Abstract
With the rapid advance of single-cell sequencing technology, cell heterogeneity in various biological processes was dissected at different omics levels. However, single-cell mono-omics results in fragmentation of information and could not provide complete cell states. In the past several years, a variety of single-cell multimodal omics technologies have been developed to jointly profile multiple molecular modalities, including genome, transcriptome, epigenome, and proteome, from the same single cell. With the availability of single-cell multimodal omics data, we can simultaneously investigate the effects of genomic mutation or epigenetic modification on transcription and translation, and reveal the potential mechanisms underlying disease pathogenesis. Driven by the massive single-cell omics data, the integration method of single-cell multi-omics data has rapidly developed. Integration of the massive multi-omics single-cell data in public databases in the future will make it possible to construct a cell atlas of multi-omics, enabling us to comprehensively understand cell state and gene regulation at single-cell resolution. In this review, we summarized the experimental methods for single-cell multimodal omics data and computational methods for multi-omics data integration. We also discussed the future development of this field.
Keywords: Single-cell sequencing, Single-cell multimodal omics, Multi-omics, Data integration, Multi-omics data integration
Introduction
Single-cell sequencing, in particular single-cell RNA sequencing (scRNA-seq), dramatically enhanced our understanding of tissue heterogeneity in various biology activities including development, immunity, tumor, and aging. In the last decade, many single-cell mono-omics sequencing approaches were developed, including scRNA-seq for profiling gene expression level at single-cell level, single-cell DNA sequencing (scDNA-seq) for detecting alteration of DNA sequence among cells, single-cell epigenome for identifying the regulatory differences among cells, single-cell proteome for revealing the phenotype differences among cells, and so on. However, these single-cell sequencing technologies only provide one modality or mono-omics, leading to fragmented information and a poor understanding of cell status. In the past several years, many single-cell multimodal omics methods have been developed to capture the information of multiple modalities of the same cell (Ma et al., 2020; Chen et al., 2022; Fiskin et al., 2022; Xu et al., 2022; Liu et al., 2023). Meanwhile, the accumulation of various huge number of single-cell omics data in many databases contain many precious information, which provides the potential to integrate these data for better understanding of cellular states (Cao et al., 2017; Regev et al., 2017; Abugessaisa et al., 2018; Franzen et al., 2019; Yuan et al., 2019; Domcke et al., 2020; Fan et al., 2020; Moreno et al., 2021; Zhang et al., 2021). In recent several years, many single-cell data integration tools have been developed to comprehensively characterize cell state or analyze gene regulation (Argelaguet et al., 2018; Stuart et al., 2019; Gayoso et al., 2021; Kang et al., 2021; Cao and Gao, 2022; Lakkis et al., 2022). In this review, we summarized the experimental approaches for joint profiling multimodalities and computational integration approaches for single-cell multi-omics data and their applications.
Joint profiling of multimodalities in single cell by experiments
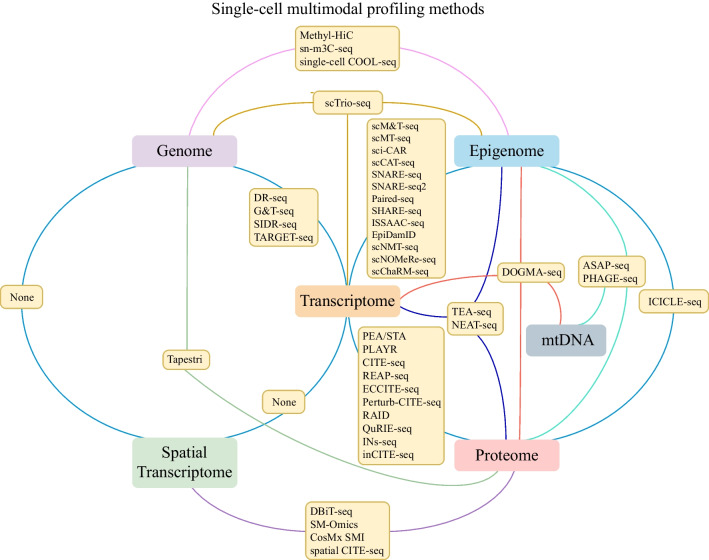
As Central Dogma indicated, the genetic information starts from genome and flows to the transcriptome, and subsequently to the proteome. Each modality of information, such as genome mutation, epigenomic modification, gene expression, and protein change, represents different aspect of cell status. Simultaneously detection of multiple modalities in single cell could be the most direct approach for comprehensive investigation of cellular status and gene regulation. Here, we summarized single-cell multimodal omics experimental approaches, which is developed quickly and gained a lot attention (Fig. 1, Table 1).
Fig. 1.
Single-cell multimodal profiling methods. For the single-cell multimodal profiling methods, transcriptome is the linkage center to connect with other modalities. Joint profiling of genome and transcriptome could dissect the association between genomic alteration and gene expression. While combining transcriptome with epigenome could dissect the impact of genome methylation or chromatin accessibility on gene expression. Joint profiling of epigenetic and genomic data reveals the association between genomic mutations and epigenetic decorations. Besides, joint measuring of transcriptome and proteome could indicate the dynamic gene mRNA and protein expression level. Meanwhile, joint profiling of epigenome, transcriptome, and proteome provides more comprehensive information about gene regulation in single-cell level. Additionally, spatial transcriptome provides spatially resolved gene expression and its combination with proteome could find the spatial temporal dynamics of gene expression level and epitopes existence
Table 1.
The comprehensive information of single-cell multimodal profiling methods
| Multimodal | Method | Genome | Transcriptome | Epigenome | Proteome | Object | Physical separation before amplification | High-throughput | Metrics | Time | Journal | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transcriptome + genome | DR-seq | gDNA | mRNA | - | - | 13 cells | No | No | CNVs; gene expression | 2015 | Nat Biotechnol | Dey et al. |
| G&T-seq | gDNA | Full-length mRNA | - | - | ~200 cells | Yes; oligo-dT-coated magnetic beads | No | Chromosomal aneuploidies; inter-chromosomal fusions; SNVs; chromosomal rearrangements; gene expression | 2015 | Nat Methods | Macaulay et al. | |
| SIDR-seq | gDNA | Total RNA (including non-coding RNA) | - | - | 43 cells | Yes; hypotonic lysis and antibody-conjugated magnetic microbeads | No | Genomic variations; transcriptomic patterns | 2018 | Genome Res | Han et al. | |
| TARGET-seq | Target gDNA | mRNA; target mRNA | - | - | 4559 cells | No | Yes | Known target somatic mutations; SNVs,small indels; gene expression | 2019 | Mol Cell | Rodriguez-Meira et al. | |
| Transcriptome + genome +methylome | scTrio-seq | gDNA | Cytosolic mRNA | gDNA methylation | - | 25 cells | Yes; centrifugation | No | CNVs; promoter and gene body methylation levels; gene expression | 2016 | Cell Res | Hou et al. |
| Transcriptome + methylome | scM&T-seq | - | Full-length mRNA | gDNA methylation | - | 61 cells | Yes; oligo-dT-coated magnetic beads | Yes | DNA methylome data; transcriptome data | 2016 | Nat Methods | Angermueller et al. |
| scMT-seq | - | Cytosolic mRNA | gDNA methylation | - | 9 cells | Yes; micropipette | No | Methylome, SNPs and transcriptome data | 2016 | Genome Biol | Hu et al. | |
| Transcriptome + chromatin accessibility | sci-CAR | - | Nuclear mRNA | chromatin accessibility | - | ~10,000 nuclei | No | Yes | Chromatin accessibility and gene expression | 2018 | Science | Cao et al. |
| scCAT-seq | - | Cytosolic mRNA | Chromatin accessibility | - | 176 cells | Yes; mild lysis and physical dissociation | Yes | 2019 | Nat Commun | Liu et al. | ||
| SNARE-seq | - | Nuclear mRNA | Chromatin accessibility | - | ~10,000 nuclei | No | Yes | 2019 | Nat Biotechnol | Chen et al. | ||
| SNARE-seq2 | - | mRNA | Chromatin accessibility | - | >80,000 nuclei or cells | No | Yes | 2021 | Nat Protocols | Plongthongkum et al. | ||
| Paired-seq | - | Nuclear mRNA | Chromatin accessibility | - | >10,000 nuclei | No | Yes | 2019 | Nat Struct Mol Biol | Zhu et al. | ||
| SHARE-seq | - | mRNA | Chromatin accessibility | - | >10,000 cells or nuclei | No | Yes | 2020 | Cell | Ma et al. | ||
| ISSAAC-seq | - | Nuclear mRNA | Chromatin accessibility | - | >10,000 nuclei | No | Yes | 2022 | Nat Methods | Xu et al. | ||
| Transcriptome + histone modification | EpiDamID | - | Total mRNA | Histone modification | - | 2,943 cells | No | No | Histone modification and gene expression | 2022 | Mol Cell | Rang et al. |
| Genome + methylome | Methyl-HiC | gDNA | - | gDNA methylation | - | 150 nuclei | No | No | General features of chromosomal architecture (chromatin loops, TADs); DNA methylome | 2019 | Nat Methods | Li et al. |
| sn-m3C-seq | gDNA | - | gDNA methylation | - | ~4200 nuclei | No | No | 3D chromatin conformation; Cytosine DNA methylation | 2019 | Nat Methods | Lee et al. | |
| Single-cell COOL-seq | gDNA | - | gDNA methylation | - | 24 nuclei | No | No | Chromatin state; nucleosome position; DNA methylation; CNVs and ploidy | 2017 | Cell Res | Guo et al. | |
| Transcriptome + methylome + chromatin accessibility | scNMT-seq | - | Cytosolic mRNA | gDNA methylation; chromatin accessibility | - | 70 cells | Yes; oligo-dT-coated magnetic beads | No | Chromatin accessibility, DNA methylation; transcriptome | 2018 | Nat Commun | Clark et al. |
| scNOMeRe-seq | - | Cytosolic mRNA | gDNA methylation; chromatin accessibility | - | 233 cells | Yes; oligo-dT-coated magnetic beads | No | Single-cell nucleosome occupancy; DNA methylome; RNA expression | 2021 | Nat Commun | Wang et al. | |
| scChaRM-seq | - | mRNA | gDNA methylation; chromatin accessibility | - | 148 cells | Yes; oligo-dT-coated magnetic beads | No | DNA methylome, chromatin accessibility and transcriptome | 2021 | Cell Stem Cell | Yan et al. | |
| Transcriptome + proteome | PEA/STA | - | Target mRNA | - | 38 surface proteins | 87 cells | No | No | Gene and protein expression | 2016 | Genome Biol | Genshaft et al. |
| PLAYR | - | 40 target mRNA | - | 40 surface proteins | 10,000 cells | No | Yes | Protein epitopes and multiple RNA | 2016 | Nat Methods | Frei et al. | |
| CITE-seq | - | mRNA | - | >100 surface proteins | 8,005 cells | No | Yes | Protein epitopes and transcriptomes | 2017 | Nat Methods | Stoeckius et al. | |
| REAP-seq | - | mRNA | - | 82 surface proteins | 7,271 cells | No | Yes | Protein epitopes and transcriptomes | 2017 | Nat Biotechnol | Peterson et al. | |
| ECCITE-seq | - | mRNA, V(D)J regions,perturbation gsRNA | - | >100 surface proteins | 5935 cells | No | Yes | Proteome, V(D)J regions, transcriptome, clonotype and CRISPR perturbation information | 2019 | Nat Methods | Mimitou et al. | |
| Perturb-CITE-seq | - | mRNA,perturbation gsRNA | - | 20 surface proteins | ~218,000 cells | No | Yes | Proteome, transcriptome and CRISPR perturbation information | 2021 | Nat Genet | Frangieh et al. | |
| RAID | - | mRNA | - | 6 intracellular (phospho-) proteins | 384 fixed cells | No | No | Gene and protein expression | 2019 | Sci Rep | Gerlach et al. | |
| QuRIE-seq | - | mRNA | - | 80 intra- or extracellular (phospho)proteins | 4,754 cells | No | Yes | Gene and protein expression | 2021 | Cell Rep Methods | Rivello et al. | |
| INs-seq | - | mRNA | - | Several intracellular proteins | 5000 cells | No | Yes | Single-cell transcriptional, signaling, TF, and metabolism maps | 2020 | Cell | Katzenelenbogen et al. | |
| inCITE-seq | - | Nuclear mRNA | - | Several intra-nuclear protein | ~10,000 nuclei | No | Yes | Transcription factors (TFs) and gene expression | 2021 | Nat Methods | Chung et al. | |
| Epigenome + proteome | ICICLE-seq | - | - | Chromatin accessibility | 46 surface proteins | >10,000 cells | No | Yes | Chromatin accessibility; protein levels | 2021 | Elife | Swanson et al. |
| Transcriptome + epigenome + proteome | TEA-seq | - | mRNA | Chromatin accessibility | 46 surface proteins | >10,000 cells | No | Yes | Chromatin accessibility; protein levels; gene expression | 2021 | Elife | Swanson et al. |
| NEAT-seq | - | mRNA | Chromatin accessibility | Several intra-nuclear protein | 6000 cells | No | Yes | TF gene locus chromatin accessibility, RNA expression, protein abundance and genome-wide TF binding motif accessibility | 2022 | Nat Methods | Chen et al. | |
| Genome + proteome | Tapestri | gDNA | - | - | 45 surface proteins | ~10,000 cells | No | Yes | SNV, CNV and protein levels | 2022 | Methods Mol Biol | Ruff et al. |
| Genome + epigenome + proteome | PHAGE-ATAC | mtDNA | Chromatin accessibility | Several intra- or extracellular proteins | 8366 cells | No | Yes | Surface and intracellular proteins, chromatin accessibility and mtDNA | 2021 | Nat Biotechnol | Fiskin et al. | |
| ASAP-seq | mtDNA | - | Chromatin accessibility | >200 intra- or extracellular proteins | >10,000 cells | No | Yes | Chromatin accessibility; protein expression; somatic mtDNA mutations | 2021 | Nat Biotechnol | Mimitou et al. | |
| Transcriptome + genome + epigenome + proteome | DOGMA-seq | mtDNA | mRNA | Chromatin accessibility | >200 intra- or extracellular proteins | >10,000 cells | No | Yes | Chromatin accessibility; gene and protein expression; somatic mtDNA mutations | 2021 | Nat Biotechnol | Mimitou et al. |
| Spatial transcriptome + proteome | DBiT-Seq | - | Spatial mRNA - 10μm | - | 22 surface proteins | Pre-fixed tissue section | No | Yes | Spatial transcriptomics and proteins | 2020 | Cell | Liu et al. |
| SM-Omics | - | Spatial mRNA - 100μm | - | multiplex proteins | Pre-fixed tissue section | No | Yes | 2022 | Nat Commun | Vickovic et al. | ||
| CosMx SMI | - | Spatial mRNA - 10μm | - | 64 surface proteins | Pre-fixed tissue section | No | No | 2022 | Blood | Gui et al. | ||
| spatial CITE-seq | - | Spatial mRNA - 50μm | - | 189 surface proteins | Pre-fixed tissue section | No | Yes | 2022 | Preprint | Liu et al. |
Joint profiling of transcriptome and genome at single-cell resolution
It is reported that genomic alterations, including single nucleotide variants (SNVs) and copy number variations (CNVs), are associated with gene expression, human health, disease, and evolution (Zhang et al., 2009; Xu et al., 2012; Jamal-Hanjani et al., 2017; Turajlic et al., 2018). scDNA-seq and scRNA-seq are powerful methods to investigate genomic mutations and transcriptomic alterations at single-cell resolution, respectively. Several methods have been developed to capture and amplify genomic DNA and RNA within the same cell for understanding how genetic variation influences gene expression at single-cell resolution. Genomic DNA (gDNA)-mRNA sequencing (DR-seq) (Dey et al., 2015) was developed to quantify gDNA and mRNA simultaneously without physical separation before amplification, thus minimizing noise and improving sensitivity. DR-seq detected CNVs in cancer cells and found genes with high cell-to-cell variation were associated with lower genomic copy number. Macaulay et al. developed genome and transcriptome sequencing (G&T-seq) (Macaulay et al., 2015) to separate gDNA and full-length mRNA in a single cell by oligo-dT-coated magnetic beads. G&T-seq not only helps us to detect single nucleotide variants (SNVs) and chromosomal rearrangements through deep single-cell sequencing, but also distinguish expression dosage, chromosomal aneuploidies, and inter-chromosomal fusions.
The aforementioned techniques only detected mRNA, while the other types of RNAs including non-coding RNAs were missed. In order to obtain comprehensive transcriptome information, Han et al. developed a method called simultaneous isolation of genomic DNA and total RNA sequencing (SIDR-seq) (Han et al., 2018) from single cells to capture total RNA instead of polyadenylated RNAs. Thus SIDR-seq could reveal a wide range of unknown correlations between genomic variations and transcriptomic patterns. Besides, to precisely identify specific gene mutations and their potential impacts on gene expression, TARGET-seq (Rodriguez-Meira et al., 2019) was developed to sensitively detect target somatic mutations in parallel whole-transcriptome, by protease digestion for improving gDNA release and enzyme modification for efficient amplification. Through TARGET-seq, specific molecular signatures in distinct subclones of tumor cells could be identified, and genetic and transcriptional heterogeneity and characteristics of treatment-resistant tumor subclones could be analyzed. Therefore, TARGET-seq paved the way to directly link somatic mutations with transcriptional signatures in distinct cancer cell populations. These approaches usually need manual cell pickup or sorting of cells into wells/microwell, which is inefficient and limits its application. Furthermore, the high cost of whole genome sequencing and whole transcriptome also limits the application of these methods.
Jointly profiling of transcriptome and epigenome at single-cell resolution
Epigenetics, which includes DNA methylation, histone modification, chromatin accessibility, and nucleosome occupancy, is the key regulator of gene expression. Many single-cell epigenome sequencing methods have been developed, including single-cell bisulfite sequencing (scBS-seq) (Smallwood et al., 2014) and single-cell reduced representation bisulfite sequencing (scRRBS-seq) (Guo et al., 2013), single-cell DNase sequencing (scDNase-seq) (Jin et al., 2015), single-cell assay for transposase-accessible chromatin using sequencing (scATAC-seq) (Buenostro et al., 2015; Chen et al., 2018; Xu et al., 2021), and single-cell micrococcal nuclease sequencing (scMNase-seq) (Lai et al., 2018). Single-cell epigenomics sequencing has emerged as an effective technique to investigate heterogeneity of gene regulation in various biological process. Nevertheless, to dissect gene expression variation regulated by epigenetic factors, the joint profiling of transcriptomics and epigenomics data at single cells is necessary, and several methods have been developed and provided more comprehensive information about the cell states.
By integrating G&T-seq and scBS-seq, Angermueller et al. developed single-cell methylome and transcriptome sequencing (scM&T-seq) (Angermueller et al., 2016) to parallel quantify methylome and transcriptome from the same cells. scM&T-seq enables us to discover the associations between heterogeneously methylated remote regulatory elements and the expression of key pluripotency genes. Furthermore, single-cell methylome and transcriptome sequencing (scMT-seq) (Hu et al., 2016) was developed based on cost-effective scRRBS-seq, which can simultaneously detect methylome, SNPs and transcriptome. Simultaneously, single-cell triple omics sequencing technique (scTrio-seq) (Hou et al., 2016) was developed to simultaneously investigate the CNVs, DNA methylome, and transcriptome in single cells. Through analyzing scTrio-seq data, they revealed that promoter methylation and gene body methylation are negatively correlated with gene expression, while DNA copy number is positively correlated with its gene expression but had no effect on DNA methylation level.
A large number of methods have been developed to simultaneously measure chromatin accessibility and transcriptome from the same cells. For instance, single-cell combinatorial indexing chromatin accessibility and mRNA (sci-CAR) (Cao et al., 2018) was established to jointly detect chromatin accessibility and polyadenylated mRNA in single nucleus, associating cis-regulatory sites to their target genes, while single-cell chromatin accessibility and transcriptome sequencing (scCAT-seq) (Liu et al., 2019) simultaneously assays chromatin accessibility in nucleus and transcriptome in the cytoplasm from the same cell to reveal regulatory relationships between cis-regulatory elements and their target genes. Furthermore, single-nucleus chromatin accessibility and mRNA expression sequencing (SNARE-seq) (Chen et al., 2019) was developed to detect chromatin accessibility and mRNA in single nucleus, and was applied to reveal the lineage-specific chromatin accessible sites and to provide insight into the relationship between the dynamics of promoter accessibility and expression level during neurogenesis. SNARE-seq is a droplet-based method with the characteristics of high throughput and high sensitivity, and captures 4–5 times more accessible sites than sci-CAR, which allows for a wider range of application. SNARE-seq2 (Plongthongkum et al., 2021), as an extension of SNARE-seq, significantly improves the throughput by 10- to 100-fold and allows process of multiple samples in the same batch to minimize technical noises using combinatorial barcoding. Parallel analysis of individual cells for RNA expression and DNA accessibility by sequencing (Paired-seq) (Zhu et al., 2019) could simultaneously profile the transcriptome and chromatin accessibility in millions of cells by ligation-based combinatorial indexing (Rosenberg et al., 2018). It was used to uncover the dynamic cellular composition and identified target genes for candidate cis-regulatory elements during mouse forebrain development. Paired-seq has significantly improved the throughput of single cells compared to sci-CAR and SNARE-seq. In addition, simultaneous high-throughput ATAC and RNA expression with sequencing (SHARE-seq) (Ma et al., 2020) was established to jointly detect chromatin accessibility and gene expression with high sensitivity and low cost. Through analyzing SHARE-seq data, researchers found that the regulatory domains of chromatin were opened before gene transcription and covered many lineage-driving genes, indicating the possibility to predict cell fate through chromatin accessibility. Due to the huge scalability of this method, SHARE-seq can be used to identify RNA barcodes, especially for CRISPR-based perturbation screening. In Situ SHERRY (Sequencing hetero RNA-DNA-hybrid) After ATAC-seq (ISSAAC-seq) (Xu et al., 2022) was developed to simultaneously detect the gene expression and chromatin accessibility in individual cells with the characteristics of highly sensitive, flexible workflow, and much lower cost. In ISSAAC-seq, chromatin and RNA were processed in situ within nucleus without physical separation, which largely simplified the experimental process and could generate high quality data. ISSAAC-seq was applied to identify major and rare cell types and cell-type specific regulatory elements in mouse cerebral cortex, and to reveal the dynamics of gene expression and chromatin accessibility during oligodendrocyte maturation. Furthermore, EpiDamID (Rang et al., 2022) was developed to simultaneously detect gene expression and histone modifications in the same cell, providing a comprehensive understanding of gene regulatory mechanisms underlying cellular processes. It is demonstrated the utility of EpiDamID in understanding cellular heterogeneity and identifying cell-type-specific gene expression and epigenetic signatures, such as revealing the Polycomb occupancy and hierarchical gene regulatory networks in mouse embryoid bodies.
Jointly profiling of 3D-genome and epigenome at single-cell resolution
We refer to the genome well organized in three-dimensional space as the 3D-genome. Distal genomic regions could be spatially adjacent for gene regulation in 3D-genome. To reveal the dynamics relationship between 3D-genome and methylation, several laboratory methods have been established to simultaneously measure 3D genomic structure and epigenetic state. Firstly, Methyl-HiC (Li et al., 2019) was developed to simultaneously and accurately detect chromosomal architecture and DNA methylome in single-cell based on in situ Hi-C and whole-genome bisulfite sequencing (WGBS) protocols. It was applied to identify similar sets of chromatin loops and comparable topologically associating domains (TADs) in mouse embryonic stem cells, and to delineate the heterogeneity of cell-type-specific chromatin structure and methylation in complex tissues. Single-nucleus methyl-3C sequencing (sn-m3C-seq) (Lee et al., 2019) was developed to detect chromatin conformation and Cytosine DNA methylation (mC) in individual cells, which facilitates researchers to identify cell types based on mC profiles and its association with the specific 3D chromatin structures. Furthermore, single-cell multi-omics sequencing technology (single-cell COOL-seq) (Guo et al., 2017) was developed to jointly detect the chromatin state or nucleosome position, DNA methylation, CNVs, and ploidy at single-cell levels. It has been applied to analyze chromatin state and the dynamics of DNA methylation, revealing the heterogeneous but highly ordered features of epigenomic reprogramming during mouse embryo development. The single-cell nucleosome, methylation, and transcription sequencing (scNMT-seq) (Clark et al., 2018) is based on scM&T-seq and scNOMe-seq. Applying scNMT-seq, Clark et al. illuminated the connections among nucleosomes, DNA methylation, and transcriptional expression level and dynamics coupling of these molecular layers during the development of mouse embryonic stem cells. Single-cell nucleosome occupancy, methylome, and RNA expression sequencing (scNOMeRe-seq) (Wang et al., 2021) could jointly profile the genome-wide chromatin accessibility, DNA methylation, and RNA expression in the same cell. It was applied to depict a single-cell multi-omics map of mice preimplantation development and revealed that the genome-wide DNA methylation remodeling promotes the reconstruction of genetic lineages in early embryos. Yan et al. developed scChaRM-seq (Yan et al., 2021) which could simultaneously measure DNA methylome, chromatin accessibility, and transcriptome, and they illuminated that the overall increase of DNA methylation during human oocyte growth is associated with chromatin accessibility, while the increase of DNA methylation at specific features correlates to transcription activity.
Joint profiling of transcriptome and proteome at single-cell resolution
In recent years, several methods were developed to jointly assay transcriptome and proteome at single-cell resolution, which has greatly enhanced our understanding of the relationship between mRNA and protein expression in complex biological processes. Proximity extension assay/specific RNA target amplification (PEA/STA) (Genshaft et al., 2016) and proximity ligation assay for RNA (PLAYR) (Frei et al., 2016) were developed to jointly measure specific target proteins and target mRNAs. PEA/STA used the Fluidigm C1 platform to isolate single cells, whereas PLAYR used flow cytometry or mass cytometry to isolate hundreds of cells. By applying PEA/STA, cellular protein-level metadata was produced to better interpret the single-cell RNA-seq results, while PLAYR was applied to interpret the interaction between transcription and translation.
Besides, there are several methods based on droplet systems to simultaneously detect protein epitopes and transcriptomes in large scale, including cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) (Stoeckius et al., 2017), RNA expression and protein sequencing assay (REAP-seq) (Peterson et al., 2017), expanded CRISPR-compatible cellular indexing of transcriptomes and epitopes by sequencing (ECCITE-seq) (Mimitou et al., 2019) and Perturb-CITE-sequencing (Perturb-CITE-seq) (Frangieh et al., 2021). Bost CITE-seq and REAP-seq adopted DNA-barcoded antibodies to label target surface proteins, allowing for high-throughput but low-cost measurement of proteome and transcriptome. CITE-seq enables us to characterize immune cell phenotypes and explore the post-transcriptional gene regulation. REAP-seq, different from CITE-seq, utilized unidirectional chemistry to generate small, stable covalent bonds between the DNA barcode and the antibody, thereby minimizing steric hindrance and potential crosstalk, which could be extensible to detect intracellular proteins. REAP-seq was used to detect the response of a subset of T cells to a drug which activates the immune system to eliminate cancer cells in mice by targeting a cell surface protein. And the ECCITE-seq was extended from CITE-seq which could simultaneously detect at least five modalities including proteome, transcriptome, V(D)J regions, clonotype, and CRISPR perturbation. Importantly, it could be applied to multimodal CRISPR screening with steady direct sgDNA capturing and recognizing the multimodal phenotyping of clone types from tumor tissues. Perturb-CITE-seq was a combination of Perturb-seq and CITE-seq which could detect surface proteins under perturbations and transcriptome and help us to understand potentially clinical-related and complex mechanisms of immune evasion.
Previous methods were mainly designed to detect surface protein and mRNA, while there remains a need to assay the intercellular proteins and intranuclear proteins. Single-cell RNA and immunodetection (RAID) (Gerlach et al., 2019) was developed to detect 6 intracellularly (phosphor-) proteins and mRNAs through Antibody RNA-barcode Conjugates (ARCs), and was applied to illuminate the heterogeneous cellular responses to different environmental signals. Quantification of RNA and intracellular epitopes by sequencing (QuRIE-seq) (Rivello et al., 2021) was established to quantify 80 intra- or extracellular proteins and mRNA expression, and was used to trace the temporal activation of the B-cell receptor pathway and reveal the mechanism of immune inhibitory drug. Furthermore, INs-seq (Katzenelenbogen et al., 2020) with the ability to jointly profile intracellular protein activity and mRNA expression, was used to define T cell subsets in specific states through TF combinations and metabolic activity, and identify novel Trem2+ suppressive myeloid cells. Additionally, intranuclear cellular indexing of transcriptomes and epitopes (inCITE-seq) (Chung et al., 2021) was established to measure multiplexed intranuclear proteins and transcriptome in thousands of nuclei helping to interpret the relationship between TF and gene expression in vivo, and offering means to decipher complex phenotypes and regulatory mechanisms during the dynamic development process.
Several techniques were developed to jointly profile the epigenomic, proteome, and transcriptome. ICICLEseq (Swanson et al., 2021) could simultaneously detect chromatin accessibility and surface proteins. TEA-seq (Swanson et al., 2021) which is based on ICICLE-seq could jointly profile surface markers, chromatin accessibility, and transcriptome to analyze peak-to-gene (and peak-to-protein) correlations among the proteins and identify the cis-regulatory modules for regulating cell state. Sequencing of nuclear protein epitope abundance, chromatin accessibility, and the transcriptome in single cells (NEAT-seq) (Chen et al., 2022) simultaneously assays intra-nuclear proteins, chromatin accessibility, and transcriptome in single-cell. It was applied to identify the TF regulation models through comparing the TF gene locus chromatin accessibility, RNA expression, protein abundance, and genome-wide TF binding motif accessibility across cells.
Several other multi-omics single-cell joint profiling techniques are available, such as Tapestri (Ruff et al., 2022), ATAC with selected antigen profiling by sequencing (ASAP-seq) (Mimitou et al., 2021) and DOGMA-seq (Mimitou et al., 2021), and PHAGE-ATAC (Fiskin et al., 2022). Tapestri platform was developed by Mission Bio to jointly profile genome heterogeneity and surface protein expression in high throughput, which provided deep insights into clonal evolution, tumor heterogeneity, and other biological processes. ASAP-seq/DOGMA-seq could simultaneously detect chromatin accessibility, gene expression, surface and intracellular proteins, and somatic mtDNA mutations, revealing the distinct cellular programming during trajectory and the heterogeneous response to stimulation. PHAGE-ATAC simultaneously measures surface and intracellular proteins, chromatin accessibility, and mtDNA in which robust capture of mtDNA fragments and detection of mitochondrial mutations can be used for cloning tracing and integrating lineage information with protein expression and cell status.
Spatially resolved omics
The biological function of cells is closely related to its surrounding environment. However, scRNA-seq can only profile the gene expression level of each cell but lacks gene’s spatial location. Recently, spatial transcriptomics (ST) emerged as a powerful tool to detect the spatially resolved transcriptomics. Fluorescence in situ hybridization (FISH) (Raj et al., 2008) is a classical method to detect spatial gene expression in situ and regarded as golden standard. With the development of technologies, many FISH-based ST imaging methods have been published, including branched DNA single-molecule FISH (bDNA sm-FISH) (Battich et al., 2013), sequential barcoded Fluorescence in situ Hybridization (seqFISH) (Lubeck et al., 2014; Shah et al., 2016), multiplexed error-robust fluorescence in situ hybridization (MERFISH) (Chen et al., 2015; Moffitt et al., 2016), single-molecule fluorescence in situ hybridization (smFISH) (Haimovich and Gerst, 2018), osmFISH (Codeluppi et al., 2018), and spatially resolved transcript amplicon readout mapping (STARmap) (Wang et al., 2018). Furthermore, 10X Genomics Visium was developed to obtain ST data with higher throughput, while Slide-seq (Rodriques et al., 2019) and HDST (Vickovic et al., 2019) significantly improved the spatial resolution.
With the emergence of spatial sequencing and imaging technologies, it is possible to simultaneously profile spatially resolved multi-omics sequencing. In recent years, Deterministic Barcoding in Tissue for spatial omics sequencing (DBiT-seq) (Liu et al., 2020) and Spatial Multiomics (SM-Omics) (Vickovic et al., 2022) have been established to profile spatial transcriptome and surface proteins through DNA-barcoded sequencing or immunofluorescence (IF). Liu et al. have applied DBiT-seq to establish a spatial multi-omics atlas of mouse embryo and mouse embryonic brain and revealed that the expression patterns of the selected famous genes are consistent with the previous knowledge. Similarly, Vickovic et al. constructed the SM-Omics automatic platform and applied the system to reveal the dynamic spatially expression patterns of key genes in transcriptomics and proteomics for diverse models. Besides, Nanostring have established CosMX spatial molecular Imager (CosMx SMI) to concurrently image 1000 RNA and 64 validated proteins. Meanwhile, Gui et al. (Gui et al., 2022) have applied CosMX combined with 10X 3′ RNA-seq and GEOMx Digital Spatial Profiler to dissect the cell-cell interaction mechanism of T cells with leukemia cells, especially for the T cells adjacent to cancer. Furthermore, spatial CITE-seq (Liu et al., 2023) was developed based on the DBiT-seq platform, and was applied to investigate the changes of spatial expression patterns of transcriptomics and proteomics for 200–300 genes in the skin where the COVID-19 mRNA vaccine was injected.
Transforming single-cell mono-omics data into multi-omics data by information extraction
Last but not least, single-cell omics data usually contains additional information. The sequencing data generate by scRNA-seq data and single-cell epigenomic data also could be used to identify the genetic variations. For example, scRNA-seq data were not only used for profiling gene expression in single cell, but also were used for CNV inference in single cell (Patel et al., 2014; Puram et al., 2017; Venteicher et al., 2017; Maynard et al., 2020). Based on scRNA-seq data of leukemia patients, we identified cancer cell specific genetic variations and genetic variations carried by cancer cells during relapse (Qin et al., 2021), which significantly enhanced our understanding of leukemiagensis. We further identified the monoallelic expression based on the genetic variants in scRNA-seq data and found the increase of monoallelic expression during leukemogenesis (Fu et al., 2021). We identified the genetic variants in scDNase-seq of cancer cells in thyroid cancer patients, among which one mutation (chr18: 52417839G>C) affects the binding of the tumor suppressor protein p53 and correlates with decreased expression of its target gene TXNL1 (Jin et al., 2015). Therefore, extracting and integrating multimodal omics information from single-cell mono-omics data is an economic alternation for comprehensive understanding of cell status and gene regulatory mechanisms.
Computational integration of single-cell multi-omics data
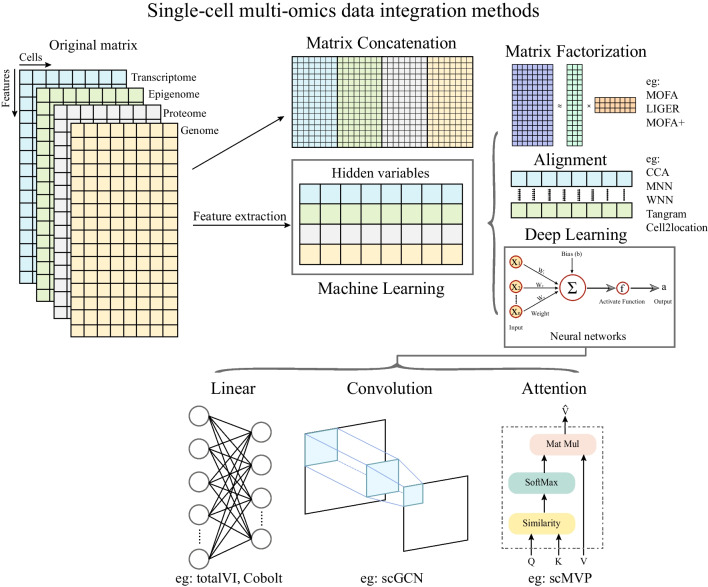
The rapid development of single-cell multimodal sequencing methods possessed a crucial need to integrate various types of data for biological insight. Some researchers integrated multiple modalities data by applying batch effect correction methods for bulk RNA-seq and scRNA-seq (Stuart and Satija, 2019). Meanwhile, others focused on developing integration methods based on machine learning which extracts features from original profiles and calculates their correlation scores. These methods typically employ algorithms such as matrix factorization, correlation analysis, and manifold alignment. For instance, matrix factorization-based integration methods, such as multi-omics factor analysis (MOFA) (Argelaguet et al., 2018) and linked inference of genomic experimental relationships (LIGER) (Welch et al., 2019), usually extract the specific features as factors which are related to the expression of different modalities and would be used in subsequent integration. However, the key challenge faced by these models is to distinguish the biological variation from technical noise, which leads to highly computational costs and, even computer crashes when handling large-scale high-dimensional data. Recently, deep learning has been applied to process large-scale multi-modality data in area such as text-to-image generation, allowing to dissect the complex relationship among different data types. With the advent of cutting-edge AI technology, multi-modality fusion models have been applied in this field. For instance, scMVP (Li et al., 2022) was designed to integrate scRNA-seq and scATAC-seq data generated from jointly profiling experimental methods, such as SNARE-seq, sci-CAR, Paired-seq, SHARE-seq and 10X multiome. scMVP shows better performance in prediction of cis-regulatory elements and data imputation from paired omics data than that of from mono-omics alone, indicating its advantages in utilizing the information from multi-modalities. Overall, researchers are striving to develop a computational integration framework that leverages the association of single-cell multi-omics profiles (Fig. 2).
Fig. 2.
Single-cell multi-omics data integration methods. The categorical hierarchical structure shows single-cell multi-omics data integration approaches. The easiest way to integrate multi-omics is to concatenate the original feature matrix of various omics data, but the noise and distinct meaning of values confuse the results of the integration. Researchers also establish many methods based on machine learning which extract features from original matrix and then combine the features across multi-modalities. For instance, MOFA, LIGER is based on matrix factorization, while CCA, MNN, WNN, Tangram and Cell2location are based on correlation alignment and anchoring. Additionally, these promising deep learning algorithms have been applied in single-cell multi-omics data integration based on various types of networks, including linear layer, convolution, and self-attention mechanism
Integrative analysis of paired single-cell multi-omics data
Several multi-omics data integration methods have been implemented in Seurat, one of the most popular single-cell analysis tool kits. These methods include canonical correlation analysis (CCA, V2.0) (Butler et al., 2018), mutual nearest neighbors (MNN, V3.0) (Stuart et al., 2019), and weighted nearest neighbors (WNN, V4.0) (Hao et al., 2021). All of these methods aim to align single cells across multi-omics profiles. In Seurat V4.0, Satija et al. employed WNN not only to integrate scRNA-seq and scATAC-seq data such as 10x multiome and SHARE-seq data, but also to integrate proteomic data and transcriptomic data such as CITE-seq data. This approach enabled the identification of cell-type-specific signatures and the projection of proteomic data onto a scRNA-seq atlas of bone marrow. Moreover, the ability to map cells from multi-omics profiles has facilitated studies in various field, including cancer therapy, developmental biology, immunology, and neural biology. For instance, Mimitou et al. (2021) applied WNN to identify a subset of CD4 T cell with functional alternation both in scATAC-seq and scRNA-seq data, and to analyze the contribution of each modality to identification of the clusters. Additionally, they captured a cluster of CD138+ T cells that could only be recognized when combined with protein profiling. Bakken et al. (2021) applied CCA in combination with shared nearest neighbor (SNN) to integrate RNA expression and chromatin accessibility generated by SNARE-Seq2 across different species and determine whether the cell cluster is conserved in evolution. Herrera et al. (2021) used ECCITE-seq to jointly analyze transcriptome, proteome, and epitope expression, and revealed the heterogeneity in cutaneous T cell lymphoma (CTCL) in Sézary syndrome patients in which indexing is designed for measuring V(D)J repertoire of T cells, from ECCITE-seq. To integrate ECCITE-seq data, they co-embedded the multi-modalities profiles and then dissected the microenvironment in skin and blood from Sézary syndrome to illuminate the diverse subclones of CTCL.
Besides, there are several tools designed to analyze CITE-seq data, which allows to the simultaneous measurement of gene expression and interested proteins levels in single cells. However, the limited number of overlapping proteins captured across samples can introduce noise into the proteomics data, which may lead to deviation in the clustering and identification of cell types when merging experimental batches. Hence, it is a huge challenge to combat batch effects and standardize the protein panels for integrating CITE-seq data. To address this issue, Gayoso et al. established totalVI (Gayoso et al., 2021) to correct and impute the protein profiles. They employed the co-embedding latent variables to identify cell clusters which are ambivalent when using proteomics data alone. TotalVI has also been applied to infer the development trajectory of B cells in the spleen and lymph nodes. Given that the limitation of totalVI in time-consuming and low result consistence, Justin Lakkis et al. developed sciPENN (Lakkis et al., 2022) to integrate multiple CITE-seq and paired scRNA-seq datasets. This method was implemented in the joint analysis of PBMC datasets generated by CITE-seq and scRNA-seq, and successfully correct the epitope expression of CD8 T cells markers, which facilitates to identify CD8 T cells subclusters in cardiovascular disease.
Integrative analysis of unpaired single-cell multi-omics data
While the paired single-cell multi-omics methods offer valuable insights to interpret biological activities, their high cost and noise level limit their widespread application. To overcome these limitations, researchers developed integrative analysis tools implemented on various mono-omics datasets. These tools, including Cobolt (Gong et al., 2021), MOFA+ (Argelaguet et al., 2020), MultiVI (Ashuach et al., 2021), scJoint (Lin et al., 2022), GLUE (Cao and Gao, 2022), scGCN (Song et al., 2021), and Symphony (Kang et al., 2021), enable to the integration of newly data generated in one modality with previously collected datasets. Furthermore, many of these approaches are capable of atlas-level integration for large-scale multi-omics datasets. For instance, the increased accuracy has been shown when applying label transfer from SNARE-seq datasets into unpaired scRNA-seq and scATAC-seq data of mouse primary motor cortex (MOp) and PBMC through Cobolt compared to using SNARE-seq alone (Gong et al., 2021). MOFA+ was applied to reveal the expression signatures alongside the trajectory and trends of scNMT-seq data which jointly profiled mRNA expression, DNA methylation, and chromatin accessibility (Argelaguet et al., 2020). MultiVI focuses on imputing missing values of one modality by integrating unpaired data with paired reference datasets and estimating the accurate values of multi-omics profiles. scJoint adopted the labels from annotated reference of scRNA-seq datasets to obtain the accurate label of unpaired multi-omics data via transfer learning. GLUE was applied to integrate tri-omics datasets from different experiments and inferred some previously unknown regulatory mechanisms in PBMC data (Cao and Gao, 2022). scGCN (Song et al., 2021) has been applied to integrate scRNA-seq and scATAC-seq data from different datasets, and has been proved to perform well in various experiments. Symphony was applied to analyze CITE-seq data of PBMC, which could be extended to integrate large-scale scRNA-seq data and multi-omics data (Kang et al., 2021). Kang et al. inferred the cell states of reference data and mapped the query cells on it based on the co-embedding result of CCA, and they found that the predicted surface protein expressions of query cells are corresponding to the previous studies.
Spatial multi-omics data integration
The proliferation of laboratory spatially resolved multi-omics methods, which enable the simultaneous measurement of transcriptome, chromatin accessibility, methylation, protein, and others, indicates a necessity to consider spatial coordinates in the development of analytical approaches. Most of the spatial transcriptomics methods require paired scRNA-seq data to diminish the high noise in ST data. Recently, several studies implemented spatially resolved multi-omics data integration analysis to overcome these limitations, including deconvolution and mapping analysis. For instance, Cell2location (Kleshchevnikov et al., 2022), developed by Bayraktar et al., applied negative-binomial regression in variation inference, and it has been widely used in mapping cell-types in scRNA-seq onto ST. Besides, Biancalani et al. developed Tangram (Biancalani et al., 2021), which implements strategy to minimizing the embedding vectors of ST and scRNA-seq data. Furthermore, Kuppe et al. (2022) applied Cell2location to map ST to snRNA-seq and snATAC-seq, constructing a single-cell map of human myocardial infarction at distinct disease stages. By integrating different omics data through SNN, they identified two rare cell clusters involved in the process of myocardial infarction, explored the activity of signaling pathways and regulatory factors in various cell types of cardiac tissues and presented it on spatial location. Ravi et al. (2022) constructed a spatially resolved multi-omics cell atlas of glioblastoma by combining transcriptome, metabolome, and proteome, and used MNN to decipher integrative multi-modality profiles, revealing the transition to reactive state of neuroglia cells in hypoxia condition.
Prospects
Cell is a fundamental unit of life that contains abundant information to subtly regulate all biological processes. To uncover the mysteries of life, it is crucial to gain a comprehensive understanding of the functions of various cellular components and their interrelationships. With continuous advance of single-cell technology, it will be possible to measure larger number of cells and diverse molecules per cell. To combine multimodal information of individual cells, more single-cell multimodal omics experimental methods are required. However, it will be difficult to capture more than 3 modalities from the same cells due to molecule loss increase with the complexity of experiments increase. Meanwhile, different models and deep-learning algorithms can be utilized to boost computationally integration of single-cell omics data from different samples, different laboratories, different platforms, and different modalities. In the future, researchers will be able to make significant strides in understanding the intricacies of cellular biology and their implications in health and disease.
Acknowledgements
We thank all members from the Jin lab for the helpful discussion. We acknowledge the assistance of Core Facilities of SUSTech. The computational work was supported by Center for Computational Science and Engineering at SUSTech.
Author contributions
Conceptualization: Xuefei Wang and Wenfei Jin; writing—original draft preparation: Xuefei Wang and Xinchao Wu; writing—review and editing: Xuefei Wang, Xinchao Wu, Ni Hong, and Wenfei Jin; visualization: Xuefei Wang and Xinchao Wu; supervision: Wenfei Jin.
Funding
This study was supported by the National Key R&D Program of China (2021YFF1200900, 2021YFA0909300), the National Natural Science Foundation of China (32170646), the Guangdong Basic and Applied Basic Research Foundation (2023A1515011908), the Shenzhen Science and Technology Program (KQTD20180411143432337), the Shenzhen Innovation Committee of Science and Technology (JCYJ20220818100401003, ZDSYS20200811144002008), and the Medical Scientific Research Foundation of Guangdong Province (A2021450).
Data Availability
No data was generated for this manuscript.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors (not applicable).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abugessaisa I, Noguchi S, Bottcher M, et al. SCPortalen: human and mouse single-cell centric database. Nucleic Acids Res. 2018;46(D1):D781–D787. doi: 10.1093/nar/gkx949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermueller C, Clark SJ, Lee HJ, et al. Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nat Methods. 2016;13(3):229–232. doi: 10.1038/nmeth.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argelaguet R, Velten B, Arnol D, et al. Multi-omics factor analysis-a framework for unsupervised integration of multi-omics data sets. Mol Syst Biol. 2018;14(6):e8124. doi: 10.15252/msb.20178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argelaguet R, Arnol D, Bredikhin D, et al. MOFA+: a statistical framework for comprehensive integration of multi-modal single-cell data. Genome Biology. 2020;21(1):111. doi: 10.1186/s13059-020-02015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashuach T, Gabitto MI, Jordan MI, et al. MultiVI: deep generative model for the integration of multi-modal data. bioRxiv; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken TE, Jorstad NL, Hu Q, et al. Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature. 2021;598(7879):111–119. doi: 10.1038/s41586-021-03465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battich N, Stoeger T, Pelkmans L. Image-based transcriptomics in thousands of single human cells at single-molecule resolution. Nat Methods. 2013;10(11):1127–1133. doi: 10.1038/nmeth.2657. [DOI] [PubMed] [Google Scholar]
- Biancalani T, Scalia G, Buffoni L, et al. Deep learning and alignment of spatially resolved single-cell transcriptomes with Tangram. Nat Methods. 2021;18(11):1352–1362. doi: 10.1038/s41592-021-01264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenostro JD, Wu BJ, Litzenburger UM, et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523(7561):486–U264. doi: 10.1038/nature14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, et al. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao ZJ, Gao G. Multi-omics single-cell data integration and regulatory inference with graph-linked embedding. Nat Biotechnol. 2022;40(10):1458–1466. doi: 10.1038/s41587-022-01284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhu J, Jia P, et al. scRNASeqDB: a database for RNA-Seq based gene expression profiles in human single cells. Genes (Basel) 2017;8(12):368. doi: 10.3390/genes8120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JY, Cusanovich DA, Ramani V, et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science. 2018;361(6409):1380–1385. doi: 10.1126/science.aau0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Boettiger AN, Moffitt JR, et al. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348(6233):aaa6090. doi: 10.1126/science.aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Miragaia RJ, Natarajan KN, et al. A rapid and robust method for single cell chromatin accessibility profiling. Nat Commun. 2018;9(1):5345. doi: 10.1038/s41467-018-07771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lake BB, Zhang K. High-throughput sequencing of the transcriptome and chromatin accessibility in the same cell. Nat Biotechnol. 2019;37(12):1452–1457. doi: 10.1038/s41587-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AF, Parks B, Kathiria AS, et al. NEAT-seq: simultaneous profiling of intra-nuclear proteins, chromatin accessibility and gene expression in single cells. Nat Methods. 2022;19(5):547–553. doi: 10.1038/s41592-022-01461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Parkhurst CN, Magee EM, et al. Joint single-cell measurements of nuclear proteins and RNA in vivo. Nat Methods. 2021;18(10):1204–1212. doi: 10.1038/s41592-021-01278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SJ, Argelaguet R, Kapourani CA, et al. scNMT-seq enables joint profiling of chromatin accessibility DNA methylation and transcription in single cells. Nat Comm. 2018;9:781. doi: 10.1038/s41467-018-03149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codeluppi S, Borm LE, Zeisel A, et al. Spatial organization of the somatosensory cortex revealed by osmFISH. Nat Methods. 2018;15(11):932–935. doi: 10.1038/s41592-018-0175-z. [DOI] [PubMed] [Google Scholar]
- Dey SS, Kester L, Spanjaard B, et al. Integrated genome and transcriptome sequencing of the same cell. Nat Biotechnol. 2015;33(3):285–289. doi: 10.1038/nbt.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domcke S, Hill AJ, Daza RM, et al. A human cell atlas of fetal chromatin accessibility. Science. 2020;370(6518):eaba7612. doi: 10.1126/science.aba7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Chen R, Chen X. SpatialDB: a database for spatially resolved transcriptomes. Nucleic Acids Res. 2020;48(D1):D233–D237. doi: 10.1093/nar/gkz934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskin E, Lareau CA, Ludwig LS, et al. Single-cell profiling of proteins and chromatin accessibility using PHAGE-ATAC. Nat Biotech. 2022;40(3):374–381. doi: 10.1038/s41587-021-01065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangieh CJ, Melms JC, Thakore PI, et al. Multimodal pooled Perturb-CITE-seq screens in patient models define mechanisms of cancer immune evasion. Nat Genet. 2021;53(3):332–341. doi: 10.1038/s41588-021-00779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen O, Gan LM, Bjorkegren JLM. PanglaoDB: a web server for exploration of mouse and human single-cell RNA sequencing data. Database (Oxford) 2019;2019:baz046. doi: 10.1093/database/baz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei AP, Bava FA, Zunder ER, et al. Highly multiplexed simultaneous detection of RNAs and proteins in single cells. Nat Methods. 2016;13(3):269–275. doi: 10.1038/nmeth.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Qin P, Zou X, et al. A comprehensive characterization of monoallelic expression during hematopoiesis and leukemogenesis via single-cell RNA-sequencing. Front Cell Dev Biol. 2021;9:702897. doi: 10.3389/fcell.2021.702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayoso A, Steier Z, Lopez R, et al. Joint probabilistic modeling of single-cell multi-omic data with totalVI. Nat Methods. 2021;18(3):272–282. doi: 10.1038/s41592-020-01050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genshaft AS, Li S, Gallant CJ, et al. Multiplexed, targeted profiling of single-cell proteomes and transcriptomes in a single reaction. Genome Biol. 2016;17:1–15. doi: 10.1186/s13059-016-1045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach JP, van Buggenum JAG, Tanis SEJ, et al. Combined quantification of intracellular (phospho-)proteins and transcriptomics from fixed single cells. Scie Rep. 2019;9:1469. doi: 10.1038/s41598-018-37977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Zhou Y, Purdom E. Cobolt: integrative analysis of multimodal single-cell sequencing data. Genome Biol. 2021;22(1):351. doi: 10.1186/s13059-021-02556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui G, Wong-Rolle A, Dillon LW, et al. Spatial-temporal multiomic analysis of tumor-immune interactions in patients with AML receiving pembrolizumab and decitabine. Blood. 2022;140:3427–3428. doi: 10.1182/blood-2022-168191. [DOI] [Google Scholar]
- Guo HS, Zhu P, Wu XL, et al. Single-cell methylome landscapes of mouse embryonic stem cells and early embryos analyzed using reduced representation bisulfite sequencing. Genome Res. 2013;23(12):2126–2135. doi: 10.1101/gr.161679.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Li L, Li JY, et al. Single-cell multi-omics sequencing of mouse early embryos and embryonic stem cells. Cell Res. 2017;27(8):967–988. doi: 10.1038/cr.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich G, Gerst JE. Single-molecule fluorescence in situ hybridization (smFISH) for RNA detection in adherent animal cells. Bio Protoc. 2018;8(21):e3070. doi: 10.21769/BioProtoc.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KY, Kim KT, Joung JG, et al. SIDR: simultaneous isolation and parallel sequencing of genomic DNA and total RNA from single cells. Genome Res. 2018;28(1):75–87. doi: 10.1101/gr.223263.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Hao S, Andersen-Nissen E, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–3587 e3529. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A, Cheng A, Mimitou EP, et al. Multimodal single-cell analysis of cutaneous T-cell lymphoma reveals distinct subclonal tissue-dependent signatures. Blood. 2021;138(16):1456–1464. doi: 10.1182/blood.2020009346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Guo HH, Cao C, et al. Single-cell triple omics sequencing reveals genetic, epigenetic, and transcriptomic heterogeneity in hepatocellular carcinomas. Cell Res. 2016;26(3):304–319. doi: 10.1038/cr.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YJ, Huang K, An Q, et al. Simultaneous profiling of transcriptome and DNA methylome from a single cell. Genome Biol. 2016;17:1–11. doi: 10.1186/s13059-016-0950-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376(22):2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- Jin W, Tang Q, Wan M, et al. Genome-wide detection of DNase I hypersensitive sites in single cells and FFPE tissue samples. Nature. 2015;528(7580):142–146. doi: 10.1038/nature15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JB, Nathan A, Weinand K, et al. Efficient and precise single-cell reference atlas mapping with symphony. Nat Commun. 2021;12(1):5890. doi: 10.1038/s41467-021-25957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenelenbogen Y, Sheban F, Yalin A, et al. Coupled scRNA-Seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell. 2020;182(4):872–885.e819. doi: 10.1016/j.cell.2020.06.032. [DOI] [PubMed] [Google Scholar]
- Kleshchevnikov V, Shmatko A, Dann E, et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat Biotechnol. 2022;40(5):661–671. doi: 10.1038/s41587-021-01139-4. [DOI] [PubMed] [Google Scholar]
- Kuppe C, Ramirez Flores RO, Li Z, et al. Spatial multi-omic map of human myocardial infarction. Nature. 2022;608(7924):766–777. doi: 10.1038/s41586-022-05060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai B, Gao W, Cui K, et al. Principles of nucleosome organization revealed by single-cell micrococcal nuclease sequencing. Nature. 2018;562(7726):281–285. doi: 10.1038/s41586-018-0567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkis J, Schroeder A, Su K, et al. A multi-use deep learning method for CITE-seq and single-cell RNA-seq data integration with cell surface protein prediction and imputation. Nat Mach Intell. 2022;4(11):940–952. doi: 10.1038/s42256-022-00545-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Luo C, Zhou J, et al. Simultaneous profiling of 3D genome structure and DNA methylation in single human cells. Nat Methods. 2019;16(10):999–1006. doi: 10.1038/s41592-019-0547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Liu Y, Zhang Y, et al. Joint profiling of DNA methylation and chromatin architecture in single cells. Nat Methods. 2019;16(10):991–993. doi: 10.1038/s41592-019-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Fu S, Wang S, et al. A deep generative model for multi-view profiling of single-cell RNA-seq and ATAC-seq data. Genome Biol. 2022;23(1):20. doi: 10.1186/s13059-021-02595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wu TY, Wan S, et al. scJoint integrates atlas-scale single-cell RNA-seq and ATAC-seq data with transfer learning. Nat Biotechnol. 2022;40(5):703–710. doi: 10.1038/s41587-021-01161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LQ, Liu CY, Quintero A, et al. Deconvolution of single-cell multi-omics layers reveals regulatory heterogeneity. Nature Comm. 2019;10:470. doi: 10.1038/s41467-018-08205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang M, Deng Y, et al. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell. 2020;183(6):1665–1681 e1618. doi: 10.1016/j.cell.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, DiStasio M, Su G et al (2023) High-plex protein and whole transcriptome co-mapping at cellular resolution with spatial CITE-seq. Nature Biotech:1–5. 10.1038/s41587-023-01676-0 [DOI] [PMC free article] [PubMed]
- Lubeck E, Coskun AF, Zhiyentayev T, et al. Single-cell in situ RNA profiling by sequential hybridization. Nat Methods. 2014;11(4):360–361. doi: 10.1038/nmeth.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Zhang B, LaFave LM, et al. Chromatin potential identified by shared single-cell profiling of RNA and chromatin. Cell. 2020;183(4):1103–1116 e1120. doi: 10.1016/j.cell.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay IC, Haerty W, Kumar P, et al. G&T-seq: parallel sequencing of single-cell genomes and transcriptomes. Nat Methods. 2015;12(6):519–522. doi: 10.1038/nmeth.3370. [DOI] [PubMed] [Google Scholar]
- Maynard A, McCoach CE, Rotow JK, et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell. 2020;182(5):1232–1251 e1222. doi: 10.1016/j.cell.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Cheng A, Montalbano A, et al. Multiplexed detection of proteins, transcriptomes, clonotypes and CRISPR perturbations in single cells. Nat Methods. 2019;16(5):409–412. doi: 10.1038/s41592-019-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Lareau CA, Chen KY, et al. Scalable, multimodal profiling of chromatin accessibility, gene expression and protein levels in single cells. Nat Biotechnol. 2021;39(10):1246–1258. doi: 10.1038/s41587-021-00927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt JR, Hao JJ, Wang GP, et al. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc Natl Acad Sci U S A. 2016;113(39):11046–11051. doi: 10.1073/pnas.1612826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno P, Fexova S, George N, et al. Expression Atlas update: gene and protein expression in multiple species. Nucleic Acids Res. 2021;50(D1):D129–D140. doi: 10.1093/nar/gkab1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson VM, Zhang KX, Kumar N, et al. Multiplexed quantification of proteins and transcripts in single cells. Nat Biotechnol. 2017;35(10):936–939. doi: 10.1038/nbt.3973. [DOI] [PubMed] [Google Scholar]
- Plongthongkum N, Diep D, Chen S, et al. Scalable dual-omics profiling with single-nucleus chromatin accessibility and mRNA expression sequencing 2 (SNARE-seq2) Nat Protoc. 2021;16(11):4992–5029. doi: 10.1038/s41596-021-00507-3. [DOI] [PubMed] [Google Scholar]
- Puram SV, Tirosh I, Parikh AS, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171(7):1611–1624 e1624. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Pang Y, Hou W, et al. Integrated decoding hematopoiesis and leukemogenesis using single-cell sequencing and its medical implication. Cell Discov. 2021;7(1):2. doi: 10.1038/s41421-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, van den Bogaard P, Rifkin SA, et al. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. 2008;5(10):877–879. doi: 10.1038/Nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang FJ, de Luca KL, de Vries SS, et al. Single-cell profiling of transcriptome and histone modifications with EpiDamID. Mol Cell. 2022;82(10):1956–1970 e1914. doi: 10.1016/j.molcel.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi VM, Will P, Kueckelhaus J, et al. Spatially resolved multi-omics deciphers bidirectional tumor-host interdependence in glioblastoma. Cancer Cell. 2022;40(6):639–655 e613. doi: 10.1016/j.ccell.2022.05.009. [DOI] [PubMed] [Google Scholar]
- Regev A, Teichmann SA, Lander ES, et al. The Human Cell Atlas. Elife. 2017;6:e27041. doi: 10.7554/eLife.27041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivello F, van Buijtenen E, Matuła K, et al. Single-cell intracellular epitope and transcript detection reveals signal transduction dynamics. Cell Rep Methods. 2021;1(5):100070. doi: 10.1016/j.crmeth.2021.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Meira A, Buck G, Clark SA, et al. Unravelling intratumoral heterogeneity through high-sensitivity single-cell mutational analysis and parallel RNA sequencing. Mol Cell. 2019;73(6):1292–1305.e1298. doi: 10.1016/j.molcel.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriques SG, Stickels RR, Goeva A, et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019;363(6434):1463–1467. doi: 10.1126/science.aaw1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AB, Roco CM, Muscat RA, et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018;360(6385):176–182. doi: 10.1126/science.aam8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff DW, Dhingra DM, Thompson K, et al. High-throughput multimodal single-cell targeted DNA and surface protein analysis using the mission Bio Tapestri platform. Methods Mol Biol. 2022;2386:171–188. doi: 10.1007/978-1-0716-1771-7_12. [DOI] [PubMed] [Google Scholar]
- Shah S, Lubeck E, Zhou W, et al. In situ transcription profiling of single cells reveals spatial organization of cells in the mouse hippocampus. Neuron. 2016;92(2):342–357. doi: 10.1016/j.neuron.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood SA, Lee HJ, Angermueller C, et al. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat Methods. 2014;11(8):817–820. doi: 10.1038/Nmeth.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Su J, Zhang W. scGCN is a graph convolutional networks algorithm for knowledge transfer in single cell omics. Nat Comm. 2021;12(1):3826. doi: 10.1038/s41467-021-24172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckius M, Hafemeister C, Stephenson W, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017;14(9):865–868. doi: 10.1038/nmeth.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart T, Satija R. Integrative single-cell analysis. Nat Rev Genet. 2019;20(5):257–272. doi: 10.1038/s41576-019-0093-7. [DOI] [PubMed] [Google Scholar]
- Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–1902 e1821. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson E, Lord C, Reading J, et al. Simultaneous trimodal single-cell measurement of transcripts, epitopes, and chromatin accessibility using TEA-seq. Elife. 2021;10:e63632. doi: 10.7554/eLife.63632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turajlic S, Xu H, Litchfield K, et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell. 2018;173(3):581–594 e512. doi: 10.1016/j.cell.2018.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteicher AS, Tirosh I, Hebert C, et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017;355(6332):eaai8478. doi: 10.1126/science.aai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickovic S, Eraslan G, Salmen F, et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat Methods. 2019;16(10):987–990. doi: 10.1038/s41592-019-0548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickovic S, Lötstedt B, Klughammer J, et al. SM-Omics is an automated platform for high-throughput spatial multi-omics. Nat Comm. 2022;13(1):795. doi: 10.1038/s41467-022-28445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Allen WE, Wright MA, et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science. 2018;361(6400):eaat5691. doi: 10.1126/science.aat5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yuan P, Yan Z, et al. Single-cell multiomics sequencing reveals the functional regulatory landscape of early embryos. Nat Comm. 2021;12(1):1247. doi: 10.1038/s41467-021-21409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JD, Kozareva V, Ferreira A, et al. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell. 2019;177(7):1873–1887 e1817. doi: 10.1016/j.cell.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hou Y, Yin X, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148(5):886–895. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wen Y, Liang Y, et al. A plate-based single-cell ATAC-seq workflow for fast and robust profiling of chromatin accessibility. Nat Protoc. 2021;16(8):4084–4107. doi: 10.1038/s41596-021-00583-5. [DOI] [PubMed] [Google Scholar]
- Xu W, Yang W, Zhang Y, et al. ISSAAC-seq enables sensitive and flexible multimodal profiling of chromatin accessibility and gene expression in single cells. Nat Methods. 2022;19(10):1243–1249. doi: 10.1038/s41592-022-01601-4. [DOI] [PubMed] [Google Scholar]
- Yan R, Gu C, You D, et al. Decoding dynamic epigenetic landscapes in human oocytes using single-cell multi-omics sequencing. Cell Stem Cell. 2021;28(9):1641–1656 e1647. doi: 10.1016/j.stem.2021.04.012. [DOI] [PubMed] [Google Scholar]
- Yuan H, Yan M, Zhang G, et al. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 2019;47(D1):D900–D908. doi: 10.1093/nar/gky939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gu W, Hurles ME, et al. Copy number variation in human health, disease, and evolution. Annu Rev Genomics Hum Genet. 2009;10:451–481. doi: 10.1146/annurev.genom.9.081307.164217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Hocker JD, Miller M, et al. A single-cell atlas of chromatin accessibility in the human genome. Cell. 2021;184(24):5985–6001 e5919. doi: 10.1016/j.cell.2021.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Yu M, Huang H, et al. An ultra high-throughput method for single-cell joint analysis of open chromatin and transcriptome. Nat Struct Mol Biol. 2019;26(11):1063–1070. doi: 10.1038/s41594-019-0323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was generated for this manuscript.