Abstract
The menopausal transition is a pivotal time of cardiovascular risk, but knowledge is limited in HIV. We studied longitudinal carotid artery intima-media thickness (CIMT) in the Women's Interagency HIV Study (2004–2019; 979 women/3247 person-visits; 72% with HIV). Among women with HIV only, those who transitioned had greater age-related CIMT progression compared to those remaining premenopausal (difference in slope = 1.64 µm/year, P = .002); and CIMT increased over time in the pretransition (3.47 µm/year, P = .002) and during the menopausal transition (9.41 µm/year, P < .0001), but not posttransition (2.9 µm/year, P = .19). In women with HIV, menopause may accelerate subclinical atherosclerosis as measured by CIMT.
Keywords: HIV, menopause, cardiovascular disease, atherosclerosis
Among women with HIV, longitudinal progression of carotid artery intima-media thickness was accelerated during the menopausal transition compared to the pre- or postmenopause periods, suggesting menopause may accelerate subclinical atherosclerosis in women with HIV.
The menopausal transition is recognized as a pivotal time of cardiovascular disease (CVD) risk [1], during which indicators of subclinical atherosclerosis (ie, carotid artery intima-media thickness [CIMT] and stiffness [2, 3]) have been shown to increase beyond what is expected with aging alone. CVD is of particular concern in women with human immunodeficiency virus (HIV), as HIV infection has been associated with increased risk of CVD, more so in women than in men [4]. However, whether menopause increases CVD risk in women with HIV remains unknown.
Leveraging longitudinal menopause ascertainment and carotid artery B-mode ultrasound imaging in the Women's Interagency HIV Study (WIHS), we examined the association of the menopausal transition with CIMT, carotid artery stiffness, and carotid artery plaque in women with and without HIV using 2 complementary approaches (Supplementary Figure 1). This study provides the first insights into the contribution of the menopausal transition to subclinical atherosclerosis in women with HIV, beyond the influence of chronological aging alone.
METHODS
Study Population
The WIHS was a multicenter cohort of women with and without HIV in the United States, described previously [5]. Institutional review boards at all WIHS sites approved the study and participants provided written informed consent.
Women in the current analysis participated in a vascular disease substudy at 6 WIHS sites (Bronx, New York; Brooklyn, New York; Chicago, Illinois; San Francisco, California; Los Angeles, California; Washington, District of Columbia) [6, 7], featuring high-resolution B-mode carotid artery ultrasound at a baseline visit (2004–2005; wave 1) and follow-up visits every 2–3 years through 2012 (waves 2–4). An additional visit (wave 5) was conducted in 2017–2019 at the Bronx, Brooklyn, and Chicago sites (Supplementary Figure 1A).
After exclusions for pregnancy, missing menopause status, hormone therapy use, hormonal contraceptive use, age, HIV seroconversion, clinical CVD, or only 1 visit in the vascular substudy (Supplementary Methods), 3247 person-visits from 979 participants remained, with further exclusions for different outcomes/analyses detailed below and in Supplementary Figure 1B. Detailed sample sizes are shown in Supplementary Table 1.
Menopause Definition
We used each participant's longitudinal survey history to identify if/when they reached their natural or surgical final menstrual period (FMP), using a multistep process [8] (described in Supplementary Methods). Briefly, we obtained date of FMP from the last visit with reported menses. All visits after the FMP were classified as postmenopausal, while visits prior were considered premenopausal.
Subclinical Atherosclerosis
As previously described [7], high-resolution B-mode ultrasound with automated computerized edge detection software assessed carotid artery arteriosclerosis. Outcomes were intima-media thickness of the right common carotid artery (CIMT; µm, continuous), presence/absence of carotid artery lesions (plaque) defined as focal CIMT > 1.5 mm in any imaged segment (common and internal carotid arteries and bifurcation), and Young's modulus of elasticity (105×N/m2, continuous, an index of arterial stiffness) measured at the common carotid artery [9]. CIMT was measured in all 5 waves of the vascular substudy, stiffness at waves 1–3 and 5, and plaque at waves 1, 4, and 5 (sample sizes for each outcome in Supplementary Table 1 and Supplementary Figure 1B).
Statistical Analysis
General Principles
We used 2 approaches (analysis 1 and analysis 2) to investigate the relationship of the menopausal transition with subclinical atherosclerosis. Analysis 1 includes all women with longitudinal data, including women who have not yet reached an FMP or women with a surgical FMP; while analysis 2 restricts to women with an observed natural FMP. In all analyses, we considered nested models to serially adjust for potential confounders/mediators including age and sociodemographic, behavioral, cardiometabolic, and HIV-related factors (described in Supplementary Methods). Covariates were time varying, excepting time-fixed variables (eg, race/ethnicity), unless otherwise specified. Analyses were carried out among all participants, as well as stratified by HIV serostatus. Young's modulus was log-transformed. P < .05 was considered statistically significant. Analyses were conducted in R (version 4.2.2). Analysis code is provided in Supplementary Methods.
Analysis 1
The purpose was to determine whether the effect of age on longitudinal progression of CIMT, stiffness, and carotid artery plaque differs for women who, during follow-up, remained premenopausal, transitioned from pre- to postmenopause, or were postmenopausal. For CIMT and stiffness outcomes, we used linear mixed-effects models with a random intercept per participant and terms for menopausal transition status (remained premenopausal, transitioned from pre- to postmenopausal, postmenopausal), age, and the interaction of age and menopausal transition status.
For the outcome of incident carotid artery plaque, we additionally excluded women with plaque at baseline (n = 45 women), and women with plaque reversal over time (ie, developed plaque during follow-up but plaque was not found at subsequent visit; n = 5 women) (Supplementary Figure 1B). We used accelerated failure time models with the Weibull distribution to estimate the association of baseline age with time to carotid artery plaque (interval censored) by menopausal transition status. Models included a term for menopausal transition status, baseline age, and the interaction of baseline age and menopausal transition status. Covariates were based on baseline data.
Analysis 2
The purpose was to differentiate whether CIMT and stiffness increase at a constant rate over time, or accelerate during the menopausal transition, suggesting an effect of ovarian aging beyond chronological aging. We used previously described methods [2, 10] involving piece-wise linear mixed-effects models to estimate associations of years before/after the FMP with CIMT and stiffness in time segments of pretransition, menopausal transition, and posttransition. We excluded women without an observed natural FMP, and removed person-visits below the fifth or above the 95th percentile of years before/after FMP due to sparsity. We chose a priori cut-points of 2 years before and after the FMP to delineate time segments, because estradiol begins to decline approximately 2 years before, and stabilizes approximately 2 years after, the FMP [11]. All models included a random intercept per participant, a term for years before/after the FMP, and terms for the interaction of the second time segment (>2 years before FMP) and third time segment (>2 years after FMP) with years before/after FMP. Age at FMP was adjusted instead of age. Likelihood ratio tests compared piece-wise models to respective linear models (ie, without interaction of time segments and years before/after FMP).
RESULTS
Participant Characteristics
Among 979 participants at the first vascular substudy visit (ie, baseline), 810 (83%) were premenopausal and 703 (72%) were women with HIV (Supplementary Table 2). During follow-up, 247 women transitioned from pre- to postmenopause, while 563 remained premenopausal and 169 were already postmenopausal (Supplementary Table 3); however, given different follow-up patterns for each outcome (Supplementary Figure 1A), the numbers of women remaining premenopausal or transitioning differed by outcome (Supplementary Table 3). At baseline, women who remained premenopausal were younger than transitioning women and postmenopausal women (median age 35, 44, and 52 years, respectively; Supplementary Tables 2 and 4).
Analysis 1: Age-Related Progression of Subclinical Atherosclerosis by Menopausal Transition Status
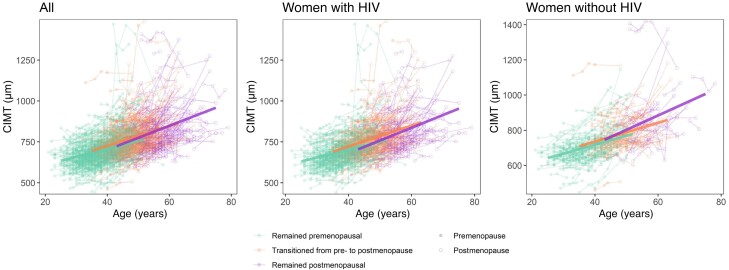
Median follow-up time was 6.6 years (interquartile range [IQR], 4.9–9.5 years) for CIMT, 4.6 years (IQR, 4.3–12.3 years) for stiffness, and 6.9 years (IQR, 6.5–13.1 years) for plaque. The association of age with CIMT was greater for transitioning women (β = 1.40; 95% confidence interval [CI], .53–2.27 µm/year; P = .002) and postmenopausal women (β = 3.42; 95% CI, 2.26–4.57 µm/year; P < .0001) compared to women who remained premenopausal (Figure 1 and Supplementary Table 5). This pattern was somewhat similar in women with and without HIV, although the difference in the effect of age for women transitioning versus remaining premenopausal was not significant in women without HIV (Figure 1 and Supplementary Table 5).
Figure 1.
Association of age with longitudinal progression of carotid artery intima-media thickness (CIMT) among women who remained premenopausal, transitioned from pre- to postmenopause, or were postmenopausal. Thin lines represent progression of CIMT within individuals. Thick lines represent estimates from a linear mixed-effect model, with random intercept and terms for menopausal transition status (remained premenopausal, transitioned from pre- to postmenopausal, postmenopausal), age, and the interaction of age and menopausal transition status. Sample sizes (number of women/person-visits): all (979/3247), women with HIV (703/2321), women without HIV (276/926).
We did not observe any difference in the association of age with carotid artery stiffness or time to carotid artery plaque by menopausal transition status, either in the combined study population nor in women with or without HIV separately (Supplementary Tables 5 and 6).
Analysis 2: Years Before/After the FMP and Subclinical Atherosclerosis
Median age at FMP for women with versus without HIV was 49 and 50 years, respectively (P = .10) (Supplementary Table 7). Median follow-up time was 6.8 years (IQR, 6.3–12.6 years) for CIMT and 4.7 years (IQR, 4.4–12.7 years) for stiffness.
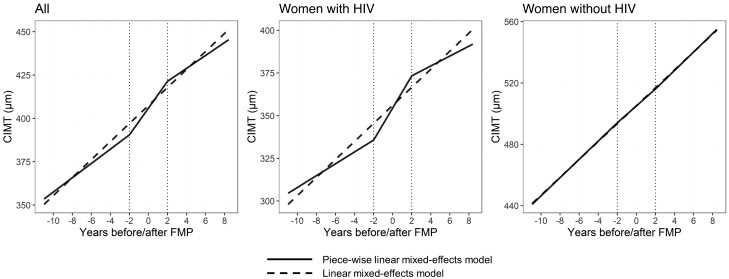
In piece-wise linear mixed effects models with cut-points of 2 years before and after the FMP, we observed that CIMT increased over time in the pretransition (β = 4.12; 95% CI, 2.38–5.86 µm/year; P < .0001), menopausal transition (β = 7.72; 95% CI, 4.69–10.75; P < .0001), and posttransition (β = 3.73; 95% CI, .43–7.03; P = .02) time segments (Figure 2 and Supplementary Table 8). The pattern of accelerated CIMT progression during the menopausal transition was more pronounced in women with HIV, where the slope in the menopausal transition was greater than that of the pretransition (P = .02) and posttransition (P = .07); furthermore, the piece-wise model provided a better fit than a linear model (P = .05), consistent with an effect of ovarian aging beyond chronological aging (Figure 2 and Supplementary Table 8). In contrast, in women without HIV, the slopes of CIMT increase over time did not differ for the pretransition, menopausal transition, or posttransition, consistent with a linear effect of chronological aging (Figure 2 and Supplementary Table 8).
Figure 2.
Relationship of years before/after the final menstrual period (FMP) with carotid artery intima-media thickness (CIMT). Estimates are from a linear model (dashed line) or a piece-wise linear mixed-effects model (solid line) with 3 time segments: pretransition (>2 years before FMP), menopausal transition (2 years before to 2 years after FMP), and posttransition (>2 years after FMP). The linear model included a term for years before/after the FMP, while the piece-wise model included terms for years before/after the FMP, and the interaction of the second time segment (>2 years before FMP) and the third time segment (>2 years after FMP) with years before/after FMP. All models included a random intercept per participant and were adjusted for age at FMP, HIV status, study site, race/ethnicity, income, educational attainment, employment, smoking status, alcohol use status, substance use, hepatitis C virus serostatus, hormonal contraceptive use, body mass index, systolic and diastolic blood pressure, diabetes, use of lipid-lowering medications, and use of hypertension medications. Among participants with HIV, model additionally adjusted for HIV viral load, CD4+ cell count, and antiretroviral therapy. Sample sizes (number of women/person-visits): all (284/977), women with HIV (209/705), women without HIV (75/272).
For the outcome of carotid artery stiffness, the slope over time did not differ for the pretransition, menopausal transition, or posttransition time segments, either in the combined study population nor in women with or without HIV separately (Supplementary Table 9), consistent with a linear effect of chronological aging.
DISCUSSION
In this large longitudinal study of women with and without HIV, increases in CIMT over time were accelerated during the menopausal transition for women with HIV, but not for women without HIV. CIMT progression has long been considered a surrogate marker of CVD risk in non-HIV populations [12], although its implications in people with HIV are less clear [13]. Women with HIV may be particularly vulnerable to the cardiovascular ramifications of hormonal changes accompanying menopause, for several possible reasons: (1) women with HIV have lower estradiol than women without HIV in premenopause [14], which has been related to CVD risk; and (2) estrogens may protect against viral replication [15]. Taken together, estrogen depletion combined with its effects on HIV control could contribute to an exacerbation of menopause-related CVD risk in women with HIV.
We did not observe a relationship of the menopausal transition with CIMT, stiffness, or plaque in women without HIV, in contrast to some previous studies [2, 3]. However, women without HIV in the WIHS are not representative of the general population, as they are recruited based on sociodemographic similarity to women with HIV, and thus are likely influenced by social determinants of health and may have a higher burden of CVD risk factors. Additionally, our sample size of women without HIV was smaller than that of women with HIV, potentially reducing power to observe significant associations.
This study was strengthened by the large sample size, length of longitudinal follow-up, and the wealth of cohort data, which allowed for extensive adjustment of potential confounders. Our study was limited by use of self-reported menopause status, which could result in some misclassification, although we minimized misclassification risk by using longitudinal data to define menopause; lack of hormonal measures (ie, follicle-stimulating hormone, anti-Mullerian hormone) to confirm menopause status; the observational nature of the study, which precludes any assumption of causality and provides little mechanistic understanding; lesser follow-up for the outcomes of carotid artery stiffness and plaque, which may have reduced power; and lastly, results may not be generalizable given the unique characteristics of the WIHS study population (eg, racial/ethnic diversity, low income, low educational attainment, etc.).
In summary, in this first report of subclinical atherosclerosis across the menopausal transition in women with and without HIV, we found that CIMT progression increased during the menopausal transition in women with HIV. Because greater CIMT progression is associated with higher risk of clinical CVD events in non-HIV populations [12], future research should examine whether CIMT progression in people with HIV confers the same risk. In context, a CIMT increase of 5.94 µm/year during the menopausal transition compared to the pretransition in women with HIV (Supplementary Table 8), over 4 years (approximately 24 µm excess), may confer 1.32–1.45 fold increased risk of CVD events [12]. The CIMT progression related to menopause in women with HIV, combined with prior observations that menopausal hormone therapy may reduce CIMT progression in women with HIV [8], suggests potential cardiovascular benefits of menopausal hormone therapy in this unique high–CVD-risk population.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Brandilyn A Peters, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA.
Adam Whalen, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA.
Xiaonan Xue, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA.
Elizabeth F Topper, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Kathleen M Weber, CORE Center of Cook County Health and Hospital System/Hektoen Institute of Medicine, Chicago, Illinois, USA.
Phyllis C Tien, Division of Infectious Diseases, University of California San Francisco, San Francisco, California, USA; Medical Service, Department of Veterans Affairs, San Francisco, California, USA.
Seble G Kassaye, Department of Medicine, Georgetown University Medical Center, Washington, District of Columbia, USA.
Howard Minkoff, Department of Obstetrics and Gynecology, State University of New York Downstate Health Sciences University, Brooklyn, New York, USA.
Ervin Fox, Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, USA.
Margaret A Fischl, Division of Infectious Diseases, University of Miami Miller School of Medicine, Miami, Florida, USA.
Lauren F Collins, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, USA.
Michelle Floris-Moore, Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, North Carolina, USA.
Howard N Hodis, Departments of Medicine and Population and Public Health Sciences, Atherosclerosis Research Unit, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Qibin Qi, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA.
David B Hanna, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA.
Anjali Sharma, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA.
Kathryn Anastos, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA.
Robert C Kaplan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA; Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA.
Notes
Acknowledgments. The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MACS/WIHS Combined Cohort Study (MWCCS) sites.
Disclaimer . The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Heart, Lung, and Blood Institute (NHLBI; grant numbers K01HL160146 to B. A. P.; R01HL148094 to R. C. K.; K01HL137557 to D. B. H.; U01HL146204-04S1 to R. C. K. and D. B. H.; and R01HL140976 to Q. Q. and R. C. K.). MWCCS is funded primarily by the NHLBI, with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute On Aging, National Institute Of Dental and Craniofacial Research, National Institute Of Allergy And Infectious Diseases, National Institute Of Neurological Disorders And Stroke, National Institute Of Mental Health, National Institute On Drug Abuse, National Institute Of Nursing Research, National Cancer Institute, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Minority Health and Health Disparities, and in coordination and alignment with the research priorities of the NIH, Office of AIDS Research. MWCCS data collection is also supported by NIH grants UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), P30-MH-116867 (Miami CHARM), UL1-TR001409 (DC CTSA), KL2-TR001432 (DC CTSA), and TL1-TR001431 (DC CTSA).
Data in this article were collected by the Women’s Interagency HIV Study (WIHS), now the MACS/WIHS Combined Cohort Study (MWCCS). MWCCS site (Principal Investigators), NIH grant numbers: Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos, David Hanna, and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D'Souza, Stephen Gange, and Elizabeth Topper), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky, Frank Palella, and Valentina Stosor), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, Deborah Konkle-Parker, and James B. Brock), U01-HL146192; UNC CRS (Adaora Adimora and Michelle Floris-Moore), U01-HL146194.
References
- 1. El Khoudary SR, Aggarwal B, Beckie TM, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation 2020; 142:e506–32. [DOI] [PubMed] [Google Scholar]
- 2. Samargandy S, Matthews Karen A, Brooks Maria M, et al. Arterial stiffness accelerates within 1 year of the final menstrual period. Arterioscler Thromb Vasc Biol 2020; 40:1001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause 2013; 20:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone L, Looby SE, Zanni MV. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr Opin HIV AIDS 2017; 12:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adimora AA, Ramirez C, Benning L, et al. Cohort profile: the Women's Interagency HIV study (WIHS). Int J Epidemiol 2018; 47:393–4i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015; 61:640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 2008; 22:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters BA, Hanna DB, Sharma A, et al. Menopausal hormone therapy and subclinical cardiovascular disease in women with and without human immunodeficiency virus. Clin Infect Dis 2023; 76:e661–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis 2001; 154:185–93. [DOI] [PubMed] [Google Scholar]
- 10. Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 2009; 54:2366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Randolph JF Jr, Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab 2011; 96:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Willeit P, Tschiderer L, Allara E, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation 2020; 142:621–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanna DB, Moon J-Y, Haberlen SA, et al. Carotid artery atherosclerosis is associated with mortality in HIV-positive women and men. AIDS 2018; 32:2393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karim R, Mack WJ, Kono N, et al. Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women’s Interagency HIV study (WIHS). J Clin Endrocrinol Metab 2013; 98:E610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Das B, Dobrowolski C, Luttge B, et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 2018; 115:E7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.