Abstract
Newborn gnotobiotic pigs were inoculated twice perorally (p.o.) (group 1) or intramuscularly (i.m.) (group 2) or three times i.m. (group 3) with inactivated Wa strain human rotavirus and challenged with virulent Wa human rotavirus 20 to 24 days later. To assess correlates of protection, antibody-secreting cells (ASC) were enumerated in intestinal and systemic lymphoid tissues from pigs in each group at selected postinoculation days (PID) or postchallenge days. Few virus-specific ASC were detected in any tissues of group 1 pigs prior to challenge. By comparison, groups 2 and 3 had significantly greater numbers of virus-specific immunoglobulin M (IgM) ASC in intestinal and splenic tissues at PID 8 and significantly greater numbers of virus-specific IgG ASC and IgG memory B cells in spleen and blood at challenge. However, as for group 1, few virus-specific IgA ASC or IgA memory B cells were detected in any tissues of group 2 and 3 pigs. Neither p.o. nor i.m. inoculation conferred significant protection against virulent Wa rotavirus challenge (0 to 6% protection rate), and all groups showed significant anamnestic virus-specific IgG and IgA ASC responses. Hence, high numbers of IgG ASC or memory IgG ASC in the systemic lymphoid tissues at the time of challenge did not correlate with protection. Further, our findings suggest that inactivated Wa human rotavirus administered either p.o. or parenterally is significantly less effective in inducing intestinal IgA ASC responses and conferring protective immunity than live Wa human rotavirus inoculated orally, as reported earlier (L. Yuan, L. A. Ward, B. I. Rosen, T. L. To, and L. J. Saif, J. Virol. 70:3075–3083, 1996). Thus, more efficient mucosal delivery systems and rotavirus vaccination strategies are needed to induce intestinal IgA ASC responses, identified previously as a correlate of protective immunity to rotavirus.
Rotaviruses are the most important cause of infant and childhood dehydrating gastroenteritis worldwide (11). Several strategies for developing an effective vaccine for preventing severe rotaviral disease have been pursued (16, 18). To date, all candidate human vaccines tested have been live replicating attenuated rotaviruses delivered orally. Such candidate vaccines have shown inconsistent efficacies in clinical trials (20, 32, 35), indicating the need for improved or alternative vaccine strategies to obtain more consistent and efficacious results. Recent studies of active immunity indicate that parenteral inoculation (intramuscular [i.m.] or intraperitoneal [i.p.]) of mice and rabbits with inactivated rotavirus or rotavirus-like particles, with or without adjuvant, generated complete or significant partial protection against rotavirus shedding following homotypic and heterotypic rotavirus challenge (9, 10, 22). These results suggest that nonreplicating-rotavirus vaccines may offer alternative approaches for immunization against rotavirus.
Although mice and rabbits serve as useful models for evaluation of immune responses to rotavirus, older mice and rabbits are refractory to disease after both homologous and heterologous rotavirus inoculations (4, 5, 9), which restricts assessment of protective immunity to prevention of virus shedding only. Gnotobiotic pigs remain susceptible to heterologous (human) and homologous (porcine) rotavirus infections and rotavirus-associated diarrhea for at least 6 weeks (6, 27–29, 36, 37, 41). Neonatal pigs and human infants also have many similarities in their gastrointestinal physiology, milk diets, and mucosal immune development (19, 25). Thus, to better understand the immunogenicity of inactivated human rotavirus (HRV), we examined the relative capacities of peroral (p.o.) or parenteral (i.m.) inoculation of gnotobiotic piglets with inactivated HRV to induce virus-specific antibody-secreting cell (ASC) responses in intestinal and systemic lymphoid tissues. The ability of each inactivated rotavirus inoculum to protect against disease was assessed against subsequent challenge with the same strain of virulent HRV.
MATERIALS AND METHODS
Virus.
The attenuated (cell culture-adapted) Wa strain (G1P1A [8];[;]) of HRV derived from a cell lysate from the 27th passage in fetal rhesus monkey kidney (MA104) cells (36, 37, 40) was used to prepare the inactivated virus inoculum. A pool of intestinal contents from the 16th gnotobiotic pig passage of virulent Wa rotavirus was diluted in minimal essential medium (GIBCO, Life Technologies, Grand Island, N.Y.) for use as the challenge inoculum (36, 37, 40). The 50% infective dose (ID50) of the virulent Wa rotavirus inoculum for gnotobiotic pigs was previously determined to be at least 1 fluorescent focus-forming unit (FFU) (36).
The rotavirus antigen used for in vitro stimulation of the cultured mononuclear cells (MNC) to enumerate memory B cells was prepared from the cell culture-attenuated Wa HRV. Rotavirus from infected MA104 cell lysates (titer, ∼107 FFU/ml) was semipurified by centrifugation (112,700 × g) through a 40% (wt/vol) sucrose cushion. The viral pellets were resuspended to ∼1/25 of the original volume in 0.05 M Tris buffer (pH 7.5) containing 0.1 M NaCl and 0.002 M CaCl2 (Tris-buffered saline–CaCl2), aliquoted, and stored at −70°C (6). The protein concentration was 1.33 mg/ml. Control antigen from mock-infected MA104 cell lysates was prepared in an identical manner and stored.
Virus inactivation.
The attenuated Wa rotavirus was inactivated by using binary ethylenimine (BEI) as previously described (1, 12). The supernatant of rotavirus-infected MA104 cell lysates (the virus titer before inactivation was approximately 107 FFU/ml) was treated with 10% BEI for 18 h at 37°C with continuous agitation. Sodium thiosulfate solution (1 M) was added to the virus-BEI mixture to a final concentration of 10% to inactivate residual BEI (21). Inactivation was verified by loss of rotavirus infectivity in MA104 cells. The inactivated virus was aliquoted and stored at −70°C.
Inoculation and challenge of gnotobiotic pigs.
Near-term pigs from five sows were derived and maintained in gnotobiotic isolation units as described previously (23). At 3 to 5 days of age, 17 pigs (group 1) were fed 5 ml each of 100 mM sodium bicarbonate to reduce gastric acidity (14) and were p.o. inoculated 10 min later with 5 ml each of inactivated Wa rotavirus without adjuvant; 20 pigs (group 2) were i.m. inoculated at multiple sites with 5 ml each of inactivated Wa rotavirus mixed with an equal volume of incomplete Freund’s adjuvant (IFA). Both group 1 and 2 pigs were reinoculated by the same route and with the same dose 10 days later. Four additional pigs (group 3) were treated the same as group 2 but were given a third i.m. inoculation 7 days after the second i.m. inoculation. Eight pigs from group 1, 12 pigs from group 2, and 2 pigs from group 3 were challenged at postinoculation days (PID) 20 to 24 with ∼106 FFU (∼106 ID50s) of virulent Wa rotavirus (36, 37, 41). Seventeen pigs (challenge controls) were given equal volumes of diluent p.o. (n = 11) or i.m. (n = 6) and challenged with the same dose of virulent Wa rotavirus as mentioned above at PID 20 to 24. Another nine age-matched naive pigs were mock inoculated and mock challenged with diluent and served as negative controls.
Pigs were observed daily for diarrhea postchallenge. Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid. Pigs with daily fecal consistency scores of ≥2 were considered diarrheic. The mean cumulative score was calculated as [∑ daily fecal scores from postchallenge days (PCD) 1 to 7]/n. Rectal swabs were collected daily and virus shedding was determined by antigen capture enzyme-linked immunosorbent assay and cell culture immunofluorescence assay with rectal swab fluids as described previously (2, 36, 41). Weekly blood samples were collected from all pigs before and after inoculation and challenge. The virus-neutralizing (VN)-antibody titers in serum were determined by plaque reduction virus neutralization assay as described previously (29, 36, 41). To collect the MNC for the enzyme-linked immunospot (ELISPOT) assay, one to six pigs from each group were euthanized at PID 8 and at PCD 0 (PID 20 to 24), PCD 4, and PCD 7.
Isolation of MNC.
The small intestines (duodenum and ileum), mesenteric lymph nodes (MLN), spleen, and blood were collected from each pig at euthanasia and their MNC were isolated as previously described (36, 41). The MNC were resuspended at a concentration of 5 × 106 cells/ml of complete medium consisting of RPMI 1640 (GIBCO BRL) supplemented with 8% fetal bovine serum, 20 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 μg of gentamicin per ml, 10 μg of ampicillin per ml, and 50 μM 2-mercaptoethanol.
In vitro stimulation of cultured, virus-sensitized MNC.
To determine the number of memory B cells in lymphoid tissues of virus-sensitized pigs, the isolated MNC were restimulated in vitro with semipurified attenuated Wa rotavirus antigen as previously described (33, 34) and then tested by ELISPOT assay. Briefly, optimized amounts of semipurified Wa rotavirus antigen or mock-infected MA104 cell control antigen were added (∼40 to 50 μg of protein/well) to triplicate wells of each cell preparation (2.5 × 106 MNC in 1 ml of E-RPMI per well of a 24-well tissue culture plate [Corning Glass Works, Corning, N.Y.]) at the beginning of incubation. After 3 days of culture (5% CO2, 37°C), 450 μl of supernatant fluid was removed and 600 μl of fresh medium was added to each well. On the fifth day, MNC were rinsed once with wash medium and virus-specific ASC were enumerated by ELISPOT assay at three (5 × 105, 5 × 104, and 5 × 103) or more dilutions.
ELISPOT assay for virus-specific ASC and total IgSC.
ELISPOT assays to enumerate isotypes of virus-specific ASC on acetone-fixed, rotavirus-infected cell plates and total-immunoglobulin (Ig)-secreting cells (IgSC) on anti-Ig-coated plates were conducted by using previously published methods and reagents (6, 41). Briefly, Wa rotavirus-infected fixed-cell plates (for virus-specific ASC) and plates coated with affinity-purified goat anti-pig IgM (25 μg/ml) (Kirkegaard & Perry Laboratories [KPL] Inc., Gaithersburg, Md.), goat anti-pig IgA (30 μg/ml) (Bethyl Laboratories Inc., Montgomery, Tex.), and goat anti-pig IgG (1 μg/ml) (Bethyl Laboratories Inc.) (for total isotype-specific IgSC) were washed with deionized water prior to use. Single cell suspensions of MNC from each tissue were added to duplicate wells (5 × 105, 5 × 104, and 5 × 103 cells/well). Plates were incubated for ∼12 h at 37°C in 5% CO2 and then washed and incubated with biotinylated mouse monoclonal antibody (purified ascites fluids) to pig IgG (derived from hybridoma 3H7; 0.03 μg/ml), pig IgA (derived from hybridoma 6D11; 0.04 μg/ml), or pig IgM (derived from hybridoma 5C9; 0.35 μg/ml) (hybridomas provided by P. Paul, Iowa State University, Ames) (24) for 2 h at room temperature. Plates were washed and horseradish peroxidase-conjugated streptavidin (KPL Inc.) was added (diluted 1:30,000 in phosphate-buffered saline). After incubation for 1 h at room temperature, the plates were washed, and spots were developed with tetramethylbenzidine and the H2O2 peroxidase substrate system (KPL Inc.). The numbers of virus-specific ASC and total IgSC were determined by counting blue spots in the wells and were reported as the number of virus-specific ASC or total IgSC per 5 × 105 MNC.
Statistical analyses.
The mean numbers of ASC and IgSC were calculated for treatment groups 1, 2, and 3 and controls at the following times: PID 8, PCD 0 (PID 20 to 24), PCD 4, and PCD 7. The statistical analyses of the virus-specific ASC in each group at the selected times were done by two-way analysis of variance with statistical analysis systems (SAS Institute Inc., Cary, N.C.) and Student’s t test. The serum VN-antibody titers, virus shedding, and diarrhea data were analyzed by Kruskall-Wallis one-way analysis of variance and the Mann-Whitney U test. The correlations between protection from challenge and numbers of virus-specific ASC at PCD 0 (PID 20 to 24) were determined by Spearman’s correlation. Statistical significance was assessed at a P value of <0.05.
RESULTS
Clinical and serologic responses to virulent Wa rotavirus challenge.
The serum VN-antibody titers at challenge and the clinical responses of each group postchallenge are summarized in Table 1. All pigs from each group shed virus as detected by enzyme-linked immunosorbent assay after challenge. Seven of 8 (88%) group 1 pigs, 10 of 12 (83%) group 2 pigs, and 2 of 2 (100%) group 3 pigs developed diarrhea. Hence, the two p.o. inoculations of pigs with inactivated Wa rotavirus conferred no protection (0%) and the two or three i.m. inoculations conferred little protection (6 and 0%, respectively) against diarrhea following virulent Wa HRV challenge (Table 1). Only the duration of virus shedding was significantly lower in the virus-inoculated pigs than in the challenged diluent-inoculated controls (Table 1). No significant differences in diarrhea and virus shedding data between group 3 pigs and group 2 pigs were found, although the tendency was toward a lower mean peak fecal titer of virus shed and a lower mean cumulative diarrhea score in group 3 pigs than in group 2 pigs.
TABLE 1.
Fecal virus shedding and clinical disease in Wa HRV-inoculated gnotobiotic pigs following oral challenge with live virulent Wa HRVa
| Exptl groupb | n | Serum VN GMT prechallengec | Virus sheddingd
|
Diarrheae
|
Rate of protection against diarrhea (%)f | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % shedding | Mean time (days) to onset ± SEM | Mean duration (days) ± SEM | Avg peak titer shed (FFU/ml) | % with diarrhea | Mean duration (days) ± SEM | Mean cumulative score ± SEM | ||||
| BEI-inactivated Wa HRV | ||||||||||
| Group 1 | 8 | 4A | 100 | 1.8 ± 0.2A | 3.3 ± 0.3A | 3.2 × 105AB | 88 | 2.0 ± 0.5AB | 8.3 ± 1.6A | 0 |
| Group 2 | 12 | 4,000B | 100 | 1.7 ± 0.2A | 3.2 ± 0.3A | 1.3 × 105A | 83 | 2.8 ± 0.4AB | 8.4 ± 0.8A | 6 |
| Group 3 | 2 | 10,951B | 100 | 2.0 ± 0.0A | 3.5 ± 0.7ABC | 3.0 × 104AB | 100 | 2.5 ± 0.7AB | 7.3 ± 1.1AB | 0 |
| Live Wa HRV | ||||||||||
| Attenuated | 18 | 46C | 83 | 1.9 ± 0.2A | 2.1 ± 0.3B | 5.4 × 104B | 61 | 1.7 ± 0.4B | 6.6 ± 0.9BC | 31 |
| Virulent | 25 | 79C | 0 | NA | NA | <250C | 12 | 0.5 ± 0.2C | 5.0 ± 0.4CD | 86 |
| Controls | ||||||||||
| Diluent inoculated | 17 | <4A | 100 | 2.0 ± 0.2A | 4.4 ± 0.3C | 3.5 × 105A | 88 | 3.2 ± 0.5A | 9.6 ± 0.9A | NA |
| Mock challenged | 9 | <4A | NA | NA | NA | NA | 11 | 0.2 ± 0.2C | 4.8 ± 0.8D | NA |
A, B, C, and D indicate values that are significantly different at a P value of <0.05. NA, not applicable.
Gnotobiotic pigs were inoculated p.o. or i.m. at 3 to 5 days of age with BEI-inactivated Wa HRV (two doses, 10 days apart, or three doses, 10 and 7 days apart); controls were sham inoculated with diluent. IFA was used with all i.m. inoculations. Pigs given live Wa HRV were inoculated and challenged as described previously (34, 37). Nine age-matched sham-inoculated gnotobiotic pigs were mock challenged to serve as controls.
Determined by plaque reduction neutralization assay at PCD 0 [PID 21 to 24]. The numbers of pigs tested for serum VN antibody in each group were 17 for group 1, 20 for group 2, 4 for group 3, 54 for virulent Wa HRV, 45 for attenuated Wa HRV, 10 for diluent-inoculated controls, and 8 for mock-challenged controls.
Determined by cell culture immunofluorescent infectivity assays.
Diarrhea was present if the fecal consistency score was ≥2 during the first week postchallenge. Fecal consistency was scored daily as described in Materials and Methods. Mean cumulative score = [(∑ fecal scores for 1 week postchallenge)/n].
Protection rate = [1 − (percentage of Wa HRV-inoculated pigs in each group with diarrhea/percentage of diluent-inoculated control pigs with diarrhea)] × 100.
In groups 2 and 3, 100% of pigs seroconverted, with high geometric mean titers (GMT) of VN antibody in serum (VN GMT = 4,000 and 10,951, respectively) to rotavirus at challenge (PID 20 to 24), whereas only 20% of group 1 seroconverted at challenge, resulting in a group 1 VN GMT of 4 (Table 1). The VN GMT of group 1 and 2 pigs increased about 20- and 2-fold by PCD 7, respectively, over the prechallenge VN GMT (data not shown). No correlation between the VN GMT in serum and protection was found.
Rotavirus-specific ASC responses in vivo.
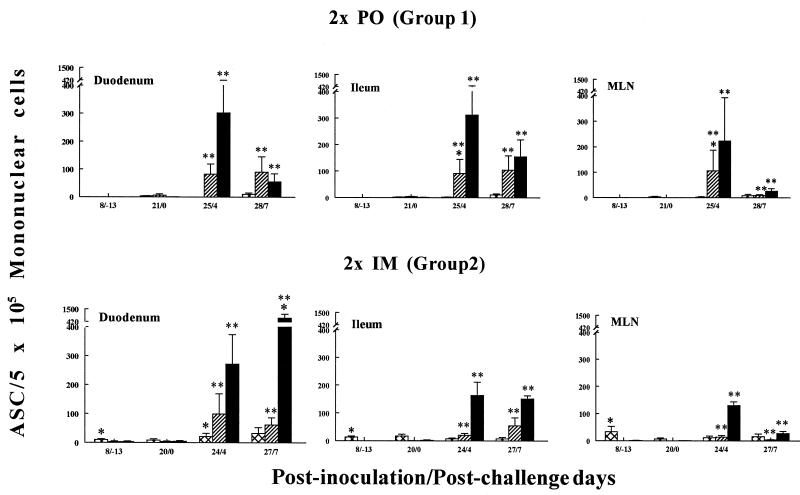
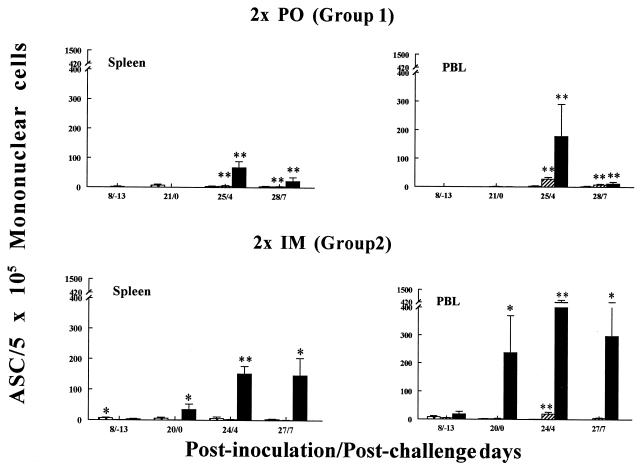
The kinetics and tissue distribution of virus-specific ASC isotypes in pigs p.o. or i.m. inoculated twice with inactivated Wa rotavirus are depicted in Fig. 1 and 2 (data for group 3 pigs was not included because too few pigs were tested [n = 1 or 2] at each time point). Each group’s peak virus-specific IgG and IgA ASC responses for each tissue are summarized in Tables 2 and 3. For the (p.o.-inoculated) group 1 pigs, few virus-specific ASC were detected before challenge in all tissues tested (<2 ASC per 5 × 105 MNC at PID 8 and <7 ASC per 5 × 105 MNC at PID 21). In contrast, significant virus-specific IgM ASC responses were induced in all tissues except blood in group 2 pigs at PID 8, with the greatest numbers of virus-specific IgM ASC induced in the MLN (33 ASC per 5 × 105 MNC). IgM ASC prevailed over IgA and IgG ASC in intestinal tissues of these pigs at challenge (PID 20) (Fig. 2). The virus-specific IgG ASC numbers were significantly greater in the systemic tissues of the group 2 pigs at PID 20 than in those of the group 1 pigs (Fig. 2 and Table 3) and were predominate in the blood of the group 2 pigs at all time points (Fig. 1 and 2). For group 3 pigs, few virus-specific ASC were detected before challenge in all tissues tested except for spleen and peripheral blood lymphocytes (PBL) (Tables 2 and 3), and as for group 2, virus-specific IgG ASC prevailed over IgA ASC in all tissues. Virus-specific IgM ASC constituted the major isotype detected among MNC from intestinal tissues prior to challenge (Table 2).
FIG. 1.
Isotype-specific ASC induced by Wa HRV in gnotobiotic pigs following p.o. or i.m. inoculation with inactivated Wa HRV and challenge with virulent Wa HRV. MNC from duodena, ilea, and MLN of pigs were collected and tested by ELISPOT assay on PID 8, 20 to 21, 24 to 25, and 27 to 28 (PCD −13, 0, 4, and 7, respectively). Each value is the mean number of Wa HRV-specific ASC per 5 × 105 MNC for four to six pigs at a given time point. Symbols: ∗, significant difference (P < 0.05) in ASC numbers between p.o.- and i.m.-inoculated pigs at the same PID and PCD; ∗∗, significant difference (P < 0.05) in ASC numbers before challenge (PID 20 to 21 [PCD 0]) and after challenge (PID 24 to 25 [PCD 4] and PID 27 to 28 [PCD 7]) in the same group. Crosshatched bars, IgM; hatched bars, IgA; solid bars, IgG.
FIG. 2.
Isotype-specific ASC induced by Wa HRV in gnotobiotic pigs following p.o. or i.m. inoculation with inactivated Wa HRV and challenge with virulent Wa HRV. MNC from spleens and PBL of pigs were collected and tested by ELISPOT assay on PID 8, 20 to 21, 24 to 25, and 27 to 28 (PCD −13, 0, 4, and 7, respectively). Each value is the mean number of Wa HRV-specific ASC per 5 × 105 MNC for four to six pigs at a given time point. Symbols: ∗, significant difference (P < 0.05) in ASC numbers between p.o.- and i.m.-inoculated pigs at the same PID and PCD; ∗∗, significant difference (P < 0.05) in ASC numbers before challenge (PID 20 to 21 [PCD 0]) and after challenge (PID 24 to 25 [PCD 4] and PID 27 to 28 [PCD 7]) in the same group. Crosshatched bars, IgM; hatched bars, IgA; solid bars, IgG.
TABLE 2.
Peak isotype-specific ASC responses to rotavirus in intestinal tissues after inoculation and challenge of gnotobiotic pigs with Wa HRV
| Wa rotavirus inoculum groupb and ASC isotype | Peak no. of ASC (mean ± SEM)/5 × 105 MNCa
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duodenum
|
Ileum
|
MLN
|
||||||||||
| PID 21–25
|
PCD 4
|
PID 21–25
|
PCD 4
|
PID 21–25
|
PCD 4
|
|||||||
| In vivo | In vitro | In vivo | In vitro | In vivo | In vitro | In vivo | In vitro | In vivo | In vitro | In vivo | In vitro | |
| Group 1 | ||||||||||||
| IgG | 0.4 ± 0.3 | 0 | 302 ± 120 | 3,500 | 1 ± 0.7 | 0 | 312 ± 168 | 410 | 0.4 ± 0.4 | 2.5 | 223 ± 169 | 613 |
| IgA | 6 ± 5 (0.1) | 0 (NDc) | 83 ± 36 (3.6) | 21.2 (165) | 3 ± 1 (0.3) | 0 (ND) | 92 ± 53 (3.4) | 51 (8) | 1 ± 0.4 (0.4) | 0 (ND) | 106 ± 82 (2.1) | 113 (5.4) |
| IgM | 3 ± 2 | ND | 0 | ND | 2 ± 1 | ND | 1 ± 1 | ND | 3 ± 2 | ND | 3 ± 2 | ND |
| Group 2 | ||||||||||||
| IgG | 5 ± 3 | 0 | 271 ± 103 | 8,000 | 2 ± 1 | 0 | 164 ± 47 | 160 | 1 ± 0.3 | 2.5 | 130 ± 14 | 333 |
| IgA | 4 ± 2 (1.3) | 0 (ND) | 99 ± 70 (2.7) | 900 (8.9) | 1 ± 0.4 (2) | 3 (ND) | 20 ± 7 (8.2) | 10 (16) | 0 (ND) | 5 (0.5) | 14 ± 5 (9.3) | 60 (5.6) |
| IgM | 8 ± 5 | ND | 21 ± 12 | ND | 17 ± 7 | ND | 7 ± 4 | ND | 7 ± 3 | ND | 12 ± 6 | ND |
| Group 3 | ||||||||||||
| IgG | 4 | ND | 18 | ND | 6 | 3 | 60 | ND | 3 | 20 | 55 | ND |
| IgA | 1 (8) | ND (ND) | 4 (5) | ND (ND) | 0 (ND) | 0 (ND) | 8 (8) | ND (ND) | 1 (6) | 0 (ND) | 16 (3) | ND (ND) |
| IgM | 8 | ND | 38 | ND | 9 | 0 | 11 | ND | 11 | 0 | 3 | ND |
Numbers of Wa rotavirus-specific ASC were assessed by ELISPOT assay at PID 21 to 25 (PCD 0) and PCD 4 (PID 25) in the duodenum, ileum, MLN, spleen, and PBL. In most of the tissues tested, the highest virus-specific IgG and IgA numbers were detected at PCD 4, except IgG ASC in the duodenum and IgA ASC in the ileum from group 2 pigs, in which virus-specific ASC numbers at PCD 7 were higher than those at PCD 4. Values in parentheses are ratios of IgG to IgA ASC based on numbers of ASC per 5 × 105 MNC.
Data groups 1 and 2 represent four to six pigs for in vivo ASC responses and 1 or 2 pigs for in vitro ASC responses. For the latter responses, MNC from the indicated tissues were restimulated in vitro with semipurified rotavirus antigen at the time points noted. Data for group 3 pigs represents one or two pigs for in vivo and in vitro ASC responses. No standard errors of the means were calculated for the in vivo or in vitro ASC responses of the group 3 pigs because of the unavailability of adequate numbers of MNC from only one or two pigs at the time points shown.
ND, not determined.
TABLE 3.
Peak isotype-specific ASC responses to rotavirus in systemic lymphoid tissues after inoculation and challenge of gnotobiotic pigs with Wa HRV
| Wa rotavirus inoculum groupb and ASC isotype | Peak no. of ASC (mean ± SEM)/5 × 105 MNCa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Spleen
|
PBL
|
|||||||
| PID 21–25
|
PCD 4
|
PID 21–25
|
PCD 4
|
|||||
| In vivo | In vitro | In vivo | In vitro | In vivo | In vitro | In vivo | In vitro | |
| Group 1 | ||||||||
| IgG | 0.1 ± 0.1 | 16 | 68 ± 21 | 305 | 0.5 ± 0.4 | 44 | 179 ± 113 | 1,522 |
| IgA | 0 (NDc) | 4 (4) | 5 ± 2 (13.6) | 13 (23) | 1 ± 0.61 (0.5) | 2 (22) | 28 ± 6 (6.4) | 270 (5.6) |
| IgM | 7 ± 4 | ND | 4 ± 2 | ND | 0.3 ± 0.3 | ND | 3 ± 2 | ND |
| Group 2 | ||||||||
| IgG | 34 ± 18 | 2,017 | 152 ± 24 | 3,450 | 237 ± 133 | 2,730 | 441 ± 166 | 11,500 |
| IgA | 0.3 ± 0.3 (113) | 4.5 (448) | 2 ± 1 (76) | 0 (ND) | 2 ± 1 (119) | 14 (195) | 20 ± 6 (22) | 85 (135) |
| IgM | 6 ± 3 | ND | 6 ± 5 | ND | 2 ± 1 | ND | 1 ± 0.6 | ND |
| Group 3 | ||||||||
| IgG | 80 | 225 | 370 | ND | 1,450 | 3,550 | 310 | ND |
| IgA | 1 (80) | 0 (ND) | 20 (19) | ND (ND) | 11 (131.8) | 0 (ND) | 44 (7) | ND (ND) |
| IgM | 6 | 0 | 17 | ND | 5 | 0 | 0 | ND |
Numbers of Wa rotavirus-specific ASC were assessed by ELISPOT assay at PID 21 to 25 (PCD 0) and PCD 4 (PID 25) in the duodenum, ileum, MLN, spleen, and PBL. In most of the tissues tested, the highest virus-specific IgG and IgA numbers were detected at PCD 4. Values in parentheses are ratios of IgG to IgA ASC based on numbers of ASC per 5 × 105 MNC.
Data groups 1 and 2 represent four to six pigs for in vivo ASC responses and one or two pigs for in vitro ASC responses. For the latter responses, MNC from the indicated tissues were restimulated in vitro with semipurified rotavirus antigen at the time points noted. Data for group 3 pigs represents one or two pigs for in vivo and in vitro ASC responses. No standard errors of the means were calculated for the in vivo or in vitro ASC responses of the group 3 pigs because of the unavailability of adequate numbers of MNC from only one or two pigs at the time points shown.
ND, not determined.
After challenge, both group 1 and 2 pigs exhibited significant anamnestic IgG and IgA ASC responses (except for IgA ASC in the spleens of group 2 pigs), with IgG ASC responses predominating in all tissues (Fig. 1 and 2 and Tables 2 and 3). The greatest numbers of virus-specific IgG ASC were induced after challenge in the small intestinal lamina propriae (726 ASC per 5 × 105 MNC in the duodenum at PCD 7) and blood (441 ASC per 5 × 105 MNC at PCD 4) of group 2 pigs. At PCD 7, the numbers of virus-specific IgG ASC in the duodena, spleens, and blood of group 2 pigs were significantly greater than those for the (p.o.-inoculated) group 1 pigs (Fig. 2). The numbers of virus-specific IgA ASC increased 14- to 106-fold for group 1 pigs and 10- to 25-fold for group 2 pigs in the intestinal lymphoid tissues and blood by PCD 4 (Tables 2 and 3). The numbers of virus-specific IgA ASC in the ilea and MLN of group 1 pigs were significantly greater than those in the ilea and MLN of group 2 pigs at PCD 4. The IgG/IgA ASC ratios were lower overall in group 1 pigs (except in the duodenum) than in group 2 pigs because of the relatively greater numbers of virus-specific IgA ASC induced in group 1 pigs (Tables 2 and 3). Group 3 pigs also showed significant anamnestic IgG and IgA ASC responses postchallenge, with IgG ASC responses predominating in all tissues (as seen with group 2 pigs). No significant differences between the IgG, IgA, and IgM ASC responses of group 2 and 3 pigs were observed.
Virus-specific B-cell memory responses after in vitro stimulation of virus-sensitized MNC.
Following 5 days of in vitro culture and virus or mock-antigen stimulations, duodenal and ileal MNC were generally less viable (16 and 23% viable, respectively) than MLN (57%), spleen (40%), and PBL (39%). No spots were detected in control wells in which mock-infected MA104 cell antigen was added to the MNC during culture. The tissue and isotype distribution of virus-specific IgG and IgA memory B cells correlated with the in vivo virus-specific ASC responses observed at and after challenge (Tables 2 and 3). Little or no in vitro data was available for group 3 pigs because of the low number of pigs tested. The numbers of IgG and IgA memory cells were low (<5 per 5 × 105 MNC) in the intestinal lymphoid tissues of all groups tested at challenge (PCD 0). At PCD 0 (PID 21), no memory IgA ASC were detected in the intestines of group 1 pigs, whereas a few memory IgA ASC were present in the intestines (3 per 5 × 105 MNC) and more were present in the blood (14 per 5 × 105 MNC) of group 2 pigs (Tables 2 and 3). The numbers of memory IgA ASC in the blood of group 2 pigs at challenge were similar to the numbers of in vivo secondary IgA ASC at PCD 4 (14 versus 20 ASC per 5 × 105 MNC) (Table 3). However, numbers of memory IgG ASC were 6- to 13-fold higher than the numbers of in vivo secondary IgG ASC postchallenge (PCD 4) in the systemic lymphoid tissues of the group 2 pigs at challenge (PCD 0) (Table 3). After challenge, the IgG and IgA memory B cells increased markedly in the intestines and blood of both groups 1 and 2. The numbers of memory IgA ASC in the blood of the group 1 pigs at PCD 4 were ∼10-fold higher than the numbers of in vivo IgA ASC in the blood at PCD 4 (Table 3). The greatest numbers of memory B cells were in the duodena of i.m.-inoculated pigs (groups 2 and 3) at PCD 4, with numbers of memory IgG ASC and IgA ASC 30- and 9-fold higher, respectively, than the numbers of corresponding in vivo virus-specific ASC at PCD 4 (Table 2). In the spleens and blood of i.m.-inoculated pigs, the numbers of memory IgG ASC were 23- to 26-fold higher than the numbers of in vivo IgG ASC at PCD 4 (Table 3).
Total in vivo IgSC response.
The magnitude of total IgSC responses for group 1 and 2 pigs was much greater than but paralleled the virus-specific ASC responses of the different tissues at each time point. Total IgSC responses for group 3 pigs were not determined. For group 2 pigs, 1.5- to 10-fold-greater numbers of total IgM IgSC were induced in all tissues, and significantly greater numbers of total IgG IgSC were induced in the systemic lymphoid tissues by PID 21 than for group 1. However, the numbers of total IgA IgSC were 1.3- to 8-fold greater in group 1 pigs than in group 2 pigs. At PCD 4, the numbers of total IgSC increased 61- to 391-fold for IgG and 4- to 27-fold for IgA in the intestines for both groups 1 and 2. The total numbers of IgG IgSC remained greatly elevated both pre- and postchallenge in the systemic lymphoid tissues (spleens and PBL) of the group 2 pigs compared to the group 1 pigs. Conversely, the total numbers of IgA IgSC in the ileum and MLN were greatly elevated in group 1 pigs compared to group 2 pigs at all times. The virus-specific IgG, IgA, and IgM ASC constituted 2 to 78% of the total IgG, IgA, and IgM IgSC for both groups 1 and 2 at different times in the various tissues.
DISCUSSION
Previous studies of mice and rabbits suggested that parenteral inoculation with UV-psoralen- or formalin-inactivated rotavirus induced at least partial protection against rotavirus shedding, but protection against diarrhea could not be assessed in models with these animals. Furthermore, in our previous studies we confirmed that inactivation of rotavirus with 10% BEI did not result in the loss of rotavirus antigenicity as verified by similar antigen titers with neutralizing monoclonal (VP4- and VP7-specific) and polyclonal antibodies before and after inactivation (12). In addition, BEI-inactivated rotavirus was effective in significantly enhancing titers of antibody to rotavirus in serum and milk in parenterally inoculated cows (12). Therefore, in the present study we investigated the ability of BEI-inactivated Wa rotavirus (attenuated strain) administered p.o. or i.m. to induce virus-specific ASC responses and protection. The numbers of virus-specific IgA ASC induced in pigs inoculated with inactivated Wa rotavirus at challenge were very low (≤6 ASC per 5 × 105 MNC) and did not differ significantly between the i.m.- and p.o.-inoculated groups. In prior studies (27, 28, 41), we showed that p.o. inoculation of gnotobiotic pigs with live Wa human rotavirus (either virulent or attenuated) induced greater numbers of virus-specific IgA and IgG ASC in intestinal lymphoid tissues at PID 21 than did either p.o. or i.m. inoculation with inactivated Wa rotavirus in the present study. These findings concur with the significantly higher rotavirus-specific IgA antibody responses observed in culture supernatants of intestinal lamina propriae from mice inoculated p.o. with live rhesus rotavirus (RRV) (7). The p.o. inoculation of mice with inactivated RRV induced ∼32-fold-less virus-specific IgA (22 versus 706 ng/ml) in intestinal lamina propria organ culture supernatants than that found after p.o. inoculation of mice with live RRV (7), and the i.m. inoculation of mice with live or inactivated RRV induced ∼10-fold less (71 versus 706 ng/ml). In this study, the p.o. inoculation of pigs with inactivated Wa rotavirus induced ∼11-fold-fewer virus-specific IgA ASC in the lamina propria (duodenum and ileum) at challenge than previously found after oral inoculation with live virulent Wa rotavirus (41) (5 versus 53 ASC per 5 × 105 MNC), and the i.m. inoculation with inactivated Wa rotavirus induced ∼27- to 53-fold fewer (1 to 2 versus 53 ASC per 5 × 105 MNC). These findings reinforce our previous conclusion that the intensity of an intestinal IgA ASC response is directly related to the magnitude of viral replication, presumably reflecting, in part, substantially increased virus quantities in the intestine (27, 28, 41). Thus, the oral delivery of a live virus or the administration of virus with an effective immunostimulatory mucosal adjuvant should generate a greater mucosal antibody response.
Whereas the p.o. inoculation of gnotobiotic pigs with virulent Wa rotavirus induced the greatest numbers of intestinal virus-specific IgA ASC at challenge (41), the two-time or three-time i.m. inoculation of pigs with inactivated Wa rotavirus induced the greatest numbers of systemic IgG ASC. In spite of high systemic IgG ASC numbers, the mean numbers of IgG ASC induced in the lamina propria (duodenum and ileum) at challenge by inoculation of pigs with inactivated Wa rotavirus were low (4 to 5 ASC per 5 × 105 MNC) compared to the mean IgG ASC numbers induced by live virulent (64 ASC per 5 × 105 MNC) or attenuated (51 ASC per 5 × 105 MNC) Wa rotavirus (41). In this context, the mouse model differs from the pig model in that the intestinal IgG antibody response elicited following i.m. inoculation of mice with inactivated RRV was of a magnitude similar to that elicited by live oral RRV (7). The total antigen dose of inactivated rotavirus administered relative to the size of the animal’s intestine may play a role in the apparently weaker immune response of the pig to inactivated rotavirus. Although a mouse’s total intestinal mass is considerably less than that of a pig, the doses of antigen were similar or even higher in the mice (virus titers before inactivation: ∼6.8 × 108 PFU of RRV per mouse [7] and 4 × 106 FFU of epidemic diarrhea of infant mice rotavirus per mouse [22] versus ∼107 FFU of Wa HRV per pig). Thus, administration of similar antigenic inactivated virus doses relative to total intestinal masses for each animal model may be needed for true comparative studies to be performed. In this regard, the neonatal pig may be a more suitable vaccine model for human infants because of its similarity in intestinal physiology and mass.
Neither p.o. nor i.m. inoculation of pigs with inactivated Wa rotavirus elicited significant intestinal IgA or IgG ASC responses in this study or protected pigs against diarrhea or virus shedding after challenge. Our results concur with the outcome of previous studies evaluating the protective efficacy of i.m. immunization of gnotobiotic pigs with a bovine rotavirus vaccine (42) but conflict with findings from parenteral-immunization studies of mice (i.p.) (22) and rabbits (i.m.) (10). Although i.m. inoculation of pigs with formalin-inactivated bovine rotavirus (without adjuvant; preinactivation virus titer of 107.5 50% tissue culture infective doses) induced high virus-specific antibody titers in serum, pigs were not protected against subsequent challenge with HRV (42). In contrast, the i.p. inoculation of mice (22) and the i.m. inoculation of rabbits (10) with either live or inactivated (with UV-psoralen or formalin) homologous or heterologous rotaviruses conferred complete or nearly complete protection against rotavirus shedding (diarrhea could not be assessed in these animal models) upon homotypic or heterotypic rotavirus challenge. The protective immunity was associated with high levels of intestinal antibody to rotavirus of the IgG but not the IgA isotype (10). It is possible that the neutralizing effect of the virus-specific IgG induced in large quantities in the intestines of mice and rabbits played an important role in protection against rotavirus infection in these models.
In this study, no correlation between the serum neutralizing-antibody titers and protection was found. This result is consistent with our previous findings (31, 41) and with findings reported for studies of rotavirus infections in mice and humans (38, 39).
Antibody responses to rotavirus were increased by using adjuvants with the virus inoculum, but the absence of adjuvants and the use of different adjuvants did not influence the protection conferred by the virus vaccination. In rabbits, significant protection was conferred by two doses of live or inactivated rotavirus in either Freund’s adjuvant (complete [CFA] and IFA) or aluminum phosphate (10). In mice inoculated i.p., with CFA for the first inoculation and IFA for the second inoculation, antibody responses were increased four- to eightfold compared to the responses of mice inoculated without adjuvant, but even without adjuvant, the mice were still significantly protected against virus shedding after challenge (22). One must also question whether the i.p. route of infection is a true parenteral route of immunization. Studies of reovirus infections in mice have suggested that viral antigen may be taken up into the gut via the serosal surface after i.p. inoculation, eliciting intestinal antigen processing and stimulation (26). Similarly in ruminants and pigs, i.p. immunization can induce IgA-containing cells in the intestine or prime for local intestinal or respiratory antibody responses when an irritant adjuvant (CFA) is administered with antigen (17, 30). Presumably, inflammation associated with use of CFA enhances antigen uptake into the intestine via the serosal surface. Therefore, immune responses induced by i.p. inoculation with CFA may be more similar to the mucosal route of antigen processing and presentation than responses induced by i.m. immunization.
The differences observed after oral and parenteral immunizations in the clinical and immunological responses of mice, rabbits, and pigs may also be related to differences in the pathogenesis of rotavirus in these animals. In the adult mouse and rabbit models, rotavirus infects and replicates within the intestine but induces no diarrhea and little or no cytopathology (i.e., villous atrophy). Further, both infection and induction of disease by heterologous rotaviruses in the neonatal mouse appear to be dose dependent in that only relatively high doses of HRV (13) (i.e., ≥105 50% tissue culture infective doses) or other rotaviruses (5) are capable of infecting the animal and inducing disease. In contrast, significant villous atrophy is observed in the intestines of gnotobiotic pigs following infection with even low doses (∼103 ID50) of virulent Wa HRV (37). Thus, the gnotobiotic pig appears to be uniquely susceptible to Wa HRV infection and disease. Consequently, it is likely that a different type of immune response (intestinal IgA antibody) and/or an immune response of much greater magnitude is required to protect the piglet against rotavirus infection and disease than is required to protect mice and rabbits against rotavirus infection (i.e., shedding) only. Further study is needed to elucidate the potential differences that are involved in the pathogenesis of rotavirus in these various animal species.
Memory B cells play an important role in secondary immune responses (15). During the secondary immune response, the immune system can prevent reinfection or disease or eliminate a pathogen which has been encountered previously by the individual more rapidly and efficiently. Hence, the magnitude of memory B-cell responses at the local site of infection at challenge may be a predictor for the protective efficacy of a vaccine against disease (33, 34). In vitro stimulation of intestinal MNC at PCD 0 from pigs inoculated p.o. with inactivated virus elicited no memory IgA ASC, which coincided with the inactivated virus’ 0% rate of protection against diarrhea after challenge. Similarly, the i.m. inoculation of pigs with inactivated virus in IFA induced few memory IgA ASC in the intestines and blood (Tables 2 and 3), and the protection that these i.m. inoculations conferred against diarrhea following challenge was minimal (0 to 6% protection rate). Also, the high numbers of memory IgG ASC in the systemic lymphoid tissues of these pigs were not correlated with protection. Studies of memory B-cell responses in the protected animals (or animals which had recovered from virulent rotavirus infection) at challenge are in progress in our laboratory. Preliminary data shows that high numbers of memory IgA ASC are induced in the MLN, spleens, and blood of pigs p.o. inoculated with live virulent Wa rotavirus at challenge (data not shown). The potential correlation between memory B cells quantitated by in vitro ELISPOT assay and protection needs to be further investigated.
In this study, the p.o. administration of inactivated virus greatly increased total IgA IgSC numbers in the ileum, MLN, and PBL at almost all time points compared to the numbers in the analogous tissues of parenterally (i.m.) inoculated pigs (data not shown). In contrast, parenteral inoculation greatly increased total IgG IgSC numbers in systemic lymphoid tissues (spleen and PBL) at almost all time points compared to p.o. inoculation with inactivated rotavirus. Thus, the rotavirus inoculum was a polyclonal B-cell activator of IgSC, but the total numbers and isotypes induced were related to the route of antigen administration. Others (3) observed similar polyclonal IgA and IgG activation in the gut-associated lymphoid tissues of mice orally inoculated with microencapsulated rotavirus.
In conclusion, this study has demonstrated that i.m. inoculation of naive pigs with inactivated Wa rotavirus induced high numbers of IgG ASC and of memory IgG ASC in systemic but not intestinal lymphoid tissues. However, the systemic IgG ASC responses and the serum neutralizing-antibody titers did not correlate with protection of these pigs against subsequent virulent rotavirus challenge. These results support the hypothesis that in this animal model, the use of mucosal immunization routes and live replicating virus to induce an intestinal IgA antibody response may be the most efficient regimen for rotavirus vaccination to induce protective immunity. Whether parenteral administration of inactivated rotavirus vaccines to animals previously exposed to rotavirus might be an effective method to boost rotavirus immune responses, especially IgA antibodies, is unclear and requires additional study.
ACKNOWLEDGMENTS
We thank Bert Bishop for conducting the statistical analysis and Kathy Gadfield and Peggy Lewis for technical assistance.
This work was supported by grants from the National Institutes of Health (RO1AI33561 and RO1AI37111) and the World Health Organization (GPV/V27/181/24). Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
Footnotes
Approved as Ohio Agricultural Research and Development Center article 52-97.
REFERENCES
- 1.Bahnemann H G. Inactivation of viral antigens for vaccine preparation with particular reference to the application of binary ethylenimine. Vaccine. 1990;8:299–303. doi: 10.1016/0264-410X(90)90083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohl E H, Saif L J, Theil K W, Agnes A G, Cross R F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982;15:312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown K A, Moser C A, Speaker T J, Khoury C A, Kim J E, Offitt P A. Enhancement by microencapsulation of rotavirus-specific intestinal immune responses in mice assessed by ELISPOT assay and intestinal fragment culture. J Infect Dis. 1995;171:1334–1338. doi: 10.1093/infdis/171.5.1334. [DOI] [PubMed] [Google Scholar]
- 4.Burns J W, Siadat-Pajouh M, Krishnaney A A, Greenberg H B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 5.Burns J W, Krishnaney A A, Vo P T, Rouse R V, Anderson L J, Greenberg H B. Analyses of homologous rotavirus infection in the mouse model. Virology. 1995;207:143–153. doi: 10.1006/viro.1995.1060. [DOI] [PubMed] [Google Scholar]
- 6.Chen W K, Campbell T, VanCott J, Saif L J. Enumeration of isotype-specific antibody-secreting cells derived from gnotobiotic piglets inoculated with porcine rotaviruses. Vet Immunol Immunopathol. 1995;45:265–284. doi: 10.1016/0165-2427(94)05343-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffin S E, Klinek M, Offit P A. Induction of virus-specific antibody production by lamina propria lymphocytes following intramuscular inoculation with rotavirus. J Infect Dis. 1995;172:874–878. doi: 10.1093/infdis/172.3.874. [DOI] [PubMed] [Google Scholar]
- 8.Conner, M., S. Crawford, C. Barone, C. O’Neal, Y. Zhou, F. Fernandez, A. Parwani, L. J. Saif, J. Cohen, and M. Estes. 1996. Rotavirus subunit vaccines. Arch. Virol. 12(Suppl.):199–206. [DOI] [PubMed]
- 9.Conner M E, Estes M K, Graham D Y. Rabbit model of rotavirus infection. J Virol. 1988;62:1625–1633. doi: 10.1128/jvi.62.5.1625-1633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conner M E, Crawford S E, Barone C, Estes M K. Rotavirus vaccine administered parenterally induces protective immunity. J Virol. 1993;67:6633–6641. doi: 10.1128/jvi.67.11.6633-6641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cukor G, Blacklow N R. Human viral gastroenteritis. Microbiol Rev. 1984;48:157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez F M, Conner M E, Parwani A V, Todhunter D, Smith K L, Crawford S E, Estes M K, Saif L J. Isotype-specific antibody responses to rotavirus and virus proteins in cows inoculated with subunit vaccines composed of recombinant SA 11 rotavirus core-like particles (CLP) or virus-like particles (VLP) Vaccine. 1996;14:1303–1312. doi: 10.1016/S0264-410X(96)00065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouvea V S, Alencar A A, Barth O M, De Castro L, Fialho M, Araujo H P, Majerowicz S, Pereira H G. Diarrhoea in mice infected with a human rotavirus. J Gen Virol. 1986;67:577–581. doi: 10.1099/0022-1317-67-3-577. [DOI] [PubMed] [Google Scholar]
- 14.Graham D Y, Dufour G R, Estes M K. Minimal infective dose of rotavirus. Arch Virol. 1987;92:261–271. doi: 10.1007/BF01317483. [DOI] [PubMed] [Google Scholar]
- 15.Guan S, Qi A. Contributions of memory B cells to secondary immune response. Bull Math Biol. 1995;57:713–731. doi: 10.1007/BF02461848. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino Y, Kapikian A Z. Rotavirus vaccine development for the prevention of severe diarrhea in infants and young children. Trends Microbiol. 1994;2:242–249. doi: 10.1016/0966-842x(94)90629-7. [DOI] [PubMed] [Google Scholar]
- 17.Husband A J, Beh K J, Lascelles A K. IgA-containing cells in the ruminant intestine following intraperitoneal and local immunization. Immunology. 1979;37:597–601. [PMC free article] [PubMed] [Google Scholar]
- 18.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, editors. Virology. 2nd ed. New York, N.Y: Raven Press; 1990. pp. 1353–1404. [Google Scholar]
- 19.Kim Y M. Development immunity in the piglet. Birth Defects. 1980;11:549–557. [PubMed] [Google Scholar]
- 20.Kobayashi M, Thompson J, Tollefson S J, Reed G W, Wright P F. Tetravalent rhesus rotavirus vaccine in young infants. J Infect Dis. 1994;170:1260–1263. doi: 10.1093/infdis/170.5.1260. [DOI] [PubMed] [Google Scholar]
- 21.Larghi O P, Savy V L, Nebel A E, Rodriguez A. Ethylenimine-inactivated rabies vaccine of tissue culture origin. J Clin Microbiol. 1976;3:26–33. doi: 10.1128/jcm.3.1.26-33.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeal M M, Sheridan J F, Ward R L. Active protection against rotavirus infection of mice following intraperitoneal immunization. Virology. 1992;191:150–157. doi: 10.1016/0042-6822(92)90176-p. [DOI] [PubMed] [Google Scholar]
- 23.Meyer R C, Bohl E H, Kohler E M. Procurement and maintenance of germ-free swine for microbiological investigations. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul P, Mengeling W L, Malstrom C E, van Deusen R A. Production and characterization of monoclonal antibodies to porcine immunoglobulin gamma, alpha, and light chains. Am J Vet Res. 1989;50:471–475. [PubMed] [Google Scholar]
- 25.Phillips R W, Tumbleson M E. Models. In: Tumbleson M E, editor. Swine in biomedical research. New York, N.Y: Plenum Press; 1986. pp. 437–440. [Google Scholar]
- 26.Rubin P H, Eaton M A, Anderson A O. Reovirus infection in adult mice: the virus hemagglutinin determines the site of intestinal disease. Microb Pathog. 1986;1:79. doi: 10.1016/0882-4010(86)90034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saif L J, Yuan L, Ward L, To T L. Comparative studies of the pathogenesis, antibody responses and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. Adv Exp Med Biol. 1997;412:397–403. doi: 10.1007/978-1-4899-1828-4_62. [DOI] [PubMed] [Google Scholar]
- 28.Saif, L. J., L. A. Ward, L. Yuan, B. I. Rosen, and T. L. To. 1996. The gnotobiotic pig as a model for studies of disease pathogenesis and immunity to human rotavirus. Arch. Virol. 12(Suppl.):153–161. [DOI] [PubMed]
- 29.Schaller J P, Saif L J, Cordle C T, Chandler E J, Winship T R, Smith K L. Prevention of human rotavirus-induced diarrhea in gnotobiotic piglets using bovine antibody. J Infect Dis. 1992;165:623–630. doi: 10.1093/infdis/165.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheldrake R F, Romalis L F, Saunders M. Specific antibody containing cells in the porcine respiratory tract following intraperitoneal and intratracheal immunisation. Res Vet Sci. 1988;45:369–373. [PubMed] [Google Scholar]
- 31.To, T. L., L. A. Ward, L. Yuan, and L. J. Saif. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. Submitted for publication. [DOI] [PubMed]
- 32.Treanor J J, Clark H F, Pichichero M, Christy C, Gouvea V, Shrager D, Palazzo S, Offit P. Evaluation of the protective efficacy of a serotype 1 bovine-human rotavirus reassortant vaccine in infants. Pediatr Infect Dis J. 1995;14:301–307. doi: 10.1097/00006454-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 33.VanCott J, Brim T A, Simkins R S, Saif L J. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J Immunol. 1993;150:3990–4000. [PubMed] [Google Scholar]
- 34.VanCott J, Brim T A, Lunnery J K, Saif L J. Contribution of immune responses induced in mucosal lymphoid tissues of pigs inoculated with respiratory or enteric strains of coronavirus to immunity against enteric coronavirus challenge. J Immunol. 1994;152:3980–3990. [PubMed] [Google Scholar]
- 35.Vesikari T. Clinical trials of live oral rotavirus vaccines: the Finnish experience. Vaccine. 1993;11:255–261. doi: 10.1016/0264-410x(93)90026-t. [DOI] [PubMed] [Google Scholar]
- 36.Ward L A, Yuan L, Rosen B I, Tô T L, Saif L J. Development of mucosal and systemic lymphoproliferative responses and protective immunity to human group A rotaviruses in a gnotobiotic pig model. Clin Diagn Lab Immunol. 1996;3:342–350. doi: 10.1128/cdli.3.3.342-350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward L A, Rosen B I, Yuan L, Saif L J. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77:1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 38.Ward, R. L. 1996. Mechanisms of protection against rotavirus in humans and mice. J. Infect. Dis. 174(Suppl. 1):51–58. [DOI] [PubMed]
- 39.Ward R L, McNeal M M, Sheridan J F. Evidence that active protection following oral immunization of mice with live rotavirus is not dependent on neutralizing antibody. Virology. 1992;188:57–66. doi: 10.1016/0042-6822(92)90734-7. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt R G, James W D, Bohl E H, Theil K W, Saif L J, Kalica A R, Greenberg H B, Kapikian A Z, Chanock R M. Human rotavirus type 2: cultivation in vitro. Science. 1980;207:189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- 41.Yuan L, Ward L A, Rosen B I, To T L, Saif L J. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zissis G, Lambert J P, Marbehant P, Marissens D, Lobmann M, Charlier P, Delem A, Zygraich N. Protection studies in colostrum-deprived piglets of a bovine rotavirus vaccine candidate using human rotavirus strains for challenge. J Infect Dis. 1983;148:1061–1068. doi: 10.1093/infdis/148.6.1061. [DOI] [PubMed] [Google Scholar]