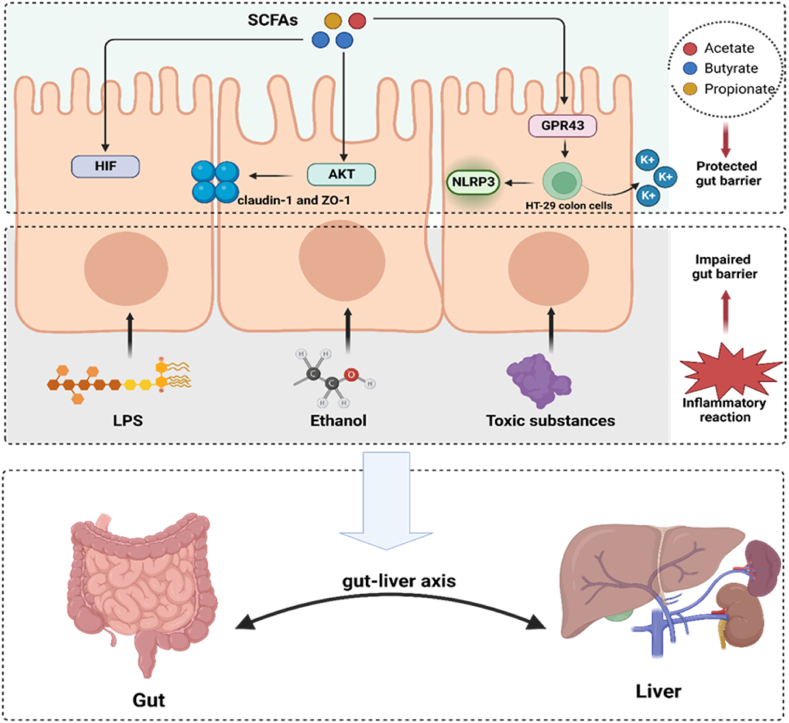
As a “new virtual metabolic organ”, the gut-liver axis, has received increasing attention in recent years [4,30]. The gut-liver axis refers to a bidirectional relationship established between the gut and the liver through the portal circulation [31]. Although GM mainly exist in the intestine, they also regulate liver function via microbial components and metabolites, acting on the liver through the gut-liver axis [32]. Due to the bidirectional communication of the gut-liver axis, the liver may continue to be influenced by gut derived metabolites and components. Research has revealed that distruption of the gut barrier allowed harmful substances such as LPS, ethanol and other toxic media to enter the liver, which damage liver function. Accumulating evidence shown that SCFAs maintained the integrity of gut barrier by regulating hypoxia-inducible factor (HIF), enhancing intestinal tight junctions, and immune cell activity. The detailed protection mechanisms of SCFAs are shown in Fig. 2.
Fig. 2.
SCFAs play a protective role as an intestinal barrier. Ethanol, LPS and toxic substances act on the intestinal epithelial barrier which could trigger an inflammatory response and cause damage to the intestinal barrier. Butyrate upregulates HIF target gene expression to enhance the epithelial barrier. Butyrate might enhance the abundance of tight junction proteins claudin-1 and ZO-1 through activation of Akt/mTOR mediated protein synthesis, which enhance the epithelial barrier. SCFAs bind GPR43 on colonic epithelial cells to stimulates K (+) efflux and hyperpolarization, which lead to NLRP3 inflammasome activation. The inflammasome pathway maintain the integrity of the intestinal barrier by ensuring the repair and cell survival under stress conditions. LPS, lipopolysaccharide; HIF, hypoxia-inducible factor; ZO-1, zonula occludens-1; GPR43, G protein-coupled receptor 43; NLRP3, NOD-like receptor thermal protein domain associated protein 3.
First, butyrate increases the level of HIF in epithelial cell lines, and the stability of different levels of HIF is crucial for enhancing the epithelial barrier [33]. Kelly et al. reported that butyrate treated can enhance the barrier function of HIF-1β knockdown cells, demonstrating the compensatory function of SCFAs on HIF [34]. Regrettably, the specific mechanisms of SCFAs and HIF are still unclear. Moreover, butyrate also can selectively upregulate tight junction protein claudin-1 and zonula occludens-1, activating the Akt signalling pathway to promote epithelial barrier [35]. Finally, the acetate/GPR43 pathway stimulates potassium efflux and hyperpolarization in HT-29 colon cells, thereby activating inflammatory NOD-like receptor thermal protein domain associated protein 3 (NLRP3) to protect the gut barrier [36]. It is well known that the barrier function of epithelial cells is the first line of defence of the intestine. Therefore, SCFAs can serve as protective messengers for the “first line of defence”, inhibiting the inflammatory response of the liver, and alleviating the occurrence and development of NAFLD.
4. Molecular mechanisms of SCFAs in the protection against hepatic steatosis in NAFLD
Hepatic steatosis is a disease characterized by excessive accumulation of lipids in the liver, mainly in the form of triglycerides (TGs), which is an important hallmark of NAFLD [37,38]. Here, we summarize various functions of SCFAs during hepatic steatosis progression, which contribute to understand the potential role of SCFAs in the progression of NAFLD and provides potential targets for the treatment of NAFLD (Table 3).
Table 3.
Target genes and related signalling pathways activated by SCFAs.
| Target genes | Complexes/pathway | Function | References |
|---|---|---|---|
| SREBP-1 | ATP-CL | Reducing the supply of substrate | [39] |
| CYP7A1 | / | Promoting the conversion of cholesterol into bile acids | [40] |
| SREBP2 | / | Augmenting cholesterol uptake in vascular cells | [40,41] |
| LDLR | / | Augmenting cholesterol uptake in vascular cells | [40,41] |
| ABCA1 | / | Accelerating cholesterol transport from the liver | [42] |
| ApoA-1 | / | Promoting cellular cholesterol efflux | [43] |
| NPC1L1 | / | Playing a vital role in intestinal cholesterol absorption | [44] |
| ABCG5/8 | / | Promoting cholesterol efflux from the duodenum | [44,45] |
| UCP2 | AMPK | Inducing liver autophagyvia | [46] |
| PPARγ | UCP2 | Inducing liver autophagyvia | [46] |
| FFAR2 | TNF-α, Gi | Reducing the inflammatory response of the liver and inhibiting lipolysis and plasma FFA levels | [47,48] |
| FFAR3 | PPARα | Affecting lipid metabolism | [49] |
| GPR109A | / | Improving the hepatic inflammatory response | [50,51] |
Abbreviations: SREBP-1, sterol regulatory element binding protein-1; CYP7A1, cholesterol 7α-hydroxylase; SREBP2, sterol regulatory element-binding protein 2; LDLR, LDL receptor; ABCA1, ATP binding cassette transporter protein A1; ApoA-1, apolipoprotein A1; NPC1L1, Niemann-Pick C1-like 1; ABCG5/8, ATP-binding cassette transport proteins G5 and G8, GPR43, G protein-coupled receptor 43; UCP2, uncoupling protein 2; AMPK, activating the 5′-adenosine monophosphate-activated protein ktinase; PPARγ, peroshorthorxisome proliferator-activated; receptor γ, FFAR2, free fatty acid receptor 2; TNF-α, tumor necrosis factor alpha; FFAR3, free fatty acid receptor 3; PPARα, pshort-chaineroxisome proliferator-activated receptor α; GPR109A, G protein-coupled receptor 109A
4.1. SCFAs reduce cholesterol to modulate hepatic steatosis
The liver is the central organ that regulates systemic cholesterol homeostasis, and abnormal cholesterol metabolism in hepatic not only lead to NAFLD progression, but also drive the development of atherosclerotic dyslipidemia [52]. Recent studies have revealed that in the high-cholesterol diet (HCD) induced NAFLD hamster model, acetate, propionate and butyrate significantly reduced total cholesterol (TC) by 24%, 18% and 17%, respectively, further demonstrating the central role of SCFAs in cholesterol reduction [40]. However, it remains unclear how SCFAs regulate cholesterol synthesis and secretion, and little is known about the mechanisms.
Here, we summarized five potential pathways for SCFAs to reduce cholesterol. First, SCFAs inhibit gene expression in the cholesterol synthesis pathway through sterol regulatory element binding protein-1 (SREBP-1) (Fig. 3a), of which SREBP-1 plays a key role in regulating fatty acid synthesis in the liver [53,54]. Fushimi et al. reported that acetic acid reduced the supply of acetyl-CoA (a substrate of cholesterol) by reducing mRNA level and activity of ATP citrate lyase (ATP-CL) caused by the suppression of SREBP-1 gene expression [39]. Second, SCFAs promote the conversion of cholesterol into bile acids (BAs) to reduce cholesterol levels by upregulating cholesterol 7α-hydroxylase (CYP7A1) expression (Fig. 3b). CYP7a1 is the key rate-limiting enzyme that converts cholesterol to BAs. Zhao et al. further confirmed that adding Ac, Pr or Bu to diet can significantly increase the expression of CYP7A1 and promote fecal excretion of BAs to reduce cholesterol levels [40]. Third, SCFAs play a vital role in accelerating cholesterol transport from the liver by regulating ATP binding cassette transporter protein A1 (ABCA1) (Fig. 3c). Based on animal studies, Du et al. found that butyrate treatment significantly inhibited HFD-induced atherosclerosis and hepatic steatosis by promoting ABCA1-mediated cholesterol efflux from macrophages [42]. In addition, SCFAs enhance the effectiveness of apolipoprotein A1 (ApoA-1) modification to promote cellular cholesterol efflux through the ABCA1 pathway [43,55]. Fourth, SCFAs reduce cholesterol levels by regulating the expression of cholesterol transport proteins such as Niemann-Pick C1-like 1 (NPC1L1) and ATP-binding cassette transport proteins G5 and G8 (ABCG5/8) (Fig. 3d). The NPC1L1 plays a vital role in intestinal cholesterol absorption, while ABCG5/8 mainly responds to cholesterol efflux in the duodenum [44]. Yamanashi et al. reported that coincubation of Caco-2 cells (a human intestinal cell line) with butyrate downregulated the expression of NPC1L1 but increased the mRNA level of ABCG5/8 [45]. Fifth, SCFAs act on GPR43 to mediate the leptin response in controlling cholesterol levels (Fig. 3e). Leptin is a signalling molecule that reduces hepatic lipogenesis and cholesterol synthesis by inhibiting the expression of SREBP-1 and cholesterol-related genes, thereby reducing cholesterol levels and alleviating hepatic steatosis [56,57].
Fig. 3.
The mechanism of SCFAs madiate a decrease in cholesterol levels. (a) SCFAs down-regulate gene expression in the cholesterol synthesis pathway through SREBP-1. (b) SCFAs promote the conversion of cholesterol into BAs by upregulating the expression of CYP7A1, thereby reducing cholesterol levels. (c) SCFAs accelerate cholesterol export and transport cholesterol out of the Kupffer cell in the NAFLD by regulating ApoA-I to affect ABCA1. (d) NPC1L1 abundantly expresses in the duodenum and mediates cholesterol absorption, while ABCG5/8 forms heterodimers, leading to cholesterol efflux. SCFAs downregulate NPC1L1 expression and upregulate ABCG5/8 expression, thereby reducing cholesterol levels. (e) SCFAs act on GPR43 to stimulate leptin production and inhibit SREBP-1 expression, thereby down-regulating cholesterol levels. SCFAs, short-chain fatty acids; SREBP-1, sterol regulatory element binding protein-1; Bas, bile acids; CYP7A1, cholesterol 7α-hydroxylase; NAFLD, Nonalcoholic fatty liver disease; ABCA1, ATP-binding cassette transporter protein A1; NPC1L1, Niemann-Pick C1-like 1; ABCG5/8, ATP-binding cassette transport proteins G5 and G8; GPR43; G protein-coupled receptor 43.
In conclusion, SCFAs reduce cholesterol levels to alleviate hepatic steatosis through genes such as SREBP-1, ATP-CL, CYP7A1, ABCA1, NPC1L1 and ABCG5/8 to improve NAFLD.
4.2. SCFAs induce autophagy to regulate hepatic steatosis
Research has implied that SCFAs alleviate NAFLD progression by activating the autophagy pathway. Autophagy is imperative for intracellular homeostasis and plays an instrumental role in the progression of NAFLD via mediating metabolic regulation, maintaining lipid homeostasis, and suppressing hepatic inflammation [58,59]. SCFAs induce uncoupling protein 2 (UCP2)-mediate autophagy of hepatocytes to improve hepatic steatosis [60]. Propionate and butyrate in SCFAs could directly induce hepatic autophagy through the activation of peroxisome proliferator-activated receptor γ (PPARγ), leading to the transcription UCP2. In addition, the increased activity of UCP2 result in the uncoupling of the respiratory chain, which in turn reducing hepatic ATP and activating the 5′-adenosine monophosphate-activated protein kinase (AMPK) pathway and autophagy [46]. In brief, SCFAs induce liver autophagyvia the PPARγ-UCP2-AMPK pathway to alleviate the development of NAFLD, which may become a new therapeutic strategy.
4.3. SCFAs regulate hepatic steatosis through GPCRs
GPCRs are the largest receptor family in humans and the most successful drug targets in history, regulating the physiological functions of almost all tissues and cells throughout the body [61,62]. GPCRs specifically bind substances in the extracellular environment, including hormones, chemokines, lipids, and proteins. Presently, the main receptors for SCFAs in the GPCRs family are GPR41, GPR43, and GPR109A. Among them, GPR41 and GPR43 are the most important receptors for SCFAs that also named free fatty acid receptor 3 (FFAR3) and free fatty acid receptor 2 (FFAR2), respectively. The schematic diagram of the interaction between SCFAs and their GPCRs in the gut and liver are shown in Fig. 4. FFAR2 can be activated by stimulating SCFAs in anti-inflammatory M2 macrophages, the expression of which contributes to upregulate tumor necrosis factor-α (TNF-α) expression to reduce the inflammatory response of the liver [47] (Fig. 4a). Besides, acetate and propionate act as external signalling molecules specifically binding to the FFAR2 receptor, coupling it to the Gi pathway in adipocytes and inhibiting lipolysis and plasma FFA levels [48]. Moreover, plasma SCFAs may indirectly affect lipid metabolism related genes in the liver through the expression FFAR3 in other tissues, such as fatty acid synthase (Fas) and peroxisome proliferator-activated receptor α (PPARα) in the liver (Fig. 4b). Additionally, in the research of Samuel et al., FFAR3 knockout mice gained less weight than wild-type mice, but this difference disappeared under germ-free conditions [63]. Therefore, SCFAs are likely to mediate this differential change through the change of FFAR3. With a deeper understanding of SCFAs, it became known that SCFAs activate GPR109A to induce differentiation of Treg cells and T cells that produce interleukin 10 (IL-10), thereby improving hepatic lipid degeneration [50] (Fig. 4c). It has been reported that butyrate increased the Regulatory cell (Treg) population of adipose tissue in obese mice via GPR109A signalling, which contributed to improving the hepatic inflammatory response [51]. Altogether, leveraging GPR signals in the treatment of liver metabolic diseases is a potential research field that deserves further investigation.
Fig. 4.
SCFAs bind with FFAR2, FFAR3 and GPR109A to improve hepatic steatosis, respectively. (a) SCFAs stimulate FFAR2 to activate M2 macrophages, which upregulate TNF-α expression and inhibit liver inflammation. Meanwhile, under the action of SCFAs, FFAR2 can decrease FFA content to improve hepatic steatosis by Gi pathway. (b) SCFAs activate FFAR3 to down-regulate lipid synthesis genes (Fas and PPARα). (c) SCFAs induce T cell differentiation into Treg cells and IL-10-producing T cells via GPR109A signalling, which alleviate liver inflammation. SCFAs, short-chain fatty acids; FFAR2, free fatty acid receptor 2; TNF-α, tumor necrosis factor-α; FFA, Free fat acid; FFAR3, free fatty acid receptor 3; GPR109A, G protein-coupled receptor 109A.
5. Epigenetic mechanisms of SCFAs in improving NAFLD
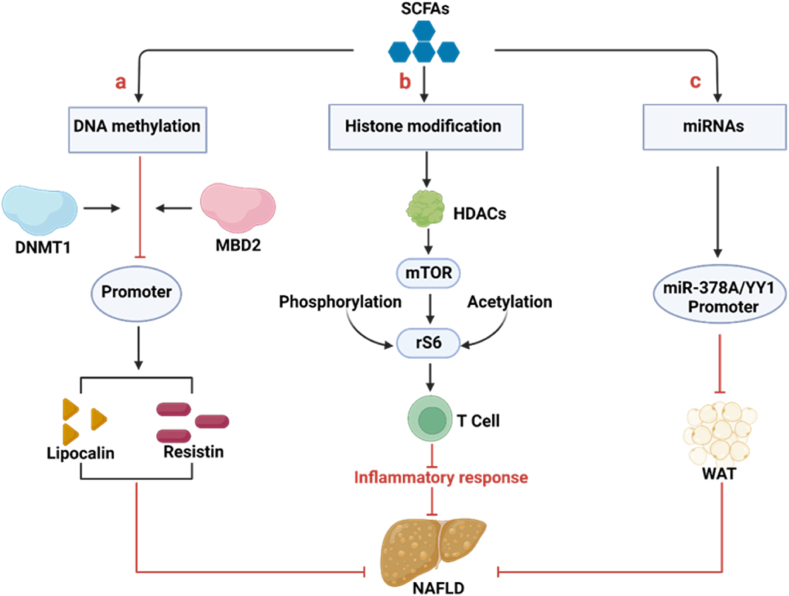
Epigenetic includes DNA methylation, histone modification, microRNA (miRNA) regulation, and chromosome remodeling [64]. NAFLD is a representative metabolic disease that is susceptible to environmental factors and epigenetic modifications. Epigenetic modifications integrate microbial signals to calibrate host cell transcriptional programs without altering the underlying genetic code [65]. In particular, microbial metabolites derived from the diet (e.g., SCFAs) can produce epigenetically modified substrates and enzyme regulators [66]. The specific mechanisms of SCFAs inducing epigenetic modifications are depicted in Fig. 5.
Fig. 5.
Possible mechanisms of SCFAs-induced epigenetic modifications to improve NAFLD. (a) SCFAs reduce the DNA methylation portion of the adiponectin and resistin promoters, which was mediated by reducing the expressions of DNMT1 and MBD2, suppressing the binding of these enzymes to the promoters of adiponectin and resistin. The mRNA expression of adiponectin and resistin is upregulated due to demethylation of gene promoter, thus restoring gene transcription. Among them, adiponectin and resistin can effectively improve NAFLD. (b) SCFAs modulate chromatin by inhibiting HDACs to increase acetylation and phosphorylation of the p70 S6 kinase and phosphorylation rS6, while regulating the mTOR pathway required for T-cell production, thereby suppressing the T-cell immune response to regulate metabolic inflammatory responses in the liver. (c) SCFAs inhibit WAT production by regulating the methylation level of the miR-378A/YY1 promoter in the host and the expression of the miR-181. SCFAs, short-chain fatty acids; DNMT1, DNA methyltransferases 1; MBD2, methyl CpG-binding structural domain protein 2; NAFLD, Nonalcoholic fatty liver disease; mTOR, mechanistic target of rapamycin; WAT, white adipose tissue.
5.1. DNA methylation
The change in DNA methylation induced by SCFAs is an important epigenetic modification [67]. During the occurrence and development of NAFLD, the hypo-and hypermethylation genes such as growth factor-α (PDGFα), phospholipase C gamma 1 (PLCG1), caspase 1 (CASP1) are epigenetic regulated and exacerbate the progression of NAFLD by participating in immune response, oxidative stress, and liver lipid degeneration [68]. It is notably that SCFAs reduce the expression of DNA methyltransferases 1 (DNMT1) and methyl CpG binding structural domain protein 2 (MBD2), inhibiting the binding of these enzymes toadiponectin and resistin promoters. Among them, adiponectin and resistin are the main adipokines secreted by white adipose tissue, and their abnormal expression promotes the progression of NAFLD [69,70] (Fig. 5a). The expression of adiponectin and resistin are upregulated in the liver of mice fed with high-fat diet and treated with antibiotics, as well as genes related to fat oxidation and heat production, such as PPARα, peroxisome proliferator-activated receptor-γ coactivator-1α (Pgc-1α), and increased adipose triglyceride lipase (Atgl) in the liver [[71], [72], [73]]. The potential mechanism is that antibiotics reduce the number of GM producing SCFAs. In addition, SCFAs upregulate the expression of adiponectin and resistin mRNA and restored it due to the reduced in DNA methylation at the gene promoter.
5.2. Histone modification
Histone modifications play a key role in the epigenetic regulation of gene expression [74]. Among that, the epigenetic role of SCFAs is mainly to activating acetylation, which acted as HDACs inhibitors has been widely demonstrated [[75], [76], [77]]. SCFAs promote T-cell differentiation by inhibiting the acetylation of p70 S6 kinase promoted by HDAC in T cells, thereby acting as a mechanism target of the rapamycin (mTOR) pathway to regulate inflammatory response in the liver [22] (Fig. 5b). Furthermore, butyrate induces Treg differentiation in vitro and in vivo by enhancing histone H3 acetylation in the forkhead box p3 (Foxp3) promoter and conserved noncoding sequence region, which may be beneficial for improving liver inflammatory response [78]. Besides, SCFAs effectively can inhibit HDACs, and the higher the concentration, the more significant the effect. Specifically, SCFAs indirectly affect HDACs through the sodium-coupled monocarboxylate transporter-1 (SMCT-1), a transporter that binds to HDACs and FFAR3, thereby inhibiting butyrate-induced histone acetylation [79,80]. Paradoxically, as previously reported, SCFAs regulated chromatin by inhibiting HDACs, but Thomas and Denu demonstrated that SCFAs induced histone acetylation by activating activates histone acetyltransferases (HATs) rather than inhibiting HDACs [81]. Altogether, the evidence for SCFAs in activating acetylation is widely accepted, which may greatly broaden the potential strategies for treating NAFLD.
5.3. MicroRNA
miRNAs are a family of post transcriptional gene inhibitors that do not encode proteins but exert a vital role in regulating gene expression. Studies have revealed that SCFAs influence the development of NAFLD by mediating miRNAs [82] (Fig. 5c). Acetate and butyrate regulate NAFLD progression through the miR-378a-YY1 axis. Overexpression of Yin yang 1 (YY1), a direct target gene of miR-378a family, significantly enhances liver lipid metabolism. Meanwhile, acetate and butyrate could affect miR-378a family via regulating the DNA methylation of its promoter [83]. Intriguingly, it is worth noting that Pant et al. provided conclusive evidence that butyrate induces cell apoptosis by regulating miR-22 in hepatic cancer cells [84]. In view of the fact that the advanced stage of NAFLD often progresses to cancer, investigating the role of miR-22 in NAFLD seems provide valuable insights into its mechanism. In summary, SCFAs regulate the metabolic processes of liver by regulating the expression of miRNAs as signalling molecules.
6. Future and outlook: SCFAs as new targets for the treatment of NAFLD
The dysregulation of GM promotes the development of NAFLD, as some bacteria can produce toxic substances, leading to liver inflammation or metabolic disorder [85]. However, as the metabolites of GM, how to link SCFAs with the prevention and treatment of NAFLD remains a crucial issue. Here, we mainly focus on the three potential applications of SCFAs (Table 4), with the aim of providing new targets for the prevention and treatment of NAFLD.
Table 4.
Different applications of SCAFs as potential therapy for the treatment of NAFLD.
| Categories | Specific measures | Results | References |
|---|---|---|---|
| Individual application |
|
|
[86] |
|
|
[87] | |
|
|
[88] | |
|
|
[89] | |
| Prebiotics application |
|
|
[90] |
|
|
[91] | |
|
|
[92] | |
| Clinical drugs application |
|
|
[93] |
|
|
[94] | |
|
|
[95] | |
|
|
[96] |
Abbreviations: SCFAs, short-chain fatty acid salts; FOS, Fructo-oligosaccharide; SP, seabuckthorn polysaccharide; OLA, Olanzapine; NAFLD, Nonalcoholic fatty liver disease; FTA, Forsythiaside A; ECD, Erchen decoction; SJP, Jiang Powder; TG, triglyceride; TC, total cholesterol; IL-6, interleukin-6; TNF-α, tumour necrosis factor-α; LPS, lipopolysaccharide; LDL-c, low-density lipoprotein cholesterol; GLP-1, glucagon-like peptide-1; PYY, Peptide YY; LDL, low-density lipoprotein; HDL, high-density lipoprotein; GM, gut microbiota; MIP-1α, macrophage inflammatory protein-1alpha; TLR-4, toll-like receptor 4; IL-1β, interleukin-1β; NF-κB, nuclear factor-kappaB; IETJ, intestinal epithelial tight junction; HFD, high fatty diet, ALT, alanine aminotransferase; AST, aspartate aminotransferase; PPARγ, peroxisome proliferator-activated receptor γ.
6.1. SCFAs improve NAFLD directly
The use of probiotics to interfere with non-alcoholic steatohepatitis (NASH) may be a novel therapeutic strategy for anti-NAFLD drug discovery. However, due to the susceptibility of probiotics direct administration to environmental and in vivo active factors, many studies have adopted SCFAs as supplements, focusing on whether they can improve NAFLD. Currently, butyrate has been widely used in experimental models to improve NAFLD, while clinical research remains scarce due to the difficulty in controlling dosage and duration. In an animal study, Fang et al. showed that sodium butyrate administration altered the composition of HFD-induced intestinal microbiota, improved the gut barrier and lowered serum LPS concentration, further ameliorating lipid accumulation associated with obesity [86]. Nevertheless, it appears that unlike SCFAs administered as a single ingredient, full-ingredient application of SCFAs seems to be more effective in improving liver fat deposition. Jiao et al. reported that oral administration of SCFAs reduced liver fat deposition in weaned pigs by reducing lipogenesis and enhancing lipolysis in different tissues [87]. Deng et al. found that SCFAs treatment significantly reduced alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride and cholesterol levels in mice with methionine and choline deficient (MCD) diet, thereby alleviating hepatic steatosis [88]. Jiao et al. also reported that SCFAs reduced adipogenesis and enhanced lipolysis in different tissues of pigs by regulating hormones and genes [89]. Although SCFAs are rarely used in clinical trials, there has been clear research in recent years indicating a strong correlation between SCFAs and the progression of NAFLD. A clinical trial showed that although the levels of SCFAs in fecal samples of patients with cirrhosis were lower, these functional abnormalities became more pronounced as the severity of liver disease increases [97]. Altogether, the feasibility of SCFAs alone treating NAFLD is of great significance for further investigation.
6.2. Prebiotics as means to increase SCFAs to improve NAFLD
Numerous studies have shown that prebiotics can selectively stimulate the growth and activity of beneficial bacteria in the intestinal tract, thereby alleviate the progression of NAFLD. The potential mechanism of prebiotics for improving NAFLD may attribute to increasing of SCFAs. In 2016, the International Scientific Association of Probiotics and Prebiotics (ISAPP) defined prebiotics as “a beneficial substrate selectively utilized by host microorganisms” [98]. Prebiotics mainly include three types: inulin, fructo-oligosaccharides, seabuckthorn polysaccharide and other polysaccharide. Firstly, inulin is a natural soluble dietary fibre that serves as a reserve biopolysaccharide for plants and is fermented by GM to produce butyrate [99]. Guo et al. reported that inulin-induced GM remodeling led to increased production of SCFA and expression of giopoietin-like protein 4 (ANGPTL4), which improved glucose and lipid metabolism [90]. Similarly, another research revealed that ingesting probiotic inulin improved fat oxidation in obese man while promoting the production of SCFAs in their body [100]. Secondly, Fructo-oligosaccharides (FOSs) are consistence of short fructose chains of various oligosaccharides with different chain lengths. Numerous studies have indicated that FOSs restore normal gastrointestinal microbiota and intestinal epithelial barrier function, and alleviate steatohepatitis [101]. Furthermore, it was found that FOSs intake increased the level of SCFAs in the body and significantly improved hepatic steatosis and inflammatory cell infiltration [102]. FOSs reversed the accumulation of high fat and high sugar (HFS) induced liver lipids in vivo by promoting the production of SCFAs by GM, reducing serum lipid levels, and improving HFS induced liver inflammation by promoting the generation of SCFAs by the GM [91]. Thirdly, seabuckthorn polysaccharide (SP) as a typical polysaccharide, which also played a similar role in upregulation of SCFAs to improved liver steatosis. In an experiment based on obese mice, continuous 12 weeks of SP dietary supplements significantly reduced body weight and increased fecal SCFAs, indicating that the regulation of SP on liver lipid metabolism may be induced by changes in GM and increase in SCFAs production [92].
These evidences suggest that prebiotics improve NAFLD by restoring the GM and increasing SCFAs levels, mainly including three types of inulin, fructo-oligosaccharides, seabuckthorn polysaccharide and other polysaccharide. Therefore, compared to administering prebiotics alone, combining prebiotics with SCFAs to improve NAFLD may enhance the therapeutic efficacy to some extent.
6.3. Clinical drugs for upregulating SCFA levels to improve NAFLD
Numerous studies have demonstrated the ability of SCFAs to ameliorate NAFLD, while no definitive medications that can be utilized for the clinical treatment of NAFLD with SCFAs. As a result, researchers have become dedicated to the search for what appears to be the existence of effective drugs for upregulating the expression of SCFAs and thereby ameliorating NAFLD. Now, probiotics have been shown to modulate the gut microbiota and produce SCFAs to inhibit lipid deposition in the liver. Nevertheless, since probiotics are categorized only as nutraceuticals and not as drugs, public acceptance and regulatory rules have also limited their use in the clinical field [103]. Until recently, some researchers have suggested proposing the term “probacine” (PRObiotic BActerial mediCINE) emphasizes the role of probiotics in the prevention, alleviation, and treating diseases, and further promotes the clinical application of probiotics [104]. Therefore, the use of probiotics in clinical medicine to improve NAFLD can be seen as a promising prospect.
In addition to probiotics, a host of approved clinical drugs can also upregulate SCAFs to some extent. Olanzapine (OLA) is a commonly used drug for the treatment of schizophrenia. OLA exposure altered the composition of carp GM, increased the abundance of SCFAs producing bacteria, and affected lipid metabolism signalling pathways. The potential mechanism can be explained by the regulation of the GM-SCFA-PPAR signalling pathway [93]. Forsythiaside A (FTA) is isolated from the traditional Chinese medicine Forsythiae fructus (Lian Qiao) and is a natural liver protective agent. Studies on mice have shown that FTA can improve liver fibrosis by inhibiting inflammation and oxidative stress, modulating GM and increasing SCFAs levels [94]. Phillygenin (PHI) is also an important fingerprint lignan component of Forsythiae fructus, with has significant liver protection, anti-inflammatory, and antioxidant effects. Wang et al. showed that PHI promoted the production of SCFAs in the intestine of mice and ameliorated carbon tetrachloride induced liver fibrosis [105]. Erchen decoction (ECD) is a classic Chinese herbal formula consisting of citrus reticulate, pine, poria cocos, and ural licorice, which has been widely used to treat NAFLD. Researchers have found that ECD effectively improved NAFLD, leading to a significant increase in SCFAs in faeces. Similar to the former, Sheng-Jiang Powder (SJP) is an empirical traditional Chinese medicine formula for treating NAFLD, commonly used in clinical. Li et al. found that liver lipid deposition in mice was effectively improved after administration of SJP [96]. Next, they further investigated whether there were changes in the gut microbiota of mice. The results were unexpected: the relative abundance of SCFAs producing bacteria was upregulated after SJP treatment.
Therefore, SJP can effectively attenuate HFD-induced NAFLD, which may be due to changes in SCFAs content in vivo. Overall, developing clinical drugs to upregulate SCFAs for the treatment of NAFLD seems to be an exciting approach. However, considering the side effects of drugs and patients compliance, it is still necessary to further clarify the safety of drug.
7. Conclusions
SCFAs play an important role in regulating inflammation, glucose and lipid metabolism, which have been revealed as one of the most prevalent therapeutic targets in management of NAFLD. In this review, we specifically discussed the production and function of SCFAs, as well as their crosstalk coordination in the gut liver axis, summarized the potential molecular and epigenetic mechanisms of SCFAs in improving NAFLD, and explained the new prospects of their therapeutic measures. It is worth noting that both direct and indirect use of SCFAs are effective methods for improving NAFLD. And prebiotics and clinical agents that increase the content of SCFAs may be valuable in improving clinical efficacy. However, the following issues still need to be further considered. First, the management of SCFAs as a treatment for NAFLD remains limited due to the lack of clinical data on how SCFAs regulate lipid metabolism. Second, the safety of prebiotics or related clinical drugs remains obscure, and their dosage should be further evaluated based on individual differences to determine potential adverse reactions. Altogether, further consideration of these issues may provide significant progress for the effective application of SCFAs in the prevention and treatment of NAFLD.
Funding
This study was supported by National Natural Science Foundation of China, Grant/Award Number: (82300661); Natural Science Foundation of Anhui province, Grant/Award Number: (2308085QH246); Natural Science Foundation of the Anhui Higher Education Institutions, Grant/Award Number: (KJ2021A0205); Basic and Clinical cooperative research program of Anhui Medical University, Grant/Award Number: (2022xkjT013); Scientific Research Foundation of Anhui Medical University (2023xkj002). Figures created with BioRender.com and published with permission.
Data availability statement
No data was used for the research described in the article.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CRediT authorship contribution statement
Xinyu Li: Writing – original draft. Maozhang He: Investigation. Xinrui Yi: Writing – review & editing. Xuejin Lu: Writing – review & editing. Meizi Zhu: Writing – review & editing. Min Xue: Writing – review & editing. Yunshu Tang: Supervision. Yaling Zhu: Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Yaling Zhu reports financial support was provided by National Natural Science Foundation of China. Yaling Zhu reports financial support was provided by National College Students Innovation and Entrepreneurship Training Program. Yaling Zhu reports financial support was provided by Natural Science Foundation of the Anhui Higher Education Institutions. Yaling Zhu reports financial support was provided by Basic and Clinical cooperative research program of Anhui Medical University.
Contributor Information
Yunshu Tang, Email: tangyunshu@ahmu.edu.cn.
Yaling Zhu, Email: zhuyaling@ahmu.edu.cn.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- GM
Gut microbiota
- NASH
Nonalcoholic steatohepatitis
- SCFAs
Short-chain fatty acids
- APS
Astragalus polysaccharides
- SA
Sodium alginate
- HFD
High-fat diet group
- HAS
HFD with 150 mg/kg/d sodium alginate group
- HDAC
Histone deacetylase
- GPCRs
G protein-coupled receptors
- GPR41
G protein-coupled receptor 41
- GPR43
G protein-coupled receptor 43
- GPR109A
G protein-coupled receptor 109A
- GLP-1
Glucagon-like peptide-1
- LPS
Lipopolysaccharide
- HIF
Hypoxia-inducible factor
- ZO-1
Zonula occludens-1
- TGs
Triglycerides
- TC
Total plasma cholesterol
- HCD
High-cholesterol diet
- SREBP-1
Sterol regulatory element binding protein-1
- SREBP-2
Sterol regulatory element binding protein-2
- ATP-CL
ATP citrate lyase
- BAs
Bile acids
- ABCA1
ATP-binding cassette transporter protein A1
- ApoA-I
Apolipoprotein A1
- NPC1L1
Niemann-Pick C1-like 1
- ABCG5
ATP-binding cassette transport proteins G5
- ABCG8
ATP-binding cassette transport proteins G8
- UCP2
Uncoupling protein 2
- PPARγ
Peroxisome proliferator-activated receptor γ
- AMPK
Adenosine 5‘-monophosphate-activated protein kinase
- FFAR3
Free fatty acid receptor 3
- FFAR2
Free fatty acid receptor 2
- TNF-α
Tumor necrosis factor-α
- FAS
Fatty acid synthase
- PPAR-α
Peroxisome proliferator-activated receptor α
- IL-10
Interleukin 10
- ncRNA
Noncoding RNA
- DNMT1
DNA methyltransferases 1
- MBD2
Methyl CpG-binding structural domain protein 2
- Pgc-1α
Peroxisome proliferator-activated receptor γ coactivator 1
- Atgl
Adipose triglyceride lipase
- Foxp3
Forkhead box p3
- HATs
Histone acetyltransferases
- WAT
White adipose tissue
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- MCD
Methionine- and choline-deficient
- ANGPTL4
Angiopoietin-like protein 4
- SP
Seabuckthorn polysaccharide
- FOSs
Fructo-oligosaccharides
- HFS
High-fat/high-sugar
- OLA
Olanzapine
- FTA
Forsythiaside A
- PHI
Phillygenin
- ECD
Erchen decoction
- SJP
Sheng-Jiang Powder
- FASN
Fatty acid synthase
- LDL-C
Low-density lipoprotein cholesterol
- LDLR
LDL receptor
References
- 1.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Maestri M., Santopaolo F., Pompili M., Gasbarrini A., Ponziani F.R. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: effects of current treatments and future strategies. Front. Nutr. 2023;10 doi: 10.3389/fnut.2023.1110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiri P., Arefhosseini S., Bakhshimoghaddam F., Jamshidi Gurvan H., Hosseini S.A. Mechanistic insights into the pleiotropic effects of butyrate as a potential therapeutic agent on NAFLD management: a systematic review. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pabst O., Hornef M.W., Schaap F.G., Cerovic V., Clavel T., Bruns T. Gut-liver axis: barriers and functional circuits. Nat. Rev. Gastroenterol. Hepatol. 2023;20:447–461. doi: 10.1038/s41575-023-00771-6. [DOI] [PubMed] [Google Scholar]
- 5.Sun C., Qiu C., Zhang Y., Yan M., Tan J., He J., Yang D., Wang D., Wu L. Lactiplantibacillus plantarum NKK20 alleviates high-fat-diet-induced nonalcoholic fatty liver disease in mice through regulating bile acid anabolism. Molecules. 2023;28:4042. doi: 10.3390/molecules28104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu H., Masujima Y., Ushiroda C., Mizushima R., Taira S., Ohue-Kitano R., Kimura I. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-53242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung C., Rivera L., Furness J.B., Angus P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S., Zhao J., Xie F., He H., Johnston L.J., Dai X., Wu C., Ma X. Dietary fiber-derived short-chain fatty acids: a potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021;22 doi: 10.1111/obr.13316. [DOI] [PubMed] [Google Scholar]
- 9.Forlano R., Sivakumar M., Mullish B.H., Manousou P. Gut microbiota-A future therapeutic target for people with non-alcoholic fatty liver disease: a systematic review. Int. J. Mol. Sci. 2022;23:8307. doi: 10.3390/ijms23158307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park G., Jung S., Wellen K.E., Jang C. The interaction between the gut microbiota and dietary carbohydrates in nonalcoholic fatty liver disease. Exp. Mol. Med. 2021;53:809–822. doi: 10.1038/s12276-021-00614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J., Zhu N., Su X., Gao Y., Yang R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. 2023;12:793. doi: 10.3390/cells12050793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 13.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 14.Reichardt N., Duncan S.H., Young P., Belenguer A., McWilliam Leitch C., Scott K.P., Flint H.J., Louis P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 16.Hong Y., Sheng L., Zhong J., Tao X., Zhu W., Ma J., Yan J., Zhao A., Zheng X., Wu G., Li B., Han B., Ding K., Zheng N., Jia W., Li H. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microb. 2021;13:1–20. doi: 10.1080/19490976.2021.1930874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H., Gao X., Liu Z., Zhang L., Fang X., Sun J., Zhang Z., Sun Y. Sodium alginate prevents non-alcoholic fatty liver disease by modulating the gut–liver Axis in high-fat diet-fed rats. Nutrients. 2022;14:4846. doi: 10.3390/nu14224846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu W., Yan M., Cao H., Zhou J., Xu Z. Effects of Clostridium butyricum capsules combined with rosuvastatin on intestinal flora, lipid metabolism, liver function and inflammation in NAFLD patients. Cell. Mol. Biol. Noisy--Gd. Fr. 2022;68:64–69. doi: 10.14715/cmb/2022.68.2.10. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Aceituno L., Esteban-Torres M., James K., Moreno F.J., van Sinderen D. Metabolism of biosynthetic oligosaccharides by human-derived Bifidobacterium breve UCC2003 and Bifidobacterium longum NCIMB 8809. Int. J. Food Microbiol. 2020;316 doi: 10.1016/j.ijfoodmicro.2019.108476. [DOI] [PubMed] [Google Scholar]
- 20.Horiuchi H., Kamikado K., Aoki R., Suganuma N., Nishijima T., Nakatani A., Kimura I. Bifidobacterium animalis subsp. lactis GCL2505 modulates host energy metabolism via the short-chain fatty acid receptor GPR43. Sci. Rep. 2020;10:4158. doi: 10.1038/s41598-020-60984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis P., Young P., Holtrop G., Flint H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 22.Park J., Kim M., Kang S.G., Jannasch A.H., Cooper B., Patterson J., Kim C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivaprakasam S., Prasad P.D., Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016;164:144–151. doi: 10.1016/j.pharmthera.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Q., Lin S., Li Y., Liu L., Li X., Gao X., Yan J., Gu B., Chen X., Li W., Tang X., Chen C., Guo L. A novel GLP-1 and FGF21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mio K., Ogawa R., Tadenuma N., Aoe S. Arabinoxylan as well as β-glucan in barley promotes GLP-1 secretion by increasing short-chain fatty acids production. Biochem. Biophys. Rep. 2022;32 doi: 10.1016/j.bbrep.2022.101343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z., Yi C.-X., Katiraei S., Kooijman S., Zhou E., Chung C.K., Gao Y., van den Heuvel J.K., Meijer O.C., Berbée J.F.P., Heijink M., Giera M., Willems van Dijk K., Groen A.K., Rensen P.C.N., Wang Y. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67:1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- 28.Mollica M.P., Mattace Raso G., Cavaliere G., Trinchese G., De Filippo C., Aceto S., Prisco M., Pirozzi C., Di Guida F., Lama A., Crispino M., Tronino D., Di Vaio P., Berni Canani R., Calignano A., Meli R. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes. 2017;66:1405–1418. doi: 10.2337/db16-0924. [DOI] [PubMed] [Google Scholar]
- 29.Hu J., Lin S., Zheng B., Cheung P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018;58:1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- 30.Konturek P.C., Harsch I.A., Konturek K., Schink M., Konturek T., Neurath M.F., Zopf Y. Gut−Liver Axis: how do gut bacteria influence the liver? Med. Sci. 2018;6:79. doi: 10.3390/medsci6030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Ohtani N., Kawada N. Role of the gut–liver Axis in liver inflammation, fibrosis, and cancer: a special focus on the gut microbiota relationship. Hepatol. Commun. 2019;3:456–470. doi: 10.1002/hep4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang P., Li T., Niu C., Sun S., Liu D. ROS-activated MAPK/ERK pathway regulates crosstalk between Nrf2 and Hif-1α to promote IL-17D expression protecting the intestinal epithelial barrier under hyperoxia. Int. Immunopharm. 2023;116 doi: 10.1016/j.intimp.2023.109763. [DOI] [PubMed] [Google Scholar]
- 34.Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J., Wilson K.E., Glover L.E., Kominsky D.J., Magnuson A., Weir T.L., Ehrentraut S.F., Pickel C., Kuhn K.A., Lanis J.M., Nguyen V., Taylor C.T., Colgan S.P. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan H., Ajuwon K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macia L., Tan J., Vieira A.T., Leach K., Stanley D., Luong S., Maruya M., Ian McKenzie C., Hijikata A., Wong C., Binge L., Thorburn A.N., Chevalier N., Ang C., Marino E., Robert R., Offermanns S., Teixeira M.M., Moore R.J., Flavell R.A., Fagarasan S., Mackay C.R. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 37.Vergani L. Fatty acids and effects on in vitro and in vivo models of liver steatosis. Curr. Med. Chem. 2019;26:3439–3456. doi: 10.2174/0929867324666170518101334. [DOI] [PubMed] [Google Scholar]
- 38.Rong L., Zou J., Ran W., Qi X., Chen Y., Cui H., Guo J. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD) Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.1087260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fushimi T., Suruga K., Oshima Y., Fukiharu M., Tsukamoto Y., Goda T. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br. J. Nutr. 2006;95:916–924. doi: 10.1079/BJN20061740. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y., Liu J., Hao W., Zhu H., Liang N., He Z., Ma K.Y., Chen Z.-Y. Structure-specific effects of short-chain fatty acids on plasma cholesterol concentration in male Syrian hamsters. J. Agric. Food Chem. 2017;65:10984–10992. doi: 10.1021/acs.jafc.7b04666. [DOI] [PubMed] [Google Scholar]
- 41.Gopoju R., Panangipalli S., Kotamraju S. Metformin treatment prevents SREBP2-mediated cholesterol uptake and improves lipid homeostasis during oxidative stress-induced atherosclerosis. Free Radic. Biol. Med. 2018;118:85–97. doi: 10.1016/j.freeradbiomed.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 42.Du Y., Li X., Su C., Xi M., Zhang X., Jiang Z., Wang L., Hong B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020;177:1754–1772. doi: 10.1111/bph.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao B. Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. Biochim. Biophys. Acta. 2012;1821:490–501. doi: 10.1016/j.bbalip.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Boer J.F., Kuipers F., Groen A.K. Cholesterol transport revisited: a new turbo mechanism to drive cholesterol excretion. Trends Endocrinol. Metab. TEM. 2018;29:123–133. doi: 10.1016/j.tem.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Yamanashi Y., Takada T., Shoda J.-I., Suzuki H. Novel function of Niemann-Pick C1-like 1 as a negative regulator of Niemann-Pick C2 protein. Hepatol. Baltim. Md. 2012;55:953–964. doi: 10.1002/hep.24772. [DOI] [PubMed] [Google Scholar]
- 46.Iannucci L.F., Sun J., Singh B.K., Zhou J., Kaddai V.A., Lanni A., Yen P.M., Sinha R.A. Short chain fatty acids induce UCP2-mediated autophagy in hepatic cells. Biochem. Biophys. Res. Commun. 2016;480:461–467. doi: 10.1016/j.bbrc.2016.10.072. [DOI] [PubMed] [Google Scholar]
- 47.Nakajima A., Nakatani A., Hasegawa S., Irie J., Ozawa K., Tsujimoto G., Suganami T., Itoh H., Kimura I. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ge H., Li X., Weiszmann J., Wang P., Baribault H., Chen J.-L., Tian H., Li Y. Activation of G Protein-Coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 49.Kimura I., Ichimura A., Ohue-Kitano R., Igarashi M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020;100:171–210. doi: 10.1152/physrev.00041.2018. [DOI] [PubMed] [Google Scholar]
- 50.Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H., Lee J.R., Offermanns S., Ganapathy V. Activation of the receptor (Gpr109a) for niacin and the commensal metabolite butyrate suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sato F.T., Yap Y.A., Crisma A.R., Portovedo M., Murata G.M., Hirabara S.M., Ribeiro W.R., Marcantonio Ferreira C., Cruz M.M., Pereira J.N.B., Payolla T.B., Guima S.E.S., Thomas A.M., Setubal J.C., Alonso-Vale M.I.C., Santos M.F., Curi R., Marino E., Vinolo M.A.R. Tributyrin attenuates metabolic and inflammatory changes associated with obesity through a GPR109A-dependent mechanism. Cells. 2020;9 doi: 10.3390/cells9092007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ioannou G.N. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol. Metab. TEM. 2016;27:84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Foufelle F., Ferré P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem. J. 2002;366:377–391. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Y., Lei L., Wang X., Jiang Q., Loor J.J., Kong F., Chen L., Li J., Zhao C., Liu M., Liu G., Li X. Low abundance of insulin-induced gene 1 contributes to SREBP-1c processing and hepatic steatosis in dairy cows with severe fatty liver. J. Dairy Sci. 2023;106:5626–5635. doi: 10.3168/jds.2022-22895. [DOI] [PubMed] [Google Scholar]
- 55.Popeijus H.E., Zwaan W., Tayyeb J.Z., Plat J. Potential contribution of short chain fatty acids to hepatic apolipoprotein A-I production. Int. J. Mol. Sci. 2021;22:5986. doi: 10.3390/ijms22115986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stern J.H., Rutkowski J.M., Scherer P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metabol. 2016;23:770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaibi M.S., Stocker C.J., O'Dowd J., Davies A., Bellahcene M., Cawthorne M.A., Brown A.J.H., Smith D.M., Arch J.R.S. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010;584:2381–2386. doi: 10.1016/j.febslet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 58.Qian H., Chao X., Williams J., Fulte S., Li T., Yang L., Ding W.-X. Autophagy in liver diseases: a review. Mol. Aspect. Med. 2021;82 doi: 10.1016/j.mam.2021.100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khambu B., Yan S., Huda N., Liu G., Yin X.-M. Autophagy in non-alcoholic fatty liver disease and alcoholic liver disease. Liver Res. 2018;2:112–119. doi: 10.1016/j.livres.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.den Besten G., Bleeker A., Gerding A., van Eunen K., Havinga R., van Dijk T.H., Oosterveer M.H., Jonker J.W., Groen A.K., Reijngoud D.-J., Bakker B.M. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 61.He J., Zhang P., Shen L., Niu L., Tan Y., Chen L., Zhao Y., Bai L., Hao X., Li X., Zhang S., Zhu L. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020;21:6356. doi: 10.3390/ijms21176356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duncan E.M., Vita L., Dibnah B., Hudson B.D. Metabolite-sensing GPCRs controlling interactions between adipose tissue and inflammation. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K., Hammer R.E., Williams S.C., Crowley J., Yanagisawa M., Gordon J.I. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y.-L., Lin Z.-J., Li C.-C., Lin X., Shan S.-K., Guo B., Zheng M.-H., Li F., Yuan L.-Q., Li Z.-H. Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study. Signal Transduct. Targeted Ther. 2023;8:98. doi: 10.1038/s41392-023-01333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.V. Woo, T. Alenghat, Epigenetic regulation by gut microbiota, Gut Microb..14 (n.d.) 2022407. 10.1080/19490976.2021.2022407.. [DOI] [PMC free article] [PubMed]
- 66.Li D., Li Y., Yang S., Lu J., Jin X., Wu M. Diet-gut microbiota-epigenetics in metabolic diseases: from mechanisms to therapeutics. Biomed. Pharmacother. Biomed. Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113290. [DOI] [PubMed] [Google Scholar]
- 67.Alsharairi N.A. Therapeutic potential of gut microbiota and its metabolite short-chain fatty acids in neonatal necrotizing enterocolitis. Life. 2023;13:561. doi: 10.3390/life13020561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vachher M., Bansal S., Kumar B., Yadav S., Burman A. Deciphering the role of aberrant DNA methylation in NAFLD and NASH. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boyraz M., Cekmez F., Karaoglu A., Cinaz P., Durak M., Bideci A. Serum adiponectin, leptin, resistin and RBP4 levels in obese and metabolic syndrome children with nonalcoholic fatty liver disease. Biomarkers Med. 2013;7:737–745. doi: 10.2217/bmm.13.13. [DOI] [PubMed] [Google Scholar]
- 70.Francisco V., Sanz M.J., Real J.T., Marques P., Capuozzo M., Ait Eldjoudi D., Gualillo O. Adipokines in non-alcoholic fatty liver disease: are we on the road toward new biomarkers and therapeutic targets? Biology. 2022;11:1237. doi: 10.3390/biology11081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao H., Fan C., Lu Y., Fan X., Xia L., Li P., Wang R., Tang T., Wang Y., Qi K. Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice. Genes Nutr. 2020;15:12. doi: 10.1186/s12263-020-00671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu Y., Fan C., Liang A., Fan X., Wang R., Li P., Qi K. Effects of SCFA on the DNA methylation pattern of adiponectin and resistin in high-fat-diet-induced obese male mice. Br. J. Nutr. 2018;120:385–392. doi: 10.1017/S0007114518001526. [DOI] [PubMed] [Google Scholar]
- 73.Y L., F C., A L., X F., R W., P L., K Q. Effects of SCFA on the DNA methylation pattern of adiponectin and resistin in high-fat-diet-induced obese male mice. Br. J. Nutr. 2018;120 doi: 10.1017/S0007114518001526. [DOI] [PubMed] [Google Scholar]
- 74.Gomathi K., Akshaya N., Srinaath N., Rohini M., Selvamurugan N. Histone acetyl transferases and their epigenetic impact on bone remodeling. Int. J. Biol. Macromol. 2021;170:326–335. doi: 10.1016/j.ijbiomac.2020.12.173. [DOI] [PubMed] [Google Scholar]
- 75.Bultman S.J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201500902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirzaei R., Afaghi A., Babakhani S., Sohrabi M.R., Hosseini-Fard S.R., Babolhavaeji K., Khani Ali Akbari S., Yousefimashouf R., Karampoor S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. Biomed. Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111619. [DOI] [PubMed] [Google Scholar]
- 77.Xie L., Alam M.J., Marques F.Z., Mackay C.R. A major mechanism for immunomodulation: dietary fibres and acid metabolites. Semin. Immunol. 2023;66 doi: 10.1016/j.smim.2023.101737. [DOI] [PubMed] [Google Scholar]
- 78.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., Takahashi M., Fukuda N.N., Murakami S., Miyauchi E., Hino S., Atarashi K., Onawa S., Fujimura Y., Lockett T., Clarke J.M., Topping D.L., Tomita M., Hori S., Ohara O., Morita T., Koseki H., Kikuchi J., Honda K., Hase K., Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 79.Sun M., Wu W., Liu Z., Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu J., Zhou Z., Hu Y., Dong S. Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J. Genet. Genomics. 2012;39:375–384. doi: 10.1016/j.jgg.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 81.Thomas S.P., Denu J.M. Short-chain fatty acids activate acetyltransferase p300. Elife. 2021;10 doi: 10.7554/eLife.72171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agbu P., Carthew R.W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 2021;22:425–438. doi: 10.1038/s41580-021-00354-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Du J., Zhang P., Luo J., Shen L., Zhang S., Gu H., He J., Wang L., Zhao X., Gan M., Yang L., Niu L., Zhao Y., Tang Q., Tang G., Jiang D., Jiang Y., Li M., Jiang A., Jin L., Ma J., Shuai S., Bai L., Wang J., Zeng B., Wu D., Li X., Zhu L. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microb. 2021;13:1–19. doi: 10.1080/19490976.2020.1862612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pant K., Yadav A.K., Gupta P., Islam R., Saraya A., Venugopal S.K. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017;12:340–349. doi: 10.1016/j.redox.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lau L.H.S., Wong S.H. Microbiota, obesity and NAFLD. Adv. Exp. Med. Biol. 2018;1061:111–125. doi: 10.1007/978-981-10-8684-7_9. [DOI] [PubMed] [Google Scholar]
- 86.Fang W., Xue H., Chen X., Chen K., Ling W. Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high-fat diet-induced obesity in mice. J. Nutr. 2019;149:747–754. doi: 10.1093/jn/nxy324. [DOI] [PubMed] [Google Scholar]
- 87.Jiao A.R., Diao H., Yu B., He J., Yu J., Zheng P., Huang Z.Q., Luo Y.H., Luo J.Q., Mao X.B., Chen D.W. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196867. [DOI] [PubMed] [Google Scholar]
- 88.Deng M., Qu F., Chen L., Liu C., Zhang M., Ren F., Guo H., Zhang H., Ge S., Wu C., Zhao L. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J. Endocrinol. 2020;245:425–437. doi: 10.1530/JOE-20-0018. [DOI] [PubMed] [Google Scholar]
- 89.Jiao A., Yu B., He J., Yu J., Zheng P., Luo Y., Luo J., Mao X., Chen D. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Food Funct. 2020;11:1845–1855. doi: 10.1039/c9fo02585e. [DOI] [PubMed] [Google Scholar]
- 90.Guo J., Zhang M., Wang H., Li N., Lu Z., Li L., Hui S., Xu H. Gut microbiota and short chain fatty acids partially mediate the beneficial effects of inulin on metabolic disorders in obese ob/ob mice. J. Food Biochem. 2022;46 doi: 10.1111/jfbc.14063. [DOI] [PubMed] [Google Scholar]
- 91.Yu R., Yin Y., Cao M., Ye D., Zhang Y., Zhou Q., Mei Y. Fructo-oligosaccharides lower serum lipid levels and suppress high-fat/high-sugar diet-induced inflammation by elevating serum and gut levels of short-chain fatty acids. J. Int. Med. Res. 2020;48 doi: 10.1177/0300060519896714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lan Y., Sun Q., Ma Z., Peng J., Zhang M., Wang C., Zhang X., Yan X., Chang L., Hou X., Qiao R., Mulati A., Zhou Y., Zhang Q., Liu Z., Liu X. Seabuckthorn polysaccharide ameliorates high-fat diet-induced obesity by gut microbiota-SCFAs-liver axis. Food Funct. 2022;13:2925–2937. doi: 10.1039/d1fo03147c. [DOI] [PubMed] [Google Scholar]
- 93.Chang X., Shen Y., Yun L., Wang X., Feng J., Yang G., Meng X., Zhang J., Su X. The antipsychotic drug olanzapine altered lipid metabolism in the common carp (Cyprinus carpio L.): insight from the gut microbiota-SCFAs-liver axis. Sci. Total Environ. 2022;856 doi: 10.1016/j.scitotenv.2022.159054. [DOI] [PubMed] [Google Scholar]
- 94.Fu K., Ma C., Wang C., Zhou H., Gong L., Zhang Y., Li Y. Forsythiaside A alleviated carbon tetrachloride-induced liver fibrosis by modulating gut microbiota composition to increase short-chain fatty acids and restoring bile acids metabolism disorder. Biomed. Pharmacother. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113185. [DOI] [PubMed] [Google Scholar]
- 95.Liu H., Xu J., Li H., Zhang L., Xu P. Network pharmacology-based investigation to explore the effect and mechanism of Erchen decoction against the nonalcoholic fatty liver disease. Anat. Rec. 2021;304:2605–2619. doi: 10.1002/ar.24770. [DOI] [PubMed] [Google Scholar]
- 96.Li J., Hu Q., Xiao-Yu D., Zhu L., Miao Y.-F., Kang H.-X., Zhao X.-L., Yao J.-Q., Long D., Tang W.-F., Wan M.-H. Effect of Sheng-Jiang powder on gut microbiota in high-fat diet-induced NAFLD, Evid.-based Complement. Altern. Med. 2020;2020 doi: 10.1155/2020/6697638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin M., Kalainy S., Baskota N., Chiang D., Deehan E.C., McDougall C., Tandon P., Martínez I., Cervera C., Walter J., Abraldes J.G. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. Off. J. Int. Assoc. Study Liver. 2019;39:1437–1447. doi: 10.1111/liv.14106. [DOI] [PubMed] [Google Scholar]
- 98.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., Verbeke K., Reid G. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 99.Du M., Cheng X., Qian L., Huo A., Chen J., Sun Y. Extraction, physicochemical properties, functional activities and applications of inulin polysaccharide: a review. Plant Foods Hum. Nutr. Dordr. Neth. 2023;78:243–252. doi: 10.1007/s11130-023-01066-6. [DOI] [PubMed] [Google Scholar]
- 100.van der Beek C.M., Canfora E.E., Kip A.M., Gorissen S.H.M., Olde Damink S.W.M., van Eijk H.M., Holst J.J., Blaak E.E., Dejong C.H.C., Lenaerts K. Fructo-oligosaccharides lower serum lipid levels and suppress high-fat/high-sugar diet-induced inflammation by elevating serum and gut levels of short-chain fatty acids. Metabolism. 2018;87:25–35. doi: 10.1016/j.metabol.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 101.Matsumoto K., Ichimura M., Tsuneyama K., Moritoki Y., Tsunashima H., Omagari K., Hara M., Yasuda I., Miyakawa H., Kikuchi K. Fructo-oligosaccharides and intestinal barrier function in a methionine-choline-deficient mouse model of nonalcoholic steatohepatitis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takai A., Kikuchi K., Ichimura M., Tsuneyama K., Moritoki Y., Matsumoto K., Tsunashima H., Onda T., Kuniyoshi N., Nariyama T., Ohyatsu S., Kubota J., Nagumo K., Sato S., Hara M., Miyakawa H. Fructo-oligosaccharides ameliorate steatohepatitis, visceral adiposity, and associated chronic inflammation via increased production of short-chain fatty acids in a mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol. 2020;20:46. doi: 10.1186/s12876-020-01194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carpi R.Z., Barbalho S.M., Sloan K.P., Laurindo L.F., Gonzaga H.F., Grippa P.C., Zutin T.L.M., Girio R.J.S., Repetti C.S.F., Detregiachi C.R.P., Bueno P.C.S., Mazuqueli Pereira E. de S.B., Goulart R. de A., Haber J.F.D.S. The effects of probiotics, prebiotics and synbiotics in non-alcoholic fat liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. Int. J. Mol. Sci. 2022;23:8805. doi: 10.3390/ijms23158805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ji J., Jin W., Liu S.-J., Jiao Z., Li X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm. 2023;4 doi: 10.1002/mco2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang C., Ma C., Fu K., Liu Y., Gong L., Peng C., Li Y. Hepatoprotective effect of phillygenin on carbon tetrachloride-induced liver fibrosis and its effects on short chain fatty acid and bile acid metabolism. J. Ethnopharmacol. 2022;296 doi: 10.1016/j.jep.2022.115478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.