Abstract
Mpox has spread rapidly to many countries in nonendemic regions. After reviewing detailed exposure histories of 109 pairs of mpox cases in the Netherlands, we identified 34 pairs where transmission was likely and the infectee reported a single potential infector with a mean serial interval of 10.1 days (95% credible interval, 6.6–14.7 days). Further investigation into pairs from 1 regional public health service revealed that presymptomatic transmission may have occurred in 5 of 18 pairs. These findings emphasize that precaution remains key, regardless of the presence of recognizable symptoms of mpox.
Keywords: Monkeypox virus, mpox, presymptomatic transmission, reproduction number, serial interval
Exposure histories of 109 pairs of mpox cases in the Netherlands were investigated. We identified 34 pairs where transmission was likely and the infectee reported a single potential infector with a mean serial interval of 10.1 days.
The current mpox outbreak was declared a public health emergency of international concern by the World Health Organization on 23 July 2022 [1]. Monkeypox virus infection is spreading predominantly among men who have sex with men (MSM) in countries that have not reported cases of the disease previously [2].
Many key characteristics of mpox are unknown for this new mode of transmission. One such characteristic is the serial interval, defined as the time between symptom onset of primary and secondary cases [3]. Knowledge of the serial interval is key, as it informs the reproduction number and the required intensity of control measures to stop an outbreak and of the possibility of presymptomatic transmission. Current estimates of the mean serial interval of mpox vary with a recent study estimating the mean serial interval as 5.6 days [4], whereas estimates have been reported of 8.5 days in the United States [5], 9.5 days in the United Kingdom [6], and 12.5 days in Italy [7]. There is no general consensus on an estimate of mean serial interval for the current mpox outbreak, largely due to the limited availability of reliable data.
In this work, we investigate paired cases in the recent mpox outbreak in the Netherlands to estimate the mode, the mean, and the standard deviation (SD) of serial intervals and to estimate the proportion of transmission events that occur before the reported symptom onset date of the infector.
METHODS
We identified 109 pairs of laboratory-confirmed and notified mpox cases in the national registry with a symptom onset for the reported infector from 20 May to 3 September 2022, and a symptom onset date for the reported infectee from 24 May to 6 September 2022. All paired cases self-identified as MSM. The data were collected using contact tracing. The regional public health services that collected the data rated the reliability of self-reported symptom onset dates (into 3 categories: unreliable, plausible, or reliable), and assessed the likelihood of transmission between 2 cases (into 3 categories: unlikely, likely and the infectee selected an infector among several contacts, or likely and the infector is the only contact reported for the infectee). The reported symptom onset was defined for any symptom associated with Monkeypox virus infection [8]. We report descriptive statistics of the classified data, and details about statistical methods are described in the Supplementary Materials.
RESULTS
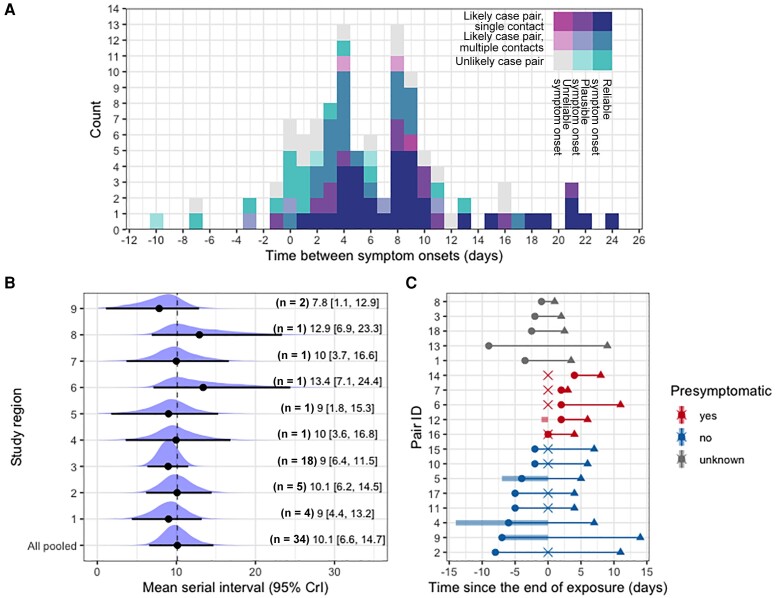
Using all 109 pairs of notified mpox cases in the national registry, the mean of observed interval between symptom onsets was 6.3 days with a SD of 6.1 days (Figure 1A). The intervals ranged from −10 to 24 days, with multiple modes at 0, 4, and 8 days. The observed variation in interval duration was explained to a large extend by the likelihood of transmission between the paired cases (Supplementary Table 1 and Supplementary Materials). After categorizing the likelihood of transmission between 2 cases, 34 pairs with reliable symptom onset dates were classified as likely and reported only 1 infector. The crude mean serial interval for those 34 pairs from all public health services was 9.4 days (SD, 6.2 days). The serial intervals ranged from 1 to 24 days, with a mode at 8 days. To allow for potential differences between public health services in detecting, classifying, and reporting, we used a hierarchical Bayesian framework where each public health service was treated as a random effect. The pooled mean serial interval over all public health services was 10.1 days (95% credible interval [CrI], 6.6–14.7 days) with SD of 6.1 days (95% CrI, 4.6–8.0 days) (Figure 1B). These results were obtained using a normal distribution to describe the serial interval distribution, and similar results were obtained when repeating the analysis using a gamma distribution (mean, 10.3 days [95% CrI, 7.6–14.1] days; SD, 6.3 days [95% CrI, 4.5–9.0 days]).
Figure 1.
Time scale of observed transmissions. A, Reported time differences between symptom onsets (n = 109). Colors show the reliability of reporting; the reliability of self-reported symptom onset dates was rated (unreliable, plausible, and reliable) and the likelihood of transmission between 2 cases was categorized (contact is unlikely, contact is likely and the most plausible one among several reported contacts, contact is likely and the only contact reported for the infectee). B, The pooled serial interval is estimated as the average duration between symptom onset dates of a pair, incorporating random effects specific for regional public health services. Black plots represent mean values of posterior distributions, and whiskers show the 95% credible intervals (CrIs). C, Transmission pairs notified by a single regional public health service (n = 18). Circles and triangles indicate symptom onset of infectors and infectees, and the cross-point is the exact date of exposure between the paired cases (if available). If the exposure date was reported as consecutive days, the time interval is visualized as a shaded bar.
Given the estimated pooled mean serial interval of 10.1 days (SD, 6.1 days) based on the subset of 34 pairs, we can translate the observed doubling time into the effective reproduction number R (ie, the number of secondary cases produced by a typical primary case) [9]. The range of values for the reproduction number R was estimated to be 1.3–1.6, using the average doubling time of 11.2–20.5 days during June 2022 in the Netherlands, before implementing the mass vaccination campaign (see Supplementary Materials for a detailed derivation of the reproduction number).
For a subset of 18 pairs from a single public health service, the exposure dates were further investigated. Among the 18 pairs, 5 pairs (28%) reported contact with an infector prior to the self-reported symptom onset date of the infector and 8 pairs (44%) reported contact with an infector after the self-reported symptom onset date of the infector; for the remaining 5 pairs (28%), the time of exposure was reported as unknown (Figure 1C). The close investigation of timing of exposure and symptom onset in these 18 pairs revealed that transmission can occur from 4 days before to 8 days after symptom onset of the infector, with an average duration from symptom onset to onward transmission of 2.2 days (SD, 3.9 days). Additionally, we estimated the average time between exposure and symptom onset (ie, incubation period) for these 18 pairs (mean, 8.1 days [SD, 4.4 days]), and the mean serial interval can be calculated as the sum of these mean durations, which was 10.3 days (SD, 5.9 days).
DISCUSSION
The present study offers empirical evidence that the average duration of the serial interval of mpox was around 10 days based on the most reliable reported transmission pairs (34 of 109 pairs) in the Netherlands. Without strict conditions on the reliability of reporting and likelihood of transmission of infection, the mean interval between symptom onsets among all 109 pairs had a shorter duration of about 6 days.
Our observations showed that the time intervals between symptom onsets of reported pairs were highly variable and covered a wide range, without a clearly defined single mode. The wide range is consistent with variable mean values reported in earlier studies [5, 7]. These observations could be explained to a large extent by the likelihood of transmission of infection, as reported by the public health services. For the most reliable reported transmission pairs, the range of serial intervals is consistent with an infectious period that starts before and ends after the entire duration of symptoms as reported by the case. Many cases might refrain from at-risk contacts while symptomatic, either from pain or to reduce the risk of transmitting to their partners. As a consequence, transmission could occur before symptom onset and for a certain fraction of cases possibly after symptoms have disappeared. This behavioral factor gives a shorter mean and flatter distribution of the serial interval for mpox compared to smallpox, a related Orthopoxvirus, although epidemiological characteristics for those 2 viruses were often considered to be comparable [10]. The difference in the serial interval could be facilitated by high intensity of exposures to mpox via sexually associated transmission routes during the current outbreak—in fact, the incubation periods for human mpox and invasive smallpox infections are remarkably similar [10, 11].
The frequency of transmission before a case has recognized symptoms is considerably lower than a previous report suggested [6], but the existence of this presymptomatic transmission has important implications for the outbreak control. There is a substantial risk of onward transmissions if infected individuals are unknowingly infectious. Mpox cases without any noticeable symptoms have been reported in Belgium [12], and a high viral load has been observed around the time of symptom onset among patients in the United Kingdom [13]. It is likely that infected individuals are capable of sustaining a high viral load even before symptom onset; thus, additional effort on monitoring and informing high-risk contacts without symptoms to adhere to temporary preventive measures may be required.
The duration of the mpox serial interval implies that the growth of the epidemic in the Netherlands was caused by the range of reproduction numbers between 1.3 and 1.6, which is consistent with other studies [14, 15]. This estimate, in turn, suggests that control measures should be sufficiently effective to prevent (1 – 1/1.6) × 100% = 38% of all potential secondary cases on average. Even if control measures such as contact tracing fail to catch the majority of contacts, they might still be highly effective in contributing to the prevention of further spread.
Our results should be interpreted with several caveats. Our analysis is restricted to cases who identified only a single infector, which may cause selection bias toward longer serial intervals because the excluded cases with multiple reported sexual contacts might have a higher frequency of sexual contact resulting in a shorter time to transmission. The analysis relies on self-reported contact history and symptom onset by notified cases. It is possible that co-primary infection pairs are incorrectly classified as primary–secondary infection pairs, resulting in a bias toward lower values. Heterogeneity in case finding, contact tracing, and reporting was mitigated by categorizing the pairs by the reporting public health service and treating the difference among public health services as a random effect in the analysis. Serial intervals could vary over the course of an epidemic, and the estimate could be biased due to right-censoring of observations induced by increasing epidemic growth, vaccination coverage, or behavioral changes due to heightened awareness. This effect is expected to be small as the study period covers both the growing and declining phases of the epidemic, and as the mass vaccination campaign started from 25 July onward when incidence was already low [8].
In conclusion, we have estimated the mean serial interval and showed that the current mpox outbreak in the Netherlands was driven by a moderate range of effective reproduction numbers. The estimate of the mean serial interval is conditional on the increased awareness of the disease, concomitant behavior change, and increased immunity from natural infection and vaccination. If activity in the affected community goes back to the pre-outbreak level, and if immunity is insufficient among those at risk, there remains a risk of outbreaks or reintroduction of the virus. Our study also found that a minority of the cases might transmit infection before recognizable symptoms. This highlights that awareness remains key, regardless of the presence of recognizable symptoms, to mitigate the public health impact of resurging mpox.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Fuminari Miura, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; Center for Marine Environmental Studies, Ehime University, Ehime, Japan.
Jantien A Backer, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Gini van Rijckevorsel, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; Department of Infectious Diseases, Public Health Service Amsterdam.
Roisin Bavalia, Department of Infectious Diseases, Public Health Service Amsterdam.
Stijn Raven, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; Department of Infectious Diseases, Public Health Service Region Utrecht, Zeist.
Mariska Petrignani, Department of Infectious Diseases, Public Health Service Haaglanden, Den Haag, The Netherlands.
Kylie E C Ainslie, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; School of Public Health, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Jacco Wallinga, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; Department of Biomedical Data Sciences, Leiden University Medical Center, The Netherlands.
for the Dutch Mpox Response Team:
Birgit van Benthem, Diederik Brandwagt, Hanna Bos, Colette van Bokhoven-Rombouts, Lian Bovée, Chantal P Rovers, Brigitte van Cleef, Alje P van Dam, Rik van Dael, Annemiek A van der Eijk, Pauline Ellerbroek, Catharina van Ewijk, Eelco Franz, Corine GeurtsvanKessel, Joke van der Giessen, Hannelore Götz, Josette M W Häger, Susan van den Hof, Elske Hoornenborg, Putri Hintaran, Jorgen de Jonge, Rosa Joosten, Marion Koopmans, Kevin Kosterman, Jente Lange, Tjalling Leenstra, Daisy Ooms, Danielle Oorsprong, Eline Op de Coul, Demi Reurings, Gini van Rijckevorsel, Gregorius J Sips, Sacha F de Stoppelaar, Albert Vollaard, Bettie Voordouw, Harry Vennema, Henry J C de Vries, Karin Ellen Veldkamp, Klaartje Weijdema, Geert Westerhuis, Margreet J M te Wierik, Matthijs R A Welkers, Toos Waegemaekers, Jacco Wallinga, and Paul Zantkuijl
Notes
Dutch Mpox Response Team. Birgit van Benthem, Diederik Brandwagt, Hanna Bos, Colette van Bokhoven-Rombouts, Lian Bovée, Chantal P. Rovers, Brigitte van Cleef, Alje P. van Dam, Rik van Dael, Annemiek A. van der Eijk, Pauline Ellerbroek, Catharina van Ewijk, Eelco Franz, Corine GeurtsvanKessel, Joke van der Giessen, Hannelore Götz, Josette M.W. Häger, Susan van den Hof, Elske Hoornenborg, Putri Hintaran, Jorgen de Jonge, Rosa Joosten, Marion Koopmans, Kevin Kosterman, Jente Lange, Tjalling Leenstra, Daisy Ooms, Danielle Oorsprong, Eline Op de Coul, Demi Reurings, Gini van Rijckevorsel, Gregorius J. Sips, Sacha F. de Stoppelaar, Albert Vollaard, Bettie Voordouw, Harry Vennema, Henry J. C. de Vries, Karin Ellen Veldkamp, Klaartje Weijdema, Geert Westerhuis, Margreet J. M. te Wierik, Matthijs R. A. Welkers, Toos Waegemaekers, Jacco Wallinga, and Paul Zantkuijl.
Author contributions. Conceptualization: F. M. and J. W. Data curation: F. M., G. v. R., R. B., S. R., M. P., and J. W. Formal analysis: F. M., J. A. B., and J. W. Investigation: F. M. and J. W. Methodology: F. M., J. A. B., and J. W. Software: F. M., J. A. B., and J. W. Validation: F. M., J. A. B., and J. W. Visualization: F. M., J. A. B., and J. W. Writing–original draft: F. M. and J. W. Writing–review and editing: All authors.
Acknowledgments. We thank the public health services (Gemeentelijke Gezondheidsdienst [GGD] Amsterdam, GGD Brabant Zuid-Oost, GGD Flevoland, GGD Fryslân, GGD Gelderland-Midden, GGD Gelderland-Zuid, GGD Gooi en Vechtstreek, GGD Groningen, GGD Haaglanden, GGD Hart voor Brabant, GGD Hollands Noorden, GGD Kennemerland, GGD region Utrecht, GGD Rotterdam-Rijnmond, GGD West-Brabant, GGD Zaanstreek-Waterland, GGD Zuid-Limburg, and GGD Zuid-Holland-Zuid) for their effort to collect the epidemiological data.
Data availability. Anonymized data and all codes used for analysis and visualization are available on Github (https://github.com/fmiura/MpxSI_2022).
Financial support. The work was supported by the Netherlands Ministry of Health, Welfare and Sport. F. M. acknowledges funding from the Japan Society for the Promotion of Science (JSPS KAKENHI, grant number 20J00793). Funding to pay the Open Access publication charges for this article was provided by the Netherlands Ministry of Health, Welfare and Sport.
References
- 1. World Health Organization . WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox 2022. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHRemergency-committee-regarding-the-multi–country-outbreak-of-monkeypox–23-july-2022. Accessed 10 August 2022.
- 2. Kraemer MUG, Tegally H, Pigott DM, et al. Tracking the 2022 monkeypox outbreak with epidemiological data in real-time. Lancet Infect Dis 2022; 22:941–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fine PEM. The interval between successive cases of an infectious disease. Am J Epidemiol 2003; 158:1039–47. [DOI] [PubMed] [Google Scholar]
- 4. Guo Z, Zhao S, Sun S, He D, Chong KC, Yeoh EK. Estimation of the serial interval of monkeypox during the early outbreak in 2022. J Med Virol 2023; 95:e28248. [DOI] [PubMed] [Google Scholar]
- 5. Madewell ZJ, Charniga K, Masters NB, et al. Serial interval and incubation period estimates of monkeypox virus infection in 12 jurisdictions, United States, May–August 2022. Emerg Infect Dis 2023; 29:818–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ward T, Christie R, Paton RS, Cumming F, Overton CE. Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ 2022; 379:e073153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guzzetta G, Mammone A, Ferraro F, et al. Early estimates of monkeypox incubation period, generation time, and reproduction number, Italy, May–June 2022. Emerg Infect Dis 2022; 28:2078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Ewijk CE, Miura F, van Rijckevorsel G, et al. Mpox outbreak in the Netherlands, 2022: public health response, characteristics of the first 1000 cases and protection of the first-generation smallpox vaccine. Emerg Infect Dis 2023; 28:2200772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wallinga J, Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. Proc Biol Sci 2007; 274:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis 2006; 194:773–80. [DOI] [PubMed] [Google Scholar]
- 11. Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill 2022; 27:2200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Baetselier I, Van Dijck C, Kenyon C, et al. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat Med 2022; 28:2288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis 2022; 22:1153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du Z, Shao Z, Bai Y, et al. Reproduction number of monkeypox in the early stage of the 2022 multi-country outbreak. J Travel Med 2022; 29:taac099. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention . Technical report 4: multi-national monkeypox outbreak, United States, 2022. 2022. https://www.cdc.gov/poxvirus/monkeypox/cases-data/technical-report/report-4.html. Accessed 2 November 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.