Abstract
Diabetes is a significant global health concern that increases the vulnerability to various chronic illnesses. In view of this issue, the current research aimed to examine the effects of administering an extract derived from the tubers of Cyperus rotundus L (CrE) on obesity, type 1 diabetes, and liver-kidney toxicity. Through the utilization of HPLC-DAD analysis, it was discovered that the extract contained several components, including quercetin (47.8%), luteolin glucoside (17%), luteolin (7.56%), apigenin-7-glucoside (6.29%), naringinin (4.52%), and seven others. In vitro experiments they have demonstrated that CrE effectively inhibited key digestive enzymes associated with obesity and type 2 diabetes, such as DPP-4, PTP1B, lipase, and α-amylase, as evidenced by their respective IC50 values are about 23, 51,83, and 67 μg/ml respectively. Furthermore, when diabetic rats were administered CrE, the activity of pancreatic enzymes linked to inflammation, namely 5-lipoxygenase (5-LO), hyaluronidase (HAase), and myeloperoxidase (MPO), was significantly suppressed by 48, 41, 75, and 47%, respectively. Moreover, CrE exhibited protective effects on pancreatic β-cells by inhibiting the formation of thiobarbituric acid reactive substances (TBARS) by 65% and the induction of superoxide Dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) activities by 62, 108, and 112% respectively as compared to diabetic untreated rat. Additionally, CrE significantly inhibited the activities of intestinal, pancreatic, and serum lipase and α-amylase activities. In diabetic rats, CrE administration suppressed glycogen phosphorylase (GP) stimulated glycogen synthase (GS) activities by 45 and 30%; and this increased liver glycogen content by 45%. Furthermore, CrE modulated key hepatic enzymes involved in carbohydrate metabolism, including hexokinase (HK), glucose-6-phosphate dehydrogenase (G6PD), glucose-6-phosphatase (G6P), and fructose-1,6-bisphosphatase (FBP). Notably, the average food and water intake (AFI and AWI) of diabetic rats treated with CrE was reduced by 15 and 16% respectively as compared to those without any treatment. Therefore, this study demonstrated the effectiveness of Cyperus rotundus tubers in preventing and treating obesity and diabetes.
Keywords: Disacchridases, Diabetes, Cyperus rotundus, Inflammation, Pancreas, Obesity
1. Introduction
In 2014, the World Health Organization (WHO) published a report that revealed a staggering number of over 600 million adults worldwide suffering from obesity, while an additional 1.9 billion individuals were classified as overweight [1]. Type 2 diabetes (T2D) is a chronic condition that arises due to insufficient or ineffective insulin, accompanied by various metabolic abnormalities. The prevalence of T2D is on the rise globally. According to the International Diabetes Federation (IDF), there were 382 million people diagnosed with diabetes worldwide in 2013, and this figure is projected to increase to 592 million by the year 2035 [2]. T2D accounts for approximately 90% of all diabetes cases globally and is the most common form of the disease. T2D is characterized by elevated blood sugar levels (hyperglycemia) and abnormal lipid levels (dyslipidemia), which result from the failure of insulin to activate signaling pathways (insulin resistance) in metabolic target tissues [[3], [4], [5], [6], [7], [8], [9], [10]].
T2D can lead to severe consequences such as cardiovascular disease, impaired vision, kidney failure, and delayed wound healing. According to the International Diabetes Federation (T2D), over half a billion people worldwide had diabetes in 2021, with type 2 diabetes accounting for 90% of cases [11], resulting in health spending of US$966 billion worldwide, with an expected increase to almost $1054 billion by 2045 [12]. In the Middle East and North Africa, T2D is most common, with a regional prevalence of 16.2%, or 73 million people. An 87% increase to 19.3%, or 136 million people, is predicted for this amount by 2045 [13]. Furthermore, Saudi Arabia ranks fourth globally in terms of the incidence of T2D. In a cross-sectional study conducted in 2011 with 9149 participants [14]. found that the prevalence T2D in Saudi Arabia was 31.6% [[15], [16], [17]]. T2D, also known as non-insulin-dependent diabetic mellitus (NIDDM), accounts for approximately 90% of all diabetes cases. It is characterized by abnormalities in pancreatic β-cell activity, insulin secretion, and insulin sensitivity. The high prevalence of diabetes mellitus in the Middle East can be attributed to factors such as the high obesity rate, population aging, and urbanization. Given that diabetes mellitus is the most prevalent disease in Saudi Arabia and imposes significant financial burdens, public health interventions should prioritize behavioral changes to prevent and control T2D effectively. Hyperglycemia is known to trigger an elevated generation of free radicals [18,19], which subsequently leads to the occurrence of oxidative stress. This oxidative stress can inflict harm upon multiple organs that are closely linked to disturbances in carbohydrate metabolism, such as the pancreas. Apart from the primary complications associated with diabetes, there exist various supplementary risk factors that play a role in the progression of the disease. These risk factors encompass hyperglycemia, hypertension, dyslipidemia, impaired fibrinolytic activity, severe atherosclerosis, and heightened platelet aggregation. If effective measures for prevention and management are not put in place, the increasing prevalence of obesity and type 2 diabetes will have significant adverse effects on the overall health and longevity of the global population. One approach to treating and preventing obesity and T2D involves several methods, including inhibiting protein tyrosine phosphatase 1B (PTP1B) and dipeptidyl peptidase-4 (DPP-4), which play crucial roles in obesity and Type 2 diabetes [[13], [14], [15], [16]]. PTP1B is a key factor in insulin resistance and is one of the most promising targets for treating type 2 diabetes. DPP-4 is an enzyme that breaks down GLP-1, which is essential for maintaining glucose homeostasis [[20], [21]]. Another method is to suppress intestinal digestive enzymes, primarily lipase and glucosidases, while activating catalytic enzymes and inhibiting carbohydrate anabolic enzymes [18,22,23]. Furthermore, protecting the pancreas from oxidative damage and inflammation is crucial since it is the only organ involved in insulin secretion and metabolic regulation. Pancreatic lipase is an essential enzyme that helps the digestive system break down dietary fat, and inhibiting its activity can prevent fat absorption in the gastrointestinal tract, thereby controlling the incidence of obesity, particularly diet-induced obesity [19]. Current gastrointestinal lipase inhibitors, such as orlistat, are useful in treating obesity in humans, but their benefits take time to become apparent, and when treatment is stopped, patients regain their weight. Moreover, some of these anti-obesity medications have been taken off the market due to side effects like diarrhea, oily stools, bloating, flatulence, abdominal pain, and decreased absorption of fat-soluble vitamins [24,25].
Hence, the development of an alternative medical supply derived from natural products or plant-based sources is of utmost importance as it will facilitate the creation of innovative pharmaceuticals without any adverse effects [[26], [27], [28], [29], [30], [31], [32]]. Numerous natural products possess the ability to reduce weight and combat obesity caused by dietary factors. These natural products contain inhibitors of digestive enzymes that hinder the hydrolysis and absorption of dietary lipids and carbohydrates. The primary enzymes responsible for the breakdown of fats and carbohydrates are α-amylase, α-glucosidase, and pancreatic lipase [33].
There have been several reports on the utilization of medicinal plants as an alternative treatment for various pathological conditions, including diabetes mellitus and the provision of antioxidants. One such plant is C. rotundus, commonly known as Nutgrass and belonging to the Cyperaceae family; which employed in traditional medicine to cure a variety of conditions, including genotoxicity disorders, hirsutism, nociception, cancer, atherosclerosis, aging, and cystitis [34]. According to research conducted by Azimi et al. [35] and Amro et al. [36], this plant compound exhibits anti-Alzheimer's disease by the suppression of inflammation and induction of antioxidant capacity. In addition, Badgujar and Bandivdekar [37] have reported that Cyperus rotundus aqueous extract stimulates milk production in female ratsby about 40%. In fact, Cyperus rotundus, commonly known as nutgrass or purple nutsedge, has been traditionally used in various cultures for its potential health benefits. However, it's important to note that while some traditional uses exist, scientific research on its health effects is still limited, and not all traditional uses may be supported by robust scientific evidence. No previous studies concerning the effects of Cyperus rotundus on diabetes and obesity. In this context, this study aims to evaluates the potential benefits effect of Cyperus rotundus in by investigating the effect of CrE on key enzymes related to carbohydrate digestion and absorption such as α-amylase and lipase as well as the insulin signaling enzymes as PTP1B and DPP-4 activities in vitro and in rats with obesity and type 2 diabetes.
2. Materials and methods
2.1. Plant materials
2.1.1. Fresh Cyperus rotundus L. Tubers collection, preparation, and extraction
Cyperus rotundus rhizomes were collected from Hail (KSA). The plant was identified and authenticated by the Aromatic and Medicinal Plants Research Institute, KSA. Voucher specimens were kept in the herbarium of the Analytical Chemistry Department, College of Science, Ha'il University, KSA (number 19010).
2.1.2. Quantification of CrE phenolic compounds
The HPLC-DAD method (Agilent, USA) utilizing a diode array detector (DAD) was employed to detect and quantify CrE phenolic compounds. Separation of the CrE phenolic compounds was achieved using a C18 column at 40 °C, with the mobile phase consisting of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) during a 60-min running time. The injection volume was 10 μL, and detection of the CrE phenolic compounds was accomplished by comparing the relative elution order and UV spectra. Identification of each peak was established through LC-MS analysis, which was performed on an Agilent 1100 LC system.
2.2. In vitro experiments
2.2.1. In vitro inhibition of α-amylase activity
The method previously described [19] was utilized to evaluate the inhibition of -amylase activity. A solution of 0.5 mg -amylase from swine pancreas (Sigma, ref. A4268) was dissolved in 1 ml of 20-mM phosphate buffer (pH 6.9). The reaction mixture contained 1 ml of the -amylase mixture (12 U/ml) and 1 ml of CrE. The reaction was allowed to proceed at 37 °C for 15 min, after which 1 ml of 0.5 percent starch was added and the mixture was incubated for an additional 15 min. The reaction was stopped by adding 2 ml of dinitrosalicylic acid (DNS), and the absorbance was measured at 565 nm after cooling.
2.2.2. In vitro determination of lipase activity
In order to assess the lipase activity, a combination of 1 ml of extract and 1 ml of pig pancreas lipase (Sigma, ref. L3126) with a concentration of 0.1 mg/ml was prepared in a 0.1 mM potassium phosphate buffer (pH 6.0). The resulting mixture was then incubated for a duration of 10 min at a temperature of 37 °C. Following the addition of 0.1 ml of p-NPP substrate, the mixture was further incubated at 37 °C for a period of 15 min. Subsequently, the optical density was measured at a wavelength of 410 nm [22].
2.2.3. In vitro activity of DPP-4 assay
The in-vitro DPP-4 assay was performed using the standard protocol for identifying chromatophore formation by cleaving Gly-Pro p-nitroanilide hydrochloride [38]. The inhibition of DPP-4 by CrE was measured by measuring the release of 4-nitroaniline from an assay mixture containing 0.1 M Tris-HCl (pH 8.0) and 2 mM Gly-Pro p-nitroanilide (substrate). The reaction mixture was mixed with a pH 4.5 sodium acetate buffer and incubated at 37 °C. Utilizing a UV–VIS Spectrophotometer, absorbance at 405 nm was found.
2.2.4. In vitro activity of PTP1B assay
Protein tyrosine phosphatase 1B activity was measured using p-nitrophenyl phosphate (p-NPP) as a substrate. 2 mM pNPP and PTP1B were introduced to each well (final volume 110 μL) in a buffer containing 50 mM citrate (pH 6.0), 0.1 M NaCl, 1 mM EDTA, and 1 mM dithiotheritol (DTT), with or without sample. 50 μLofp-NPP in buffer was added after a 10-min preincubation time at 37 °C, and 10 M NaOH was added to halt the reaction [39].
2.3. In vivo experiments
2.3.1. Animals
In this research, a total of 26 male Wistar rats (Rattus norvegicus) were used as subjects. The rats were 8 weeks old and had an average weight of 176g ± 17. The animal study protocol followed in this research was approved by the Monastir University Ethics Committee; with the approval number MU-86/609/EEC. The study was conducted in accordance with international standards for animal experimentation. The rats were housed individually in cages that were equipped with stainless steel wire mesh. They were provided with unlimited access to food and water throughout the duration of the study. The laboratory environment in which the rats were kept maintained a humidity level of 55 ± 5%. The temperature in the laboratory ranged between 25 and 30 °C. Additionally, a 12-h light-dark cycle was maintained to simulate natural day and night conditions. These controlled environmental conditions ensured that the rats were kept in a suitable and consistent environment throughout the study. This allowed for accurate and reliable data collection and analysis.
2.3.2. Induction of obesity
The investigation was carried out in accordance with a prior study [25]. The HFF-diet consisted of 60% standard diet, 11.69% sheep fats, 10% fructose, and 0.5% cholic acid, as detailed in the aforementioned study [25]. Rats were provided with unrestricted access to either a standard chow and their solid and liquid intake was monitored on a weekly basis. Obesity and type 2 diabetes were induced by a significant rise in body weight and blood glucose levels in comparison to normal rats [19].
2.3.3. Experimental design and procedure
Five experimental groups were formed by randomly assigning thirty rats. The first group, named (con), consisted of healthy rats that were given a regular diet for ninety days. The second group, referred to as (O), comprised obese rats. The third and fourth groups, (O-CrE100) and (O-CrE200) [40,41] respectively, were also obese rats that received CrE at doses of 100 and 200 mg/kg bw daily dissolving in 2 ml of distilled water with 5% DMSO. Group 5, (O-Ort), consisted of obese rats treated with orlistat at dose 10 mg/kg bw [41] (O-Ort). All groups of rats were given a solution of 5% DMSO in 2 ml of distilled water for 90 days by gastric gavage route as described by our previous study [19]. At the end of the experimentation, the animals were decapitated after receiving an intraperitoneal injection of 30 mg/kg pentobarbital sodium to induce anesthesia [23]. The serum was obtained by blood centrifugation 15 min at 4 °C and 1.500×g. The samples were kept at 80 °C until they biochemical analysis (α-amylase, lipase, DPP-4 and PTP1B activities and glucose and Hb levels). The pancreas and the intestinal mucosal tissue were combined and homogenized using centrifugation at 5000×g. The supernatant was stored at −80 °C before biochemical testing [42]. An oral glucose tolerance test (OGTT) was run at the end of the study. Rats were given 2 g/kg of glucose solution by gastric gavage. Also, insulin tolerance test (regular insulin 0.75 UI/kg, Regular Humulin, Lilly France, S.A., France) [43] was performed. An ACCU-CHEK Go glucometer (Roche, Germany) was used to measure the blood glucose levels in the tail blood.
2.3.4. Biochemical analysis
The α-amylase activity was assessed by measuring the rate at which glucose was extracted from the CNPG3 substrate. To do this, 1 mL of CNPG3 was mixed with 20 μL of serum and incubated for 5 min at 37 °C. The absorbance was measured at 405 nm using a spectrophotometer (Kits Biomaghreb, Tunisia, ref 20033). In order to determine the lipase activity, the conversion of 1,2-diglyceride into glycerol and free fatty acids was measured using the Biolabo Kit (France, ref 99881). Protein quantity was determined using the Biuret colorimetric method (Kit biolabo, France, ref K2016). Pancreatic proteins carbonyls (PCOs) were measured using the methodology of Reznick et al. [44]. The pancreas thiol groups (-SH) measurement was evaluated according to Ellman's method [45]. The Buege and Aust methodology [46] was followed in order to calculate the pancreasl TBARS rates. The H2O2 level in the pancreas was measured in accordance with Dingeon et al. [47]. In short, in the presence of peroxidase, hydrogen peroxide combines with p-hydroxybenzoic acid and 4-aminoantipyrine to create quinoneimine, which is detected at 505 nm and has a pink color. The procedure of Marklund and Marklund [48] was used to calculate the superoxide dismutase activity. The glutathione peroxidase (GPx) activity, using the protocol of by Pagila and Valentine [49]; and the catalase (CAT) activity according to the protocol of Aebi [50]. To quantify MPO activity, 1 g of pancreas was crushed in 50 mg of phosphate buffer (pH 6.0) and then centrifuged. The resulting supernatant was cultured in 80 mM phosphate buffer (pH 5.4), containing 1.6 mM tetramethylbenzidine and 0.3 mM hydrogen peroxide. The quantity of MPO activity in the pancreas was measured by determining the absorbance at 460 nm after the addition of a 0.2 M sulfuric acid solution, with duration of 2 min [51]. The absorbance at 585 nm was measured in order to determine the 5-LO activity, according to Wu [52]. The CRP level was determined using the Biolabo kit (France, ref CRP620E). The G6-PDH activity was measured using a commercial kit (Ref 97089) obtained from Bolabo, France. The hepatic activity of glucose metabolism enzymes, including HK, PK, G6P, and FBP, was measured using techniques described in Refs. [[40], [41], [42]]. The liver glycogen content, liver glycogen synthase activity, and glycogen phosphorylase activity were measured using the techniques described by Cornblath et al. [53] and Leloir et al. [54] respectively. The intestine and pancreas maltase and sucrase activities were measured using the protocol outlined in our previous study [55]. The serum levels of glucose, HbA1c and total HbA were determined by following the instructions of the commercial kits (Kit Biolabo, France, ref LP87809, K2010 and K3502200). The protocol previously described by Almasri et al. [56] was utilized to conduct the serum DPP-4 assay. Sigma P6244-50UG was the source of Tyrosine phosphatase 1B protein, and the inhibitory activities of the tested samples were evaluated using p-nitrophenyl phosphate (p-NPP) as a substrate. The hydrolysis of p-NPP by PTP1B was calculated based on the increase in absorbance at 405 nm [39].
2.4. Statistical analysis
The data are presented as mean ± SEM. The determination was done using six animals in every group. The data obtained from each analysis were entered into StatView 5.0 software for statistical anaysis. The Fisher test and one-way analysis of variance (ANOVA) were used to identify any significant differences (P < 0.05).
3. Results
3.1. Quantification of phytochemicals through HPLC-DAD analysis
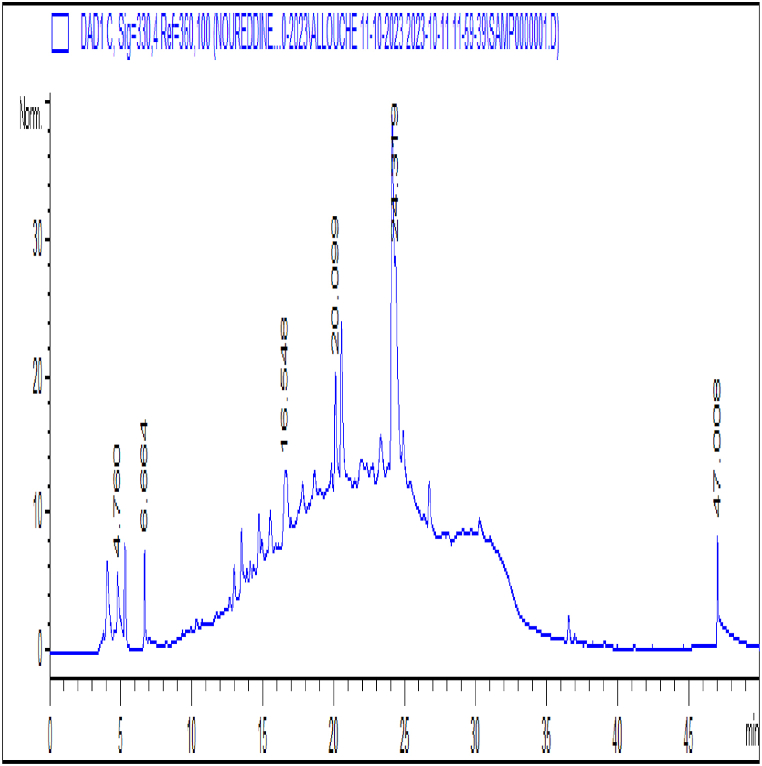
The ethanol extract of Cyperus rotundus L. tubercules has been found to contain a total of twelve phenolic compounds. Our research shows that the extract has significant constituents, with quercetin being the most abundant at 47.8%. Following quercetin, luteolin glucoside is present at 17%, luteolin at 7.56%, and apigenin-7-glucoside at 6.29%. Additionally, the extract contains naringinin at 4.52% and seven other phenolic acids or compounds at lower concentrations or as traces (Table 1, Fig. 1).
Table 1.
Phenolic compounds in ethanol Cyperus rotundus Linn tubers extract were identified by HPLC-DAD analysis.
| Peak compound | RT (min) | Compounds | (%) |
|---|---|---|---|
| 1 | 7.107 | Gallic acid | 1.2 |
| 2 | 11.503 | Chorogenic acid | 1.62 |
| 3 | 12.919 | Tyrosol | 2.75 |
| 4 | 13.489 | Cafeic acid | 0.56 |
| 5 | 14.221 | Vanillic acid | 0.42 |
| 6 | 15.235 | Rutin | 5.02 |
| 7 | 15.526 | Verbascoside | 4.03 |
| 8 | 16.584 | Luteolin glucoside | 18.17 |
| 9 | 17.783 | Apigenin-7-glucoside | 6.29 |
| 10 | 20.974 | Naringinin | 4.52 |
| 11 | 23.123 | Quercetin | 47.80 |
| 12 | 23.290 | Luteolin | 7.56 |
Fig. 1.
HPLC chromatogram of ethanol Cyperus rotundus Linn tubers extract.
3.2. In vitro evaluation of CrE on α-amylase and lipase activity
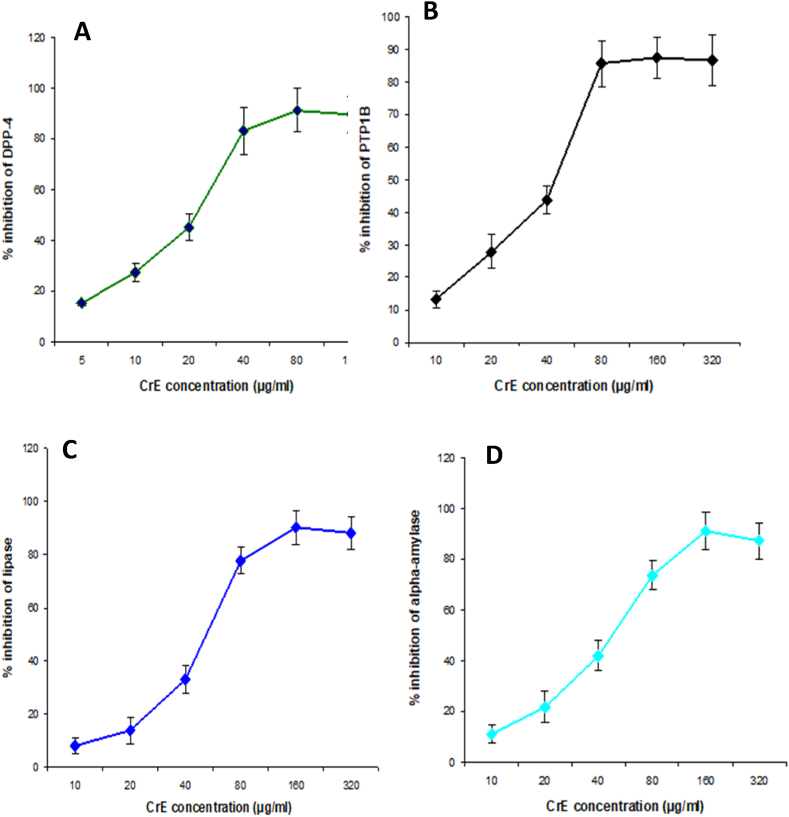
Table 2 illustrates the potential of CrE to mitigate hypercholesterolemia and obesity through the inhibition of α-amylase and lipase activities. Our study shows that there is an increasing inhibition as a function of the increase in the concentration of CrE, of which the IC50 value of 67 and 83 μg/ml respectively suggests that CrE is a highly effective inhibitor of carbohydrate and lipid lipase hydrolyzing enzymes, with its inhibitory action being comparable to acarbose and orlistat, which has an IC50 value of 17 and 59 μg/ml respectively (Table 2, Fig. 2).
Table 2.
Effect of ethanol Cyperus rotundus Linn tubers extract on DPP-4, PTP1B, α-amylase and lipase activities. In vitro study by determining the inhibitory concentration IC50 compared to standard drugs (IC50) of an in vitro study to commercially available standard medications (IC50, μg/mL) (n = 3).
| IC50 | IC50 | ||
|---|---|---|---|
| DPP-4 | 23.9 ± 1.3 | α-amylase | 67.3 ± 3.7 |
| PTP1B | 51.9 ± 3.1 | Lipase | 83.2 ± 4.6 |
| Acarbose | 17 ± 1.3 | Sitagliptin | 23.9 ± 1.3 |
| Orlisat | 59 ± 7 |
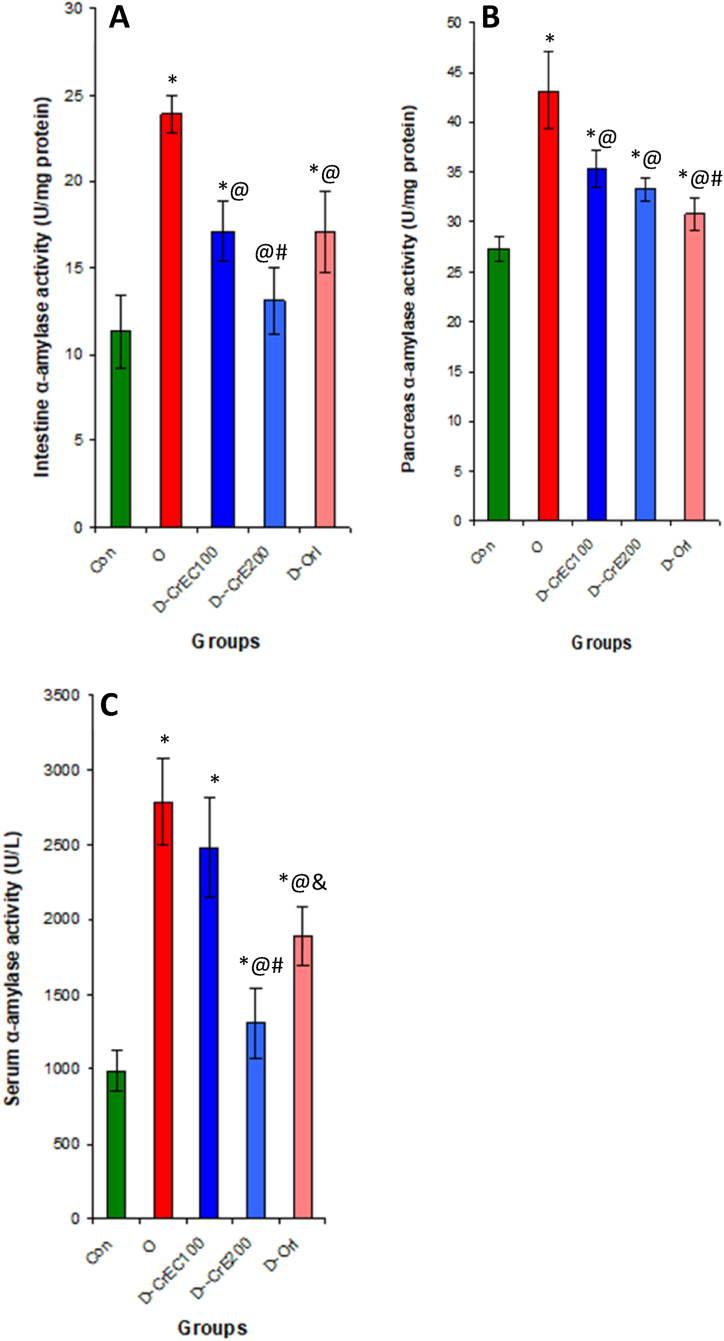
Fig. 2.
Assessing the impact of elevated CrE concentrations on the functioning of pivotal enzymes associated with insulin signaling, specifically DPP-4 (A) and PTP1B (B), as well as on the processing and assimilation of lipids and glucose, namely lipase (C) and α-amylase (D). (In vitro assessment). Our findings indicate that CrE exhibits potent inhibitory effects on these enzymes, leading to the promotion of insulin signaling and the suppression of lipid and monosaccharide digestion and absorption.
3.3. In-vitro inhibition of DPP-4 and PTP1B activities by CrE
This study shows that CrE potentially inhibits, in vitro, key enzymes that suppress insulin signaling in a concentration-dependent manner as DPP-4 and PTP1B, with IC50 values of 23.9 and 51.9 μg/ml respectively. These values are comparable to the specific inhibitor Sitagliptin, which has an IC50 value of approximately 24 μg/ml (Table 2, Fig. 2).
3.4. CrE and stress oxidant in pancreas of rat with obesity and type 2 diabetes
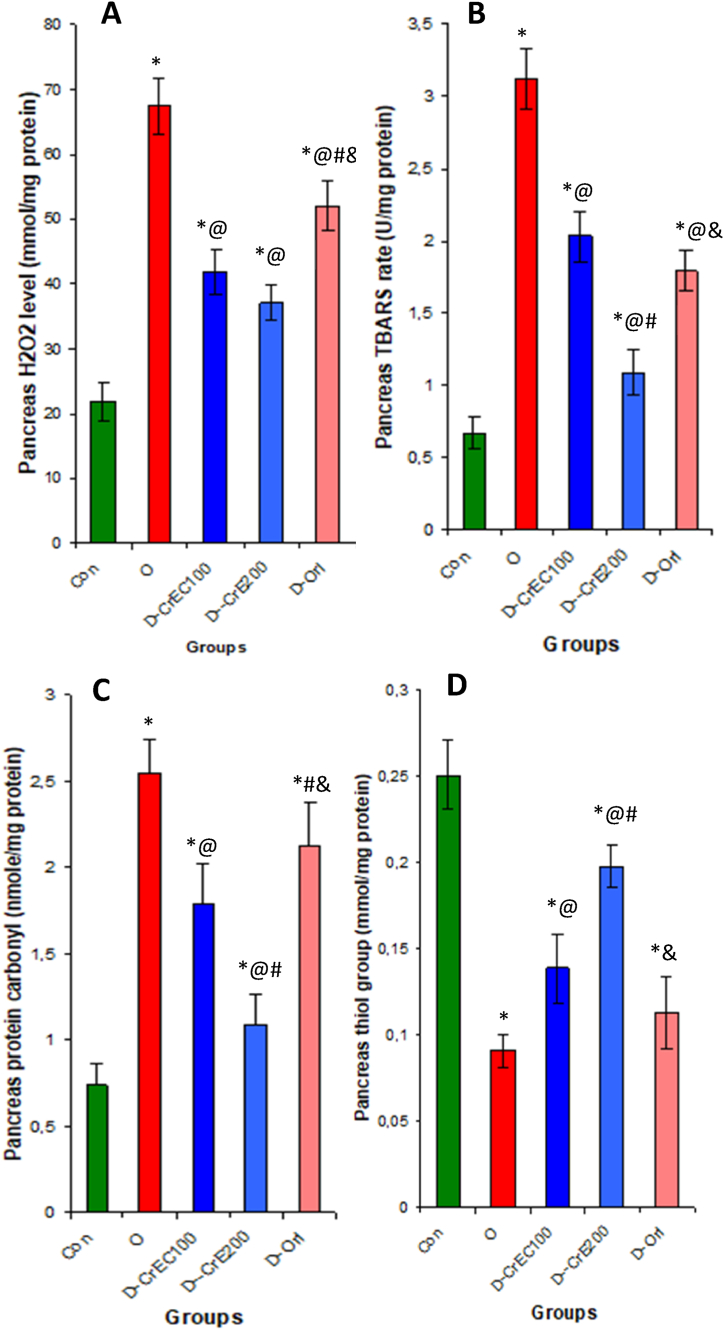
Our research has revealed that the presence of type 2 diabetes in individuals with obesity can lead to stress on the pancreas. This is supported by a significant decrease in the activity of antioxidant enzymes such as SOD, CAT, and GPX, as well as a reduction in the concentrations of H2O2, TBARS, and the thiol group. However, our findings also suggest that CrE may have a protective effect on obese rats. In fact, the consumption of CrE by obese rats resulted in a notable increase in antioxidant indices such as SOD, CAT, and GPX activities by 68%, 134%, and 112%, respectively, as well as an increase in thiol group levels by 117% compared to obese rats that were not treated with CrE. Additionally, the levels of TBARS, H2O2, and protein carboxyl were significantly reduced by 44.9%, 65%, and 57%, respectively (as shown in Table 3 and Fig. 3).
Table 3.
Effect of CrE ingestion by obese rats on AFI, AWI, pancreatic activity of antioxidant enzymes (SOD, CAT and GPX) and metabolic catabolism enzymes (HK, PK and 6PD and carbohydrate metabolism (G6P and FBP) in the liver. Our results clearly show that CrE stimulates glucose degradation and inhibits monossaccharide biosynthesis. Statistical analyses as given as: *P < 0.05 significant differences compared to controls; @P < 0.05 significant differences compared to diabetic rats; #P < 0.05 significant differences compared to diabetic rats treated with CPC100. &P < 0.05 significant differences compared to diabetic rats treated with CPC200.
| Con | O | D-CrE100 | D-CrE200 | D-Orl | |
|---|---|---|---|---|---|
| AFI | 17.3 ± 1.5 | 21.9 ± 1.6* | 19.3 ± 1*@ | 18.3 ± 1.3 | 17.9 ± 2.1*@ |
| AWI | 18.5 ± 1.5 | 23.9 ± 2.1* | 21.7 ± 1.7*@ | 20.3 ± 1.4*@ | 21.7 ± 1.8*@ |
| SOD | 1.35 ± 0.21 | 0.73 ± 0.13*# | 0.97 ± 0.14#@ | 1.23 ± 0.17#@ | 0.93 ± 0.11*#@ |
| CAT | 31.9 ± 3.9 | 11.2 ± 0. 97**# | 21.9 ± 2.3#@ | 26.3 ± 3.1#@ | 23.9 ± 1.9#@$ |
| GPX | 2.19 ± 0.21 | 0.93 ± 0.1*# | 1.56 ± 0.19* | 1.98 ± 0.39* | 1.32 ± 0.19*#@ |
| HK | 251 ± 29 | 137 ± 17* | 197 ± 31*@ | 209 ± 23*@ | 129 ± 17*@ |
| PK | 201 ± 31 | 104 ± 9* | 163 ± 19*@ | 173 ± 35*@ | 141 ± 19*@ |
| G6PD | 497 ± 39 | 207 ± 29* | 421 ± 47*@ | 477 ± 29*@ | 397 ± 53*@ |
| G6P | 697 ± 29 | 1537 ± 86* | 1147 ± 81*@ | 789 ± 47*@ | 1137 ± 65*@# |
| FBP | 349 ± 25 | 803 ± 51* | 549 ± 49*@ | 402 ± 63*@ | 671 ± 59*@# |
Fig. 3.
The impact of CrE ingestion on pancreatic oxidative stress indices was investigated. Our findings reveal that CrE serves as a protective agent against oxidative stress in the pancreas, as evidenced by a notable reduction in oxidative stress indicators, including H202 (A), TBARS (B), and carbonyl (C) levels. Additionally, there was an observed increase in antioxidants, particularly thiol groups (D). The corresponding statistical analyses are detailed in Table 3.
3.5. Effect of CRE on key enzymes related to inflammation and pancreatitis in diabetic rats
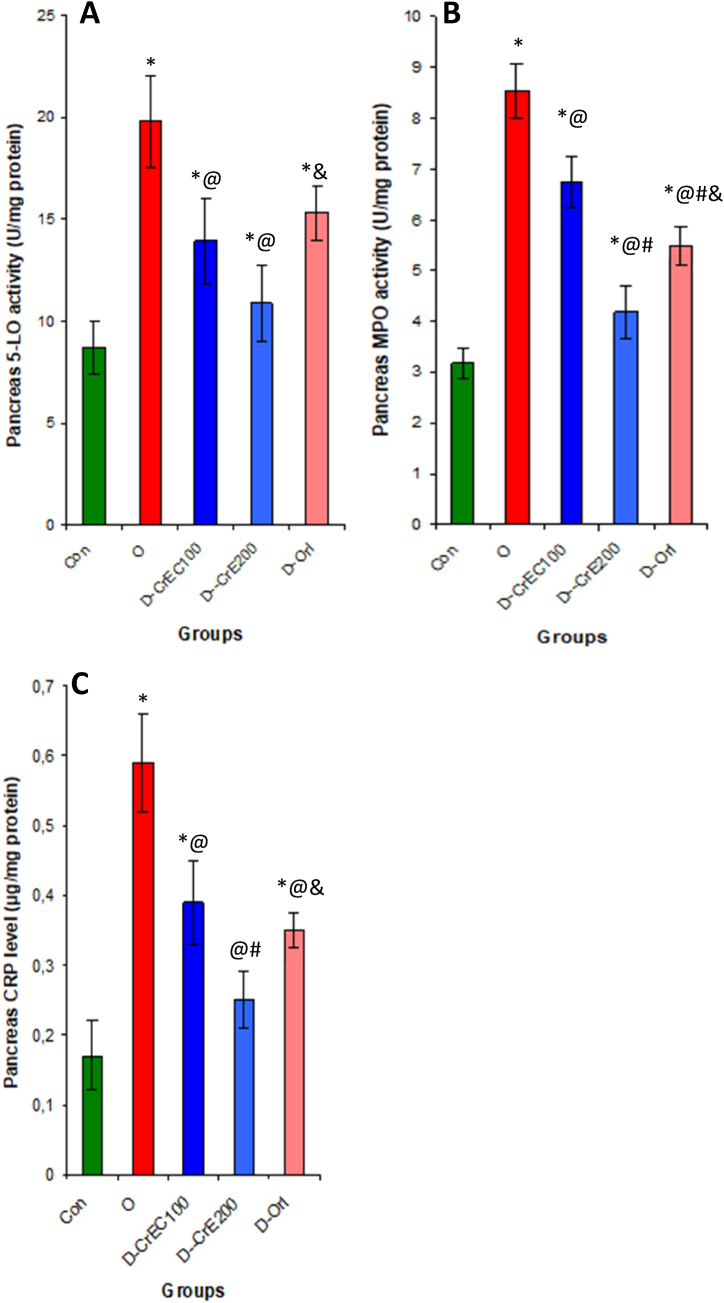
The impact of CRE on key enzymes associated with inflammation and pancreatitis in diabetic rats was observed in the pancreas of type 2 diabetic rats, which potentially exhibited elevated levels of CRP and increased activity of the enzymes myeloperoxidase (MPO) and hyaluronidase (HAase) compared to normal rats. It has been revealed that administering oral CrE supplementation at a dosage of 200 mg/kg bw effectively mitigates pancreas toxicity induced by type 2 diabetes and significantly protects the organ from inflammation and damage. This was evidenced by a significant reduction in the activities of inflammatory enzymes such as HAase and MPO by about 44 and 50% and the CRP level by 57%, respectively, in comparison to untreated rats (Fig. 4).
Fig. 4.
The influence of CrE consumption on pancreas inflammation indices, including pancreas 5-LO (A) and MPO (B) activities, as well as CRP levels (C), was examined. Our study outcomes reveal that the intake of CrE leads to a reduction in the activities of crucial enzymes associated with lymphocyte infiltration and inflammation in the pancreas. Detailed statistical analyses are presented in Table 3.
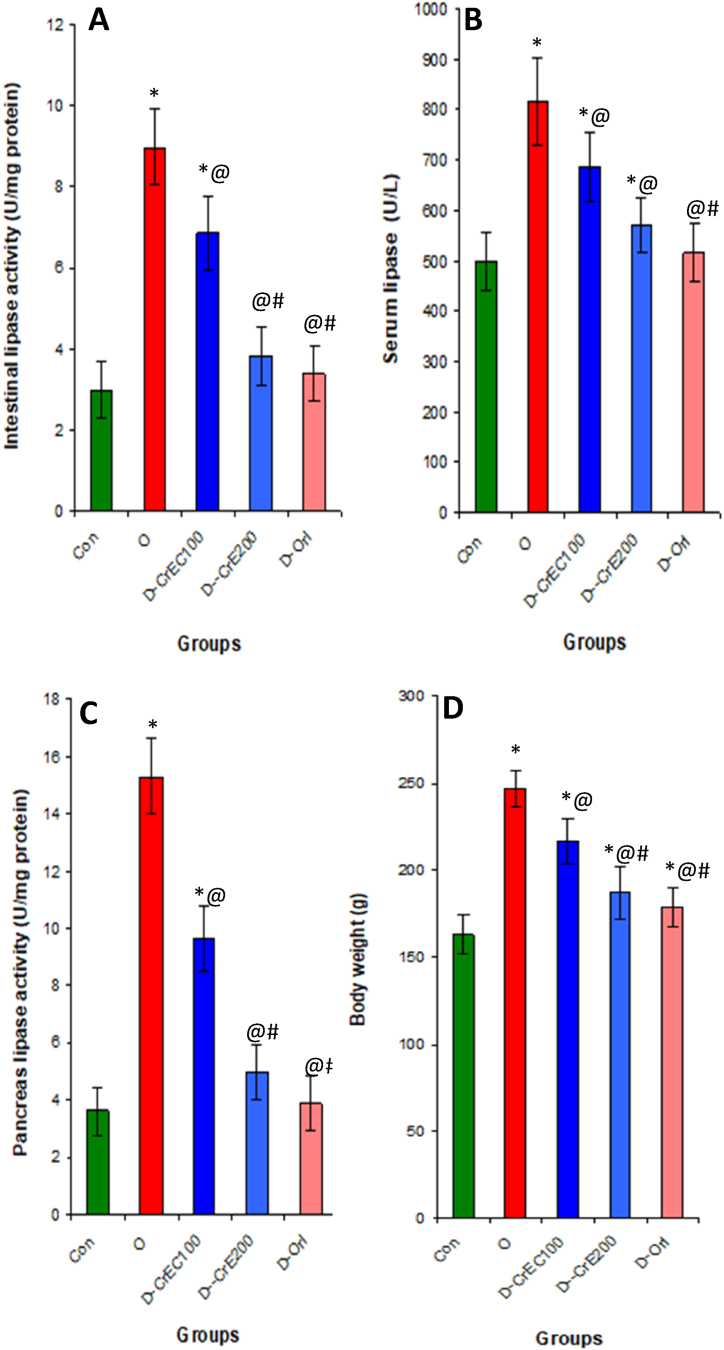
3.6. Effects of CrE on intestinal, pancreas and serum lipase activity, body weight gain, AFI and AWI
According to the results of our investigation, a significant correlation exists between a high-fat diet and the increased activity of lipase. This heightened lipase activity leads to a considerable elevation in body weight, abdominal fat index (AFI), and adipose weight index (AWI) due to the enhanced digestion and absorption of lipids. However, our research has demonstrated that the administration of CrE as a supplement can effectively impede lipase activity. This inhibition was observed in the intestines, serum, and pancreas, with a reduction of 57%, 30%, and 67% respectively, compared to obese rats that were not treated. As a result, this inhibition of lipase activity leads to a deceleration of lipid absorption, resulting in a decrease in body weight, AFI, and AWI by 24%, 16%, and 15% respectively (as shown in Table 3, Fig. 5).
Fig. 5.
Effect of CrE supplementation on intestinal (A), serum (B), and pancreatic (C) lipase activity and body weight (D). Indeed, this administration significantly inhibits the activity of this enzyme and subsequently suppresses the digestion and absorption of lipids at the intestinal level, inhibits the accumulation of lipids in the body, or even an anti-obesity activity. Statistical analyses as given in Table 3.
3.7. Obesity, α-amylase activity and CrE
Fig. 5 depicts a significant surge in the activity of intestinal, pancreatic, and serum α-amylase in obese rats, resulting in a corresponding increase of 238% in blood glucose levels compared to healthy rats. Conversely, when obese rats were given CrE, the activity of this digestive enzyme in the intestines, blood, and pancreas was notably diminished by approximately 45%, 53%, and 30% respectively (Fig. 6).
Fig. 6.
Impact of CrE supplementation on alpha-amylase activity in the intestines (A), serum (B) and pancreas (C) of obese rats. CrE drops-down the activity of this digestive enzyme and subsequently reduces the transformation of non-absorbable polysaccharides into absorbable monosaccharides by the intestines and this leads to a reduction in blood sugar. Statistical analyses as given in table 3.
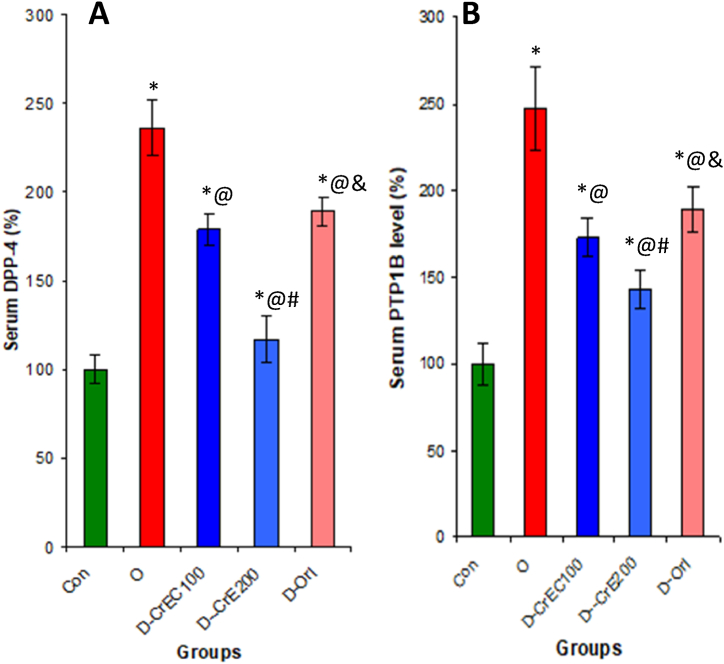
3.8. Effects of CrE on blood DPP-4 and PTP1B activities in serum of obese rat
The results of our study indicate that diabetes associated with obesity leads to a substantial increase in the activity of DPP-4 in the blood. This increase, in turn, results in the breakdown of GLP-1 or even the suppression of insulin signaling. Conversely, when obese rats were treated with CrE, a significant decrease of 50% in DPP-4 activity in the blood was observed compared to untreated obese rats. In addition, the current study reveals a correlation between obesity and the inhibition of insulin signaling pathways, as evidenced by a significant increase of 147% in PTP1B activity in the blood of obese rats compared to their healthy counterparts. However, administering CrE to obese rats resulted in a reversal of this activity by 42.1%, thereby stimulating insulin signaling pathways and preventing the onset of type 2 diabetes or insulin resistance (Fig. 7).
Fig. 7.
Effect of CrE administration on the activity of key enzymes of the insulin signaling pathway in serum, namely DPP-4 (A) and PTP1B (B). This inhibition increases the level of GLP-1 and the cascades of phosphorylation reactions leading to the degradation of glucose and subsequently anti-diabetic effects. Statistical analyses as given in Table 3.
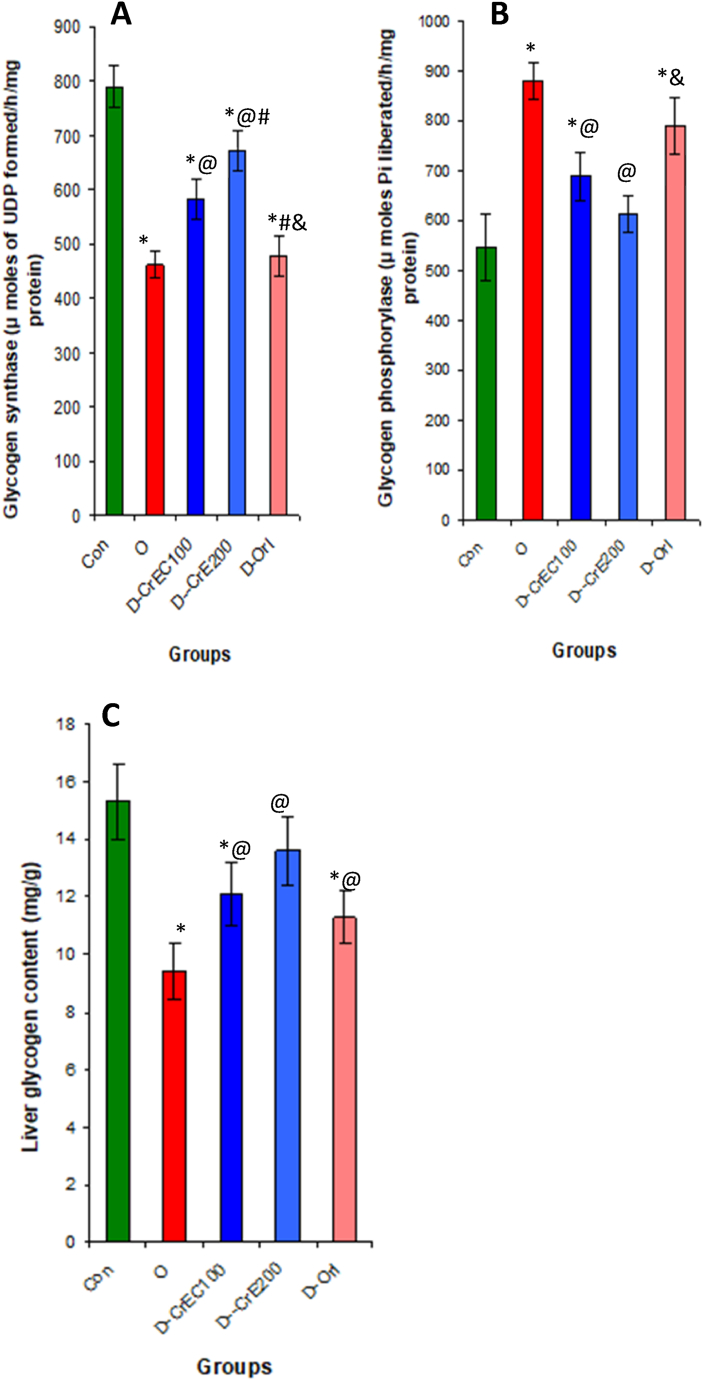
3.9. CrE ingestion, GP and GS activities, and liver glycogen level
Fig. 6 illustrates the correlation between obesity and the reduced activity of the enzyme GS, which plays a crucial role in glycogen synthesis. Additionally, it highlights the heightened activity of the enzyme GP, which responds to increased blood glucose levels, and the declining levels of glycogen in the liver. In comparison to obese rats that were not administered CrE, obese rats treated with CrE experienced a noteworthy 77% increase in hepatic glycogen levels. This notable increase can be attributed to the significant suppression of GP by 30% and the substantial elevation of GS by 45% at the hepatic level (Fig. 8).
Fig. 8.
Evaluation of the effect of CrE on the activity of GS (A), GP (B) and hepatic glycogen levels (C). An induction of glycogen synthesis by CrE was clearly observed. Statistical analyses as given in Table 3.
3.10. Obesity, CrE ingestion and liver key enzymes related to glucose anabolism and catabolism
Table 4 presents a depiction of the relationship between obesity and type 2 diabetes, highlighting the characteristic of reduced insulin activity. This diminished insulin activity triggers the activation of enzymes involved in the production of glucose, namely FBP and G6P, while simultaneously suppressing the activity of enzymes associated with glucose metabolism, such as HK, PK, and G6PD. Consequently, this imbalance leads to elevated blood glucose levels and a decrease in glycogen levels. Conversely, when rats were administered CrE, there was a notable increase in the activity of enzymes responsible for glucose breakdown, including HK, PK, and G6PD, which exhibited activity levels of 52%, 66%, and 130% respectively. Additionally, there was a decrease in the activity of enzymes involved in glucose production, specifically FBP and G6P, which displayed activity levels of 50% and 48% respectively (Table 3).
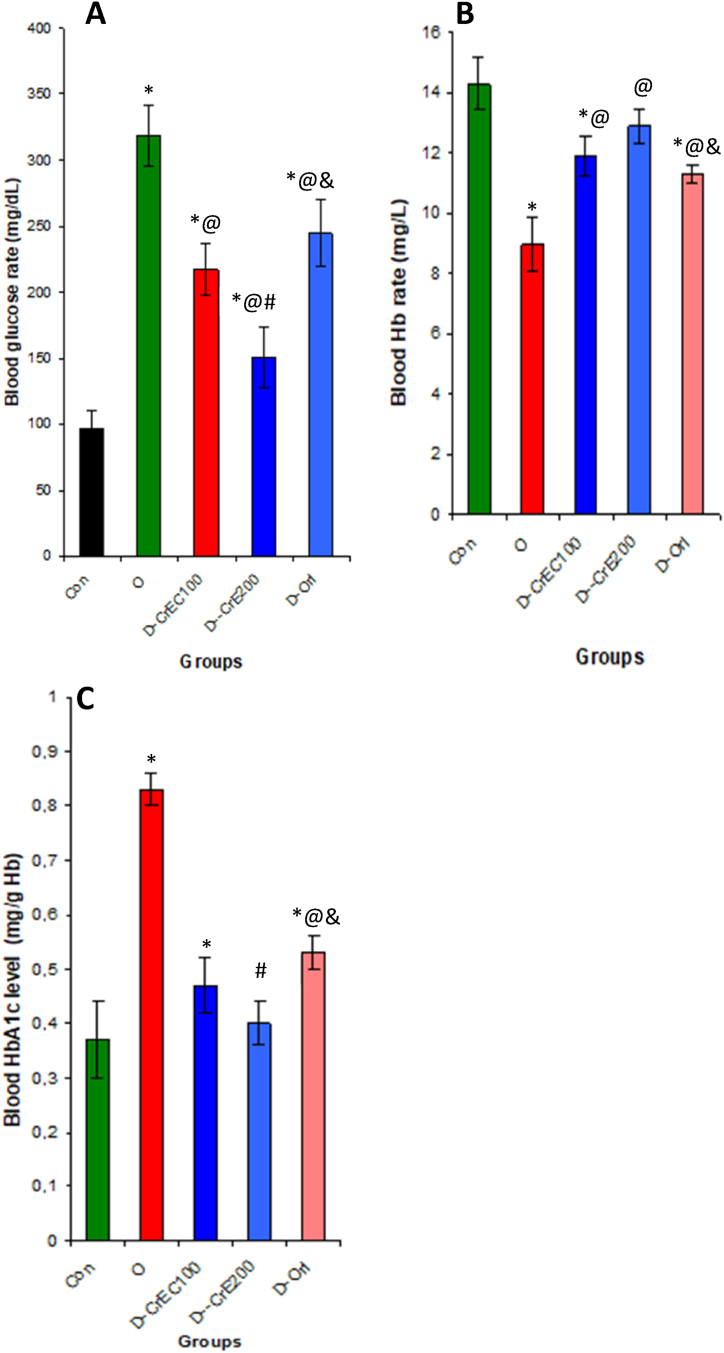
3.11. CrE ingestion, blood glucose, Hb and HbAc levels
Our results clearly show that the ingestion of CrE by obese rats suppresses glucose levels by 52%. This reduction in blood glucose levels leads to an increase in Hb levels and a reduction in Hb glycosylation, evidenced by a significant reduction in HbAc in the blood (Fig. 9).
Fig. 9.
Effect of obesity and CrE ingestion on blood glucose (A) and Hb (B) and HbAc (C) levels. An increase in blood sugar causes glycosylation of haemoglobin and subsequently an increase in HbAc and a reduction in total Hb in diabetics. On the contrary, a reduction in blood sugar leads to a reduction in glycosylated hemoglobin (HbAc) and an increase in free haemoglobin (Hb). Statistical analyses as given in Table 3.
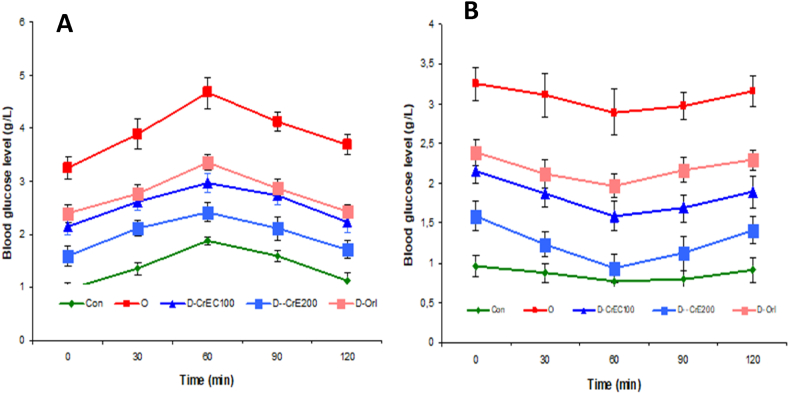
3.12. CrE ingestion, OGGT and ITT
Our results clearly show that the administration of a glucose solution at a concentration of 2 g/kg bw in normal rats is associated with a slight increase in blood sugar levels for 60 min followed by a return to the normal state (Fig. 10A). The injection of insulin causes a slow decrease in blood sugar (Fig. 10B) clearly shows the effectiveness of the homeostasis system in maintaining stable blood sugar levels. In obese people, supplementation with a glucose solution leads to a significant increase in blood sugar levels which goes from 3.25 g/l at t0 to 4.67 g/l 60 min later. after 60 min, there is a gradual and slow decrease in blood sugar, clearly showing a state of insulin resistance. Also, this study shows that the injection of insulin into these rats leads to a slow and weak decrease in glucose levels. In obese rats treated with CrE at a dose of 200 mg/kg bw, the administration of the glucose solution led to an increase in the blood glucose level from 1.59 g/l at t0 to a level of 2.42 g/l, 60min later. Also, the ingestion of CrE improves the effectiveness of insulin treatment, evidenced by a significant and significant reduction in blood sugar following the injection of this medication.
Fig. 10.
Effect of obesity and CrE ingestion blood glucose tolerance test (OGGT) (A) and insulin tolerance test (ITT) (B). Our results show that a HFFD diet induces a state of insulin resistance and CrE suppresses this state.
4. Discussion
This study investigated, for the first time, that CrE ingestion by obese rats associated with type 2 diabetes caused an improvement in insulin signaling through inhibition of DPP-4 and PTP- 1B; an inhibition of the digestive system responsible for the hydrolysis and absorption of lipids and carbohydrates, namely lipase and alpha-amylase, as well as the induction of catabolic enzymes and the inhibition of anabolic enzymes at the hepatic level. In addition, this functional food inhibits inflammation and oxidative stress at the pancreatic level and improves insulin activity, highlighted by OGGT and ITT.
In fact, Obesity is primarily caused by an increase in calorie intake, a decrease in physical activity, and various other factors such as oxidative stress and a high-fat-fructose diet. In fact, HFF diet enhanced functioning of these two digestive enzymes as lipase and α-amylase, which stimulate the breakdown of fats and carbohydrates within the intestines. This process leads to a notable increase in glucose and fats that accumulate in the body, resulting in a discernible rise in both body weight and blood sugar levels. Consequently, the development of obesity and type 2 diabetes occurs. Therefore, one of the most widely utilized approaches to combat obesity involves inhibiting fatty acid and monosaccharides absorption and activating the metabolism of fats and carbohydrates. The inhibitory effects of CrE on these enzymes can be attributed to the presence of various bioactive substances. The HPLC-DAD analysis of the ethanol extract obtained from Cyperus rotundus L. tubercules revealed the significant presence of constituents such as quercetin (47.8%), luteolin glucoside (17%), luteolin (7.56%), apigenin-7-glucoside (6.29%), and naringinin (4.52%) (Table 1). Various analytical techniques, including gas chromatography-mass spectrometry (GC-MS), high performance liquid chromatography (HPLC), and liquid chromatography-mass spectrometry (LC-MS), have been used to study the phytochemical analysis of CrE showed the presence of bioactive compounds such as quercetin and luteolin [57,58]. Consistent with our results, Sayed et al. [59] showed the presence of luteolin in Cyperus rotundus.
In obese rat, the results of this investigation indicate that obesity contributes to an increase in oxidative stress markers in the pancreas. Specifically, the levels of thiol groups decrease, while the levels of TBARS, protein carbonyls, and H2O2 rise. However, obese rats that received CrE through gastric gavage exhibited a significant protective effect against this oxidative stress. This was evident through a notable increase in thiol groups and a significant decrease in TBARS, protein carbonyls, and H2O2. These findings align with a study conducted by Jaikumkao et al. [60], which demonstrated that rats fed a high-fat diet experienced pancreatic injury and reduced kidney autophagy. Numerous studies have consistently shown that a long-term high-fat diet induces histopathological changes in the pancreas that closely resemble the early stages of type 2 diabetes, as well as obesity and insulin resistance [61,62]. In contrast, obese rats treated with CrE exhibited significant suppression of oxidative stress indices in the pancreas, highlighting the effectiveness of CrE in preventing oxidative stress and preserving pancreatic function. The rhizomes of Cyperus rotundus have been extensively studied and have been found to possess various medicinal properties, including antioxidants [63]. The high concentration of antioxidant substances, such as quercetin, luteolin glucoside, lutein, and apigenin-7-glucoside, contribute to their antioxidant activity. Similar to our findings, Oyedemi et al. [64] demonstrated that administering 25 mg/kg of quercetin to type 2 diabetic rats increased antioxidant activity in the pancreas by inducing SOD and CAT, enhancing reduced glutathione levels, and suppressing the rate of TBARS.
A persistent, low-grade pancreatic inflammatory state has been linked to obesity in previous research, which may contribute to insulin resistance and -cell dysfunction. Hermsdorff et al. [65] discovered a correlation between the accumulation of abdominal fat and levels of CRP and IL-6. Our study revealed that obesity led to an increase in key enzymes associated with inflammation in the pancreas, such as 5-LO and MPO activities, as well as CRP levels. However, the administration of CrE to obese rats suppressed this pancreatic inflammation. Interestingly, the administration of CrE to diabetic rats inhibited the recruitment of neutrophils to the pancreas and almost completely suppressed the infiltration of lymphocytes. This suppression was accompanied by a significant decrease in MPO and 5-LO activities, as well as CRP levels, indicating a reduction in pancreatic inflammation. Recent studies [[66], [67], [68]] have provided evidence that quercetin possesses the ability to hinder the synthesis of lipoxygenase (LOX) and cyclooxygenase (COX). Additionally, quercetin has demonstrated its potential to mitigate pancreatic histopathological damage in rats, along with reducing the levels of NF-κB, IL-1β, IL-6, and TNF-α mRNA and protein [69,70].
This study presented evidence that the supplementation of obese male rats with CrE resulted in a significant reduction in the activity of lipase in the intestine, serum, and pancreas. Lipase is an enzyme found in the brush-border surface membrane of intestinal cells and plays a crucial role in the digestion of lipids. The decrease in lipase activity suggests a decrease in the levels of absorbable free fatty acids and triglycerides, which ultimately leads to a decrease in body weight [33]. Analysis using HPLC-DAD revealed that CrE contains various bioactive molecules that are likely responsible for its anti-obesity effects. For instance, quercetin, which constitutes 47% of CrE, has been reported by Zhou et al. [71] to possess several pharmacological effects, including the prevention of insulin resistance, inhibition of α-amylase activity, stimulation of insulin secretion, and prevention of atherosclerosis. Others studies by Rivera et al. [72] and Barrios-Nolasco et al. [73] have shown that the ingestion of quercetin by obese rats resulted in reduced body weight, obesity, insulin resistance, dyslipidemia, and hypertension. Another bioactive molecule found in CrE is apigenin, a flavonoid present at a concentration of 6.29%. Gentile et al. [74] reported that the administration of apigenin to mice fed with a high-fat diet led to a decrease in body weight and epididymal fat weight, as well as reductions in blood total cholesterol, triglycerides, and glucose levels. Furthermore, apigenin has been found to exert anti-diabetic effects by decreasing the activity of enzymes responsible for insulin resistance and hepatic gluconeogenic enzymes, while also protecting against liver and kidney dysfunctions and toxicities [40].
Moreover, our study demonstrates that the administration of a CrE supplement significantly reduced the activity of α-amylase in the intestine, serum, and pancreas of obese rats. α-amylase is an enzyme found in the brush-border surface membrane of intestinal cells and plays a crucial role in carbohydrate digestion and hyperglycemia. It is believed that inhibiting the activity of these enzymes in the human digestive system can effectively manage diabetes by reducing the absorption of glucose derived from starch [33]. Furthermore, it has been demonstrated that the roots of C. rotundus possess antidiabetic properties in animal models. In rats with induced hyperglycemia using alloxan, an aqueous ethanol extract of C. rotundus significantly lowered blood glucose levels by inhibiting the activity of α-glucosidase [75]. Additionally, compounds such as quercetin, luteolin glucoside, luteolin, naringinin, and apigenin-7-glucoside found in C. rotundus have been reported to act as natural inhibitors of α-amylase [[76], [77], [78]]. One of the primary consequences of diabetes is the occurrence of oxidative stress. Exposure to oxidative stress directly affects insulin signaling and glucose transport in the liver and skeletal muscle, leading to decreased functionality.
The present study has demonstrated that CrE exhibits the potential to inhibit DPP-4 and PTP1B in vitro, with IC50 values of 23.9 and 51.9 μg/ml, respectively. Furthermore, CrE effectively suppresses the activity of lipase and α-amylase, with IC50 values of 67 and 83 μg/ml, respectively. In obese rats, the ingestion of CrE potentially supressed serum DPP-4 and PTP1B. According of our results, previous research has indicated that chronic consumption of a high-fat diet and the activation of the ER-stress response, whether through acute or pharmacological means, lead to an increase in both PTP1B mRNA expression and activity [39,79], and conseuquently decline of glucagon-like peptide-1 (GLP-1) level. Furthermore, the gastrointestinal hormone as GLP-1 known as plays a crucial role in regulating insulin secretion and glucose metabolism in the gut. However, the enzyme dipeptidyl peptidase-4 (DPP-4) inhibits the effectiveness of GLP-1, causing its levels to decrease to suboptimal levels in individuals with diabetes mellitus. DPP-4 rapidly degrades GLP-1, thereby shortening its lifespan. In obese and diabetic individuals, inhibiting DPP-4 can elevate the levels of GLP-1, which in turn stimulates insulin secretion and chute-down the breakdown of fats and carbohydrates. According to the findings of this study, it has been observed that the bioactive compounds present in the tubers of Cyperus rotundus L have the ability to inhibit DPP-4 in the serum of obese rats by 50% when compared to obese rats that were not treated. This inhibition leads to an improvement in the levels of the intestinal hormone GLP-1. As a result, there is an acceleration in the activity of phosphatidylinositol (PI) 3-kinase (PI–3K), which in turn enhances insulin biosynthesis and cell proliferation. Consequently, this leads to a decrease in both body weight and blood glucose levels, as demonstrated in our study. In a similar vein, Ideta et al. [80] have also reported that a combination of a high-fat diet (HFD) and DPP-4 inhibitor therapy can reduce hepatic lipogenesis by upregulating the genes involved in lipogenesis and activating AMPK. These findings align with the research conducted by Iqbal et al. [81], who discovered that Cyperus has the ability to inhibit α-glucosidase and dipeptidyl peptidase-4 (DPP-4). The potential inhibitory effect against DPP-4 observed in this study can be attributed to the presence of bioactive compounds in CrE. These compounds, such as quercetin [82], apigenin-7-glucoside [83] and naringenin [83], have been shown to inhibit the activity of DPP-4.
It is worth noting that blocking the enzyme PTP1B activates the insulin signaling pathway, leading to more efficient carbohydrate utilization and the activation of glycogen synthesis. Consequently, inhibiting both PTP1B and DPP-4 is considered a potential therapeutic strategy for enhancing insulin sensitivity. In the current study, it was demonstrated that a 12-week high-fat and high-fructose (HFF) diet significantly increased the levels of serum PTP1B and DPP-4, resulting in insulin resistance. This, in turn, led to an increase in the activities of lipase and α-amylase. However, in obese rats, the administration of 200 mg/kg of CrE resulted in a significant reduction of PTP1B levels in the serum by 42% compared to untreated obese rats. This inhibition of PTP1B may lead to an increase in beta cell mass, ultimately resulting in enhanced release of insulin in response to glucose stimulation. Consequently, there is a notable decrease in the activities of lipase and α-amylase, leading to a decrease in body weight and regulation of blood glucose levels. It is widely recognized that PTP1B activates extracellular signal-regulated protein kinase 1/2 (ERK1/2). Additionally, AKT phosphorylation plays a crucial role in the survival of β-cells and the degradation of proapoptic proteins. Various bioactive substances present in CrE are believed to contribute to the inhibition of PTP1B. Quercetin, which constitutes 47% of CrE, has been shown to inhibit PTP1B both in vivo and in vitro, as demonstrated by the research conducted by Nasrollahi et al. [84] and Almasri et al. [85]. Furthermore, luteolin and apigenin, which are also present in CrE, have been identified as PTP1B inhibitors in previous studies [71,[72], [86]].
Our study discovered that diabetes enhances GP while reducing GS activities. Nevertheless, the supplementation of CRE at a dose of 200 mg/kg bw daily promotes GS activity and suppresses GP activity at the hepatic level. This results in increased hepatic glycogen levels and decreased circulating glucose levels. In fact, type 2 diabetes is a medical condition characterized by impaired insulin secretion from pancreatic β-cells, leading to increased hepatic glucose production. The liver plays a crucial role in maintaining blood glucose homeostasis through the activity of key hepatic enzymes involved in carbohydrate metabolism, such as GP and GS. In individuals without diabetes, insulin suppresses GP and stimulates GS, ensuring hepatic glycogen synthesis and stable blood glucose levels. Srivastava et al. [87] showed that the constituents of C. rotundus rhizomes as quercetin and phenolic acids increase insulin secretion; and consequently increase in glycogen rate. In fact, C. rotundus flavonoids have been shown to repair damaged cells in diabetic rats administered alloxan.
The enzyme HK plays a vital role in regulating carbohydrate metabolism. The administration of alloxan has been found to cause significant damage to pancreatic beta cells, leading to decreased circulating insulin levels and subsequent inactivation of hepatic HK. This impairment leads to a reduction in glycolysis, ultimately causing a significant increase in hyperglycemia. Previous research studies have shown a correlation between decreased insulin levels and altered HK, PK and G6PD activities, which aligns with our findings. In contrast, our study observed a stimulation of hepatic HK, PK and G6PD activities in diabetic rats treated with CRE. This effect is attributed to the anti-inflammatory activity of CRE at the pancreatic level, which promotes the regenerative effect of pancreatic β-cells. Furthermore, the administration of CRE to diabetic rats resulted in an increase in hepatic activity of HK, PK and G6PD, stimulating glucose metabolism and energy production. Previous research findings have consistently supported our results, which indicate that consuming substances that enhance the functioning of glycolytic enzymes like HK, PK and G6PD can have anti-diabetic effects. Our study further demonstrates that diabetes leads to an increase in G6P and FBP activities, which in turn hampers carbohydrate metabolism and insulin secretion by β-cells. This mechanism directly contributes to the observed decline in insulin secretion. However, the administration of CRE has been shown to inhibit these enzymes responsible for glycogenesis and hyperglycemia, resulting in the suppression of insulin secretion and the activation of glycolysis enzymes. Ultimately, this leads to the normalization of blood glucose levels.
Glycosylated hemoglobin (HbA1C), which accounts for 4–6% of hemoglobin in healthy individuals, is largely composed of HbA1 [88]. Measuring glycosylated hemoglobin is a common practice for long-term blood glucose control. Studying glycosylated hemoglobin is more important for diabetes diagnosis than fasting blood glucose [89]. The amount of glycosylated hemoglobin indicates the average blood glucose during the preceding few weeks. In this study, an increase in HbA1C by 124% was observed as compared to healthy rat confirms type 2 diabetes. In obese rat treated with CrE, a protective effect against hyperglycemia by by different mechanisms, namely 1) protection of the pancreas against oxidative stress and inflammation and subsequently more circulating active insulin; 2) inhibition of key enzymes responsible for hyperglycemia and hyperlipidemia; 3) activation and stimulation of the insulin signaling pathway by inhibition of the activity of DPP-4, PTP1-B; and GP and activation of GS and finally activation of catabolism and inhibition of carbohydrate anabolism. All of these mechanisms cause blood glucose levels similar to those in healthy rats; and therefore, a normal HbA1C level. Despite our study suggest eating Cyperus rotundus reduces fat and type 2 diabetes by increasing metabolism and inhibiting digestive enzymes like lipase and amylase. There are certain restrictions on this study, though.
-
1.
It would be interesting to test these findings with a clinical trial as well.
-
2.
Additional research is necessary to identify the active medications that have anti-obesity and anti-type 2 diabetes properties.
5. Conclusion
The primary objective of the present study was to investigate the impact of administering a specific extract obtained from the tubers of Cyperus rotundus L (CrE) on obesity, type 1 diabetes by the inhibition of the digestives enzymes in the intestine as α-amylase and lipase; and also stimulation of carbohydrate catabolism by the inhibition of DPP-4 and PTP-B activities. The outcomes of this research endeavour hold the potential to make significant contributions towards the advancement of innovative natural remedies for the prevention and management of obesity and type 2 diabetes.
Funding
This research has been funded by the Deputy for Research & Innovation, Ministry of Education through Initiative of Institutional Funding at University of Ha'il Saudi Arabia through project number RG–23 065.
Data availability statement
The data utilized to underpin the findings of this study can be obtained from the corresponding author upon request.
CRediT authorship contribution statement
Faiza I.A. Abdella: Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Amani Toumi: Validation, Software, Resources. Sarra Boudriga: Visualization, Validation. Tahani Y.A. Alanazi: Visualization, Validation, Software, Resources, Methodology. Asma K. Alshamari: Validation, Software, Resources, Funding acquisition, Formal analysis, Data curation. Ahlam Abdulrahman Alrashdi: Writing – original draft, Visualization, Validation, Supervision. Khaled Hamden: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Acknowledgments
We would like to acknowledge the support provided by the Deputy for Research & Innovation, Ministry of Education through Initiative of Institutional Funding at University of Ha'il Saudi Arabia through project number RG–23 065.
Contributor Information
Faiza I.A. Abdella, Email: fai.ahmed@uoh.edu.sa.
Amani Toumi, Email: amani.toumi@univ-lorraine.fr.
Sarra Boudriga, Email: sarra.boudriga@fsm.rnu.tn.
Tahani Y.A. Alanazi, Email: ty.alanazi@uoh.edu.sa.
Asma K. Alshamari, Email: ak.alshamari@uoh.edu.sa.
Ahlam Abdulrahman Alrashdi, Email: a.alrshedi@uoh.edu.sa.
Khaled Hamden, Email: khaled.hamden@issatmh.rnu.tn.
References
- 1.Mohajan D., Mohajan H.K. Obesity and its related diseases: a new Escalating Alarming in global health. J. Int. Med. Res. 2023;2:12–23. [Google Scholar]
- 2.da Rocha Fernandes J., Ogurtsova K., Linnenkamp U., Guariguata L., Seuring T., Zhang P., et al. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res. Clin. Pract. 2016;117:48–54. doi: 10.1016/j.diabres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Iwara I.A., Mboso E.O., Ibor O.R., Elot K., Igajah C., Bassey A.A., et al. Modulatory effects of extract of Heinsia crinita against fructose/streptozotocin-induced oxidative stress in diabetic rat models. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e21308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C., Wang W., Gu J. Targeting ferroptosis: new perspectives of Chinese herbal medicine in the treatment of diabetes and its complications. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma N., Yu X., Yang T., Zhao Y., Li H. A hypoglycemia early alarm method for patients with type 1 diabetes based on multi-dimensional sequential pattern mining. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekakitie L.I., Oyinloye B.E., Ajiboye B.O. The ameliorative activity of Chrysobalanus orbicularis in streptozotocin-induced type II diabetes mellitus rat model. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrivastava S., Sharma A., Saxena N., Bhamra R., Kumar S. Addressing the preventive and therapeutic perspective of berberine against diabetes. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhen D., Ding L., Wang B., Wang X., Hou Y., Ding W., et al. Oral administration of kynurenic acid delays the onset of type 2 diabetes in Goto-Kakizaki rats. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e17733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Njan A.A., Adenuga F.O., Ajayi A.M., Sotunde O., Ologe M.O., Olaoye S.O., et al. Neuroprotective and memory-enhancing effects of methanolic leaf extract of Peristrophe bicalyculata in rat model of type 2 diabetes mellitus. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidarianpour A., Keshvari M., Shahidi S., Zarei M. Modulation of GPC-4 and GPLD1 serum levels by improving glycemic indices in type 2 diabetes: resistance training and hawthorn extract intervention. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soares Andrade C.A., Shahin B., Dede O., Akpeji A.O., Ajene C., Albano Israel F.E., et al. The burden of type 2 diabetes mellitus in states of the European Union and United Kingdom at the national and subnational levels: a systematic review. Obes. Rev. 2023;24 doi: 10.1111/obr.13593. [DOI] [PubMed] [Google Scholar]
- 12.Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183 doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magliano D.J., Boyko E.J., Atlas I.D.F.D. tenth ed. International Diabetes Federation; 2021. What Is Diabetes? IDF DIABETES ATLAS [Internet. [Google Scholar]
- 14.Fallatah A.H.I. 2020. Exploring Type Two Diabetes Mellitus in the Kingdom of Saudi Arabia: Studying the Socio-Economic Environment. [Google Scholar]
- 15.Albarakat M., Guzu A. Prevalence of type 2 diabetes and their complications among home health care patients at Al-Kharj military industries corporation hospital. J. Fam. Med. Prim. Care. 2019;8:3303. doi: 10.4103/jfmpc.jfmpc_634_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AlOtaibi A.A., Almesned M., Alahaideb T.M., Almasari S.M., Alsuwayt S.S. Assessment of diabetes-related distress among type 2 diabetic patients, Riyadh, Saudi Arabia. J. Fam. Med. Prim. Care. 2021;10:3481. doi: 10.4103/jfmpc.jfmpc_488_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsaidan A.A., Ghoraba M. Awareness of diabetic retinopathy among patients with type 2 diabetes mellitus in primary health care in security forces hospital Riyadh, Saudi Arabia. J. Fam. Med. Prim. Care. 2019;8:2433. doi: 10.4103/jfmpc.jfmpc_324_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamed T., Souiy Z., Achour L., Hamden K. Anti-obesity, anti-hyperglycaemic, anti-antipyretic and analgesic activities of Globularia alypum extracts. Arch. Physiol. Biochem. 2022;128:1453–1460. doi: 10.1080/13813455.2020.1773865. [DOI] [PubMed] [Google Scholar]
- 19.Tiss M., Souiy Z., Abdeljelil N ben, Njima M., Achour L., Hamden K. Fermented soy milk prepared using kefir grains prevents and ameliorates obesity, type 2 diabetes, hyperlipidemia and Liver-Kidney toxicities in HFFD-rats. J. Funct.Foods. 2020;67 doi: 10.1016/J.JFF.2020.103869. [DOI] [Google Scholar]
- 20.Hariftyani A.S., Kurniawati L.A., Khaerunnisa S., Veterini A.S., Setiawati Y., Awaluddin R. In silico analysis of potential antidiabetic phytochemicals from Matricaria chamomilla L. against ptp1b and aldose reductase for type 2 diabetes mellitus and its complications. Nat. Prod. Sci. 2021;27:99–114. [Google Scholar]
- 21.You H., Zhang Y., Wu T., Li J., Wang L., Yu Z., et al. Identification of dipeptidyl peptidase IV inhibitory peptides from rapeseed proteins. LWT. 2022;160 doi: 10.1016/j.lwt.2022.113255. [DOI] [Google Scholar]
- 22.Tiss M., Souiy Z., Achour L., Hamden K. Ephedra alata extracts exerts anti-obesity, anti-hyperglycemia, anti-antipyretic and analgesic effects. Nutr. Food Sci. 2022;52:119–128. [Google Scholar]
- 23.Tiss M., Hamden K. Globularia alypum extracts Attenuate hyperglycemia and protect against various organ toxicities in alloxan-induced experimental diabetic rats. Evidence-Based Complement Altern Med. 2022;2022 doi: 10.1155/2022/6816942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldhafiri A.J. Pharmacotherapeutic options for the management of obesity in children. J Adv Biomed Pharm Sci. 2023;6:223–227. [Google Scholar]
- 25.Aydin B., Onbasi K. Lipase inhibitor orlistat: an old but still effective weapon. Med. Sci. 2021;10:1406–1411. [Google Scholar]
- 26.Abdel-Mohsen D.M., Akabawy A., El-Khadragy M.F., Abdel Moneim A.E., Amin H.K. Green coffee bean extract potentially ameliorates liver injury due to HFD/STZ-induced diabetes in rats. J. Food Biochem. 2023:2023. [Google Scholar]
- 27.Safaei R., Sakhaee K., Saberifar M., Fadaei M.S., EdalatJoo S., Fadaei M.R., et al. Mechanistic Insights into the Xanthones present in mangosteen fruit (Garcinia mangostana) and their applications in diabetes and related complications. J. Food Biochem. 2023:2023. [Google Scholar]
- 28.Vajdi M., Hassanizadeh S., Shojaei-Zarghani S., Bagherniya M., Askari G. The effects of purslane consumption on obesity indices: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. J. Food Biochem. 2023:2023. [Google Scholar]
- 29.Pan R., Yuan J., Bai J., Zhang J., Zhang J., Gu Y., et al. Antiobesity effect of lactiplantibacillus plantarum fermented barley extracts via the interactions with gut microbiota of the obese adult humans. J. Food Biochem. 2023:2023. [Google Scholar]
- 30.Nikrad N., Mokhtari Ardekani A., Farhangi M.A., Zahiri Tousi A., Fard Tabrizi F Pourteymour, Vaezi M., et al. The effect of thylakoid membranes of spinach extract supplementation on atherogenic, glycemic, and anthropometric indices and renal function in obese PCOS women under a hypo-caloric diet: a randomized, double-blind, controlled trial. J. Food Biochem. 2023:2023. [Google Scholar]
- 31.Saad F., Al-Shaikh T.M., Zouidi F., Taher M.A., Saidi S.A., Hamden K. Betalain-enriched beetroots exhibit antiulcer and anti-inflammatory potentials. J. Food Process. Preserv. 2023;2023 doi: 10.1155/2023/9522830. [DOI] [Google Scholar]
- 32.Abdelkader Saidi S., Al-Shaikh T.M., Hamden K. Evaluation of gastroprotective effect of betalain-rich ethanol extract from opuntia stricta var. Dillenii employing an in vivo rat model. J. Food Qual. 2023:2023. [Google Scholar]
- 33.Hamden K., Boujibiha M.A., Ben Abdeljelil N., Njima M., Selmi B., Achour L. Phytoestrogens inhibit key-enzymes linked to obesity, type 2 diabetes and liver-kidney toxicity in high fructose-fat diet in mice. Arch. Physiol. Biochem. 2019;125:423–429. doi: 10.1080/13813455.2018.1479427. [DOI] [PubMed] [Google Scholar]
- 34.Kamala A., Middha S.K., Karigar C.S. Plants in traditional medicine with special reference to Cyperus rotundus L.: a review. 3 Biotech. 2018;8:309. doi: 10.1007/s13205-018-1328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azimi A., Ghaffari S.M., Riazi G.H., Arab S.S., Tavakol M.M., Pooyan S. α-Cyperone of Cyperus rotundus is an effective candidate for reduction of inflammation by destabilization of microtubule fibers in brain. J. Ethnopharmacol. 2016;194:219–227. doi: 10.1016/j.jep.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 36.Amro M.S., Teoh S.L., Norzana A.G., Srijit D. The potential role of herbal products in the treatment of Parkinson's disease. Clin. Ter. 2018;169:e23–e33. doi: 10.7417/T.2018.2050. [DOI] [PubMed] [Google Scholar]
- 37.Badgujar S.B., Bandivdekar A.H. Evaluation of a lactogenic activity of an aqueous extract of Cyperus rotundus Linn. J. Ethnopharmacol. 2015;163:39–42. doi: 10.1016/j.jep.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Wang R., Zhao H., Pan X., Orfila C., Lu W., Ma Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019;7:1848–1856. doi: 10.1002/fsn3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen P.-H., Zhao B.-T., Ali M.Y., Choi J.-S., Rhyu D.-Y., Min B.-S., et al. Insulin-mimetic selaginellins from Selaginella tamariscina with protein tyrosine phosphatase 1B (PTP1B) inhibitory activity. J. Nat. Prod. 2015;78:34–42. doi: 10.1021/np5005856. [DOI] [PubMed] [Google Scholar]
- 40.Saidi S.A., Al-Shikh T.M., Hamden K. Ephedra alata subsp. alenda (Ephedraceae) leaf extracts: phytochemical screening, anti-diabetic, anti-obesity and anti-toxic activities on diabetic-induced liver-kidney-testes toxicities and inhibition of α-amylase and lipase enzymes. Heliyon. 2022 doi: 10.1016/j.heliyon.2022.e11954. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Oraon R., Mahmood T.A., Shamim A., Ahsan F., Shariq M., Parveen S., et al. Antihyperlipidemic and antiobesity potential of aquilaria agallocha and borago officinalis in fixed-dose combination; A contingent probe with atorvastatin and orlistat. Curr. Bioact. Compd. 2021;17:22–34. [Google Scholar]
- 42.Dallali D., Fakhfakh J., Paris C., Hamden K., Varbanov M., Allouche N. Fructooligosaccharides from Cynoglossum tubiflorus: effect of the molecular size on their antidiabetic activity in high-fat diet and alloxan induced diabetic rats. Bioorg. Chem. 2024 doi: 10.1016/j.bioorg.2024.107100. [DOI] [PubMed] [Google Scholar]
- 43.Yang H.-J., Zhang T., Wu X.-G., Kim M.-J., Kim Y.-H., Yang E.-S., et al. Aqueous blackcurrant extract improves insulin sensitivity and secretion and modulates the gut microbiome in non-obese type 2 diabetic rats. Antioxidants. 2021;10:756. doi: 10.3390/antiox10050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reznick A.Z. Packer LBT-M in E. [38] Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Oxyg. Radicals Biol. Syst. Part C. 1994;233:357–363. doi: 10.1016/S0076-6879(94)33041-7. Academic Press. [DOI] [PubMed] [Google Scholar]
- 45.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 46.Buege J.A., Aust S.D. [30] Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. Elsevier. [DOI] [PubMed] [Google Scholar]
- 47.Dingeon B., Ferry J.P., Roullet A. Automatic assay of blood sugar by Trinder's method. Ann. Biol. Clin. 1975;33:3–13. [PubMed] [Google Scholar]
- 48.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 49.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 50.Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. Elsevier. [DOI] [PubMed] [Google Scholar]
- 51.Meurer M., de Oliveira B.M.M., Cury B.J., Jerônimo D.T., Venzon L., França T.C.S., et al. Extract of Tagetes erecta L., a medicinal plant rich in lutein, promotes gastric healing and reduces ulcer recurrence in rodents. J. Ethnopharmacol. 2022;293 doi: 10.1016/j.jep.2022.115258. [DOI] [PubMed] [Google Scholar]
- 52.Wu H. Affecting the activity of soybean lipoxygenase-1. J. Mol. Graph. 1996;14:331–337. doi: 10.1016/s0263-7855(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 53.Cornblath M., Randle P.J., Parmeggiani A., Morgan H.E. Regulation of glycogenolysis in muscle: effects of glucagon and anoxia on lactate production, glycogen content, and phosphorylase activity in the perfused isolated rat heart. J. Biol. Chem. 1963;238:1592–1597. [PubMed] [Google Scholar]
- 54.Leloir L.F., Goldemberg S.H. [14] Glycogen synthetase from rat liver:(Glucose) n+ (UDPG)→(Glucose) n+ 1+ UDP. Methods Enzymol. 1962;5:145–147. Elsevier. [Google Scholar]
- 55.Hamden K., Jaouadi B., Salami T., Carreau S., Bejar S., Elfeki A. Modulatory effect of fenugreek saponins on the activities of intestinal and hepatic disaccharidase and glycogen and liver function of diabetic rats. Biotechnol. Bioproc. Eng. 2010;15 doi: 10.1007/s12257-009-3159-0. [DOI] [Google Scholar]
- 56.Almasri I.M., Mohammad M.K., Taha M.O. Inhibition of dipeptidyl peptidase IV by fexofenadine: virtual screening study. J. Appl. Pharmaceut. Sci. 2019;9:28–32. [Google Scholar]
- 57.Kilani-Jaziri S., Neffati A., Limem I., Boubaker J., Skandrani I., Sghair M Ben, et al. Relationship correlation of antioxidant and antiproliferative capacity of Cyperus rotundus products towards K562 erythroleukemia cells. Chem. Biol. Interact. 2009;181:85–94. doi: 10.1016/j.cbi.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Beldar G., Somani S., Rode A. Development and evaluation of Cyperus rotundus based hair removal cream. Int J Pharm Sci. 2024;2:1. [Google Scholar]
- 59.Sayed H.M., Mohamed M.H., Farag S.F., Mohamed G.A., Proksch P. A new steroid glycoside and furochromones from Cyperus rotundus L. Nat. Prod. Res. 2007;21:343–350. doi: 10.1080/14786410701193056. [DOI] [PubMed] [Google Scholar]
- 60.Jaikumkao K., Promsan S., Thongnak L., Swe M.T., Tapanya M., Htun K.T., et al. Dapagliflozin ameliorates pancreatic injury and activates kidney autophagy by modulating the AMPK/mTOR signaling pathway in obese rats. J. Cell. Physiol. 2021;236:6424–6440. doi: 10.1002/jcp.30316. [DOI] [PubMed] [Google Scholar]
- 61.Tirkes T., Jeon C.Y., Li L., Joon A.Y., Seltman T.A., Sankar M., et al. Association of pancreatic steatosis with chronic pancreatitis, obesity, and type 2 diabetes mellitus. Pancreas. 2019;48:420. doi: 10.1097/MPA.0000000000001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khatua B., El-Kurdi B., Singh V.P. Obesity and pancreatitis. Curr. Opin. Gastroenterol. 2017;33:374. doi: 10.1097/MOG.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bezerra J.J.L., Pinheiro A.A.V. Traditional uses, phytochemistry, and anticancer potential of Cyperus rotundus L.(Cyperaceae): a systematic review. South Afr. J. Bot. 2022;144:175–186. [Google Scholar]
- 64.Oyedemi S.O., Nwaogu G., Chukwuma C.I., Adeyemi O.T., Matsabisa M.G., Swain S.S., et al. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: in silico studies of molecular interaction of quercetin with hexokinase and catalase. J. Food Biochem. 2020;44 doi: 10.1111/jfbc.13127. [DOI] [PubMed] [Google Scholar]
- 65.Hermsdorff H.H.M., Zulet M.Á., Puchau B., Martínez J.A. Central adiposity rather than total adiposity measurements are specifically involved in the inflammatory status from healthy young adults. Inflammation. 2011;34:161–170. doi: 10.1007/s10753-010-9219-y. [DOI] [PubMed] [Google Scholar]
- 66.Li Y., Yao J., Han C., Yang J., Chaudhry M.T., Wang S., et al. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhaskar S., Kumar K.S., Krishnan K., Antony H. Quercetin alleviates hypercholesterolemic diet induced inflammation during progression and regression of atherosclerosis in rabbits. Nutrition. 2013;29:219–229. doi: 10.1016/j.nut.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 68.Lesjak M., Beara I., Simin N., Pintać D., Majkić T., Bekvalac K., et al. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct.Foods. 2018;40:68–75. [Google Scholar]
- 69.Xiong G., Ji W., Wang F., Zhang F., Xue P., Cheng M., et al. Quercetin inhibits inflammatory response induced by LPS from Porphyromonas gingivalis in human gingival fibroblasts via suppressing NF-κB signaling pathway. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/6282635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng S.-C., Huang W.-C., Pang J-H S., Wu Y.-H., Cheng C.-Y. Quercetin inhibits the production of IL-1β-induced inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int. J. Mol. Sci. 2019;20:2957. doi: 10.3390/ijms20122957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou J.-F., Wang W.-J., Yin Z.-P., Zheng G.-D., Chen J.-G., Li J.-E., et al. Quercetin is a promising pancreatic lipase inhibitor in reducing fat absorption in vivo. Food Biosci. 2021;43 doi: 10.1016/j.fbio.2021.101248. [DOI] [Google Scholar]
- 72.Rivera L., Morón R., Sánchez M., Zarzuelo A., Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity. 2008;16:2081–2087. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 73.Barrios-Nolasco A., Domínguez-López A., Miliar-García A., Cornejo-Garrido J., Jaramillo-Flores M.E. Anti-inflammatory effect of ethanolic extract from tabebuia rosea (bertol.) DC., quercetin, and anti-obesity drugs in adipose tissue in wistar rats with diet-induced obesity. Molecules. 2023;28:3801. doi: 10.3390/molecules28093801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gentile D., Fornai M., Colucci R., Pellegrini C., Tirotta E., Benvenuti L., et al. The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deng S., Xia L., Zhu X., Zhu J., Cai M., Wang X. Natural alpha-glucosidase inhibitors rapid fishing from Cyperus rotundus using immobilized enzyme affinity screening combined with UHPLC-QTOF MS. Iran J Pharm Res IJPR. 2019;18:1508. doi: 10.22037/ijpr.2019.1100753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ćorković I., Gašo-Sokač D., Pichler A., Šimunović J., Kopjar M. Dietary polyphenols as natural inhibitors of α-amylase and α-glucosidase. Life. 2022;12:1692. doi: 10.3390/life12111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proença C., Ribeiro D., Freitas M., Fernandes E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: a review. Crit. Rev. Food Sci. Nutr. 2022;62:3137–3207. doi: 10.1080/10408398.2020.1862755. [DOI] [PubMed] [Google Scholar]
- 78.Zhu J., Chen C., Zhang B., Huang Q. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit. Rev. Food Sci. Nutr. 2020;60:695–708. doi: 10.1080/10408398.2018.1548428. [DOI] [PubMed] [Google Scholar]
- 79.Al-Qabbaa S.M., Qaboli S.I., Alshammari T.K., Alamin M.A., Alrajeh H.M., Almuthnabi L.A., et al. Sitagliptin mitigates diabetic nephropathy in a rat model of streptozotocin-induced type 2 diabetes: possible role of PTP1B/JAK-STAT pathway. Int. J. Mol. Sci. 2023;24:6532. doi: 10.3390/ijms24076532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ideta T., Shirakami Y., Miyazaki T., Kochi T., Sakai H., Moriwaki H., et al. The dipeptidyl peptidase-4 inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK activation in non-alcoholic fatty liver disease model mice. Int. J. Mol. Sci. 2015;16:29207–29218. doi: 10.3390/ijms161226156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choudhary M.I., Azizuddin Jalil S., Nawaz S.A., Khan K.M., Tareen R.B. Antiinflammatory and lipoxygenase inhibitory compounds from vitex agnus‐castus. Phyther Res An Int J Devoted to Pharmacol Toxicol Eval Nat Prod Deriv. 2009;23:1336–1339. doi: 10.1002/ptr.2639. [DOI] [PubMed] [Google Scholar]
- 82.Miranda PH. de A., Lacerda K.C.D., Araújo C.M., Barichello J.M., Lima W.G., Costa D.C. Oral formulation of DPP-4 inhibitor plus Quercetin improves metabolic homeostasis in type 1 diabetic rats. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-33727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aboulaghras S., Sahib N., Bakrim S., Benali T., Charfi S., Guaouguaou F.-E., et al. Health benefits and pharmacological aspects of chrysoeriol. Pharmaceuticals. 2022;15:973. doi: 10.3390/ph15080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nasrollahi Z., ShahaniPour K., Monajemi R., Ahadi A.M. Abelmoschus esculentus (L.) Moench improved blood glucose, lipid, and down‐regulated PPAR‐α, PTP1B genes expression in diabetic rats. J. Food Biochem. 2022;46 doi: 10.1111/jfbc.14097. [DOI] [PubMed] [Google Scholar]
- 85.Almasri I., Othman H., Bashaer A.-I., Mohammad M., Bustanji Y. Flavonoids from plant source as protein tyrosine phosphatase 1B inhibitors: in silico update. ACTA Pharm Sci. 2021;59 [Google Scholar]
- 86.Ali M.Y., Jannat S., Rahman M.M. Investigation of C-glycosylated apigenin and luteolin derivatives' effects on protein tyrosine phosphatase 1B inhibition with molecular and cellular approaches. Comput Toxicol. 2021;17 [Google Scholar]
- 87.Srivastava R.K., Singh A., Shukla S.V. Chemical investigation and pharmaceutical action of Cyperus rotundus-a review. J Biol Act Prod from Nat. 2013;3:166–172. [Google Scholar]
- 88.Shad F.S., Haghighi M.J. Study of the effect of the essential oil (extract) of rhubarb stem (shoot) on glycosylated hemoglobin and fasting blood glucose levels in patients with type II diabetes. Biomedicine. 2018;8 doi: 10.1051/bmdcn/2018080424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Selvin E., Steffes M.W., Zhu H., Matsushita K., Wagenknecht L., Pankow J., et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data utilized to underpin the findings of this study can be obtained from the corresponding author upon request.