Abstract
Doxorubicin (DOX) is a widely used chemotherapeutic drug known to cause dose-dependent myocardial toxicity, which limits its clinical potential. DL-3-n-butylphthalide (NBP), a substance extracted from celery seed species, has a number of pharmacological properties, such as antioxidant, anti-inflammatory, and anti-apoptotic actions. However, whether NBP can protect against DOX-induced acute myocardial toxicity is still unclear. Therefore, this study was designed to investigate the potential protective effects of NBP against DOX-induced acute myocardial injury and its underlying mechanism. By injecting 15 mg/kg of DOX intraperitoneally, eight-week-old male C57BL6 mice suffered an acute myocardial injury. The treatment group of mice received 80 mg/kg NBP by gavage once daily for 14 days. To mimic the cardiotoxicity of DOX, 1uM DOX was administered to H9C2 cells in vitro. In comparison to the DOX group, the results showed that NBP improved cardiac function and decreased serum levels of cTnI, LDH, and CK-MB. Additionally, HE staining demonstrated that NBP attenuated cardiac fibrillar lysis and breakage in DOX-treated mouse hearts. Western blotting assay and immunofluorescence staining suggested that NBP attenuated DOX-induced oxidative stress, apoptosis, and inflammation both in vivo and in vitro. Mechanistically, NBP significantly upregulated the Nrf2/HO-1 signaling pathway, while the Nrf2 inhibitor ML385 prevented NBP from protecting the myocardium from DOX-induced myocardial toxicity in vitro. In conclusion, Our results indicate that NBP alleviates DOX-induced myocardial toxicity by activating the Nrf2/HO-1 signaling pathway.
Keywords: Doxorubicin, Myocardial toxicity, Oxidative stress, Nrf2, DL-3-n-Butylphthalide
1. Introduction
Doxorubicin (DOX) is a broad-spectrum anthracycline antibiotic commonly used in the treatment of various cancers, including carcinoma, sarcoma, and hematological malignancies [1]. However, the use of DOX therapy is associated with significant side effects, particularly cardiotoxicity, which can result in reduced cardiac ejection fraction, arrhythmias, and even heart failure [2,3]. Due to the dose-dependent myocardial toxicity, the clinical use of DOX is currently limited, with studies suggesting that the maximum safe dose for adults is 550 mg/m2 [4]. Dexrazoxane, an FDA-approved drug, is the only available treatment for anthracycline cardiotoxicity [5]. However, it is crucial to remember that there is a potential risk of developing secondary malignancies associated with its use [6]. Therefore, it really matters to acquire a secure and efficient replacement medication for the treatment of DOX-induced myocardial toxicity.
The mechanism of DOX-induced myocardial toxicity involves various aspects, including oxidative stress [7], autophagy [8], pyroptosis [9], endoplasmic reticulum stress [10], and iron death [11]. However, it is widely acknowledged that oxidative stress is primarily responsible for DOX-induced myocardial toxicity [12]. The transcription factor nuclear factor E2-associated factor 2 (Nrf2), which is a crucial redox-sensitive transcription factor, controls the expression of downstream antioxidant enzymes including heme oxygenase 1 (HO-1), superoxide dismutase 2 (SOD2), and quinone oxidoreductase 1 (NQO1) to mitigate oxidative stress and maintain cellular redox homeostasis [13]. Genetic interventions targeting Nrf2 or using Nrf2 activators found in natural compounds have demonstrated the ability to reverse pathological changes in various cardiovascular disease models, including myocardial infarction [14], heart failure [15], sepsis-induced myocardial dysfunction [16], hypertension [17], atherosclerosis [18], diabetic cardiomyopathy [19], and DOX-induced cardiomyopathy [20]. Importantly, multiple investigations established that the Nrf2/HO-1 signaling pathway shows reduced expression in DOX-induced cardiomyopathy, and suppressing Nrf2 or blocking its activity can exacerbate DOX-induced cardiotoxicity while upregulating Nrf2 can attenuate DOX-induced cardiotoxicity [21]. These findings imply that the modulation of Nrf2 could be a crucial therapeutic approach to treating DOX-induced myocardial toxicity.
DL-3-n-butylphthalide (NBP), a synthetic racemate of L-3-n-butylphthalide extracted from celery seeds, was approved by the State Food and Drug Administration of China in 2002 for the treatment of ischemic stroke [22]. In recent years, it has been shown that NBP plays crucial roles in a number of physiological processes, such as the regulation of autophagy and the prevention of inflammation, apoptosis, endoplasmic reticulum stress, and oxidative stress [[23], [24], [25], [26], [27]]. Wang et al. demonstrated that NBP improved pathological alterations in mice with Alzheimer's disease by upregulating Nrf2, inhibiting TXNIP-TrX interactions, maintaining redox homeostasis, and reducing inflammation [28]. NBP has also been identified to inhibit oxidative stress and ameliorate diabetes-associated cognitive decline by upregulating Nrf2/HO-1 signaling pathway [24]. Notably, NBP has demonstrated protective effects against cardiovascular diseases. Previous studies have reported that NBP protects rat cardiomyocytes from ischemia/reperfusion injury through its anti-oxidative stress properties and influence on the mitochondrial apoptotic pathway [22,29]. NBP also prevents the formation of atherosclerotic plaques in ApoE−/− mice by activating the Keap-1/Nrf-2 signaling pathway to reduce oxidative stress [30]. Furthermore, NBP has been found to prevent cardiac remodeling and arrhythmia in rats with myocardial infarction by attenuating fibrosis and Cx43 gap junction remodeling via modulation of the PI3k/Akt/Nrf2/ARE signaling pathway [31]. Additionally, NBP treatment reduced cardiac hypertrophy and dysfunction in mice [32]. Nevertheless, the extent to which NBP contributes to the development of DOX-triggered cardiac damage remains largely unexplored. Consequently, the aim of this investigation was to assess the potential of NBP to alleviate myocardial injury induced by DOX and shed light on the mechanisms involved.
2. Materials and methods
2.1. Drugs and antibodies
Doxorubicin (DOX, #HY-15142), Butylphthalide (NBP, #HY-B0647), ML385 (#HY-100523), Polyethylene glycol 300 (PEG300, #HY-Y0873), and Dimethyl sulfoxide (DMSO, #HY-Y0320) were obtained from MedChemExpress (New Jersey, USA). Anti-Nuclear factor E2-related factor 2 (Nrf2, #GTX103322), anti-heme oxygenase 1 (HO-1, #GTX101147), anti–NF–κB P65 (P65, #GTX102090), and anti-phospho–NF–κB P65 (P–P65, #GTX133899) were purchased from GeneTex (California, USA). Anti-interleukin 6 (IL-6, #YT5348) and antitumor necrosis factor α (TNF-α, #YT4689) were obtained from ImmunoWay (Texas, USA). Anti-IL-1β (#ab283822) was purchased from Abcam (Cambridge, UK). Anti-cleaved caspase3 (C-Caspase3, #9664) was obtained from Cell Signaling Technology (Massachusetts, USA). Anti-NADPH quinone oxidoreductase-1 (NQO1, #A19586), anti-superoxide dismutase-2 (SOD2, #A1340), anti-B cell lymphoma 2 (BCL-2, #A20777), and anti-BCL-2-associated X protein (BAX, #A11931) were purchased from ABclonal Technology (Wuhan, China). Anti-α-Tubulin antibody and HRP conjugated Goat Anti-Rabbit IgG were obtained from Servicebio Technology (Wuhan, China).
2.2. Animals and treatment
In line with the specifications detailed in the document for ethical approval (Approval number: SY2023-004), all experimental procedures and protocols adhered to the established guidelines. These procedures were duly authorized by the Animal Ethics Committee of Wuhan Third People's Hospital. Wuhan Buzz Biological Technology Co., Ltd provided male C57BL/6 mice, which were aged eight weeks and weighed 20–25g. The mice were accommodated in a regulated environment with a temperature of 20–25 °C, humidity levels ranging from 45% to 55%, and a 12-h light-dark cycle. Unrestricted access to water and food were provided. Following a period of seven days for acclimation, the mice were randomly divided into three groups. (1) The control group received intragastric gavage of 50% PEG300 + 50% physiological saline once a day for 14 consecutive days along with a single intraperitoneal injection of physiological saline on day 8. (2) The DOX group received an intraperitoneal injection of 15 mg/kg DOX dissolved in physiological saline on the 8th day of the experiment to induce acute myocardial toxicity. (3) The DOX + NBP group was pretreated with 80 mg/kg of NBP dissolved in a solution of 50% PEG300 and 50% saline by gavage once a day for 7 days. On day 8, the mice in this group also received a single intraperitoneal injection of DOX at a dose of 15 mg/kg. The gavage of NBP continued for an additional 7 days after the DOX injection. The dosages of doxorubicin and NBP used in the experiment were based on previous literature reports, with appropriate adjustments [7,33]. The changes in body weight of the mice were recorded, and at the end of the investigation, their hearts and blood samples were collected for further analysis.
2.3. Echocardiography
Following anesthesia with 1.5% isoflurane inhalation, the mice's chest hair was removed using depilatory cream. The mice's cardiac function was assessed using a small animal ultrasound instrument (Visual Sonics Inc., Canada). Measurements were taken from a transverse axis section adjacent to the sternum to evaluate key cardiac parameters, such as left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), left ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume (LVESV). The researchers who performed the measurements were unaware of the experimental groups.
2.4. Cardiac injury analysis
Blood samples were collected from mice using the retro-orbital bleeding technique. These blood samples were centrifuged for 15 min using a 4° centrifuge to obtain serum, which was then stored in a −80° refrigerator for a short period of time. The serum levels of creatine kinase isoenzyme (CK-MB) and lactate dehydrogenase (LDH) were measured using an automated biochemical analyzer (Lei du, China) and specific assay kits. The lactate dehydrogenase assay kit (Leidu, S03034) and the creatine kinase isoenzyme assay kit (Changchun Huili, C060) were used. The serum's cardiac troponin I (cTnI) concentration was determined using an ELISA kit (Uersheng, SEA478Mu). All procedures were conducted following the protocols provided by the respective manufacturers.
2.5. Histological analysis
Following the echocardiographic assessment, the hearts of mice from various experimental groups were collected and preserved in 4% paraformaldehyde. The heart tissues were then embedded in paraffin and sliced into 4–5 μm sections. Hematoxylin and eosin (H&E) staining was used to evaluate the histological morphology.
2.6. Western blotting
The levels of Nrf2, HO-1, NQO1, SOD2, IL-6, IL-1β, TNF-α, P65, P–P65, Bax, Bcl-2, and cleaved caspase 3 expression in cardiac tissues and H9C2 cells were assessed by Western blotting. Briefly, mouse cardiac tissues or H9C2 cells were subjected to lysis using RIPA lysis buffer containing 1% phosphatase inhibitor and PMSF (Servicebio, G2008), while being kept on ice for 30 min. The lysates were then centrifuged and the protein quantification was performed using the BCA assay kit. Afterward, the denatured protein samples were separated by SDS-PAGE gel electrophoresis and transferred onto NC membranes. The membranes were blocked and incubated with primary antibodies overnight at 4 °C, followed by incubation with HRP-conjugated secondary antibodies. The bands were visualized and a semi-quantitative analysis was performed using ImageJ software. All protein expression levels were normalized to α-Tubulin.
2.7. Oxidative stress testing
To measure the levels of reactive oxygen species (ROS) in mouse cardiac tissues and H9C2 cells, we employed the DHE assay kits. The experiments were performed in accordance with the manufacturer's instructions and observed using a fluorescence microscope. The levels of SOD, MDA, and GSH in heart tissue homogenates and H9C2 cardiomyocyte supernatants were measured using a superoxide dismutase kit (Nanjing Jiancheng, A001-1), malondialdehyde assay kit (Servicebio, G4300) glutathione peroxidase assay kit (Nanjing Jiancheng, A005), respectively.
2.8. TUNEL testing
Apoptosis was detected using a TUNEL kit (Servicebio, G1501). Paraffin sections of mouse hearts and cell crawls of H9C2 were prepared following the previously described methods. TUNEL staining was carried out as per the manufacturer's protocol, and fluorescence images were captured using a fluorescence microscope. The acquired images were then analyzed for fluorescence intensity using ImageJ software.
2.9. Cell Culture and treatment
Cultures of rat cardiomyocytes H9C2(Procell Life Technologies Co., Ltd., Wuhan, China) were conducted at 37 °C in a 5% CO2 incubator. The medium used in the experiment was modified Eagle's medium (DMEM) with the addition of 10% fetal bovine serum (Gibco, USA) and 1% penicillin and streptomycin. When the confluent area of the cells reached approximately 80%, they were passaged at a 1:3 ratio. DOX and NBP were dissolved in ddH2O at concentrations of 1 mM and 10 mM, respectively. ML385, a classic Nrf2 selective inhibitor, was dissolved in DMSO to create a 10 mM stock solution. The experiment consisted of four groups: (1) the control group, (2) the DOX group, (3) the DOX + NBP group, and (4) the DOX + NBP + ML385 group. Each mL of culture medium contained 0.5 μL of DMSO as vehicle control in the control group, DOX group, and DOX + NBP group. In the DOX group, the H9C2 cells were treated with 1 μM DOX for 24 h. In the DOX + NBP group, the cells were pretreated with 20 μM NBP for 24 h, followed by changing the culture medium and treating it with a culture medium containing 1 μM DOX and 20 μM NBP for 24 h. Similarly, in the DOX + NBP + ML385 group, the cells were first pretreated with 20 μM NBP for 24 h, followed by co-incubation with 1 μM DOX, 20 μM NBP, and 5 μM ML385 for 24 h. The supernatant and cellular sediments were collected for subsequent assays.
2.10. CCK-8 assay
Inoculate 100 μL of suspension containing approximately 104 H9C2 cells per well in a 96 well plate. Culture the cells for 12 h until they fully adhere to the wall. Pretreat the cells with 0, 1, 10, 20, 50, and 100 μM NBP for 24 h. Then incubate the cells with 0, 1, 10, 20, 50, and 100 μM NBP along with 1 μM DOX for 24 h. After completing the experiment, 10 μL of CCK-8 reagent (Servicebio, G4103) was added to each well.Subsequently, the plate underwent incubation at 37 °C for a duration of 1.5 h to allow for reactions to occur. To determine cell viability within each group, an enzyme-linked immunosorbent assay reader (TECAN, Infinite M200 PRO, Switzerland) was employed to measure the absorbance values at a wavelength of 450 nm.
2.11. Statistical analysis
All datas were analyzed using GraphPad Prism software (San Diego, USA). Quantitative datas are presented as mean ± standard error. Comparisons among multiple groups were conducted using non-paired one-way analysis of variance (ANOVA) and post hoc t-tests. p < 0.05 was considered statistically significant.
3. Result
3.1. NBP protected against DOX-induced cardiac dysfunction and myocardial injury in mice
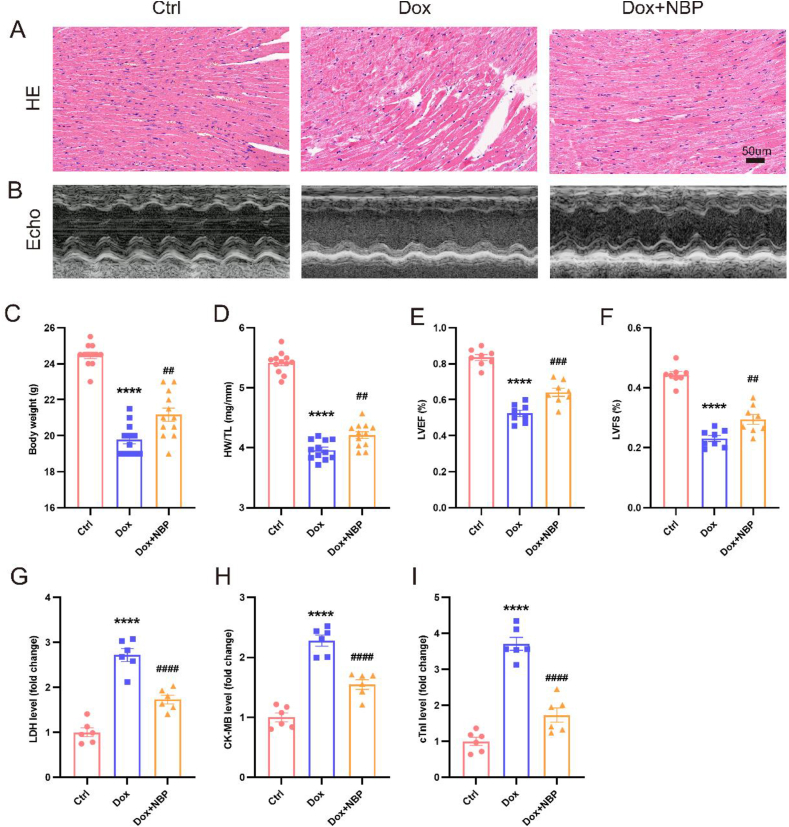
Our data demonstrated a noteworthy decline in body weight in mice after 7 days of exposure to DOX, which is consistent with other observations. However, NBP gavage mitigated this DOX-induced weight loss (Fig. 1C). Additionally, we observed that the DOX group's heart weight to tibia length ratio was much lower than that of the control group. Nevertheless, NBP administration attenuated the extent of this reduction (Fig. 1D). To evaluate the impact of NBP on DOX-induced cardiac dysfunction, we assessed cardiac function using transthoracic echocardiography. Our results revealed that the left ventricular ejection fraction in mice treated with DOX for seven days was significantly lower (52.383 ± 5.080%) compared to the control group (83.426 ± 4.689%). However, NBP treatment ameliorated this decrease by increasing the ejection fraction to 64.114 ± 6.292% (Fig. 1B, E). Moreover, the DOX group exhibited a lower short-axis shortening rate than the control group, indicating impaired cardiac function. Notably, NBP pretreatment attenuated this DOX-induced decrease in the shortening rate (Fig. 1F). These findings suggest that NBP can potentially improve DOX-induced cardiac dysfunction in mice. HE staining revealed standard myocardial structure in the control group, whereas the DOX group displayed myocardial fiber lysis, breakage, and vacuolar degeneration. However, these effects were improved in the NBP group (Fig. 1A). Considering that lactate dehydrogenase (LDH), cardiac troponinase (cTnI), and creatine kinase isoenzyme (CK-MB) are commonly used as diagnostic markers for myocardial injury, we examined the levels of LDH, cTnI, and CK-MB in the serum of mice [21]. The levels of cTnI, CK-MB, and LDH were discovered to be elevated in the DOX group when compared to the control group. However, NBP treatment reversed the DOX-induced elevation of these markers (Fig. 1G–I). These findings indicate that NBP treatment has a beneficial effect in ameliorating DOX-induced myocardial injury.
Fig. 1.
NBP improves DOX-induced cardiac dysfunction and myocardial injury (A) Representative histological images of the HE staining in three groups. (B) Representative echocardiogram images in three groups. (C) Body weight in the indicated groups 7 days after DOX injection (n = 12). (D) The ratio of heart weight to tibia length (n = 12). (E) LVEF in the indicated groups (n = 8). (F) LVFS in the indicated groups (n = 8). (G–I) The LDH, CK-MB, and cTnI levels among groups (n = 6). Values represent mean ± SEM. ****p < 0.0001 vs. Ctrl group; ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs. DOX group.
3.2. NBP attenuates DOX-induced oxidative stress in mouse cardiac tissues
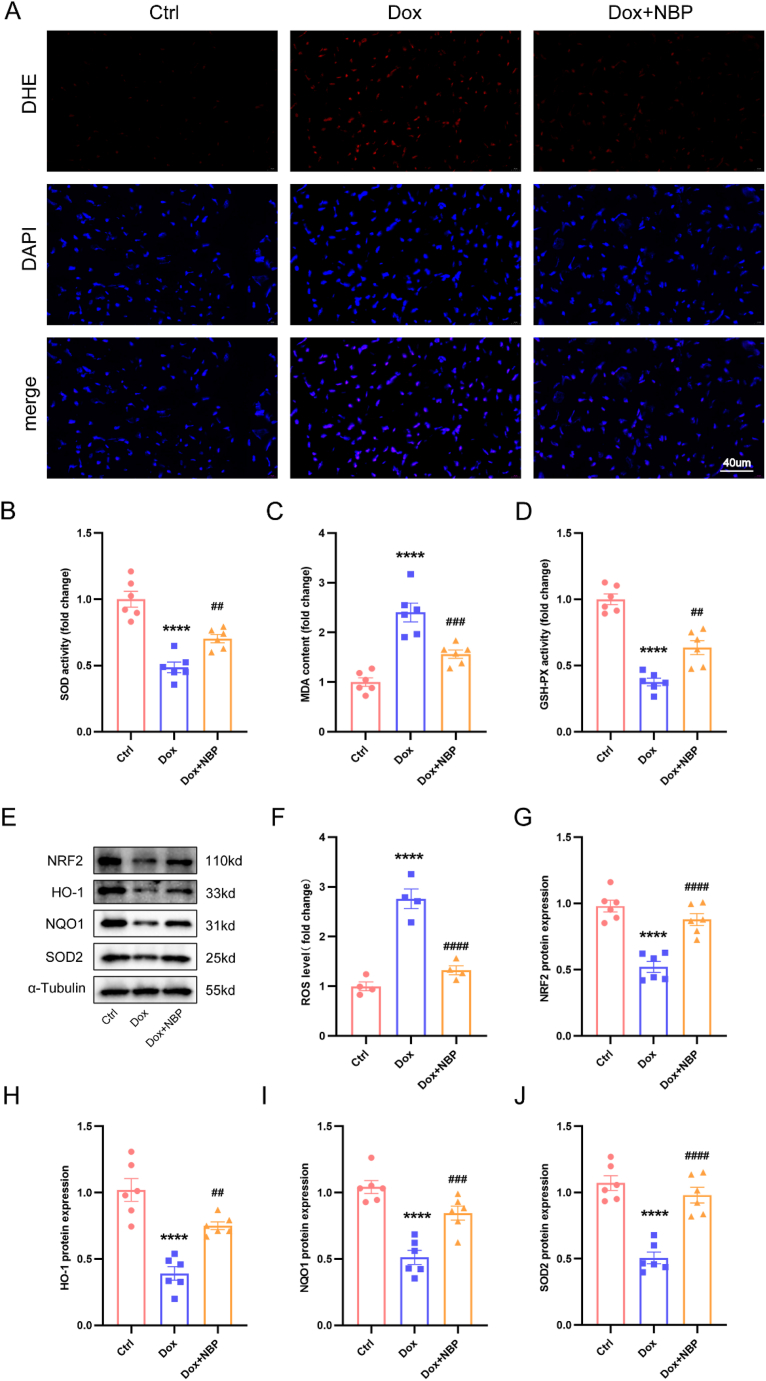
We investigated the levels of ROS in mouse myocardial tissues using DHE staining. Our findings revealed that the control group had low ROS production. However, the DOX group showed a substantial increase in ROS production, which was significantly reduced after treatment with NBP (Fig. 2A and F). Additionally, we examined the levels of SOD, MDA, and GSH-PX in cardiac tissues. The results demonstrated that the DOX group had lower levels of SOD and GSH-PX and higher levels of MDA than the control group. Treatment with NBP effectively improved these DOX-induced changes in these metrics (Fig. 2B–D). Nrf2, an essential transcription factor involved in the regulation of oxidative stress, plays a crucial role in regulating the expression levels of downstream antioxidant enzymes. Western blotting results indicated that the expression of Nrf2, HO-1, NQO1, and SOD2 was significantly lower in the DOX group compared to the control group. Conversely, NBP treatment for 14 days increased the expression levels of these proteins (Fig. 2E, G-J). Based on our findings, it can be inferred that NBP has the potential to mitigate the oxidative stress harm caused by DOX in an in vivo setting.
Fig. 2.
NBP inhibited DOX-induced oxidative damage in mice (A) Representative DHE staining images in three groups (n = 4). (B–D) The level of SOD, MDA, and GSH-PX among groups. (E) Representative Western blots of Nrf2, HO-1, NQO1, and SOD2 protein levels in three groups (n = 6). (F) Quantitative analysis of fluorescence intensity of DHE staining. (G–J) Statistical analysis of Nrf2, HO-1, NQO1, and SOD2 protein levels in three groups (n = 6). ****p < 0.0001 vs. Ctrl group; ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs. DOX group.
3.3. NBP attenuated DOX-induced inflammation and apoptosis in mouse heart tissues
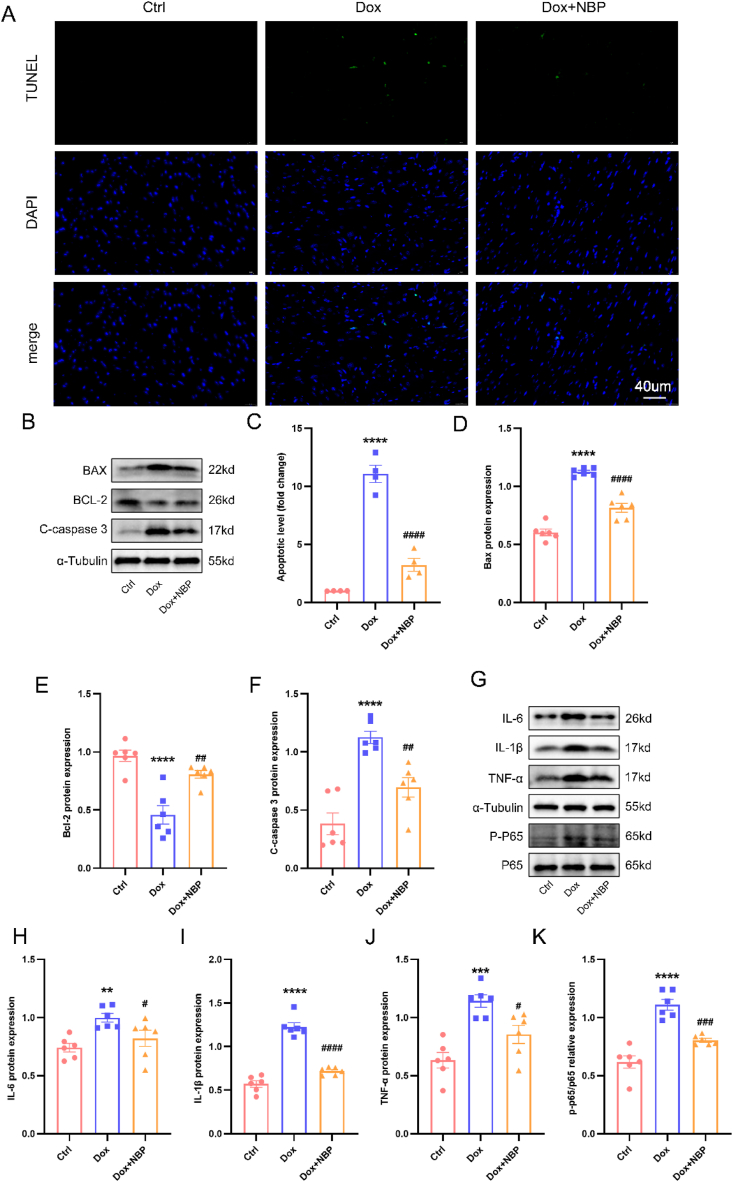
TUNEL staining revealed a deficiency in green fluorescence manifestation in the control group, while a noteworthy upsurge in green fluorescence manifestation was observed in the DOX group, indicating an elevation in DOX-induced apoptosis. Conversely, the NBP-treated group exhibited a notable decrease in apoptosis levels (Fig. 3A–C). Furthermore, our findings demonstrated elevated levels of BAX and Cleaved-caspase3 proteins in the DOX group when compared to the control group. On the contrary, the expression of BCL-2 was lower in the DOX group compared to the control group. Notably, NBP treatment mitigated the DOX-induced elevation of BAX and Cleaved-caspase3 and partially restored the DOX-induced decrease in BCL-2 expression (Fig. 3B,D-F). Additionally, we examined the impact of DOX on inflammatory markers. Western blot analysis revealed that DOX led to increased protein expression of TNF-α, IL-1β, and IL-6, whereas NBP interventions attenuated the DOX-induced elevation of inflammatory molecules. Moreover, DOX was found to induce phosphorylation of NF-κB, a transcription factor that translocates to the nucleus to regulate downstream inflammatory factor expression [34]. Notably, NBP treatment reduced the phosphorylation level of NF-κB P65 (Fig. 3G–K). Overall, these findings appear to indicate that NBP can ameliorate inflammation and apoptosis levels in DOX-induced mice in vivo.
Fig. 3.
NBP attenuated cardiac apoptosis and inflammation in DOX-treated mice (A) Representative TUNEL staining images in heart tissues. (B) The indicated groups showed representative Western blots of BAX, BCL-2, and Cleaved-caspase3. (C) The quantitative results of TUNEL staining (n = 4). (E–F) Statistical results of BAX, BCL-2, and Cleaved-caspase3 protein levels in the indicated groups (n = 6). (G) In three groups, representative Western blots of IL-6, IL-1β, TNF-α, and P–P65 protein levels. (H–J) Statistical analysis of IL-6, IL-1β, TNF-α, and P–P65 protein levels in heart tissues (n = 6). **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. Ctrl group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs. DOX group.
3.4. NBP ameliorates DOX-induced oxidative stress in cardiomyocytes through the Nrf2/HO-1 signaling pathway in vitro
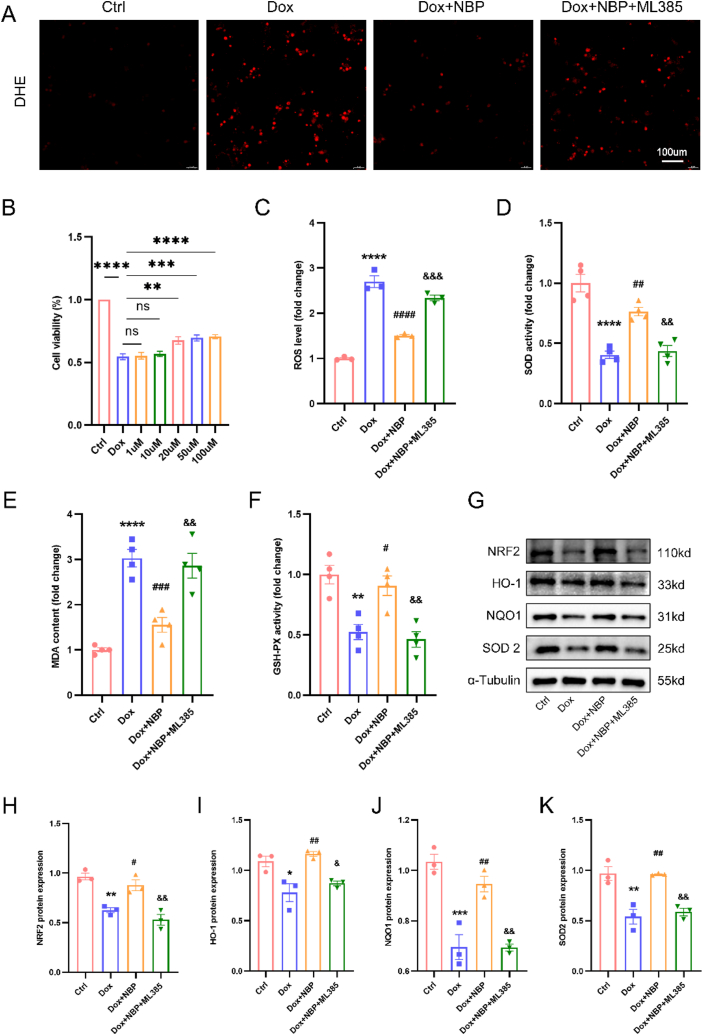
To further investigate the protective effect of NBP against DOX-induced cardiomyocytes in vitro, we exposed H9C2 cells to 1μM DOX for 24 h to simulate DOX-induced myocardial injury in vivo. Using the CCK8 assay to assess cell viability, we observed a significant reduction in cell viability after 24 h of DOX treatment. Subsequently, we examined the impact of different concentrations of NBP (1 μM, 10 μM, 20 μM, 50 μM, and 100 μM) on the viability of DOX-exposed H9C2 cells. Our findings indicated that the effects of 1 μM and 10 μM NBP on cell viability were not statistically different from the DOX group. However, the interventions with 20 μM, 50 μM, and 100 μM NBP increased cell viability, although these three concentrations did not differ statistically significantly from one another (Fig. 4B). Based on the previous study and our CCK8 results, we chose a concentration of 20 μM for the ensuing experiments [29]. In our vivo experiments, we made a revelation that myocardial injury induced by DOX is closely linked to the signaling pathway of Nrf2/HO-1. In order to delve deeper into understanding the protective impact of NBP against DOX-induced myocardial injury, we employed ML385, a specific Nrf2 blocker, to validate the involvement of Nrf2/HO-1 in the safeguarding effect of NBP on cardiomyocytes. Corresponding to our vivo experiments, we observed that 20 μM of NBP could attenuate DOX-induced ROS production in H9C2 cells. However, the inhibitory effect of NBP on ROS production disappeared after the Nrf2 blockade (Fig. 4A–C). Furthermore, the changes in SOD, MDA, and GSH-PX levels in the cell supernatants demonstrated that ML385 attenuated the protective effect of NBP against oxidative stress in vitro (Fig. 4D–F). Additionally, Western blotting experiments confirmed that ML385 administration decreased the protein expression of Nrf2 and its downstream targets HO-1, NQO1, and SOD2 compared to the DOX + NBP group (Fig. 4G–K). In conclusion, our results suggest that NBP attenuated DOX-induced oxidative stress in vitro, while this protective effect was abolished after blocking Nrf2.
Fig. 4.
NBP ameliorates DOX-induced oxidative stress in cardiomyocytes through the Nrf2/HO-1 signaling pathway in vitro (A) Representative TUNEL staining images in H9C2 cells. (B) Cell viability after DOX treatment (n = 5). (C) Quantitative analysis of DHE staining in H9C2 cells. (D–F) SOD, MDA, and GSH-PX levels in H9C2 cell supernatants. (G–K) Representative Western blots and statistical analysis of Nrf2, HO-1, NQO1, and SOD2 protein levels in four groups (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. Ctrl group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs. DOX group; &p < 0.05, &&p < 0.01, &&&p < 0.0001 vs. DOX + NBP group.
3.5. NBP ameliorates DOX-induced apoptosis and inflammation in cardiomyocytes through the Nrf2/HO-1 signaling pathway in vitro
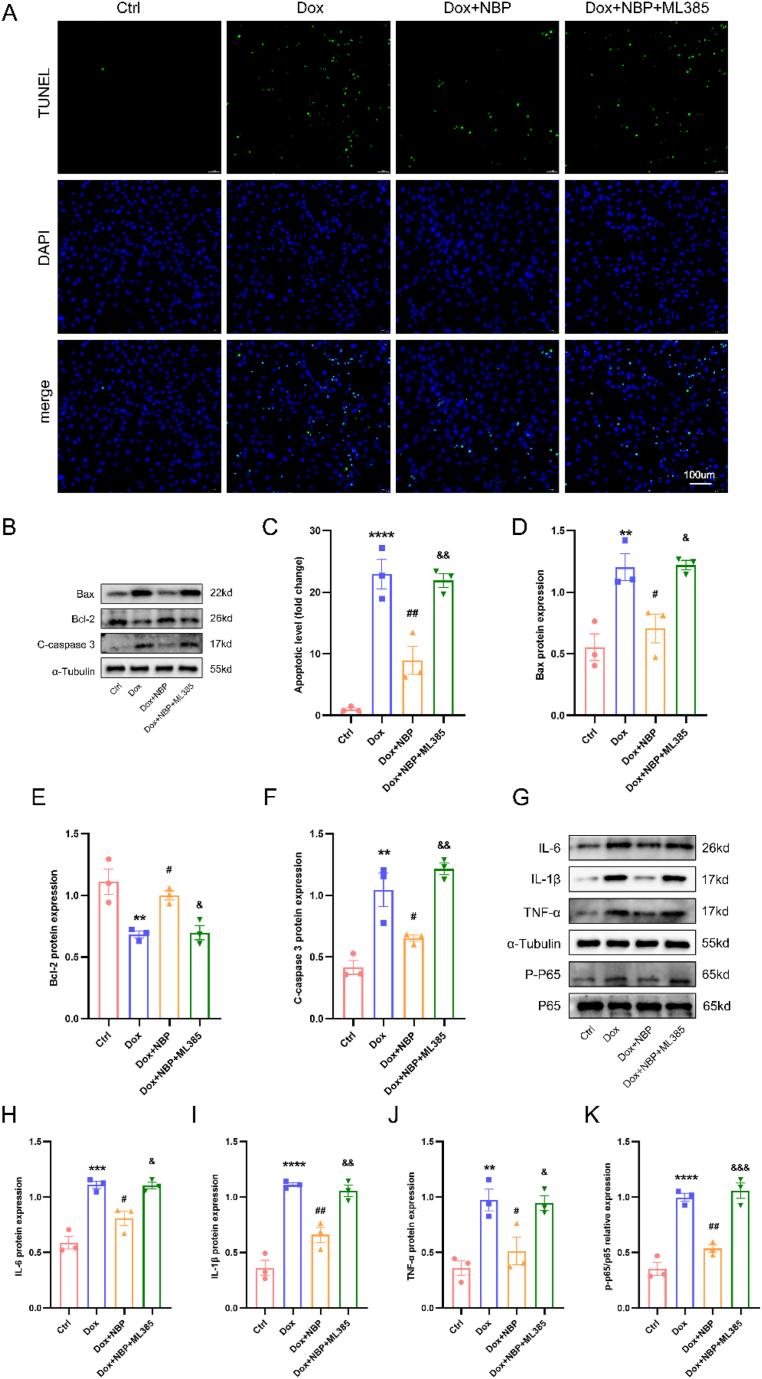
Apoptosis and inflammation are important factors in DOX-induced myocardial injury. TUNEL staining revealed that NBP reduced apoptosis in DOX-treated H9C2 cells, but ML385 hindered the protective effect of NBP (Fig. 5A–C). The Western blotting outcomes also presented evidence that NBP partially reinstated the decreased quantities of BCL-2 and raised levels of cleaved-caspase 3 and BAX initiated by DOX in cardiomyocytes, while ML385 blocked this effect (Fig. 5B–D-F). Furthermore, in line with the anti-inflammatory properties of NBP observed in vivo, protein immunoblotting revealed that NBP decreased DOX-induced phosphorylation of NF-κB P65 and expression of IL-1β, TNF-α, and IL-6 in H9C2 cells. Once again, ML385 administration reversed these changes induced by NBP (Fig. 5G–K). These findings suggest that NBP alleviates DOX-induced apoptosis and inflammation in H9C2 cells by potentially upregulating the Nrf2/HO-1 signaling pathway.
Fig. 5.
NBP ameliorates DOX-induced apoptosis and inflammation in cardiomyocytes through the Nrf2/HO-1 signaling pathway in vitro. (A) Representative TUNEL staining images in H9C2 cells (n = 3). (B) Representative Western blots of BAX, BCL-2, and Cleaved-caspase3 protein levels in four groups. (C) Quantitative analysis of TUNEL staining in H9C2 cells. (D–F) Statistical analysis of BAX, BCL-2, and Cleaved-caspase3 proteins levels (n = 3). (G–K) Representative Western blots and statistical analysis of IL-6, IL-1β, TNF-α, and P–P65 protein levels in four groups (n = 3). **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. Ctrl group; #p < 0.05, ##p < 0.01 vs. DOX group; &p < 0.05, &&p < 0.01, &&&p < 0.0001 vs. DOX + NBP group.
4. Discussion
In this study, we examined the impact of NBP on DOX-induced myocardial toxicity. Our findings indicated that NBP administration effectively mitigated cardiac dysfunction and myocardial injury caused by DOX exposure. Furthermore, NBP significantly reduced oxidative stress, apoptosis, and inflammation induced by DOX both in vivo and in vitro. We also discovered that NBP increased the expression of Nrf2/HO-1 while inhibiting the activation of NF-κB P65. Importantly, when Nrf2 was blocked, the protective function of NBP against DOX-triggered toxicity in cardiomyocytes in vitro was abolished. In summary, our results demonstrate that NBP protects against DOX-triggered damage by upregulating the Nrf2/HO-1 signaling pathway.
DOX has been a cornerstone in treating various cancers since its discovery in the 1970s. However, DOX therapy is known to cause several organ toxicities, including hepatotoxicity, nephrotoxicity, neurotoxicity, gonadotoxicity, and cardiotoxicity [[35], [36], [37], [38]]. With the increasing use of DOX chemotherapy, cardiovascular toxicity has emerged as a significant cause of premature death in cancer survivors [39]. Many scientists have been working tirelessly to find drugs capable of treating DOX cardiotoxicity, such as resveratrol and melatonin [40,41]. However, additional effort is required prior to their practical implementation in clinics, highlighting the need for further exploration of drugs for DOX cardiotoxicity treatment. NBP, primarily used in the clinic for the treatment of ischemic stroke and cognitive dysfunction, has shown a wide range of bioactivities and a high degree of safety [42,43]. Over the past few years, scientists have discovered that NBP also has a protective effect on cardiac ischemia-reperfusion injury, atherosclerosis, and arrhythmia, which has attracted our attention [[29], [30], [31]]. In the present study, we observed that NBP administration ameliorated the DOX-induced reduction in ejection fraction and the elevation of myocardial injury markers cTnI, CK-MB, and MDA. Furthermore, NBP attenuated DOX-induced ROS production and subsequent apoptosis both in vivo and in vitro, and it also reduced the downstream expression of inflammation factor by decreasing the phosphorylation of NF-κB P65. In conclusion, our data suggest that NBP has a protective effect against DOX-induced cardiomyocyte injury.
The significance of DOX-induced cardiotoxicity has been highlighted by an increasing amount of research, which emphasizes the role of oxidative stress [12]. Multiple studies have shown that diminishing the generation of ROS can mitigate DOX-induced damage to the heart [44,45]. Nrf2, an essential antioxidant transcription factor encoded by the NFE2L gene, plays a crucial role in this process [46]. In its normal state, Nrf2 forms a complex with the Kelch-like ECH-related protein (Keap1) through the double glycine repeat sequence (DGR) and remains in the cytoplasm. However, when the cell experiences oxidative stress, Nrf2 disengages from Keap1 and translocates into the nucleus. Once within the nucleus, Nrf2 attaches to antioxidant response elements (AREs), leading to the activation of downstream antioxidant enzymes such as HO-1, NQO1, and glutathione S-transferase (GST). These enzymes enhance the defense against oxidative stress [47]. Our study found that NBP significantly increased the expression of Nrf2 and its downstream target genes, HO-1 and NQO1, in cardiomyocytes treated with DOX. This increase in Nrf2 activity resulted in reduced ROS generation and elevated levels of SOD, GSH-PX, and SOD in the serum. These findings align with previous research demonstrating that NBP can mitigate diabetes-associated cognitive decline and pathological changes in Alzheimer's disease by activating the Nrf2-mediated antioxidant response system [24,28]. To further investigate the role of the Nrf2/HO-1 signaling pathway in NBP's effect on DOX-induced myocardial injury, we used the Nrf2 blocker ML385. Our results showed that ML385 nullified the beneficial effects of NBP on cardiomyocytes in vitro and attenuated the NBP-induced upregulation of the Nrf2/HO-1 signaling pathway. Therefore, our findings suggest that NBP ameliorates DOX-induced myocardial injury and reduces oxidative stress through the Nrf2/HO-1 signaling pathway.
Apoptosis is one of the essential pathogenic events in myocardial toxicity of DOX, and reduction of apoptosis levels in the heart has been recommended as a protective measure against DOX-induced myocardial injury. DOX leads to decreased mitochondrial membrane potential due to excessive production of ROS and lipid peroxidation, resulting in the increased permeability of the mitochondrial membrane. This, in turn, triggers the release of cytochrome C from the mitochondria into the cytoplasm, activating a cascade reaction of cysteine asparaginase and ultimately leading to apoptosis [48]. In order to thoroughly investigate the impact of NBP on DOX-induced myocardial toxicity, we also examined changes in apoptosis levels. Our findings revealed that DOX significantly increased the expression of BAX in cardiac tissues while decreasing the level of BCL-2, which aligns with the findings of Pan et al. [49]. Interestingly, NBP treatment altered the DOX-induced changes in BCL-2 and BAX, suggesting that NBP has the ability to counteract DOX-induced apoptosis in cardiomyocytes. This hypothesis was further supported by the analysis of cleaved-caspase3 expression and the quantification of TUNEL-stained positive cells. Our results are also consistent with the study conducted by Xu et al. in which NBP attenuated podocyte apoptosis and delayed the progression of diabetic nephropathy in mouse kidneys by reducing endoplasmic reticulum stress and inhibiting the activation of the renin-angiotensin system [50]. Moreover, the in vitro experiments conducted in this study further demonstrated that NBP prevented DOX-induced apoptosis, and blocking Nrf2 abolished the protective effect of NBP against apoptosis in H9C2 cells. Therefore, our study suggests that the inhibitory effect of NBP on DOX-induced apoptosis is mediated through the activation of Nrf2.
In addition to oxidative stress and apoptosis, DOX-induced inflammation has been well-reported in DOX-induced cardiotoxicity. The overexpression of inflammatory factors is closely associated with DOX-induced myocardial toxicity, and inhibiting the expression of these factors can partially alleviate DOX-induced myocardial toxicity [51]. Several natural compounds, including dihydrotanshinone I, cardamonin, and nerolidol, have been suggested as potential therapeutics to inhibit NF-κB activation and mitigate DOX cardiotoxicity [[52], [53], [54]]. The team of Wang et al. has reported that dihydrotanshinone I inhibited NF-κB activation by targeting the TFEB-IKK–NF–κB signaling axis, thereby inhibiting M1 macrophage polarisation and excessive release of pro-inflammatory factors, achieving improved cardiac function in zebrafish and mice [52]. In our study, we observed a significant increase in the phosphorylation level of NF-κB P65 in the DOX group. However, NBP treatment reduced the phosphorylation of NF-κB P65 and the expression of downstream inflammatory factors IL-1β, TNF-α, and IL-6. These findings are consistent with previous reports that NBP attenuates inflammation in other injury models, such as renal ischemia-reperfusion injury and LPS-induced lung injury, by down-regulating IL-6, TNF-α, and IL-1β. This suggests that NBP has excellent potential for anti-inflammatory effects [55,56]. In our next experiment, we observed that the intervention of ML385 compromised the anti-inflammatory effect of NBP against DOX, indicating that the protective effect of NBP against DOX-induced myocardial injury is at least partially mediated by the inhibition of NF-κB activation and downstream inflammatory factor expression through the activation of Nrf2.
5. Conclusion
To summarize, our investigation demonstrated that the administration of NBP displays a safeguarding impact against myocardial injury caused by DOX. This protective mechanism is achieved through the activation of the Nrf2/HO-1 signaling pathway. Furthermore, NBP has been observed to augment the expression of Nrf2 and its subsequent target genes, effectively mitigating oxidative stress and inhibiting apoptosis. Notably, NBP also exhibits a capacity to reduce the phosphorylation level of NF-κB P65 and the expression of inflammatory mediators, thereby attenuating inflammation. These significant findings indicate the potential suitability of using NBP as a therapeutic approach for managing DOX-induced myocardial injury.
Funding
This work was supported by grants from the Technical Innovation Project of Hu Bei Province of China (Grant No. 2016ACA153) and the Fundamental Research Funds for the Central Universities (Grant No. 2042021kf0112).
Availability of data and materials
Data will be made available on request.
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Wuhan Third People's Hospital (SY2023-004). All methods were performed in accordance with the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health. Informed consent was not required for this study because it does not involve human subjects.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Dengke Li: Writing – original draft, Investigation, Data curation, Conceptualization. Wei Zhang: Software, Methodology, Formal analysis. Hui Fu: Validation, Investigation, Data curation. Xi Wang: Visualization, Supervision, Conceptualization. Yanhong Tang: Writing – review & editing, Supervision, Project administration, Conceptualization. Congxin Huang: Writing – review & editing, Resources, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27644.
Contributor Information
Dengke Li, Email: dengkeldk@163.com.
Congxin Huang, Email: huangcongxin@vip.163.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Kciuk M., Gielecinska A., Mujwar S., Kolat D., Kaluzinska-Kolat Z., Celik I., et al. Doxorubicin-an agent with multiple mechanisms of Anticancer activity. Cells. 2023;12(4) doi: 10.3390/cells12040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjanuwattra J., Siri-Angkul N., Chattipakorn S.C., Chattipakorn N. Doxorubicin and its proarrhythmic effects: a comprehensive review of the evidence from experimental and clinical studies. Pharmacol. Res. 2020;151 doi: 10.1016/j.phrs.2019.104542. [DOI] [PubMed] [Google Scholar]
- 3.Narayan H.K., Finkelman B., French B., Plappert T., Hyman D., Smith A.M., et al. Detailed echocardiographic Phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 Years of follow-up. Circulation. 2017;135(15):1397–1412. doi: 10.1161/CIRCULATIONAHA.116.023463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henriksen P.A. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104(12):971–977. doi: 10.1136/heartjnl-2017-312103. [DOI] [PubMed] [Google Scholar]
- 5.Cvetkovic R.S., Scott L.J. Dexrazoxane : a review of its use for cardioprotection during anthracycline chemotherapy. Drugs. 2005;65(7):1005–1024. doi: 10.2165/00003495-200565070-00008. [DOI] [PubMed] [Google Scholar]
- 6.De Baat E.C., Van Dalen E.C., Mulder R.L., Hudson M.M., Ehrhardt M.J., Engels F.K., et al. Primary cardioprotection with dexrazoxane in patients with childhood cancer who are expected to receive anthracyclines: recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Child Adolesc Health. 2022;6(12):885–894. doi: 10.1016/S2352-4642(22)00239-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Hu C., Kong C.Y., Song P., Wu H.M., Xu S.C., et al. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020;27(2):540–555. doi: 10.1038/s41418-019-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D.L., Wang Z.V., Ding G., Tan W., Luo X., Criollo A., et al. Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation. 2016;133(17):1668–1687. doi: 10.1161/CIRCULATIONAHA.115.017443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang G., Li X., Yang F., Huang T., Qiu C., Peng K., et al. Amentoflavone mitigates doxorubicin-induced cardiotoxicity by suppressing cardiomyocyte pyroptosis and inflammation through inhibition of the STING/NLRP3 signalling pathway. Phytomedicine. 2023;117 doi: 10.1016/j.phymed.2023.154922. [DOI] [PubMed] [Google Scholar]
- 10.Yarmohammadi F., Rezaee R., Haye A.W., Karimi G. Endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity may be therapeutically targeted by natural and chemical compounds: a review. Pharmacol. Res. 2021;164 doi: 10.1016/j.phrs.2020.105383. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Yan S., Liu X., Deng F., Wang P., Yang L., et al. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ. 2022;29(10):1982–1995. doi: 10.1038/s41418-022-00990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong C.Y., Guo Z., Song P., Zhang X., Yuan Y.P., Teng T., et al. Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. Int. J. Biol. Sci. 2022;18(2):760–770. doi: 10.7150/ijbs.65258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Q. Ma, Role of nrf2 in oxidative stress and toxicity, Annu Rev Pharmacol Toxicol. 532013) 401-426. [DOI] [PMC free article] [PubMed]
- 14.Yao H., He Q., Huang C., Wei S., Gong Y., Li X., et al. Panaxatriol saponin ameliorates myocardial infarction-induced cardiac fibrosis by targeting Keap1/Nrf2 to regulate oxidative stress and inhibit cardiac-fibroblast activation and proliferation. Free Radic. Biol. Med. 2022;190:264–275. doi: 10.1016/j.freeradbiomed.2022.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Chen X., Wan W., Guo Y., Ye T., Fo Y., Sun Y., et al. Pinocembrin ameliorates post-infarct heart failure through activation of Nrf2/HO-1 signaling pathway. Mol Med. 2021;27(1):100. doi: 10.1186/s10020-021-00363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan Y., Wan H.H., Sun M.M., Zhang W.J., Dong M., Ge W., et al. Cardamonin protects against lipopolysaccharide-induced myocardial contractile dysfunction in mice through Nrf2-regulated mechanism. Acta Pharmacol. Sin. 2021;42(3):404–413. doi: 10.1038/s41401-020-0397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Huang J., Lv Y., Chen Y., Rao J. Crocin exhibits an antihypertensive effect in a rat model of gestational hypertension and activates the Nrf-2/HO-1 signaling pathway. Hypertens. Res. 2021;44(6):642–650. doi: 10.1038/s41440-020-00609-7. [DOI] [PubMed] [Google Scholar]
- 18.Yu J., Li W., Xiao X., Huang Q., Yu J., Yang Y., et al. (-)-Epicatechin gallate blocks the development of atherosclerosis by regulating oxidative stress in vivo and in vitro. Food Funct. 2021;12(18):8715–8727. doi: 10.1039/d1fo00846c. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Luo W., Qian Y., Zhu W., Qian J., Li J., et al. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine. 2019;59 doi: 10.1016/j.phymed.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Q., Chen X., Tian X., Zhang J., Xue S., Jiang Y., et al. Tanshinone I inhibits doxorubicin-induced cardiotoxicity by regulating Nrf2 signaling pathway. Phytomedicine. 2022;106 doi: 10.1016/j.phymed.2022.154439. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y., Wu X., Nie X., Wu Y., Zhang C., Lee S.M., et al. Natural compound glycyrrhetinic acid protects against doxorubicin-induced cardiotoxicity by activating the Nrf2/HO-1 signaling pathway. Phytomedicine. 2022;106 doi: 10.1016/j.phymed.2022.154407. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y.G., Li Y., Wang C.Y., Ai J.W., Dong X.Y., Huang H.Y., et al. L-3-n-Butylphthalide protects rats' cardiomyocytes from ischaemia/reperfusion-induced apoptosis by affecting the mitochondrial apoptosis pathway. Acta Physiol. 2014;210(3):524–533. doi: 10.1111/apha.12186. [DOI] [PubMed] [Google Scholar]
- 23.Yang C.S., Guo A., Li Y., Shi K., Shi F.D., Li M. Dl-3-n-butylphthalide reduces neurovascular inflammation and ischemic brain injury in mice. Aging Dis. 2019;10(5):964–976. doi: 10.14336/AD.2019.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B.N., Wu C.B., Chen Z.M., Zheng P.P., Liu Y.Q., Xiong J., et al. DL-3-n-butylphthalide ameliorates diabetes-associated cognitive decline by enhancing PI3K/Akt signaling and suppressing oxidative stress. Acta Pharmacol. Sin. 2021;42(3):347–360. doi: 10.1038/s41401-020-00583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng B., Zhou Y., Zhang H., Yang G., Hong Z., Han D., et al. Dl-3-n-butylphthalide prevents the disruption of blood-spinal cord barrier via inhibiting endoplasmic reticulum stress following spinal cord injury. Int. J. Biol. Sci. 2017;13(12):1520–1531. doi: 10.7150/ijbs.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang A.L., Liu X.Y., Gao N., Hu T.P., Yan S.T., Zhang G.Q. Dl-3-n-butylphthalide improves intestinal microcirculation disorders in septic rats by regulating the PI3K/AKT signaling pathway and autophagy. Int Immunopharmacol. 2023;118 doi: 10.1016/j.intimp.2023.110049. [DOI] [PubMed] [Google Scholar]
- 27.Chen X.Q., Qiu K., Liu H., He Q., Bai J.H., Lu W. Application and prospects of butylphthalide for the treatment of neurologic diseases. Chin Med J (Engl). 2019;132(12):1467–1477. doi: 10.1097/CM9.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C.Y., Xu Y., Wang X., Guo C., Wang T., Wang Z.Y. Dl-3-n-Butylphthalide inhibits NLRP3 inflammasome and mitigates alzheimer's-like pathology via nrf2-TXNIP-TrX Axis. Antioxid Redox Signal. 2019;30(11):1411–1431. doi: 10.1089/ars.2017.7440. [DOI] [PubMed] [Google Scholar]
- 29.Tian X., He W., Yang R., Liu Y. Dl-3-n-butylphthalide protects the heart against ischemic injury and H9c2 cardiomyoblasts against oxidative stress: involvement of mitochondrial function and biogenesis. J. Biomed. Sci. 2017;24(1):38. doi: 10.1186/s12929-017-0345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J., Shi X., Xu J., Lin W., Chen Y., Han B., et al. DL-3-n-butylphthalide prevents oxidative stress and atherosclerosis by targeting Keap-1 and inhibiting Keap-1/Nrf-2 interaction. Eur J Pharm Sci. 2022;172 doi: 10.1016/j.ejps.2022.106164. [DOI] [PubMed] [Google Scholar]
- 31.Qiu H., Ma J., Wu H., Ding C. DL-3-n-butylphthalide improves ventricular function, and prevents ventricular remodeling and arrhythmias in post-MI rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018;391(6):627–637. doi: 10.1007/s00210-018-1490-8. [DOI] [PubMed] [Google Scholar]
- 32.Han B., Xu J., Shi X., Zheng Z., Shi F., Jiang F., et al. DL-3-n-Butylphthalide attenuates myocardial hypertrophy by targeting gasdermin D and inhibiting gasdermin D mediated inflammation. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.688140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou P.T., Wang L.P., Qu M.J., Shen H., Zheng H.R., Deng L.D., et al. Dl-3-N-butylphthalide promotes angiogenesis and upregulates sonic hedgehog expression after cerebral ischemia in rats. CNS Neurosci. Ther. 2019;25(6):748–758. doi: 10.1111/cns.13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alanazi A.M., Fadda L., Alhusaini A., Ahmad R., Hasan I.H., Mahmoud A.M. Liposomal resveratrol and/or carvedilol attenuate doxorubicin-induced cardiotoxicity by modulating inflammation, oxidative stress and S100A1 in rats. Antioxidants. 2020;9(2) doi: 10.3390/antiox9020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa Godinho L.R.L., Cella P.S., Guimaraes T.a.S., Palma G.H.D., Nunes J.H.C., Deminice R. Creatine supplementation potentiates exercise protective effects against doxorubicin-induced hepatotoxicity in mice. Antioxidants. 2023;12(4) doi: 10.3390/antiox12040823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takenaka T., Inoue T., Miyazaki T., Kobori H., Nishiyama A., Ishii N., et al. Klotho suppresses the renin-angiotensin system in adriamycin nephropathy. Nephrol. Dial. Transplant. 2017;32(5):791–800. doi: 10.1093/ndt/gfw340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaminska K., Cudnoch-Jedrzejewska A. A review on the neurotoxic effects of doxorubicin. Neurotox. Res. 2023;41(5):383–397. doi: 10.1007/s12640-023-00652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawicki K.T., Sala V., Prever L., Hirsch E., Ardehali H., Ghigo A. Preventing and treating anthracycline cardiotoxicity: new insights. Annu. Rev. Pharmacol. Toxicol. 2021;61:309–332. doi: 10.1146/annurev-pharmtox-030620-104842. [DOI] [PubMed] [Google Scholar]
- 39.Zamorano J.L., Lancellotti P., Rodriguez Munoz D., Aboyans V., Asteggiano R., Galderisi M., et al. ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur. J. Heart Fail. 2016;19(1):9–42. doi: 10.1002/ejhf.654. 2017. [DOI] [PubMed] [Google Scholar]
- 40.Gu J., Hu W., Zhang D.D. Resveratrol, a polyphenol phytoalexin, protects against doxorubicin-induced cardiotoxicity. J. Cell Mol. Med. 2015;19(10):2324–2328. doi: 10.1111/jcmm.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Attachaipanich T., Chattipakorn S.C., Chattipakorn N. Potential roles of melatonin in doxorubicin-induced cardiotoxicity: from cellular mechanisms to clinical application. Pharmaceutics. 2023;15(3) doi: 10.3390/pharmaceutics15030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S., Li F., Yang J., Xie D., Yue C., Luo W., et al. Efficacy and safety of 3-n-butylphthalide combined with endovascular treatment in acute ischemic stroke due to large vessel occlusion. CNS Neurosci. Ther. 2022;28(12):2298–2307. doi: 10.1111/cns.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q., Han C., Xia Y., Wan F., Yin S., Li Y., et al. Efficacy and safety of 3-n-butylphthalide for the treatment of cognitive impairment: a systematic review and meta-analysis. CNS Neurosci. Ther. 2022;28(11):1706–1717. doi: 10.1111/cns.13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L., Tao X., Qi Y., Xu L., Yin L., Peng J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 2018;16:189–198. doi: 10.1016/j.redox.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manna P., Dewanjee S., Joardar S., Chakraborty P., Bhattacharya H., Bhanja S., et al. Carnosic acid attenuates doxorubicin-induced cardiotoxicity by decreasing oxidative stress and its concomitant pathological consequences. Food Chem. Toxicol. 2022;166 doi: 10.1016/j.fct.2022.113205. [DOI] [PubMed] [Google Scholar]
- 46.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91(21):9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X., Yu Y., Lei H., Cai Y., Shen J., Zhu P., et al. The nrf-2/HO-1 signaling Axis: a ray of hope in cardiovascular diseases. Cardiol. Res. Pract. 2020 doi: 10.1155/2020/5695723. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christidi E., Brunham L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021;12(4):339. doi: 10.1038/s41419-021-03614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan J.A., Tang Y., Yu J.Y., Zhang H., Zhang J.F., Wang C.Q., et al. miR-146a attenuates apoptosis and modulates autophagy by targeting TAF9b/P53 pathway in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2019;10(9):668. doi: 10.1038/s41419-019-1901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J., Tang Z., He Y., Cai S., Wang B., Zhang S., et al. Dl-3-n-Butylphthalide ameliorates diabetic nephropathy by ameliorating excessive fibrosis and podocyte apoptosis. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.628950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yarmohammadi F., Karbasforooshan H., Hayes A.W., Karimi G. Inflammation suppression in doxorubicin-induced cardiotoxicity: natural compounds as therapeutic options. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021;394(10):2003–2011. doi: 10.1007/s00210-021-02132-z. [DOI] [PubMed] [Google Scholar]
- 52.Wang X., Wang Q., Li W., Zhang Q., Jiang Y., Guo D., et al. TFEB-NF-kappaB inflammatory signaling axis: a novel therapeutic pathway of Dihydrotanshinone I in doxorubicin-induced cardiotoxicity. J. Exp. Clin. Cancer Res. 2020;39(1):93. doi: 10.1186/s13046-020-01595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qi W., Boliang W., Xiaoxi T., Guoqiang F., Jianbo X., Gang W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed. Pharmacother. 2020;122 doi: 10.1016/j.biopha.2019.109547. [DOI] [PubMed] [Google Scholar]
- 54.Arunachalam S., Nagoor Meeran M.F., Azimullah S., Sharma C., Goyal S.N., Ojha S. Nerolidol attenuates oxidative stress, inflammation, and apoptosis by modulating Nrf2/MAPK signaling pathways in doxorubicin-induced acute cardiotoxicity in rats. Antioxidants. 2021;10(6) doi: 10.3390/antiox10060984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong Y., Yin J., Chen T., Wen J., Zhang Q., Li X., et al. Dl-3-n-butylphthalide pretreatment attenuates renal ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2021;557:166–173. doi: 10.1016/j.bbrc.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Gong Q., Xue Y., Li X., Song L., Zhu L. DL-3-n-butylphthalide attenuates lipopolysaccharide-induced acute lung injury via SIRT1-dependent and -independent regulation of Nrf2. Int Immunopharmacol. 2019;74 doi: 10.1016/j.intimp.2019.05.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.