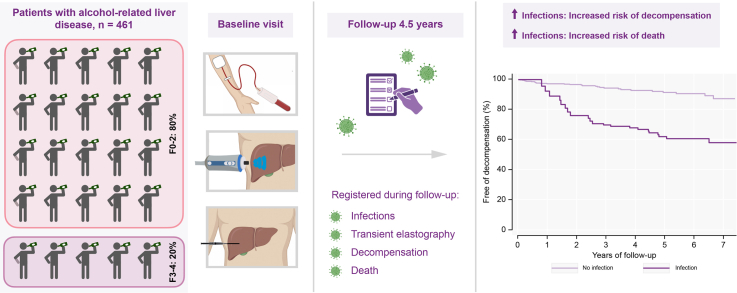
Abstract
Background & Aims
Infections are frequent in patients with cirrhosis and worsen prognosis. We evaluated the incidence of infections and their impact on decompensation and death in patients with early alcohol-related liver disease (ALD) during long-term follow-up.
Methods
We performed a prospective cohort study of patients in secondary care with a history of excess alcohol intake, no prior decompensation, and with liver biopsies along with clinical investigations conducted at baseline. During follow-up, we reviewed the patients’ electronic healthcare records for cases of infections, hospitalizations, transient elastography measurements, decompensations, all-cause mortality, and alcohol intake.
Results
We included 461 patients with a mean age of 56±10 years (76% males; fibrosis stage F0-1/F2/F3-4 = 259/107/93 [56%/23%/20%]). During a median follow-up of 4.5 years (IQR 2.9-6.3), 134 patients (29%) developed a total of 312 infections, most frequently pneumonia (106/312, 34%) and urinary tract infections (57/312, 18%). Excessive alcohol intake during follow-up, smoking ≥30 pack years, MELD score and elevated liver stiffness during follow-up were independent predictors of infections. Patients who developed at least one infection had a significantly increased risk of subsequent decompensation (hazard ratio 4.98, 95% CI 2.47-10.03) and death (hazard ratio 8.24, 95% CI 4.65-14.59). Infections increased the risk of decompensation and death independently of baseline fibrosis stage, age, gender, and MELD score.
Conclusions
Almost one-third of patients with early ALD develop an infection, which worsens their prognosis by increasing the risk of decompensation and death. The risk of infections increases with liver disease severity and ongoing harmful use of alcohol.
Impact and implications
This study reveals that infections significantly worsen the prognosis of patients with early alcohol-related liver disease (ALD), increasing the likelihood of decompensation and death by up to eight times. These findings, pertinent to healthcare providers, researchers, and policymakers, emphasize the importance of early prevention and management of infections in patients with ALD, even those in early stages who may be asymptomatic. It was observed that nearly one-third of patients with early-stage ALD developed infections over 4.5 years, with risk factors including alcohol overuse, smoking, and higher MELD scores. The research underscores the critical need to incorporate these insights into clinical practice and public health policies to improve patient outcomes and mitigate the impact of infections in patients with ALD.
Keywords: Alcoholic liver disease, Decompensation, Fibrosis, Cirrhosis, Prognosis
Graphical abstract
Highlights
-
•
Almost one-third of patients with early-stage alcohol-related liver disease developed infections during 4.5 years of follow-up.
-
•
Alcohol overuse, smoking, MELD score and a high transient elastography measurement independently predicted subsequent infections.
-
•
In patients with early-stage alcohol-related liver disease, infections increase the risk of death by up to 8-fold.
Introduction
The incidence of alcohol use disorder is on the rise globally, with an estimated prevalence of 100 million in 2016. This translates to 99 million disability-adjusted life years lost.1 Excessive alcohol intake predisposes to cirrhosis, the end stage of alcohol-related liver disease (ALD), which accounts for an estimated 370,000 deaths per year globally.2 Infections are frequent in both compensated and decompensated cirrhosis 3,4 and incidence of infections increases with severity of liver disease.5 Similarly, infections worsen prognosis in patients with cirrhosis by increasing the risk of acute decompensation three times and mortality 4-7 times.4,6 Both alcohol consumption and cirrhosis induce susceptibility to infections.[7], [8], [9] Alcohol damages mucosal surfaces throughout the body, thereby impairing barrier function against pathogens.7 Moreover, both cell-mediated and humoral responses of the innate and adaptive immune system are affected by alcohol, promoting a hyperinflammatory state while reducing efficacy against pathogens.7 In cirrhosis, impaired immunity is a result of local damage to the reticuloendothelial system and Kupffer cells, impaired production of circulating immune factors, and compromised function of innate and adaptive immune cells.9 Furthermore, both alcohol and cirrhosis are associated with gut dysbiosis and a leaky gut barrier, resulting in translocation of bacterial products and pathogens.8,9
However, the role of infections in the early stages of ALD, before cirrhosis and decompensation, has not been investigated. We hypothesized that infections occur frequently across the spectrum of ALD, including in early-stage disease, and that the risk increases with disease severity. Moreover, we hypothesized that infections worsen prognosis. Therefore, we aimed to investigate the incidence of infections in a cohort of patients with biopsy-verified alcohol-related fibrosis, covering the full spectrum of ALD before decompensation and with a long-term follow-up outcome assessment. Furthermore, we aimed to investigate the risk factors for developing infections and determine whether infections predict subsequent decompensation and death.
Patients and methods
Study design
We performed a single-center, prospective cohort study. The Danish Data Protection Agency (13/8204) and the Ethics Committee for the Region of Southern Denmark (S-20120071, S-20160021) approved the study. All participants signed an informed consent form and the study adhered to the Declaration of Helsinki. We report results according to the STROBE checklist.10
Patients
The study cohort has previously been described.11 We recruited and consecutively included patients in the Region of Southern Denmark from municipal alcohol rehabilitation centers, patients referred to three outpatient liver clinics and patients who responded to online advertisements. Inclusion criteria were age 18-75 years and a history of alcohol overuse defined as >24 g/day for women and >36 g/day for men for a minimum of 1 year. Exclusion criteria were previous or current decompensation, concurrent liver disease other than ALD, debilitating disease (including cancer) with an expected survival of <1 year, severe alcohol-related hepatitis, ultrasonic evidence of hepatic congestion or bile duct dilatation and contraindication to a liver biopsy.
Investigations
All patients underwent clinical investigations by trained personnel. We performed standard anthropometric measurements, blood sampling, and liver stiffness measurements by transient elastography (TE) using the FibroScan 502 Touch (Echosens, France). Initially, we obtained a percutaneous liver biopsy from all patients independently of TE measurements, but in 2016, we found that no patients with TE<6 kPa had advanced fibrosis.12 For ethical reasons, we therefore abstained from biopsies in cases of TE<6 kPa, and all patients with TE<6 were labelled as fibrosis stage F0-1.
An experienced liver pathologist (S.D.) assessed all biopsies and scored them according to Kleiner fibrosis stage.13 Lobular inflammation, ballooning, and steatosis were graded in accordance with the NASH Clinical Research Network activity score.13,14 We used the sum of ballooning and lobular inflammation for a combined inflammatory activity score (0-5) referred to in this manuscript as histological inflammation. A biopsy was considered representative if it was at least 10 mm in length and contained at least six portal tracts or showed regeneration nodules.
Follow-up data
We reviewed all participants’ electronic healthcare files for inpatient and outpatient follow-up data on infections, hospitalizations, alcohol intake, TE measurements, decompensations, and death. Since Danish electronic patient files are nationwide, we evaluated all contacts to any hospital in Denmark during the follow-up period. Follow-up started at inclusion and ended on October 1st, 2020, if the patient died, or was lost to follow-up.
We defined decompensation of cirrhosis in accordance with the Baveno VII recommendations as overt ascites, overt hepatic encephalopathy, or variceal bleeding.15 We defined further decompensation as a new development of the first type of decompensation, or jaundice, recurrent variceal bleeding, recurrent ascites (≥3 large-volume paracenteses within a year), recurrent overt encephalopathy, spontaneous bacterial peritonitis (SBP), or hepatorenal syndrome.15
Progression of TE during follow-up was defined as a 20% increase from the TE at baseline to the TE at follow-up. If TE was measured at multiple points in time for the same patients, the last measurement was used.
Infections
There is no generally accepted definition of infection based on electronic health care records. Instead, we followed best practice, aiming to balance specificity and sensitivity in the definition of infections.16 First, we selected all diagnoses of infections documented by a clinician in the electronic healthcare files for evaluation. Next, we assessed the following: 1) biochemical signs of infection including elevated C-reactive protein, leukocytosis, and neutrophilia; 2) relevant clinical symptoms including fever and organ-specific symptoms; 3) radiological signs of infection; 4) prescription of antibiotic/antiviral/antifungal medication; and 5) positive cultures. Finally, only infections that met at least three of the above criteria are reported here.
Sepsis was defined based on the attending clinician’s diagnosis. Most clinicians use the Sepsis-3 criteria,17 however, since blood pressure, respiratory rate and Glasgow Coma Scale were not consistently documented in the electronic health records, we could not verify the Sepsis-3 criteria.
Infections were characterized as severe infections when requiring in-hospital treatment, if treated with intravenous antibiotics, and/or if supportive treatment was necessary because of the infection. Hence, not all infections treated in the hospital were characterized as requiring in-hospital treatment. We registered pathogens and antibiotic resistance when a positive culture from blood, stool, urine, airway secretions, or ascites was available. For each positive culture, an expert microbiologist retrospectively reviewed the laboratory report, including antibiotic susceptibility, to exclude non-significant results.
Statistical analysis
We report normally distributed continuous data as mean (standard deviation) and non-normally distributed continuous data as median (IQR). The median follow-up time was calculated as the median number of months from inclusion until the last day of follow-up (October 1st, 2020, if the patient was lost to follow-up or died) for all patients.
We chose potential predictors of infections based on subject-matter knowledge together with availability in clinical care and applied univariable competing risk regression to assess factors associated with time to first infection. All variables were then included in a multivariable competing risk regression analysis to identify independent predictors of infections. Prioritizing clinical relevance and to allow for comparison of subhazard ratios (sHRs), continuous variables were stratified using prespecified cut-offs or by using the upper limit of normal for blood tests. For years of smoking, we chose a cut-off of 30 pack years as this has previously been associated with worse infection outcomes.18 For the model for end-stage liver disease (MELD) score, we chose a cut-off of 9 equivalent to the lower cut-off for predicting mortality.19 Analyses were performed in the entire cohort and in subgroups dividing the cohort according to Kleiner fibrosis stage (F0-2 vs. F3-4). In case of missing data, we used complete case analysis. Possible multicollinearity in the multivariable models was assessed by calculating the variance inflation factor. For variance inflation factors below 5 we did not suspect significant collinearity between variables in the models. We plotted the cumulative incidence curves for developing infections, calculated sHRs using competing risk regression20 (with death without an infection as the competing event) and tested for significant differences between groups using the Pepe-Mori test.21
We analyzed the impact of infections on the risk of later decompensation and death using Cox regression models where developing an infection was considered a time-dependent covariate. All patients were included in the model as non-infected patients and could be reclassified when developing an infection. In the analyses evaluating the impact of infections on later decompensation, we only included infections that occurred prior to decompensation. Between-group comparisons for survival data with a time-dependent covariate were performed using the Mantel-Byar test.22 To avoid immortal time bias, we illustrated the survival analyses with a time-dependent covariate (infection status) using a Simon & Makuch plot.23
Patients were censored from the survival analyses if they were lost to follow-up or if no event had occurred at the end of follow-up.
In sensitivity analyses, we stratified patients into three groups based on their baseline liver stiffness measurements (using cut-offs of 10 and 15 kPa), by their fibrosis stage, as well as by their alcohol intake during follow-up. Statistical analyses were conducted using STATA 17 (Statacorp TX, US). A p value <0.05 was considered statistically significant.
Results
Patient and infection characteristics
We included 461 patients with alcohol-related liver disease between April 2013 and September 2018, of whom 363 underwent liver biopsy (Table 1). The vast majority of patients (80%) had fibrosis stage F2 or below at inclusion and none were previously or currently decompensated. Patients reported a median of 16 years (IQR 8-26) of excess drinking, and 42% were abstinent at inclusion. During a median follow-up of 4.5 years (IQR 2.9-6.3), 134 patients (29%) had at least one infection, 56 (12%) progressed to decompensated cirrhosis, 35 (8%) of the decompensated patients developed at least one further decompensation, and 75 (16%) died. Nine were lost to follow-up due to moving abroad or to regions not included in the ethical permissions.
Table 1.
Patient characteristics
| All patients N = 461 | |
|---|---|
| Age, years | 56 ±10 |
| Male | 351 (76%) |
| BMI (kg/m2) | 27 ±5 |
| Comorbidities | |
| Type 2 diabetes | 47 (10%) |
| Metabolic syndrome | 114 (25%) |
| HOMA-IR ≥2.5 | 262 (57%) |
| Chronic obstructive pulmonary disease | 14 (3%) |
| Alcohol history | |
| Abstinent at inclusion | 192 (42%) |
| Duration of excess drinking (years) | 16 (8-26) |
| Drinks in the week leading up to inclusion, for ongoing drinkers (units) | 21 (7-35) |
| Smoking history | |
| Smoking/ex-smoker/non-smoker | 55%/26%/18% |
| Years of smoking | 30 (10-40) |
| Histology | |
| Fibrosis stage∗ | 36/126/107/27/66 |
| 0/1/2/3/4 | 8%/27%/23%/6%/14% |
| Steatosis score 0/1/2/3∗∗ | 157/85/73/39 |
| Ballooning 0/1/2∗∗ | 181/108/65 |
| Lobular inflammation 0/1/2/3∗∗ | 81/162/84/27 |
| Liver stiffness | |
| Transient elastography (kPa)∗∗∗ | 6.5 (4.8-11.7) |
| ≤10 kPa | 311 (67%) |
| >10 - ≤15 kPa | 38 (8%) |
| >15 - ≤25 kPa | 33 (7%) |
| >25 kPa | 66 (13%) |
| Biochemistry | |
| ALT (U/L) | 31 (22-48) |
| AST (U/L) | 34 (25-51) |
| GGT (U/L) | 72 (34-190) |
| Bilirubin (μmol/L) | 10 (7-14) |
| Platelet count (109/L) | 238 ±90 |
| Albumin (g/L) | 42 (40-45) |
| Creatinine (μmol/L) | 74 ±16 |
| Lymphocytes (109/L) | 7.1 ±2.3 |
| C-reactive protein (mg/L) | 2.6 (1-6) |
| Follow-up | |
| Excessive alcohol intake during follow-up∗∗∗∗ | 265 (57%) |
| Decompensation | 56 (12%) |
| Further decompensation (at least one) | 35 (8%) |
| Death | 75 (16%) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; HOMA-IR, homeostatic model assessment for insulin resistance.
Mean (SD) or median (IQR) or n (%).
Fibrosis stage missing in 99 patients: we refrained from a biopsy in patients with TE <6 kPa (n = 97) from 2016; one had an inconclusive biopsy, and biopsy was not technically possible in one.
Steatosis score, lobular inflammation and ballooning is missing in 107 patients (98 missing, 9 inconclusive).
Transient elastography was unavailable in 13 patients.
Data on alcohol intake during follow-up was available in 403 patients.
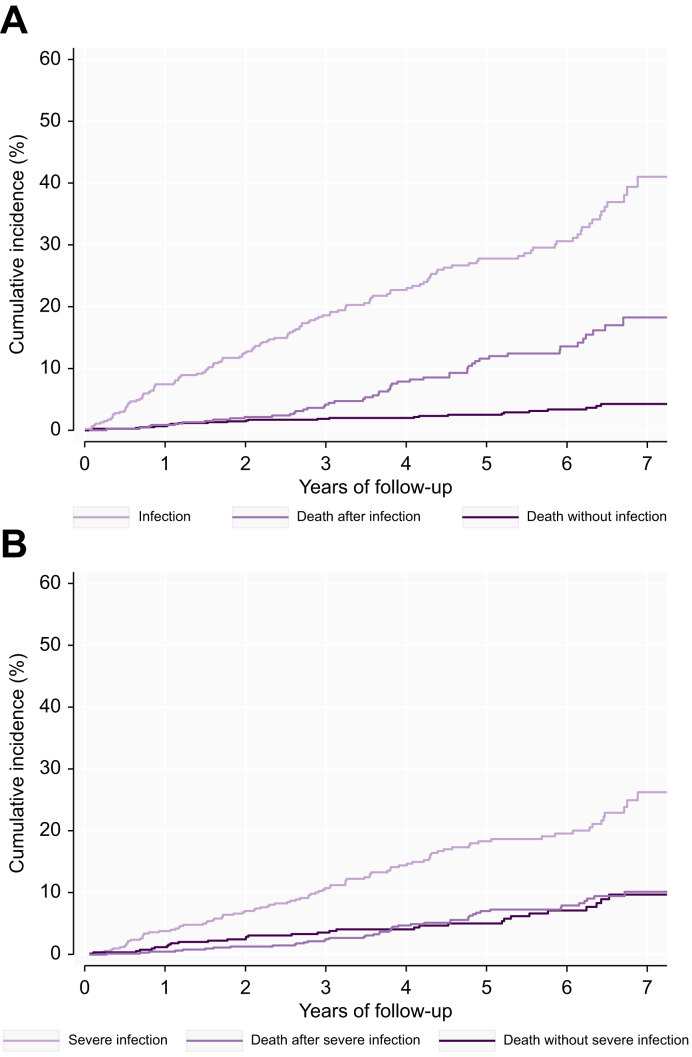
Overall, 134 patients developed a total of 312 infections (Fig. 1A), with a median number of two (range 1-11, IQR 1-3). More than half of the infections (n = 185, 59%) were severe, requiring in-hospital treatment (Fig. 1B, Table 2). The median time from inclusion to first infection was 2.3 years (IQR 0.9-4.1). The most frequent site of infection was pulmonary followed by urinary tract (Table 2).
Fig. 1.
Cumulative incidence of infections and severe infections.
(A) Cumulative incidence of infections. (B) Cumulative incidence of severe infections.
Table 2.
Characteristics of infections
| All infections (n = 312) | Severe infections∗ (n = 185) | |
|---|---|---|
| Patients with min. 1 infection | 134 (29%) | 91 (20%) |
| Site of infection | ||
| Pulmonary, n | 106 (34%) | 79 (43%) |
| Urinary tract, n | 57 (18%) | 15 (8%) |
| Skin, n | 29 (9%) | 10 (5%) |
| Sepsis, n | 25 (8%) | 25 (14%) |
| Gastrointestinal tract, n | 22 (7%) | 14 (8%) |
| SBP, n | 11 (4%) | 11 (6%) |
| Other, n | 45 (14%) | 21 (11%) |
| Unknown, n | 17 (5%) | 10 (5%) |
| Type of infection | ||
| Bacterial | 128 (41%) | 79 (43%) |
| Viral | 9 (3%) | 4 (2%) |
| Fungal | 6 (2%) | 2 (1%) |
| Unknown (not cultured) | 169 (54%) | 100 (54%) |
| Treatment | ||
| No treatment | 8 (3%) | 0 |
| Intravenous treatment | 183 (59%) | 171 (92%) |
| Peroral treatment | 120 (38%) | 14 (8%) |
| Agent known | 149 | 83 |
| Escherichia coli | 45 (30%) | 25 (30%) |
| Other gram-neg. rods | 21 (14%) | 15 (18%) |
| Staphylococcus species | 27 (18%) | 15 (18%) |
| Streptococcus species | 13 (9%) | 8 (10%) |
| Other/including virus + fungus | 31/43 (21%/29%) | 16/20 (19%/24%) |
| Resistance detected, yes | 74 (50%) | 50 (60%) |
| Infection when hospitalized, yes | 243 (78%) | 182 (98%) |
Infections were characterized as severe infections when demanding in-hospital treatment, if treated with intravenous antibiotics and/or if supportive treatment was necessary as a result of the infection.
The majority (243, 78%) of all infections were diagnosed at hospital admission or during hospitalization. Twenty-five (8%) resulted in sepsis. Specimen cultures revealed a relevant pathogen in 45% of all infections (Table 2). Bacteria with antibiotic resistance to one or more of the tested antibiotics were detected in 74 patients (50%) and resistance had a clinically significant impact on treatment in six (4%). The antibiotic resistance mechanisms detected in the isolated bacteria were beta-lactamases including AmpC and extended-spectrum beta-lactamase. None of the bacteria with antibiotic resistance were multidrug-resistant organisms.
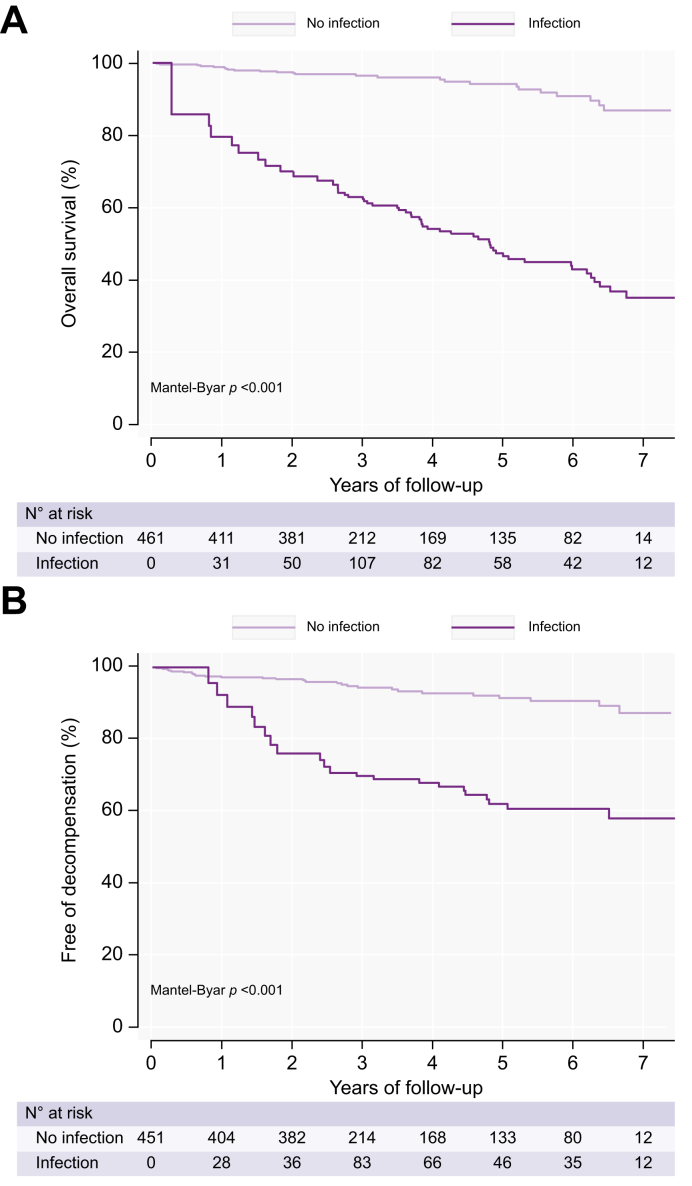
Infections and the risk of death
Patients with at least one registered infection during follow-up had a more than 8-fold higher risk of death (HR 8.24, 95% CI 4.65-14.59, p < 0.001) (Fig. 2A), independent of age, gender, baseline fibrosis stage, and MELD score (HR 5.57, 95% CI 3.03-10.23, p < 0.001) (Table S1). Decompensation and infections did not significantly interact on the risk of death, as the effect of decompensation on death did not depend on infections, and vice versa. Patients who remained compensated in the follow-up period also had an increased risk of death (HR 5.35, 95% CI 2.57-11.12, p < 0.001). Of the 134 patients who developed at least one infection, 50 patients (37%) died during follow-up, compared to 25 out of 328 (8%) who did not develop an infection. The median time from first infection to death was 17.5 (IQR 1.4-61.8) months. Out of the 75 patients who died during follow-up, 29 (38%) were infected during the hospital admission where they died, and another 21 patients, adding up to a total of 53%, had an infection within 3 months of dying. The most common infections in the months prior to death were pneumonia (17 of 40 patients, 43%) and sepsis (8 out of 40, 20%). Patients with minimal or moderate fibrosis (F0-2) had the same 30-day mortality rate after an infection (11%) as patients with severe fibrosis or cirrhosis (F3-4) (Table S2).
Fig. 2.
Risk of death and decompensation in patients developing infections.
(A) Risk of death in patients developing infections (Simon & Makuch plot). (B) Risk of decompensation in patients developing infections (Simon & Makuch plot).
Between-group comparisons for survival data with a time-dependent covariate was performed using the Mantel-Byar test.
Infections and the risk of decompensation
Infections were associated with a significantly increased risk of subsequent decompensation (HR 4.98, 95% CI 2.47-10.03, p < 0.001) (Fig. 2B). In a multivariable analysis including infections during follow-up, age, gender, baseline fibrosis stage, and MELD score, infections were independently associated with an increased risk of death (adjusted HR 2.60, 95% CI 1.18-5.73, p = 0.018) (Table S3). The 47 patients who decompensated and had at least one infection, had a total of 125 infections with a mean of 2.7±2 infections each.
The 56 patients who decompensated were admitted to the hospital 330 times in total and 108 of the admissions (33%) were with an infection, either diagnosed at hospital admission or during hospitalization. In comparison, infections occurred in 23% of hospital admissions for the patients who never decompensated. The most frequent types of infection in compensated patients were pneumonia (40%), urinary tract infections (16%) and gastrointestinal infections (8%). In decompensated patients, we observed the same types of infection, except for SBP, which now comprised of 13% of all infections.
Predictors associated with development of infections
The following factors all predicted infections in univariable competing risk regression analysis (Table 3): alcohol overuse during follow-up, smoking ≥30 pack years, MELD score ≥9, presence of type 2 diabetes and liver stiffness at baseline and during follow-up. In multivariable analysis, independent predictors of developing infections were alcohol overuse during follow-up, smoking ≥30 pack years, MELD score ≥9 and liver stiffness >15 kPa measured by TE during follow-up (Table 3, Fig. 3). Alcohol overuse, smoking and baseline liver stiffness measurements were also independent predictors of developing severe infections (Table S4).
Table 3.
Factors associated with developing infections assessed by univariable and multivariable competing risk regression analysis.
| Fibrosis, all stages F0-4 n = 461 |
Fibrosis stage F0-2 n = 366 |
Fibrosis stage F3-4 n = 93 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis |
p | Multivariable analysis |
p | Univariable analysis |
p | Multivariable analysis |
p | Univariable analysis |
p | Multivariable analysis |
p | |
| sHR (95% CI) | asHR (95% CI) | sHR (95% CI) | asHR (95% CI) | sHR (95% CI) | asHR (95% CI) | |||||||
| Age ≥50, yes | 1.25 (0.82-1.90) | 0.303 | 0.987 (0.59-1.64) | 0.959 | 1.17 (0.71-1.93) | 0.528 | 1.29 (0.70-2.37) | 0.417 | 0.57 (0.27-1.41) | 0.144 | 0.39 (0.13-1.20) | 0.100 |
| Gender, male | 1.08 (0.73-1.59) | 0.699 | 1.30 (0.85-1.99) | 0.225 | 0.86 (0.52-1.43) | 0.574 | 1.03 (0.57-1.86) | 0.931 | 1.91 (1.05-3.47) | 0.035 | 1.87 (0.92-3.79) | 0.082 |
| Alcohol overuse at baseline, yes | 1.43 (0.98-2.08) | 0.060 | 1.10 (0.71-1.69) | 0.672 | 1.79 (1.13-2.83) | 0.013 | 1.16 (0.64-2.09) | 0.630 | 1.05 (0.49-2.24) | 0.902 | 0.76 (0.27-2.13) | 0.595 |
| Alcohol overuse during follow-up, yes | 2.35 (1.54-3.60) | <0.001 | 2.39 (1.45-3.95) | 0.001 | 2.96 (1.64-5.34) | <0.001 | 3.06 (1.49-6.29) | 0.002 | 1.74 (0.93-3.28) | 0.086 | 1.56 (0.66-3.73) | 0.313 |
| Smoking ≥30 years | 1.62 (1.32-2.31) | 0.008 | 1.60 (1.06-2.43) | 0.026 | 1.46 (0.94-2.28) | 0.095 | 1.44 (0.84-2.48) | 0.184 | 1.46 (0.82-2.62) | 0.201 | 1.52 (0.84-2.76) | 0.170 |
| BMI ≥30 | 0.78 (0.53-1.16) | 0.229 | 0.76 (0.46-1.27) | 0.301 | 0.85 (0.52-1.39) | 0.529 | 0.68 (0.36-1.29) | 0.242 | 0.75 (0.38-1.48) | 0.402 | 0.94 (0.41-2.11) | 0.872 |
| Type 2 diabetes | 1.95 (1.23-3.11) | 0.005 | 1.43 (0.74-2.74) | 0.286 | 1.94 (0.98-3.83) | 0.057 | 1.34 (0.49-3.71) | 0.569 | 1.13 (0.59-2.14) | 0.710 | 2.09 (0.89-4.89) | 0.089 |
| HOMA-IR ≥2.5 | 1.13 (0.80-1.60) | 0.491 | 0.74 (0.48-1.13) | 0.159 | 1.07 (0.69-1.66) | 0.759 | 0.91 (0.53-1.58) | 0.748 | 0.60 (0.32-1.13) | 0.112 | 0.42 (0.18-0.93) | 0.034 |
| Leukocytes ≥8.8 | 1.26 (0.88-1.79) | 0.208 | 1.41 (0.94-2.13) | 0.099 | 1.25 (0.80-1.95) | 0.327 | 1.60 (0.92-2.78) | 0.095 | 1.22 (0.67-2.21) | 0.511 | 1.16 (0.60-2.25) | 0.655 |
| CRP ≥6 | 1.21 (0.85-1.72) | 0.282 | 0.90 (0.59-1.36) | 0.615 | 1.14 (0.73-1.78) | 0.573 | 0.67 (0.37-1.19) | 0.173 | 1.18 (0.68-2.04) | 0.202 | 1.06 (0.46-2.44) | 0.900 |
| MELD score ≥9 | 3.18 (2.03-4.99) | <0.001 | 2.37 (1.33-4.22) | 0.003 | 4.42 (2.04-9.62) | <0.001 | 6.89 (2.45-19.38) | <0.001 | 1.37 (0.76-2.44) | 0.292 | 1.17 (0.62-2.21) | 0.629 |
| TE at baseline∗ | ||||||||||||
| ≤10 kPa | 1 | — | — | — | 1 | — | — | — | — | — | — | — |
| >10 - ≤15 kPa | 2.49 (1.40-4.42) | 0.002 | 1.46 (0.73-2.90) | 0.282 | 2.38 (1.32-4.31) | 0.004 | 1.22 (0.55-2.72) | 0.622 | — | — | — | — |
| >15 kPa | 3.69 (2.53-5.40) | <0.001 | 1.39 (0.62-3.12) | 0.417 | 2.28 (1.05-4.94) | 0.038 | 1.13 (0.34-3.72) | 0.838 | — | — | — | — |
| TE at follow-up | ||||||||||||
| ≤10 kPa | — | — | — | — | — | — | — | — | — | — | — | — |
| >10 - ≤15 kPa | 2.29 (1.22-4.29) | 0.009 | 1.76 (0.83-3.76) | 0.120 | 2.52 (1.29-4.89) | 0.002 | 1.88 (0.79-4.46) | 0.154 | 0.71 (0.06-7.89) | 0.784 | 1.53 (0.12-18.86) | 0.738 |
| >15 kPa | 3.59 (2.51-5.13) | <0.001 | 2.27 (1.07-4.80) | 0.032 | 2.39 (1.39-4.11) | 0.002 | 1.83 (0.64-5.23) | 0.040 | 3.14 (0.91-10.79) | 0.069 | 4.96 (1.11-22.20) | 0.036 |
| Progression of TE during follow-up, yes | 0.94 (0.63-1.42) | 0.785 | 0.82 (0.48-1.41) | 0.483 | 1.17 (0.72-1.92) | 0.524 | 0.85 (0.43-1.67) | 0.642 | 0.67 (0.31-1.46) | 0.313 | 0.57 (0.20-1.62) | 0.294 |
CRP, C-reactive protein; HOMA-IR, homeostatic model assessment for insulin resistance; MELD, model for end-stage liver disease; (a)sHR, (adjusted) subdistribution hazard ratio; TE, transient elastography.
Values in bold denote statistical significance.
The analyses were not possible in the group of patients with fibrosis stage F3-4 due to a low number of patients with TE <10 (n = 5) and TE 10-15 (n = 4).
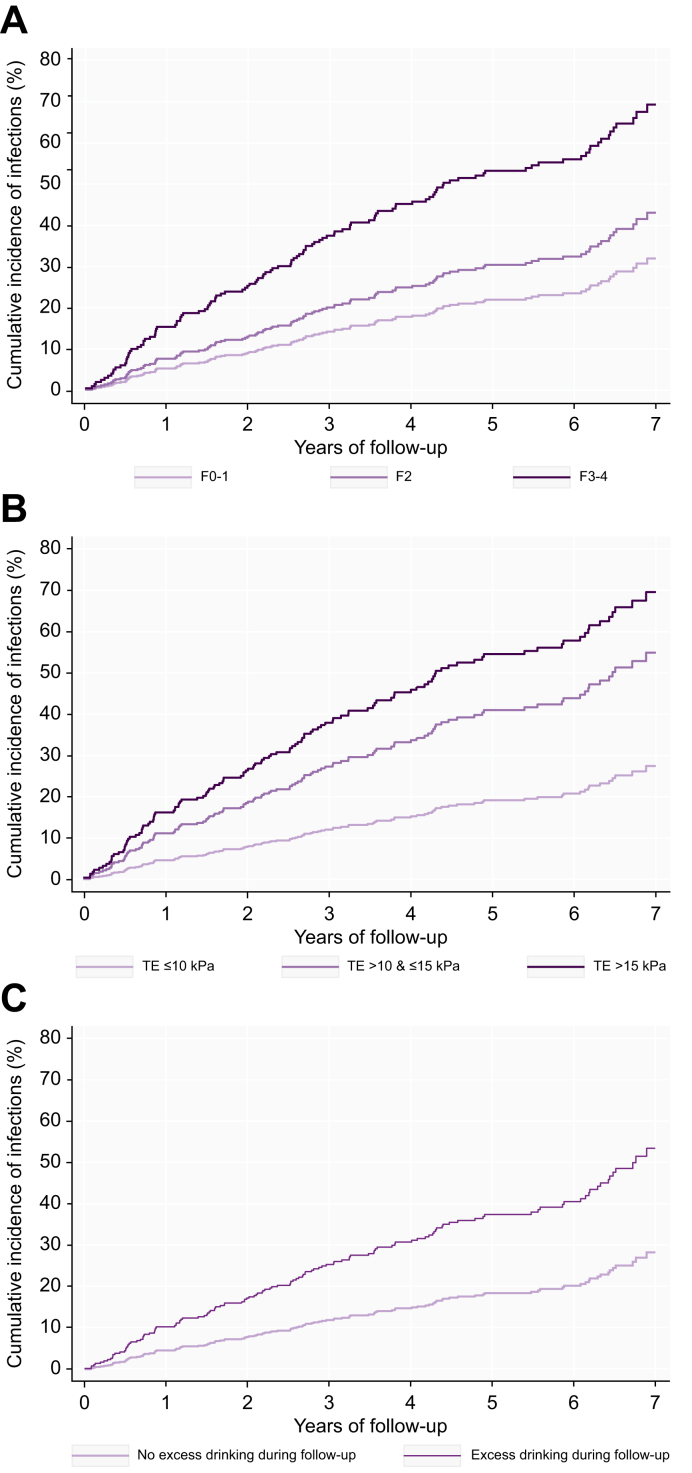
Fig. 3.
Cumulative incidence of infections.
(A) Cumulative incidence of infections stratified by Kleiner fibrosis stage. (B) Cumulative incidence of infections stratified by liver stiffness; The cumulative incidence plot is based on 447 patients as liver stiffness measurements are missing in 14 patients. (C) Cumulative incidence of infections stratified by excessive alcohol use during follow-up; The cumulative incidence plot is based on 403 patients as data on alcohol intake was not available in 58 patients.
When stratifying patients by their baseline liver stiffness measurements, we could divide patients into groups based on their risk of developing infections (Fig. 3B). Patients in the intermediate group with TE between 10 and 15 kPa had a cumulative incidence of infections of 42% and a more than 2-fold higher risk of developing infections than patients with TE <10 kPa (sHR 2.48, 95% CI 1.39-4.39, p = 0.002). For patients in the high-risk group with TE >15 kPa, the cumulative incidence increased to 53% (sHR 3.70 95% CI 2.54-5.40, p <0.001) compared to the low-risk group.
Subgroup and sensitivity analyses
When restricting the analyses to include only patients with baseline fibrosis stage F0-2, we found a 9-fold increased risk of death in patients with at least one infection during follow-up (HR 9.18, 95% CI 4.46-18.90, p <0.001). In this group of patients, 18 patients decompensated during follow-up, and infections increased the risk of later decompensation (HR 6.22, 95% CI 1.89-20.54, p = 0.003). In patients with F0-2 at baseline, report of alcohol overuse during follow-up and MELD score ≥9 were independent predictors of developing an infection (Table 3 and Table S5).
Among patients with baseline fibrosis stage F3-4 (n = 93), 38 patients decompensated during follow-up. In F3-4 patients with at least one registered infection in the follow-up period, there was an increased risk of death (3.65, 95% CI 1.46-9.10, p = 0.005) and an increased risk (though not statistically significant) of later decompensation (HR 2.19, 95% CI 0.91-5.25, p = 0.080).
The percentage of patients that developed an infection during follow-up was more than twice as high in patients with baseline fibrosis stage F3-4 compared to F0-2 (55% vs. 22%), and a larger percentage of the total number of infections were severe (66% vs. 54%) (Table S6).
Discussion
In this prospective study of 461 patients with early ALD, we found that almost one-third of patients developed infections during a median period of 4.5 years. Patients who developed infections had a nearly 5-fold higher risk of decompensation and an 8-fold higher risk of death. In adjusted analyses including age, gender, fibrosis stage, and MELD score, infections were an independent predictor of later decompensation and death. These results were mainly driven by the group of patients with no to moderate fibrosis at baseline, highlighting the prognostic impact of infections on ALD, even in the early stages of fibrosis. Alcohol overuse, smoking ≥30 pack years, MELD score ≥9 and high liver stiffness independently predicted subsequent infections.
We are the first to investigate the impact of infections on decompensation and death in patients with liver disease prior to severe fibrosis and cirrhosis. Infections in patients with compensated and decompensated cirrhosis have been widely investigated.4,6,[24], [25], [26], [27] In this group of patients with advanced disease, infections have a marked impact on prognosis by precipitating the transition from a compensated to a decompensated state, and by reducing survival.4,6,24,25,27 However, as 80% of patients in our study had a baseline fibrosis stage of F2 or below, we extensively report on the risk and characteristics of infections in patients with early-stage ALD. The risk of infections increased with disease severity but even patients with liver stiffness below 10 kPa had a cumulative incidence of infections of 21% during the 4.5-year follow-up period, increasing to 42% for patients with liver stiffness between 10 and 15 kPa, and 53% for patients above 15 kPa. Progression to a liver stiffness measurement >15 kPa in the follow-up period was an independent predictor of developing infections while baseline liver stiffness measurements were not. This suggests disease progression as a key risk factor for infections.
Interestingly, we found that infections did not significantly increase the risk of decompensation in F3-4 patients, suggesting that this finding in the full cohort was driven by the change found in the early-stage patients. The analyses in the F3-4 patients were, however, limited by a lower number of patients.
A recent registry-based Swedish study investigated the risk of infections in patients who underwent liver biopsy and had an ICD-code of ALD compared to matched controls from the general population.5 In that study, patients with ALD had a 3-fold higher risk of infections, and patients with alcohol-related liver fibrosis, but not cirrhosis, still had a 2-fold higher risk of infections. While the study included patients from the entire spectrum of liver disease, nearly 60% of patients had cirrhosis and 30% were decompensated prior to inclusion. Further, the diagnoses of infection relied on ICD codes assigned from 1969-2017.5
There was a high incidence of infections in our cohort of patients with ALD, as almost one-third of patients developed an infection during 4.5 years of follow-up. In another study of 201 patients with compensated cirrhosis, of whom one in five had alcohol as a contributing factor, 17% developed an infection during 3 years of follow-up.6 Consequently, in our study, alcohol in itself and a history of excessive drinking were also strong predictors of infections. This might be due to the known detrimental effects of alcohol on the immune system and mucosal barrier functions leading to increased susceptibility to infections compared to other etiologies of liver disease. However, we are not aware of studies on infection incidences in early stages of fibrosis of other etiologies, and therefore lack comparators.
In a study by Reichert et al., bacterial infections in patients with cirrhosis of mixed etiologies who remained compensated did not have an impact on survival.28 This conflicts with our study, where infections increased the risk of death in the full cohort of patients with ALD, both after decompensation and in patients who never decompensated. The impact of infections on survival was, however, lower in compensated patients than in those who died after decompensating.
Our study further highlights that patients with early stages of ALD develop infections at other sites compared to those with compensated or decompensated cirrhosis. A large study of hospitalized patients with cirrhosis and infections reported that the most common type of infection was SBP, accounting for more than a quarter of all infections.3 Other studies in patients with compensated liver disease at baseline report that SBP represented 11-13% of infections.6,25 In our study, just 4% of all infections were SBP. In decompensated patients, the proportion of SBP increased to 13%. Pneumonia was the most frequent infection in our population. Active smoking was highly prevalent in our study population (55%) and more than 30 pack years of smoking was an independent predictor of developing infections. It is well documented that smoking increases risk of infections, especially in the respiratory tract, by inhibiting systemic immune responses and by direct harm to the respiratory epithelium.29 Furthermore, new evidence suggests that smoking, especially heavy smoking, increases the risk of fibrosis progression.30 This highlights the importance of smoking cessation in this group to prevent infections and fibrosis progression. Prevention of infections might improve the long-term health of these patients and should include interventions to obtain abstinence from alcohol along with smoking cessation. Furthermore, the recent SARS-CoV-2 pandemic has revealed highly efficient ways to limit the spread of contagious diseases through vaccination along with hygienic practices that limit the exposure to pathogens.31
Strengths of this study include the wide spectrum of biopsy-proven ALD, from no fibrosis to compensated cirrhosis. We rigorously identified and ascertained the incidence of infections by manually reading all electronic healthcare records. Consequently, the data have high granularity with information on not only the presence of infections but also treatment, type of infection, causative agent, and whether resistance was detected. Our data is limited to infections diagnosed during hospitalization or in outpatient clinics, with no information from primary care. This will inevitably lead to a selection bias towards infections of greater severity, but most likely also greater clinical importance. Furthermore, patients with more severe underlying disease are more likely to be hospitalized, increasing the chance of diagnosing an infection and the risk of acquiring a nosocomial infection. Limiting our study to secondary care also allowed us to systematically validate all infections following a standardized combination of clinical symptoms, biochemical testing, microbiology, imaging and need for antibiotics. While this method may be less specific than hospital codes or positive cultures only, it is far more sensitive. This study was not based on highly specific infection criteria but balanced specificity and sensitivity in the definition of infections. As such, it reflects real-life data. For example, not all diagnoses of infections are supported by positive cultures. However, the aim of this study was not to investigate infections and ALD from a pathophysiological perspective but instead to study the impact of infections on patient prognosis. When assessing the association between fibrosis progression during follow-up and the development of infections, we used TE measured both prior to and after development of infections. In future studies, an optimized study design for prospectively assessing infections and their impact on later decompensation would include continuous in-person visits with prespecified infection screenings and assessments of liver disease severity at fixed time-points.
In conclusion, infections are frequent in early ALD. Our results highlight the negative prognostic impact of infections on patients with ALD, as developing infections increases their risk of later decompensation and death. Prevention of infections may improve the long-term health of these patients.
Financial support
This project received funding through the GALAXY project from the European Union’s Horizon 2020 Framework Program for Research and Innovation, Grant Agreement number 668031, through the MicrobLiver project from the Novo Nordic Foundation Challenge Program, grant number NNF15OC0016692, and from the Research Foundations at University of Southern Denmark, Odense University Hospital and Region of Southern Denmark. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Authors’ contributions
SJ, SL, AK, and MT conceptualized the study. SJ and SL drafted the manuscript and performed the data analyses. SJ created the visual elements. SJ, SL, DNR, MI, NT, KL, MK, JKH, CDH, KT, SD, HBJ, USJ, TH, and MT conducted the study. PA oversaw administration. MT and AK were supervisors. MT and AK obtained funding for the study. MT is the guarantor of the article. All authors revised the manuscript for important intellectual content.
Data availability statement
The full dataset is available by contact to open@rsyd.dk, after approval from the Danish Data Protection Agency.
GALAXY consortium
Ema Anastasiadou, Manimozhian Arumugam, Peer Bork, Torben Hansen, Jenny Presto, Hans Israelsen, Morten Karsdal, Cristina Legido-Quigley, Hans Olav Melberg, Maja Thiele, Jonel Trebicka, Aleksander Krag (coordinator).
MicrobLiver consortium
Peer Bork, Mathias Mann, Jelle Matthijnssens, Aleksander Krag, Torben Hansen (coordinator).
Acknowledgements
We wish to thank the research nurses and medical student team at FLASH and the administrators of the GALAXY and MicrobLiver consortia: Louise Skovgaard Just, Lise Ryborg, and Vibeke Nielsen.
Conflict of interest
MT: Speaker’s fee for Echosens, Siemens Healthcare, Norgine, Madrigal, Takeda, and Tillotts Pharma. Advisory fee from GE Healthcare, Boehringer Ingelheim, GSK and AstraZeneca. Co-founder and board member for Evido. Board member for Alcohol & Society (non-governmental organisation). AK has received grants from EU Horizon 2020, Novo Nordisk Foundation, Innovationfund Denmark, Danish National Research Foundation, Region of Southern Denmark, and AstraZeneca, receives royalties from Gyldendal as a co-author on a textbook of internal medicine, served as speaker for Novo Nordisk, Norgine, Siemens and Nordic Bioscience, and participated in advisory boards for Norgine, Siemens, Resalis Therapeutics, Boehringer Ingelheim and Novo Nordisk. Research support; Norgine, Siemens, Nordic Bioscience, AstraZeneca, Echosens. Consulting Takeda, Resalis Therapeutics, Zealand Pharma, AlphaSights. Board member and co-founder of Evido. JKH: Speaker’s fee from Norgine. MK: Speaker’s fee from Siemens Healthcare. SJ, SL, DNR, MI, KL, CDH, KT, NT, PA, USJ, SD, HBJ, and TH have nothing to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2024.101016.
Contributor Information
Aleksander Krag, Email: aleksander.krag@rsyd.dk.
GALAXY and MicrobLiver consortia:
Ema Anastasiadou, Manimozhian Arumugam, Peer Bork, Torben Hansen, Jenny Presto, Hans Israelsen, Morten Karsdal, Cristina Legido-Quigley, Hans Olav Melberg, Maja Thiele, Jonel Trebicka, and Aleksander Krag
MicrobLiver consortium:
Peer Bork, Mathias Mann, Jelle Matthijnssens, Aleksander Krag, and Torben Hansen
Supplementary data
The following are the supplementary data to this article:
References
- 1.Alcohol G.B.D., Drug Use C. The global burden of disease attribuTable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987–1012. doi: 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IHME Global Burden of disease. 2019. http://ghdx.healthdata.org/
- 3.Piano S., Singh V., Caraceni P., et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology. 2019;156(5):1368–1380 e1310. doi: 10.1053/j.gastro.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Arvaniti V., D'Amico G., Fede G., et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139(4):1246–1256. doi: 10.1053/j.gastro.2010.06.019. 1256 e1241-1245. [DOI] [PubMed] [Google Scholar]
- 5.Hagstrom H., Thiele M., Simon T.G., et al. Risk of infections and their role on subsequent mortality in biopsy-proven alcohol-related liver disease. United Eur Gastroenterol J. 2022;10(2):198–211. doi: 10.1002/ueg2.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villanueva C., Albillos A., Genesca J., et al. Bacterial infections adversely influence the risk of decompensation and survival in compensated cirrhosis. J Hepatol. 2021;75(3):589–599. doi: 10.1016/j.jhep.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Szabo G., Saha B. Alcohol's effect on host defense. Alcohol Res. 2015;37(2):159–170. doi: 10.35946/arcr.v37.2.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan C., Levitsky J. Infection and alcoholic liver disease. Clin Liver Dis. 2016;20(3):595–606. doi: 10.1016/j.cld.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Albillos A., Lario M., Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61(6):1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 10.von Elm E., Altman D.G., Egger M., et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bmj. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen D.N., Thiele M., Johansen S., et al. Prognostic performance of 7 biomarkers compared to liver biopsy in early alcohol-related liver disease. J Hepatol. 2021;75(5):1017–1025. doi: 10.1016/j.jhep.2021.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiele M., Detlefsen S., Sevelsted Moller L., et al. Transient and 2-dimensional shear-wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology. 2016;150(1):123–133. doi: 10.1053/j.gastro.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Kleiner D.E., Brunt E.M., Van Natta M., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 14.Brunt E.M., Janney C.G., Di Bisceglie A.M., et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 15.de Franchis R., Bosch J., Garcia-Tsao G., et al. BAVENO VII - renewing consensus in portal hypertension: report of the Baveno VII Consensus Workshop: personalized care in portal hypertension. J Hepatol. 2022;76(4):959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churpek M.M., Dumanian J., Dussault N., et al. Determining the electronic signature of infection in electronic health record data. Crit Care Med. 2021;49(7):e673–e682. doi: 10.1097/CCM.0000000000004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe K.E., Zein J., Hatipoglu U., et al. Association of smoking and cumulative pack-year exposure with COVID-19 outcomes in the Cleveland clinic COVID-19 registry. JAMA Intern Med. 2021;181(5):709–711. doi: 10.1001/jamainternmed.2020.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamath P.S., Wiesner R.H., Malinchoc M., et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 20.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 21.Pepe M.S., Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12(8):737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 22.Mantel N., Byar D.P. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc. 1974;69(345):81–86. [Google Scholar]
- 23.Simon R., Makuch R.W. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35–44. doi: 10.1002/sim.4780030106. [DOI] [PubMed] [Google Scholar]
- 24.Dionigi E., Garcovich M., Borzio M., et al. Bacterial infections change natural history of cirrhosis irrespective of liver disease severity. Am J Gastroenterol. 2017;112(4):588–596. doi: 10.1038/ajg.2017.19. [DOI] [PubMed] [Google Scholar]
- 25.Nahon P., Lescat M., Layese R., et al. Bacterial infection in compensated viral cirrhosis impairs 5-year survival (ANRS CO12 CirVir prospective cohort) Gut. 2017;66(2):330–341. doi: 10.1136/gutjnl-2015-310275. [DOI] [PubMed] [Google Scholar]
- 26.Otero Sanchez L., Karakike E., Njimi H., et al. Clinical course and risk factors for infection in severe forms of alcohol-associated liver disease. Hepatology. 2021;74(5):2714–2724. doi: 10.1002/hep.31984. [DOI] [PubMed] [Google Scholar]
- 27.Trebicka J., Fernandez J., Papp M., et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74(5):1097–1108. doi: 10.1016/j.jhep.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Reichert M.C., Schneider C., Greinert R., et al. Isolated bacterial infection without decompensation has no impact on survival of compensated patients with cirrhosis. Liver Int. 2021;41(6):1370–1378. doi: 10.1111/liv.14842. [DOI] [PubMed] [Google Scholar]
- 29.Feldman C., Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect. 2013;67(3):169–184. doi: 10.1016/j.jinf.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Marti-Aguado D., Clemente-Sanchez A., Bataller R. Cigarette smoking and liver diseases. J Hepatol. 2022;77(1):191–205. doi: 10.1016/j.jhep.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Chow E.J., Uyeki T.M., Chu H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. 2023;21(3):195–210. doi: 10.1038/s41579-022-00807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset is available by contact to open@rsyd.dk, after approval from the Danish Data Protection Agency.