Abstract
Background
Afatinib can be started at a dose lower than the recommended starting dose of 40 mg/day for the treatment of epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC), however treatment outcomes in real-world clinical practice remains unclear.
Methods
This retrospective study of patients with NSCLC from 18 major hospitals (public, private or university teaching hospitals) enrolled in Malaysia’s National Cardiovascular and Thoracic Surgical Database (NCTSD) assessed the efficacy of lower doses of afatinib on treatment outcomes in a real-world clinical practice. Data on clinical characteristics, afatinib dosing, and treatment outcomes for patients included in NCTSD from 1st January 2015 to 31st December 2020 were analyzed.
Results
Of the 133 patients studied, 94.7% had adenocarcinoma. Majority of the patients (60.9%) had EGFR exon 19 deletion and 23.3% had EGFR exon 21 L858R point mutation. The mean age of patients was 64.1 years and majority (83.5%) had Eastern Cooperative Oncology Group performance status of 2–4 at diagnosis. The most common afatinib starting doses were 40 mg (37.6%), 30 mg (29.3%), and 20 mg (26.3%) once daily (OD), respectively. A quarter of patients had dose reduction (23.3%) due to side effects or cost constraints. Majority of the patients had partial response to afatinib (63.2%) whilst 2.3% had complete response. Interestingly, the objective response rate was significantly higher (72.3%) with afatinib OD doses of less than 40 mg compared to 40 mg (54.0%) (P=0.032). Patients on lower doses of afatinib were two times more likely to achieve an objective response [odds ratio =2.64; 95% confidence interval (CI): 1.20–5.83; P=0.016]. These patients had a numerically but not statistically longer median time to treatment failure (TTF). Median TTF (95% CI) for the overall cohort was 12.4 (10.02–14.78) months. Median overall survival (95% CI) was 21.30 (15.86–26.75) months.
Conclusions
Lower afatinib doses (<40 mg OD) could be equally effective as standard dose in patients with EGFR-mutant advanced NSCLC and may be more suited to Asian patients, minimizing side effects that may occur at higher dosages of afatinib leading to dose interruptions and affecting treatment outcomes.
Keywords: Adenocarcinoma, resource-limited settings, survival, treatment outcome, tyrosine kinase inhibitors (TKIs)
Introduction
In 2020, the World Health Organization (WHO) estimated that globally, lung cancer was the second most common cancer (2.21 million cases) and the leading cause of cancer death (1.80 million deaths) (1). In Malaysia, 5,139 new cases of lung cancer were reported in 2020, which was 10.2% of all cancers in the country for that year (2). The 1-year survival rates of patients with lung cancer in Malaysia between 2007 and 2016 were 63.3% for stage I disease and 29.6% for stage IV disease (3). The 5-year survival rates were 37.1% for stage I disease and 6.3% for stage IV disease (3). Majority of lung cancer cases are non-small cell lung cancer (NSCLC) (4,5). Patients with NSCLC harboring epidermal growth factor receptor (EGFR) mutations are common, with Asian patients having a higher rate of EGFR mutation (EGFRm+) than Caucasians (6).
The recommended first-line treatment for EGFRm+ NSCLC is an EGFR tyrosine kinase inhibitor (TKI) (7,8). Afatinib, an irreversible second generation EGFR TKI, has activity against common as well as rare EGFR mutations (9). Two major randomized controlled phase III trials (the global LUX-Lung 3 and the Asian LUX-lung 6 trials) demonstrated afatinib’s efficacy in EGFRm+ NSCLC patients with significant improvements in progression-free survival (PFS) and overall survival (OS) versus chemotherapy (10-12). In another major trial, patients on afatinib had significantly longer PFS and time to treatment failure (TTF) compared to gefitinib, a first generation EGFR TKI (13,14).
The recommended starting dose of afatinib is 40 mg/day, which can be reduced if there are adverse reactions (15). Evidence from clinical trials and real-world clinical practice showed that with dose adjustments, adverse events (AEs) associated with afatinib can be managed safely (16). In one study, maintenance doses of afatinib at 40 or 30 mg once daily (OD) were effective and tolerable for Malaysian patients with EGFRm+ NSCLC (17).
The objective of this retrospective study was to assess the efficacy of lower doses of afatinib on treatment outcomes in patients with EGFRm+ NSCLC in real-world clinical practice. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-691/rc).
Methods
Study design and data source
This was a retrospective, observational study of adult patients (18 years or older) with stage IIIB, IIIC and IV (8th edition AJCC) NSCLC who received standard of care treatment following international guidelines. The patients were identified from the National Cardiovascular and Thoracic Surgical Database (NCTSD) between 1st January 2015 and 31st December 2020. Patients with incomplete staging or treatment information were excluded from the analysis. Figure 1 shows the flowchart of patients included in the study.
Figure 1.
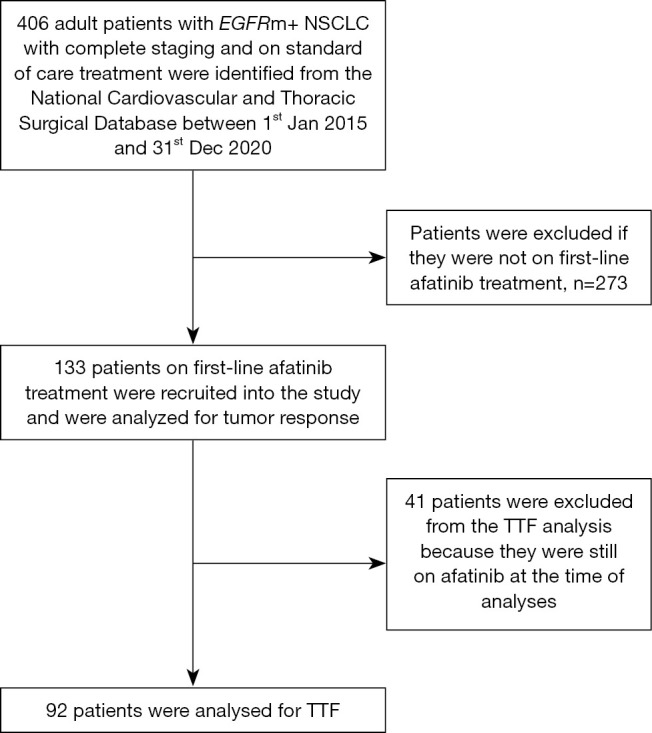
Flowchart of patients who met the inclusion and exclusion criteria for the study population. EGFRm+ NSCLC, epidermal growth factor receptor mutated non-small cell lung cancer; TTF, time to treatment failure.
The NCTSD is a nationwide hospital database set up in 18 major public, university, and private hospitals in Malaysia, which compiles detailed information on the diagnosis and management of adult patients with cytologically or histologically confirmed lung cancer. The participating hospitals continuously entered data into the web-based registry. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Universiti Malaya Medical Centre Medical Research Ethics Committee (MECID 20201115–9217). The Universiti Malaya Medical Centre Medical Research Ethics Committee waived the need for informed consent as patient confidentiality was preserved using identification code numbers.
Outcome variables
The demographic and clinical characteristics of patients were obtained from the NCTSD’s electronic database. These included data on age, gender, ethnicity, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, comorbidities, tumor histology, tumor stage, presence of brain metastases, and EGFR mutation. Data on afatinib starting dose, dose reductions, and treatment status were also captured.
Objective tumor response according to RECIST 1.1 [complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), objective response rate (ORR), disease control rate (DCR), TTF, OS, site of disease progression, and resistance mechanism] (18) were analyzed.
Disease control status was defined as the “best status to date”, specifically if patients had CR, PR or SD. The TTF was defined as the duration from the first day of therapy to the last day of therapy with afatinib. The OS was defined as the time from initiation of afatinib treatment until death from any cause.
Resistance mechanism after failure of first-line afatinib was assessed with tissue and liquid biopsy to look for histology transformation and emergence of new alterations such as T790M, BRAF, HER2 and KRAS mutations; c-MET amplification; and NRTK and RET fusions.
Statistical analysis
Data were retrieved from the registry and screened for missing values. Any missing data were cross-examined with the site investigators. Data analysis was performed using IBM SPSS Statistics version 23. Categorical data are presented as percentages while continuous data are presented as mean ± standard deviation or median with interquartile range. Between-group comparisons for categorical variables were performed using the Chi-squared test. For inferential data analysis, the patients were categorized according to ORR and DCR status. Multivariate analysis was performed using binary logistic regression that included covariates that had been shown to significantly affect treatment outcome in previous studies, such as EGFR subtype, symptomatic brain metastases and ECOG performance status (15,17,19,20). The Kaplan-Meier method was used to estimate the OS and TTF, followed by Cox regression to determine the hazard ratio (HR). The duration of time is described in months. The HRs are given with 95% confidence intervals (CIs) and P values. All P values reported are two-sided and considered significant at the 0.05 threshold.
Results
Demographic and clinical characteristics
Of 406 patients with EGFRm+ NSCLC in the registry, the study included 133 patients treated with first-line afatinib (Figure 1). Majority had EGFR exon 19 deletion (60.9%) and 23.3% had EGFR exon 21 L858R point mutation. Most of the patients had adenocarcinoma (94.7%) and either stage IVA (52.6%) or IVB (39.8%) disease. The mean age of the patients was 64.1 years and majority (83.5%) had ECOG performance status of 2–4 at diagnosis. At diagnosis, 34.6% had symptomatic brain metastases (Table 1). The afatinib 40 mg OD starting dose group was significantly younger and had more patients with symptomatic brain metastases than the lower dose group (Table 2).
Table 1. Demographic and clinical characteristics of patients treated with afatinib (n=133).
| Demographic and clinical characteristics | Values |
|---|---|
| Age (years), mean ± SD | 64.1±10.5 |
| Gender, n (%) | |
| Female | 78 (58.6) |
| Male | 55 (41.4) |
| Ethnicity, n (%) | |
| Malay | 44 (33.1) |
| Chinese | 80 (60.2) |
| Indian | 1 (0.8) |
| Others† | 8 (6.0) |
| Smoking history, n (%) | |
| Never smoker | 111 (83.5) |
| Previous or current smoker | 22 (16.5) |
| ECOG performance status at diagnosis, n (%) | |
| ECOG 0–1 | 22 (16.5) |
| 0 | 0 |
| 1 | 22 (16.5) |
| ECOG 2–4 | 111 (83.5) |
| 2 | 64 (48.1) |
| 3 | 25 (18.8) |
| 4 | 22 (16.6) |
| Comorbidities n (%) | |
| No | 84 (63.2) |
| Yes | 49 (36.8) |
| Diabetes mellitus | 35 (26.3) |
| Hypertension | 19 (14.3) |
| Ischemic heart disease | 5 (3.8) |
| Stroke | 4 (3.0) |
| Chronic kidney disease | 1 (0.8) |
| Others‡ | 21 (15.8) |
| Tumor histology, n (%) | |
| Adenocarcinoma | 126 (94.7) |
| Squamous cell carcinoma | 4 (3.0) |
| Others§ | 3 (2.3) |
| Tumor stage, n (%) | |
| IIIC | 10 (7.5) |
| IVA | 70 (52.6) |
| IVB | 53 (39.8) |
| Symptomatic baseline brain metastases, n (%) | |
| No | 87 (65.4) |
| Yes | 46 (34.6) |
| EGFR mutation subtype, n (%) | |
| Exon 19 deletion | 81 (60.9) |
| Exon 21 L858R point mutation | 31 (23.3) |
| Resistant mutation | 4 (3.0) |
| Exon 20 insertion | 3 (2.2) |
| Exon 20 insertion and Exon 20 S768I | 1 (0.8) |
| Rare or compound mutations | 17 (12.8) |
| Exon 18 G719X | 6 (4.5) |
| Exon 21 L861Q | 6 (4.5) |
| Exon 18 G719X and exon 20 S768I | 5 (3.8) |
†, others: native from Sabah and Sarawak; ‡, others: 10 hyperlipidemia, 4 hyperparathyroid, 2 gouts, 2 orthopedic problem, 1 benign prostatic hyperplasia, 1 asthma, 1 hepatitis B infection; §, others: 1 adenosquamous, 1 favor adenocarcinoma, 1 adenocarcinoma not otherwise specified. SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
Table 2. Demographic and clinical characteristics of patients according to the starting dose of afatinib.
| Demographic and clinical characteristics | Afatinib starting dose | P value | |
|---|---|---|---|
| 40 mg once daily (n=50) | Less than 40 mg once daily (n=83) | ||
| Age (years), mean ± SD | 61.6±11.6 | 65.5±9.5 | 0.036 |
| Gender, n (%) | 0.331 | ||
| Female | 32 (64.0) | 46 (55.4) | |
| Male | 18 (36.0) | 37 (44.6) | |
| Ethnicity, n (%) | 0.301 | ||
| Malay | 10 (20.0) | 34 (41.0) | |
| Chinese | 37 (74.0) | 43 (51.8) | |
| Indian | 1 (2.0) | 0 (0.0) | |
| Others† | 2 (4.0) | 6 (7.2) | |
| Smoking history, n (%) | 0.337 | ||
| Never smoker | 43 (86.0) | 68 (81.9) | |
| Previous or current smoker | 7 (14.0) | 15 (18.1) | |
| ECOG performance status at diagnosis, n (%) | 0.188 | ||
| ECOG 0–1 | 11 (22.0) | 11 (13.3) | |
| ECOG 2–4 | 39 (78.0) | 72 (86.7) | |
| Comorbidities n (%) | 0.598 | ||
| No | 33 (66.0) | 51 (61.4) | |
| Yes | 17 (34.0) | 32 (38.6) | |
| Tumor histology, n (%) | 0.165 | ||
| Adenocarcinoma | 45 (90.0) | 81 (97.6) | |
| Squamous cell carcinoma | 3 (6.0) | 1 (1.2) | |
| Others | 2 (4.0) | 1 (1.2) | |
| Tumor stage, n (%) | 0.433 | ||
| IIIC | 5 (10.0) | 5 (6.0) | |
| IVA | 23 (46.0) | 47 (56.6) | |
| IVB | 22 (44.0) | 31 (37.3) | |
| Symptomatic baseline brain metastases, n (%) | 0.032 | ||
| No | 23 (46.0) | 60 (72.3) | |
| Yes | 27 (54.0) | 23 (27.7) | |
| EGFR mutation subtype, n (%) | 0.729 | ||
| Exon 19 deletion | 30 (60.0) | 51 (61.4) | |
| Exon 21 L858R point mutation | 10 (20.0) | 21 (25.3) | |
| Resistant mutation | 2 (4.0) | 2 (2.4) | |
| Rare or compound mutations | 8 (16.0) | 9 (10.8) | |
†, others: native from Sabah and Sarawak. SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
Afatinib treatment
The starting doses of afatinib were 40 mg (37.6%), 30 mg (29.3%), 25 mg (6.8%) and 20 mg (26.3%) OD, respectively (Table 3). The main reason for starting on lower than the recommended 40 mg dose was due to low body mass index (BMI; 85.5%). Ten percent of the patients began lower doses due to financial constraints and 5% because the patients were worried about potential side effects.
Table 3. Afatinib starting dose and adjustment with its reasons (n=133).
| Afatinib treatment | Values |
|---|---|
| Starting dose, n (%) | |
| 40 mg once daily | 50 (37.6) |
| 30 mg once daily | 39 (29.3) |
| 25 mg once daily | 9 (6.8) |
| 20 mg once daily | 35 (26.3) |
| Reasons for lower starting dose, n (%)† | |
| Physicians’ decision based on patient’s low BMI | 71 (85.5) |
| Financial constraint | 8 (9.6) |
| Patient was worried about side-effects | 4 (4.8) |
| Current treatment status, n (%) | |
| Continued | 41 (30.8) |
| Discontinued | 92 (69.2) |
| Reasons for discontinuation | |
| Disease progression | 66 (49.6) |
| Death | 4 (3.0) |
| Financial constraint | 8 (6.0) |
| Side-effects | 7 (5.3) |
| Patients’ request | 5 (3.8) |
| Others | 2 (1.5) |
| Dose reduction, n (%) | |
| No | 102 (76.7) |
| Yes | 31 (23.3) |
| Reasons for dose reduction | |
| Side-effects | 26 (19.5) |
| Financial constraint | 5 (3.8) |
†, number of patients =83. BMI, body mass index.
At the time of analysis, about two-thirds of patients (92 patients) had discontinued treatment with afatinib, and this was primarily due to disease progression (Table 3). Only 23.3% of the patients had dose reductions during the course of their treatment, and these were due to side effects or financial constraints. Of 31 patients with afatinib dose reductions, 17 started on a dosage of 40 mg OD (55%) while 14 started on <40 mg OD (45%). None of the patients had their afatinib dose increased. The discontinuation rates were lower in the <40 mg OD group (16.9%) than in the 40 mg OD group (34.0%). A closer look at the reasons behind discontinuation of afatinib reveal AEs to be the major reason, but this was lower in the <40 mg OD group (57.1%) than in the 40 mg OD group (94.1%).
Tumor response
Majority of the patients had PR to afatinib (63.2%) and 2.3% had CR (Table 4). The ORR was 65.4% and the DCR was 79.7% (Table 4). One-fifth of patients (20.3%) had PD and did not respond to afatinib (Table 4). Of the 92 (69%) patients who had disease progression with afatinib at the time of writing, 17 patients (12.8%) had new brain metastases whilst 75 patients (56.4%) had new metastatic lesions at other sites (Table 4). The resistance mechanism upon disease progression was EGFR exon 20 T790M mutation in 23 (53.5%) of 43 patients who were investigated by liquid or repeat tissue biopsy (Table 4).
Table 4. Treatment outcome with first-line afatinib and resistance mechanism identified at disease progression (n=133).
| Treatment outcome | Values |
|---|---|
| Best tumor response, n (%) | |
| Complete response | 3 (2.3) |
| Partial response | 84 (63.2) |
| Stable disease | 19 (14.3) |
| Progressive disease | 27 (20.3) |
| Objective response, n (%) | |
| Yes | 87 (65.4) |
| No | 46 (34.6) |
| Disease control, n (%) | |
| Yes | 106 (79.7) |
| No | 27 (20.3) |
| Disease progression site, n (%) | |
| None | 41 (30.8) |
| New brain metastases | 17 (12.8) |
| New metastatic lesions at other sites | 75 (56.4) |
| Investigation for resistance mechanism, n (%) | |
| No progression | 41 (30.8) |
| Not investigated | 49 (36.8) |
| Investigated | 43 (32.3) |
| Resistance mechanism identified | |
| Exon 20 T790M mutation† | 23 (53.5) |
| Small cell lung cancer transformation† | 2 (4.7) |
| Other resistant mechanisms† | 3 (7.0) |
| No resistance mechanism detected† | 15 (34.9) |
†, number of patients investigated for resistance mechanism =43. Other resistant mechanisms: two MET amplification, one exon 18 G719X.
The ORR was significantly higher (72.3%) with afatinib starting doses of less than 40 mg dose OD compared to 40 mg OD (54.0%) (P=0.032) (Table 5). Patients on the lower doses were more than two times likely to achieve an objective response [odds ratio (OR) =2.64; 95% CI: 1.20–5.83); P=0.016]. The DCR was higher (84.3%) with afatinib starting doses of less than 40 mg dose OD compared to 40 mg OD (72.0%) but the difference was not statistically significant (P=0.087, Table 5). Focusing solely on the lower dose group, the ORR was 69.6% for patients with brain metastases versus 73.3% for patients without brain metastases (P=0.731). The DCR in this group was 78.3% for patients with brain metastases versus 86.7% for patients without brain metastases (P=0.346).
Table 5. Univariate and multivariate analyses of ORR and DCR with first-line afatinib according to clinical and treatment characteristics.
| Characteristics | Objective response | Disease control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ORR, n (%) | Univariate analysis | Multivariate analysis | DCR, n (%) | Univariate analysis | Multivariate analysis | ||||
| *P value | †OR (95% CI), P value | *P value | †OR (95% CI), P value | ||||||
| EGFR mutation subtype, n (%) | 0.071 | 0.588 | |||||||
| Exon 19 deletion (n=81) | 56 (69.1) | – | 65 (80.2) | – | |||||
| Exon 21 L858R (n=31) | 20 (64.5) | 0.72 (0.29–1.82), 0.490‡ | 25 (80.6) | 1.29 (0.40–4.13), 0.666‡ | |||||
| Resistant mutations (n=4) | 4 (100.0) | – | 4 (100.0) | – | |||||
| Rare and complex mutations (n=17) | 7 (41.2) | 0.27 (0.09–0.85), 0.025‡ | 12 (70.6) | 0.33 (0.09–1.22), 0.096‡ | |||||
| Baseline symptomatic brain metastases, n (%) | 0.423 | 0.163 | |||||||
| No (n=87) | 59 (67.8) | – | 73 (83.9) | – | |||||
| Yes (n=46) | 28 (60.9) | 0.54 (0.22–1.34), 0.184‡ | 33 (71.7) | 0.37 (0.14–0.96), 0.041‡ | |||||
| ECOG performance status, n (%) | 0.096 | 0.142 | |||||||
| 0–1 (n=22) | 11 (50.0) | – | 15 (68.2) | – | |||||
| 2–4 (n=111) | 76 (68.5) | 1.99 (0.69–5.77), 0.205‡ | 91 (82.0) | 2.36 (0.78–7.11), 0.127‡ | |||||
| Tumor stage, n (%) | 0.317 | 0.124 | |||||||
| IVB (n=53) | 37 (69.8) | – | 44 (83.0) | – | |||||
| IVA (n=70) | 42 (60.0) | 0.47 (0.21–1.09), 0.078‡ | 52 (74.3) | 0.41 (0.15–1.10), 0.076‡ | |||||
| IIIC (n=10) | 8 (80.0) | 2.00 (0.35–11.55), 0.436‡ | 10 (100.0) | – | |||||
| Comorbidities, n (%) | 0.984 | 0.672 | |||||||
| No (n=84) | 55 (65.5) | – | 66 (78.6) | – | |||||
| Yes (n=49) | 32 (65.3) | 1.00 (0.43–2.29), 0.993‡ | 40 (81.6) | 1.39 (0.50–3.89), 0.526‡ | |||||
| Afatinib starting dose, n (%) | 0.032 | 0.087 | |||||||
| 40 mg once daily (n=50) | 27 (54.0) | – | 36 (72.0) | – | |||||
| Less than 40 mg once daily (n=83) | 60 (72.3) | 2.64 (1.20–5.83), 0.016‡ | 70 (84.3) | 2.10 (0.77–5.75), 0.149‡ | |||||
| Afatinib dose adjustment, n (%) | 0.158 | 0.881 | |||||||
| No (n=102) | 70 (68.6) | – | 81 (79.4) | – | |||||
| Yes (n=31) | 17 (54.8) | 1.11 (0.43–2.82), 0.833‡ | 25 (80.6) | 2.10 (0.68–6.55), 0.200‡ | |||||
*, P value of Chi-squared test. †, multivariate analysis with cox regression; ‡, the first parameter for each characteristic was the reference group. ORR, objective response rate; DCR, disease control rate; OR, odds ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group.
There were no significant differences in ORR in terms of mutation subtypes, baseline symptomatic brain metastases, ECOG performance status, tumor stage, comorbidities, or afatinib dose adjustments (Table 5). However, patients with rare and compound mutations had a lower ORR compared to common mutations (OR =0.27; 95% CI: 0.09–0.85; P=0.025). There were also no significant differences in DCR in terms of mutation subtypes, ECOG performance status, tumor stage, comorbidities, afatinib starting dose, or afatinib dose adjustments.
Out of 92 patients who had disease progression on afatinib, 56 patients (61%) went on to receive second-line treatment (Table 6), the majority of whom received osimertinib (n=43) and the rest had chemotherapy (n=13). Only 32 patients (57%) of the 56 patients who received second-line treatment had a resistance mechanism checked upon disease progression to afatinib. Of those who received second-line osimertinib (n=43), the resistance mechanisms were investigated in 28 patients, more than half of whom (n=19) had EGFR exon 20 T790M mutation as a resistance mechanism to afatinib (Table 6).
Table 6. Resistance mechanisms of 56 patients who received osimertinib versus chemotherapy as second-line treatment after disease progression.
| Resistance mechanisms, n (%) | Second-line treatment | P value | |
|---|---|---|---|
| Osimertinib (n=43) | Chemotherapy (n=13) | ||
| Investigated | 28 (65.1) | 4 (30.8) | 0.076 |
| Exon 20 T790M mutation | 19 (44.2) | 1 (7.7) | |
| No resistance mechanism identified | 9 (20.9) | 3 (23.1) | |
| Not investigated | 15 (34.9) | 9 (69.2) | – |
TTF and survival outcomes with first-line afatinib
Only patients who discontinued afatinib (n=92) were included in the TTF analysis (Figures 1,2 and Table 7). Patients who were still on afatinib were excluded from this analysis. The median TTF with first-line afatinib (95% CI) was 12.4 (10.02–14.78) months (Figure 2). Multivariate analysis revealed no significant difference in TTF in terms of mutation subtypes, presence or absence of baseline symptomatic brain metastases, ECOG performance status, tumor stage, comorbidities, afatinib starting dose, or afatinib dose adjustments (Table 7). Patients on afatinib dose lower than 40 mg OD had a numerically but not statistically longer median TTF [12.50 (95% CI: 10.44–14.56) months] than patients on 40 mg OD [9.80 (95% CI: 5.72–13.88) months] (HR =0.76; 95% CI: 0.49–1.20; P=0.238) (Figure 3).
Figure 2.
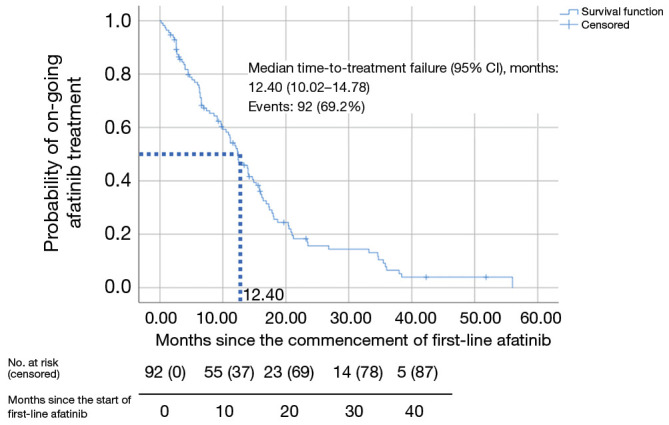
Kaplan-Meier plot for time-to-treatment failure of patients on first-line afatinib. CI, confidence interval.
Table 7. Univariate and multivariate analyses of TTF according to clinical and treatment characteristics (n=92).
| Characteristics | Patients, n (%)† | mTTF (months) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| EGFR mutation subtype, n (%) | |||||||
| Exon 19 deletion | 55 (59.8) | 10.5 | – | – | – | – | |
| Exon 21 L858R point mutation | 23 (25.0) | 13.9 | 1.21 (0.72–2.06)‡ | 0.471‡ | 1.22 (0.72–2.06)‡ | 0.464‡ | |
| Resistant mutations | 1 (1.1) | 12.4 | 0.93 (0.12–7.26)‡ | 0.946‡ | 0.89 (0.12–6.82)‡ | 0.910‡ | |
| Rare and complex mutation | 13 (14.1) | 6.6 | 0.70 (0.36–1.37)‡ | 0.301‡ | 0.71 (0.37–1.38)‡ | 0.312‡ | |
| Baseline symptomatic brain metastases, n (%) | |||||||
| No | 56 (60.9) | 10.0 | – | – | – | – | |
| Yes | 36 (39.1) | 11.2 | 0.91 (0.56–1.47)‡ | 0.701‡ | 0.91 (0.56–1.47)‡ | 0.701‡ | |
| ECOG performance status, n (%) | |||||||
| 0–1 | 15 (16.3) | 14.0 | – | – | – | – | |
| 2–4 | 77 (83.7) | 11.1 | 2.30 (1.22–4.36)‡ | 0.010‡ | 1.75 (0.97–3.16)‡ | 0.062‡ | |
| Tumor stage, n (%) | |||||||
| IVB | 38 (41.3) | 7.9 | – | – | – | – | |
| IVA | 51 (55.4) | 12.3 | 0.86 (0.53–1.40)‡ | 0.543‡ | 0.76 (0.49–1.18)‡ | 0.219‡ | |
| IIIC | 3 (3.3) | 4.7 | 4.84 (1.35–17.37)‡ | 0.015‡ | 3.37 (1.00–11.34)‡ | 0.050‡ | |
| Comorbidities, n (%) | |||||||
| No | 59 (64.1) | 11.2 | – | – | – | – | |
| Yes | 33 (35.9) | 10.9 | 1.39 (0.86–2.25)‡ | 0.174‡ | 1.3 (0.85–2.09)‡ | 0.214‡ | |
| Afatinib starting dose, n (%) | |||||||
| 40 mg once daily | 29 (31.5) | 9.8 | – | – | – | – | |
| Less than 40 mg once daily | 63 (68.5) | 12.5 | 0.62 (0.38–1.02)‡ | 0.062‡ | 0.76 (0.49–1.20)‡ | 0.238‡ | |
| Afatinib dose adjustment, n (%) | |||||||
| No | 67 (72.8) | 11.1 | – | – | – | – | |
| Yes | 25 (27.2) | 11.2 | 0.77 (0.47–1.26)‡ | 0.301‡ | 0.77 (0.48–1.26)‡ | 0.307‡ | |
†, number and percentage of subgroup of patients who failed treatment with afatinib; ‡, the first group was the reference category in logistic regression analysis. mTTF, median time to treatment failure; HR, hazard ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group.
Figure 3.
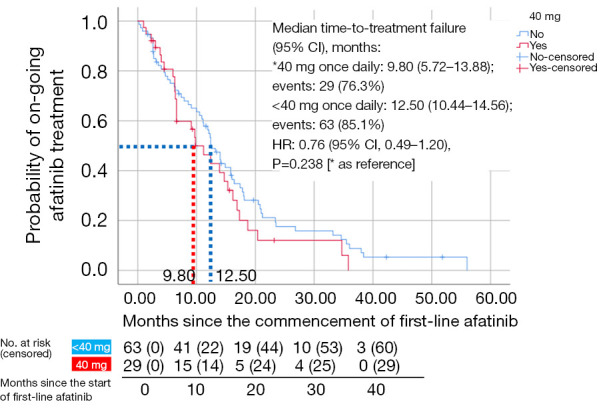
Kaplan-Meier curves for time-to-treatment failure for patients who received afatinib 40 mg once daily and those who received afatinib less than 40 mg once daily. CI, confidence interval; HR, hazard ratio.
The median OS (95% CI) for patients treated with first-line afatinib was 21.30 (15.86–26.75) months (Figure 4).
Figure 4.
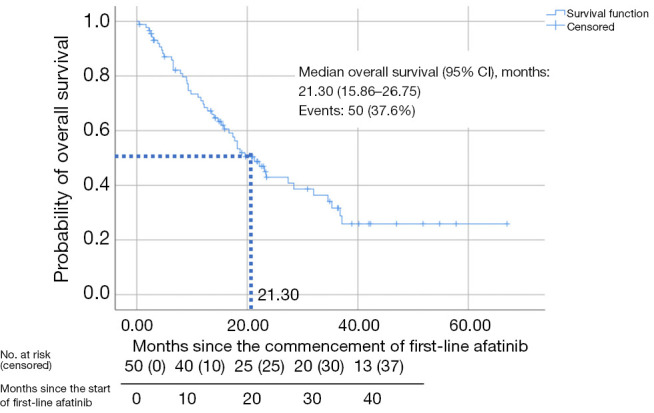
Kaplan-Meier plot for overall survival of patients on first-line afatinib. CI, confidence interval.
TTF and survival outcomes with second-line treatment
Fifty-six patients (61%) in the study received second-line treatment (Table 6, Figure 5). Median TTF (95% CI) with osimertinib as a second-line treatment was 14.20 (8.94–19.46) months (Figure 5). Patients on chemotherapy as second-line treatment had a shorter median TTF [3.50 (95% CI: 0.01–7.02) months]. Patients on second-line osimertinib had significantly longer TTF than those on second-line chemotherapy (HR =0.12; 95% CI: 0.05–0.28; P<0.001) (Figure 5). The cumulative OS for patients on sequential afatinib and osimertinib treatments was 25.6 (±12.3) months. Three patients developed C797S mutation after failure of second-line osimertinib, with TTF of 16.6, 6.9, and 5.9 months, respectively.
Figure 5.
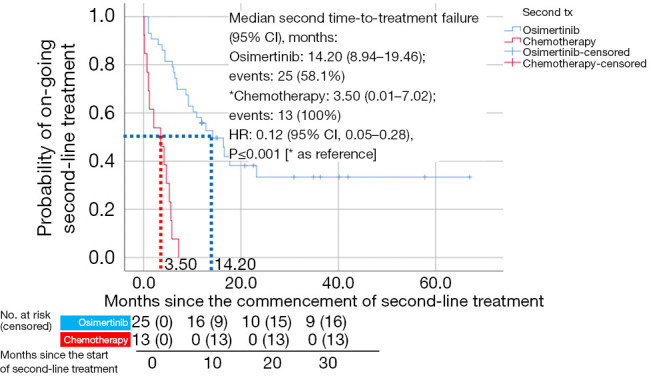
Kaplan-Meier curves for time-to-treatment failure of patients on second-line osimertinib and that of patients treated with chemotherapy. CI, confidence interval; HR, hazard ratio; tx, treatment.
Clinical outcomes in patients with brain metastases
Forty-six patients had symptomatic brain metastases at diagnosis. The ORR of patients with baseline symptomatic brain metastases was 69.6% with afatinib less than 40 mg OD and 52.2% with afatinib 40 mg OD (P=0.701).
Patients with baseline symptomatic brain metastases on first-line afatinib were significantly less likely to achieve disease control compared to those without baseline brain metastases (71.7% versus 83.9%; OR =0.37; 95% CI: 0.14–0.96; P=0.041) (Table 5).
Discussion
Our real-world study showed that lower doses of afatinib could be equally effective as standard dose in EGFR-mutant advanced NSCLC patients with good response and survival outcomes. Patients on afatinib doses of less than 40 mg OD had significantly higher ORR (72.3%) than those on 40 mg or higher (54.0%) (P=0.032). Both groups had similar DCR. This is in keeping with afatinib’s effectiveness as first-line treatment in patients with EGFR-mutant advanced NSCLC, which has been well established in clinical trials and real-world studies (4,6-9,16,17,21-25). Afatinib also has a good tolerability profile but as some patients do experience gastrointestinal and cutaneous AEs, tolerability-guided dose reduction has been suggested (8,9,22,25). In our study, patients on lower doses of afatinib had lower discontinuation rate due to AEs than those on standard dose.
Our study showed that afatinib with a dosage of less than 40 mg OD is equally effective in patients with brain metastases, as there was no significant difference in ORR between patients with baseline symptomatic brain metastases and those without. In contrast to first generation EGFR-TKI, afatinib has been shown to be effective in patients with brain metastases (7), and this has also been reported by real world studies (16,23). As afatinib is able to penetrate the blood-brain barrier, it has the potential to be effective in treating brain metastases as well as reducing the risk of central nervous system progression (16,26). A Korean real-world study showed that majority of patients with baseline brain metastases no longer had brain metastases after afatinib treatment (16). The same study also showed that dose reductions did not affect efficacy for patients with or without baseline brain metastases (16). The data from our study, however, has to be interpreted with caution as the response rate may have been affected by local radiation therapy to the brain metastases, which was not captured and reported in the study. Patients in our study who received sequential afatinib followed by osimertinib (n=43) were significantly less likely to fail treatment compared to sequential afatinib followed by chemotherapy. This could be because a majority of these patients (68% of patients who were investigated for resistance mechanism upon disease progression) harbored the resistant exon 20 T790M mutation and therefore would have responded better to osimertinib. This result was consistent with previous studies (20,27-29). Evidence from a South Korean real-world study showed that median time on treatment for patients on sequential afatinib-osimertinib regimen was over 3.5 years (28). The authors of the study attributed this to afatinib’s effect in producing a long-term, chemotherapy-free state (28). The shorter OS with sequential treatment in our real-world study versus the FLAURA study (30) using osimertinib in the first-line setting could be due to the characteristics of our patients (poorer ECOG status, symptomatic brain metastases). However, there was no significant difference between patients starting afatinib on 40 mg and <40 mg in terms of their ECOG performance status.
An afatinib dosage of less than 40 mg may be more suited for Asian patients. This could be due to several reasons. Firstly, Asian patients generally have lower BMI than Caucasian patients (31). In the post hoc analyses of LUX-Lung 6 trial (Asian patients), the efficacy of lower doses of afatinib was demonstrated, and there were more patients with lower BMI on a final dose of 30 mg OD compared to patients with higher BMI (32). Thus, despite not being able to capture the exact BMI of our patients in the database, we would generally assume the BMI of these patients studied to be in keeping with the overall population. Based on a lower BMI, a lower dose of afatinib may be equally or more effective than a dosage of 40 mg OD as shown in our studies, whilst minimizing the side effects experienced by patients leading to dose interruptions, affecting survival outcomes.
Asian patients may only need lower doses of afatinib for the drug to be effective, while minimizing its side effects. Many studies have demonstrated that drug metabolism in Asian patients are reduced compared to Caucasians (33-35). However, a study showed that the pharmacokinetic profile of afatinib did not exhibit statistically significant differences between Asian (including the tested subpopulations, i.e., Chinese, Japanese, Korean, Southeast Asian, Taiwanese and other Asian) and Caucasian patients (36).
The key strengths of our study are its real-world clinical practice setting and inclusion of patients who would otherwise be excluded from randomized controlled trials (i.e., those with poor ECOG performance scores and those with brain metastases). One-fifth of patients had an ECOG performance status of 4. They were given afatinib in addition to standard palliative care because they were keen on active treatment after discussing with their treating clinician. In addition, there are very few real-world studies on afatinib from Southeast Asia, where patients are likely to have a lower BMI (31). Patients on afatinib with lower BMI have been shown to have better disease control (37). Therefore, individualized titration of dosage of afatinib is recommended to optimally balance the risk and benefit of treatment.
Some limitations of our study include the retrospective nature of the study, and the lack of information on the types of treatment used for brain metastases (e.g., radiation therapy) which may have confounded response to afatinib. One major limitation is patients defined as having a low BMI in the study were based on the treating clinician’s visual assessment; therefore, the exact BMI was not captured in the database. We also did not compare treatment outcomes based on the lowest afatinib maintenance dose and its duration. Inadequate sample size of patients on afatinib 40 mg OD could have led to the lack of statistically significant difference for TTF. In addition, the duration of response and PFS were not captured in our database. Therefore, the findings of this study should be interpreted with caution.
Despite the retrospective nature of the study, efficacy was encouraging in the overall cohort. A bigger sample size is needed to establish the efficacy of doses below 40 mg OD across patient subgroups including those with baseline brain metastases and specific EGFR subtype. Future studies can be focused on patients with brain metastases on afatinib 40 mg OD or lower doses, with baseline brain radiation therapy as a stratification factor. In addition, studies should look into the efficacy of different doses according to BMI scores with objective assessments (such as PFS) in a prospective study with adequate sample size for both comparative arms.
Conclusions
Overall, lower doses of afatinib were equally effective as standard dose in producing good response and prolonging TTF in patients with EGFR-mutant advanced NSCLC. Irrespective of age, gender, stage of disease, ECOG performance status, and even more interestingly, baseline symptomatic brain metastases, a dosage of less than 40 mg may be more suited for Asian patients, minimizing the potential side effects that may occur at higher dosages of afatinib leading to dose interruptions and affecting treatment outcomes. We recommend future studies in a more controlled and objective setting to check the validity of our current hypothesis.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors wish to thank the Malaysian Association for Thoracic and Cardiovascular Surgery for maintaining the lung cancer database, study coordinators at each of the participating centers, and Anne John Michael (MedPhrase) for help in preparing the manuscript.
Funding: This work was supported by the Malaysian Thoracic Society.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Universiti Malaya Medical Centre Medical Research Ethics Committee (MECID 20201115–9217). The Universiti Malaya Medical Centre Medical Research Ethics Committee waived the need for informed consent as patient confidentiality was preserved using identification code numbers.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-691/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-691/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-691/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-691/coif). G.F.H. reports grants or contracts from AUXI Therapeutics and Taiho; payment or honoraria from AstraZeneca and Boehringer Ingelheim; support for attending meetings and/or travel from MSD; and participation on a Data Safety Monitoring Board or Advisory Board from Adipolab, Amgen, Astrazeneca, Boehringer Ingelheim, Dr Reddy, Eisai, MSD, Pfizer, Servier. Y.K.P. reports payments as speaker from AstraZeneca, GSK, and Pfizer; support for attending the European Respiratory Society Congress 2022 from EuroDrug; participation on Advisory Boards for Amgen, Eurodrug Laboratories, Boehringer Ingelheim, MSD, Novartis, Orient Europharma, Pfizer, Sanofi-Aventis, and Specialised Therapeutics; research funding from AstraZeneca, MSD, and Sanofi; duty as trustee for Lung Foundation of Malaysia, and role as the Immediate Past President for the Malaysian Thoracic Society. L.M.T. reports payment or honoraria from AstraZeneca, Boehringer Ingelheim, Fresenius Kabi, Janssen, Novartis, Pfizer, and Roche; and support for attending meetings and/or travel from AstraZeneca, Boehringer Ingelheim, Fresenius Kabi, and Roche. I.M.N. reports speaker honoraria from Eisei, MSD, Novartis, and Pfizer; Principal Investigator fees under Clinical Research Malaysia from Amgen, AstraZeneca, Mirati, MSD, and Novartis; travel grant for educational meetings/investigator meeting from Arcus, Eisei, ESMO, MSD, Novartis, and Roche; and monthly payments as Board of Director for Syarikat Hospital PUSRAWI Sdn. Bhd/ Syarikat MAIWP Healthcare Sdn Bhd from June 2021 to June 2023. K.F.H. reports payment or honoraria from EISAI; support for attending meetings and/or travel from Merck, and participation on a Data Safety Monitoring Board or Advisory Board for MSD. M.T. reports honoraria from Boehringer Ingelheim for a lecture in 2023 and is the current President of the Malaysian Oncological Society. S.H.H. reports funding from Boehringer Ingelheim for medical writing; grants or contracts from Arcus, AstraZeneca, Janssen, Merck, MSD, Novartis, and Pfizer; payment or honoraria from AstraZeneca, Janssen, MSD, Novartis, Pfizer, and Takeda; support for attending meetings and/or travel from MSD; participation on a Data Safety Monitoring Board or Advisory Board from AstraZeneca, Janssen, MSD, Novartis, Pfizer, and Takeda, as well as receipt of equipment, materials, drugs, medical writing, gifts or other services from AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, and Takeda. The other authors have no conflicts of interest to declare.
References
- 1.World Health Organization. Cancer [cited 2022 Feb 3]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer
- 2.Globocan 2020. Malaysia [cited 2022 Mar]. Available online: https://gco.iarc.fr/today/data/factsheets/populations/458-malaysia-fact-sheets.pdf
- 3.Ministry of Health Malaysia. National Strategic Plan for Cancer Control Programme 2021-2025; 2021. Available online: https://www.moh.gov.my/moh/resources/Penerbitan/Rujukan/NCD/Kanser/National_Strategic_Plan_for_Cancer_Control_Programme_2021-2025.pdf
- 4.Tanaka H, Taima K, Itoga M, et al. Real-world study of afatinib in first-line or re-challenge settings for patients with EGFR mutant non-small cell lung cancer. Med Oncol 2019;36:57. 10.1007/s12032-019-1278-9 [DOI] [PubMed] [Google Scholar]
- 5.How SH, Liam CK, Zainal Abidin MA, et al. Outcomes of Patients with EGFR-Mutant Advanced NSCLC in a Developing Country in Southeast Asia. Cancer Manag Res 2022;14:1995-2005. 10.2147/CMAR.S364713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura K, Nukiwa T, Gemma A, et al. Real-world treatment of over 1600 Japanese patients with EGFR mutation-positive non-small cell lung cancer with daily afatinib. Int J Clin Oncol 2019;24:917-26. 10.1007/s10147-019-01439-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passaro A, de Marinis F, Tu HY, et al. Afatinib in EGFR TKI-Naïve Patients with Locally Advanced or Metastatic EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Pooled Analysis of Three Phase IIIb Studies. Front Oncol 2021;11:709877. 10.3389/fonc.2021.709877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park K, Wan-Teck Lim D, Okamoto I, et al. First-line afatinib for the treatment of EGFR mutation-positive non-small-cell lung cancer in the 'real-world' clinical setting. Ther Adv Med Oncol 2019;11:1758835919836374. 10.1177/1758835919836374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang SK, Hsieh MS, Lee MR, et al. Real-world experience of afatinib as a first-line therapy for advanced EGFR mutation-positive lung adenocarcinoma. Oncotarget 2017;8:90430-43. 10.18632/oncotarget.19563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 11.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 12.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 13.Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 14.Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. 10.1093/annonc/mdw611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim CK, Wei YF, Tsai MS, et al. Treatment effectiveness and tolerability of afatinib at different doses in patients with EGFR-mutated lung adenocarcinoma: How low can we go? Eur J Cancer 2018;103:32-40. 10.1016/j.ejca.2018.07.128 [DOI] [PubMed] [Google Scholar]
- 16.Lee SY, Choi CM, Chang YS, et al. Real-world experience of afatinib as first-line therapy for advanced EGFR mutation-positive non-small cell lung cancer in Korea. Transl Lung Cancer Res 2021;10:4353-67. 10.21037/tlcr-21-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho GF, Chai CS, Alip A, et al. Real-world experience of first-line afatinib in patients with EGFR-mutant advanced NSCLC: a multicenter observational study. BMC Cancer 2019;19:896. 10.1186/s12885-019-6107-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19.Liang SK, Lee MR, Liao WY, et al. Prognostic factors of afatinib as a first-line therapy for advanced EGFR mutation-positive lung adenocarcinoma: a real-world, large cohort study. Oncotarget 2018;9:23749-60. 10.18632/oncotarget.25255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey RD, Adams VR, Beardslee T, et al. Afatinib for the treatment of EGFR mutation-positive NSCLC: A review of clinical findings. J Oncol Pharm Pract 2020;26:1461-74. 10.1177/1078155220931926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu S, Shih JY, Jang TW, et al. Afatinib as First-Line Treatment in Asian Patients with EGFR Mutation-Positive NSCLC: A Narrative Review of Real-World Evidence. Adv Ther 2021;38:2038-53. 10.1007/s12325-021-01696-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Luo Y, Chen J, et al. Efficacy and Safety of Afatinib in the Treatment of Advanced Non-Small-Cell Lung Cancer with EGFR Mutations: A Meta-Analysis of Real-World Evidence. J Oncol 2021;2021:8736288. 10.1155/2021/8736288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon SH, Kim YS, Chung JH, et al. 485P - A real-world experience of first-line afatinib in Korean patients with EGFR-mutant non-small cell lung cancer. Ann Oncol 2019;30:ix163. [Google Scholar]
- 24.Agraso S, Lázaro M, Firvida XL, et al. Real-world data with afatinib in Spanish patients with treatment-naïve non-small-cell lung cancer harboring exon 19 deletions in epidermal growth factor receptor (Del19 EGFR): Clinical experience of the Galician Lung Cancer Group. Cancer Treat Res Commun 2022;33:100646. 10.1016/j.ctarc.2022.100646 [DOI] [PubMed] [Google Scholar]
- 25.Halmos B, Tan EH, Soo RA, et al. Impact of afatinib dose modification on safety and effectiveness in patients with EGFR mutation-positive advanced NSCLC: Results from a global real-world study (RealGiDo). Lung Cancer 2019;127:103-11. 10.1016/j.lungcan.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 26.Hochmair M. Medical Treatment Options for Patients with Epidermal Growth Factor Receptor Mutation-Positive Non-Small Cell Lung Cancer Suffering from Brain Metastases and/or Leptomeningeal Disease. Target Oncol 2018;13:269-85. Erratum in: Target Oncol 2018;13:667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CH, Chang JW, Chang CF, et al. Real-world Afatinib Outcomes in Advanced Non-small Cell Lung Cancer Harboring EGFR Mutations. Anticancer Res 2022;42:2145-57. 10.21873/anticanres.15697 [DOI] [PubMed] [Google Scholar]
- 28.Jung HA, Hong MH, Lee HW, et al. Totality outcome of afatinib sequential treatment in patients with EGFR mutation-positive non-small cell lung cancer in South Korea (TOAST): Korean Cancer Study Group (KCSG) LU-19-22. Transl Lung Cancer Res 2022;11:1369-79. 10.21037/tlcr-22-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim T, Jang TW, Choi CM, et al. Sequential treatment of afatinib and osimertinib or other regimens in patients with advanced non-small-cell lung cancer harboring EGFR mutations: Results from a real-world study in South Korea. Cancer Med 2021;10:5809-22. 10.1002/cam4.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 31.Tham KW, Abdul Ghani R, Cua SC, et al. Obesity in South and Southeast Asia-A new consensus on care and management. Obes Rev 2023;24:e13520. 10.1111/obr.13520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang JC, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 2016;27:2103-10. 10.1093/annonc/mdw322 [DOI] [PubMed] [Google Scholar]
- 33.Lin SK. Racial/Ethnic Differences in the Pharmacokinetics of Antipsychotics: Focusing on East Asians. J Pers Med 2022;12:1362. 10.3390/jpm12091362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo C, Nguyen S, Yang C, et al. Pharmacogenomics in Asian Subpopulations and Impacts on Commonly Prescribed Medications. Clin Transl Sci 2020;13:861-70. 10.1111/cts.12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phan VH, Moore MM, McLachlan AJ, et al. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol 2009;5:243-57. 10.1517/17425250902800153 [DOI] [PubMed] [Google Scholar]
- 36.Freiwald M, Schmid U, Fleury A, et al. Population pharmacokinetics of afatinib, an irreversible ErbB family blocker, in patients with various solid tumors. Cancer Chemother Pharmacol 2014;73:759-70. 10.1007/s00280-014-2403-2 [DOI] [PubMed] [Google Scholar]
- 37.Wu CE, Chang CF, Huang CY, et al. Feasibility and effectiveness of afatinib for poor performance status patients with EGFR-mutation-positive non-small-cell lung cancer: a retrospective cohort study. BMC Cancer 2021;21:859. 10.1186/s12885-021-08587-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


