Abstract
A well-documented side effect of cannabis and Δ9-tetrahydrocannabinol (THC) acute administration is deficits in cognition and attention. Cannabidiol (CBD), a non-intoxicating constituent of cannabis, may modulate THC’s impairing effects. A goal of this study was to determine the effects of THC and CBD, alone and in combination, on performance in the rodent Psychomotor Vigilance Test (rPVT), a translational paradigm used to quantify sustained attention. Outcome measures in the rPVT include motor speed, premature responding, and lapses in attention. Sprague-Dawley rats were trained to perform the rPVT to the acquisition criteria and then received oral doses (mg/kg) of THC (1–17.6), CBD (1–100), and combinations of THC + CBD in sesame oil prior to rPVT sessions, administered in a within-subject randomized design. Blood was collected from rats receiving selected doses of THC alone or THC + CBD for analysis of THC and its metabolites. THC alone produced significant decreases in accuracy and increases in lapses in attention at higher doses (10 mg/kg; p’s < 0.05). The co-administration of CBD (10 mg/kg) with THC (3 or 10 mg/kg) caused greater impairments to sustained attention compared with administration of THC alone (p’s < 0.05). The rPVT is a translational platform sensitive to detect impairments in attention associated with THC and other cannabis constituents. Further work is necessary to determine the mechanism of THC and CBD interactions on impairments in sustained attention.
Keywords: Δ9-tetrahydrocannabinol, cannabidiol, cannabis, attention, cognition
Introduction
Cannabis products containing Δ9-tetrahydrocannabinol (THC) and/or cannabidiol (CBD) are widely used for medical and recreational purposes. THC is the main psychoactive cannabinoid in cannabis, and acute administration of cannabis or THC produces euphoria, relaxed mood, and ‘drug liking’. THC also produces negative effects that include increased heart rate, impairments in neurocognitive function and motor skills, feelings of paranoia and anxiety, and dependence in some users (D’Souza et al., 2004; Haney, 2005; Lichtman, Varvel, & Martin, 2002; Spindle et al., 2018). CBD, which has minimal purported psychoactive effects on its own, is thought to modulate the effects of THC (Boggs, Nguyen, Morgenson, Taffe, & Ranganathan, 2018; Gibson et al., 2022). Specifically, the addition of CBD is often reported to reduce the aversive or abuse liability effects of THC and cannabis (MacCallum & Russo, 2018). However, data from controlled human and animal laboratory studies are mixed; in certain cases, CBD exacerbates, while in other cases it mitigates, THC’s acute effects (for review, see Boggs et al., 2018).
A well-documented side effect of acute administration of cannabis and THC are impairments in sustained attention (Crane, Schuster, Fusar-Poli, & Gonzalez, 2013; Hart, van Gorp, Haney, Foltin, & Fischman, 2001; Sewell et al., 2013). Synthetic THC formulations, such as dronabinol (Marinol) and nabilone (Cesamet) are FDA approved for treatment of nausea in cancer chemotherapy and inappetence in AIDS patients with cachexia, and THC-induced impairments can limit therapeutic use. Further, concomitant with its legalization in many US states, there has been a rise in recreational and medical use of cannabis and THC products that are widely available for inhaled or oral administration. A majority of commercially-available cannabis products contain both THC and CBD in varying concentrations. CBD on its own does not produce impairments in cognition or attention (Spindle et al., 2020). Some human laboratory studies report a mitigating effect of CBD on THC-induced impairments in memory or cognition (Morgan, Schafer, Freeman, & Curran, 2010; Osborne, Solowij, & Weston-Green, 2017), though there are also many studies showing that the co-administration of CBD either has no effect, or exacerbates the negative effects of THC (for review, see Freeman et al., 2019).
The goal of this pilot study was to use a translational paradigm to assess the effects of oral THC and CBD, alone and in combination, on cognitive behavior in rodents. We chose to use the Rodent Psychomotor Vigilance Test (rPVT)(Davis, Roma, & Hienz, 2016), a previously developed rodent analogue of the human PVT, which is utilized as an objective risk assessment tool in laboratory, clinical, and operational settings (Davis et al., 2016). The rPVT requires continuous monitoring and detection of a brief, randomly-occurring visual target. When the target appears, the subject must quickly depress the response key to earn a food pellet reinforcer. It measures sustained attention (correct responses and lapses in attention), changes in inhibitory control (premature responses), and motor speed (reaction times). Inhibitory control contributes to anticipation, planning, and goal setting, which are key executive functions. Likewise, slowing of motor speed provides information on potential sedating or motor disrupting effects of drugs. An advantage of this technique is that it provides a stable performance baseline that is sensitive to experimental manipulations (e.g. effects of drugs, sleep deprivation or restriction, radiation exposure; Davis, DeCicco-Skinner, & Hienz, 2015; Davis et al., 2016; Loomis, McCarthy, Edgar, Tricklebank, & Gilmour, 2013).
Materials and Methods
Subjects
Adult male and female Sprague Dawley rats (N=5, 60% female; Charles River, Wilmington, MA) were single housed in wire-topped, plastic cages (27 × 48 × 20 cm) with standard enrichment. The vivarium was on a 12-hr reverse light cycle (lights off at 8:00 a.m.) and was humidity and temperature controlled. Diet was a corn-based chow (Teklad product no. 2018, Global 18% Protein Rodent Diet; Harlan, Indianapolis, IN). Rats were food maintained to ~90% of free feeding weight, individually adjusted to maintain optimal performance in the rPVT. Daily home cage food was given at the end of behavioral testing, water was available ad libitum except during test procedures. All procedures used in this study were approved by the Johns Hopkins Institutional Animal Care and Use Committee. The facilities adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were AAALAC-approved.
Drugs
Ampules of (−)-trans-THC (50–200 mg/ml in 95% USP ethyl alcohol) were provided by the U.S. National Institute on Drug Abuse (NIDA) Drug Supply Program. Synthetic CBD was obtained from Albany Molecular Research Inc. (Rensselaer, NY, USA); CBD was confirmed to not contain THC or other contaminants by independent testing. THC and CBD were dissolved in 100% USP sesame oil (Spectrum Chemical; New Brunswick, NJ, USA) and administered via oral gavage (1 ml/kg, p.o.). Doses were 0, 1, 3, 5.6, 10, 17.6 mg/kg THC; 0, 1, 3, 10, 30, 60, and 100 mg/kg CBD. Combinations of THC and CBD were 3 mg/kg THC + 3 mg/kg CBD (denoted as 3:3), 3 mg/kg THC + 10 mg/kg CBD (3:10), 10 mg/kg THC + 3 mg/kg CBD (10:3), 10 mg/kg THC + 10 mg/kg CBD (10:10).
Rodent psychomotor vigilance test
Training and testing in the rPVT were performed as previously described (Davis et al., 2016). Briefly, sessions began with the onset of the house light and after a variable delay of 3–10 sec, the light behind the nose-poke key was illuminated. A correct response was defined as a response on the nose poke key within 1.5 sec after light onset and was reinforced with a pellet. A response prior to the light onset (premature response) was not reinforced and produced an 8 sec time out signaled by extinguishing the house light. A trial with no response (miss) or response after the 1.5-sec interval had elapsed was not reinforced. The delay period for the next trial began after a 1-sec inter-trial interval, timed either after the response or the end of the 1.5-sec stimulus duration, whichever occurred first.
Reaction times (RT) were recorded for all trials with a response and reflect the elapsed time in milliseconds from stimulus onset to the occurrence of a response. To count as a valid RT, this response needed to occur after the stimulus light was illuminated for 150 ms and before the end of the 1500 ms response window. Summary RT measures were calculated that included the fastest 10% (Q10), quartiles 25, 50 (median) and 75%, as well as the slowest 10% of reaction times (90th percentile, Q90). Analysis of RT percentiles were employed as consistent with the human PVT, as RT distributions are often skewed due to the physiological limits on reaction times (Stebbins & Miller, 1964). Premature responses are defined as responses prior to and within 150 ms of the onset of the stimulus light. The 150 ms cutoff was determined based on physiological limits to the minimal speed with which an organism can respond. Premature responses are further dissociable into false alarms, where responding occurs during the 3–10 s interval when a stimulus could have appeared, and early responses, which occur prior to the 3s interval. Lapses in attention are defined by trials not responded to (i.e., misses or errors of omission) plus trials where the RT is greater than twice an animal’s mean RT for that session. This lapse criterion is well established in the literature (Christie, Bolortuya, et al., 2008; Christie, McKenna, Connolly, McCarley, & Strecker, 2008) and similar to that employed in human studies (Lim & Dinges, 2008). Attention-related impairments are indicated by significant decreases in accuracy and/or increases in RTs, premature responses, or lapses when compared to baseline performances and/or to the performances of a control group.
Rats were trained to perform the rPVT to the acquisition criteria (75% correct responses and <25% false alarms). A total of N=8 (50% female) rats were initially trained on rPVT procedures, but 3 did not meet acquisition criteria (Final N=5; 60% female). After acquisition was established, drug or vehicle was administered 90-min prior to rPVT sessions. The 90-min pretreatment time was selected based on our prior studies showing antinociceptive and hypothermic effects of oral THC were observed at this time point (Moore & Weerts, 2022). Doses were administered in a randomized design for each drug: THC was tested first (1–17.6 mg/kg), with each dose being given twice, then CBD (1–100 mg/kg), and then THC + CBD combinations (as detailed above). Drug and vehicle administrations occurred on two separate days each week (e.g., Tuesday and Thursday), with non-drug rPVT sessions occurring on subsequent days to ensure maintenance of baseline performance criteria. These criteria were established prior to the next drug administration, and stability was assessed prior to each subsequent drug administration (accuracy above 75% and within ±10% of the individual’s baseline, no increasing or decreasing trends for 3 days prior to drug).
Analysis of THC and key metabolites after THC and CBD administration
Following completion of rPVT behavioral testing with all doses and dose combinations, rats were administered oral THC (10 mg/kg) alone or oral THC (10 mg/kg) + CBD (10 mg/kg) in a randomized order prior to blood collection. Blood was collected into EDTA tubes 90-min after drug administration, matched for timing with the rPVT sessions. Tubes were then placed on wet ice for 30 min followed by centrifugation at 3000×g for 10 min. Plasma was transferred to low protein binding microcentrifuge tubes and stored at −80°C until shipment on dry ice to iC42 Clinical Research and Development (Aurora, CO) where THC and its key metabolites 11-Hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) and 11-Nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) were quantified using an established validated high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay. Details of the assay and validation results are as previously described (Sempio et al., 2022). The results of blank, zero, calibrators and quality control samples included in the study sample batch met all predefined acceptance criteria: the calibration range for THC and THC-COOH was 0.78– 400 ng/mL and that for 11-OH-THC 1.56–400 ng/ml. There was no carry over and no matrix interferences. Accuracy in the study sample batch was within the ±15% acceptance criterion and imprecision was <15%.
Data Analysis
Vehicle administrations for each drug condition were analyzed by a two-way, random effect intraclass correlations of absolute agreement (Shrout & Fleiss, 1979) to evaluate stability of individual performances. Intraclass correlation coefficients (ICC) range between 0 and 1, with values closer to 1 denoting smaller within-subject variation across sessions. For each drug, a repeated one-way ANOVA was conducted to evaluate effects when administered alone (dose as within-subjects effects). A repeated two-way ANOVA was conducted to evaluate the effects of THC + CBD combinations (THC Dose and CBD Dose as within-subjects effects). The following rPVT outcome metrics were assessed: 1) % correct trials (accuracy), 2) % premature responses (inhibitory control), 3) % lapses (sustained attention), and 4) reaction times (e.g., fastest 10%, median RT, slowest 10%). Two-way ANOVAs (for THC and CBD alone) or three-way ANOVAs (for THC + CBD combinations) were conducted to analyze outcomes across different stimulus intervals and RTs by percentile. Dunnett’s post hoc tests were used to analyze differences between drug dose and vehicle (for THC and CBD alone) or THC + CBD dose and no CBD/THC alone condition (for THC + CBD combinations). Metabolites in blood after THC or THC + CBD administration were compared with unpaired, two-tailed t-tests. Statistics were performed in GraphPad Prism with p ≤ 0.05 significance level.
Results
Individual performance on the rPVT is stable
ICC analysis showed good internal consistency for accuracy [single measure ICC (2,15)=0.66, F(4, 76)=44.32, p<0.001], premature responding [single measure ICC (2,15)=0.72, F(4, 76)=51.23, p<0.001], and lapses [single measure ICC (2,15)=0.47, F(4, 76)=19.20, p<0.001] across 15 vehicle test days.
CBD did not impair sustained attention in the rPVT
When administered alone, CBD (1–100 mg/kg) had no effects on accuracy in the rPVT (Fig 1A, open circles; F(6,34)=0.23, p=0.78; see also Supplemental Fig 1A). CBD also did not affect % of lapses in attention (Fig 1B; F(6,34)=0.34, p=0.75), premature responses (Fig 1C; F(6,34)=0.49, p=0.58), nor reaction times (Fig 2A; F(6,96)=1.05, p=0.40).
Fig 1.
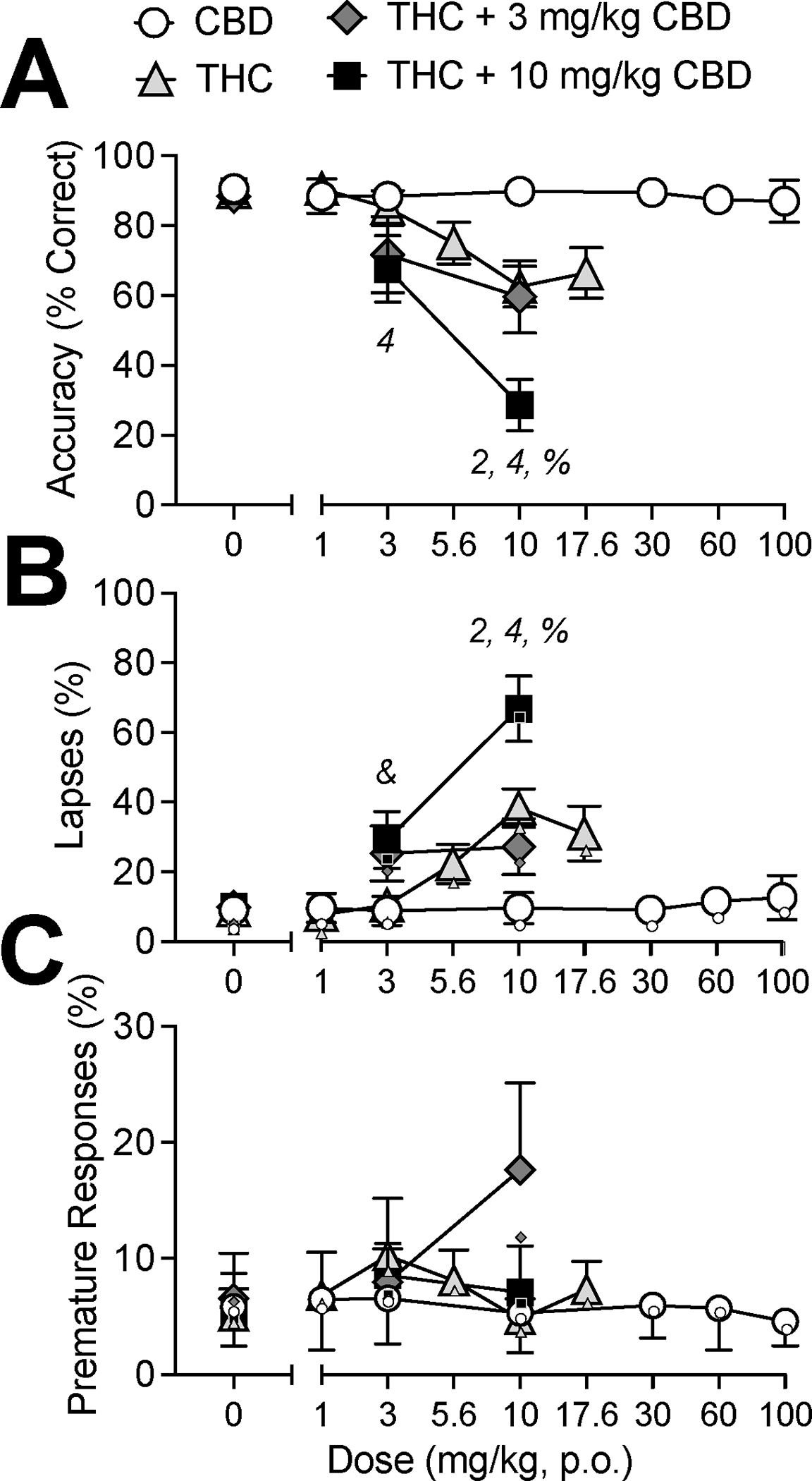
Effects of vehicle (0 mg/kg), CBD alone (1–100 mg/kg, p.o.), THC alone (1–17.6 mg/kg, p.o.), or THC (3, 10 mg/kg, p.o.) in combination with CBD (3, 10 mg/kg, p.o.) on performance in the rPVT. (A) Total accuracy (% correct trials) (B) Percent of lapses (C) Percent of premature responses. Data are mean ±SEM. For lapses (B), small data points indicate the proportion of missed trials. For premature responses (C), small data points indicate the proportion of false alarms. Numbers denote differences from vehicle within different groups: CBD alone (1), THC alone (2), 3 mg/kg THC + CBD (3) and 10 mg/kg THC + CBD (4). Symbols denote a difference between THC alone and THC + CBD group (THC + 3 mg/kg CBD vs. 3 mg/kg CBD alone: “&”; THC + 10 mg/kg CBD vs. 10 mg/kg CBD alone: “%”).
Fig 2.
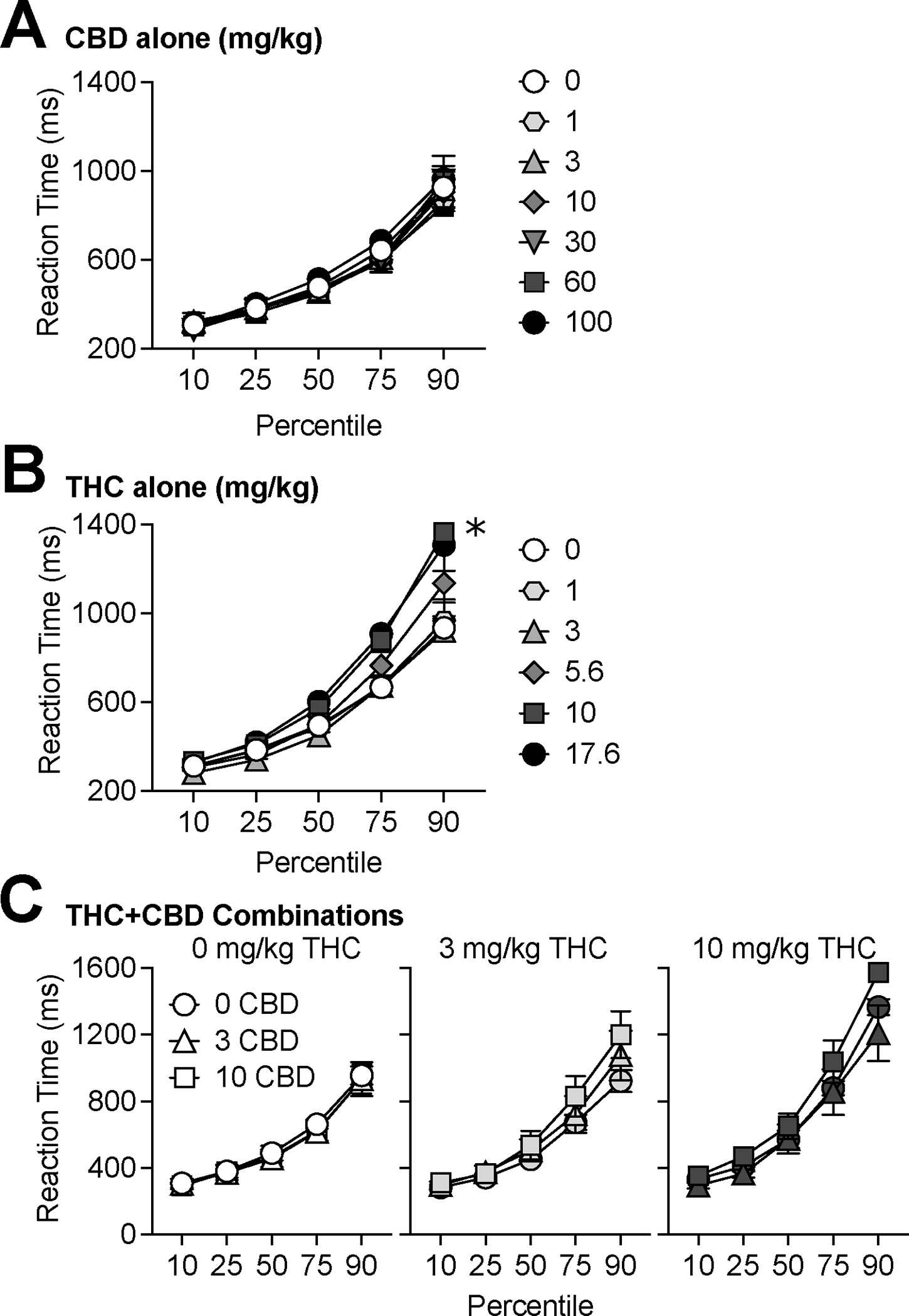
Reaction times by percentile for (A) CBD alone (0–100 mg/kg, p.o.), (B) THC alone (0–17.6 mg/kg, p.o.), or (C) THC (0, 3, 10 mg/kg, p.o.) in combination with CBD (0, 3, 10 mg/kg, p.o.). Asterisks (*) represent significant difference from vehicle (p<0.05).
THC impaired sustained attention in the rPVT
When THC (1–17.6 mg/kg) was administered alone, there was a dose-dependent decrease in accuracy in the rPVT (Fig 1A, gray triangles; F(5,29)=6.20, p<0.05). Specifically, 10 mg/kg THC reduced accuracy compared to vehicle (p<0.05). Reductions in accuracy were observed across all response stimulus intervals (i.e., no interaction between Interval × THC Dose, p=0.12; Supplemental Fig 1B). THC produced a dose-dependent increase in lapses in attention (Fig 1B; F(5,29)=10.61, p<0.01); 10 mg/kg THC significantly increased lapses compared to vehicle (p<0.05). There was no effect of THC on premature responses (Fig 1C; F(5,29)=1.04, p=0.40). Analysis of THC effects on RTs across percentiles demonstrated that THC increased RTs (Fig 2B; Percentile × THC interaction: F(20,80)=6.30, p<0.05); Q90 RTs were slower after treatment with 10 mg/kg THC compared with vehicle (p<0.05). Interestingly, treatment with 17.6 mg/kg THC did not significantly reduce accuracy (p=0.08); increase lapses (p=0.08), nor increase Q90 RTs (p=0.11).
THC + CBD combination effects on rPVT performance
The co-administration of selected doses of CBD and THC caused greater impairments to sustained attention than when THC was administered alone (Fig 1A; gray diamonds and black squares). Specifically, there was an interaction of THC × CBD on accuracy, F(4,16)=3.30, p<0.05), in which THC + CBD (10:10) administration resulted in poorer accuracy than 10 mg/kg THC alone (p<0.01). Impairments in accuracy produced by co-administration of THC + CBD were equivalent across response stimulus intervals (i.e. no interaction of response stimulus interval × CBD Dose for 0, 3, or 10 mg/kg THC; Supplemental Fig 1C).
We also observed an interaction of THC × CBD on lapses in attention (Fig 1B; F(4,16)=5.00, p<0.01), THC + CBD (3:10 and 10:10) resulted in a greater percentage of lapses compared with 3 or 10 mg/kg THC alone, respectively (p’s<0.05). There were no effects of THC, CBD, nor a CBD × THC interaction on premature responding (Fig 1C; THC × CBD, F(4,16) = 2.22, p=0.11). CBD did not modulate the effects of THC on increasing reaction times (i.e. no interaction of percentile × CBD Dose for 0, 3, or 10 mg/kg THC; Fig 2C).
Blood levels of THC, 11-OH-THC, and THC-COOH
Levels of THC in plasma collected 90-min after administration of THC alone (10 mg/kg) did not differ from THC levels after administration of THC + CBD (10:10) [THC alone: 40.7±16.9 ng/ml, THC + CBD: 43.66 ± 30.9 ng/ml; t(7)=0.09, p=0.93]. Similarly, levels of 11-OH-THC in blood were not significantly different after THC alone (10 mg/kg) and THC + CBD (10:10) administration [THC alone: 40.1 ±11.0 ng/ml, THC + CBD: 26.3 ±11.8 ng/ml; t(7)=0.85, p=0.42]. Levels of the metabolite THC-COOH in blood were higher after THC alone (10 mg/kg) compared with THC + CBD (10:10) administration [THC alone: 80.2 ±17.3 ng/ml, THC + CBD: 22.6 ±2.4 ng/ml; t(7)=2.92, p=0.02]. One sample (THC + CBD) was excluded from analysis due to classification as an outlier [THC: 346.9 ng/ml (<8 SD above the mean)], 11-OH-THC: 23.8 ng/ml, THC:COOH: 17.9 ng/ml]. This subject’s THC alone blood sample and behavioral data (all collected on separate days) were included in all other analyses.
Discussion
This study demonstrated that orally-administered THC caused disruptions in attention which were further exacerbated by the co-administration of CBD. THC and THC + CBD combinations, but not CBD alone, reduced overall accuracy in the rPVT through an increase in the number of lapses. Lapses are trials not responded to (i.e., misses) and trials where the RT is greater than twice an animal’s mean RT for that session. The increase in lapses following THC and THC + CBD administration was entirely due to increases in missed trials. THC also increased RTs, particularly the slowest 10%. This effect was not modulated by CBD. Reaction times are only included in the analysis if the response occurs within 1500 ms following the onset of the stimulus. The evidence that THC alone and THC + CBD selectively affected the slowest 10% of RTs indicates that rats could perform the task, which includes responding within a short timeframe. This is relevant, as THC has previously been shown to alter time estimation in rats and humans (Han & Robinson, 2001; Sewell et al., 2013); however, the increases in lapses in attention that occurred after THC + CBD combinations were primarily due to misses (i.e. no response), indicating that more likely, THC and THC + CBD effects were due to either inattention to the stimulus or sedation.
In humans, acute THC causes deficits on a variety of cognitive tasks including information processing, divided attention, tracking performance, and driving tests (for review, see Broyd, van Hell, Beale, Yucel, & Solowij, 2016). Prior results from clinical studies investigating the effects of THC + CBD combinations have been mixed, with the direction of CBD modulatory effects on THC-induced impairments in cognitive abilities being inconsistent across outcomes (for review, see Freeman et al., 2019; Osborne et al., 2017). In a clinical study of psychomotor performance, similar to the task in the present study, oral administration of 2.5 mg THC + 1.35 mg CBD, but not THC alone (2.5 mg), impaired performance on a finger tapping task (Roser et al., 2009), a well-established test of motor disturbances and inter-manual coordination. In another human laboratory study, administration of vaporized cannabis containing 13.75 mg of THC alone or 13.75 mg THC + 13.75 mg CBD induced greater impairment on the Divided Attention Task and Paced Auditory Serial Addition Task, when compared with cannabis containing 13.75 mg THC alone (Arkell et al., 2019). However, in this same study, there was no difference between THC or THC + CBD conditions on the Digit Symbol Substitution Task. In contrast, (Englund et al., 2013) gave subjects oral CBD (600 mg) prior to intravenous THC (1.5 mg/kg) and assessed verbal learning and memory, symbol coding (processing speed), and digit-span forward and reverse (working memory). CBD co-administration did not affect THC-induced impairments in the symbol coding or digit span tasks; however, CBD mitigated the THC-induced performance deficit on the delayed recall test (verbal memory) (Englund et al., 2013). Incongruent findings from these studies likely underscore the importance of dose of CBD and dose ratio of THC + CBD.
Several nonhuman primate studies have observed a mitigation of THC effects by CBD. In rhesus macaques performing a stop signal task, THC (0.32 mg/kg, intramuscular, IM) impaired accuracy; CBD co-administration (1 mg/kg, IM) attenuated this impairment (Jacobs et al., 2016). However, CBD did not alter the effects of THC when administered in a 1:1 dose ratio (Jacobs et al., 2016). A study using adolescent squirrel monkeys found that co-administration of CBD (0.3 mg/kg, IM) did not affect THC-induced (0.1 mg/kg, IM) cognitive impairment; however THC + CBD co-administration mitigated the slowing of reaction times produced by THC alone (Withey et al., 2021). In a study with rhesus macaques, CBD (0.5 mg/kg, IM) co-administered with THC (0.2 or 0.5 mg/kg, IM) blocked THC-induced impairments in a visuospatial memory task (Wright, Vandewater, & Taffe, 2013). This study also examined other cognitive tasks, including self-ordered search and progressive-ratio test of motivated responding, and CBD had no effect on THC-induced impairments (Wright et al., 2013). Overall, the limited available data from nonhuman primate studies indicate either no effect or a mitigation of THC effects by CBD.
Similar to the present study, a recent study in rats found no effects of low dose THC alone (3 mg/kg, IP) on attention, impulsivity, perseverations, or response latencies in the 5-choice serial reaction time task (5-CSRTT) (Barnard et al., 2022). This study also assessed effects of acute smoke exposure of cannabis containing primarily THC (19.5%, CBD <0.07%) or primarily CBD (17.9%, 0.7% THC), observing no effects of either cannabis strain on attention (Barnard et al., 2022). These doses were likely not high enough to observe effects that were seen in the present study at 10 mg/kg THC alone or with combinations of THC + CBD. Interestingly, in the Barnard et al. (2022) study, plasma THC concentrations were higher at the time of testing (124 ng/ml, 30 minutes post-IP injection) compared with the present study (40.7 ng/ml, 90 minutes post-oral gavage). This highlights differences in pharmacokinetics and pharmacodynamics based on route of administration (Baglot et al., 2021), as well as commonly observed lack of correlation between THC blood levels and behavior (Ginsburg, Hruba, Zaki, Javors, & McMahon, 2014; Grotenhermen, 2003; Hollister et al., 1981).
Following THC or THC + CBD administration, levels of THC and metabolites, 11-OH-THC and THC-COOH observed in the present study were comparable to other preclinical studies (Baglot et al., 2021; Moore, Davis, Harvey, Taffe, & Weerts, 2021), and these levels are similar to those observed in clinical studies following THC administration (Grotenhermen, 2003). The levels of THC and metabolites in blood were variable, consistent with studies of oral cannabinoid administration in humans (Newmeyer et al., 2017), and were not observed to be correlated with any behavioral outcomes. Evidence from other studies suggest that the modulation of THC effects by CBD are due, at least in part, to pharmacokinetic interactions (for review, see Boggs et al., 2018). The addition of CBD likely delays the metabolism and elimination of THC through actions on shared CYP450 metabolic pathways. In the present study, THC and metabolites were measured for verification of relevant levels, but this pilot study was underpowered to detect pharmacokinetic interactions. While we did not see statistical differences in THC levels between groups at 90-min post-treatment, the metabolite THC-COOH levels were significantly lower in the THC + CBD group, suggesting delayed metabolism. These data are in agreement with prior studies of THC + CBD co-administration in rats (Klein et al., 2011). Future studies are needed to further explore the pharmacokinetic interactions of orally administered THC + CBD in rats.
Supplementary Material
Public Health Significance:
A well-documented side effect of cannabis and its primary active constituent, Δ9-tetrahydrocannabinol (THC), is deficits in cognition and attention. Cannabidiol (CBD), a non-intoxicating constituent of cannabis, is thought to modulate THC’s impairing effects. Using laboratory animals, we tested effects of THC and CBD alone and in combination, on attention and found that CBD exacerbated the impairing effects of THC.
References
- Arkell TR, Lintzeris N, Kevin RC, Ramaekers JG, Vandrey R, Irwin C, … McGregor IS (2019). Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology (Berl), 236(9), 2713–2724. doi: 10.1007/s00213-019-05246-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglot SL, Hume C, Petrie GN, Aukema RJ, Lightfoot SHM, Grace LM, … Hill MN (2021). Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Sci Rep, 11(1), 23990. doi: 10.1038/s41598-021-03242-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard IL, Onofrychuk TJ, Sandini TM, McElroy DL, Zagzoog A, Roebuck AJ, … Howland JG (2022). The effects of acute Cannabis smoke or Delta(9)-THC injections on the trial-unique, nonmatching-to-location and five-choice serial reaction time tasks in male Long-Evans rats. Neurobiology of Learning and Memory, 192, 107624. doi: 10.1016/j.nlm.2022.107624 [DOI] [PubMed] [Google Scholar]
- Boggs DL, Nguyen JD, Morgenson D, Taffe MA, & Ranganathan M (2018). Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Delta(9)-Tetrahydrocannabinol. Neuropsychopharmacology, 43(1), 142–154. doi: 10.1038/npp.2017.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, Yucel M, & Solowij N (2016). Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biol Psychiatry, 79(7), 557–567. doi: 10.1016/j.biopsych.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Christie MA, Bolortuya Y, Chen LC, McKenna JT, McCarley RW, & Strecker RE (2008). Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task. Sleep, 31(10), 1393–1398. [PMC free article] [PubMed] [Google Scholar]
- Christie MA, McKenna JT, Connolly NP, McCarley RW, & Strecker RE (2008). 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. Journal of Sleep Research, 17(4), 376–384. doi: 10.1111/j.1365-2869.2008.00698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, & Gonzalez R (2013). Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev, 23(2), 117–137. doi: 10.1007/s11065-012-9222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, … Krystal JH (2004). The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology, 29(8), 1558–1572. doi: 10.1038/sj.npp.1300496 [DOI] [PubMed] [Google Scholar]
- Davis CM, DeCicco-Skinner KL, & Hienz RD (2015). Deficits in Sustained Attention and Changes in Dopaminergic Protein Levels following Exposure to Proton Radiation Are Related to Basal Dopaminergic Function. PLoS One, 10(12), e0144556. doi: 10.1371/journal.pone.0144556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CM, Roma PG, & Hienz RD (2016). A rodent model of the human psychomotor vigilance test: Performance comparisons. Journal of Neuroscience Methods, 259, 57–71. doi: 10.1016/j.jneumeth.2015.11.014 [DOI] [PubMed] [Google Scholar]
- Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, … Kapur S (2013). Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol, 27(1), 19–27. doi: 10.1177/0269881112460109 [DOI] [PubMed] [Google Scholar]
- Freeman AM, Petrilli K, Lees R, Hindocha C, Mokrysz C, Curran HV, … Freeman TP (2019). How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neuroscience & Biobehavioral Reviews, 107, 696–712. doi: 10.1016/j.neubiorev.2019.09.036 [DOI] [PubMed] [Google Scholar]
- Gibson LP, Karoly HC, Ellingson JM, Klawitter J, Sempio C, Squeri JE, … Hutchison KE (2022). Effects of cannabidiol in cannabis flower: Implications for harm reduction. Addiction Biology, 27(1), e13092. doi: 10.1111/adb.13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Hruba L, Zaki A, Javors MA, & McMahon LR (2014). Blood levels do not predict behavioral or physiological effects of Delta(9)-tetrahydrocannabinol in rhesus monkeys with different patterns of exposure. Drug and Alcohol Dependence, 139, 1–8. doi: 10.1016/j.drugalcdep.2014.02.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F (2003). Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet, 42(4), 327–360. doi: 10.2165/00003088-200342040-00003 [DOI] [PubMed] [Google Scholar]
- Han CJ, & Robinson JK (2001). Cannabinoid modulation of time estimation in the rat. Behavioral Neuroscience, 115(1), 243–246. doi: 10.1037/0735-7044.115.1.243 [DOI] [PubMed] [Google Scholar]
- Haney M (2005). The marijuana withdrawal syndrome: diagnosis and treatment. Curr Psychiatry Rep, 7(5), 360–366. doi: 10.1007/s11920-005-0036-1 [DOI] [PubMed] [Google Scholar]
- Hart CL, van Gorp W, Haney M, Foltin RW, & Fischman MW (2001). Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology, 25(5), 757–765. doi: 10.1016/S0893-133X(01)00273-1 [DOI] [PubMed] [Google Scholar]
- Hollister LE, Gillespie HK, Ohlsson A, Lindgren JE, Wahlen A, & Agurell S (1981). Do plasma concentrations of delta 9-tetrahydrocannabinol reflect the degree of intoxication? Journal of Clinical Pharmacology, 21(S1), 171S–177S. doi: 10.1002/j.1552-4604.1981.tb02593.x [DOI] [PubMed] [Google Scholar]
- Jacobs DS, Kohut SJ, Jiang S, Nikas SP, Makriyannis A, & Bergman J (2016). Acute and chronic effects of cannabidiol on Delta(9)-tetrahydrocannabinol (Delta(9)-THC)-induced disruption in stop signal task performance. Exp Clin Psychopharmacol, 24(5), 320–330. doi: 10.1037/pha0000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, … McGregor IS (2011). Cannabidiol potentiates Delta(9)-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl), 218(2), 443–457. doi: 10.1007/s00213-011-2342-0 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Varvel SA, & Martin BR (2002). Endocannabinoids in cognition and dependence. Prostaglandins Leukot Essent Fatty Acids, 66(2–3), 269–285. doi: 10.1054/plef.2001.0351 [DOI] [PubMed] [Google Scholar]
- Lim J, & Dinges DF (2008). Sleep deprivation and vigilant attention. Annals of the New York Academy of Sciences, 1129, 305–322. doi: 10.1196/annals.1417.002 [DOI] [PubMed] [Google Scholar]
- Loomis S, McCarthy A, Edgar D, Tricklebank M, & Gilmour G (2013). Behavioural evidence that modafinil and amphetamine do not produce equivalent qualities of wake promotion in sleep-restricted rats. Sleep Medicine, 14, e185. doi: 10.1016/j.sleep.2013.11.436 [DOI] [Google Scholar]
- MacCallum CA, & Russo EB (2018). Practical considerations in medical cannabis administration and dosing. Eur J Intern Med, 49, 12–19. doi: 10.1016/j.ejim.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Moore CF, Davis CM, Harvey EL, Taffe MA, & Weerts EM (2021). Appetitive, antinociceptive, and hypothermic effects of vaped and injected Delta-9-tetrahydrocannabinol (THC) in rats: exposure and dose-effect comparisons by strain and sex. Pharmacology Biochemistry and Behavior, 202, 173116. doi: 10.1016/j.pbb.2021.173116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CF, & Weerts EM (2022). Cannabinoid tetrad effects of oral Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in male and female rats: sex, dose-effects and time course evaluations. Psychopharmacology (Berl), 239(5), 1397–1408. doi: 10.1007/s00213-021-05995-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, & Curran HV (2010). Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. British Journal of Psychiatry, 197(4), 285–290. doi: 10.1192/bjp.bp.110.077503 [DOI] [PubMed] [Google Scholar]
- Newmeyer MN, Swortwood MJ, Andersson M, Abulseoud OA, Scheidweiler KB, & Huestis MA (2017). Cannabis Edibles: Blood and Oral Fluid Cannabinoid Pharmacokinetics and Evaluation of Oral Fluid Screening Devices for Predicting Delta(9)-Tetrahydrocannabinol in Blood and Oral Fluid following Cannabis Brownie Administration. Clinical Chemistry, 63(3), 647–662. doi: 10.1373/clinchem.2016.265371 [DOI] [PubMed] [Google Scholar]
- Osborne AL, Solowij N, & Weston-Green K (2017). A systematic review of the effect of cannabidiol on cognitive function: Relevance to schizophrenia. Neuroscience & Biobehavioral Reviews, 72, 310–324. doi: 10.1016/j.neubiorev.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Roser P, Gallinat J, Weinberg G, Juckel G, Gorynia I, & Stadelmann AM (2009). Psychomotor performance in relation to acute oral administration of Delta9-tetrahydrocannabinol and standardized cannabis extract in healthy human subjects. Eur Arch Psychiatry Clin Neurosci, 259(5), 284–292. doi: 10.1007/s00406-009-0868-5 [DOI] [PubMed] [Google Scholar]
- Sempio C, Almaraz-Quinones N, Jackson M, Zhao W, Wang GS, Liu Y, … Klawitter J (2022). Simultaneous Quantification of 17 Cannabinoids by LC-MS-MS in Human Plasma. Journal of Analytical Toxicology, 46(4), 383–392. doi: 10.1093/jat/bkab030 [DOI] [PubMed] [Google Scholar]
- Sewell RA, Schnakenberg A, Elander J, Radhakrishnan R, Williams A, Skosnik PD, … D’Souza DC (2013). Acute effects of THC on time perception in frequent and infrequent cannabis users. Psychopharmacology (Berl), 226(2), 401–413. doi: 10.1007/s00213-012-2915-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, & Fleiss JL (1979). Intraclass correlations: uses in assessing rater reliability. Psychol Bull, 86(2), 420–428. doi: 10.1037//0033-2909.86.2.420 [DOI] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Goffi E, Weerts EM, Mitchell JM, Winecker RE, … Vandrey R (2020). Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users. Drug and Alcohol Dependence, 211, 107937. doi: 10.1016/j.drugalcdep.2020.107937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, … Vandrey R (2018). Acute Effects of Smoked and Vaporized Cannabis in Healthy Adults Who Infrequently Use Cannabis: A Crossover Trial. JAMA Netw Open, 1(7), e184841. doi: 10.1001/jamanetworkopen.2018.4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins WC, & Miller JM (1964). Reaction Time as a Function of Stimulus Intensity for the Monkey. Journal of the Experimental Analysis of Behavior, 7, 309–312. doi: 10.1901/jeab.1964.7-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withey SL, Kangas BD, Charles S, Gumbert AB, Eisold JE, George SR, … Madras BK (2021). Effects of daily Delta(9)-Tetrahydrocannabinol (THC) alone or combined with cannabidiol (CBD) on cognition-based behavior and activity in adolescent nonhuman primates. Drug and Alcohol Dependence, 221, 108629. doi: 10.1016/j.drugalcdep.2021.108629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ Jr., Vandewater SA, & Taffe MA (2013). Cannabidiol attenuates deficits of visuospatial associative memory induced by Delta(9) tetrahydrocannabinol. British Journal of Pharmacology, 170(7), 1365–1373. doi: 10.1111/bph.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


