Abstract
Various preclinical and a limited number of clinical studies of CAR-NK cells have shown promising results: efficient elimination of target cells without side effects similar to CAR-T therapy. However, the homing and infiltration abilities of CAR-NK cells are poor due to the inhibitory tumor microenvironment. From the perspective of clinical treatment strategies, combined with the biological and tumor microenvironment characteristics of NK cells, CAR-NK combination therapy strategies with anti-PD-1/PD-L1, radiotherapy and chemotherapy, kinase inhibitors, proteasome inhibitors, STING agonist, oncolytic virus, photothermal therapy, can greatly promote the proliferation, migration and cytotoxicity of the NK cells. In this review, we will summarize the targets selection, structure constructions and combinational therapies of CAR-NK cells for tumors to provide feasible combination strategies for overcoming the inhibitory tumor microenvironment and improving the efficacy of CAR-NK cells.
Keywords: Chimeric antigen receptor, Natural killer cells, Cancer immunotherapy
1. Introduction
Chimeric antigen receptor (CAR)-modified immune effector cell (CAR-T/NK) therapy is a tumor adoptive therapy developed in recent years. At present, CAR-T cell therapy has been proven to be an effective tumor therapy, especially the application of CD19-targeted CAR-T cells in B cell originated leukemia and lymphoma [1]. However, extra-target toxicity, cytokine release syndrome (CRS) and graft-versus-host disease (GVHD) have limited their further clinical applications [2]. CAR-T cell therapy is highly personalized because the cells used come from the patients themselves, which can be expensive, time-consuming, and sometimes fail to achieve the best results due to the poor quality and/or quantity of patient-derived T cells [3]. Natural killer (NK) cells are considered an alternative to modified T cells because NK cells inherently lack these disadvantages and have a wide range of sources. CAR-NK cell therapy is therefore safer than CAR-T cell therapy and is becoming a research hotspot in the field of tumor immunity [4]. NK cells express significantly lower levels of PD-1 and hardly cause immune suppression, which makes NK cells a good candidate for fighting solid tumors, establishing the way for their wide applications in allogeneic environments and the generation of nonspecific cell therapeutic products [5]. In addition, NK cells promote the migration of dendritic cells into tumors, thereby enhancing the effect of anti-PD-1/PD-L1/CTLA4 immunotherapy [6]. Therefore, NK cells play an important role in tumor killing.
CAR-NK cell therapy has been proven to kill malignant hematological tumors and solid tumor cells in preclinical and clinical trials, showing its potential as an off-the-shelf product with wide clinical applications [7]. These encouraging findings demonstrate that CAR-NKs are an attractive cancer treatment and pave the way for their further development. In solid tumors, although preclinical data are encouraging, clinical data are still in their infancy. In this case, there are still some challenges to using CAR cell therapy, such as how to effectively deliver CAR-NK cells to tumor tissues, how to improve CAR-NK cell persistence and activity within tumors, and how to deal with tumor immune escape. New technologies and improvements are being explored both preclinically and clinically, aiming to solve the obstacles of CAR cell therapy for solid tumors or to explore new methods centered on cellular immunotherapy and combined with other antitumor strategies that may achieve better therapeutic effects [8]. In this review, we will summarize the target selection, structure construction and combination therapy strategies of CAR-NKs for cancer.
1.1. Selection of CAR-NK targets
To achieve the effective treatment of tumors by CAR-NK cells, it is particularly important to select the appropriate target antigen in the first step of CAR molecular design [9]. A properly selected target plays a central role in determining the success of CAR-NK cell therapy. Ideally, target antigen should be overexpressed on tumor tissue, with no or low expression on normal cells. At the same time, antigens also need to have cell membrane localization able to be recognized by the CAR-NK cells [10]. CAR targets can be broadly classified into four categories, namely, tumor-specific antigen (TSA), tumor-associated antigen (TAA) [11], cancer-associated stromal cell (CASC) surface antigen, and glycolipid antigen. This category covers almost all targets currently under study [10,12]. Therefore, the potential targets of CAR therapy can be flexibly selected over a wide range. The key to target antigen selection is to find one with better safety, specificity and effectiveness for the modified NK cell therapy. At present, CD19, B cell maturation antigen (BCMA) and CD33 are the most common CAR targets in malignant hematological diseases, while MSLN, GD2, HER2, carcinoembryonic antigen (CEA), GPC3 and EGF receptor variant III (EGFRvIII) are the most common CAR targets in solid cancers [11]. However, CD19 CAR therapy for lymphoma or lymphoblastic leukemia is the most studied indication in clinical practice and the only target of current FDA-approved therapies. Therefore, the selection of appropriate targets is an important challenge in the clinical application of CAR-NK cell immunotherapy in cancer research [13,14].
1.2. Design and construction of CAR-NK cells
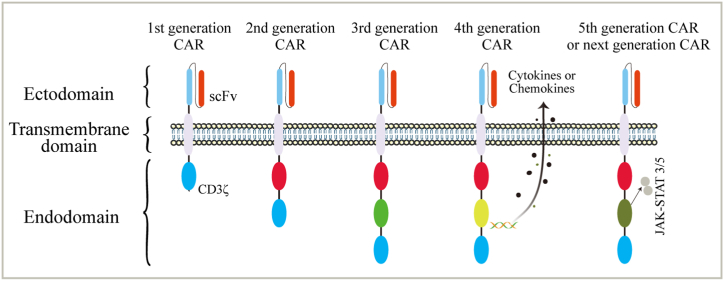
CAR is the core component of CAR-T/CAR-NK cells, which are generally composed of three structures: the extracellular domain, transmembrane domain and intracellular domain [15]. At present, CAR cell therapy is widely used in targeted immunotherapy. Based on the intracellular activation domain, five generations of CAR vector designs have been produced [16] (Fig. 1). The first generation of CARs contains only antigen recognition domains derived from scFv antibodies (targeting the cell membrane antigens) and CD3ζ signal domains (effector cell activation) [17]. On the basis of the original intracellular signal region, one or two costimulatory signal elements, such as CD28, OX-40 and 4-1BB, are connected in series to derive the second- and third-generation CARs, and the CAR structure with the introduction of cytokines and costimulatory ligands is called the fourth-generation CAR [18]. The second generation can improve the cytotoxicity, proliferation activity and survival time of NK cells, increase the release of cytokines induced by antigens, and upregulate antiapoptotic proteins, which enhance the antitumor effect [19]. The third-generation CAR contains a structure with two costimulatory domains [20] to further improve the signal transduction function so that CAR-NK cells have greater in vivo expansion capacity and longer persistence [21]. The fourth generation of CAR, also known as TRUCK (T cells redirected for universal cytokine killing), encodes CAR or its promoters, suicide genes, cytokines, chemokines, etc., and further produces effective secondary signals under the stimulation of the above molecules. These secondary signals are recognized by other components of the immune system, which further amplifies the antitumor immune effect, prolongs the survival time of cells in the body and enhances the killing ability of the related immunological cells. It effectively reduces toxicity and side effects and becomes more controllable [16,[22], [23], [24]].
Fig. 1.
Evolution of chimeric antigen receptor (CAR)-NK cell structure over time: The structural components of the 1st, 2nd, 3rd, 4th and 5th generation of CAR. The number and composition of intracellular activation domains determines CAR generation. Various combinations of activating domains are used to mount a strong antitumor response. One example of a “fourth-generation” CAR is shown, coexpressing stimulating cytokines or chemokines. The 5th CAR generation or next is based on the second generation CAR, but they contain a truncated cytoplasmic IL-2 receptor β-chain domain with a transcription factor STAT3 binding site. The different CAR generations are designed to further improve the therapeutic activity of the CAR-based immunotherapies. Abbreviations: scFv, single-chain variable fragment; TM, transmembrane.
At present, fifth-generation CARs are being explored. These CARs are based on the second-generation CARs, but they contain a truncated cytoplasmic IL-2 receptor β-chain domain with a transcription factor STAT3 binding site. The antigen-specific activation of the receptor triggers TCR (through CD3ζ), costimulatory (CD28) and cytokine (JAK-STAT3/5) signals, which effectively provide all three physiologically necessary cooperative signals to drive T cell activation and proliferation [16]. Other variants of CAR, such as double CAR, dissociated CAR and inducible segregated CAR, are also being explored to further improve the specificity and controllability of T cell infusion. At present, second- and third-generation CAR designs are mainly used in clinical research and achievement transformation, and fourth- and fifth-generation CARs have gradually attracted increasing attention.
1.3. Database analysis of CAR-NK cell applications
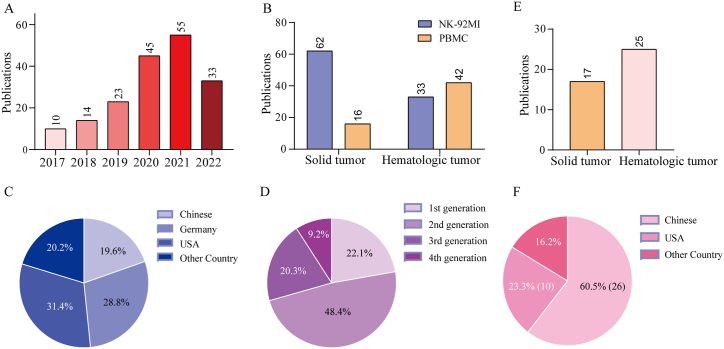
We searched the PubMed database and found that there were 153 CAR-NK preclinical studies, of which 95 CAR-NK cells were derived from cell lines (mainly NK-92 or NK-92MI) and 58 study cells were from primary cells (as of August 31, 2022) (Fig. 2). All of these studies introduced foreign antigen-binding motifs from tumor-targeted monoclonal antibodies into cells. The common targets of solid tumors are HER2 (16 items); GD2 (7 items); GPC3 (7 items); EGFR (5 items); EGFRvIII (5 items); PDL1 (5 items); MSLN (5 items); EpCAM (5 items); PMSA (6 items); NKG2D (3 items); B7H3 (2 items); PSCA (3 items); and CD147 (2 items), among which HER2, GD2, GPC3, EGFRvIII, PDL1, MSLN and EpCAM have been more studied. In addition, there are also B7H6, FRa, DLL3, CD44, CD73, CEA1, CD25, HLA-G, Robo and other target studies. The common research targets of hematological tumors are CD123, CD138, CD20, CD33, CD38, CD5, CD7, CD19, CD22, BCMA, etc., among which CD19 is the most common, up to 36, followed by CD123 and CD20. The analysis revealed that Her2 (expressed on a subset of cancer cells of breast, gastric and colon) was the most commonly used target of solid tumors, whereas CD19 antigen (B-cell malignancies) was the most common in hematologic diseases. The number of preclinical studies on CAR-NK cells is increasing year by year (Fig. 2A). Statistically, it has been found that the proportion of studies on solid tumors and hematologic diseases is similar (78 cases of solid tumors and 75 cases of hematologic malignancies). Interestingly, the number of CAR-NK cell lines studied in solid tumors is approximately twice that of hematological malignancies, which are mostly studied using primary NK cells (Fig. 2B). In terms of research area, 30 projects (19.6%) were from China, 44 projects (28.8%) were from Germany, and 48 projects (31.4%) were from the United States. The total proportion of other countries was 20.2% (Fig. 2C). From the analysis of the CAR molecular domain, the first generation of CAR molecular research is rare and now mainly focuses on the second and third generations of CAR research. The second generation of CAR research is the majority, accounting for approximately 48.4%. The third-generation CAR accounted for approximately 20.3%, showing an increasing trend year by year. The studies on fourth-generation CAR molecules have also been emerging, accounting for approximately 9.2% at present (Fig. 2D).
Fig. 2.
CAR-NK cells: growing interest and data from preclinical and clinical studies. (A) Bar graph showing the number of manuscripts reporting experimental data on human CAR-NK cells until August 2022. (B) Bars show the number of publications in all CAR-NK cells derived from NK-92MI cell lines (blue) and primary NK cells (yellow). (C) Pie charts showing the percentage of publications in different countries. (D) Pie charts showing the percentage of publications in different generations. (E) Bars show the number of CAR-NK clinical trials in all patients with CAR-NK cells derived from solid tumors (yellow) and hematologic tumors (pink). (F) Pie charts showing the percentage of CAR-NK clinical trials in different countries.
Forty-three registered CAR-NK clinical trials are currently available from the clinicaltrials.gov database. As of August 31, 2022, many studies are still in early planning or are enrolling in ClinicalTrials.gov. Of these, 25 were hematologic diseases and 17 were solid tumors; 26 were in China and 10 were in the United States (Fig. 2E–F). Trials of CAR NK cells have focused on lymphomas and leukemia, followed by solid tumors, including ovarian, prostate, brain, liver, bowel, lung and pancreatic cancers. As a new anticancer weapon, CAR-NK cell therapy has been applied in early clinical trials with increasingly rich research results and some breakthroughs [25]. However, its efficacy and safety are mostly still in an argumentation stage, the treatment effects of individual patients are very different, and there is a risk of serious complications [26]. In recent years, the number and type of CAR-NK clinical trials are growing rapidly, rendering its clinical practice more challenging.
CAR-NK cell therapy has many advantages compared with CAR-T cell therapy, and many preclinical studies have proven that CAR-NK cell therapy has great potential not only in the treatment of hematological tumors but also in the treatment of solid tumors [4]. In a recent study, 11 patients with recurrent or refractory CD19-positive hematological tumors, most of them had a good response to CAR-NK cell therapy and no major side effects, and the IL-6 level was not higher than baseline [25]. Although the efficacy and advantages of CAR-NK cells in tumor killing have made great progress, there are still some technical or clinical problems that remain to be solved to optimize antitumor immunotherapy based on CAR-NK cells. The tumor microenvironment is a major obstacle to the success of CAR-NK cell therapy, including immunosuppressive substances, immunosuppressive cells, and adverse environments for normal immune cell function. In addition, the homing ability of NK cells is poor, which is disadvantageous to their intratumoral infiltration. The heterogeneity of solid tumors, limited infiltration from blood to the tumor site, and immunosuppression caused by metabolic disorders completely inhibit the function of CAR-NK cells [27,28]. From the perspective of clinical treatment strategies, combined with the biological characteristics of NK cells and the tumor microenvironment, CAR-NK combination therapy can greatly promote the proliferation, persistence, migration and invasion of NK cells into tumors.
1.4. Advantages and challenges of CAR-NK in anti-tumor therapy
CAR-NK cell therapy has many advantages compared with CAR-T cell therapy, and many preclinical studies have proved that CAR-NK cell therapy has great potential not only in the treatment of hematological tumors, but also in the treatment of solid tumors [4]. CAR-NK cells have many advantages, such as the safety of clinical application, the mechanism of identifying cancer cells and the richness of clinical samples [29]. CAR-NK cell therapy does not cause graft-versus-host disease, nor does it secrete inflammatory factors that cause cytokine release syndrome, such as IL-1, IL-6, etc [25]. At the same time, CAR-NK cells also express a variety of activated receptors, which can specifically recognize the ligands expressed on tumor cells and improve the effect of immunotherapy [30]. NK cells are rich in a variety of NK cell lines, umbilical cord blood and peripheral blood, which are sufficient to meet the needs of patients' products [31]. In addition, CAR-NK cells have more anti-tumor pathways, such as cell degranulation, release of cytokines, activation of apoptosis pathway and mediated antibody-dependent cytotoxicity (ADCC), which are faster and more direct than CAR-T cells [32,33]. The survival cycle of CAR-NK cells in vivo is short, and it is not easy to produce long-term adverse reactions. CAR-NK cells have a defensive effect against pathogens and help to prevent and treat infection. CAR-NK cells have immunomodulatory function, which can regulate the function of B cells and CTL cells [34,35]. In short, CAR-NK cells have good anti-tumor activity, high safety and can be produced on a large scale. They are general-purpose and ready-to-use products. The establishment of good CAR-NK cells is of great significance to improve the therapeutic effect of cancer.
Compared with CAR-T, CAR-NK has some technical problems to be solved. Due to tumor heterogeneity and lack of cytokines, insufficient expansion of CAR-NK cells in vivo after transplantation is a serious challenge. In particular, the number of NK cells from a single donor is not sufficient for treatment, and the expansion of primary NK cells in vitro is the main challenge of CAR-NK cell therapy [36]. In addition, the transfection efficiency of CAR-NK cells is low [37]. Through the unremitting research on CAR-NK cell therapy, it is believed that the existing problems will find a solution. CAR-NK cell therapy or combined with other treatments may become a powerful strategy for tumor immunotherapy in the future.
1.5. Application strategy of CAR-NK cells in tumor combination therapy
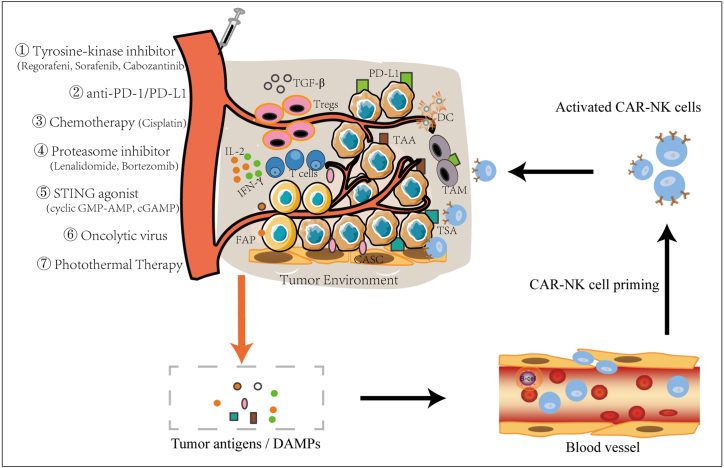
Immunotherapy has become the fourth major type of tumor therapy after surgery, radiotherapy and chemotherapy. CAR-NK is the most advanced research field of applied research and clinical medical practice in recent years and its clinical application in tumor immunotherapy has increasingly shown great potential. However, tumor immunosuppressive microenvironment and the shift of NK cell receptor/ligand group spectrum seriously inhibit the function of CAR-NK cells. Therefore, according to different tumor types and individual characteristics of different patients, reasonable and effective combination of CAR-NK cell therapy with other immunotherapy methods, radiotherapy and chemotherapy to reshape tumor microenvironment or correct the deviation of NK cell receptor/ligand group spectrum can not only improve the anti-tumor activity of CAR-NK cells, but also enhance the sensitivity of tumor cells, thus achieving better therapeutic effect [38,39] (Fig. 3,Table 1).
-
(1)
Combined immunotherapy strategy based on correcting the spectral shift of NK receptor/ligand groups
Fig. 3.
Application strategy of CAR-NK cells in tumor combination therapy. The TME is the major hindrance of CAR-NK therapy due to many factors such as immunosuppressive components secreted by a variety of inhibitory cells in the TME. Overcoming the immunosuppressive function of the TME in CAR-NK therapy is the key point to be considered for combination therapy, such as anti-PD-1/PD-L1, chemotherapy, kinase inhibitors, proteasome inhibitors, STING agonist, oncolytic virus, photothermal therapy. These methods can stimulate the release of tumor antigens and DAMPs, thus promoting the initiation, expansion and killing of CAR-NK cells. Numerous targetable molecules exist that can be categorized as TSA, TAA, CASC expressed antigen.
Table 1.
Review of current research on CAR-NK combination drugs against various solid tumors.
| Serial NO. | Tumor Type and cell lines | Specific Target | Source of NK cells | CAR composition | Drug Category | Examples | Advantages | References PMID |
|---|---|---|---|---|---|---|---|---|
| 1 | Castration‐resistant prostate cancer | PMSA | NK-92 | NKG2D-2B4-CD3ζ | Immune checkpoint inhibitors | Anti‐PD‐L1 mAb; Atezolizumab | Anti‐PD‐L1 mAb significantly enhanced the anti-tumour effect of CAR NK‐92 cells. | 35696531 |
| 2 | Cisplatin-resistant lung cancer | N | NK-92MI,primary NK | N | Immune checkpoint inhibitors | Anti-PD-1, anti-PD-L1, MEK/Erk pathway inhibitors | Enhance NK cell cytotoxicity to cisplatin-resistant cells. | 28801607 |
| 3 | K562 myeloid leukemia cells | N | PBMC | N | Immune checkpoint inhibitors | Atezolizumab | Anti-PD-L1 mAb augments human PD-L1+ NK cell antitumor activity in vivo | 31340937 |
| 4 | Multiple myeloma (MM) cells | N | NK, PBMC | N | Immune checkpoint inhibitors | anti-PD-1 mAb | PD-1 blocking enhanced exNK cell degranulation and cytolytic activity. | 27356741 |
| 5 | MDA-MB-231、H460 and HTB1 | PD-L1 | high- affinity natural killer (t- haNK),NK-92 | Not detailed. | Immune checkpoint inhibitors | anti- PD-1, N-803 (IL-15 superagonist) | These studies demonstrate the antitumor efficacy of PD-L1 thaNK cells and provide a rationale for the potential use of these cells in clinical studies. | 32439799 |
| 6 | Digestive cancers | N | NK-92, NKL | N | Immune checkpoint inhibitors | Anti-PD-1, anti-PD-L1 | Blocking PD-1/PD-L1 signaling markedly enhances cytokines production and degranulation and suppresses apoptosis of NK cells in vitro. | 28692048 |
| 7 | Head and neck cancer | N | PBMC | N | Immune checkpoint inhibitors | Nivolumab,Cetuximab | PD-1 blockade on NK cells enhances cetuximab mediated ADCC of PD-L1high tumor cells. | 30282672 |
| 8 | Hematopoietic tumor | N | Primary,NK-92 | N | Immune checkpoint inhibitors | anti-PD-L1 mAb | Blocking PD-L1 increases susceptibility to NK cell killing. | 26155422 |
| 9 | Ovarian cancer | N | PM21-NK, PBMC | N | Immune checkpoint inhibitors | anti-PD-L1 | PD-L1 blockade enhances anti-tumor efficacy of NK cells. | 30377572 |
| 10 | PD-L1- positive cancer cell lines | N | NK-92-CD16, PBMC | N | Immune checkpoint inhibitors | Atezolizumab, An anti- hPD- L1- hIgG1 | NK cells induce an ADCC response in combination with anti- PD-L1 mAbs, which helps promote ADCC antitumor activity against PD-L1- positive tumors. | 32830112 |
| 11 | K562 | N | NK-92 | N | Immune checkpoint inhibitors | Anti-PD-1, anti-PD-L1 | PD-1 and PD-L1 blockade elicited a strong NK cell response that was indispensable for the full therapeutic effect of immunotherapy. | 30198904 |
| 12 | Cisplatin resistant ovarian cancer | N | NK-92MI | N | Chemotherapy | Cisplatin | Overcome immunoresistance of chemoresistant ovarian cancers. | 34058473 |
| 13 | Ovarian cancer | CD44 | NK-92 | CD28−CD28-4-1BB-CD3ζ | Chemotherapy | Cisplatin | The simultaneous treatment with CD44NK and cisplatin showed higher anti-tumor activity than sequential treatment. | 34680456 |
| 14 | Gastric cancer | NKG2D | PBMC | 4-1BB- CD3ζ | Chemotherapy | Cisplatin | Enhance cytototoxicity. | 30132099 |
| 15 | Ovarian cancer | CD133 | NK-92 | CD28−CD28-4-1BB-CD3ζ | Chemotherapy | Cisplatin | The sequential treatment with cisplatin followed by CAR-NK cells led to the strongest killing effect. | 28836469 |
| 16 | Pancreatic Carcinoma | Robo1 | NK-92 | N | Radiotherapy | 125I Seed Brachytherapy | Robo1 specific CAR-NK immunotherapy enhances efficacy of 125I seed brachytherapy in an orthotopic pancreatic cancer mouse model. | 31704816 |
| 17 | Colorectal Cancer | EpCAM | NK-92 | CD8-4-1BB-CD3ζ | Tyrosine-kinase inhibitor | Regorafenib | Enhance the therapeutic efficacy of CAR-modified immune effector cells for solid tumors. | 30410941 |
| 18 | Hepatocellular Carcinoma | GPC3 | PBMC | CD8−CD28−CD3ζ | Tyrosine-kinase inhibitor | Sorafenib | The upregulated tumor cell apoptosis induced by the combined treatment. | 31078430 |
| 19 | Renal Cell Carcinoma (RCC) | EGFR | NK-92 | CD8−CD28−CD137-CD3ζ | Tyrosine kinase inhibitor | Cabozantinib | Enhance the killing ability of CAR-NK-92 cells against the RCC cells. | 29423418 |
| 20 | Advanced renal cell carcinoma | CAIX | NK-92 | CD8−CD28-4-1BB- CD3ζ | Proteasome inhibitor | Bortezomib | Bortezomib can enhance the effects of the CAR-NK92 cells against RCC in vitro and in vivo. | 30272343 |
| 21 | Pancreatic cancer | Mesothelin | NK-92 | CD8-4-1BB-CD3ζ | STING agonists | Cyclic GMP-AMP (cGAMP) | Combination of CAR-NK-92 cells targeting mesothelin and cGAMP displayed greater antitumor efficacy. | 35371622 |
| 22 | Hematologic malignancies and solid tumors | CD16a | Human iPSCs | N | Antibodybased drugs | anti-CD20 mAb, anti-HER2 mAb | HnCD16-iNK cells combined with mAbs are highly effective against hematologic malignancies and solid tumors that are typically resistant to NK cell–mediated killing. | 31856277 |
| 23 | Glioblastoma | EGFR | PBMCs | N | Oncolytic virus | An oncolytic virus expressing IL-15/IL-15Rα | OV-IL15C plus EGFR-CAR NK cells synergistically suppressed tumor growth and significantly improved survival. | 34006525 |
| 24 | lung cancer | B7–H3 | NK-92MI | N | Photothermal Therapy (PTT) | Near infrared (NIR-II) | synergistic CAR-NK immunotherapy is carried out specifically to eradicate any possible residual tumor cells after PTT. | 34159726 |
| 25 | Human Pancreatic Carcinoma | Robo1 | Primary NK cells | N | Brachytherapy | Iodine 125 seed brachytherap-y (125I IBT) | Robo1 specific CAR-NK immunotherapy enhances efficacy of125I IBT in an orthotopic pancreatic cancer mouse model. | 31704816 |
Data were obtained from the PubMed database. N-denotes information not provided in the study. Other abbreviations: iPSC, induced pluripotent stem cell. PBMCs, Peripheral Blood Mononuclear Cells.
Although NK cells have a strong ability to kill tumor cells, tumor cells and the tumor immune microenvironment gradually evolve many mechanisms, such as NK receptor/ligand group spectrum shifts, to inhibit the activation and killing function of NK cells and weaken the antitumor effect mediated by NK cells [40,41]. An increasing number of studies have shown that tumor cells can change the expression of NK cell receptors and ligands through the tumor immune microenvironment, reduce the expression of activated receptors, and promote or upregulate the expression of inhibitory receptors, thus inhibiting the activation and killing function of NK cells and even leading to the loss or depletion of NK cells and promoting tumor immune escape [42,43]. Therefore, regulating or correcting the shift in the NK receptor/ligand group spectrum is a potential target of therapeutic intervention.
Immune checkpoint blockade therapy targeting T cell inhibitory receptors has become a hot spot of tumor immunotherapy, especially CTLA-4, PD-1 or PD-L1 monoclonal antibodies, which have achieved remarkable efficacy in melanoma and other solid tumors, opening a new era of tumor immunotherapy. It was ranked as number one on the top 10 scientific breakthroughs of 2013 by Science magazine [44]. Especially in the tumor environment, PD-1 may be more accurately regarded as a marker of immune cell activation [45]. There are many reports on PD-1 on CAR-T cells, and related application studies are constantly making breakthroughs, such as CRISPR-Cas9 knocking out PD-1 on CAR-T cells [[46], [47], [48]], introducing a genetically engineered switch receptor structure to interfere with PD-1 signaling [49,50], CAR-T cells combined with PD-1 blockers [[51], [52], [53]] or genetically modified CAR-T cell autocrine PD-1 blockers [[54], [55], [56]]. All of these studies are aimed at blocking PD-1 receptors on CAR-T cells, reducing immunosuppressive function and improving the antitumor activity of CAR-T cells. At present, researchers are paying increasing attention to the immune checkpoints of inhibitory receptors on NK cells [57]. A large number of studies have shown that NK cells can also express the inhibitory receptor PD-1, so the therapeutic application of immune checkpoint inhibitors may increase the killing effect of NK cells against PD-L1+ tumors [[57], [58], [59], [60], [61]]. In a review of the study showing that NK cells may play an important role in antitumor responses blocked by immune checkpoints, Jeffrey Miller et al. also emphasized the importance of immune checkpoints beyond T cells, suggesting that the PD-1 axis is important for NK cells in the tumor microenvironment [62]. PD-1 is mainly expressed on activated T and NK cells [63,64], PD-L1 is mainly expressed in tumor and myeloid cells, and PD-L2 is mainly expressed in dendritic cells (DC) [65]. Many studies have found that there are NK cell subsets with high expression of PD-1 in the peripheral blood of some people and confirmed that PD-1 antibody can reverse its functional depletion [[66], [67], [68]], which further suggests that the effect of PD-1 inhibitors on NK cells in immunotherapy is also worthy of attention. In addition, some NK cells present in the tumor microenvironment express PD-1, and these NK cells have a more active and reactive phenotype than cells that do not express PD-1, which does not mark the depletion phenotype of NK cells [69], and they are more sensitive to PD-1 inhibitors [62]. The high proportion of PD-1+ NK cells in ascites of patients with ovarian cancer suggests that they may be induced or expanded in the tumor microenvironment, and the tumor site is significantly abundant. The proliferation and activation function of these NK cells are decreased but can be reversed by an anti-PD-L1 antibody [66]. Meanwhile, in a study of a mouse tumor model, it was also found that the expression of PD-1 on NK cells was upregulated in the tumor microenvironment, which inhibited the degranulation and cytotoxic function of NK cells. PD-1 and PD-L1 blockers can stimulate a strong NK cell response [69]. It is worth noting that PD-1-negative NK cells may not be able to kill tumor cells because they cannot be activated or have become incompetent. NK cells have both phenotypic and functional heterogeneity [70]. It has been reported that NK cells of patients with multiple myeloma have high expression of PD-1. Treatment with an anti-PD-1 antibody can restore the antitumor activity of NK cells and enhance cytotoxicity [58]. Since we know that the PD-1 pathway is essential for the tumor escape of T cells and NK cells [71] and PD-1 expression is also significantly increased in continuously activated CAR-NK-92 cells [72], we can consider combining PD-1 antibodies to overcome tumor escape, reverse CAR-NK cell functional depletion and enhance cytotoxicity [73].
There are different reports on the level of PD-1 expression on the cell line NK-92. Joy Hsu et al. mentioned the lack of PD-1 expression on NK-92 cells in their study on the role of NK cells in PD-1/PD-L1 blockade-mediated immunotherapy. They then overexpressed PD-1 on NK-92 cells for functional studies [69]. Other studies verified that PD-1 on NK-92 cells could be upregulated in response to the tumor microenvironment or cytokine stimulation [74,75]. In addition, Nadia Mensali et al. proposed an alternative strategy in which genetically modified built-in PD-1 blockers rescued NK-92 activity from PD-L1-mediated tumor escape by modifying NK-92 cells with intracellular segment removed PD-1 (TPD-1), eliminating PD-1/PD-L1 inhibition [76]. This indirectly indicates that PD-1 is expressed on NK-92 cells. In a study of continuous stimulation of CAR-NK-92 cells by target cells, Oelsner et al. reported that the expression of PD-1 was significantly increased after continuous activation of NK-92/63.z and NK-92/63.28.z cells but not in NK92/63.137.z cells. It is worth noting that the killing activity of NK-92/63.z and NK-92/63.28.z CAR cells was significantly better than that of NK92/63.137.z [72].
The expression of PD-L1 in tumor cells leads to a reduction in the NK cytotoxic response and the generation of more aggressive tumors in vivo [62], highlighting the importance of immune checkpoints in NK cells in addition to T cells and suggesting that the PD-1/PD-L1 axis is important for NK cells in the tumor microenvironment [69]. Using a mouse model, researchers confirmed that NK cells responded to PD-1 and PD-L1 inhibitors, enhancing the immunotherapy of NK cells against cancer [62]. Studies have reported that the addition of an anti-PD-L1 antibody to the coculture of macrophages and NK cells can significantly reduce the inhibitory effect of macrophages on NK cell proliferation and ADCC, and it is worth noting that it can achieve complete reversal [77]. Increased PD-L1 expression leads to increased resistance to NK cell lysis, and blocking JAK pathway activation prevents increased PD-L1 expression, thereby increasing the susceptibility of tumor cells to NK cell activity [78]. The combination of anti-PD-L1 and NK cell therapy, blocking PD-L1, could increase the number and persistence of NK cells in vivo and retain its cytotoxic phenotype [79,80]. Therefore, blocking PD-L1 can enhance the sensitivity of NK cells to tumor cells. Recent studies have reported that genetic susceptibility genes can cause cancer not only by increasing the probability of gene mutation but also by shaping immune function. Impaired migration and adhesion of immune cells in tumor patients may lead to inefficient antitumor immunity [81]. These factors can explain why immune checkpoint inhibitors are not as effective in the treatment of ovarian cancer.
There are also rare reports that PD-L1 expression can be detected on NK cells [75,82,83]. This may be because tumors induce PD-L1 expression on NK cells through AKT signaling. Anti-PD-L1 monoclonal antibody can also enhance the antitumor effect of NK cells, only through anti-PD-L1 monoclonal antibody activating PD-L1+ NK cells to exert an antitumor effect, rather than a PD-1-dependent mechanism, which provides new insight into NK cell activation. This may also provide a potential explanation for why clinically some patients lacking PD-L1 expression on tumor cells still respond to anti-PD-L1 monoclonal antibody therapy.
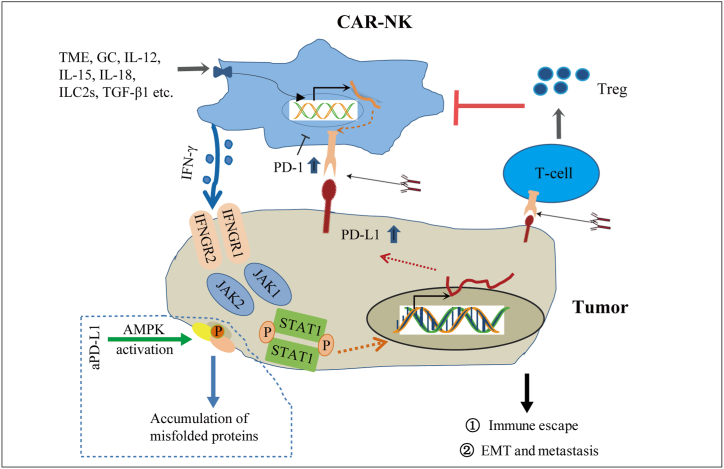
In addition, glucocorticoids (GCs), ILC2s, PMA, TGF-β, IL-12, IL-15 and IL-18 in the tumor microenvironment can induce the expression of PD-1 on NK cells [75,[84], [85], [86], [87], [88]]. PD-L1 on tumor cells can also interact with PD-1 on T cells, thus promoting the expansion of Treg cells. Treg cells have been known to inhibit the function and persistence of NK cells [79,89], and blocking PD-1/PD-L1 may prevent the induction of Tregs. Energy status determines the abundance of PD-L1 protein and antitumor immunity. Studies on immune checkpoint blockade have revealed the key role of AMPK in regulating the immune response to immune checkpoint blockade [90]. It is speculated that anti-PD-L1 may promote AMPK activation and the accumulation of false proteins in tumors, thus enhancing the sensitivity of the tumor to NK cells (Fig. 4).
Fig. 4.
Depict the mechanism of the interaction between tumor cells and NK cells. NK cells bind to target cells, secrete IFN-γ, up-regulate PD-L1 on target cells through JAK/STAT pathway, directly inhibit the function of NK cells through binding with PD-1, or indirectly enhance tumor resistance to NK cells through Treg cells, thus promoting tumor immune escape. In addition, PD-L1, GC, interleukin, TGF and other factors in the tumor microenvironment can induce the up-regulation of PD-1 on NK cells. PD-1/PD-L1 antibody can inhibit the interaction between PD-1 and PD-L1, and blocking PD-L1 may activate AMPK and enhance the sensitivity of tumor to NK cells.
NK cells also express inhibitory receptors such as TIM-3, LAG-3 and TIGIT [[91], [92], [93], [94]]. As an immune checkpoint blocking target, these inhibitory receptors are expected to be combined with CAR-NK cells to activate the antitumor immune response of NK cells and be used for tumor treatment [95]. Based on the correct NK receptor/ligand group of immunotherapy spectral migration strategies (especially inhibitory receptor checkpoint block therapy and CAR modification of NK cell combination therapy), it is more advantageous to correct the dysfunctional state of NK cells in the tumor microenvironment, enhance the activity of NK cells and antitumor immune response, and have good application prospects.
-
(2)
Combination with radiotherapy and chemotherapy to enhance the antitumor activity of CAR-NK cells
Recent studies have found that chemoradiotherapy can not only directly inhibit the proliferation of cancer cells and promote the apoptosis of cancer cells but also regulate the expression of NK cell receptors/ligands and enhance the sensitivity of immune cells to tumor cell killing. Many studies have reported that CAR-T/NK cells are still cytotoxic in the presence of cisplatin, and cisplatin can enhance CAR-T/NK cell-mediated cytotoxicity and has good antitumor activity [96,97]. Recent studies have reported that CAR-NK cells targeting CD44 remain cytotoxic in the presence of cisplatin, and most importantly, the combination of CD44-CAR-NK cells and cisplatin shows higher antitumor activity than sequential therapy [98]. The combination of CAR-NK92 cells targeting CD133 and cisplatin has the strongest antitumor effect on ovarian cancer, while cisplatin does not affect the cytotoxicity and viability of CAR-NK cells [97]. In addition, the synergistic effect of CAR-T/NK cells and radiotherapy in glioblastoma and pancreatic cancer has also been verified [99,100]. Therefore, the combination of radiotherapy and chemotherapy and immunotherapy has a synergistic antitumor effect.
Conventional intravenous chemotherapy has a good killing effect on tumor cells, but it also has very serious toxic and side effects. In order to reduce systemic toxicity, the concept of using immune cells as carriers to deliver anti-tumor drugs directly to the tumor site has received much attention [101,102]. A high level of free sulfhydryl groups can be detected on the surface of NK cells. Siegler et al. encapsulated paclitaxel in synthetic cross-linked multilayer liposome vesicles (cMLV) nanoparticles [103]. cMLV binds stably to merhydryl groups on the surface of NK cells through Maleimide, a functional group on its surface lipid bilayer. In this way, paclitaxel-loaded cMLV was transported to the vicinity of tumor cells by utilizing the targeting property of CAR-NK cells to exert the combined effect of tumor cell immunotherapy and chemotherapy.
-
(3)
Combination with a variety of kinase inhibitors regulates the tumor microenvironment and promotes antitumor immunity
Recent studies have shown that some tyrosine kinase inhibitors (TKIs) not only have direct antitumor activity but also regulate the tumor microenvironment and promote antitumor immunity. Studies have shown that regorafenib [8] and sorafenib [104,105] can reshape the immunosuppressive microenvironment and enhance the antitumor immune response, and the combination of these drugs can be used to enhance the efficacy of immunotherapy. Sorafenib combined with GPC3-CAR T cells can synergistically induce tumor cell apoptosis, demonstrating the clinical potential of sorafenib combined with targeted GPC3-CAR T cells in the treatment of hepatocellular carcinoma [106]. The combination of CAR-NK cells targeting epithelial cell adhesion molecule (EpCAM) and regorafenib in the treatment of a human colon cancer model has a synergistic effect [8], showing a good cytotoxic effect, which provides a new strategy for the treatment of solid tumors. In addition, cabotinib can increase the expression of EGFR in renal cancer cells, reduce the expression of PD-L1 on the membrane surface, enhance the killing ability of CAR-NK-92 cells to renal cancer cells in vitro, and have a synergistic effect with CAR-NK-92 cells targeting EGFR [106].
-
(4)
Combined with proteasome inhibitors to enhance the function of CAR-NK cells
Proteasome inhibitors have been shown to inhibit tumors directly or indirectly by enhancing the function of NK cells or enhancing the sensitivity of tumor cells to killing. For example, lenalidomide, which has been approved by the FDA, can enhance NK cell killing and proliferation by inducing peripheral T cells and dendritic cells to release IL-2 and IFN-γ [107]. A number of studies have shown that lenalidomide can enhance the activity of CAR-T cells, and the combination of the two has a good clinical effect [108,109]. Bortezomib can enhance the sensitivity of tumor cells to NK cell killing. For example, the inhibitory effect of bortezomib combined with CAR-NK-92 cells on established carbonic anhydrase IX (CAIX)-positive xenografts is more significant than that of CAR-NK-92 cells alone or bortezomib alone [110]. The elucidation of the regulatory effect of these drugs on NK cells has laid a theoretical basis for the subsequent combined application of NK cellular immunotherapy [111,112].
-
(5)
Combined with STING agonists, they activate the STING signaling pathway in CAR-NK cells and enhance antitumor activity
Stimulator of Interferon Genes (STING) agonists can directly activate the STING signaling pathway in NK cells and enhance the antitumor activity of NK cells by activating NK cells, upregulating the expression of NK cell activating receptors and downregulating the expression of NK cell inhibitory receptors, which provides a new idea and basis for tumor immunotherapy [113,114]. CAR T cells combined with STING agonists can stimulate immune responses to eliminate tumor cells that are not recognized by adoptively transferred lymphocytes. Therefore, these therapies may improve the efficacy of CAR-T cells in the treatment of solid tumors and help prevent the emergence of escape mutations [115]. Modulation of the TME by activating the cGAS-STING pathway changes the balance of immune stimulatory signals so that low-dose CAR-T cell therapy can induce effective tumor regression [116,117]. Therefore, STING agonists combined with CAR-T/NK cells can provide a new therapeutic strategy for tumor immunotherapy [118]. Although immune cell combination therapy is a possible therapeutic breakthrough for solid tumors at present, the clinical treatment regimen, including treatment sequence, treatment window, dose and frequency, needs to be explored in more clinical studies.
-
(6)
Combined with oncolytic virus, reshape tumor microenvironment and enhance anti-tumor immunity
Oncolytic virus (OV) provides a novel and promising treatment option for cancer patients who are resistant to traditional therapy. Natural or genetically modified OV is a versatile tumor killer. They directly cleave tumor cells while preserving normal cells, and indirectly enhance anti-tumor immunity by releasing antigens and activating inflammatory responses in the tumor microenvironment [119,120]. OV-IL15C plus EGFR-CAR NK cells synergistically suppressed tumor growth and significantly improved survival compared with either monotherapy, correlating with increased intracranial infiltration and activation of NK and CD8+ T cells and elevated persistence of CAR-NK cells in an immunocompetent model [121]. Another study confirmed that a combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus-1 for breast cancer brain metastases could kill MDA-MB-231 tumor cells more effectively and significantly prolong the survival time of tumor-bearing mice [122]. Vaccinia virus expressing CCL5 induces more NK cell aggregation in tumor foci, and oncolytic vaccinia virus expressing specific chemokine can enhance the efficacy of NK cell therapy [123]. CAdVEC (binary oncolytic adenovirus) combined with HER2-specific CAR-T cells in the treatment of advanced solid tumors (NCT03740256) and VCN-01 (oncolytic adenovirus expressing hyaluronidase) combined with mesothelin-specific CAR-T cells in the treatment of pancreatic cancer, serous ovarian cancer (NCT05057715) are in phase I clinic trial. OVV-01 (oncolytic vaccinia virus) combined with trained immunity NK cells IBR900 in the treatment of advanced solid tumors including lymphoma has been terminated due to treatment program adjustment (NCT05271279). CAR-NK cells as an immunotherapy for refractory malignant tumors, although there are many shortcomings, but it has great potential, the current clinical research will contribute to the further development and improvement of CAR-NK therapy. In addition, a nanoparticle RNA vaccine aims to transmit CAR antigen to the lymphatic compartment in the human body, thereby promoting the homologous and selective expansion of CAR cells. At the dose of subtherapeutic CAR-T cells, it can improve the implantation rate of CAR-T cells and regression of large tumors in refractory mouse models [124].
-
(7)
Combined with photothermal therapy to release antigens and promote the recruitment of endogenous immune cells
Mild hyperthermia of a tumor can reduce its dense structure and interstitial fluid pressure, increase blood perfusion, release antigens, and promote the recruitment of endogenous immune cells. Therefore, the combination of mild hyperthermia with adoptive transfer of CAR cells could potentially improve the therapeutic index of these cells in solid tumors [125]. Based on the Temperature-Feedback Nanoplatform for NIR-II Penta-Modal Imaging-Guided, photothermal therapy (PTT) combined with CAR-NK cells immunotherapy has good therapeutic effect on lung cancer. Meanwhile, synergistic CAR-NK immunotherapy is carried out specifically to eradicate any possible residual tumor cells after PTT [126,127]. The mice were treated with 125I seed implantation alone or the combination of 125I seeds with Robo1-specific CAR-NK cells. Robo1 specific CAR-NK immunotherapy enhances efficacy of 125I seed brachytherapy in an orthotopic pancreatic cancer mouse model [100]. Microwave ablation (MWA) enhances the activation, infiltration and persistence of AXL-CAR T cells in xenografts derived from AXL positive lung cancer patients through TME remodeling. The synergistic therapeutic effect of MWA and AXL-CAR T cells may be valuable for NSCLC therapy [128].
1.6. Prospects and challenges
With the in-depth study of immunotherapy, CAR-NK as a rising star will bring new development and opportunities for tumor immunotherapy. It is urgent to develop more efficient transfection methods and more efficient and safer non-viral transfection techniques in both preclinical and clinical studies, which may be an improvement direction in the future [29]. Even if the best CAR-NK cells can be made, they will always encounter the inhibitory tumor microenvironment, which may be the greatest challenge to overcome [129]. According to the pyroptosis signal pathway of tumor cells, combined therapy strategies need to be further explored, so as to enhance the immune identifiability of tumor cells, reshape the tumor microenvironment, and then improve the persistence and anti-tumor activity of CAR cells [[130], [131], [132]]. Many solid tumors are characterized by hypoxic cores that may affect NK cell function, or on the other hand by using HIF1α-driven CAR to take advantage of the hypoxic environment to improve anti-tumor activity and persistence. In addition, reducing the large number of immunosuppressive cells (Treg, MDSC, TAM) present in TME is also a strategy to improve the activity of CAR-NK cells and enhance the anti-tumor effect. With the update of CAR-T technology and the accumulation of clinical experience, the combined application of CAR-NK cells with other anti-tumor therapies will be another research direction for tumor therapy in the future.
2. Conclusions
In this review, we make a comprehensive summary of the target selection, structure construction and combined anti-tumor strategies of CAR-NK, focusing on the strategy of combined application in solid tumors. In addition, we also analyzed the current research status, existing problems and challenges of CAR-NK cells. It is hoped that these information can provide some help for better CAR-NK cell research, provide more reasonable joint strategy suggestions for overcoming the inhibitory tumor microenvironment and improving the efficacy of CAR-NK cells, and bring new hope to cancer patients and their families.
Funding information
This study was supported by Faculty Development Grants of Xiangyang No.1 People's Hospital Affiliated to Hubei University of Medicine (Grants number: XYY2023D02) and Innovative Research Program of Xiangyang No.1 People's Hospital (Grants number: XYY2023QT01).
Ethics statement
Not applicable. Approval by an ethics committee was not needed for this study because no applicants/patients were enrolled for this review article.
Data availability statement
Data associated with the study has not been deposited into a publicly available repository. Data included in article/referenced in article. The data of this manuscript will be made available on request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Junping Li: Writing – original draft, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Hong Hu: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization. Kai Lian: Writing – review & editing, Formal analysis. Dongdong Zhang: Writing – review & editing, Methodology. Pengchao Hu: Writing – review & editing, Visualization, Software. Zhibing He: Writing – review & editing, Software. Zhenfeng Zhang: Writing – review & editing, Supervision, Conceptualization. Yong Wang: Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Zhenfeng Zhang, Email: zhangzhf@gzhmu.edu.cn.
Yong Wang, Email: 563963149@qq.com.
References
- 1.Zhao J., Wu M., Li Z., et al. Chimeric antigen receptor therapy in hematological malignancies: antigenic targets and their clinical research progress[J] Ann. Hematol. 2020;99(8):1681–1699. doi: 10.1007/s00277-020-04020-7. [DOI] [PubMed] [Google Scholar]
- 2.Chow V.A., Gopal A.K., Maloney D.G., et al. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy[J] Am. J. Hematol. 2019;94(8):E209–E213. doi: 10.1002/ajh.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Li P., Fang H., et al. Paving the way towards universal chimeric antigen receptor therapy in cancer treatment: current landscape and progress[J] Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.604915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C., Oberoi P., Oelsner S., et al. Chimeric antigen receptor-engineered NK-92 cells: an off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity[J] Front. Immunol. 2017;8:533. doi: 10.3389/fimmu.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daher M., Rezvani K. Outlook for new CAR-based therapies with a focus on CAR NK cells: what lies beyond CAR-engineered T cells in the race against cancer[J] Cancer Discov. 2021;11(1):45–58. doi: 10.1158/2159-8290.CD-20-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barry K.C., Hsu J., Broz M.L., et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments[J] Nat. Med. 2018;24(8):1178–1191. doi: 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadelain M. Chimeric antigen receptors: driving immunology towards synthetic biology[J] Curr. Opin. Immunol. 2016;41:68–76. doi: 10.1016/j.coi.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q., Zhang H., Ding J., et al. Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models[J] J Immunol Res. 2018;2018 doi: 10.1155/2018/4263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maus M.V., June C.H. Zoom Zoom: racing CARs for multiple myeloma[J] Clin. Cancer Res. 2013;19(8):1917–1919. doi: 10.1158/1078-0432.CCR-13-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B., Yan L., Zhou M. Target selection of CAR T cell therapy in accordance with the TME for solid tumors[J] Am. J. Cancer Res. 2019;9(2):228–241. [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend M.H., Shrestha G., Robison R.A., et al. The expansion of targetable biomarkers for CAR T cell therapy[J] J. Exp. Clin. Cancer Res. 2018;37(1):163. doi: 10.1186/s13046-018-0817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanindratarn P., Dean D.C., Nelson S.D., et al. vol. 82. Cancer Treat Rev; 2020. (Chimeric Antigen Receptor T (CAR-T) Cell Immunotherapy for Sarcomas: from Mechanisms to Potential Clinical applications[J]). [DOI] [PubMed] [Google Scholar]
- 13.Xin Y.J., Hubbard-Lucey V.M., Tang J. Immuno-oncology drug development goes global[J] Nat. Rev. Drug Discov. 2019;18(12):899–900. doi: 10.1038/d41573-019-00167-9. [DOI] [PubMed] [Google Scholar]
- 14.Yu J.X., Upadhaya S., Tatake R., et al. Cancer cell therapies: the clinical trial landscape[J] Nat. Rev. Drug Discov. 2020;19(9):583–584. doi: 10.1038/d41573-020-00099-9. [DOI] [PubMed] [Google Scholar]
- 15.Walsh Z., Yang Y., Kohler M.E. Immunobiology of chimeric antigen receptor T cells and novel designs[J] Immunol. Rev. 2019;290(1):100–113. doi: 10.1111/imr.12794. [DOI] [PubMed] [Google Scholar]
- 16.Tokarew N., Ogonek J., Endres S., et al. Teaching an old dog new tricks: next-generation CAR T cells[J] Br. J. Cancer. 2019;120(1):26–37. doi: 10.1038/s41416-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen M.C., Riddell S.R. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells[J] Immunol. Rev. 2014;257(1):127–144. doi: 10.1111/imr.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khawar M.B., Sun H. CAR-NK cells: from natural basis to design for kill[J] Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.707542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savoldo B., Ramos C.A., Liu E., et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients[J] J. Clin. Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.June C.H., O'Connor R.S., Kawalekar O.U., et al. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 21.Zhong X.S., Matsushita M., Plotkin J., et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication[J] Mol. Ther. 2010;18(2):413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L., Dou M., Ma Q., et al. Chimeric antigen receptor (CAR)-modified NK cells against cancer: opportunities and challenges[J] Int. Immunopharm. 2019;74 doi: 10.1016/j.intimp.2019.105695. [DOI] [PubMed] [Google Scholar]
- 23.Pegram H.J., Lee J.C., Hayman E.G., et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning[J] Blood. 2012;119(18):4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chmielewski M., Hombach A.A., Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma[J] Immunol. Rev. 2014;257(1):83–90. doi: 10.1111/imr.12125. [DOI] [PubMed] [Google Scholar]
- 25.Liu E., Marin D., Banerjee P., et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors[J] N. Engl. J. Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michels A., Hartmann J., Buchholz C.J. [Chimeric antigen receptors in oncology: clinical applications and new developments][J] Bundesgesundheitsblatt - Gesundheitsforsch. - Gesundheitsschutz. 2020;63(11):1331–1340. doi: 10.1007/s00103-020-03222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y., Wang A.Y. Novel immune check-point regulators in tolerance maintenance[J] Front. Immunol. 2015;6:421. doi: 10.3389/fimmu.2015.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J.R., Digiusto D.L., Slovak M., et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma[J] Mol. Ther. 2007;15(4):825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 29.Gong Y., Klein W.R., Wang J., et al. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy[J] J. Hematol. Oncol. 2021;14(1):73. doi: 10.1186/s13045-021-01083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basar R., Daher M., Rezvani K. Next-generation cell therapies: the emerging role of CAR-NK cells[J] Blood Adv. 2020;4(22):5868–5876. doi: 10.1182/bloodadvances.2020002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W., Jiang J., Wu C. CAR-NK for tumor immunotherapy: clinical transformation and future prospects[J] Cancer Lett. 2020;472:175–180. doi: 10.1016/j.canlet.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 32.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy[J] Nat. Rev. Drug Discov. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 33.Xie G., Dong H., Liang Y., et al. CAR-NK cells: a promising cellular immunotherapy for cancer[J] EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee M.Y., Robbins Y., Sievers C., et al. Chimeric antigen receptor engineered NK cellular immunotherapy overcomes the selection of T-cell escape variant cancer cells[J] J Immunother Cancer. 2021;9(3) doi: 10.1136/jitc-2020-002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachiller M., Perez-Amill L., Battram A.M., et al. NK cells enhance CAR-T cell antitumor efficacy by enhancing immune/tumor cells cluster formation and improving CAR-T cell fitness[J] J Immunother Cancer. 2021;9(8) doi: 10.1136/jitc-2021-002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu E., Tong Y., Dotti G., et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity[J] Leukemia. 2018;32(2):520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iyer R.K., Bowles P.A., Kim H., et al. Industrializing autologous adoptive immunotherapies: manufacturing advances and challenges[J] Front. Med. 2018;5:150. doi: 10.3389/fmed.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esfahani K., Elkrief A., Calabrese C., et al. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 2020;17(8):504–515. doi: 10.1038/s41571-020-0352-8. [DOI] [PubMed] [Google Scholar]
- 39.Xu J., Tian K., Zhang H., et al. Chimeric antigen receptor-T cell therapy for solid tumors require new clinical regimens[J] Expert Rev. Anticancer Ther. 2017;17(12):1099–1106. doi: 10.1080/14737140.2017.1395285. [DOI] [PubMed] [Google Scholar]
- 40.Karmakar S., Pal P., Lal G. Key activating and inhibitory ligands involved in the mobilization of natural killer cells for cancer immunotherapies[J] ImmunoTargets Ther. 2021;10:387–407. doi: 10.2147/ITT.S306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantovani S., Oliviero B., Varchetta S., et al. Natural killer cell responses in hepatocellular carcinoma: implications for novel immunotherapeutic approaches[J] Cancers. 2020;12(4) doi: 10.3390/cancers12040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun C., Sun H., Zhang C., et al. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma[J] Cell. Mol. Immunol. 2015;12(3):292–302. doi: 10.1038/cmi.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krijgsman D., Roelands J., Andersen M.N., et al. Expression of NK cell receptor ligands in primary colorectal cancer tissue in relation to the phenotype of circulating NK- and NKT cells, and clinical outcome[J] Mol. Immunol. 2020;128:205–218. doi: 10.1016/j.molimm.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade[J] Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauken K.E., Wherry E.J. Overcoming T cell exhaustion in infection and cancer[J] Trends Immunol. 2015;36(4):265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi B.D., Yu X., Castano A.P., et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma[J] J Immunother Cancer. 2019;7(1):304. doi: 10.1186/s40425-019-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo X., Jiang H., Shi B., et al. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma[J] Front. Pharmacol. 2018;9:1118. doi: 10.3389/fphar.2018.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu G., Zhang Q., Li D., et al. PD-1 silencing improves anti-tumor activities of human mesothelin-targeted CAR T cells[J] Hum. Immunol. 2021;82(2):130–138. doi: 10.1016/j.humimm.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Liu X., Ranganathan R., Jiang S., et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors[J] Cancer Res. 2016;76(6):1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen N., Morello A., Tano Z., et al. CAR T-cell intrinsic PD-1 checkpoint blockade: a two-in-one approach for solid tumor immunotherapy[J] OncoImmunology. 2017;6(2) doi: 10.1080/2162402X.2016.1273302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song W., Zhang M. Use of CAR-T cell therapy, PD-1 blockade, and their combination for the treatment of hematological malignancies[J] Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108382. [DOI] [PubMed] [Google Scholar]
- 52.Kato D., Yaguchi T., Iwata T., et al. GPC1 specific CAR-T cells eradicate established solid tumor without adverse effects and synergize with anti-PD-1 Ab[J] Elife. 2020;9 doi: 10.7554/eLife.49392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J., Deng Q., Jiang Y.Y., et al. CAR-T 19 combined with reduced-dose PD-1 blockade therapy for treatment of refractory follicular lymphoma: a case report[J] Oncol. Lett. 2019;18(5):4415–4420. doi: 10.3892/ol.2019.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou J.T., Liu J.H., Song T.T., et al. EGLIF-CAR-T cells secreting PD-1 blocking antibodies significantly mediate the elimination of gastric cancer[J] Cancer Manag. Res. 2020;12:8893–8902. doi: 10.2147/CMAR.S260915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rafiq S., Yeku O.O., Jackson H.J., et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo[J] Nat. Biotechnol. 2018;36(9):847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X., Yang S., Li S., et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-beta trap enhances antitumor efficacy of CAR-T cell therapy[J] Mol Ther Oncolytics. 2021;21:144–157. doi: 10.1016/j.omto.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pesce S., Greppi M., Grossi F., et al. PD/1-PD-Ls checkpoint: insight on the potential role of NK cells[J] Front. Immunol. 2019;10:1242. doi: 10.3389/fimmu.2019.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benson D.J., Bakan C.E., Mishra A., et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody[J] Blood. 2010;116(13):2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sivori S., Pende D., Quatrini L., et al. NK cells and ILCs in tumor immunotherapy[J] Mol. Aspect. Med. 2021;80 doi: 10.1016/j.mam.2020.100870. [DOI] [PubMed] [Google Scholar]
- 60.Abdolahi S., Ghazvinian Z., Muhammadnejad S., et al. Adaptive NK cell therapy modulated by anti-PD-1 antibody in gastric cancer model[J] Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.733075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vacca P., Pietra G., Tumino N., et al. Exploiting human NK cells in tumor therapy[J] Front. Immunol. 2019;10:3013. doi: 10.3389/fimmu.2019.03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.NK cells respond to checkpoint blockade[J] Cancer Discov. 2018;8(12):1498. doi: 10.1158/2159-8290.CD-NB2018-131. [DOI] [PubMed] [Google Scholar]
- 63.Morvan M.G., Lanier L.L. NK cells and cancer: you can teach innate cells new tricks[J] Nat. Rev. Cancer. 2016;16(1):7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 64.Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age[J] Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zou W., Wolchok J.D., Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations[J] Sci. Transl. Med. 2016;8(328):324r–328r. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pesce S., Greppi M., Tabellini G., et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization[J] J. Allergy Clin. Immunol. 2017;139(1):335–346. doi: 10.1016/j.jaci.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 67.Romero D. Immunotherapy: PD-1 says goodbye, TIM-3 says hello[J] Nat. Rev. Clin. Oncol. 2016;13(4):202–203. doi: 10.1038/nrclinonc.2016.40. [DOI] [PubMed] [Google Scholar]
- 68.Della C.M., Pesce S., Muccio L., et al. Features of memory-like and PD-1(+) human NK cell subsets[J] Front. Immunol. 2016;7:351. doi: 10.3389/fimmu.2016.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu J., Hodgins J.J., Marathe M., et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade[J] J. Clin. Invest. 2018;128(10):4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vivier E., Raulet D.H., Moretta A., et al. Innate or adaptive immunity? The example of natural killer cells[J] Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beldi-Ferchiou A., Lambert M., Dogniaux S., et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma[J] Oncotarget. 2016;7(45):72961–72977. doi: 10.18632/oncotarget.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oelsner S., Friede M.E., Zhang C., et al. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma[J] Cytotherapy. 2017;19(2):235–249. doi: 10.1016/j.jcyt.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 73.Fabian K.P., Padget M.R., Donahue R.N., et al. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations[J] J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang L., Shen M., Xu L.J., et al. Enhancing NK cell-mediated cytotoxicity to cisplatin-resistant lung cancer cells via MEK/Erk signaling inhibition[J] Sci. Rep. 2017;7(1):7958. doi: 10.1038/s41598-017-08483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y., Cheng Y., Xu Y., et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers[J] Oncogene. 2017;36(44):6143–6153. doi: 10.1038/onc.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mensali N., Dillard P., Fayzullin A., et al. "Built-in" PD-1 blocker to rescue NK-92 activity from PD-L1-mediated tumor escape mechanisms[J] Faseb. J. 2021;35(9) doi: 10.1096/fj.202100025R. [DOI] [PubMed] [Google Scholar]
- 77.Su S., Zhao J., Xing Y., et al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages[J] Cell. 2018;175(2):442–457. doi: 10.1016/j.cell.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 78.Bellucci R., Martin A., Bommarito D., et al. Interferon-gamma-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression[J] OncoImmunology. 2015;4(6) doi: 10.1080/2162402X.2015.1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oyer J.L., Gitto S.B., Altomare D.A., et al. PD-L1 blockade enhances anti-tumor efficacy of NK cells[J] OncoImmunology. 2018;7(11) doi: 10.1080/2162402X.2018.1509819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J., Larrocha P.S., Zhang B., et al. Antibody targeting tumor-derived soluble NKG2D ligand sMIC provides dual co-stimulation of CD8 T cells and enables sMIC(+) tumors respond to PD1/PD-L1 blockade therapy[J] J Immunother Cancer. 2019;7(1):223. doi: 10.1186/s40425-019-0693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mastrogiovanni M., Vargas P., Rose T., et al. The tumor suppressor adenomatous polyposis coli regulates T lymphocyte migration[J] Sci. Adv. 2022;8(15):l5942. doi: 10.1126/sciadv.abl5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong W., Wu X., Ma S., et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector[J] Cancer Discov. 2019;9(10):1422–1437. doi: 10.1158/2159-8290.CD-18-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iraolagoitia X.L., Spallanzani R.G., Torres N.I., et al. NK cells restrain spontaneous antitumor CD8+ T cell priming through PD-1/PD-L1 interactions with dendritic cells[J] J. Immunol. 2016;197(3):953–961. doi: 10.4049/jimmunol.1502291. [DOI] [PubMed] [Google Scholar]
- 84.Quatrini L., Vacca P., Tumino N., et al. Glucocorticoids and the cytokines IL-12, IL-15, and IL-18 present in the tumor microenvironment induce PD-1 expression on human natural killer cells[J] J. Allergy Clin. Immunol. 2021;147(1):349–360. doi: 10.1016/j.jaci.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 85.Jung K.H., Lee J.H., Kim M., et al. 89)Zr immuno-PET imaging of tumor PD-1 reveals that PMA upregulates lymphoma PD-1 through NFkappaB and JNK signaling[J] Mol. Imag. 2022 doi: 10.1155/2022/5916692. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen C., Liu C., Zhang Z., et al. PD-1 affects the immunosuppressive function of group 2 innate lymphoid cells in human non-small cell lung cancer[J] Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.680055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bao S., Jiang X., Jin S., et al. TGF-beta1 induces immune escape by enhancing PD-1 and CTLA-4 expression on T lymphocytes in hepatocellular carcinoma[J] Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.694145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Concha-Benavente F., Kansy B., Moskovitz J., et al. PD-L1 mediates dysfunction in activated PD-1(+) NK cells in head and neck cancer patients[J] Cancer Immunol. Res. 2018;6(12):1548–1560. doi: 10.1158/2326-6066.CIR-18-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurebayashi Y., Olkowski C.P., Lane K.C., et al. Rapid depletion of intratumoral regulatory T cells induces synchronized CD8 T- and NK-cell activation and IFNgamma-dependent tumor vessel regression[J] Cancer Res. 2021;81(11):3092–3104. doi: 10.1158/0008-5472.CAN-20-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai X., Bu X., Gao Y., et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade[J] Mol. Cell. 2021;81(11):2317–2331. doi: 10.1016/j.molcel.2021.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu H., Zhou S., Liu J., et al. Lirilumab and avelumab enhance anti-HPV+ cervical cancer activity of natural killer cells via vav1-dependent NF-kappaB disinhibition[J] Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.747482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li F., Chen Y., Pang M., et al. Immune checkpoint inhibitors and cellular treatment for lymphoma immunotherapy[J] Clin. Exp. Immunol. 2021;205(1):1–11. doi: 10.1111/cei.13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brauneck F., Seubert E., Wellbrock J., et al. Combined blockade of TIGIT and CD39 or A2AR enhances NK-92 cell-mediated cytotoxicity in AML[J] Int. J. Mol. Sci. 2021;22(23) doi: 10.3390/ijms222312919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen X., Xue L., Ding X., et al. An fc-competent anti-human TIGIT blocking antibody ociperlimab (BGB-A1217) elicits strong immune responses and potent anti-tumor efficacy in pre-clinical models. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.828319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Q., Bi J., Zheng X., et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity[J] Nat. Immunol. 2018;19(7):723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 96.Tao K., He M., Tao F., et al. Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment[J] Cancer Chemother. Pharmacol. 2018;82(5):815–827. doi: 10.1007/s00280-018-3670-0. [DOI] [PubMed] [Google Scholar]
- 97.Klapdor R., Wang S., Hacker U., et al. Improved killing of ovarian cancer stem cells by combining a novel chimeric antigen receptor-based immunotherapy and chemotherapy[J] Hum. Gene Ther. 2017;28(10):886–896. doi: 10.1089/hum.2017.168. [DOI] [PubMed] [Google Scholar]
- 98.Klapdor R., Wang S., Morgan M.A., et al. NK cell-mediated eradication of ovarian cancer cells with a novel chimeric antigen receptor directed against CD44[J] Biomedicines. 2021;9(10) doi: 10.3390/biomedicines9101339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weiss T., Weller M., Guckenberger M., et al. NKG2D-Based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma[J] Cancer Res. 2018;78(4):1031–1043. doi: 10.1158/0008-5472.CAN-17-1788. [DOI] [PubMed] [Google Scholar]
- 100.Xia N., Haopeng P., Gong J.U., et al. Robo1-specific CAR-NK immunotherapy enhances efficacy of (125)I seed brachytherapy in an orthotopic mouse model of human pancreatic carcinoma[J] Anticancer Res. 2019;39(11):5919–5925. doi: 10.21873/anticanres.13796. [DOI] [PubMed] [Google Scholar]
- 101.Luo H., Zhou Y., Zhang J., et al. NK cell-derived exosomes enhance the anti-tumor effects against ovarian cancer by delivering cisplatin and reactivating NK cell functions[J] Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1087689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng W., Zhu T., Tang L., et al. Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer[J] J. Transl. Med. 2023;21(1):383. doi: 10.1186/s12967-023-04206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siegler E.L., Kim Y.J., Chen X., et al. Combination cancer therapy using chimeric antigen receptor-engineered natural killer cells as drug carriers[J] Mol. Ther. 2017;25(12):2607–2619. doi: 10.1016/j.ymthe.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Busse A., Asemissen A.M., Nonnenmacher A., et al. Immunomodulatory effects of sorafenib on peripheral immune effector cells in metastatic renal cell carcinoma[J] Eur. J. Cancer. 2011;47(5):690–696. doi: 10.1016/j.ejca.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 105.Yang J., Eresen A., Scotti A., et al. Combination of NK-based immunotherapy and sorafenib against hepatocellular carcinoma[J] Am. J. Cancer Res. 2021;11(2):337–349. [PMC free article] [PubMed] [Google Scholar]
- 106.Wu X., Luo H., Shi B., et al. Combined antitumor effects of sorafenib and GPC3-CAR T cells in mouse models of hepatocellular carcinoma[J] Mol. Ther. 2019;27(8):1483–1494. doi: 10.1016/j.ymthe.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benson D.J., Cohen A.D., Jagannath S., et al. A phase I trial of the anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma[J] Clin. Cancer Res. 2015;21(18):4055–4061. doi: 10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao G., Wei R., Feng L., et al. Lenalidomide enhances the efficacy of anti-BCMA CAR-T treatment in relapsed/refractory multiple myeloma: a case report and revies of the literature[J] Cancer Immunol. Immunother. 2022;71(1):39–44. doi: 10.1007/s00262-021-02959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang L., Jin G., Chen Z., et al. Lenalidomide improves the antitumor activity of CAR-T cells directed toward the intracellular Wilms Tumor 1 antigen[J] Hematology. 2021;26(1):818–826. doi: 10.1080/16078454.2021.1981534. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Q., Xu J., Ding J., et al. Bortezomib improves adoptive carbonic anhydrase IXspecific chimeric antigen receptormodified NK92 cell therapy in mouse models of human renal cell carcinoma[J] Oncol. Rep. 2018;40(6):3714–3724. doi: 10.3892/or.2018.6731. [DOI] [PubMed] [Google Scholar]
- 111.Luna J.I., Grossenbacher S.K., Sturgill I.R., et al. Bortezomib augments natural killer cell targeting of stem-like tumor cells[J] Cancers. 2019;11(1) doi: 10.3390/cancers11010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guillerey C., Huntington N.D., Smyth M.J. Targeting natural killer cells in cancer immunotherapy[J] Nat. Immunol. 2016;17(9):1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 113.Yan X., Yao C., Fang C., et al. Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer[J] Int. J. Biol. Sci. 2022;18(2):585–598. doi: 10.7150/ijbs.65019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xagoraris I., Farrajota N.D.S.P., Kokaraki G., et al. Sting is commonly and differentially expressed in T- and nk-cell but not B-cell non-hodgkin lymphomas[J] Cancers. 2022;14(5) doi: 10.3390/cancers14051186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith T.T., Moffett H.F., Stephan S.B., et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors[J] J. Clin. Invest. 2017;127(6):2176–2191. doi: 10.1172/JCI87624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ji F., Zhang F., Zhang M., et al. Targeting the DNA damage response enhances CD70 CAR-T cell therapy for renal carcinoma by activating the cGAS-STING pathway[J] J. Hematol. Oncol. 2021;14(1):152. doi: 10.1186/s13045-021-01168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu N., Palmer D.C., Robeson A.C., et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer[J] J. Exp. Med. 2021;218(2) doi: 10.1084/jem.20200844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Da Y., Liu Y., Hu Y., et al. STING agonist cGAMP enhances anti-tumor activity of CAR-NK cells against pancreatic cancer[J] OncoImmunology. 2022;11(1) doi: 10.1080/2162402X.2022.2054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma R., Li Z., Chiocca E.A., et al. The emerging field of oncolytic virus-based cancer immunotherapy[J] Trends Cancer. 2023;9(2):122–139. doi: 10.1016/j.trecan.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mamola J.A., Chen C.Y., Currier M.A., et al. Opportunities and challenges of combining adoptive cellular therapy with oncolytic virotherapy[J] Mol Ther Oncolytics. 2023;29:118–124. doi: 10.1016/j.omto.2023.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma R., Lu T., Li Z., et al. An oncolytic virus expressing IL15/IL15Ralpha combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma[J] Cancer Res. 2021;81(13):3635–3648. doi: 10.1158/0008-5472.CAN-21-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen X., Han J., Chu J., et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases[J] Oncotarget. 2016;7(19):27764–27777. doi: 10.18632/oncotarget.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li F., Sheng Y., Hou W., et al. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency[J] J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reinhard K., Rengstl B., Oehm P., et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors[J] Science. 2020;367(6476):446–453. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 125.Chen Q., Hu Q., Dukhovlinova E., et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells[J] Adv. Mater. 2019;31(23) doi: 10.1002/adma.201900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu M., Xue B., Wang Y., et al. Temperature-feedback Nanoplatform for NIR-II penta-modal imaging-guided synergistic photothermal therapy and CAR-NK immunotherapy of lung cancer[J] Small. 2021;17(43) doi: 10.1002/smll.202101397. [DOI] [PubMed] [Google Scholar]
- 127.Huang X., Lu Y., Guo M., et al. Recent strategies for nano-based PTT combined with immunotherapy: from a biomaterial point of view[J] Theranostics. 2021;11(15):7546–7569. doi: 10.7150/thno.56482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cao B., Liu M., Wang L., et al. Remodelling of tumour microenvironment by microwave ablation potentiates immunotherapy of AXL-specific CAR T cells against non-small cell lung cancer[J] Nat. Commun. 2022;13(1):6203. doi: 10.1038/s41467-022-33968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodriguez-Garcia A., Palazon A., Noguera-Ortega E., et al. CAR-T cells hit the tumor microenvironment: strategies to overcome tumor escape[J] Front. Immunol. 2020;11:1109. doi: 10.3389/fimmu.2020.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu C., Guo C., Chen H., et al. A novel chimeric PD1-NKG2D-41BB receptor enhances antitumor activity of NK92 cells against human lung cancer H1299 cells by triggering pyroptosis[J] Mol. Immunol. 2020;122:200–206. doi: 10.1016/j.molimm.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 131.Yang X., Bian J., Wang Z., et al. A bio-liposome activating natural killer cell by illuminating tumor homogenization antigen properties[J] Adv. Sci. 2023;10(12) doi: 10.1002/advs.202205449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fang Y., Wang Y.J., Zhao H.L., et al. Development of FAP-targeted chimeric antigen receptor NK-92 cells for non-small cell lung cancer[J] Discov. Med. 2023;35(176):405–417. doi: 10.24976/Discov.Med.202335176.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with the study has not been deposited into a publicly available repository. Data included in article/referenced in article. The data of this manuscript will be made available on request.