Abstract
Malaria and schistosomiasis are infectious diseases that cause coagulation disorders, biochemical abnormalities, and thrombocytopenia. Malaria and Schistosoma mansoni co-infection cause exacerbations of health consequences and co-morbidities.This study aimed to compare the effect of malaria and Schistosoma mansoni co-infection and malaria infection on selected biochemical and coagulation profiles, and platelet count. An institutional-based comparative cross-sectional study was conducted from March 30 to August 10, 2022. A total of 70 individuals were enrolled in the study using a convenient sampling technique. Wet mount and Kato Katz techniques were conducted to detect Schistosoma mansoni in a stool sample. Blood films were prepared for the detection of plasmodium. The data was coded and entered into EpiData version 3.1 before being analyzed with SPSS version 25. An independent t test was used during data analysis. A P-value of less than 0.05 was considered statistically significant. The mean [SD] of alanine aminotransferase, aspartate aminotransferase, creatinine, total bilirubin, and direct bilirubin in the co-infected was higher than in malaria infected participants. However, the mean of total protein and glucose in co-infected was lower than in the malaria infected participants. The mean of prothrombin time, international normalization ratio, and activated partial thromboplastin time in co-infected was significantly higher, while the platelet count was lower compared to malaria infected participants. Biochemical and coagulation profiles, and platelet count status in co-infection were changed compared to malaria infected participants. Therefore, biochemical and coagulation profiles and platelet count tests should be used to monitor and manage co-infection related complications and to reduce co-infection associated morbidity and mortality.
Keywords: Biochemical profile, Coagulation profile, Malaria, S. mansoni, Co-infection, Dembiya, Ethiopia
Subject terms: Biochemistry, Microbiology
Introduction
Malaria is an infectious disease caused by protozoan parasites of the genus Plasmodium and transmitted by female Anopheles mosquitos1. Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi are the species that infect humans among the plasmodium species2. The majority of malaria infections are caused by P. falciparum and P. vivax in the world3. Based on the World Health Organization (WHO, 2020), malaria caused 627,000 deaths and 241,000,000 cases globally in 20204. An increase in malaria cases from 245 million in 2020 to an approximated 247 million cases globally in 84 malaria-endemic countries in 2021, with the WHO African Region causing the majority of this increase3. It was anticipated that 619,000 people would die from malaria in 2021. Ninety six percent of malaria deaths occur on the African continent, and children under five continue to account for the majority of malaria fatalities. In low-income nations, malaria is common, especially in children, pregnant women, and other susceptible groups like migrants3,5. Over 100 million people live in Ethiopia, and 68% of them are thought to be at risk for the illness. The annual reported cases of malaria are approximately 2.9 million, with 4,782,000 related deaths. During epidemics, the rate of morbidity and mortality rises rapidly6.
Malaria parasites go through a hepatocyte developmental stage. Sporozoites produced from the salivary gland of a mosquito must effectively target and penetrate hepatocytes7. These parasites replicate in the red blood cells of their human host following an initial replication phase in the liver. These erythrocytes replication cycles cause the typical disease symptoms, including fever and anemia, and eventually lead to organ failure and patient death8. Sequestration of erythrocytes with mature forms of the parasite in the deep vascular beds of vital organs is the major pathologic hallmark of severe malaria. Plasmodium falciparum malaria frequently causes life-threatening complications such as cerebral malaria, renal failure, hepatic dysfunction, jaundice, abnormal bleeding, and severe anemia9.
Malaria causes blood coagulation disorders, which contribute to inflammation and organ failure10. This is due to the sequestration of parasitized red blood cells in the microcirculation, generation of cytokines, and activation of the coagulation system in malaria patients11. Hence, malaria parasite-infected red blood cells enhance tissue factor expression by endothelial cells and support the assembly of coagulation complexes, generation of thrombin, activation of platelets, generation of microthrombi, and activation of the intrinsic pathway of coagulation12.
This causes prolongation of prothrombin time (PT) and activated partial thromboplastin time (APTT) and disseminated intravascular coagulation13. Due to peripheral platelet consumption and destruction, malaria results thrombocytopenia. Immune complexes are comprised of malaria antigens and Immunoglobulin G (IgG) and Immunoglobulin M (IgM) antibodies that cause platelets to be sequestered by macrophages in the spleen. This causes a reduction of platelet lifespan during malaria infection14.
Furthermore, malaria induces biochemical changes within the host11. Sporozoites invade hepatocytes in the liver stage, causing organ congestion, sinusoidal blockage, and cellular inflammation. Hepatocyte changes can result in the leakage of parenchyma and membrane enzymes into the general circulation15. Due to this, malaria causes biochemical abnormalities such as high bilirubin, elevated aspartate aminotransferase16, elevated alanine aminotransferase (ALT), and high creatinine, which increase the risk of disease complications17.
Schistosomiasis is a parasitic disease caused by the genus Schistosoma of blood-dwelling trematodes. According to the global burden of disease study, schistosomes infect 252,000,000 people, 90% of whom reside in sub-Saharan Africa, and are estimated to have cost the world 3,300,000 disability-adjusted life years18. Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum are the species which infect human being among the Schistosoma species19. Schistosoma species which causes disease, where it resides, and the severity of infection all influence the clinical presentation and pathology of the schistosomiasis20. The mesenteric plexus is a habitat to S. mansoni, and leads to intestinal or hepatosplenic schistosomiasis, which affect the intestine, liver, and spleen21. Schistosoma pathogenesis is mainly associated with the host’s immune responses to Schistosoma egg antigens, which results in the formation of granulomas in the intestine and the liver where the eggs are trapped22. This egg-induced granulomas causes liver failure, which leads to protein synthesis impairment, and increment of ALT and AST levels23.
Schistosomiasis also has an impact on hematological profiles, either directly through the gut or indirectly by aggravating blood loss through feces by rupturing blood vessels with the help of the egg spine24. The existence of thrombocytopenia in schistosomiasis patients may result from the association of schistosomiasis with splenomegaly, which enhances platelet destruction and filtering by the spleen24. Moreover, schistosomiasis causes coagulation disorders due to decrease hepatic synthesis of coagulation proteins, as well as decrease clearance of activated forms associated with consumption of coagulation factors. Due to the activation of the coagulation system, patients with schistosomiasis have elevated levels of coagulation activation markers, prolonged PT, and APTT, as well as extensive fibrin deposition over hepatic egg granulomas25.
Malaria and schistosomiasis are among the parasitic infections that shares common transmission areas in various tropical regions. The co-infection of malaria and schistosomiasis is common in Africa. In Ethiopia, the co-infection rate of schistosomiasis and malaria was reported to be 15%, resulting in a higher incidence of anemia when compared to individuals with malaria only26. Also, co-infections of these parasites are prevalent as a result of geographical overlap between schistosomiasis and malaria, resulting in various forms of association, exacerbated health consequences, and co-morbidities27. Moreover, the co-infection has a significant impact on the regulation of inflammatory factors associated with the progression of these infections and their respective morbidity28.
Studying on malaria and S. mansoni co-infection effect on biochemical and coagulation profiles, and platelet count is important to reduce different problems which are related to those co-infection like impaired protein synthesis, liver fibrosis, and coagulation disorders. Also, in order to put better clinical management and control of malaria, especially in malaria and Schistosoma co-endemic areas, information on the effect of malaria and S. mansoni co-infection and malaria infection on biochemical and coagulation profiles, and platelet count is needed. Furthermore, a clinician can develop a dependable diagnosis and effective treatment interventions for patients with malaria and S. mansoni co-infection by having knowledge of changes in biochemical and coagulation profiles as well as platelet count. However, study on the effect of co-infection with malaria and S. mansoni on biochemical and coagulation profiles and platelet count is still limited. Therefore, the current study attempted to compare the effect of malaria and S. mansoni co-infection and malaria infection on selected biochemical and coagulation profiles, and platelet count.
Methods and material
Study area
The study was conducted at Dembiya Primary Hospital, Chuahit Health Center, and Abrija Health Center, which are located within the Central Gondar Administrative Zone, Amhara Regional State. Dembiya Primary Hospital, Chuahit Health Center, and Abrija Health Center are found in Dembiya district. Dembiya district is located in Northwest Ethiopia, 35 km from Gondar, town of Central Gondar Administrative Zone, 183 km from Bahir Dar, capital of Amhara Region State and 762 km from Addis Ababa, capital city of Ethiopia, between 12° 39' N and 37°09' E. The southern part of the district is bordered by Lake Tana.
Dembiya district has a total population of 326,686, of whom 162,477 were men and 164,209 were women in 2017. It is located on altitude of between 1500 and 2600 m above sea-level. Its average annual rainfall and average temperature range from 995 to 1175 mm and 21.5 °C, respectively. There is one governmental primary hospital, ten health centers, nine private clinics, and 49 Health posts providing health care service for Dembiya and its surrounding people29. Rivers within this district include Angereb and Derma. These rivers serve as sources of water for bathing, washing clothes, and other domestic and recreational purposes. They may contain the major sources of malaria and S.mansoni infections. As reported by the District Health Bureau, malaria and S.mansoni are endemic in the study area.
Study design, period, and population
An institutional-based comparative cross-sectional study was conducted from March 30 to August 10/2022 at Dembiya Primary Hospital, Chuahit Health Center, and Abrija Health Center. The study populations were malaria and S. mansoni co-infected and malaria infected individuals. The study excluded pregnant women, people with multiple intestinal parasite infections, people receiving antiretroviral therapy, people with a history of chronic diseases like hypertension, cardiac disease, diabetes mellitus, chronic renal disease, and inherited bleeding disorders, people who were positive for hepatitis B and hepatitis C viruses, people who were taking anticoagulant therapy, smokers, and people who used alcohol excessively.
Sample size determination and sampling technique
Sample size was determined based on rules of thumb that have been recommended by van Voorhis and Morgan, 30 study subjects per group are required to detect real differences, which lead to about 80% power30. Thus, 70 study participants (35 infected by both malaria and S. mansoni and 35 malaria infected participants, sex and age match control) were enrolled in the study. A convenient sampling technique was used to select study participants.
Operational definition
The selected biochemical profiles were ALT, AST, creatinine, glucose, total bilirubin, direct bilirubin, and total protein. Similarly, the selected coagulation profiles were PT, INR, and APTT. The abnormalities of coagulation profiles and platelet count are, prolonged PT, PT > 16 Second, prolonged INR , INR > 1.1, prolonged APTT, APTT > 35 s, and low platelet count, platelet count < 150 × 103/µL31. Also, the normal value of ALT, AST, total bilirubin, direct bilirubin, creatinine, total protein and glucose were 0–41 U/L, 0–40 U/L, 0–1.2 mg/dl, 0–0.2 mg/dl, 0.7–1.2 mg/dl, 3.5–5.2 g/dl, and 74–109 mg/dl, respectively32.
Data collection procedures
Questionnaire survey
Socio-demographic characteristics of study participant were collected using a semi-structured questionnaire prepared in Amharic language. The questionnaire was initially written in English language and translated to Amharic language. Socio-demographic data was collected by principal investigator (PI), trained Medical Laboratory Personnel and Nurses. Trained clinicians who work at Dembiya selected health institutions Outpatient department (OPD) assessed the clinical information and patient history. Following identifying individuals who were eligible for the study, then the volunteer study participants were linked to Medical Laboratory Personnel for blood and stool samples collection.
Sample collection and laboratory examination
Microscopic detection of plasmodium
On a microscopic glass slide, 6 micro liter and 2 micro liter of capillary blood were placed separately for preparing thick and thin blood films, respectively. So blood films, both thick and thin, were prepared and air dried. Absolute methanol was used to fix thin blood films, and both films were stained for 10 min with a 10% Geimsa working solution.
An experienced malaria microscopist and PI read both thin and thick blood films with a 100 × objective lens and examine100 microscopic field’s to rule out the absence or presence of the malaria parasites. The discrepancy results were confirmed by another experienced malaria microscopist. Malaria parasitemia was determined in thick blood films along with 200 white blood cells. It was calculated by using the formula:Parasite/μL = Parasite counted/200WBC × Total white blood cells count. Parasitaemia was classified into three categories according to the number of parasites/μl of blood, low (< 1000), moderate (1000–9999) and high (≥ 10,000)33.
Microscopic detection of schistosome
Single stool specimen of about one gram was collected from each study participant. The sample was collected in a clean, dry, and leak-proof container with a unique identification number. Each stool specimen was first examined using the direct wet mount technique, and then Kato-Katz slides prepared on a template containing 41.7 mg of stool. Eggs counted for S. mansoni were recorded and later converted into eggs per gram (EPG) of stool, multiplying by a factor of 2434. Finally, infection intensity (light (1–99 EPG), moderate (100–399 EPG), and heavy (≥ 400 EPG)) was classified according to WHO criteria35.
Blood sample collection for biochemical and coagulation profiles and platelet count examination
Seven milli litre of venous blood was collected by blood collectors and PI using a sterile disposable plastic syringe after cleaning the venous puncture site with 70% alcohol. The collected blood sample was transferred into three test tubes. The first 2.7 ml of the collected blood sample was placed into 3.2 % sodium citrate anticoagulant test tube. For PT and APTT analysis, platelet-poor plasma was prepared by centrifuging at 1500 revolution per minute for 15 min34. Then plasma was separated and stored in Eppendorf tube at − 20 °C until processed. The coagulation profiles (PT, APTT, and INR) were done at Felege Hiwot Compressive Specialized Hospital Laboratory by using Semi-Auto Coagulation Analyzer (HumaClot DuoPlus Human, Germany)36.
The 2 ml of blood was transferred into ethylene diamine tetra-acetic acid (EDTA) test tube for platelet count. Platelet count was determined using Fully Auto Hematology Analyzer37.
The remaining venous blood was transferred into nonanticoagulated tube and allowed to clot at bench top. Then, blood centrifuged at 2500 revolutions per minute for four minute and serum was separated and stored in Eppendorf tube at − 20 °C until processed. Then, serum was analyzed by using Fully Auto Chemistry Analyzer38 for serum level of ALT, AST, creatinine, glucose, total bilirubin, direct bilirubin, and total protein38.
Serological tests
Immune-chromatographic assay was used to determine hepatitis B virus and hepatitis C virus to exclude individuals who were positive for these diseases34.
Urine collection and HCG examination
Urine was collected from all women whose age is 15–49 years in the study using a clean urine cup and a urine human chorionic gonadotropin (HCG) test was performed for both the cases and the controls using a rapid chromatographic immunoassay test strip to exclude pregnant women39.
Data quality control
Data collectors took appropriate training in order to maintain data quality. Quality control was performed by re-reading all slides by an expert laboratory technologist, to ensure the accuracy of the detection of Plasmodium and Schistosoma which was conducted by laboratory technologists. Standard operating procedures and manufacturer instructions were strictly followed throughout the procedures and all reagents were stored and prepared according to the manufacturer’s instructions.
Data management and analysis
Data was coded and entered into the EpiData (v3.1) statistical software, and exported to the statistical package of social science (SPSS) version 25 for analysis. The homogeneity of variance was checked by using Levene’s statistics. The Skewness, kurtosis, and Shapiro–Wilk normality tests were used for checking the distribution of continuous variables, and it revealed that some data were normally distributed and some data were not normally distributed for each group. Independent t test Difference test was used for comparison of normally distributed biochemical and coagulation profiles, and platelet count between groups. For each group, the result was provided as the mean and SD for normally distributed data. A p-value of less than 0.05 was considered statistically significant in all statistical analyses.
Ethics approval and consent to participate
This study was conducted after ethical approval was obtained from research and ethics committee of School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. Moreover, letter of support was submitted to the Dembiya Primary Hospital, Chuahit Health Center, and Abrija Health Center. Before starting the actual data collection, permission was obtained from the Hospital and Health Centers Chief Executive Officer or health facilities and the Administrator. Additionally, after explaining the purpose, benefits, and the possible risks of the study, written informed consent from the age of 16 and above and/or assent from those less than 16 years old study participants along with written informed consent from their respective parents/ caregiver/guardians was obtained. And also, written informed consent from illiterate study participants was obtained from their respective parents/guardians. All laboratory results were kept confidential. Since those were stored in a file using codes without study participants name. Apparently, those positive for parasites and with biochemical and coagulation profiles, and platelet count abnormality were linked to the hospital and health centers for appropriate treatment and management.
Result
Socio-demographic characteristics of study participants
A total of 70 study participants participated from Dembiya Primary Hospital [36 (51.4%)], Chuahit Health Center [18 (25.7%)], and Abrija Health Center [16 (22.9%)]. Participants from rural and urban were 38 (54.3%) and 32 (45.7%), respectively. Among 70 study participants, 35 were malaria and S. mansoni co-infected, and 35 were malaria infected participants. The prevalence of malaria and S. mansoni co-infection was higher in males (18, 51.4%) than females (17, 48.6%), and higher in the age group of 5–14 years (11, 31.4%) than those other age groups [15–24 years, 8 (22.9%), 25–34 years, 8 (22.9%), 35–44 years, 5 (14.3%), > 44 years, 3 (8.6%)] (Table 1).
Table 1.
Socio-demographic characteristics of the study participants at Dembiya selected health institutions, 2022.
| Socio-demographic characteristics | S. mansoni and malaria coinfection | Malaria | ||
|---|---|---|---|---|
| Frequency | % | Frequency | % | |
| Sex | ||||
| Male | 18 | 51.4 | 18 | 51.4 |
| Female | 17 | 48.6 | 17 | 48.6 |
| Age | ||||
| 5–14 | 11 | 31.4 | 7 | 20 |
| 15–24 | 8 | 22.9 | 11 | 31.4 |
| 25–34 | 8 | 22.9 | 9 | 25.7 |
| 35–44 | 5 | 14.3 | 2 | 5.7 |
| > 44 | 3 | 8.6 | 6 | 17.1 |
| Residence | ||||
| Urban | 16 | 45.7 | 16 | 45.7 |
| Rural | 19 | 54.3 | 19 | 54.3 |
| Occupation | ||||
| Government employee | 5 | 14.3 | 5 | 14.3 |
| Nongovernment employee | 2 | 5.7 | 1 | 2.9 |
| House wife | 3 | 8.6 | 6 | 17.1 |
| Student | 13 | 37.1 | 12 | 34.3 |
| Daily laborer | 3 | 8.6 | 2 | 5.7 |
| Farmer | 7 | 20 | 8 | 22.9 |
| Merchant | 2 | 5.7 | 1 | 2.9 |
| Educational status | ||||
| Illiterate | 7 | 20 | 11 | 31.4 |
| Can read and write | 4 | 11.4 | 1 | 2.9 |
| Primary school | 14 | 40 | 10 | 28.6 |
| Secondary school | 3 | 8.6 | 8 | 22.9 |
| College/university | 1 | 2.9 | 2 | 5.7 |
| Diploma and above | 6 | 17.1 | 3 | 8.6 |
| Family size | ||||
| 1–3 | 12 | 34.3 | 18 | 51.4 |
| 4–6 | 7 | 20 | 13 | 37.1 |
| 7–9 | 11 | 31.4 | 3 | 8.6 |
| > 9 | 5 | 14.3 | 1 | 2.9 |
| Income | ||||
| 500 Birr | 2 | 5.7 | 4 | 11.4 |
| 501–1000 | 2 | 5.7 | 3 | 8.6 |
| 1001–2500 | 11 | 31.4 | 13 | 37.1 |
| > 2500 | 20 | 57.1 | 15 | 42.9 |
Intensity of malaria parasitaemia
The overall mean of malaria parasitemia on S. mansoni and malaria coinfected group was 10,891.9. The mean of parasitemia of malaria in males and females were 8784.2 and 14,931.8 in S. mansoni and malaria co-infected group, respectively. From a total of 35 S. mansoni and malaria coinfected group 4 (11.4%), 18 (51.4%), and 13 (37.1%) were due to low, moderate, and high malaria parasitemia infection, respectively. The overall mean of malaria parasitemia on malaria infected group was 8539.4. The mean of parasitemia of malaria in males and females were 7866.7 and 9251.8 malaria infected group, respectively. And also, from a total of 35 malaria infected group 3 (8.6%), 24 (68.6%), and 8 (22.9%) were due to low, moderate, and high malaria parasitemia infection, respectively (Table 2).
Table 2.
Intensity of malaria parasitaemia among study participants at Dembiya selected health institutions, 2022.
| Level of parasitaemia | S. mansoni and malaria coinfected | Malaria infected |
|---|---|---|
| Low | 4 (11.4%) | 3 (8.6%) |
| Moderate | 18 (51.4%) | 24 (68.6%) |
| High | 13 (37.1%) | 8 (22.9%) |
Biochemical profiles among study participants
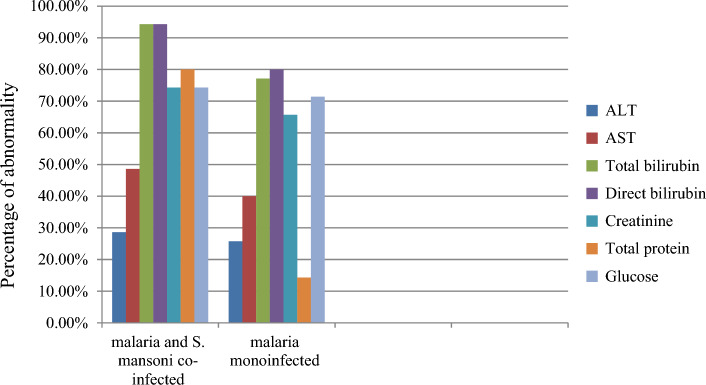
The percentages of elevated ALT, AST, total bilirubin, direct bilirubin, and creatinine were higher in malaria and S. mansoni co-infected participants than in malaria infected participants. In addition, the percentages of lowered total protein and glucose were higher in malaria and S. mansoni co-infected participants than in malaria infected participants.
Among malaria and S. mansoni co-infected participants, 10 (28.6%), 17 (48.6%), 33 (94.3%), 33 (94.3%), and 26 (74.3%) had elevated ALT, AST, total bilirubin, direct bilirubin, and creatinine, respectively. Similarly, 25 (71.4%), 17 (51.4%), 2 (5.7%), 2 (5.7%), 7 (20.0%), 7 (20%), and 8 (22.9%) of the malaria and S. mansoni co-infected participants had normal ALT, AST, total bilirubin, direct bilirubin, creatinine, total protein, and glucose, respectively. On the other hand, 28 (80%) and 26 (74.3%) of the malaria and S. mansoni co-infected participants had decreased total protein and glucose, respectively. Similarly, 9 (25.7%), 14 (40.0%), 27 (77.1%), 28 (80%) and 23 (65.7%) had elevated ALT, AST, total bilirubin, direct bilirubin, and creatinine, respectively, in malaria infected participants. However, 26 (74.3%), 21 (60%), 8 (22.9%), 7 (20%), 11 (31.4%), 18 (51.4%), and 10 (28.6%) malaria infected participants had normal ALT, AST, total bilirubin, direct bilirubin, creatinine, total protein, and glucose, respectively. Besides, 5 (14.3%) and 25 (71.4%) of the malaria infected participants had decreased total protein and glucose, respectively, (Fig. 1). Among coinfected male individuals 27.8%, 33.3%, 94.4%, 66.7%, and 88.9% had high ALT, AST, total bilirubin, creatinine, and direct bilrubin, respectively. Also, 29.4%, 64.7%, 94.3%, 82.4, and 100% co-infected females individuals had high ALT, AST, total bilirubin, creatinine, and direct bilrubin, respectively. Additionally, the percentages of elevated ALT, AST, total bilirubin, direct bilirubin, and creatinine in the age 5–14 year co-infected individuals were higher than other age groups. Also, the percentages of lowered total protein and glucose were high in the age 5–14 year group (Tables 3 and 4).
Figure 1.
Prevalence of abnormal biochemical profiles of study participants at Dembiya selected health institutions, 2022.
Table 3.
Prevalence of abnormal biochemical profiles of malaria and S. mansoni co-infected study participants at Dembiya selected health institutions, 2022.
| Profiles mean (SD) | Age | Gender | ||||||
|---|---|---|---|---|---|---|---|---|
| 5–14 N | 15–24 N | 25–34 N | 35–44 N | > 44 N | Male N | Female N | ||
| ALT | Normal | 3 | 3 | 4 | 4 | 3 | 7 | 7 |
| High | 8 | 4 | 4 | 1 | 0 | 11 | 10 | |
| AST | Normal | 2 | 7 | 5 | 2 | 2 | 8 | 6 |
| High | 9 | 1 | 3 | 3 | 1 | 10 | 11 | |
| Creatinine | Low | 0 | 0 | 2 | 0 | 0 | 1 | 1 |
| Normal | 0 | 1 | 1 | 2 | 3 | 5 | 2 | |
| High | 11 | 7 | 5 | 3 | 0 | 12 | 14 | |
| Total bilirubin | Normal | 0 | 4 | 3 | 2 | 1 | 1 | 1 |
| High | 11 | 4 | 5 | 3 | 2 | 17 | 16 | |
| Direct bilirubin | Normal | 0 | 2 | 3 | 1 | 1 | 2 | 1 |
| High | 11 | 7 | 5 | 3 | 2 | 16 | 16 | |
| Total protein | Low | 9 | 5 | 5 | 3 | 1 | 15 | 13 |
| Normal | 1 | 2 | 1 | 1 | 1 | 2 | 1 | |
| High | 1 | 1 | 2 | 1 | 1 | 1 | 3 | |
| Glucose | Low | 9 | 5 | 5 | 2 | 1 | 14 | 12 |
| Normal | 2 | 2 | 2 | 2 | 2 | 3 | 5 | |
| High | 0 | 1 | 1 | 1 | 0 | 1 | 1 | |
N number.
Table 4.
Prevalence of abnormal biochemical profiles of malaria infected study participants at Dembiya selected health institutions, 2022.
| Profiles mean (SD) | Age | Gender | ||||||
|---|---|---|---|---|---|---|---|---|
| 5–14 N | 15–24 N | 25–34 N | 35–44 N | > 44 N | Male N | Female N | ||
| ALT | Normal | 1 | 3 | 4 | 3 | 2 | 6 | 6 |
| High | 10 | 5 | 4 | 2 | 1 | 12 | 11 | |
| AST | Normal | 5 | 4 | 3 | 2 | 1 | 7 | 8 |
| High | 6 | 4 | 5 | 3 | 2 | 11 | 9 | |
| Creatinine | Low | 0 | 0 | 1 | 1 | 0 | 3 | 2 |
| Normal | 2 | 3 | 3 | 2 | 1 | 5 | 6 | |
| High | 9 | 5 | 4 | 2 | 2 | 10 | 9 | |
| Total bilirubin | Normal | 2 | 2 | 3 | 2 | 1 | 6 | 4 |
| High | 9 | 6 | 5 | 3 | 2 | 12 | 13 | |
| Direct bilirubin | Normal | 1 | 3 | 3 | 1 | 2 | 8 | 6 |
| High | 10 | 5 | 5 | 4 | 1 | 10 | 11 | |
| Total protein | Low | 10 | 5 | 4 | 3 | 1 | 8 | 7 |
| Normal | 1 | 3 | 3 | 2 | 2 | 6 | 4 | |
| High | 0 | 0 | 1 | 0 | 0 | 4 | 6 | |
| Glucose | Low | 10 | 6 | 6 | 3 | 2 | 12 | 14 |
| Normal | 1 | 2 | 1 | 2 | 1 | 5 | 1 | |
| High | 0 | 0 | 1 | 0 | 0 | 1 | 2 | |
N = number.
Comparison of biochemical profiles among study participants
In the malaria and S. mansoni co-infected participants, the mean [SD] values of ALT, AST, creatinine, total bilirubin, direct bilirubin, total protein, and glucose were 37.1 [7.17] IU/L, 46.9 [8.83] IU/L, 1.48 [0.47] mg/dL, 2.27 [0.69] mg/dL, 0.89 [0.54] mg/dL, 4.74 [1.61] g/dL, and 66.6 [14.0] mg/dL, respectively. And also, the mean [SD] of ALT, AST, creatinine, total bilirubin, direct bilirubin, total protein, and glucose were 36.5 [6.79] IU/L, 39.1 [6.76] IU/L, 1.38 [0.4] mg/dL, 1.81 [0.75] mg/dL, 0.58 [0.51] mg/dL, 4.88 [1.36] g/dL, and 74.5 [8.6] mg/dL in the malaria-infected participants, respectively, (Table 5). The biochemical profiles like ALT, AST, creatinine, total bilirubin, direct bilirubin, glucose, and total protein were normally distributed; a parametric test (Independent t test) was used to compare the mean difference of these biochemical profiles between cases and controls. As a result, an independent t test revealed higher mean values for ALT, AST, creatinine, total bilirubin, and direct bilirubin but lower mean value of Total protein and glucose in malaria and S. mansoni co-infected participants than in malaria infected participants. The mean of ALT, AST, total bilirubin, direct bilirubin, and creatinine in the age 5–14 year co-infected individuals were higher than. Also, the mean of total protein and glucose were low in the age 5–14 year group compared to other age groups, (Tables 6 and 7).
Table 5.
Comparison of biochemical profiles among study participants at Dembiya selected health institutions, 2022.
| Profiles | Malaria mono-infected participants mean (SD) | Malaria and S. mansoni co-infected participants mean (SD) | p value |
|---|---|---|---|
| ALT (IU/L) | 36.5 (6.79) | 37.1(7.17) | 0.736 |
| AST (IU/L) | 39.1 (6.76) | 46.9 (8.83) | 0.049 |
| Creatinine (mg/dL) | 1.38 (0.4) | 1.48 (0.47) | 0.325 |
| Total bilirubin (mg/dL) | 1.81 (0.75) | 2.27 (0.69) | 0.009 |
| Direct bilirubin (mg/dL) | 0.58 (0.51) | 0.89 (0.54) | 0.018 |
| Total protein (g/dL) | 4.88 (1.36) | 4.74 (1.61) | 0.685 |
| Glucose (mg/dL) | 74.5 (8.6) | 66.6 (14.0) | 0.047 |
Table 6.
Age, gender, and infection status based comparison of biochemical profiles among malaria and S. mansoni co-infected study participants at Dembiya selected health institutions, 2022.
| Profiles mean (SD) | Age | Gender | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5–14 | 15–24 | 25–34 | 35–44 | > 44 | p-value | Male | Female | p-value | |
| ALT | 39 (7) | 37 (7.5) | 36.9 (7.1) | 37.2 (4) | 30 (10) | > 0.05 | 35.9 (8) | 38.3 (5.5) | > 0.05 |
| AST | 47 (7.3) | 39 (8) | 40 (8) | 41 (4) | 32 (13) | > 0.05 | 39.8 (10) | 44.1 (7.1) | > 0.05 |
| Creatinine | 1.8(0.2) | 1.5 (0.5) | 1.4 (0.7) | 1.6 (0.6) | 1 (0.1) | > 0.05 | 1.4 (0.5) | 0.60 (0.50) | > 0.05 |
| Total bilirubin | 2.6 (0.6) | 2.1 (0.6) | 2.5 (0.5) | 1.6 (0.8) | 1.8 (0.3) | < 0.05 | 2.3 (0.8) | 12.28 (0.6) | > 0.05 |
| Direct bilirubin | 1.2 (0.5) | 0.9 (0.4) | 0.8 (0.6) | 0.6 (0.6) | 0.2 (0.06) | < 0.05 | 0.8 (0.6) | 0.94 (0.5) | > 0.05 |
| Total protein | 4 (2) | 4.8 (0.5) | 5 (1) | 5 (2) | 4.5 (0.03) | > 0.05 | 4.9 (1.5) | 4.61 (1.72) | > 0.05 |
| Glucose | 62 (12.6) | 68 (15) | 66 (11) | 68 (17) | 73 (19) | > 0.05 | 65.7 (16) | 67.5 (12.2) | > 0.05 |
Table 7.
Age, gender, and infection status based comparison of biochemical profiles among malaria infected study participants at Dembiya selected health institutions, 2022.
| Profiles mean (SD) | Age | Gender | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5–14 | 15–24 | 25–34 | 35–44 | > 44 | p-value | Male | Female | p-value | |
| ALT | 44 (7) | 34 (6) | 40.5 (7) | 38.4 (1.6) | 34.5 (8) | > 0.05 | 35.7 (6) | 37.3 (7.4) | > 0.05 |
| AST | 45 (6.5) | 38 (5.7) | 43.4 (5.8) | 42.2 (0.7) | 34.9 (8.7) | > 0.05 | 38.6 (6) | 39.5 (7.4) | > 0.05 |
| Creatinine | 1.5 (0.4) | 1.3 (0.3) | 1 (0.5) | 1.4 (0.6) | 1.3 (0.50 | > 0.05 | 1.4 (0.5) | 1.4 (0.32) | > 0.05 |
| Total bilirubin | 2.5 (0.9) | 1.6 (0.4) | 2.1 (0.9) | 2.1 (0.3) | 1.7 (0.8) | > 0.05 | 1.8(0.6) | 1.79 (0.86) | > 0.05 |
| Direct bilirubin | 0.9 (0.6) | 0.5 (0.41) | 0.8 (0.6) | 0.5 (0.2) | 0.5 (0.5) | > 0.05 | 0.4 (0.4) | 0.7 (0.56) | > 0.05 |
| Total protein | 3.5 (1.5) | 5.5 (1) | 4 (1.4) | 5.2 (1.2) | 4.5 (0.5) | > 0.05 | 5.2 (1.3) | 4.5 (1.3) | > 0.05 |
| Glucose | 60.3 (9.53) | 69.7 (5.83) | 63.6 (11) | 69.1 (2) | 72.5 (12) | > 0.05 | 70 (7.4) | 67 (11.4) | < 0.05 |
Coagulation profiles and platelet count among study participants
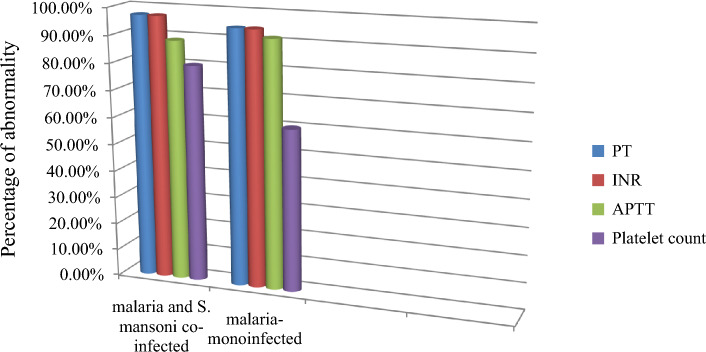
The percentages of prolonged PT, INR, APTT, and low platelet count were higher in malaria and S. mansoni co-infected participants than in malaria infected participants.
In malaria and S. mansoni co-infected participants, 34 (97.1%), 34 (97.1%), and 31 (88.6%) had prolonged PT, INR, and APTT, respectively. On the other hand, 1 (2.9%), 1 (2.9%), 1 (2.9%), and 7 (20%) of the malaria and S. mansoni co-infected participants had normal values of PT, INR, APTT, and platelet count, respectively. However, 28 (80%) of the malaria and S. mansoni co-infected participants had a low platelet count. In malaria infected participants, 33 (94.3%), 33 (94.3%) and 32 (91.4%) had prolonged PT, INR, and APTT, respectively. Likewise, 0 (0%), 1 (2.9%), 3 (8.6%), and 14 (40%) of the malaria infected participants had normal values of PT, INR, APTT, and platelet count, respectively. In addition, only 21 (60%) of the malaria infected participants had a low platelet count (Fig. 2). The percentages of prolonged PT, APTT, and INR in the age 5–14 co-infected individuals were higher than in other age groups. Also, the percentages of low platelets count were high in the age 5–14 year group (Tables 8 and 9).
Figure 2.
Prevalence of abnormal coagulation profiles and platelet count of study participants at Dembiya selected health institutions, 2022.
Table 8.
Prevalence of abnormal coagulation profiles and platelet count of malaria and S. mansoni co-infected study participants at Dembiya selected health institutions, 2022.
| Profiles mean (SD) | Age | Gender | ||||||
|---|---|---|---|---|---|---|---|---|
| 5–14 N | 15–24 N | 25–34 N | 35–44 N | > 44N | Male N | Female N | ||
| PT | Short | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Normal | 2 | 1 | 2 | 1 | 2 | 0 | 1 | |
| Prolonged | 9 | 6 | 6 | 3 | 0 | 18 | 16 | |
| INR | Short | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| Normal | 2 | 2 | 2 | 1 | 1 | 2 | 1 | |
| Prolonged | 8 | 5 | 5 | 4 | 2 | 16 | 15 | |
| APTT | Short | 0 | 1 | 2 | 1 | 0 | 1 | 0 |
| Normal | 2 | 3 | 2 | 2 | 1 | 7 | 8 | |
| Prolonged | 9 | 4 | 4 | 2 | 2 | 10 | 9 | |
| Platelet count | Low | 9 | 4 | 1 | 1 | 1 | 15 | 11 |
| Normal | 2 | 4 | 7 | 3 | 2 | 3 | 5 | |
| High | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
N number.
Table 9.
Prevalence of abnormal coagulation profiles and platelet count of malaria infected study participants at Dembiya selected health institutions, 2022.
| Profiles mean (SD) | Age | Gender | ||||||
|---|---|---|---|---|---|---|---|---|
| 5–14 N | 15–24 N | 25–34 N | 35–44 N | > 44 N | Male N | Female N | ||
| PT | Short | 1 | 1 | 1 | 0 | 1 | 2 | 1 |
| Normal | 2 | 3 | 2 | 2 | 2 | 1 | 2 | |
| Prolonged | 8 | 4 | 5 | 3 | 0 | 15 | 14 | |
| INR | Short | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Normal | 2 | 4 | 1 | 2 | 1 | 2 | 3 | |
| Prolonged | 9 | 4 | 7 | 3 | 2 | 15 | 13 | |
| APTT | Short | 0 | 2 | 3 | 1 | 0 | 1 | 1 |
| Normal | 2 | 3 | 2 | 1 | 2 | 3 | 2 | |
| Prolonged | 9 | 3 | 3 | 4 | 1 | 14 | 14 | |
| Platelet count | Low | 8 | 5 | 5 | 2 | 1 | 13 | 10 |
| Normal | 2 | 2 | 2 | 3 | 2 | 5 | 6 | |
| High | 1 | 1 | 1 | 0 | 0 | 0 | 1 | |
N number.
Comparison of coagulation profiles and platelet count among study participants
In the malaria and S. mansoni co-infected participants, the mean [SD] values of PT, INR, APTT, and platelet count were 25.3 (5.9) s, 2.43 (0.6), 42.8 (9.9) s, and 127.0 (33.9) × 103/μL, respectively. In addition, the mean [SD] values of PT, INR, APTT, and platelet count were 21.7 [10.20], 2.01 [1.19], 40.6 [5.7] sec, and 140 [64] × 103/μL, respectively, in the malaria infected participants (Table 10).
Table 10.
Comparison of coagulation profiles and platelet count among study participants at Dembiya selected health institutions, 2022.
| Profiles | Malaria mono-infected participants | malaria and S. mansoni co-infected participants | p value |
|---|---|---|---|
| PT | 21.7 (10.20) | 25.3 (5.9) | 0.048 |
| INR | 2.01 (1.19) | 2.43 (0.6) | 0.150 |
| APTT | 40.6 (5.7) | 42.8 (9.9) | 0.848 |
| Platelet count (103/μl) | 140 (64) | 127.0 (33.9) | 0.043 |
The coagulation profiles like PT, INR, APTT, and platelet count were normally distributed; a parametric test (Independent t test) was used to compare the mean difference of these coagulation profiles between cases and controls. As a result, an independent t test revealed higher mean values for PT, INR, and APTT but a lower mean value of platelet count in malaria and S. mansoni co-infected participants than in malaria infected participants. The mean of PT, INR, and APTT in the age 5–14 year co-infected individuals were higher than other age groups. Also, the mean of platelet count was low in the age 5–14 year group compared to other age groups (Tables 11 and 12).
Table 11.
Age, gender, and infection status based comparison of coagulation profiles and platelet count among malaria and S. mansoni co-infected study participants at Dembiya selected health institutions, 2022.
| Profiles mean (SD) | Age | Gender | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5–14 | 15–24 | 25–34 | 35–44 | > 44 | p-value | Male | Female | p-value | |
| PT | 27.5 (5) | 20.6 (6.7) | 26.1 (5.5) | 27.0 (3.98) | 23.9 (5.3) | > 0.05 | 26.2 (5.7) | 24.4 (6.1) | > 0.05 |
| INR | 2.7 (0.6) | 1.9 (0.7) | 2.5 (0.6) | 2.6 (0.4) | 2.2 (0.6) | > 0.05 | 2.52 (0.61) | 2.33 (0.66) | > 0.05 |
| APTT | 46 (6.4) | 41 (10) | 43 (13) | 41 (6) | 35 (10) | > 0.05 | 42.7 (10.85) | 42.8 (9.01) | > 0.05 |
| Platelet count | 101.7 (31) | 140 (33) | 135 (22) | 136 (41) | 147 (4) | < 0.05 | 124.2 (29.9) | 129.9 (38.4) | > 0.05 |
Table 12.
Age, gender, and infection status based comparison of coagulation profiles and platelet count among malaria infected study participants at Dembiya selected health institutions, 2022.
| Profiles mean (SD) | Age | Gender | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5–14 | 15–24 | 25–34 | 35–44 | > 44 | p-value | Male | Female | p-value | |
| PT | 27.6 (10.5) | 22 (5.9) | 25.9 (6) | 22.7 (4) | 21.3 (6) | > 0.05 | 3.1 (5.8) | 23.2 (8.4) | < 0.05 |
| INR | 2.8 (1) | 2 (0.7) | 2.4 (0.70) | 2.2 (0.4) | 1.9 (0.7) | > 0.05 | 2.2 (0.59) | 2.2 (0.9) | < 0.05 |
| APTT | 47 (10.5) | 41 (5.3) | 45 (7) | 46.4 (2) | 40 (8) | > 0.05 | 42.0 (7.3) | 42.7 (7.9) | > 0.05 |
| Platelet count | 150 (41) | 160 (35) | 186 (38) | 207 (68) | 193 (33) | > 0.05 | 135.9 (30.1) | 137.6 (53.4) | < 0.05 |
Discussion
Malaria and schistosomiasis co-infections are common, especially in Africa40. The co-infection of malaria and schistosomiasis has a significant impact, including exacerbated health consequences and co-morbidities.
In this study, the mean of malaria parasitemia was higher in malaria and S. mansoni co-infection compared to malaria infection. This finding was similar to a study conducted in Western Ethiopia in 2016 by Mebrate Dufera, that found the mean of malaria parasitemia was higher in malaria and S. mansoni co-infection compared to malaria mono-infection41. However, this finding was in deviation from a study conducted in Northwest Ethiopia which found higher mean malaria parasitemia in malaria monoinfection than malaria and S. mansoni co-infection42. The variation could be explained by variation in the intensity of schistosomiasis co-infection, exposure levels, and duration, and immunity status of the study participants, which would affect malaria parasitemia.
Also, in this study the percentages of elevated ALT, AST, total bilirubin , direct bilirubin, and creatinine in the age 5–14 year malaria and S. mansoni co-infected individuals were higher than other age groups (15–24 years, 25–34 years, 35–44 years, and > 44 years). In addition, the percentages of lowered total protein and glucose were high in the age 5–14 year group compared to other age groups (15–24 years, 25–34 years, 35–44 years, and > 44 years). This might be due to children's immune systems not be strong enough to combat infections, which makes them more susceptible to develop repeated and potentially fatal infections.
Moreover, in this study mean values of ALT, AST, creatinine, total bilirubin, and direct bilirubin in the malaria and S. mansoni co-infected participants were higher than in malaria infected participants. However, mean value of glucose and total protein in malaria and S. mansoni co-infected participants were lower than in malaria monoinfected participants. Furthermore, only mean values of AST, total bilirubin, and direct bilirubin were significantly higher but mean value of glucose significantly lower in the malaria and S. mansoni co-infected participants than in malaria infected participants. This finding was in consistent with a study conducted in Ethiopia that found lower mean value of glucose and total protein the malaria and S. mansoni co-infected participants as compared to malaria infected participants 42. This might be as a result of co-infection with S. mansoni and malaria, which can result in more severe and chronically devastating morbidity than a parasite infection alone. Participants with co-infections with malaria and S. mansoni can suffer from a high amount of intravascular hemolysis of parasitized red blood cells as a result of this, which results in a high level of bilirubin43. Additionally, this imbalance in the biochemical profiles may be caused by liver damage brought on by the co-infection of S. mansoni and malaria. Additionally, S. mansoni and malaria may result in hepatomegaly and jaundice, which raise liver enzymes and bilirubin levels25,44,45. Low levels of glucose and total protein could be caused by S. mansoni and malaria co-infection's decreased protein synthesis and glucose utilization46. On the contrary, this finding was in disagreement with a study conducted in Ethiopia that found higher mean value of ALT and AST the malaria infected participants as compared to malaria and S. mansoni co-infected participants. And also, This finding was contradictory with a study conducted in Western Kenya that found significantly lower median value of ALT and creatinine in the malaria and S. mansoni co-infected participants as compared to malaria mono-infected participants47.
This difference could be brought about by the differences of study participants, exposure levels and duration, infection severity, nutritional status, demographic factors, concurrent infections, parasite strains, and immunity levels.
Furthermore, in this study the percentages of prolonged PT, APTT, and INR in the age 5–14 malaria and S. mansoni co-infected individuals were higher than other age groups (15–24 years, 25–34 years, 35–44 years, and > 44 years). Also, the percentages of low platelets count were high in the age 5–14 year group compared to other age groups (15–24 years, 25–34 years, 35–44 years, and > 44 years). This may be because children's immune systems are less strong and matured, making them more vulnerable to infectious diseases and progress to severe disease.
This study showed that PT, INR, and APTT were higher in malaria and S. mansoni co-infected participants than in malaria infected participants. However, merely mean values of PT were significantly higher in the malaria and S. mansoni co-infected participants than in malaria monoinfected participants. Therefore, it might be concluded that the interaction between S. mansoni and malaria may make the coagulation problem worse. This could be as a result of having both malaria and S. mansoni infections at the same time, which had considerably higher rates of liver size enlargement than individuals who just had one of the diseases48.
In addition to producing cytokines and activating the coagulation system, malaria sequesters red blood cells in the microcirculation. Therefore, red blood cells infected with the malaria parasite stimulate the expression of tissue factors by endothelial cells and assist the formation of coagulation complexes, the production of thrombin, the activation of platelets, the production of microthrombi, and the activation of the intrinsic pathway of coagulation49. Schistosoma mansoni contributes to coagulation disorders by reducing hepatic production of coagulation proteins and decreasing clearance of activated forms related to coagulation factor consumption50,51.
In this study, the mean value of platelet count was significantly lower in malaria and S. mansoni co-infected participants compared to malaria infected participants. This finding was similar with a study conducted in western Kenya that found a significantly lower median value of platelet count in the malaria and S. mansoni co-infected group as compared to the malaria infected group47. In cases of malaria and S. mansoni coinfection, the decrease in platelet count may be brought on by the consumption and destruction of platelets by the parasites. Since malaria antigens and IgG and IgM antibodies found in immune complexes induce platelets to be trapped by macrophages in the spleen. Due to increased macrophage activity during malaria infection, this results in the loss of more platelets and a reduction in platelet lifespan52,53. Additionally, splenomegaly, which results in platelet degradation and filtering by the spleen and thrombocytopenia, may be associated with schistosomiasis24. There were limitations on this study. Pregnant women and children under five years old were not allowed to participate in this study; only individuals five years of age and older were participated. Furthermore, we solely used microscopy to measure parasite density; we did not count parasite infections using molecular methods like polymerase chain reaction.
Conclusions and recommendations
In the current study, biochemical and coagulation profiles, as well as platelet count, in malaria and S. mansoni co-infected participants were changed compared to malaria infected participants. Since mean values of ALT, AST, total bilirubin, direct bilirubin, and creatinine were higher, but glucose and total protein were lower in malaria and S. mansoni co-infected participants compared to malaria infected participants. Moreover, the mean values of PT, INR, and APTT were higher, but the platelet count was lower in malaria and S. mansoni co-infected participants compared to malaria infected participants. This implies that screening patients for biochemical and coagulation profiles, and platelet count changes has greatest role in treating malaria and S. mansoni co-infected patients. Furthermore, assessing biochemical and coagulation profiles and platelet count changes in patients with malaria and S. mansoni co-infection is an important step toward reducing malaria and S. mansoni co-infection associated morbidity and mortality.
Further study need to be conducted to elucidate the possible alteration of biochemical and coagulation profiles and platelet count consequences of malaria and S. mansoni co-infection in different epidemiological settings which includes children under the age of 5 years and pregnant women. Finally, we would like to recommend that patients be screened for malaria and S. mansoni co-infection-associated biochemical and coagulation profiles, and platelet count abnormalities to prevent biochemical and coagulation disorders.
Acknowledgements
We acknowledge the study participants, our colleagues and all staff members of Dembiya Primary Hospital, Chuahit Health Center, Abrija Health Center, and Felege Hiwot Compressive Specialized Hospital Laboratory staff.
Abbreviations
- ALT
Alanine aminotransferase
- APTT
Activated partial thromboplastin time
- AST
Aspartate aminotransferase
- EPG
Eggs per gram
- INR
International normalized ration
- ML
Milliliters
- PI
Principal investigator
- PT
Prothrombin time
- SD
Standard deviation
Author contributions
W.A. conceived, designed the study, interpretation of the data, and wrote the original draft of the manuscript. Z. A. participated in the data collection and entering data into the software. A.W. participated in the laboratory assay. M. A. participated in the data collection. A. A. performed statistical analysis of the data. W.L. and A.D. were involved in supervising the project, editing, and reviewing the draft of the manuscript. Finally, all the authors read and approved the final manuscript.
Data availability
We confirmed that all the data for this manuscript are available, if someone wants to request the data can contact the corresponding author Mr. Wagaw Abebe.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: Adane Derso and Wossenseged Lemma were omitted from the author list in the original version of this Article. Full information regarding the correction made can be found in the correction for this Article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/12/2024
A Correction to this paper has been published: 10.1038/s41598-024-64155-9
References
- 1.Crompton PD, et al. Malaria immunity in man and mosquito: Insights into unsolved mysteries of a deadly infectious disease. Annu. Rev. Immunol. 2014;32:157–187. doi: 10.1146/annurev-immunol-032713-120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recker M, Bull PC, Buckee CO. Recent advances in the molecular epidemiology of clinical malaria. F1000Research. 2018;7:1159. doi: 10.12688/f1000research.14991.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WH Organization . World malaria report 2022. World Health Organization; 2022. [Google Scholar]
- 4.Organization WH. WHO guideline for malaria 2021. World Health Organization; 2021. [Google Scholar]
- 5.Garrood WT, et al. Driving down malaria transmission with engineered gene drives. Front. Genet. 2022;13:891218. doi: 10.3389/fgene.2022.891218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girum T, Shumbej T, Shewangizaw M. Burden of malaria in Ethiopia, 2000–2016: Findings from the Global Health Estimates 2016. Trop. Dis. Travel Med. Vaccin. 2019;5:1–7. doi: 10.1186/s40794-019-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arredondo SA, et al. Secretory organelle function in the Plasmodium sporozoite. Trends Parasitol. 2021;37(7):651–663. doi: 10.1016/j.pt.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Watt A. A Pathophysiological, Clinical, and Epidemiological View of Malaria. Liberty University; 2023. [Google Scholar]
- 9.White NJ. Severe malaria. Malar. J. 2022;21(1):284. doi: 10.1186/s12936-022-04301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohiagu FO, et al. Pathophysiology of severe malaria infection. Asian J. Health Sci. 2021;7(2):ID22 –ID22. doi: 10.15419/ajhs.v7i2.492. [DOI] [Google Scholar]
- 11.Okagu IU, et al. Molecular mechanisms of hematological and biochemical alterations in malaria: A review. Mol. Biochem. Parasitol. 2022;247:111446. doi: 10.1016/j.molbiopara.2021.111446. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh K, Shetty S. Blood coagulation in falciparum malaria—A review. Parasitol. Res. 2008;102(4):571–576. doi: 10.1007/s00436-007-0832-0. [DOI] [PubMed] [Google Scholar]
- 13.Boral BM, Williams DJ, Boral LI. Disseminated intravascular coagulation. Am. J. Clin. Pathol. 2016;146(6):670–680. doi: 10.1093/ajcp/aqw195. [DOI] [PubMed] [Google Scholar]
- 14.Bayleyegn B, et al. Role of platelet indices as a potential marker for malaria severity. J. Parasitol. Res. 2021;2021:1–8. doi: 10.1155/2021/5531091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onyesom I, Onyemakonor N. Levels of parasitaemia and changes in some liver enzymes among malarial infected patients in Edo-Delta Region of Nigeria. Curr. Res. J. Biol. Sci. 2011;3(2):78–81. [Google Scholar]
- 16.Furnee C, et al. Effect of intestinal parasite treatment on the efficacy of oral iodized oil for correcting iodine deficiency in schoolchildren. Am. J. Clin. Nutr. 1997;66(6):1422–1427. doi: 10.1093/ajcn/66.6.1422. [DOI] [PubMed] [Google Scholar]
- 17.Al-Salahy M, et al. Parasitaemia and its relation to hematological parameters and liver function among patients malaria in Abs, Hajjah, Northwest Yemen. Interdiscip. Perspect. Infect. Dis. 2016 doi: 10.1155/2016/5954394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotez PJ, et al. The global burden of disease study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014;8(7):e2865. doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelwan ML. Schistosomiasis: Life cycle, diagnosis, and control. Curr. Ther. Res. 2019;91:5–9. doi: 10.1016/j.curtheres.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aagaard-Hansen BBJ. The Social Context of Schistosomiasis and its Control. World Health Organization; 2008. [Google Scholar]
- 21.Colley DG, et al. Human schistosomiasis. Lancet. 2014;383(9936):2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costain AH, MacDonald AS, Smits HH. Schistosome egg migration: Mechanisms, pathogenesis and host immune responses. Front. Immunol. 2018;9:3042. doi: 10.3389/fimmu.2018.03042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doenhoff MJ, Chiodini PL, Hamilton JV. Specific and sensitive diagnosis of schistosome infection: Can it be done with antibodies? Trends Parasitol. 2004;20(1):35–39. doi: 10.1016/j.pt.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Da’dara AA, Skelly PJ. Schistosomes versus platelets. Thromb. Res. 2014;134(6):1176–1181. doi: 10.1016/j.thromres.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Leite LAC, et al. Hemostatic dysfunction is increased in patients with hepatosplenic schistosomiasis mansoni and advanced periportal fibrosis. PLoS Negl. Trop. Dis. 2013;7(7):e2314. doi: 10.1371/journal.pntd.0002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degarege A, et al. Malaria and related outcomes in patients with intestinal helminths: A cross-sectional study. BMC Infect. Dis. 2012;12:1–9. doi: 10.1186/1471-2334-12-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinung’hi SM, et al. Coinfection of intestinal schistosomiasis and malaria and association with haemoglobin levels and nutritional status in school children in Mara region Northwestern Tanzania: A cross-sectional exploratory study. BMC Res. Notes. 2017;10(1):1–11. doi: 10.1186/s13104-017-2904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Hesran J-Y, et al. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans. R. Soc. Trop. Med. Hyg. 2004;98(7):397–399. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 29.others., Z.F.A., Dembiya district finance and economic development office annual report 2017,Koladiba: officer of finance and economic development, Amhara Region, Ethiopia,
- 30.VanVoorhis CW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor. Quant. methods Psychol. 2007;3(2):43–50. doi: 10.20982/tqmp.03.2.p043. [DOI] [Google Scholar]
- 31.Patel IJ, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J. Vasc. Interv. Radiol. 2012;23(6):727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Arrasyid NK, et al. Correlation between soil-transmitted helminths infection and serum iron level among primary school children in Medan. Open Access Maced. J. Med. Sci. 2017;5(2):117. doi: 10.3889/oamjms.2017.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atroosh WM, et al. Genetic variation of pfhrp2 in Plasmodium falciparum isolates from Yemen and the performance of HRP2-based malaria rapid diagnostic test. Parasites Vectors. 2015;8(1):1–8. doi: 10.1186/s13071-015-1008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eyayu T, et al. Basic coagulation profiles and platelet count among Schistosoma mansoni-infected adults attending Sanja primary hospital, northwest Ethiopia. Res. Rep. Trop. Med. 2020;11:27. doi: 10.2147/RRTM.S244912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO Expert Committee on the Control of Schistosomiasis. World Health Organization . Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis: Report of a WHO Expert Committee. World Health Organization; 2002. [PubMed] [Google Scholar]
- 36.HUMANGmbH. HumaClot Duoplus User Manual. HUMAN GmbH, G.
- 37.https://www.siemens-healthineers.com/cl/hematology/systems/advia-560-hematology-system, A.U.M., Germany, (2022).
- 38.www.roche.com. Roche Diagnostics GmbH, D. COBAS C 311 user manual, Mannheim, Germany, (2022).
- 39.WH Organization . Reproductive Health Indicators: Guidelines for their Generation, Interpretation and Analysis for Global Monitoring. World Health Organization; 2006. [Google Scholar]
- 40.Mwangi TW, Bethony J, Brooker S. Malaria and helminth interactions in humans: An epidemiological viewpoint. Ann. Trop. Med. Parasitol. 2006;100(7):551–570. doi: 10.1179/136485906X118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dufera M, et al. Reciprocal increase of malaria parasitaemia, S. mansoni egg count and anemia in malaria-Schistosomiasis mansoni co-infected individuals in Fincha Sugar Estate, western Ethiopia. J. Sci. Technol. Arts Res. 2016;5(4):10–21. [Google Scholar]
- 42.Getie S, et al. Prevalence and clinical correlates of Schistosoma mansoni co-infection among malaria infected patients Northwest Ethiopia. BMC Res. Notes. 2015;8:1–6. doi: 10.1186/s13104-015-1468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazigo HD, et al. Co-infections with Plasmodium falciparum, Schistosoma mansoni and intestinal helminths among schoolchildren in endemic areas of northwestern Tanzania. Parasites Vectors. 2010;3(1):1–7. doi: 10.1186/1756-3305-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onyesom I. Activities of some liver enzymes in serum of P. falciparum malarial infected humans receiving artemisinin and non-artemisinin-based combination therapy. Ann. Biol. Res. 2012;3(7):3097–3100. [Google Scholar]
- 45.Silva FLD, et al. Alterations in the lipid profiles and circulating liver enzymes in individuals infected by Schistosoma mansoni. Rev. Soc. Bras. Med. Trop. 2018;51:795–801. doi: 10.1590/0037-8682-0113-2018. [DOI] [PubMed] [Google Scholar]
- 46.Adamu J, Jigam A. Effects of malaria infection on some haematological and biochemical parameters in the general population and pregnant malaria patients attending two district hospitals in Niger State, Nigeria. Glob. J. Infect. Dis. Clin. Res. 2019;5(1):001–005. doi: 10.17352/gjidcr.000021. [DOI] [Google Scholar]
- 47.Kamau E, et al. Epidemiological and clinical implications of asymptomatic malaria and schistosomiasis co-infections in a rural community in western Kenya. BMC Infect. Dis. 2021;21:1–13. doi: 10.1186/s12879-021-06626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sangweme DT, et al. Impact of schistosome infection on Plasmodium falciparum malariometric indices and immune correlates in school age children in Burma Valley, Zimbabwe. PLoS Negl. Trop. Dis. 2010;4(11):e882. doi: 10.1371/journal.pntd.0000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akinosoglou KS, Solomou EE, Gogos CA. Malaria: A haematological disease. Hematology. 2012;17(2):106–114. doi: 10.1179/102453312X13221316477336. [DOI] [PubMed] [Google Scholar]
- 50.Tanabe M. Haemostatic abnormalities in hepatosplenic schistosomiasis mansoni. Parasitol. Int. 2003;52(4):351–359. doi: 10.1016/S1383-5769(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 51.Mebius MM, et al. Interference with the host haemostatic system by schistosomes. PLoS Pathog. 2013;9(12):e1003781. doi: 10.1371/journal.ppat.1003781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coelho HCC, et al. Thrombocytopenia in Plasmodium vivax malaria is related to platelets phagocytosis. PLoS One. 2013;8(5):e63410. doi: 10.1371/journal.pone.0063410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhan X, et al. Identification of a novel thrombospondin-related anonymous protein (BoTRAP2) from Babesia orientalis. Parasites Vectors. 2019;12(1):1–8. doi: 10.1186/s13071-019-3457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We confirmed that all the data for this manuscript are available, if someone wants to request the data can contact the corresponding author Mr. Wagaw Abebe.