Abstract
BACKGROUND
The complexity of stroke sequelae, the heterogeneity of outcome measures and rehabilitation pathways, and the lack of extensively validated prediction models represent a challenge in predicting stroke rehabilitation outcomes
AIM
To prospectively investigate a multidimensional set of variables collected at admission to inpatient post-stroke rehabilitation as potential predictors of the functional level at discharge.
DESIGN
Multicentric prospective observational study.
SETTING
Patients were enrolled in four Intensive Rehabilitation Units (IRUs).
POPULATION
Patients were consecutively recruited in the period December 2019-December 2020 with the following inclusion criteria: aged 18+, with ischemic/haemorrhagic stroke, and undergoing inpatient rehabilitation within 30 days from stroke.
METHODS
This is a multicentric prospective observational study. The rehabilitation pathway was reproducible and evidence-based. The functional outcome was disability in activities of daily living, measured by the modified Barthel Index (mBI) at discharge. Potential multidimensional predictors, assessed at admission, included demographics, event description, clinical assessment, functional and cognitive profile, and psycho-social domains. The variables statistically associated with the outcome in the univariate analysis were fed into a multivariable model using multiple linear regression.
RESULTS
A total of 220 patients were included (median [IQR] age: 80 [15], 112 women, 175 ischemic). Median mBI was 26 (43) at admission and 62.5 (52) at discharge. In the multivariable analysis younger age, along with better functioning, fewer comorbidities, higher cognitive abilities, reduced stroke severity, and higher motor functions at admission, remained independently associated with higher discharge mBI. The final model allowed a reliable prediction of discharge functional outcome (adjusted R2=77.2%).
CONCLUSIONS
The model presented in this study, based on easily collectable, reliable admission variables, could help clinicians and researchers to predict the discharge scores of the global functional outcome for persons enrolled in an evidence-based inpatient stroke rehabilitation program.
CLINICAL REHABILITATION IMPACT
A reliable outcome prediction derived from standardized assessment measures and validated treatment protocols could guide clinicians in the management of patients in the subacute phase of stroke and help improve the planning of the rehabilitation individualized project.
Key words: Rehabilitation, Treatment outcome, Stroke
The increasing effectiveness and diffusion of thrombolytic and thrombectomy treatments are not sufficient yet to completely avoid residual neurological deficits in stroke patients, and recanalization strategies are not always applicable.1, 2 Therefore, despite recent improvements in acute phase management, stroke remains a major cause of disability.3 Given these premises, post-acute rehabilitation maintains a pivotal role in allowing patients to equally access adequate rehabilitation treatment and obtain a satisfactory functional outcome.4-6
However, the economic resources available in healthcare organisations are limited,7 thus it is essential to intervene on treatment and assessment processes to maximize functional recovery and thus reduce the costs deriving from residual disabilities.8, 9 The first and necessary step is the introduction of intervention protocols within an evidence-based Integrated Care Pathway (ICP).10 Additionally, evidence-based treatments should be complemented by the standardisation of measures, including a reproducible and complete functional assessment11 performed by an interdisciplinary team12 before and after treatment. In fact, it is essential to apply standardized and validated protocols for an in-depth evaluation of rehabilitation progression and outcomes and a coherent modification of the treatment plans.
It is in this optic that predictive models of the functional outcome in stroke patients eligible for rehabilitation13 are developed. Such data-based predictive tools aim to support clinical decisions to improve several processes in the care of post-acute stroke patients. Their application would enable a more accurate selection of the most efficient intensive rehabilitation path, already in the subacute phase.14 Further, the possibility to estimate the days of hospitalisation necessary to achieve the pre-established functional goals15 and to predict the extent of the need for the assistance required by patients upon returning home16 may improve rehabilitation and service planning. Finally, identifying the profile of response to treatment may allow the customisation of the approach based on specific deficits, with targeted treatments (“tailored approach”).17-19
The functional outcome in post-stroke rehabilitation has been extensively studied in the literature, often focusing on individual functional aspects as well as on complete recovery.20-26 Many studies described the outcomes of various treatments and evaluated predictors of recovery, but with a limited ability to generalise the results obtained due to a non-comparability of the data.27-30 Multiple reasons could be attributed to this limitation, such as the heterogeneity of the applied rehabilitation approaches, or the inconsistency of the baseline and outcome assessments of the patients.
For instance, García-Rudolp et al.,31 in their paper, systematically evaluated studies assessing the effect of a wide range of interventions for stroke rehabilitation, but the inconsistency in measuring outcomes hindered the possibility to compare the results obtained from different treatments. Some studies failed to accurately describe the adopted rehabilitation protocols and the choice of outcome variables only partially reflected post-stroke patients’ independence.32, 33 It has been stated in many papers34, 35 that an improvement in methodological quality in stroke rehabilitation-related studies is essential, especially regarding the reproducibility and reliability of patients’ evaluation at different time points. Moreover, Salter et al.36 highlighted the considerable heterogeneity in functional assessment used in literature, which hinders the interpretation of data deriving from randomised controlled trials concerning stroke rehabilitation.
Within a knowledge-translation approach, Don Carlo Gnocchi Foundation, one of the largest Italian scientific research and rehabilitation, has recently developed and implemented an evidence-based interdisciplinary ICP for post-acute stroke inpatient rehabilitation,10 including a multidimensional assessment protocol based on validated tools. After a pilot study confirming the feasibility and suggesting improved outcomes of the ICP compared to previous practice, the ICP has been implemented in four of the Don Carlo Gnocchi Foundation intensive rehabilitation units (IRUs), in order to standardise the outcome definition and the process of care according to national and international stroke rehabilitation guidelines.6, 10
The RIPS (inpatient rehabilitation post-stroke) study is a multicentre prospective study, involving the above-mentioned IRUs where the evidence-based stroke ICP had been applied; the study protocol has been extensively described elsewhere.37 RIPS aimed to study multiple features and outcomes in the context of patients admitted to ICPs after stroke. This analysis is focused on answering the main question of RIPS, that is to investigate which features recorded from the multidimensional assessment performed at admission to intensive post-stroke rehabilitation, may result as independent predictors of the functional outcome at discharge, measured with the modified Barthel Index (mBI).
Materials and methods
All patients admitted to four IRUs that applied a shared stroke rehabilitation ICP (Florence, Massa, Fivizzano, and La Spezia) were systematically assessed for eligibility from December 2019 to December 2020. Patients aged 18+ years who had suffered an ischemic or haemorrhagic stroke within 30 days before admission were considered eligible for this study, and all those signing informed consent to participate were systematically enrolled. Patients with a transitory ischemic attack, and with severe hemorrhagic or ischemic stroke (inducing disorders of consciousness states and critical clinical care conditions) addressed to the severe brain injury high-complexity rehabilitation ward were excluded. Measures regarding clinical information, nutritional, functional, neurological, and neuropsychological assessments, were taken at admission (T0), at discharge (T1), and at an in-person or telephone follow-up 6 months after the stroke (T2).
The study was approved by the local ethical committee (CEAVC Em. 2021-007 ID 14513_bio) and registered on ClinicalTrials.gov (registration number: NCT03866057, https://clinicaltrials.gov/ct2/show/study/NCT03866057?term=fondazione+don+gnocchi%2C+stroke&draw=2&rank=1).
As to the sample size, calculation was performed using the following equation:
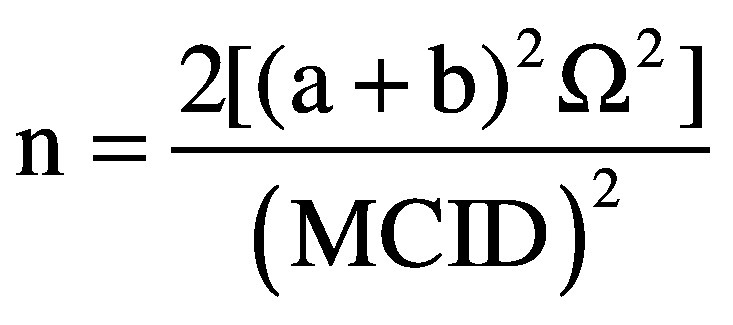 |
(1)
a = equal to 1.96 assuming a significance level of 0.05.
b = equal to 1.28 assuming a power of 90%
σ2 = population variance (standard deviation)
MCID = Minimal Clinically Important Difference for the modified Barthel Index
Data assumptions for the population variance were obtained from data retrospectively collected between 2015 and 2017 at two participating hospitals (Massa and Fivizzano). The 527 patients enrolled in the retrospective study showed a standard deviation on the modified Barthel Index at the admission of 29 points. Those data were presented at the XIX SIRN National Congress (Perugia, 4th-6th April 2019). To determine the MCID, the value proposed by Hsieh et al.38 for the Barthel Index in stroke patients was adopted. The value was adjusted according to the score range (100 points) of the modified scale version, leading to an MCID of 9 points.
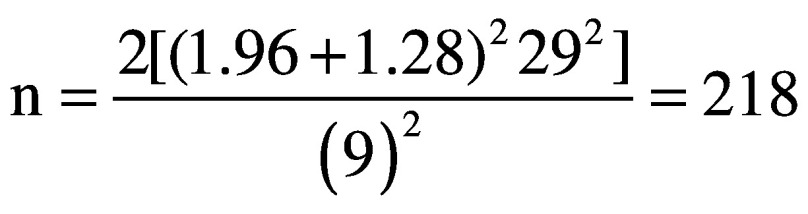 |
(2)
A sample size of 218 patients was obtained.
Considering a 10% increase to account for possible drop-outs the total sample size was estimated in 240 patients.
For this analysis, we focused on data collected in person at T0 and T1. The reporting of this study followed the STROBE checklist for observational studies.39
Measures and analyses
In this work, the functional status of patients at discharge from the IRUs (T1), as measured with the modified Barthel Index (mBI) total score, was assumed as the study outcome.
The factors analysed for association with the outcome were collected at admission, addressing five different domains: Demographics, Description of the event, Clinical features, Psycho-social features and Functional profile (body functions and activity).
Specifically, the detail of each considered variable for the identified domains is reported in Table I.
Table I. —List of variables, for each domain, considered for association with the outcome.
| Domain | Variable evaluated |
|---|---|
| Demographics | Age |
| Gender | |
| Educational level | |
| Cohabitation | |
| Description of the event | Aetiology (ischemic or hemorrhagic stroke) |
| Time from the event | |
| Recurrent event | |
| Side of the lesion | |
| Area of the lesion | |
| Acute phase treatment (i.e. thrombolysis or thrombectomy) | |
| Clinical features | Markers of complexity Pain Acute infection Dysphagia Malnutrition Nasogastric tube or percutaneous enterostomy Pressure ulcers Bladder catheter Incontinence Central venous catheter Tracheostomy cannula Reduced vigilance Delirium Clinical instability Depression |
| Anemia | |
| Dialysis | |
| Agitation | |
| Diplopia | |
| CIRS comorbidity index | |
| BMI | |
| NIHSS, Total score and item 9 sub-score (Aphasia) | |
| SDC | |
| Psycho-social features | Adjusted score of the MoCA |
| Equivalent score of the OCS heart test | |
| mFWC | |
| FAI | |
| Functional profile | mBI |
| TCT | |
| FAC | |
| mRS, baseline and anamnestic score | |
| SPPB | |
| MAS | |
| FMA |
CIRS: Cumulative Index Rating Scale; BMI: Body Mass Index; NIHSS: National Institutes of Health Stroke Scale; SDC: Communication Disability Scale; MoCA: Montreal Cognitive Assessment; OCS: Oxford cognitive screen; mFWC: modified Functional Walking Classification; FAI: Frenchay Activity Index: mBI: Modified Barthel Index; TCT: Trunk Control Test; FAC: Functional Ambulation Categories; mRS: Modified Rankin Score; SPPB: Short Physical Performance Battery; MAS: Modified Ashworth Scale; FMA: Fugl-Meyer Assessment.
Details concerning each evaluation tool reference are reported as Supplementary Digital Material 1, Supplementary Table I.
Statistical analysis
The variables were first analysed by the multi-professional clinical team for clinical imputation of missing data, wherever possible. Specifically, the variable of Montreal Cognitive Assessment (MoCA) was imputed considering values of 0, based on the assumption of the impossibility to administer the scale, in case of the absence of communication (Communication Disability Scale, SDC=0) or presence of reduced vigilance/coma (marker of complexity=1) or presence of delirium (marker of complexity=1) or presence of global aphasia (National Institutes of Health Stroke Scale, NIHSS_9=3).
Subsequently, the variables were screened according to the percentage of missing data. More in detail, variables not reaching at least 80% completeness were discarded. Moreover, categorical variables with frequencies on at least one category not reaching the minimum of ten cases were discarded.
Finally, on the remaining variables, an automatic k-Nearest Neighbours-based single imputation method40 was performed on Python for filling in missing data that could not be attributed with values during the clinical screening. Further, variables screening was clinically performed excluding those variables with overlapping information with others. Additional detail on the excluded variables is presented in Table I.
The statistical analyses were performed on IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp. Firstly, descriptive analyses were performed using the mean and standard deviation (std), or median and interquartile range (IQR) when appropriate, for numerical variables and relative frequencies for the categorical variables. Comparisons over time of numerical variables were performed using the Wilcoxon Test.
The univariate analyses were performed using Pearson’s or Spearman’s correlation and the t-test of the Mann-Whitney Test, for numerical and categorical variables, respectively. Finally, the variables that resulted statistically associated with the outcome in the univariate step were fed into a multivariable model. The multivariate analysis was performed using multiple linear regression. The normality of the distributions was tested through the Shapiro-Wilk Test.
A simple calculator is available in the Supplementary Digital Material 2, offering the mBI estimation at discharge based on the coefficients obtained in this multivariable regression model. In order to have a better understanding of where the calculator might fail or succeed the most, error analyses were computed by comparing the baseline characteristics of patients with good estimated or wrongly estimated outcomes.
Specifically, the estimated values were further analysed by dividing the patients into three groups based on the values of the differences between estimated and measured mBI scores at discharge. In the absence of an established reference to define a cut-off for a Minimal Clinical Important Difference (MCID) in the mBI, we estimated a cut-off of 10 utilising the MCID=1.85 proposed for the Barthel Index Scale (scoring 0-20).38 Then, we generated the three groups, considering the difference between the real and estimated mBI values (estimation errors). Patients with estimation errors below -10 were attributed to the group of overestimated mBI values, over 10 were identified as underestimated values, and the others were considered as correctly estimated. Lastly, the descriptive analyses of the variables in the multivariable model were repeated for each group and statistical comparisons of the variables among groups were presented. Specifically, the Kruskal-Wallis Test, with pairwise comparisons, was applied for numerical variables and the χ2 Test was used for categorical ones. For each test applied a P value <0.05 was considered statistically significant.
Results
Out of the 278 stroke patients who were eligible for the RIPS study, 235 (85%) signed the informed consent and were enrolled. Fifteen patients (0.06%) were subsequently excluded since their discharge mBI (our primary outcome) had not been recorded, due to anticipated discharge preventing discharge evaluation (T1). Thus, data on 220 patients (80% of all eligible patients) were included in the analyses of this work. Of these, 125 were recruited from the centre in Florence, 34 from the centre in Massa, 13 from the centre in Fivizzano, and 48 from La Spezia.
The main characteristics of the included sample are presented in Table II.41 The patients reported a median [IQR] age of 80.0 [15.0] years, with 50.9% of women, and a prevalence of ischemic aetiology (79.5%). The mBI of patients significantly increased between admission and discharge (P value <0.001), passing from a median value of 26.0 [43.0] to 62.5 [52.0].
Table II. —Description of the sample included in the analyses.
| Variables | Mean (std)/ Median [IQR] or Frequencies (%)a |
N. |
|---|---|---|
| Age | 80.0 [15.0] | 220 |
| Gender (male) | 108 (49.1%) | 220 |
| Educational level (years) | 8.0 [8.0] | 205 |
| Cohabitation (yes) | 144 (74.6%) | 193 |
| Center | Florence: 125 (58.8%) Massa: 34 (15.5%) Fivizzano: 13 (5.9%) La Spezia: 48 (21.8%) |
220 |
| Aetiology (ischemic) | 175 (79.5%) | 220 |
| Time from the event | 11.0 [8.0] | 220 |
| Recurrent event (yes) | 30 (13.8%) | 217 |
| Side of the lesion | Right: 96 (46.4%) Left: 96 (46.4%) Both: 15 (7.2%) |
207 |
| Area of the lesion | None: 16 (7.3%) Sub-tentorial: 24 (10.9%) Supra-tentorial: 172 (78.2%) Both: 8 (3.6%) |
220 |
| Thrombolysis (yes) | 50 (28.9%) | 173 |
| Reduced vigilance and coma (yes) | 14 (6.4%) | 220 |
| Clinical instability (yes) | 21 (9.5%) | 220 |
| Delirium (yes) | 12 (5.5%) | 220 |
| Acute infection (yes) | 26 (11.8%) | 220 |
| Depression (yes) | 58 (26.4%) | 220 |
| Dysphagia (yes) | 117 (53.2%) | 220 |
| Malnutrition (yes) | 13 (5.9%) | 220 |
| BMI | 25.1 [5.08] | 175 |
| Bedsores (yes) | 26 (11.8%) | 220 |
| Bladder catheter (yes) | 90 (40.9%) | 220 |
| Incontinence (yes) | 85 (38.6%) | 220 |
| Central venous catheter (yes) | 9 (4.1%) | 220 |
| Tracheostomy (yes) | 1 (0.5%) | 220 |
| Anaemia (yes) | 76 (34.5%) | 220 |
| Dialysis (yes) | 4 (1.8%) | 220 |
| Agitation (yes) | 13 (6.5%) | 201 |
| Pain (yes) | 47 (21.4%) | 220 |
| Diplopia (yes) | 6 (2.7%) | 219 |
| CIRS_comorbidity | 3.0 [2.0] | 212 |
| NIHSS | 7.0 [8.0] | 218 |
| Aphasia (NIHSS_9) | No aphasia: 138 (63.0%) Mild to moderate aphasia: 34 (15.5%) Severe aphasia: 33 (15.1%) Mute or global aphasia: 14 (6.9%) |
219 |
| SDC | 3.0 [2.0] | 220 |
| MoCA_adjusted_Santangelo* | 18.73 [7.53] | 191 |
| OCS Space Asymmetry_raw | 0.0 [4.0] | 115 |
| OCS Space Asymmetry_cut-off | Normal: 76 (66.1%) Right neglect: 14 (12.2%) Left neglect: 25 (21.7%) |
115 |
| mFWC | 6.0 [0.0] | 133 |
| FAI | 28.0 [8.0] | 132 |
| mBI | 26.0 [43.0] | 219 |
| TCT | 48.0 [75.0] | 209 |
| FAC | 0.0 [2.0] | 210 |
| SPPB | 0.0 [2.0] | 206 |
| mRS | 4.0 [1.0] | 209 |
| mRS_anamnestic | 0.0 [1.0] | 208 |
| MAS | 0.0 [2.0] | 200 |
| FMA | 158.0 [80.0] | 183 |
| LoS (days) | 32.0 [28.0] | 216 |
| mBI | 62.5 [52.0] | 220 |
BMI: Body Mass Index; CIRS: Cumulative Index Rating Scale; FMA: Fugl-Meyer Assessment; IQR: Interquartile Range; LoS: length of stay; MAS: Modified Ashworth Scale; mBI: Modified Barthel Index; mFWC: Modified Functional Walking Classification; MoCA: Montreal Cognitive Assessment; mRS: Modified Rankin Score; N.: numerosity; NIHSS: National Institutes of Health Stroke Scale; OCS: Oxford cognitive screen; SDC: Communication Disability Scale; SPPB: Short Physical Performance Battery; std: standard deviation; TCT: Trunk Control Test. aDichotomous variables are presented with the frequency of one class only, indicated between brackets. *MoCA adjusted score on normative values by Santangelo et al.41
For what concerns the clinical imputation, the number of missing data on the MoCA reduced from 35% to 13.2%. After this process, among the considered variables, thrombolysis, Body Mass Index (BMI), Frenchay Activity Index (FAI), modified Functional Walking Classification (mFWC), and Oxford Cognitive Screening (OCS) heart test were excluded for insufficient completeness level (Table II). In addition, diplopia, and the complexity markers central venous catheter, tracheostomy, and dialysis were excluded from the analyses for insufficient cases in one of the categories (Table II).
From the univariate analyses (Table III), all variables in the Psycho-social features and Functional and cognitive profile domains and all variables in the Demographics and Clinical features domain, except for educational level, agitation, anaemia, and depression, resulted significantly associated with the outcome. Instead, for what concerns the domain of the Description of the event, only the time from the event was significantly associated with the outcome.
Table III. —Univariate analyses. The statistically significant P values are reported in bold.
| Variables | mBI median [IQR] on the groups or Correlation coefficient (R) | P value |
|---|---|---|
| Demographics | ||
| Age | -0.361 | <0.001 |
| Gender | Male: 74.0 [48.0] Female: 52.5 [59.0] |
0.009 |
| Educational level (years) | 0.065 | 0.337 |
| Cohabitation | Yes: 72.0 [56.0] No: 52.0 [54.0] |
0.046 |
| Description of the event | ||
| Aetiology | Ischemic: 63.0 [54.0] Hemorrhagic: 60.0 [54.0] |
0.502 |
| Time from the event | -0.163 | 0.015 |
| Recurrent event | No: 62.0 [52.0] Yes: 77.5 [62.0] |
0.555 |
| Side of the lesion | Right: 58.0 [50.0] Left: 63.0 [56.0] Bilateral: 86.0 [60.0] |
0.192 |
| Area of the lesion | None: 77.5 [47.0] Sub-tentorial: 76.0 [48.0] Supra-tentorial: 57.5 [59.0] Both: 66.5 [43.0] |
0.100 |
| Clinical features | ||
| Reduced vigilance and coma | Yes: 11.5 [40.0] No: 66.5 [51.0] |
<0.001 |
| Clinical instability | Yes: 16.0 [52.0] No: 67.0 [49.0] |
<0.001 |
| Delirium | Yes: 39.50 [54.0] No: 63.0 [53.0] |
0.047 |
| Acute infection | Yes: 36.5 [65.0] No: 67.0 [51.0] |
0.003 |
| Depression | Yes: 56.5 [49.0] No: 63.0 [53.0] |
0.659 |
| Dysphagia | Yes: 48.0 [60.0] No: 79.0 [42.0] |
<0.001 |
| Malnutrition | Yes: 13.0 [48.0] No: 66.0 [51.0] |
<0.001 |
| Bedsores | Yes: 17.5 [44.0] No: 69.0 [49.0] |
<0.001 |
| Bladder catheter | Yes: 37.5 [54.0] No: 79.0 [42.0] |
<0.001 |
| Incontinence | Yes: 43.0 [59.0] No: 77.0 [46.0] |
<0.001 |
| Anaemia | Yes: 58.0 [57.0] No: 63.0 [53.0 ] |
0.596 |
| Agitation | Yes: 55.0 [37.0] No: 63.0 [54.0] |
0.864 |
| Pain | Yes: 43.0 [58.0] No: 72.0 [52.0] |
<0.001 |
| CIRS_comorbidity | -0.181 | 0.007 |
| NIHSS | -0.663 | <0.001 |
| Aphasia (NIHSS_9) | No aphasia: 74.0 [52.0] Mild to moderate aphasia: 53.0 [55.0] Severe aphasia: 52.0 [64.0]a Mute or global aphasia: 20.0 [39.0]b |
<0.001 |
| Psycho-social features | ||
| MoCA_adjusted_Santangelo* | 0.502 | <0.001 |
| Functional profile | ||
| mBI | 0.765 | <0.001 |
| TCT | 0.721 | <0.001 |
| mRS_anamnestic | -0.137 | 0.043 |
| MAS | -0.253 | <0.001 |
| FMA | 0.788 | <0.001 |
BMI: Body Mass Index; CIRS: Cumulative Index Rating Scale; FMA: Fugl-Meyer Assessment; IQR: Interquartile Range; LoS: length of stay; MAS: Modified Ashworth Scale; mBI: Modified Barthel Index; mFWC: Modified Functional Walking Classification; MoCA: Montreal Cognitive Assessment; mRS: Modified Rankin Score; N.: numerosity; NIHSS: National Institutes of Health Stroke Scale; OCS: Oxford Cognitive Screen; SDC: Communication Disability Scale; SPPB: Short Physical Performance Battery; std: standard deviation; TCT: Trunk Control Test. aPairwise comparisons highlighted significant P values in pairs of: No aphasia - Severe aphasia and Severe aphasia - Mute or global aphasia; bpairwise comparisons highlighted significant P values in all pairs involving Mute or global aphasia. *MoCA adjusted score on normative values by Santangelo et al.41
In the multivariable analysis, age, mBI, the comorbidity index of the Cumulative Illness Rating Scale (CIRS), the adjusted MoCA score, the NIHSS, and the Fugl-Meyer Assessment (FMA) resulted being significantly associated with the outcome (adjusted R2=77.2%). Specifically, younger age, higher independence in basic activities of daily living, reduced number of comorbidities, higher cognitive abilities, reduced stroke severity, and higher motor functions at admission, resulted to be independently associated with a more favourable functional outcome at discharge (Table IV).
Table IV. —Multivariate analyses. The statistically significant P values are reported in bold.
| Independent variables | Unstandardized coefficients | P value | 95% Confidence Interval | ||
|---|---|---|---|---|---|
| B | Standard Error | Lower-limit | Upper-limit | ||
| Constant | 45.268 | 23.117 | 0.052 | -0.322 | 90.857 |
| Age | -0.412 | 0.101 | <0.001 | -0.612 | -0.212 |
| Gender | -2.782 | 2.320 | 0.232 | -7.357 | 1.792 |
| Cohabitation | 4.486 | 2.631 | 0.090 | -0.704 | 9.675 |
| Time from the event | -0.012 | 0.180 | 0.949 | -0.367 | 0.344 |
| Reduced vigilance and coma | -6.882 | 5.064 | 0.176 | -16.870 | 3.106 |
| Clinical instability | 1.523 | 4.172 | 0.715 | -6.704 | 9.750 |
| Delirium | -3.571 | 5.186 | 0.492 | -13.798 | 6.656 |
| Acute infection | 4.587 | 3.839 | 0.234 | -2.984 | 12.159 |
| Dysphagia | -4.145 | 2.491 | 0.098 | -9.057 | 0.767 |
| Malnutrition | -1.908 | 4.999 | 0.703 | -11.767 | 7.950 |
| Bedsores | 6.765 | 3.705 | 0.069 | -0.542 | 14.072 |
| Bladder catheter | -1.646 | 2.790 | 0.556 | -7.148 | 3.856 |
| Incontinence | -1.865 | 2.650 | 0.483 | -7.092 | 3.362 |
| Pain | 5.468 | 2.796 | 0.052 | -0.046 | 10.981 |
| CIRS_comorbidity | -1.621 | 0.701 | 0.022 | -3.003 | -0.239 |
| NIHSS | -0.782 | 0.338 | 0.021 | -1.448 | -0.117 |
| Aphasia (NIHSS_9) | 1.494 | 1.570 | 0.342 | -1.602 | 4.589 |
| MoCA_adjusted_Santangelo* | 0.414 | 0.180 | 0.022 | 0.060 | 0.768 |
| mBI | 0.305 | 0.069 | <0.001 | 0.169 | 0.441 |
| TCT | 0.071 | 0.053 | 0.187 | -0.035 | 0.176 |
| mRS_anamnestic | -0.562 | 1.028 | 0.585 | -2.590 | 1.465 |
| MAS | 0.116 | 0.353 | 0.742 | -0.579 | 0.812 |
| FMA | 0.250 | 0.035 | <0.001 | 0.182 | 0.319 |
*MoCA adjusted score on normative values by Santangelo et al.41
The mBI at discharge was correctly estimated with our model in 111 patients, while the expected outcome value was overestimated and underestimated respectively in 54 and 55 patients. The statistical comparisons of the variables among the 3 groups thus derived showed that only baseline and discharge mBI (P<0.001) and baseline FMA (P=0.049) were statistically different in incorrectly estimated outcome groups. Specifically, baseline mBI and FMA were lower in both the incorrectly estimated mBI groups (Table V).
Table V. —Descriptive analyses on the group of patients with overestimated, underestimated, and correctly estimated mBI values at discharge.
| Variable | Mean (std)/Median [IQR] or Frequencies (%) |
P valuea | ||
|---|---|---|---|---|
| Correctly estimated (N.=111) | Overestimated (N.=54) | Underestimated (N.=55) | ||
| Age | 81.0 [15.0] | 80.5 [15.0] | 80.0 [15.0] | 0.782 |
| Gender | Male: 53 (47.7%) Female: 58 (52.3%) |
Male: 27 (50%) Female: 27 (50%) |
Male: 28 (50.9%) Female: 27 (49.1%) |
0.937 |
| Cohabitation | No: 32 (28.8%) Yes: 79 (71.2%) |
No: 12 (22.2%) Yes: 42 (77.8%) |
No: 11 (20.0%) Yes: 44 (80.0%) |
0.403 |
| Time from the event | 11.0 [9.0] | 12.0 [9.00] | 11.0 [8.00] | 0.903 |
| Reduced vigilance and coma | No: 106 (95.5%) Yes: 5 (4.5%) |
No: 49 (90.7%) Yes: 5 (9.3%) |
No: 51 (92.7%) Yes: 4 (7.3%) |
0.412 |
| Clinical instability | No: 98 (88.3%) Yes: 13 (11.7%) |
No: 49 (90.7%) Yes: 5 (9.3%) |
No: 52 (94.5%) Yes: 3 (5.5%) |
0.475 |
| Delirium | No: 106 (95.5%) Yes: 5 (4.5%) |
No: 50 (92.5%) Yes: 4 (7.4%) |
No: 52 (94.5%) Yes: 3 (5.5%) |
0.687 |
| Acute infection | No: 97 (87.4%) Yes: 14 (12.6%) |
No: 48 (88.9%) Yes: 6 (11.1%) |
No: 49 (89.1%) Yes: 6 (10.9%) |
1.000 |
| Dysphagia | No: 55 (49.5%) Yes: 56 (50.5%) |
No: 23 (42.6%) Yes: 31 (57.4%) |
No: 24 (43.6%) Yes: 31 (56.4%) |
0.555 |
| Malnutrition | No: 105 (94.6%) Yes: 6 (5.4%) |
No: 51 (94.4%) Yes: 3 (5.6%) |
No: 51 (92.7%) Yes: 4 (7.3%) |
0.930 |
| Bedsores | No: 100 (90.1%) Yes: 11 (9.9%) |
No: 47 (87.0%) Yes: 7 (13.0%) |
No: 47 (85.5%) Yes: 8 (14.5%) |
0.627 |
| Bladder catheter | No: 70 (63.1%) Yes: 41 (36.9%) |
No: 30 (55.6%) Yes: 24 (44.4%) |
No: 30 (54.5%) Yes: 25 (45.5%) |
0.492 |
| Incontinence | No: 76 (68.5%) Yes: 35 (31.5%) |
No: 29 (53.7%) Yes: 25 (46.3%) |
No: 30 (54.5%) Yes: 25 (45.5%) |
0.097 |
| Pain | No: 88 (79.3%) Yes: 23 (20.7%) |
No: 41 (75.9%) Yes: 13 (24.1%) |
No: 44 (80.0%) Yes: 11 (20.0%) |
0.886 |
| CIRS_comorbidity | 3.00 [2.00] | 3.00 [2.00] | 3.00 [2.00] | 0.857 |
| NIHSS | 6.00 [9.00] | 8.00 [10.0] | 7.00 [7.00] | 0.184 |
| Aphasia (NIHSS_9) | No aphasia: 70 (63.1%) Mild to moderate aphasia: 14 (12.6%) Severe aphasia: 18 (16.2%) Mute or global aphasia: 9 (8.1%) |
No aphasia: 35 (64.8%) Mild to moderate aphasia: 9 (16.7%) Severe aphasia: 8 (14.8%) Mute or global aphasia: 2 (3.7%) |
No aphasia: 34 (61.8%) Mild to moderate aphasia: 11 (20.0%) Severe aphasia: 7 (12.7%) Mute or global aphasia: 3 (5.5%) |
0.848 |
| MoCA_adjusted_Santangelo*** | 16.0 [21.0] | 16.0 [13.0] | 16.0 [12.0] | 0.873 |
| mBI | 35.0 [53.0] | 12.5 [39.0]** | 20.0 [33.0] | <0.001 |
| TCT | 61.0 [63.0] | 48.0 [62.0] | 48.0 [62.0] | 0.092 |
| mRS_anamnestic | 0.00 [1.00] | 0.00 [1.00] | 0.00 [1.00] | 0.481 |
| MAS | 0.00 [2.00] | 0.00 [3.00] | 0.00 [3.00] | 0.943 |
| FMA | 163 [87.0] | 135 [83.0] | 149 [74.0] | 0.049 |
| mBI dicharge | 72.0 [47.0] | 32.50 [44.0]** | 81.0 [32.0] | <0.001 |
aAll P values are connected to multiple comparisons among the three groups. The asterisks on the group of overestimated or underestimated patients are referred to the pairwise comparison of the respective groups with the correctly estimated group. Pairwise comparisons are not provided for P values not statistically significant. **P value <0.001; *P value <0.05. ***MoCA adjusted score on normative values by Santangelo et al.41
Discussion
The epochal improvements reported in acute phase treatments of stroke are not matched by reports of the same progress in the subacute phase. In addition to the innovation gap between acute and post-acute interventions, this may also depend on the poor quality of studies and inconsistency of treatments and evaluations, rather than a lack of effective rehabilitation strategies.31-33 Our aim was to design and carry out a high-quality prospective study that would address multiple aspects. First, the evidence-based stroke ICP adopted had been consistently implemented and shared among all the participating centres,10 including the standardised assessment measures and the validated treatment protocols. This would reduce the variability regarding assessments and treatments often met in multicentric observational studies involving rehabilitation. Second, this study was designed and carried out to meet the requirements of the criteria of the Quality In Prognosis Studies (QUIPS) tool,42 for the risk of bias in studies of prognostic factors. Finally, we designed the study protocol and reported our results to comply with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Guidelines.43
The results of our study identified a final model that explains the variance of the discharge mBI of the study participants with an adjusted R2 of 77.2%. This model provides a reliable estimate of the functional outcome of stroke patients at discharge from the four IRUs, outreaching studies available in the current literature.44, 45
To adequately describe the impact of rehabilitation on the outcome of stroke patients, a wide range of variables reflecting the complexity of this condition have been considered. This is essential to understand patients’ needs and promote the recovery of function and the reintegration of these patients in their pre-morbid setting, whenever possible.17, 46 In our analysis, among all the multidimensional features collected at admission, younger age, lower number of comorbidities, better cognitive function, reduced neurological deficits, and higher motor abilities were independently associated with a more favourable outcome at discharge.
Comparing our results to systematic reviews of the literature, Hakkennes et al.44 confirmed the association with outcome of stroke severity, age, cognition, and functional level before rehabilitation as shown in our work, while we did not find an independent predictive role of continence in our cohort. The very old age of our population may explain why this feature was highly common and did not show a significant relationship with the outcome. However, a more recent review by Meyer et al.,45 also did not identify urinary continence as a significant predictor of stroke discharge functional outcome. These authors reviewed multivariable predictive models of functional outcomes after post-stroke inpatient rehabilitation. Among a large number of models described, they identified 16 variables that were included in the final models in at least five studies. When considering papers investigating either by BI or by Functional Independence Measure (FIM) as the primary outcome, only 7 of the 16 variables resulted as significant predictors in more than 50% of the studies: age, admission functional level (BI or FIM), stroke severity (NIHSS), dysphasia, impulsivity, previous stroke, and neglect. Age and admission functional level (as measured with mBI), NIHSS and cognition, were significant also in our analyses, whereas previous history of stroke and dysphasia were not. This depend on their relative severity or on how these features were evaluated. For example, a previous ischemic event treated early and efficiently with recent therapies, as well as a mild production aphasia, may have a negligible impact on mBI at discharge.47 In our study, any previous stroke was reported in anamnesis, regardless of the neurological sequelae, and the presence of language impairment was attested with the NIHSS sub-scale, rather than by a stroke-specific language assessment battery. This may have hidden the prognostic power of these variables and could be a starting point for future studies to implement a more in-depth assessment of previous strokes and a better characterization of the speech disorder. Among the other variables that Meyer et al.45 identified, impulsivity was not assessed in our analysis, whereas neglect was not included because of exceeding missing values. This must be acknowledged as a limitation of the present study.
The need for a comprehensive and multidisciplinary patient evaluation is crucial to describe the result of the patient’s rehabilitation process. This concept was also highlighted by Weng et al.,48 who retrospectively enrolled stroke patients included in a post-acute care program. The authors reinforced the usefulness of a systematic multidomain evaluation before rehabilitation to predict the outcome, showing that a higher baseline and greater improvement of cognitive and physical abilities were associated with shorter lengths of stay in the IRU, fewer hospital readmissions, and reduced 1-year mortality. Indeed, promoting a shared and standardized assessment protocol is the first step concerning the integration of evidence-based treatments in rehabilitation, and a strength of the present study. Standardisation can foster these processes by improving clinical research through increased data quality, supporting better data integration and reusability, enabling data exchange with partners, increasing the use of software tools, improving team communication, and facilitating regulatory reviews and audits.46
Wei-Chieh Chen et al.49 focused on the identification of clinical factors able to predict functional independence at hospital discharge, in stroke patients who received in-hospital rehabilitation. Similarly to our findings, they showed that baseline daily activity function (measured with mBI) and motor function impairment of the hemiplegic lower limb were the most important prognostic factors of functional independence. However, the generalisability of these results is limited due to the retrospective nature of the study and the different organization of the post-stroke care pathway in Taiwan.
Another retrospective study50 exploited a multiparametric impairment assessment in stroke patients before and after inpatient rehabilitation. Plotting on a scattergram for each test the percentage of the highest score on admission and of patients whose score improved, Yagihashi et al.50 defined three patterns of change in impairment during hospitalisation without however predicting the result of the rehabilitative treatment. Understanding which deficits can improve could allow for better decisions on when and how much to insist on the rehabilitation of impairment, rather than to provide a proper discharge functional outcome prediction, needed to plan the patient’s pathway including time of discharge and return to home.
Another aspect essential for a reliable and interpretable prognostic analysis of the stroke population is the choice of standardised, reliable and reproducible assessment tools, especially for what concerns the outcome measures. Many retrospective studies aimed to identify outcome predictors in stroke patients available before undergoing an intensive rehabilitation cycle. For instance, Bertolin et al.51 retrospectively examined acute predictors of cognitive and functional outcomes six months after stroke. The authors showed that demographic factors and cognitive, physical, and functional measures at stroke onset poorly explained cognitive sequels. However, the same variables only accounted for moderate degrees of variance in functional outcomes, measured with the basic activities of daily living (ADL)/Instrumental ADL (IADL) (ΔR2=4.2%) and Participation subscales of the Stroke Impact Scale (SIS) (ΔR2=2.3%). In their paper, brief screening instruments such as NIHSS and a short screener for deficits in orientation, registration, and attention52 (the Short Blessed Test) were the most consistent individual predictors. On the other hand, these authors’ choice of a self-report instrument like the SIS Score, to measure the independence in self-care and daily activity, in household chores and small purchases and the limitation in work and recreation, can be controversial. In fact, self-reports are subjected to a risk of report bias, mainly linked to invalid answers, due to lack of sincerity and introspective ability,53 while the mBI assessed by a specialist, as we chose in our study, seems to be less prone to such bias. In fact, the choice of the primary outcome to adequately reflect the patient’s level of residual disability is also pivotal when studying rehabilitation functional outcomes. Many authors agree that it would be advisable to avoid measures focused on a narrow aspect of the function, such as binary outcomes or those describing single activities (e.g. ambulation) or measures that are possibly more related to contextual factors rather than to the rehabilitation intervention, such as whether the patient is discharged back home.54 Many of the existing predictive models in stroke are aimed to obtain an automatic prediction of clinical binary outcomes, such as independence of walking,55 or the likelihood of achieving at least one specific clinical score for independence56 or upper limb functionality.57 The results of modelling strategies using binary outcomes should be interpreted with caution, as pointed out by Dijkland et al.54 For instance, Scrutinio et al.56 developed an accurate prediction tool (area under the curve, AUC=0.866), externally validated by García-Rudolph et al.58 (AUC=0.873), which incorporates age, sex, time from stroke onset to inpatient rehabilitation admission, baseline motor and cognitive functional independency scores, and neglect. However, their predictive model aimed to predict the probability of achieving an independence level requiring no more than supervision and a motor FIM score higher than 61 points. The use of a dichotomised measure may be a viable solution while targeting a very specific outcome, but its clinical application in support of the clinical decision for a comprehensive evaluation of the patient’s status may not be optimal. Indeed, binary outcomes often are of little relevance for the patient59 and the family, whilst the continuous mBI60, 61 accurately reflects the level of independence and the burden that the caregiver will have to face at discharge. Thus, further development and validation of models with continuous outcomes should be promoted. This will allow a more granular prediction of the patient’s prognosis, with the possibility to evaluate alternative strategies to prevent the high risk of an unfavourable rehabilitative outcome, and also provide ground to improve the discharge planning with patients and families, already in the first days of rehabilitation hospitalisation. To avoid these limitations, we chose to preserve the continuous nature of our outcome variable (mBI at discharge). With this approach, our model obtains a good explanation of the outcome variance, with the possibility of distinguishing different outcomes at the end of hospitalisation such as complete recovery, sub-optimal or sufficient to continue the rehabilitation at home/assisted health service, which has a strong clinical impact.
Our descriptive analyses on the groups of patients with overestimated, underestimated, and correctly estimated mBI values at discharge showed that patients who were incorrectly predicted had significantly lower baseline mBI and FMA. This could mean that patients with a lower functional level at admission to IRU have less chance of being accurately estimated in their probability of reaching rehabilitation targets within their stay. It is possible that, while MBI and FMA provide a reliable admission assessment for some of these patients, others performed less well upon admission for reasons not strictly related to stroke severity, such as concurring acute clinical confounders, distress upon transfer, promoting agitation and lack of collaboration.62 These underlying clinical factors may either improve and resolve during the stay, explaining underestimation, or develop in full-blown clinical complications, explaining overestimation upon admission, but this hypothesis should be verified by further research. Possibly, including other assessment measures at admission, or repeating the same measures (MBI and FMA) at different times, particularly in those with lower scores upon admission, as well as integrating predicting models in time with updated clinical information of the patients may help improve the overall prediction.
Limitations of the study
This study has several limitations. Firstly, the model tested in this analysis needs external validation, which we plan to provide using datasets from ongoing prospective studies; further, we plan to compare and integrate it with machine learning-based approaches, for the analysis of the non-linear behaviour of data. In the meantime, a simple calculator, based on the coefficients obtained in the multivariable regression model, is available in the supplementary material, offering the mBI estimation at discharge based on these independent predictors, for stroke patients addressing post-acute intensive inpatient rehabilitation. The abovementioned lack of exploring impulsivity and neglect are also among our study limitations.63 Finally, the number of patients, in reference to the multifactorial complexity of the baseline evaluation, may have underestimated the predictive role of some variables. On the other hand, the strengths of our work lie in the thorough description and reproducibility of the evidence-based rehabilitation protocol, and in the systematic assessment of all stroke patients addressing the IRUs in the considered time frame, with 85% of them enrolled and with 94% of them included in this prognostic analysis. Indeed, our study fulfils the criteria of the QUIPS tool,41 which assesses the risk of bias in studies of prognostic factors, for all the six considered areas of participation, attrition, prognostic factor measurement, confounding measurement and account, outcome measurement, and analysis and reporting. In fact, we prospectively assessed and proposed the study to all patients addressing the participating centres for post-acute intensive inpatient rehabilitation after a stroke, thus reducing selection bias. Thus, we provided a systematic enrolment of a cohort of patients actually representing the post-acute stroke patients addressing intensive inpatient rehabilitation. Our final model includes a set of reliable, easily collected variables at admission to intensive inpatient rehabilitation, predicting stroke patients’ discharge functional outcomes. These results can be considered the first step in understanding potential predictors of the mBI, while future research should involve the external validation of this model and the validation of algorithms for the development of a data-driven tool. While promoting a rationalisation of economic community resources (avoiding expenses for futile treatments), refining the prediction of stroke rehabilitation outcomes has great potential to facilitate an improvement in individual rehabilitation planning (personalised approach) and to optimie rehabilitation outcomes.
Conclusions
Among multidimensional potential predictors of stroke rehabilitation outcome, we found that younger age, as well as greater independence in basic activities of daily living, fewer comorbidities, higher cognitive abilities, reduced stroke severity, and higher motor function, recorded at admission, were independently associated with the ability to perform activities of daily living at discharge from intensive inpatient rehabilitation after stroke. The final model including these variables could help clinicians to provide a granular prediction of the functional outcome of post-acute stroke patients at their admission to inpatient rehabilitation. Additional investigations, for those patients who present very low mBI and FMA, and the further development of this model in larger settings are needed to further improve its performance.
Supplementary Digital Material 1
Supplementary Table I
Supplementary material, evaluation tools references.
Supplementary Digital Material 2
Supplementary Text File 1
Calculator
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Owens Johnson C, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:439–58. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30871944&dopt=Abstract 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyden S, Wold J. Acute Treatment of Ischemic Stroke. Neurol Clin 2022;40:17–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34798968&dopt=Abstract 10.1016/j.ncl.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 3.Wafa HA, Wolfe CD, Emmett E, Roth GA, Johnson CO, Wang Y. Burden of Stroke in Europe: Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020;51:2418–27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32646325&dopt=Abstract 10.1161/STROKEAHA.120.029606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol 2020;19:348–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32004440&dopt=Abstract 10.1016/S1474-4422(19)30415-6 [DOI] [PubMed] [Google Scholar]
- 5.Duncan PW, Bushnell C, Sissine M, Coleman S, Lutz BJ, Johnson AM, et al. Comprehensive Stroke Care and Outcomes: Time for a Paradigm Shift. Stroke 2021;52:385–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33349012&dopt=Abstract 10.1161/STROKEAHA.120.029678 [DOI] [PubMed] [Google Scholar]
- 6.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016;47:e98–169. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27145936&dopt=Abstract 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 7.Rajsic S, Gothe H, Borba HH, Sroczynski G, Vujicic J, Toell T, et al. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ 2019;20:107–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29909569&dopt=Abstract 10.1007/s10198-018-0984-0 [DOI] [PubMed] [Google Scholar]
- 8.Abdul Aziz AF, Mohd Nordin NA, Muhd Nur A, Sulong S, Aljunid SM. The integrated care pathway for managing post stroke patients (iCaPPS©) in public primary care Healthcentres in Malaysia: impact on quality adjusted life years (QALYs) and cost effectiveness analysis. BMC Geriatr 2020;20:70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32070291&dopt=Abstract 10.1186/s12877-020-1453-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sulch D, Kalra LD. Integrated care pathways in stroke management. Age Ageing 2000;29:349–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10985445&dopt=Abstract 10.1093/ageing/29.4.349 [DOI] [PubMed] [Google Scholar]
- 10.Cecchi F, Diverio M, Arienti C, Corbella E, Marrazzo F, Speranza G, et al. Development and implementation of a stroke rehabilitation integrated care pathway in an Italian no profit institution: an observational study. Eur J Phys Rehabil Med 2020;56:713–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33494558&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 11.Bernhardt J, Borschmann K, Boyd L, Carmichael ST, Corbett D, Cramer SC, et al. Moving Rehabilitation Research Forward: Developing Consensus Statements for Rehabilitation and Recovery Research. Neurorehabil Neural Repair 2017;31:694–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28803534&dopt=Abstract 10.1177/1545968317724290 [DOI] [PubMed] [Google Scholar]
- 12.Jarva E, Mikkonen K, Tuomikoski AM, Kääriäinen M, Meriläinen M, Karsikas E, et al. Healthcare professionals’ competence in stroke care pathways: A mixed-methods systematic review. J Clin Nurs 2021;30:1206–35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33350004&dopt=Abstract 10.1111/jocn.15612 [DOI] [PubMed] [Google Scholar]
- 13.Walker MF, Hoffmann TC, Brady MC, Dean CM, Eng JJ, Farrin AJ, et al. Improving the Development, Monitoring and Reporting of Stroke Rehabilitation Research: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil Neural Repair 2017;31:877–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29233072&dopt=Abstract 10.1177/1545968317732686 [DOI] [PubMed] [Google Scholar]
- 14.Bernhardt J, Godecke E, Johnson L, Langhorne P. Early rehabilitation after stroke. Curr Opin Neurol 2017;30:48–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27845945&dopt=Abstract 10.1097/WCO.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 15.Durand A, D’Amours L, Giroux A, Pelletier M, Leblond J, Richards CL. Benchmarking length of stay for inpatient stroke rehabilitation without adversely affecting functional outcomes. J Rehabil Med 2020;52:jrm00113. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33000174&dopt=Abstract 10.2340/16501977-2746 [DOI] [PubMed]
- 16.Langhorne P, Ramachandra S; Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev 2020;4:CD000197. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32324916&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 17.Stinear CM. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol 2017;16:826–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28920888&dopt=Abstract 10.1016/S1474-4422(17)30283-1 [DOI] [PubMed] [Google Scholar]
- 18.Cecchi F, Cassio A, Lavezzi S, Scarponi F, Gatta G, Montis A, et al. Redefining a minimal assessment protocol for stroke rehabilitation: the new “Protocollo di Minima per l’ICtus” (PMIC2020). Eur J Phys Rehabil Med 2021;57:669–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34042407&dopt=Abstract 10.23736/S1973-9087.21.06638-7 [DOI] [PubMed] [Google Scholar]
- 19.Stinear CM. Stroke rehabilitation research needs to be different to make a difference. F1000 Res 2016;5:F1000 Faculty Rev-1467. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27408689&dopt=Abstract 10.12688/f1000research.8722.1 [DOI]
- 20.Kiper P, Szczudlik A, Agostini M, Opara J, Nowobilski R, Ventura L, et al. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch Phys Med Rehabil 2018;99:834–42. [DOI] [PubMed] [Google Scholar]
- 21.Stinear CM, Smith MC, Byblow WD. Prediction Tools for Stroke Rehabilitation. Stroke 2019;50:3314–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31610763&dopt=Abstract 10.1161/STROKEAHA.119.025696 [DOI] [PubMed] [Google Scholar]
- 22.Belagaje SR. Stroke Rehabilitation. Continuum (Minneap Minn) 2017;23(1, Cerebrovascular Disease):238–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28157752&dopt=Abstract 10.1212/CON.0000000000000423 [DOI] [PubMed]
- 23.Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One 2014;9:e87987. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24505342&dopt=Abstract 10.1371/journal.pone.0087987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornby TG, Reisman DS, Ward IG, Scheets PL, Miller A, Haddad D, et al. Locomotor CPG Appraisal Team . Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. J Neurol Phys Ther 2020;44:49–100. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31834165&dopt=Abstract 10.1097/NPT.0000000000000303 [DOI] [PubMed] [Google Scholar]
- 25.Lee PY, Huang JC, Tseng HY, Yang YC, Lin SI. Effects of Trunk Exercise on Unstable Surfaces in Persons with Stroke: A Randomized Controlled Trial. Int J Environ Res Public Health 2020;17:1–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33297451&dopt=Abstract 10.3390/ijerph17239135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijer R, Ihnenfeldt DS, de Groot IJ, van Limbeek J, Vermeulen M, de Haan RJ. Prognostic factors for ambulation and activities of daily living in the subacute phase after stroke. A systematic review of the literature. Clin Rehabil 2003;17:119–29. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12625651&dopt=Abstract 10.1191/0269215503cr585oa [DOI] [PubMed] [Google Scholar]
- 27.Nave AH, Rackoll T, Grittner U, Bläsing H, Gorsler A, Nabavi DG, et al. Physical Fitness Training in Patients with Subacute Stroke (PHYS-STROKE): multicentre, randomised controlled, endpoint blinded trial. BMJ 2019;366:l5101. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31533934&dopt=Abstract 10.1136/bmj.l5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Yu J, Bao Y, Xie Q, Xu Y, Zhang J, et al. Constraint-induced aphasia therapy in post-stroke aphasia rehabilitation: A systematic review and meta-analysis of randomized controlled trials. PLoS One 2017;12:e0183349. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28846724&dopt=Abstract 10.1371/journal.pone.0183349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwakkel G, Veerbeek JM, van Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol 2015;14:224–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25772900&dopt=Abstract 10.1016/S1474-4422(14)70160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chien W, Chong I, Tse M, Chien C, Cheng H. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: A systematic review and meta-analysis. Brain Behav 2020;10:e01742. 10.1002/brb3.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García-Rudolph A, Sánchez-Pinsach D, Salleras EO, Tormos JM. Subacute stroke physical rehabilitation evidence in activities of daily living outcomes: A systematic review of meta-analyses of randomized controlled trials. Medicine (Baltimore) 2019;98:e14501. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30813152&dopt=Abstract 10.1097/MD.0000000000014501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langhammer B, Sunnerhagen KS, Lundgren-Nilsson Å, Sällström S, Becker F, Stanghelle JK. Factors enhancing activities of daily living after stroke in specialized rehabilitation: an observational multicenter study within the Sunnaas International Network. Eur J Phys Rehabil Med 2017;53:725–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28417611&dopt=Abstract 10.23736/S1973-9087.17.04489-6 [DOI] [PubMed] [Google Scholar]
- 33.Groeneveld IF, Goossens PH, van Braak I, van der Pas S, Meesters JJ, Rambaran Mishre RD, et al. SCORE-study group . Patients’ outcome expectations and their fulfilment in multidisciplinary stroke rehabilitation. Ann Phys Rehabil Med 2019;62:21–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30053628&dopt=Abstract 10.1016/j.rehab.2018.05.1321 [DOI] [PubMed] [Google Scholar]
- 34.McIntyre A, Janzen S, Iruthayarajah J, Saikaley M, Sequeira D, Teasell R. Differences in stroke rehabilitation motor and cognitive randomized controlled trials by world region: Number, sample size, and methodological quality. NeuroRehabilitation 2020;47:191–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32716328&dopt=Abstract 10.3233/NRE-203168 [DOI] [PubMed] [Google Scholar]
- 35.Aryan R, Jagroop D, Danells CJ, Rozanski G, Unger J, Huntley AH, et al. Publication Rate and Consistency of Registered Trials of Motor-Based Stroke Rehabilitation. Neurology 2021;96:617–26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33568550&dopt=Abstract 10.1212/WNL.0000000000011660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salter KL, Teasell RW, Foley NC, Jutai JW. Outcome assessment in randomized controlled trials of stroke rehabilitation. Am J Phys Med Rehabil 2007;86:1007–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17912137&dopt=Abstract 10.1097/PHM.0b013e3181587b3d [DOI] [PubMed] [Google Scholar]
- 37.Hakiki B, Paperini A, Castagnoli C, Hochleitner I, Verdesca S, Grippo A, et al. Predictors of Function, Activity, and Participation of Stroke Patients Undergoing Intensive Rehabilitation: A Multicenter Prospective Observational Study Protocol. Front Neurol 2021;12:632672. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33897593&dopt=Abstract 10.3389/fneur.2021.632672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsieh YW, Wang CH, Wu SC, Chen PC, Sheu CF, Hsieh CL. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil Neural Repair 2007;21:233–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17351082&dopt=Abstract 10.1177/1545968306294729 [DOI] [PubMed] [Google Scholar]
- 39.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17938396&dopt=Abstract 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 40.Pedregosa F, Weiss R, Brucher M, Varoquaux G, Gramfort A, Michel V, et al. Scikit-learn: Machine Learning in Python. J Mach Learn Res 2011;12:2825–30. [Google Scholar]
- 41.Santangelo G, Siciliano M, Pedone R, Vitale C, Falco F, Bisogno R, et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol Sci 2015;36:585–91. Available from: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25380622&dopt=Abstract 10.1007/s10072-014-1995-y [DOI] [PubMed] [Google Scholar]
- 42.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23420236&dopt=Abstract 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 43.Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019;13(Suppl 1):S31–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30930717&dopt=Abstract 10.4103/sja.SJA_543_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hakkennes SJ, Brock K, Hill KD. Selection for inpatient rehabilitation after acute stroke: a systematic review of the literature. Arch Phys Med Rehabil 2011;92:2057–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22133256&dopt=Abstract 10.1016/j.apmr.2011.07.189 [DOI] [PubMed] [Google Scholar]
- 45.Meyer MJ, Pereira S, McClure A, Teasell R, Thind A, Koval J, et al. A systematic review of studies reporting multivariable models to predict functional outcomes after post-stroke inpatient rehabilitation. Disabil Rehabil 2015;37:1316–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25250807&dopt=Abstract 10.3109/09638288.2014.963706 [DOI] [PubMed] [Google Scholar]
- 46.Standardization to Enhance Data Sharing - Sharing Clinical Research Data - NCBI Bookshelf [Internet]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK137818/ [cited 2023, Jul 3].
- 47.Dhamoon MS, Moon YP, Paik MC, Sacco RL, Elkind MS. Trajectory of functional decline before and after ischemic stroke: the Northern Manhattan Study. Stroke 2012;43:2180–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22649168&dopt=Abstract 10.1161/STROKEAHA.112.658922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weng SC, Hsu CY, Shen CC, Huang JA, Chen PL, Lin SY. Combined Functional Assessment for Predicting Clinical Outcomes in Stroke Patients After Post-acute Care: A Retrospective Multi-Center Cohort in Central Taiwan. Front Aging Neurosci 2022;14:834273. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35783145&dopt=Abstract 10.3389/fnagi.2022.834273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen WC, Hsiao MY, Wang TG. Prognostic factors of functional outcome in post-acute stroke in the rehabilitation unit. J Formos Med Assoc 2022;121:670–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34303583&dopt=Abstract 10.1016/j.jfma.2021.07.009 [DOI] [PubMed] [Google Scholar]
- 50.Yagihashi K, Sonoda S, Watanabe M, Okamoto S, Okuyama Y, Okazaki H. Pattern of item score change in Stroke Impairment Assessment Set in comprehensive inpatient rehabilitation wards. Fujian Med J 2020;6:49–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35111521&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertolin M, Van Patten R, Greif T, Fucetola R. Predicting cognitive functioning, activities of daily living, and participation 6 months after mild to moderate stroke. Arch Clin Neuropsychol 2018;33:562–76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29028864&dopt=Abstract 10.1093/arclin/acx096 [DOI] [PubMed] [Google Scholar]
- 52.Carpenter CR, Bassett ER, Fischer GM, Shirshekan J, Galvin JE, Morris JC. Four sensitive screening tools to detect cognitive dysfunction in geriatric emergency department patients: brief Alzheimer’s Screen, Short Blessed Test, Ottawa 3DY, and the caregiver-completed AD8. Acad Emerg Med 2011;18:374–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21496140&dopt=Abstract 10.1111/j.1553-2712.2011.01040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenman R, Tennekoon V, Hill LG. Measuring bias in self-reported data. Int J Behav Healthc Res 2011;2:320–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25383095&dopt=Abstract 10.1504/IJBHR.2011.043414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dijkland SA, Dippel DW, Lingsma HF. Letter by Dijkland et al Regarding Article, “Development and Validation of a Predictive Model for Functional Outcome After Stroke Rehabilitation: The Maugeri Model”. Stroke 2018;49:e133. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29440583&dopt=Abstract 10.1161/STROKEAHA.117.020108 [DOI] [PubMed] [Google Scholar]
- 55.Bland MD, Sturmoski A, Whitson M, Connor LT, Fucetola R, Huskey T, et al. Prediction of discharge walking ability from initial assessment in a stroke inpatient rehabilitation facility population. Arch Phys Med Rehabil 2012;93:1441–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22446516&dopt=Abstract 10.1016/j.apmr.2012.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scrutinio D, Lanzillo B, Guida P, Mastropasqua F, Monitillo V, Pusineri M, et al. Development and validation of a predictive model for functional outcome after stroke rehabilitation the maugeri model. Stroke 2017;48:3308–15. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29051222&dopt=Abstract 10.1161/STROKEAHA.117.018058 [DOI] [PubMed] [Google Scholar]
- 57.Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G. EPOS Investigators. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke 2010;41:745–50. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20167916&dopt=Abstract 10.1161/STROKEAHA.109.572065 [DOI] [PubMed] [Google Scholar]
- 58.García-Rudolph A, Bernabeu M, Cegarra B, Saurí J, Madai VI, Frey D, et al. Predictive models for independence after stroke rehabilitation: maugeri external validation and development of a new model. NeuroRehabilitation 2021;49:415–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34542037&dopt=Abstract 10.3233/NRE-201619 [DOI] [PubMed] [Google Scholar]
- 59.Bath PM, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, et al. European Stroke Organisation Outcomes Working Group . Statistical analysis of the primary outcome in acute stroke trials. Stroke 2012;43:1171–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22426314&dopt=Abstract 10.1161/STROKEAHA.111.641456 [DOI] [PubMed] [Google Scholar]
- 60.Yang CM, Wang YC, Lee CH, Chen MH, Hsieh CL. A comparison of test-retest reliability and random measurement error of the Barthel Index and modified Barthel Index in patients with chronic stroke. Disabil Rehabil 2022;44:2099–103. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32903114&dopt=Abstract 10.1080/09638288.2020.1814429 [DOI] [PubMed] [Google Scholar]
- 61.Wang YC, Chang PF, Chen YM, Lee YC, Huang SL, Chen MH, et al. Comparison of responsiveness of the Barthel Index and modified Barthel Index in patients with stroke. Disabil Rehabil 2023;45:1097–102. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35357990&dopt=Abstract 10.1080/09638288.2022.2055166 [DOI] [PubMed] [Google Scholar]
- 62.Kulshrestha A, Singh J. Inter-hospital and intra-hospital patient transfer: recent concepts. Indian J Anaesth 2016;60:451–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27512159&dopt=Abstract 10.4103/0019-5049.186012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belanger HG. Recovery from stroke: factors affecting prognosis. Clin Neuropsychol 2019;33:813–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30882270&dopt=Abstract 10.1080/13854046.2019.1578899 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Supplementary material, evaluation tools references.
Supplementary Text File 1
Calculator


