Abstract
Tobacco (Nicotiana tabacum L.) is a widely cultivated crop of the genus Nicotiana. Due to the highly addictive nature of tobacco products, tobacco smoking remains the leading cause of preventable death and disease. There is therefore a critical need to develop tobacco varieties with reduced or non-addictive nicotine levels. Nicotine and related pyridine alkaloids biosynthesized in the roots of tobacco plants are transported to the leaves, where they are stored in vacuoles as a defense against predators. Jasmonate, a defense-related plant hormone, plays a crucial signaling role in activating transcriptional regulators that coordinate the expression of downstream metabolic and transport genes involved in nicotine production. In recent years, substantial progress has been made in molecular and genomics research, revealing many metabolic and regulatory genes involved in nicotine biosynthesis. These advances have enabled us to develop tobacco plants with low or ultra-low nicotine levels through various methodologies, such as mutational breeding, genetic engineering, and genome editing. We review the recent progress on genetic manipulation of nicotine production in tobacco, which serves as an excellent example of plant metabolic engineering with profound social implications.
Keywords: Alkaloid, biosynthesis, jasmonate, Nicotiana, nicotine, tobacco, transcription factor
Molecular and genomics studies have revealed metabolic and regulatory genes involved in nicotine biosynthesis, enabling us to develop tobacco plants with ultra-low nicotine levels through mutational breeding, genetic engineering, and genome editing.
Introduction
Tobacco (Nicotiana tabacum L.) is an economically important crop that is cultivated around the world, with a global production of ~7.1 Mt in 2020; the top producing countries include China, India, Brazil, and the USA (https://ourworldindata.org/grapher/tobacco-production?time=latest). The genus Nicotiana, which is part of the family Solanaceae and comprises 76 naturally occurring species (Knapp et al., 2004), is one of the most extensively studied genera of flowering plants, in large part due to its economic and cultural importance (Lewis, 2011). Nicotiana species arose in the Americas and Australia, where they have been traditionally used by native peoples for recreational and therapeutic purposes (Goodman, 2004).
After Columbus arrived in the Americas, tobacco smoking spread around the world due to the highly addictive nature of tobacco products, which has led to global health, economic, and social impacts ever since (Goodman, 2004). Despite efforts to reduce tobacco consumption in developed countries in recent years, smoking remains the leading preventable cause of disease and death worldwide. The health risks of smoking and other tobacco use are well established, with tobacco consumption being linked to a range of diseases, including lung cancer, cardiovascular disease, chronic obstructive pulmonary disease, and many other illnesses (U.S. Department of Health and Human Services, 2014). The World Health Organization (WHO) estimates that tobacco use is responsible for ~8 million deaths per year, with 80% of these deaths now occurring in low- and middle-income countries (https://www.who.int/news-room/fact-sheets/detail/tobacco). This number is expected to increase to >10 million deaths per year by 2030 if current trends continue.
Nicotine and related pyridine alkaloids, including nornicotine, anatabine, and anabasine, are specialized metabolites present in Nicotiana species (Kaminski et al., 2020). In most tobacco varieties, nicotine is the predominant alkaloid, typically comprising >90% of the total alkaloid pool (Sisson and Saunders, 1982). The predominant naturally occurring form of nicotine is an optically pure (S)-isomer. Nicotine was first isolated by a German chemist Wilhelm Heinrich Posselt and named in honor of Jean Nicot, a French ambassador who introduced tobacco to Europe in the 16th century (Goodman, 2004). When inhaled, nicotine is rapidly absorbed into the bloodstream through the lungs and can easily pass through the blood–brain barrier to reach the brain. Nicotine is a psychoactive substance that has multiple effects on the brain, acting as an agonist for nicotinic acetylcholine receptors (Benowitz, 2009). One of its main effects is to stimulate the release of dopamine, a neurotransmitter that is involved in the brain’s reward system. This leads to feelings of pleasure, euphoria, and stimulatory motivation, thereby contributing to the strong addictive property of tobacco alkaloids. Over time, regular nicotine use can lead to addiction and tolerance, requiring higher doses to achieve the same effects.
The WHO has recommended reducing nicotine levels in cigarettes to a non-addictive level (0.4 mg g–1), which could substantially benefit public health by reducing the number of people who become addicted to tobacco products and by making it easier for smokers to quit the harmful habit (WHO, 2015). In 2022, the US Food and Drug Administration (FDA) proposed a new rule that would establish a maximum nicotine level in cigarettes and other tobacco products sold in the USA in line with the WHO recommendation (https://www.fda.gov/news-events/press-announcements/fda-announces-plans-proposed-rule-reduce-addictiveness-cigarettes-and-other-combusted-tobacco). It is important to develop tobacco varieties with non-addictive nicotine levels to support this public health goal (Lewis, 2019).
Nicotine is produced primarily in the roots of tobacco plants and transported via the xylem to the leaves, where it is stored in vacuoles and serves as a defense mechanism against predation. Due to its potent toxicity, nicotine has historically been used as an insecticide. Insect herbivory and physical damage to the plant can increase nicotine accumulation. The plant hormone jasmonate (JA) plays a crucial signaling role in eliciting nicotine biosynthesis in response to damage, activating transcriptional regulators that coordinate the expression of downstream metabolic and transport genes. The amount of nicotine in tobacco leaves is influenced by various factors such as cultivation practices, environmental conditions, and genetic background. With advances in molecular and genomics research (Dewey and Xie, 2013; Sierro et al., 2014; Wang and Bennetzen, 2015; Shoji, 2020b), it is now possible to use mutational breeding, genetic engineering, and genome editing to generate tobacco plants with low-nicotine or ultra-low nicotine traits, which are defined here as <20% and 5% relative to the level in wild-type tobacco, respectively (Lewis, 2019). Here we overview the recent advances in genetic manipulation of nicotine production in tobacco, presenting an excellent example of plant metabolic engineering with social impacts.
Alkaloid biosynthesis in tobacco
Alkaloids are a class of nitrogen-containing, mostly alkaline chemicals that are usually biosynthesized from amino acids and their derivatives through a series of reactions catalyzed by metabolic enzymes from various protein families (Shoji, 2020a). Nicotine is composed of a pyrrolidine and a pyridine ring, which are formed from amino acid precursors early in the pathway and are condensed in later steps (Fig. 1). Most, but not all, of the metabolic genes involved in nicotine biosynthesis have been identified and intensively studied for genetic manipulation (Dewey and Xie, 2013; Shoji, 2020b).
Fig. 1.

Biosynthesis of nicotine and related alkaloids in tobacco. Dashed arrows represent undefined or multiple steps. Metabolic enzymes whose genes are regulated by ERF199 and ERF189, and their reaction steps, are shown in red. Putrescine N-methyltransferase (PMT) and N-methylputrescine oxidase (MPO) have been proposed to have evolved from spermidine synthase (SPDS) and diamine oxidase (DAO), respectively. AO, aspartate oxidase; BBL, berberine bridge enzyme-like protein; ODC, ornithine decarboxylase; QPT, quinolinate phosphoribosyltransferase; QS, quinolinate synthase.
Pyrrolidine formation
To form pyrrolidine, a five-membered ring intermediate, N-methylpyrrolinium cation, is biosynthesized from ornithine through a series of consecutive reactions catalyzed by three enzymes: ornithine decarboxylase (ODC) (Imanishi et al., 1998), putrescine N-methyltransferase (PMT) (Hibi et al., 1994), and N-methylputrescine oxidase (MPO) (Heim et al., 2007; Katoh et al., 2007) (Fig. 1).
PMT and MPO are suggested to have evolved from primary metabolic enzymes involved in polyamine biosynthesis, specifically spermidine synthase (SPDS) and diamine oxidase (DAO), respectively (Junker et al., 2013; Naconsie et al., 2014). The pyrrolidine-forming branch of nicotine biosynthesis is thought to have arisen through duplication of the polyamine pathway (Kajikawa et al., 2017a, b; Xu et al., 2017). This duplication event resulted in more ODC genes, which potentially facilitated enhanced metabolic flow into the duplicated branch. Consequently, this increase in metabolic flow allowed catalytic innovation through the neo-functionalization of the duplicate genes, leading to the evolution of PMT and MPO genes. There are two ODC genes, ODC1 and ODC2, and they exhibit differential expression patterns (Xu et al., 2004). ODC2 is co-regulated with other genes involved in nicotine biosynthesis, suggesting a coordinated regulation of nicotine production. On the other hand, ODC1 expression is nearly constitutive, indicating its involvement in basal cellular processes.
Down-regulating both ODC genes using RNAi resulted in reduced nicotine levels but increased anatabine accumulation in hairy roots and transgenic plants (DeBoer et al., 2011a; Dalton et al., 2016). Additionally, the ODC-silenced lines showed reduced accumulation of polyamines along with various physiological and morphological abnormalities, including early senescence and partial sterility (Dalton et al., 2016; Choubey and Rajam, 2017). These abnormalities are probably caused by the decreased levels of polyamines resulting from ODC down-regulation.
PMT catalyzes the first committed step of pyrrolidine formation in nicotine biosynthesis. Several studies have shown that when PMT is down-regulated using co-suppression, antisense, or RNAi techniques, there is a significant decrease in nicotine accumulation in Nicotiana species (Sato et al., 2001; Chintapakorn and Hamill, 2003; Steppuhn et al., 2004; Wang et al., 2008, 2009). Interestingly, PMT knockdown results in a simultaneous increase in anatabine levels (Chintapakorn and Hamill, 2003; Steppuhn et al., 2004; Wang et al., 2009). Additionally, in PMT-suppressed lines of Nicotiana sylvestris, abnormal leaf fusion and a significant increase in polyamine levels were observed alongside reduced nicotine production (Sato et al., 2001).
Down-regulating MPO through RNAi resulted in significant decreases in nicotine and nornicotine levels, accompanied by substantial increases in anatabine and anabasine in tobacco hairy roots (Shoji and Hashimoto, 2008). Anatabine and anabasine do not possess a pyrrolidine ring, indicating that suppressing the expression of ODC, PMT, or MPO genes, involved in pyrrolidine biosynthesis, prompts compensatory production of the pyridine-derived alkaloids.
Pyridine formation
Nicotinic acid is a primary metabolite in the biosynthesis pathway that supplies NAD, an essential cofactor for many oxidation–reduction reactions. The pyridine part of nicotine and other related alkaloids is derived from nicotinic acid. In the NAD pathway, aspartate is converted to nicotinic acid mononucleotide via quinolinate through reactions catalyzed by aspartate oxidase (AO), quinolinate synthase (QS), and quinolinate phosphoribosyltransferase (QPT) (Sinclair et al., 2000; Katoh et al., 2006) (Fig. 1). Duplication of AO and QPT genes has occurred in Nicotiana species but not in other lineages, enabling massive downstream nicotine production by increasing upstream metabolic flows (Kajikawa et al., 2017a, b; Xu et al., 2017). One of the two QPT genes, QPT2, is expressed in the roots and induced in response to JA along with other genes involved in nicotine biosynthesis, while QPT1 is constitutively expressed in all tobacco tissues, possibly involved in NAD biosynthesis (Shoji and Hashimoto, 2011a). Such differential expression patterns between the two genes suggest sub-functionalization after gene duplication. It remains unclear how nicotinic acid is supplied from nicotinic acid mononucleotide.
Down-regulating AO genes using RNAi resulted in reduced nicotine accumulation and early senescence in tobacco leaves (Hidalgo Martinez et al., 2020). However, there have been no reports on how manipulating QS affects nicotine accumulation or phenotypic traits in tobacco.
QPT is a rate-limiting enzyme in pyridine ring formation. Down-regulating QPT1 and QPT2 via RNAi significantly reduced nicotine and anabasine levels and also caused notable changes in plant growth (Khan et al., 2017). These alterations in growth could be attributed to a decreased supply of NAD. Additionally, a QPT2-knockout mutant generated using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated nuclease 9 (Cas9) genome editing showed drastically reduced nicotine production (Smith et al., 2022). It is important to note that this tobacco mutant displayed only modest growth changes in a greenhouse environment, but, when transplanted to fields, its growth and development were severely inhibited. These findings suggest that knocking out QPT2 is not a viable strategy for producing agriculturally useful tobacco with low nicotine.
Condensation of pyrrolidine and pyridine rings
In contrast to the early steps of the nicotine biosynthesis pathway, which largely overlap with or are evolutionarily related to polyamine or NAD pathways, it is unclear how the rings are condensed in the late part of the pathway. It is even unclear whether the substrate for the condensation reaction is nicotinic acid itself or one of its derivatives. Two oxidoreductases, A622 and berberine bridge enzyme-like protein (BBL), have been proposed to be involved in the later steps, but biochemical details of their reactions in the pathway remain to be determined (Hibi et al., 1994; DeBoer et al., 2009; Kajikawa et al., 2009, 2011) (Fig. 1). A622 is an NADPH-dependent reductase belonging to the PIP family (Kajikawa et al., 2009), which was named after its founding members: pinoresinol–lariciresinol reductase (Min et al., 2003), isoflavone reductase (Wang et al., 2006), and phenylcoumaran benzylic ether reductase (Min et al., 2003). BBL is primarily localized in vacuoles within tobacco roots (Kajikawa et al., 2009). A622 and BBL are required to produce all pyridine alkaloids, suggesting that steps mediated by A622 and BBL are shared among the routes leading to the different alkaloids (DeBoer et al., 2009; Kajikawa et al., 2009, 2011) (Fig. 1).
In wood tobacco (Nicotiana glauca), silencing of A622 via RNAi significantly decreased the levels of its predominant alkaloid, anabasine, in the leaves and hairy roots (DeBoer et al., 2009). Furthermore, in tobacco hairy roots, down-regulating A622 through RNAi drastically reduced the levels of nicotine and other alkaloids, and inhibited root growth (Kajikawa et al., 2009). This growth inhibition was attributed to the overaccumulation of cytotoxic nicotinic acid (Li et al., 2017), not all of which could be utilized for nicotine production.
The tobacco genome contains six BBL genes: BBLa, BBLb, BBLc, BBLd, BBLd2, and BBLe (Kajikawa et al., 2017b). Simultaneous RNAi of BBLa, BBLb, and BBLc resulted in a significant reduction of up to 94% in foliar nicotine levels (Kajikawa et al., 2011). Furthermore, careful phenotypic analysis of knockouts of all six BBL genes, generated through chemically induced mutagenesis and CRISPR/Cas9-mediated editing (Lewis et al., 2015, 2020; Schachtsiek and Stehle, 2019), revealed that combined mutations in BBLa, BBLb, and BBLc substantially reduced (up to 17-fold) nicotine accumulation (Lewis et al., 2015, 2020). However, additional mutations in BBLd, BBLd2, and BBLe did not contribute to further decreases in nicotine levels (Lewis et al., 2020). Interestingly, tobacco lines with loss of BBL function overaccumulated a minor alkaloid called dehydrometanicotine (Kajikawa et al., 2011; Lewis et al., 2015). Additionally, field-grown BBL RNAi lines showed a significant 29% reduction in yield compared with wild-type tobacco, indicating that loss of BBL function negatively affects plant growth (Lewis et al., 2020).
Nornicotine formation
Another alkaloid of tobacco, nornicotine, typically comprises ~2–5% of the total alkaloids in leaves (Sisson and Saunders, 1982). The conversion of nicotine to nornicotine occurs through a demethylation step catalyzed by nicotine N-demethylase, a cytochrome P450 enzyme belonging to the CYP82E subfamily (Fig. 1). Several CYP82E genes have been identified in tobacco, including CYP82E2, CYP82E3, CYP82E4, CYP82E5, CYP82E10, and CYP82E21. CYP82E2 and CYP82E3 encode non-functional enzymes due to substitutions in critical amino acid residues, while their counterparts in progenitor species N. sylvestris and Nicotiana tomentosiformis encode highly active demethylases (Siminszky et al., 2005; Gavilano et al., 2007). CYP82E4 is induced during leaf senescence and curing, and is responsible for the majority of nornicotine production in tobacco (Siminszky et al., 2005; Chakrabarti et al., 2008), whereas CYP82E5, CYP82E10, and CYP82E21 are primarily expressed in green leaves, roots, and flower ovaries, respectively, and contribute to nornicotine production to a lesser extent (Gavilano and Siminszky, 2007; Lewis et al., 2010; Liedschulte et al., 2016). The relaxed substrate specificity of the CYP82E4 enzyme is responsible for an increased ratio of (R)-nornicotine to (S)-nornicotine, which is higher than the ratio of the corresponding nicotine isomers (Cai et al., 2012). In a proportion of individuals in tobacco populations, nornicotine occasionally becomes the dominant alkaloid due to transcriptional reactivation of CYP82E4, which is normally silenced (Griffith et al., 1955; Siminszky et al., 2005). However, the genetic or epigenetic basis of this change remains elusive.
Knockout mutations in CYP82E4, CYP82E5, and CYP82E10 were obtained from chemically mutagenized populations (Lewis et al., 2010; Song et al., 2020), and the relative contributions of each mutation to nornicotine production were evaluated in a series of mutant genotypes (Lewis et al., 2010). In tobacco, a triple knockout line had significantly decreased nornicotine accumulation in leaves compared with the wild type, which accounted for <1% of the total alkaloid level (Lewis et al., 2010; Song et al., 2020), similar to the results of RNAi-mediated silencing of these three genes in tobacco (Lewis et al., 2008). In a cyp82e4 cyp82e5 cyp82e10 triple mutant of a flue-cured tobacco variety, along with the decreased nornicotine, nicotine levels were also significantly reduced to 73% of that in the wild type (Song et al., 2020). This reduction was attributed to the down-regulation of nicotine biosynthesis genes, indicating an unknown negative regulatory relationship between nicotine demethylation and nicotine biosynthesis.
Under conditions where the availability of one-carbon (C1) units is limited due to a restricted C1 pool, methyl groups from nicotine become important in C1 metabolism. When a key metabolic gene encoding methylenetetrahydrofolate reductase (MTHFR) involved in tetrahydrofolate-mediated C1 metabolism was down-regulated using RNAi, CYP82E4 expression was induced possibly to supply methyl groups from nicotine (Hung et al., 2013). Conversely, when MTHFR was overexpressed, CYP82E4 was suppressed, leading to a decrease in nornicotine accumulation (Hung et al., 2013).
Tobacco-specific nitrosamines
Tobacco-specific nitrosamines (TSNAs) comprise a group of carcinogenic compounds that are formed from the reaction of nitrite and secondary amines, including nicotine and nornicotine, during tobacco curing and use (Konstantinou et al., 2018). TSNAs are highly carcinogenic and mutagenic and have been linked to various types of cancer, particularly lung cancer. The most abundant TSNAs in tobacco are Nʹ-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), and N-nitrosoanatabine (NAT). NNN, which is readily formed from nornicotine, is considered one of the most carcinogenic compounds in tobacco products and more harmful than other TSNAs. Suppressing nornicotine formation is an effective strategy to restrict NNN generation (see ‘Nornicotine formation’).
Nitrate is a nitrosating agent that contributes to TSNA formation. Therefore, in addition to reducing precursor alkaloids, lowering nitrate levels in tobacco leaves is a promising strategy to reduce the formation of these carcinogenic compounds. Burley tobacco varieties accumulate high levels of nitrate (Lewis et al., 2012), making them a prime target for this strategy. Leaf nitrate and TNSA levels were effectively reduced by expressing a constitutively active variant of nitrate reductase, a key enzyme in the nitrogen assimilation pathway (Lu et al., 2016). Additionally, a member of the chloride channel (CLC) protein family mediates nitrate accumulation in the vacuole (Geelen et al., 2000). Suppressing or knocking out a tobacco CLC gene CLCNt2 resulted in decreased nitrate and TSNA levels in cured leaves without negatively impacting biomass (Bovet et al., 2022).
Membrane transporters
Membrane transporters from various families play crucial roles in facilitating the movement of natural products across biological membranes. In tobacco cells, nicotine is sequestered into vacuoles to prevent cytotoxic effects at high concentrations. Several transporters belonging to the multidrug and toxic compound extrusion (MATE) family, namely JASMONTE-INDUCIBLE ALKALOID TRANSPORTER 1 (JAT1), JAT2, MATE1, and MATE2, mediate vacuolar sequestration of nicotine (Morita et al., 2009; Shoji et al., 2009; Shitan et al., 2014). These MATE transporters are localized in the tonoplast membrane and function as proton antiporters, coupling proton gradients across the membrane with nicotine uptake into the vacuoles. JAT1 and JAT2 are expressed in the leaves and encode proteins that are phylogenetically related to the xenobiotic-transporting DETOXIFICATION1 (DTX1) transporter found in Arabidopsis (Arabidopsis thaliana) (Morita et al., 2009; Shitan et al., 2014). MATE1 and MATE2 encode homologs of flavonoid transporters and are co-expressed with nicotine biosynthesis genes in tobacco roots (Shoji et al., 2009). MATE2 is located near A622 on chromosome 12 in the tobacco genome (Kajikawa et al., 2017b). It is worth noting that this clustering of non-homologous genes is a unique case so far in the nicotine pathway. The crystal structure of MATE2 has been reported, providing valuable insights into its substrate recognition and transport mechanisms (Tanaka et al., 2021). However, it should be noted that RNAi-mediated suppression of MATE1 and MATE2 did not significantly alter alkaloid levels in the leaves and roots (Shoji et al., 2009). As for JAT1 and JAT2, it remains unknown whether manipulating these transporter genes impacts alkaloid profiles.
NICOTINE UPTAKE PERMEASE 1 (NUP1) is a member of the purine permease family localized in the plasma membrane (Hildreth et al., 2011; Kato et al., 2014) and functions as a transporter responsible for the uptake of metabolites containing a pyridine ring, including nicotine and vitamin B6 (Hildreth et al., 2011; Kato et al., 2014, 2015). NUP1 is primarily expressed in epidermal cells of roots (Kato et al., 2014). It has been suggested that NUP1 may modulate nicotine biosynthesis by participating in the transcriptional regulation of the ethylene response factor (ERF) transcription factor genes ERF199 and ERF189, as well as in root growth (Hildreth et al., 2011; Kato et al., 2014). However, the exact mechanisms linking membrane transport and these regulatory functions are still not understood.
A grafting experiment demonstrated root-to-shoot translocation of tobacco alkaloids between tobacco rootstock and tomato scion (Dawson, 1942). Nicotine moves upward through the xylem along the transpiration stream. Nicotine efflux occurs from root cells, and nicotine influx takes place in leaf cells, facilitating xylem loading and unloading, respectively. However, the membrane transporters responsible for these processes have remained elusive. Some wild Nicotiana species lack or have reduced abilities for long-distance alkaloid translocation (Pakdeechanuan et al., 2012). Exploring the genetic basis of such natural variations is intriguing and could provide valuable insights into the alkaloid transport mechanism.
Regulation
In plants, metabolic pathways that produce specialized products are subject to dynamic regulation in response to developmental and environmental cues. Transcription factors often coordinate the expression of multiple metabolic and transport genes involved in these pathways and play key roles in integrating the metabolic processes in various biological responses (Chezem and Clay, 2016; Shoji et al., 2021).
In tobacco, the JA signaling pathway up-regulates defense-related nicotine biosynthesis (Shoji et al., 2000). JAs comprise a group of plant hormones derived from fatty acids that play a central role in development and defense responses against biotic and abiotic stresses (Wasternack and Strnad, 2018). JAs have been widely used as elicitors to induce the production of natural products in plant tissue cultures. The molecular mechanism of the JA signaling pathway, from signal perception to gene induction, is conserved in a wide range of plant species (Paschold et al., 2007; Shoji et al., 2008). The JA signaling pathway involves the proteasome-dependent degradation of JASMONATE ZIM-DOMAIN (JAZ) repressors, which leads to the activation of transcription factors such as the basic helix–loop–helix (bHLH)-family MYC2 (Wasternack and Strnad, 2018) (Fig. 2). However, it remains unclear how the conserved upstream JA signaling cascade is linked to diverse downstream defense responses and metabolic pathways, such as the nicotine biosynthesis pathway in tobacco. The JA-responsive transcription factors ERF199 and ERF189 have emerged as a central molecular component linking upstream signaling with downstream metabolic processes in tobacco (Shoji and Yuan, 2021) (Fig. 2).
Fig. 2.

A model of JA- and topping-dependent induction of nicotine biosynthesis genes in tobacco. ERF199 and ERF189 up-regulate nicotine biosynthesis genes (e.g. QPT2) by recognizing GC-rich elements in their promoter regions. A bHLH-family MYC2 transcription factor mediates JA-dependent induction of ERF genes. MYC2 is a direct target of JAZ repressors. When a co-complex comprising COI1 and JAZ perceives a JA signal, JAZ proteins are removed through proteasome-dependent degradation. MYC2 also directly up-regulates nicotine biosynthesis genes by recognizing G-box elements as homo- and/or heterodimers. Topping induces nta-TMX27 expression, leading to nta-miRX27 degradation. Degradation of this miRNA relieves its inhibition of QPT2, resulting in enhanced QPT2 expression.
ERF199 and ERF189 transcription factors
In the 1930s, a mutant of a Cuban cigar tobacco variety with low nicotine content was discovered in Europe. This naturally occurring mutant has since been used to breed commercial tobacco varieties with low-nicotine traits (Legg et al., 1970), which typically have nicotine levels that are 10–20% of those found in wild-type tobacco. The low-nicotine phenotype is determined by semi-dominant mutations at two unlinked genetic loci, NICOTINE1 (NIC1) and NICOTINE2 (NIC2), which are also referred to as A and B, respectively (Legg and Collins, 1971). However, it should be noted that the low-nicotine tobacco resulting from these mutations has certain drawbacks, such as inferior leaf quality and increased susceptibility to insect pathogens (Chaplin and Weeks, 1976). As a result, the economic value and utilization of this genotype have been limited.
Molecular genetics and genomics studies have revealed the identities of the NIC genes. NIC1 was identified as ERF199, located on chromosome 7 and derived from N. sylvestris (Qin et al., 2021; Shoji et al., 2022), while NIC2 was identified as ERF189, located on chromosome 19 and derived from N. tomentosiformis (Shoji et al., 2010; Kajikawa et al., 2017b) (Fig. 2). ERF199 and ERF189 encode JA-responsive ERF transcription factors and are mostly coordinately expressed with nicotine biosynthesis genes in response to various developmental and phytohormonal cues. In the tobacco genome, ERF199 and ERF189 are present in clusters of five and ten homologous ERF genes, respectively (Kajikawa et al., 2017b) (Box 1). Based on expression and functional studies, it is reasonable to consider that ERF199 and ERF189 mainly regulate nicotine biosynthesis (Shoji and Hashimoto, 2015). ERF199 and ERF189 up-regulate numerous nicotine biosynthesis genes, including ODC2, PMT, MPO, AO2, QS, QPT2, A622, BBL, MATE1, and MATE2, by recognizing specific GC-rich cis-regulatory elements in the promoter regions of the downstream target genes (Shoji et al., 2010, 2013). ERF189, and possibly ERF199, are up-regulated by the MYC2 transcription factor, thereby linking upstream JA signaling to downstream nicotine biosynthesis (Shoji and Hashimoto, 2011b; Sui et al., 2021) (Fig. 2).
Box 1. Conserved transcriptional regulators involved in metabolism of defense compounds in multiple plant species.
A small group of ERF transcription factors, classified in clade II of group IXa, including ERF199 and ERF189 involved in nicotine biosynthesis in tobacco, have emerged as key transcriptional regulators of JA-induced metabolism of defense compounds in various plant lineages. These ERFs include ORCAs, which regulate biosynthesis of monoterpenoid indole alkaloids in periwinkle (Catharanthus roseus) (van der Fits and Memelink, 2000), JRE4, which regulates steroidal glycoalkaloid production in tomato (Solanum lycopersicum) (Cardenas et al., 2016; Thagun et al., 2016; Nakayasu et al., 2018), and ORA, which regulates artemisinin production in sweet annie (Artemisia annua) (Lu et al., 2013). Most of these ERF genes form clusters of homologs in plant genomes. As more plant genomes become available, clustered ERF genes of this group have been discovered in a wide range of eudicots (Shoji and Yuan, 2021). It remains an open question whether these newly discovered ERFs also function as regulators of defense compound metabolism or other processes. Implications of these findings on the evolution of metabolic pathways were discussed elsewhere (Shoji, 2019).
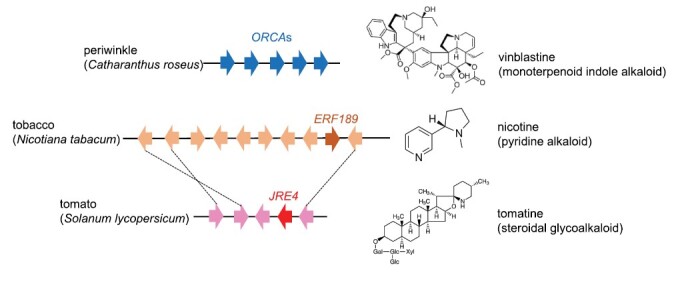
Clustered ERF transcription factor genes and their target specialized metabolites. Clusters of ERF genes in three plant species are depicted, with closely related genes in tobacco and tomato linked by broken lines. Specialized metabolites regulated by these ERF genes are shown on the right.
Disrupting ERF199 and ERF189 simultaneously using CRISPR/Cas9 gene editing resulted in an ultra-low-nicotine phenotype in tobacco, with nicotine levels reaching only 2–5% of the wild-type levels. Importantly, this was achieved without causing major growth defects, at least in a greenhouse environment (Hayashi et al., 2020). A comprehensive metabolic profiling revealed significant influences of this double knockout not only on nicotine but also on other metabolites that are not directly related to the nicotine biosynthesis pathway in tobacco. This implies the existence of unknown regulatory mechanisms within the metabolic network (Hayashi et al., 2020). To determine the potential utility of the double knockout plants in agricultural fields, it will be necessary to fully characterize their agronomic properties, including comparing them with the naturally occurring nic1 nic2 double mutant in terms of leaf quality, insect resistance, and other agronomic traits (Chaplin and Weeks, 1976). Moreover, complete loss of function of ERF199 resulted in a low-nicotine phenotype similar to that observed in the nic1 nic2 double mutant, emphasizing the importance of ERF199 as a regulator of nicotine biosynthesis (Burner et al., 2022; Shoji et al., 2022). In contrast, a null mutation of ERF189 only slightly affected nicotine levels (Shoji et al., 2022).
ERF199 and ERF189 are highly expressed in the roots, where nicotine is produced, but have nominal expression levels in the leaves (Hayashi et al., 2020; Kajikawa et al., 2017a, b). Interestingly, transient overexpression of ERF189 in Nicotiana benthamiana leaves led to the hyperaccumulation of nicotine, suggesting that ERF189, or possibly ERF199, is not only necessary but also sufficient for inducing alkaloid production in the leaves of Nicotiana plants (Hayashi et al., 2020). Further characterization is required to elucidate the molecular basis of functional redundancy and potential differentiation between these two ERFs.
ERF32, ERF91, and ERF221, which are phylogenetically related to ERF199 and ERF189, are also reported to have regulatory roles in nicotine biosynthesis, primarily based on gain-of-function and promoter binding analyses (DeBoer et al., 2011b; Sears et al., 2014; Liu et al., 2019; Sui et al., 2019). However, additional genetic evidence obtained through knockout and other experiments is required to confirm their in planta roles in regulating nicotine biosynthesis.
MYC2 transcription factor
The MYC2 transcription factor plays a central role in the JA signaling cascade, regulating a wide range of genes involved in JA-dependent responses (Luo et al., 2023). This bHLH-family transcription factor is encoded by four genes in the tobacco genome: MYC1a, MYC1b, MYC2a, and MYC2b (Shoji and Hashimoto, 2011b; Zhang et al., 2012). In addition to regulating other target genes, the tobacco MYC2 transcription factors up-regulate numerous nicotine biosynthesis genes directly by recognizing G-box elements in their promoters and indirectly through ERF199 and ERF189 (DeBoer et al., 2011b; Shoji and Hashimoto, 2011b; Zhang et al., 2012) (Fig. 2).
Disrupting MYC2a in tobacco resulted in an 80% reduction in foliar nicotine levels (Sui et al., 2021). This finding highlights the importance of MYC2a in regulating nicotine biosynthesis and suggests that there may be functional differences among the various MYC2 homologs. Alternatively, it is possible that MYC2 members in tobacco form heterodimers, similar to their homologs in other species (Fernandez-Calvo et al., 2011). In this case, knocking out any one MYC2 member could drastically affect nicotine levels, acting in a dominant-negative manner. It will be important to carefully examine the impacts of the loss of each MYC2 gene individually to determine their specific contributions to nicotine biosynthesis and overall plant physiology, along with their potential interactions.
Apart from the ERFs and MYC2 mentioned above, several other transcription factors belonging to the ARF, MYB, NAC, and WRKY families have also been implicated in regulating nicotine biosynthesis in various contexts (Fu et al., 2013; Jin et al., 2017; Hu et al., 2021; Bian et al., 2022). To understand the in planta regulatory roles of these transcription factors and their relationship with ERFs and MYC2 in JA-mediated regulation of the nicotine pathway, further genetic and other analyses are necessary.
Protein phosphorylation
Transcription factors are often regulated through protein phosphorylation. A mitogen-activated protein (MAP) kinase kinase JAM1, a MAP kinase MPK4, and a protein phosphatase PP2C2b have been proposed to mediate the regulation of nicotine biosynthesis in tobacco (DeBoer et al., 2011b; Liu et al., 2021). Further analysis is necessary to identify the specific targets of phosphorylation and their functional relationship with ERFs and the JA signaling cascade. Understanding the upstream regulatory components and their interactions is crucial for effectively manipulating the nicotine biosynthesis pathway.
Regulatory non-coding RNAs
miRNAs and long non-coding RNAs (lncRNAs) are important regulatory factors in various biological processes, including in regulating specialized metabolism in plants (Owusu Adjei et al., 2021; Wang et al., 2023; Zhan and Meyers, 2023). miRNAs are small non-coding RNAs, typically 20–24 nucleotides long, that can induce degradation of target mRNAs or repress their translation (Zhan and Meyers, 2023). lncRNAs exhibit diverse mechanisms of gene regulation, many of which are still not fully understood (Wang et al., 2023). One type of lncRNA, called endogenous target mimics (eTMs), act as decoys by mimicking the target RNAs of specific miRNAs, inhibiting the actions of the miRNAs (Wu et al., 2013).
In tobacco, comprehensive screenings of non-coding RNA species have revealed the relevance of some of these RNAs in regulating genes in the nicotine pathway (Chen et al., 2019; Jin et al., 2020; Zheng et al., 2022). One pair of regulatory RNAs, the miRNA nta-miRX27 and its corresponding eTM nta-TMX27, target a key nicotine biosynthesis gene, QPT2, to regulate nicotine levels in tobacco (Li et al., 2015). Removing the uppermost growing point of the plant (called ‘topping’) is a common practice in tobacco cultivation that stimulates nicotine production (Qin et al., 2020). Following topping, nta-TMX27 expression is induced, which leads to nta-miRX27 degradation (Li et al., 2015) (Fig. 2). Degradation of the miRNA relieves its inhibition on QPT2, resulting in enhanced QPT2 expression (Li et al., 2015). Altering the expression of nta-miRX27 and nta-TMX27 significantly affects nicotine accumulation (Li et al., 2015).
Environmental and hormonal regulations
Several studies have reported the influence of environmental stresses, including high temperature (Yang et al., 2016), flooding (Zhang et al., 2016), and salt stress (Chen et al., 2016b), on nicotine accumulation in tobacco. High temperature induces the expression of nicotine biosynthesis genes through the JA signaling pathway (Yang et al., 2016). Additionally, carbon monoxide (Cheng et al., 2018) and hydrogen sulfide (Chen et al., 2016a) have been suggested to act as signals in this induction process. However, the physiological and ecological significance of these stress-induced responses in nicotine accumulation remain unclear.
Ethylene treatment in Nicotiana attenuata and N. sylvestris roots clearly suppressed the JA-dependent induction of nicotine biosynthesis genes (Shoji et al., 2000; Winz and Baldwin, 2001). Additionally, ethylene signaling inhibits the JA-mediated induction of ERF189 in tobacco (Shoji et al., 2010). The nicotine accumulation stimulated by topping is believed to be caused by reduced auxin supply from apical tissues. Indeed, auxin application leads to rapid down-regulation of PMT and A622 expression in tobacco roots, which reduces nicotine biosynthesis (Hibi et al., 1994).
Conclusions and perspectives
Recent molecular and genomic studies have made substantial contributions to our understanding of nicotine biosynthesis and its regulation in tobacco. These studies have revealed numerous metabolic, transport, and regulatory genes that play key roles in nicotine accumulation. The discovery of these genes provides valuable genetic tools for manipulating and controlling alkaloid production and accumulation in tobacco plants.
All metabolic genes involved in the formation of pyridine and pyrrolidine rings have been identified. However, efforts to reduce alkaloid contents by manipulating these early steps in the nicotine biosynthesis pathway have not always yielded favorable outcomes. There are two main reasons for these failures: (i) interference with polyamine and NAD supplies (Sato et al., 2001; Dalton et al., 2016; Choubey and Rajam, 2017; Khan et al., 2017; Hidalgo Martinez et al., 2020; Smith et al., 2022) and (ii) the compensatory production of pyridine-derived alkaloids, anabasine and anatabine, when the supply of pyrrolidine rings is blocked (Chintapakorn and Hamill, 2003; Steppuhn et al., 2004; Shoji and Hashimoto, 2008; Wang et al., 2009; DeBoer et al., 2011a; Dalton et al., 2016). In contrast to the early steps, biochemical details of later steps in nicotine biosynthesis, including ring condensation, remain elusive. Nevertheless, a breakthrough was achieved via a triple knockout mutant involving three BBL genes that participate toward the end of the pathway (Lewis et al., 2015, 2020). This mutant exhibited a drastic reduction in alkaloid content with a moderate decrease in yield.
Nornicotine, a precursor of a carcinogenic TSNA (Konstantinou et al., 2018), is a target for alkaloid reduction in tobacco breeding programs. Nornicotine is formed from nicotine through demethylation catalyzed by CYP82Es. Disrupting three CYP82E genes significantly reduced the nornicotine content in tobacco (Lewis et al., 2010; Song et al., 2020). This finding presents a promising strategy for reducing the levels of this harmful compound in tobacco plants.
A homologous pair of JA-responsive ERF transcription factors, ERF199 and ERF189, work together to coordinate the expression of many nicotine metabolic and transport genes in tobacco. Double knockout of ERF199 and ERF189 resulted in an ultra-low-nicotine phenotype in tobacco plants without substantial growth defects under greenhouse conditions (Hayashi et al., 2020). However, it will be crucial to evaluate the performance of the double mutant in field settings to determine its suitability for practical applications.
Epigenetic mechanisms include DNA methylation, where methyl groups are added to specific regions of the DNA, and histone modification, where chemical groups are added to histone proteins to alter the chromatin structure. These modifications are heritable through cell divisions but do not involve alterations in the underlying DNA sequences, resulting in gene activation and repression. Regulatory RNA molecules, such as small and long non-coding RNAs, play a significant role in epigenetic regulation. Epigenetics and regulatory RNAs have emerged as crucial mechanisms in the regulation of specialized metabolism in plants (Hayashi et al., 2023). Although largely unexplored, it is intriguing to explore whether and, if so, how epigenetic control is significant in nicotine biosynthesis.
It is important to explore natural variations in alkaloid profiles within and between different Nicotiana species. Through such studies, we can gain valuable insights into the genetic and biochemical factors that influence the production and regulation of alkaloids in Nicotiana plants. This has been exemplified by the molecular identification of NIC1 and NIC2. A large collection of Nicotiana germplasms serves as a valuable genetic resource to investigate the natural diversity of alkaloid profiles (Sisson and Saunders, 1982; Kaminski et al., 2020). This collection encompasses a wide range of Nicotiana species and varieties, each with its own unique alkaloid profile (Sisson and Saunders, 1982; Kaminski et al., 2020). By employing powerful genomics approaches, such as high-throughput sequencing (Sierro et al., 2014, 2018; Xu et al., 2017), we can delve deeper into the genetic and epigenetic basis of alkaloid diversity and identify more key genes and regulatory elements involved in alkaloid biosynthesis and regulation.
Nicotiana plants have gained attention as a promising platform for plant-based bioproduction due to their robust growth, high biomass accumulation, established cultivation and processing infrastructure, genetic modification potential, and metabolic versatility (Jassbi et al., 2017; Molina-Hidalgo et al., 2021). One important objective in this context is to reduce the production of addictive alkaloids, aiming to decrease the presence of these harmful chemicals in the final products and the surrounding environment. Furthermore, our comprehensive understanding of the genetic framework underlying nicotine production and accumulation provides a valuable foundation for further genetic modification and metabolic engineering of these plants. By integrating synthetic biology and other methodologies, it becomes possible to manipulate the biosynthetic pathways and regulatory networks, paving the way for developing Nicotiana plants with enhanced production of the desired compounds.
Acknowledgements
We thank the members of our group for their collaboration.
Contributor Information
Tsubasa Shoji, Instutute of Natural Medicine, University of Toyama, Sugitani, Toyama, Toyama 930-0194, Japan; RIKEN Center for Sustainable Resource Science, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan.
Takashi Hashimoto, Nara Institute of Science and Technology, Ikoma, Nara 630-0192, Japan.
Kazuki Saito, RIKEN Center for Sustainable Resource Science, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan.
Yonghua Li-Beisson, French Alternative and Atomic Energy Commission, France.
Author contributions
TS: wrote a draft of the manuscript; TH and KS: revised the draft before final approval of the contents.
Conflict of interest
The authors declare no conflict of interest.
Funding
Research in the authors’ group was supported in part by a grant from the Japan Society for the Promotion of Science [Grant-in-Aid for Scientific Research (S) No. 19H05632] to KS and TS.
References
- Benowitz NL. 2009. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annual Review of Pharmacology and Toxicology 49, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Sui X, Wang J, et al. 2022. NtMYB305a binds to the jasmonate-responsive GAG region of NtPMT1a promoter to regulate nicotine biosynthesis. Plant Physiology 188, 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovet L, Campanoni P, Lu J, et al. 2022. CLCNt2 mediates nitrate content in tobacco leaf, impacting the production of tobacco-specific nitrosamines in cured leaves. Frontiers in Plant Science 13, 741078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burner N, McCauley A, Pramod S, Frederick J, Steede T, Kernodle SP, Lewis RS.. 2022. Analyses of diverse low alkaloid tobacco germplasm identify naturally occurring nucleotide variability contributing to reduced leaf nicotine accumulation. Molecular Breeding 42, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Siminszky B, Chappell J, Dewey RE, Bush LP.. 2012. Enantioselective demethylation of nicotine as a mechanism for variable nornicotine composition in tobacco leaf. Journal of Biological Chemistry 287, 42804–42811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas PD, Sonawane PD, Pollier J, et al. 2016. GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Natature Communications 7, 10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti M, Bowen SW, Coleman NP, Meekins KM, Dewey RE, Siminszky B.. 2008. CYP82E4-mediated nicotine to nornicotine conversion in tobacco is regulated by a senescence-specific signaling pathway. Plant Molecular Biology 66, 415–427. [DOI] [PubMed] [Google Scholar]
- Chaplin JF, Weeks WW.. 1976. Association between percent total alkaloids and other traits in the flue-cured tobacco. Crop Science 16, 416–418. [Google Scholar]
- Chen X, Chen Q, Zhang X, Li R, Jia Y, Ef AA, JiaA, Hu L, Hu X.. 2016a. Hydrogen sulfide mediates nicotine biosynthesis in tobacco (Nicotiana tabacum) under high temperature conditions. Plant Physiology and Biochemistry 104, 174–179. [DOI] [PubMed] [Google Scholar]
- Chen X, Sun S, Liu F, et al. 2019. A transcriptomic profile of topping responsive non-coding RNAs in tobacco roots (Nicotiana tabacum). BMC Genomics 20, 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang X, Jia A, Xu G, Hu H, Hu X, Hu L.. 2016b. Jasmonate mediates salt-induced nicotine biosynthesis in tobacco (Nicotiana tabacum L.). Plant Diversity 38, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Hu L, Wang P, Yang X, Peng Y, Lu Y, Chen J, Shi J.. 2018. Carbon monoxide potentiates high temperature-induced nicotine biosynthesis in tobacco. International Journal of Molecular Science 19, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chezem WR, Clay NK.. 2016. Regulation of plant secondary metabolism and associated specialized cell development by MYBs and bHLHs. Phytochemistry 131, 26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapakorn Y, Hamill JD.. 2003. Antisense-mediated down-regulation of putrescine N-methyltransferase activity in transgenic Nicotiana tabacum L. can lead to elevated levels of anatabine at the expense of nicotine. Plant Molecular Biology 53, 87–105. [DOI] [PubMed] [Google Scholar]
- Choubey A, Rajam MV.. 2017. Transcriptome response and developmental implications of RNAi-mediated ODC knockdown in tobacco. Functional & Integrative Genomics 17, 399–412. [DOI] [PubMed] [Google Scholar]
- Dalton HL, Blomstedt CK, Neale AD, Gleadow R, DeBoer KD, Hamill JD.. 2016. Effects of down-regulating ornithine decarboxylase upon putrescine-associated metabolism and growth in Nicotiana tabacum L. Journal of Experimental Botany 67, 3367–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson RF. 1942. Accumulation of nicotine in reciprocal grafts of tomato and tobacco. American Journal of Botany 29, 66–71. [Google Scholar]
- DeBoer KD, Dalton HL, Edward FJ, Hamill JD.. 2011a. RNAi-mediated down-regulation of ornithine decarboxylase (ODC) leads to reduced nicotine and increased anatabine levels in transgenic Nicotiana tabacum L. Phytochemistry 72, 344–355. [DOI] [PubMed] [Google Scholar]
- DeBoer KD, Lye JC, Aitken CD, Su AK, Hamill JD.. 2009. The A622 gene in Nicotiana glauca (tree tobacco): evidence for a functional role in pyridine alkaloid synthesis. Plant Molecular Biology 69, 299–312. [DOI] [PubMed] [Google Scholar]
- DeBoer K, Tilleman S, Pauwels L, Vanden Bossche R, De Sutter V, Vanderhaeghen R, Hilson P, Hamill JD, Goossens A.. 2011b. APETALA2/ETHYLENE RESPONSE FACTOR and basic helix–loop–helix tobacco transcription factors cooperatively mediate jasmonate-elicited nicotine biosynthesis. The Plant Journal 66, 1053–1065. [DOI] [PubMed] [Google Scholar]
- Dewey RE, Xie J.. 2013. Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 94, 10–27. [DOI] [PubMed] [Google Scholar]
- Fernandez-Calvo P, Chini A, Fernandez-Barbero G, et al. 2011. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. The Plant Cell 23, 701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Guo H, Cheng Z, Wang R, Li G, Huo G, Liu W.. 2013. NtNAC-R1, a novel NAC transcription factor gene in tobacco roots, responds to mechanical damage of shoot meristem. Plant Physiology and Biochemistry 69, 74–81. [DOI] [PubMed] [Google Scholar]
- Gavilano LB, Coleman NP, Bowen SW, Siminszky B.. 2007. Functional analysis of nicotine demethylase genes reveals insights into the evolution of modern tobacco. Journal of Biological Chemistry 282, 249–256. [DOI] [PubMed] [Google Scholar]
- Gavilano LB, Siminszky B.. 2007. Isolation and characterization of the cytochrome P450 gene CYP82E5v2 that mediates nicotine to nornicotine conversion in the green leaves of tobacco. Plant and Cell Physiology 48, 1567–1574. [DOI] [PubMed] [Google Scholar]
- Geelen D, Lurin C, Bouchez D, Frachisse JM, Lelievre F, Courtial B, Barbier-Brygoo H, Maurel C.. 2000. Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. The Plant Journal 21, 259–267. [DOI] [PubMed] [Google Scholar]
- Goodman J. 2004. Tobacco in history and culture: an encyclopedia. New York: Charles Scribner’s Sons. [Google Scholar]
- Griffith RB, Valleau WD, Stokes GW.. 1955. Determination and inheritance of nicotine to nornicotine conversion in tobacco. Science 121, 343–344. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Alseekh S, Fernie AR.. 2023. Genetic and epigenetic control of the plant metabolome. Proteomics 23, e2200104. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Watanabe M, Kobayashi M, Tohge T, Hashimoto T, Shoji T.. 2020. Genetic manipulation of transcriptional regulators alters nicotine biosynthesis in tobacco. Plant and Cell Physiology 61, 1041–1053. [DOI] [PubMed] [Google Scholar]
- Heim WG, Sykes KA, Hildreth SB, Sun J, Lu RH, Jelesko JG.. 2007. Cloning and characterization of a Nicotiana tabacum methylputrescine oxidase transcript. Phytochemistry 68, 454–463. [DOI] [PubMed] [Google Scholar]
- Hibi N, Higashiguchi S, Hashimoto T, Yamada Y.. 1994. Gene expression in tobacco low-nicotine mutants. The Plant Cell 6, 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo Martinez D, Payyavula RS, Kudithipudi C, Shen Y, Xu D, Warek U, Strickland JA, Melis A.. 2020. Genetic attenuation of alkaloids and nicotine content in tobacco (Nicotiana tabacum). Planta 251, 92. [DOI] [PubMed] [Google Scholar]
- Hildreth SB, Gehman EA, Yang H, et al. 2011. Tobacco nicotine uptake permease (NUP1) affects alkaloid metabolism. Proceedings of the National Academy of Sciences, USA 108, 18179–18184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Zhang H, Wang B, et al. 2021. Transcriptomic analysis provides insights into the AUXIN RESPONSE FACTOR 6-mediated repression of nicotine biosynthesis in tobacco (Nicotiana tabacum L.). Plant Molecular Biology 107, 21–36. [DOI] [PubMed] [Google Scholar]
- Hung CY, Fan L, Kittur FS, et al. 2013. Alteration of the alkaloid profile in genetically modified tobacco reveals a role of methylenetetrahydrofolate reductase in nicotine N-demethylation. Plant Physiology 161, 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi S, Hashizume K, Nakakita M, Kojima H, Matsubayashi Y, Hashimoto T, Sakagami Y, Yamada Y, Nakamura K.. 1998. Differential induction by methyl jasmonate of genes encoding ornithine decarboxylase and other enzymes involved in nicotine biosynthesis in tobacco cell cultures. Plant Molecular Biology 38, 1101–1111. [DOI] [PubMed] [Google Scholar]
- Jassbi AR, Zare S, Asadollahi M, Schuman MC.. 2017. Ecological roles and biological activities of specialized metabolites from the genus Nicotiana. Chemical Reviews 117, 12227–12280. [DOI] [PubMed] [Google Scholar]
- Jin J, Xu Y, Lu P, et al. 2020. Degradome, small RNAs and transcriptome sequencing of a high-nicotine cultivated tobacco uncovers miRNA’s function in nicotine biosynthesis. Scientific Reports 10, 11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Zhou Q, Wei Y, Yang J, Hao F, Cheng Z, Guo H, Liu W.. 2017. NtWRKY-R1, a novel transcription factor, integrates IAA and JA signal pathway under topping damage stress in Nicotiana tabacum. Frontiers in Plant Science 8, 2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker A, Fischer J, Sichhart Y, Brandt W, Drager B.. 2013. Evolution of the key alkaloid enzyme putrescine N-methyltransferase from spermidine synthase. Frontiers in Plant Science 4, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa M, Hirai N, Hashimoto T.. 2009. A PIP-family protein is required for biosynthesis of tobacco alkaloids. Plant Molecular Biology 69, 287–298. [DOI] [PubMed] [Google Scholar]
- Kajikawa M, Shoji T, Kato A, Hashimoto T.. 2011. Vacuole-localized berberine bridge enzyme-like proteins are required for a late step of nicotine biosynthesis in tobacco. Plant Physiology 155, 2010–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa M, Sierro N, Hashimoto T, Shoji T.. 2017a. A model for evolution and regulation of nicotine biosynthesis regulon in tobacco. Plant Signaling & Behavior 12, e1338225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa M, Sierro N, Kawaguchi H, Bakaher N, Ivanov NV, Hashimoto T, Shoji T.. 2017b. Genomic insights into the evolution of the nicotine biosynthesis pathway in tobacco. Plant Physiology 174, 999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski KP, Bovet L, Laparra H, Lang G, De Palo D, Sierro N, Goepfert S, Ivanov NV.. 2020. Alkaloid chemophenetics and transcriptomics of the Nicotiana genus. Phytochemistry 177, 112424. [DOI] [PubMed] [Google Scholar]
- Kato K, Shitan N, Shoji T, Hashimoto T.. 2015. Tobacco NUP1 transports both tobacco alkaloids and vitamin B6. Phytochemistry 113, 33–40. [DOI] [PubMed] [Google Scholar]
- Kato K, Shoji T, Hashimoto T.. 2014. Tobacco nicotine uptake permease regulates the expression of a key transcription factor gene in the nicotine biosynthesis pathway. Plant Physiology 166, 2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh A, Shoji T, Hashimoto T.. 2007. Molecular cloning of N-methylputrescine oxidase from tobacco. Plant and Cell Physiology 48, 550–554. [DOI] [PubMed] [Google Scholar]
- Katoh A, Uenohara K, Akita M, Hashimoto T.. 2006. Early steps in the biosynthesis of NAD in Arabidopsis start with aspartate and occur in the plastid. Plant Physiology 141, 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Pandey SS, Jyotshna, Shanker K, Khan F, Rahman LU.. 2017. Cloning and functional characterization of quinolinic acid phosphoribosyl transferase (QPT) gene of Nicotiana tabacum. Physiologia Plantarum 160, 253–265. [DOI] [PubMed] [Google Scholar]
- Knapp S, Chase MW, Clarkso JJ.. 2004. Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae). Taxon 53, 73–82. [Google Scholar]
- Konstantinou E, Fotopoulou F, Drosos A, et al. 2018. Tobacco-specific nitrosamines: a literature review. Food and Chemical Toxicology 118, 198–203. [DOI] [PubMed] [Google Scholar]
- Legg PD, Collins GB.. 1971. Inheritance of percent total alkaloids in Nicotiana tabacum L. II. Genetic effects of two loci in Burley21 × LA Burley21 populations. Canadian Journal of Genetics and Cytology 13, 287–291. [Google Scholar]
- Legg PD, Collins GB, Litton CC.. 1970. Registration of LA Burley21 tobacco germplasm. Crop Science 10, 212. [Google Scholar]
- Lewis RS. 2011. Nicotiana. In: Kole C, ed. Wild crop relatives: genomic and breeding resources. Berlin: Springer, 185–208. [Google Scholar]
- Lewis RS. 2019. Potential mandated lowering of nicotine levels in cigarettes: a plant perspective. Nicotine & Tobacco Research 21, 991–995. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Bowen SW, Keogh MR, Dewey RE.. 2010. Three nicotine demethylase genes mediate nornicotine biosynthesis in Nicotiana tabacum L.: functional characterization of the CYP82E10 gene. Phytochemistry 71, 1988–1998. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Drake-Stowe KE, Heim C, Steede T, Smith W, Dewey RE.. 2020. Genetic and agronomic analysis of tobacco genotypes exhibiting reduced nicotine accumulation due to induced mutations in berberine bridge like (BBL) genes. Fronters in Plant Science 11, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS, Jack AM, Morris JW, Robert VJ, Gavilano LB, Siminszky B, Bush LP, Hayes AJ, Dewey RE.. 2008. RNA interference (RNAi)-induced suppression of nicotine demethylase activity reduces levels of a key carcinogen in cured tobacco leaves. Plant Biotechnology Journal 6, 346–354. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Lopez HO, Bowen SW, Andres KR, Steede WT, Dewey RE.. 2015. Transgenic and mutation-based suppression of a berberine bridge enzyme-like (BBL) gene family reduces alkaloid content in field-grown tobacco. PLoS One 10, e0117273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS, Parker RG, Danehower DA, Andres K, Jack AM, Whitley DS, Bush LP.. 2012. Impact of alleles at the Yellow Burley (Yb) loci and nitrogen fertilization rate on nitrogen utilization efficiency and tobacco-specific nitrosamine (TSNA) formation in air-cured tobacco. Journal of Agricultural and Food Chemistry 60, 6454–6461. [DOI] [PubMed] [Google Scholar]
- Li F, Wang W, Zhao N, et al. 2015. Regulation of nicotine biosynthesis by an endogenous target mimicry of microRNA in tobacco. Plant Physiology 169, 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang F, Wu R, Jia L, Li G, Guo Y, Liu C, Wang G.. 2017. A novel N-methyltransferase in Arabidopsis appears to feed a conserved pathway for nicotinate detoxification among land plants and is associated with lignin biosynthesis. Plant Physiology 174, 1492–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedschulte V, Schwaar JD, Laparra H, Vuarnoz A, Philippon B, Bakaher N, Sierro N, Bovet L, Lang G, Goepfert S.. 2016. Identification of CYP82E21 as a functional nicotine N-demethylase in tobacco flowers. Phytochemistry 131, 9–16. [DOI] [PubMed] [Google Scholar]
- Liu H, Kotova TI, Timko MP.. 2019. Increased leaf nicotine content by targeting transcription factor gene expression in commercial flue-tured tobacco (Nicotiana tabacum L.). Genes (Basel) 10, 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Singh SK, Patra B, Liu Y, Wang B, Wang J, Pattanaik S, Yuan L.. 2021. Protein phosphatase NtPP2C2b and MAP kinase NtMPK4 act in concert to modulate nicotine biosynthesis. Journal of Experimental Botany 72, 1661–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang L, Lewis RS, Bovet L, Goepfert S, Jack AM, Crutchfield JD, Ji H, Dewey RE.. 2016. Expression of a constitutively active nitrate reductase variant in tobacco reduces tobacco-specific nitrosamine accumulation in cured leaves and cigarette smoke. Plant Biotechnology Journal 14, 1500–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Zhang L, Zhang F, Jiang W, Shen Q, Zhang L, Lv Z, Wang G, Tang K.. 2013. AaORA, a trichome-specific AP2/ERF transcription factor of Artemisia annua, is a positive regulator in the artemisinin biosynthetic pathway and in disease resistance to Botrytis cinerea. New Phytologist 198, 1191–1202. [DOI] [PubMed] [Google Scholar]
- Luo L, Wang Y, Qiu L, Han X, Zhu Y, Liu L, Man M, Li F, Ren M, Xing Y.. 2023. MYC2: a master switch for plant physiological processes and specialized metabolite synthesis. International Journal of Molecular Sciences 24, 3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min T, Kasahara H, Bedgar DL, et al. 2003. Crystal structures of pinoresinol-lariciresinol and phenylcoumaran benzylic ether reductases and their relationship to isoflavone reductases. Journal of Biological Chemistry 278, 50714–50723. [DOI] [PubMed] [Google Scholar]
- Molina-Hidalgo FJ, Vazquez-Vilar M, D’Andrea L, Demurtas OC, Fraser P, Giuliano G, Bock R, Orzaez D, Goossens A.. 2021. Engineering metabolism in Nicotiana species: a promising future. Trends in Biotechnolgy 39, 901–913. [DOI] [PubMed] [Google Scholar]
- Morita M, Shitan N, Sawada K, Van Montagu MC, Inze D, Rischer H, Goossens A, Oksman-Caldentey KM, Moriyama Y, Yazaki K.. 2009. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proceedings of the National Academy of Sciences, USA 106, 2447–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naconsie M, Kato K, Shoji T, Hashimoto T.. 2014. Molecular evolution of N-methylputrescine oxidase in tobacco. Plant and Cell Physiology 55, 436–444. [DOI] [PubMed] [Google Scholar]
- Nakayasu M, Shioya N, Shikata M, et al. 2018. JRE4 is a master transcriptional regulator of defense-related steroidal glycoalkaloids in tomato. The Plant Journal 94, 975–990. [DOI] [PubMed] [Google Scholar]
- Owusu Adjei M, Zhou X, Mao M, Rafique F, Ma J.. 2021. MicroRNAs roles in plants secondary metabolism. Plant Signaling & Behavior 16, 1915590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakdeechanuan P, Shoji T, Hashimoto T.. 2012. Root-to-shoot translocation of alkaloids is dominantly suppressed in Nicotiana alata. Plant and Cell Physiology 53, 1247–1254. [DOI] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT.. 2007. Co(i)-ordinating defenses: NaCOI1 mediates herbivore- induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. The Plant Journal 51, 79–91. [DOI] [PubMed] [Google Scholar]
- Qin Q, Humphry M, Gilles T, Fisher A, Patra B, Singh SK, Li D, Yang S.. 2021. NIC1 cloning and gene editing generates low-nicotine tobacco plants. Plant Biotechnology Journal 19, 2150–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Bai S, Li W, Sun T, Galbraith DW, Yang Z, Zhou Y, Sun G, Wang B.. 2020. Transcriptome analysis reveals key genes involved in the regulation of nicotine biosynthesis at early time points after topping in tobacco (Nicotiana tabacum L.). BMC Plant Biology 20, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Hashimoto T, Hachiya A, Tamura K, Choi KB, Morishige T, Fujimoto H, Yamada Y.. 2001. Metabolic engineering of plant alkaloid biosynthesis. Proceedings of the National Academy of Sciences, USA 98, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtsiek J, Stehle F.. 2019. Nicotine-free, nontransgenic tobacco (Nicotiana tabacum L.) edited by CRISPR-Cas9. Plant Biotechnology Journal 17, 2228–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears MT, Zhang H, Rushton PJ, Wu M, Han S, Spano AJ, Timko MP.. 2014. NtERF32: a non-NIC2 locus AP2/ERF transcription factor required in jasmonate-inducible nicotine biosynthesis in tobacco. Plant Molecular Biology 84, 49–66. [DOI] [PubMed] [Google Scholar]
- Shitan N, Minami S, Morita M, et al. 2014. Involvement of the leaf-specific multidrug and toxic compound extrusion (MATE) transporter Nt-JAT2 in vacuolar sequestration of nicotine in Nicotiana tabacum. PLoS One 9, e108789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T. 2019. The recruitment model of metabolic evolution: jasmonate-responsive transcription factors and a conceptual model for the evolution of metabolic pathways. Frontiers in Plant Science 10, 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T. 2020a. Alkaloid biosynthesis and regulation in plants. In: Arimura GI, Maffei M, eds. Plant specialized metabolism. New York: CRC Press, 85–118. [Google Scholar]
- Shoji T. 2020b. Nicotine biosynthesis, transport, and regulation in tobacco: insights into the evolution of a metabolic pathway. In: Ivanov NV, Sierro N, Peitsch MC, eds. The tobacco plant genome. Cham: Springer, 147–156. [Google Scholar]
- Shoji T, Hashimoto T.. 2008. Why does anatabine, but not nicotine, accumulate in jasmonate-elicited cultured tobacco BY-2 cells? Plant and Cell Physiology 49, 1209–1216. [DOI] [PubMed] [Google Scholar]
- Shoji T, Hashimoto T.. 2011a. Recruitment of a duplicated primary metabolism gene into the nicotine biosynthesis regulon in tobacco. The Plant Journal 67, 949–959. [DOI] [PubMed] [Google Scholar]
- Shoji T, Hashimoto T.. 2011b. Tobacco MYC2 regulates jasmonate-inducible nicotine biosynthesis genes directly and by way of the NIC2-locus ERF genes. Plant and Cell Physiology 52, 1117–1130. [DOI] [PubMed] [Google Scholar]
- Shoji T, Hashimoto T.. 2015. Stress-induced expression of NICOTINE2-locus genes and their homologs encoding Ethylene Response Factor transcription factors in tobacco. Phytochemistry 113, 41–49. [DOI] [PubMed] [Google Scholar]
- Shoji T, Inai K, Yazaki Y, et al. 2009. Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiology 149, 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Kajikawa M, Hashimoto T.. 2010. Clustered transcription factor genes regulate nicotine biosynthesis in tobacco. The Plant Cell 22, 3390–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Mishima M, Hashimoto T.. 2013. Divergent DNA-binding specificities of a group of ETHYLENE RESPONSE FACTOR transcription factors involved in plant defense. Plant Physiology 162, 977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Moriyama K, Sierro N, Ouadi S, Ivanov NV, Hashimoto T, Saito K.. 2022. Natural and induced variations in transcriptional regulator genes result in low-nicotine phenotypes in tobacco. The Plant Journal 111, 1768–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Ogawa T, Hashimoto T.. 2008. Jasmonate-induced nicotine formation in tobacco is mediated by tobacco COI1 and JAZ genes. Plant and Cell Physiology 49, 1003–1012. [DOI] [PubMed] [Google Scholar]
- Shoji T, Umemoto N, Saito K.. 2021. Genetic divergence in transcriptional regulators of defense metabolism: insight into plant domestication and improvement. Plant Molecular Biology 109, 401–411. [DOI] [PubMed] [Google Scholar]
- Shoji T, Yamada Y, Hashimoto T.. 2000. Jasmonate induction of putrescine N-methyltransferase genes in the root of Nicotiana sylvestris. Plant and Cell Physiology 41, 831–839. [DOI] [PubMed] [Google Scholar]
- Shoji T, Yuan L.. 2021. ERF gene clusters: working together to regulate metabolism. Trends in Plant Science 26, 23–32. [DOI] [PubMed] [Google Scholar]
- Sierro N, Battey JN, Ouadi S, Bakaher N, Bovet L, Willig A, Goepfert S, Peitsch MC, Ivanov NV.. 2014. The tobacco genome sequence and its comparison with those of tomato and potato. Nature Communications 5, 3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierro N, Battey JND, Bovet L, et al. 2018. The impact of genome evolution on the allotetraploid Nicotiana rustica—an intriguing story of enhanced alkaloid production. BMC Genomics 19, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminszky B, Gavilano L, Bowen SW, Dewey RE.. 2005. Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase. Proceedings of the National Academy of Sciences, USA 102, 14919–14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair SJ, Murphy KJ, Birch CD, Hamill JD.. 2000. Molecular characterization of quinolinate phosphoribosyltransferase (QPRtase) in Nicotiana. Plant Molecular Biology 44, 603–617. [DOI] [PubMed] [Google Scholar]
- Sisson VA, Saunders JA.. 1982. Alkaloid composition of USDA tobacco (Nicotiana tabacum L.) introduction collection. Tobacco Science 26, 117–120. [Google Scholar]
- Smith WA, Matsuba Y, Dewey RE.. 2022. Knockout of a key gene of the nicotine biosynthetic pathway severely affects tobacco growth under field, but not greenhouse conditions. BMC Research Notes 15, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Sui X, Li M, Gao Y, Li W, Zhao L, Li F, Yao X, Liu C, Wang B.. 2020. Development of a nornicotine-reduced flue-cured tobacco line via EMS mutagenesis of nicotine N-demethylase genes. Plant Signaling & Behavior 15, 1710053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT.. 2004. Nicotine’s defensive function in nature. PLoS Biology 2, E217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, He X, Song Z, Gao Y, Zhao L, Jiao F, Kong G, Li Y, Han S, Wang B.. 2021. The gene NtMYC2a acts as a ‘master switch’ in the regulation of JA-induced nicotine accumulation in tobacco. Plant Biology (Stuttgart, Germany) 23, 317–326. [DOI] [PubMed] [Google Scholar]
- Sui X, Zhang H, Song Z, Gao Y, Li W, Li M, Zhao L, Li Y, Wang B.. 2019. Ethylene response factor NtERF91 positively regulates alkaloid accumulations in tobacco (Nicotiana tabacum L.). Biochemical and Biophysical Research Communications 517, 164–171. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Iwaki S, Sasaki A, Tsukazaki T.. 2021. Crystal structures of a nicotine MATE transporter provide insight into its mechanism of substrate transport. FEBS Letters 595, 1902–1913. [DOI] [PubMed] [Google Scholar]
- Thagun C, Imanishi S, Kudo T, et al. 2016. Jasmonate-responsive ERF transcription factors regulate steroidal glycoalkaloid biosynthesis in tomato. Plant and Cell Physiology 57, 961–975. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. 2014. The health consequences of smoking—50 years of progress. A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services. [Google Scholar]
- van der Fits L, Memelink J.. 2000. ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289, 295–297. [DOI] [PubMed] [Google Scholar]
- Wang P, Liang Z, Zeng J, Li W, Sun X, Miao Z, Tang K.. 2008. Generation of tobacco lines with widely different reduction in nicotine levels via RNA silencing approaches. Journal of Biosciences 33, 177–184. [DOI] [PubMed] [Google Scholar]
- Wang P, Zeng J, Liang Z, Miao Z, Sun X, Tang K.. 2009. Silencing of PMT expression caused a surge of anatabine accumulation in tobacco. Molecular Biology Reports 36, 2285–2289. [DOI] [PubMed] [Google Scholar]
- Wang X, Bennetzen JL.. 2015. Current status and prospects for the study of Nicotiana genomics, genetics, and nicotine biosynthesis genes. Molecular Genetics and Genomics 290, 11–21. [DOI] [PubMed] [Google Scholar]
- Wang X, Fan H, Wang B, Yuan F.. 2023. Research progress on the roles of lncRNAs in plant development and stress responses. Frontiers in Plant Science 14, 1138901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, He X, Lin J, Shao H, Chang Z, Dixon RA.. 2006. Crystal structure of isoflavone reductase from alfalfa (Medicago sativa L.). Journal of Molecular Biology 358, 1341–1352. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Strnad M.. 2018. Jasmonates: news on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. International Journal of Molecular Sciences 19, 2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2015. Advisory note: global nicotine reduction strategy. Geneva: WHO Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winz RA, Baldwin IT.. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. IV. Insect-induced ethylene reduces jasmonate-induced nicotine accumulation by regulating putrescine N-methyltransferase transcripts. Plant Physiology 125, 2189–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Wang ZM, Wang M, Wang XJ.. 2013. Widespread long noncoding RNAs as endogenous target mimics for microRNAs in plants. Plant Physiology 161, 1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Sheehan MJ, Timko MP.. 2004. Differential induction of ornithine decarboxylase (ODC) gene family members in transgenic tobacco (Nicotiana tabacum L. cv. Bright Yellow 2) cell suspensions by methyl-jasmonate treatment. Plant Growth Regulation 44, 101–116. [Google Scholar]
- Xu S, Brockmoller T, Navarro-Quezada A, et al. 2017. Wild tobacco genomes reveal the evolution of nicotine biosynthesis. Proceedings of the National Academy of Sciences, USA 114, 6133–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li J, Ji J, Li P, Yu L, Abd Allah EF, Luo Y, Hu L, Hu X.. 2016. High temperature induces expression of tobacco transcription factor NtMYC2a to regulate nicotine and JA biosynthesis. Frontiers in Physiology 7, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan J, Meyers BC.. 2023. Plant small RNAs: their biogenesis, regulatory roles, and functions. Annual Review of Plant Biology 74, 21–51. [DOI] [PubMed] [Google Scholar]
- Zhang HB, Bokowiec MT, Rushton PJ, Han SC, Timko MP.. 2012. Tobacco transcription factors NtMYC2a and NtMYC2b form nuclear complexes with the NtJAZ1 repressor and regulate multiple jasmonate-inducible steps in nicotine biosynthesis. Molecular Plant 5, 73–84. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lin HM, Hu H, Hu X, Hu L.. 2016. Gamma-aminobutyric acid mediates nicotine biosynthesis in tobacco under flooding stress. Plant Diversity 38, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Wang Z, Pang L, Song Z, Zhao H, Wang Y, Wang B, Han S.. 2022. Systematic identification of methyl jasmonate-responsive long noncoding RNAs and their nearby coding genes unveils their potential defence roles in tobacco BY-2 cells. International Journal of Molecular Sciences 23, 15568. [DOI] [PMC free article] [PubMed] [Google Scholar]


