Abstract
Purpose of the Review
Early diagnosis and treatment of HIV following seroconversion improves individual and population health. Using published data on pre-treatment CD4 cell counts, we benchmarked the level of immunodeficiency at HIV diagnosis and ART initiation in the “real world” against those of the treatment and control arms of landmark controlled trials that successfully reduced HIV-related deaths (INSIGHT/START) and onward HIV transmission (HPTN 052).
Recent Findings
The median CD4 count in the treatment vs. control arms of the INSIGHT/START trial and HPTN 052 were 650 vs. 408 cells/μL and 442 vs. 221 cells/μL, respectively. In the real world, recent global estimates of the median CD4 count at start of ART range from 234 to 350 cells/μL, and only 25% of those initiating ART do so early (i.e., with CD4 > 500 cells/μL). Recent global data on trends in the median CD4 count at diagnosis and ART initiation are not encouraging.
Summary
We identify a critical need for new targets and metrics for persons newly diagnosed with HIV, newly enrolling in HIV care, and newly initiating ART, based on pre-treatment CD4 counts, to help increase the focus of implementation efforts on achieving earlier diagnosis, linkage to care, and ART initiation.
Keywords: Pre-treatment CD4 count, Implementation science, Metrics, 90-90-90 targets, Treatment as prevention
Introduction
A key tenet of implementation science is improving the uptake, engagement, and ultimately the impact of evidence-based interventions. All large-scale programmatic implementation, such as HIV care and treatment scale-up, should be tied to metrics that reflect both implementation outcomes and health outcomes. Moreover, these metrics should reflect, as closely as possible, the overarching goals of the implementation activities.
The Goals of the Response to the HIV Epidemic Are to Diagnose and Treat All Persons with HIV As Soon as Possible After Seroconversion
The overarching goals of the public health response to the HIV epidemic are to reduce, control, or eliminate: (1) HIV-related morbidity and mortality and (2) the onward spread of HIV infection. These goals are informed by evidence from landmark randomized controlled trials that have demonstrated a fundamental premise underlying the public health response to the HIV epidemics around the globe: diagnosing and treating all persons with HIV as soon as possible after seroconversion is the most effective way to reduce both the risk of HIV-related morbidity/mortality and onward transmission of the HIV virus. However, in this current era of universal test and treat [1] with millions of people on lifesaving antiretroviral therapy (ART) [2], still millions have undiagnosed or untreated HIV [3], and trends in pre-treatment CD4 count (i.e., at diagnosis and at ART initiation) are problematic [4-9], raising concerns about suboptimal impact of implementation activities and the public health response to the global HIV epidemic on HIV incidence and death. [2, 3].
Unpacking the Evidence That Supports This Fundamental Premise
Early effective combination antiretroviral treatment (ART) improves the prognosis of HIV-infected persons, reduces HIV morbidity and mortality [10-12], and also dramatically reduces the likelihood of onward HIV-transmission [13]. In the HIV Prevention Trial Network (HPTN) 052 trial [13], early initiation of ART (median CD4+ cell count 442 cells/μL) reduced HIV sexual transmission by 93% among HIV serodiscordant couples as compared with delayed ART initiation (median CD4 count 221 cells/μL) [14]. Because of this and compelling data from observational and modeling studies, the CD4 cell count threshold for ART initiation has increased from 200 cells/μL in 2006 to 350 cells/μL in 2010 to 500 cells/μL in 2013[15, 16]. Ample evidence supports early ART as an essential strategy for controlling HIV epidemic spread (i.e., treatment as prevention) [12, 13, 17-19].
More recently, the first evidence from randomized trials (i.e., strategic timing of antiretroviral therapy (START) and TEMPRANO) has shown that even earlier initiation of ART, specifically at a high baseline CD4 count > 500 cells/μL (median CD4 count 650 cells/μL), yields substantial clinical benefits, including major reductions in the risk of serious AIDS events, serious non-AIDS events, and death compared with delayed treatment initiation (median CD4 count 408 cells/μL) [10, 11]. As a result of this evidence, universal voluntary HIV counseling and testing followed by immediate initiation of ART for all persons diagnosed with HIV (universal testing and treatment, Universal Test and Treat (UTT)), irrespective of clinical stage or CD4 count, is now the global standard [3, 20-22] and increasingly the global norm in practice [3, 23••-25].
Despite Their Central Importance, the Timeliness of HIV Diagnosis and ART Initiation Are Not Reflected in the Status Quo Metrics Used to Monitor and Inform the Implementation Activities and Public Health Response to the HIV Epidemic
Efforts aimed at achieving substantial reductions in HIV incidence and mortality requires high coverage of early ART initiation [10, 11, 18, 20, 26]. Thus, it is not enough to strive for high population-level coverage of HIV diagnosis and ART initiation. Targets and metrics should also push initiatives to strive towards high population-level coverage of early diagnosis and ART initiation, outcomes for which targets or metrics do not yet exist. Metrics commonly used to monitor and inform the public health response include the following.
Epidemiologic Metrics
Global and national metrics include HIV incidence, overall HIV prevalence, the prevalence of undiagnosed HIV, and deaths (all cause and HIV-related), including year-specific estimates and trends over time for these metrics, disaggregated by sex, population (e.g., pregnant women and key populations) and broad age categories [2, 27-29]. These estimates are usually generated through a combination of surveillance and modeling. In some places with HIV case surveillance, epidemiologic metrics include individual-level, population-based data on the number with newly diagnosed HIV, number with newly diagnosed AIDS, and the ability to finely stratify indicators by age, sex, geography, and risk factor or key population group [30]. Measuring HIV serologic status is now standard on many population-based or household surveys like the demographic and health surveys (DHS) with HIV serologic testing and AIDS Indicator Surveys (AIS), making them useful for estimating HIV prevalence, including the prevalence of undiagnosed HIV, with disaggregation by sex and age [31, 32]. More recently, AIDS Indicator Surveys [33] and even prospective studies [34] have been used to generate national estimates of HIV incidence.
Programmatic and Implementation Metrics
Normative bodies—such as WHO and the Joint United Nations Programme on HIV/AIDS (UNAIDS)—and programmatic initiatives—such as President’s Emergency Plan For AIDS Relief (PEPFAR), the global fund, and national and local HIV/AIDS programs-produce global, national, and subnational metrics reflecting their programmatic coverage, outputs, and accomplishments [35]. These metrics are often limited to those diagnosed and/or receiving care and other HIV-related prevention or treatment services. Typical programmatic metrics include the number of clinics offering HIV services, the number of pregnant women tested for HIV, the number of people living with HIV (PLWH) in care, and the number of PLWH on ART. In some places where viral load testing is routine, metrics are available on the number of PLWH in HIV care with viral suppression. These metrics are often disaggregated by geography, sex, and broad age categories (see, for example, amfAR’s interactive version of PEPFAR’s monitoring, evaluation, and reporting database [36]).
Hybrid Metrics
We refer to the combination of epidemiological and programmatic or implementation metrics as “hybrid metrics.” The HIV care continuum [37-39] and metrics used to track progress towards UNAIDS 90-90-90 targets [40] are examples of hybrid metrics. The HIV care continuum, a model that depicts the sequential steps of HIV medical care for persons living with HIV, illustrates the number or proportion of persons who are estimated to be HIV infected, diagnosed, receiving medical care, prescribed ART, and virally suppressed. The UNAIDS 90-90-90 initiative is essentially a targeted care continuum, incorporating goals of 90% achievement at certain steps of the continuum. Targeted care continua have strengths from a public health perspective, since the continuum and 90-90-90 incorporate the entire denominator of people living with HIV, including those with undiagnosed HIV. Arguably, hybrid metrics are the most commonly used metrics for monitoring the status and the progress of the response to the HIV epidemic.
Other examples of hybrid metrics include those generated from AIDS Indicator Surveys with additional HIV-related biomarkers [41] and, more recently, the PEPFAR-funded population-based HIV Impact Assessments (PHIAs), which are cross-sectional household surveys that measure both epidemiologic and programmatic metrics [42].
The Problem With the Status Quo Metrics
All of the above metrics are useful for epidemiologic surveillance of the HIV epidemic, tracking HIV prevention and treatment cascade milestones, and, assessing the progress, and in some cases, the impact, of programmatic efforts. However, for the most part, these metrics do not reflect the fundamental public health implementation goal of diagnosing and treating all persons with HIV as soon as possible after seroconversion.
The hallmark of HIV infection and disease progression is continuous and progressive decline of CD4 cells in the absence of treatment [43-45]. The factors that determine disease progression depend on the individual and virus, and significant heterogeneity in CD4 decline has been documented [46-48] However, pre-treatment CD4 counts, on average, are reflective of both current immunodeficiency and time elapsed since HIV seroconversion [47]. The level of immunodeficiency reflects the risk of opportunistic illness and death; the time elapsed since seroconversion, and prior to ART initiation, reflects the period of time during which there is a risk of onward transmission of HIV.
Using data on pre-treatment CD4 counts from population-based surveillance data in the USA, the Centers of Disease Control and Prevention (CDC) estimated that the 44,450 persons diagnosed with HIV in 2011 [49] were infected with HIV for an average of 5.6 years (95% CI 5.5–5.6 years) by the time they were diagnosed [50]. Investigators in France estimated the time between steps of the HIV care cascade in 2010 and found that a median of 3.4 years (inter-quartile range (IQR) 1.1–5.7 years) elapsed between infection and viral suppression [51]. In a representative sample of patients initiating ART in Cameroon from 2007 to 2010, patients had been infected for an average of 9–10 years before starting ART. Data from seroconverter cohorts indicate that, in the absence of treatment, the average time from seroconversion to a CD4 count of 350 cells/μL is 2.8 to 4.2 years, and that that the average time from seroconversion to a CD4 count of 500 cells/μL is 1–2 years [39, 46-48, 52, 53]. CD4-based metrics that reflect both immunodeficiency (risk of morbidity/mortality) and time since seroconversion (risk of onward transmission) are valuable because they contain longitudinal information. Yet, they are largely absent among the metrics used to track and inform implementation activities and the public health response to the HIV epidemic. We argue below that such metrics are critical to the future success of the response to the HIV epidemic.
Discussion
In the remaining discussion, we: (1) review available global data on the typical levels of immunodeficiency at HIV diagnosis and ART initiation using published estimates of pre-treatment CD4 count information from the “real world,” (2) benchmark these “real world” pre-treatment CD4 counts against those from the most recent WHO recommendations for when to start ART prior to their “treat all” recommendation in late 2015 as well as landmark randomized controlled trials that serve as the evidence-base for the programmatic implementation and the global response to the HIV epidemic, and (3) conclude by proposing new CD4-based targets and metrics that can help steer global initiatives towards an even more effective, evidence-aligned response to the HIV epidemic, by increasing the focus of implementation efforts on early diagnosis and ART initiation.
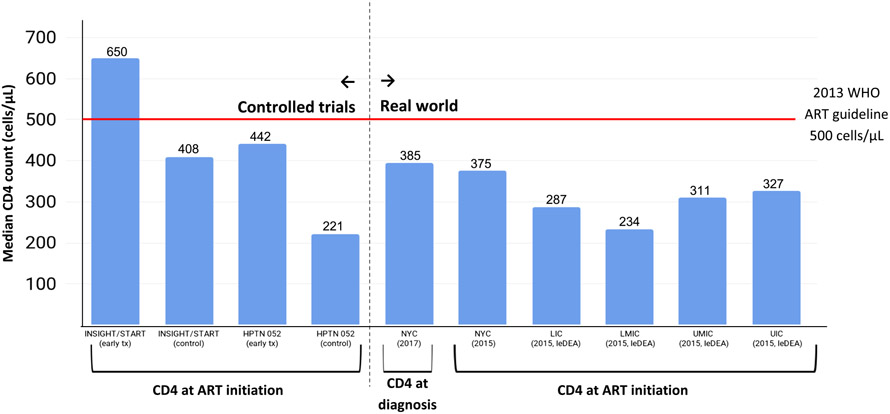
For the purposes of this paper, we define early diagnosis, linkage, and ART initiation as having a CD4 > 500 cells/μL at the time when these events occur, and “coverage” of early outcomes as the proportion of PLWH experiencing these events with CD4 > 500 cells/μL. We chose 500 cells/μL as the threshold because: (a) the current median CD4 at ART initiation is much lower globally, and evidence suggests “higher CD4 is better”; (b) 500 cells/μL was the last treatment guideline (WHO 2013), prior to which ART should be initiated; (c) the median time from seroconversion to a CD4 of 500 cells/μL is 1–2 years, which seems like an achievable time period during which an HIV diagnosis could be made for most people with HIV, assuming appropriately targeted HIV testing; and (d) 500 cells/μL is below the median cell count in between the median CD4 counts of the treatment (650 cells/μL) and control (408 cells/μL) arms of INSIGHT/START.
CD4 Counts at HIV Diagnosis and ART Initiation in the Real World
CD4 Counts at HIV Diagnosis/Linkage to Care
The median CD4 count at diagnosis and/or linkage to care is a useful metric because it represents a ceiling for the median CD4 count at ART initiation, given continuous and progressive decline of CD4 cells after seroconversion in the absence of treatment. When available, population-based (or otherwise representative) data on CD4 counts at HIV diagnosis and linkage to care are extremely useful for monitoring the timeliness of diagnosis or care enrollment and for identifying potential for improvements in earlier ART initiation that could be achieved through more focused, metric-driven implementation.
A systematic review of the median CD4 count at presentation to care in developed countries during 1992–2011 reported an increase of only 1.5 cells/μL per year, from 307 cells/μL in 2006 to 336 cells/μL in 2011 [54]. In New York City, which is an epicenter of the epidemic in the USA and where concerted efforts to “End the Epidemic” are underway, population-based data show that only 35% were diagnosed with CD4 > 500 cells/μL as recently as 2015 [55], and the median CD4 count at diagnosis has increased from 326 cells/μL in 2006 to 385 cells/μL in 2017, and the already slow trend has plateaued in recent years [56]. In New York, the median CD4 of 385 at diagnosis is the “ceiling,” meaning that at least half of New Yorkers diagnosed with HIV in 2017 will initiate ART at a CD4 count of 385 cells/μL or lower, indicative of much-needed improvements in the coverage of early HIV diagnosis (the prerequisite to improvements in early ART initiation). A meta-analysis of studies from sub-Saharan Africa of the median CD4 count at presentation to care and ART initiation during 2002–2013 estimated that the mean CD4 count at care entry (251 cells/μL) in 2002 did not increase materially over the subsequent 11-year period [57].
Population-based HIV surveillance systems, such as those in New York City [58, 59] and around the USA [30], are well positioned to monitor and report on trends in CD4 counts at diagnosis. Yet, beyond tracking immunologically defined AIDS (CD4 < 200 cells/μL), CD4 information is rarely disseminated by health authorities or used to evaluate or inform implementation activities or policies. For example, in the latest annual report from the US Centers for Disease Control and Prevention [30], CD4 count information at diagnosis was not presented at all, even though all jurisdictions in the USA report this information to the CDC. NYC has recently begun to include information on median CD4 counts in HIV annual reports and on their Ending the Epidemic (ETE) Dashboard system [56]; however, reporting on or disseminating such information remains the exception [60], despite the fact that low coverage of early diagnosis and linkage to care limits our ability to improve the coverage of early ART initiation (i.e., % with CD4 > 500 cells/μL at ART initiation) [6, 7, 61-64].
CD4 Counts at ART Initiation
Trends in median CD4 count at or immediately prior to ART initiation suggest that PLWH are increasingly starting ART earlier in the course of HIV infection, but these median CD4 counts remain low and their rate of increase, being limited by concomitant lack of improvements in median CD4 counts at diagnosis/linkage, is quite slow [4, 5, 8, 54, 57, 65-70]. In a recent global analysis of temporal trends in adult CD4 cell counts at treatment initiation among nearly 1 million patients initiating ART in the 55 countries represented in the IeDEA and COHERE cohorts, the estimated median CD4 cell count at the start of ART increased during 2002 to 2015 [5]. The rate and magnitude of increase in the median CD4 count at ART initiation varied by country income group, but notably, the median CD4 counts at ART initiation in the most recent year of the analysis (2015) were below 350 cells/μL in all settings; in other words, about 50% of people starting ART globally do so at levels below 350 cells/μL, where they are at high risk of death before and, for a time [71], after starting treatment. The coverage of early ART initiation —the proportion of patients initiating ART with CD4 > 500 cells/μL—in 2015 was < 25% for all groups except men in high income countries (for whom it was only slightly higher) [5].
The most recent analysis of median CD4 at ART initiation in New York City found an increase from 199.5 cells/μL in 2006 to 352 cells/μL in 2012 (21.8 cells per year) [9], and only 77% of persons diagnosed in 2015 initiated ART within 6 months [55]. If this CD4 trend continues to prevail, the coverage of early ART initiation should reach 50% in NYC in 2019. Though the timeliness of ART initiation relative to seroconversion (as measured by CD4 at ART start) has improved, progress is slow.
Benchmarking Estimates of Pre-Treatment CD4 Counts from the “Real World” Against Treatment Guidelines and Efficacy Studies that Successfully Reduced Onward HIV Transmission and HIV-Related Deaths
Meaningful evidence, policy, and practice benchmarks against which to compare the actual CD4 count at ART initiation in the real world to assess the progress of the response to the HIV epidemic using pre-treatment CD4 counts include: (1) the CD4 thresholds of global HIV treatment guidelines (prior to ‘treat all’ recommendations) and (2) the median CD4 counts in the treatment and control arms of the above landmark randomized controlled trials that showed substantial reductions in onward HIV transmission and risk of HIV-related death.
We took the published data from the real world on the median CD4 count at diagnosis and ART initiation from the prior section and plotted them in relation to these benchmarks, which included the CD4 counts at ART initiation in the treatment vs. control arms of the INSIGHT/START (650 vs. 408 cells/μL) and HPTN 052 (442 vs. 221 cells/μL), and the WHO treatment guideline threshold prior to expansion to “Treat all” in late 2015 (i.e., 500 cells/μL). In the real world, median CD4 counts, especially those at ART initiation, were much lower (234–327 cells/μL) than that of the control arm of the INSIGHT/START trial (408 cells/μL), but higher than the control arm of HPTN 052 (221 cells/μL). This suggests that tremendous opportunity remains to more fully realize the clinical and public health benefits of ART promised by these controlled trials; this requires diagnosing and initiating ART much earlier among people living with HIV. Benchmarking real world data against the trials and previous treatment thresholds may provide insights about why we continue to observe suboptimal improvement in trends in incidence rates and death rates globally [2, 3].
Interpreting the Recent Results of Universal Test and Treat Intervention Trials
Four large-scale community-based randomized controlled intervention trials (ANRS, Ya Tse, SEARCH and PopART) aimed to assess the impact of Universal Test and Treat (UTT) strategies, and all examined HIV incidence in the community as a primary outcome. All four trials were set in sub-Saharan Africa (SSA). The preliminary evidence from these trials relating to the impact of UTT on HIV incidence is mixed [3], with Ya Tse and PopART showing reduced incidence under UTT [72, 73] and ANRS/TasP and SEARCH showing no effect [74]. The reasons are not yet clear. Importantly, no information on the CD4 count at diagnosis and/or treatment initiation has been reported as yet from these trials. Comparing the CD4 count at ART initiation between study arms and benchmarking, the median CD4 count at ART initiation in the intervention and control arms of these trials with those of the HPTN 052 and INSIGHT/START studies (as we have done in Fig. 1) could provide useful insights as to why the intervention trials were or were not effective at reducing HIV incidence and/or mortality.
Fig. 1.
Median CD4 count at ART initiation in landmark controlled trials (left side of Figure) and at diagnosis or ART initiation in the real world (right side of Figure)
Proposed CD4-Based Targets and Metrics That Can Help Increase the Coverage of Timely Diagnosis, Linkage, and ART Initiation
Among the status quo HIV metrics there is an absence of measures related to the critical component of timely achievement (i.e., CD4 > 500 cells/μL) of key programmatic milestones relative to HIV seroconversion. In part, this may be because events like HIV seroconversion are not generally observable. However, pre-treatment CD4 counts are both observable and routinely measured. Moreover, pre-treatment CD4 counts are often captured in the population-based surveillance systems of many resource-rich settings that are now actively focused on ending their HIV epidemics. We argue that it is not only possible, but essential to construct and/or model new and equally useful metrics for monitoring the coverage of timely achievement of programmatic outcomes, milestones, and targets relative to the time of seroconversion.
Specifically, we propose that data on pre-treatment CD4 counts be systematically leveraged for top line indicators capable of reflecting the timeliness of achievement of HIV care continuum milestones, especially early treatment initiation. The best available evidence dictates that the public health goal should be to diagnosis and initiate ART when the CD4 count is > 500 cells/μL [10, 11], and the threshold of the 2013 WHO guidelines suggested that all persons with HIV should start treatment before the CD4 drops below 500 cells/μL [15].
Therefore, we propose an analog to the UNAIDS 90-90-90 targets that will better align implementation targets and metrics with the public health goals of the response to the HIV epidemic: 90% of all persons with a new diagnosis of HIV should have a CD4 count > 500 cells/μL at diagnosis; 90% of all persons enrolling into HIV care should have a CD4 count > 500 cells/μL; and 90% of all people initiating ART should have a CD4 count > 500 cells/μL at treatment initiation. In 2015, if a threshold of a CD4 count of 500 cells/μL was used as a target for early ART initiation, we would be at 25% of the target globally (with the exception of men in high income countries who are at 26%) [5, 75]. We further recommend that whenever possible, initiatives and jurisdictions strive to routinely monitor the percent of PLWH with CD4 < 200 cells/μL prior to treatment (immunologically defined AIDS), and the median pre-treatment CD4 counts in their populations at the time they reach key care continuum milestones (i.e., CD4 count at diagnosis, linkage, and ART initiation). Proposed indicator definitions are provided in Table 1.
Table 1.
Recommended new priority implementation metrics based on pro-treatment CD4 counts to inform, monitor, and evaluate progress towards achieving key goals of the public health response to the HIV epidemic
| Metrics reflecting | Notes |
|---|---|
| Timeliness of diagnosis/care linkage | |
| Number and % with a CD4 count | Denominator. All persons newly diagnosed with HIV; Useful when population-based data on HIV diagnoses are available; Useful as an outcome for community testing intervention studies aimed at earlier diagnosis: Target: 90% of all persons newly diagnosed with HIV should have a pre-treatment CD4 > 500 cells/μL |
| Number and percent with CD4 > 500 cells/μL (timely diagnosis) | |
| Number and percent with CD4 < 500 cells/μL | |
| Number and percent with CD4 < 200 cells/μL | |
| Median CD4 count | |
| Timeliness of enrollment into HIV care | |
| Number and % with a CD4 count | Denominator: All persons newly enrolling in HIV care; Useful when population-based data on HIV diagnoses are not available; Useful as an outcome for community intervention studies aimed at earlier diagnosis and linkage; Target: 90% of all persons newly enrolling in HIV care should have a pre-treatment CD4 > 500 cells/μL |
| Number and percent with CD4 > 500 cells/μL (timely care enrollment) | |
| Number and percent with CD4 < 500 cells/μL | |
| Number and percent with CD4 < 200 cells/μL | |
| Median CD4 count | |
| Timeliness ART initiation | |
| Number and % with a CD4 count | Denominator: All persons initiating ART; Useful when population-based data on HIV diagnoses are not available; Useful as an outcome for community intervention studies aimed at earlier diagnosis. Linkage and ART initiation; Target: 90% of all persons newly initiating ART should have a pre-treatment CD4 > 500 cells/uL |
| Number and percent with CD4 > 500 cells/μL (timely ART initiation) | |
| Number and percent with CD4 < 500 cells/μL | |
| Number and percent with CD4 < 200 cells/μL | |
| Median CD4 count |
For the New Metrics Skeptics
There may be reluctance to consider adding new metrics and targets for the global HIV response. The HIV care continuum and 90-90-90 targets have been embraced and have helped to simplify metrics (making them more accessible to stakeholders in the response) and to greatly enhance comparative assessment of progress across settings. We hope to create a healthy dialog around the addition of new metrics and targets and have endeavored to address some potential concerns about the addition of new metrics into the mix:
Why add new metrics and targets when we already have good ones that are serving us well? Our review has clearly highlighted that the coverage of early diagnosis and ART initiation are suboptimal, arguably in part because we have not been monitoring the timeliness with which these outcomes are achieved and do not have related targets. We need metrics and targets that better align our response with its fundamental scientific premise. We believe adding new metrics is important when the status quo metrics will not suffice. The key concept of timeliness relative to seroconversion is unequivocally missing from our current metrics.
Pre-treatment CD4 testing for ART eligibility is no longer necessary because of “treat all.” CD4 testing is no longer needed when there is viral load testing. There is a high degree of consensus that CD4 monitoring after ART initiation is not needed, when routine viral load testing is being done [76]. This could result in a decline in the routine use of post-treatment CD4 monitoring in some settings. One recent study from SSA prior to the “treat all” era reported that nearly 30% of patients initiating ART during 2005–2015 had no pre-treatment CD4 count with 3 months before or one month after ART initiation [77]. More recent data from SSA suggest that pretreatment CD4 counts are becoming rare [78, 79]. While “treat all” policies have eliminated the need to assess eligibility for treatment, assessing pre-treatment immunodeficiency remains a critical standard of care [80-82] and a prerequisite to differentiated care [77, 80, 83]. It is very concerning, therefore, that PEPFAR has steadily and progressively reduced its support of CD4 testing in favor of viral load monitoring [84]. Pretreatment CD4 count monitoring that does not delay ART initiation must continue to support differentiated care (i.e., identification and management of advanced HIV disease), while viral load testing without CD4 is adequate for monitoring patients on treatment;
New metrics likely will not tell us anything we do not already know. CD4 counts at diagnosis and ART initiation are improving globally, albeit slowly. How will new metrics and targets help? With declines in incidence and mortality slowing considerably in recent years, both globally [75] and in some of the hardest hit areas [3], there are recent concerns about a potentially suboptimal public health impact of the response to the global HIV epidemic, despite rapid scale-up of HIV treatment [3, 85]. This may be precisely because there have not been targets or metrics that systematically monitor progress towards the central goal of increasing the coverage of timely ART initiation. Further, we should not assume that the current, inadequately improving trends could not level off completely or even reverse (e.g., as global financial commitment wanes). The proposed metrics would likely represent useful early warning systems, signaling well before increases in incidence and HIV-related deaths would be detected.
Conclusion—Breaking the Spell of the Red Queen
In a 2013 editorial entitled, Under the spell of Red Queen [4], the late Joep Lange lamented the stubbornly slow increasing trend in the median CD4 count at presentation to care, which he characterized with this quote from the Red Queen in Lewis Carroll’s Through the Looking Glass [86]: “It takes all the running you can do, to keep in the same place.” Our review highlights that this situation has not materially improved since he penned those words, making Lange’s apt characterization and concerns as relevant and sobering today as they were five years ago. However, we hold out hope that we can “break the spell of the Red Queen” by making key changes to our targets and metrics that explicitly call on policy makers and implementers to strive for earlier, more timely diagnosis and ART initiation, as these are fundamental prerequisites to securing future improvements in HIV-related deaths and HIV incidence. Combined with existing global and national ART coverage targets and metrics, coverage metrics for timely diagnosis, care enrollment, and ART initiation— with targets of 90% > 500 cells/μL—will be highly useful in maximizing progress and potential impact of today’s programmatic implementation on tomorrow’s HIV incidence and mortality.
Acknowledgments
The authors would like to acknowledge Rebecca Zimba and Julia A. Schillinger for their thoughts and comments on the drafts of our manuscript.
Funding Information
DN was supported by a funding from the US National Institutes of Health, including Central Africa IeDEA (U01AI096299), the Einstein, Rockefeller, CUNY Center for AIDS Research (ERC CFAR, P30 AI124414), and the HIV Center for Clinical and Behavioral Studies (P30 MH043520). DN and MR were both supported by the CUNY Institute for Implementation Science in Population Health.
Footnotes
Conflict of Interest Dr. Nash reports grants from NIH, during the conduct of the study. Ms. Robertson has nothing to disclose.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV (http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/). In: HIV/AIDS, editor. Geneva: ISBN: 978 92 4,150,956 52,015. Accessed 22 April 2019. [Google Scholar]
- 2.UNAIDS. UNAIDS Data Book Geneva: UNAIDS; 2018. [Available from: http://www.unaids.org/en/resources/documents/2018/unaids-data-2018. Accessed 22 April 2019. [Google Scholar]
- 3.Nash D, Yotebieng M, Sohn AH. Treating all people with HIV infection in sub-Saharan Africa: a new era calling for new approaches. J Viral Erad. 2018;4(Suppl 2):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lange JM. Editorial commentary: Under the spell of the red queen. Clin Infect Dis. 2013;57(7):1048–50. [DOI] [PubMed] [Google Scholar]
- 5.••. IeDEA, Collaboration. Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis. 2018;66(6):893–903. This study generated global estimates of the trend in CD4 counts at ART initiation.
- 6.Lahuerta M, Ue F, Hoffman S, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of HIV care and treatment scale-up or a long-term phenomenon? Journal of Health Care for the Poor and Underserved. 2013;24(1):359–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahuerta M, Wu Y, Hoffman S, Elul B, Kulkarni SG, Remien RH, et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011: findings from four sub-saharan African countries. Clin Infect Dis. 2014;58(3):432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahuerta M, Ue F, Hoffman S, Elul B, Kulkarni SG, Wu Y, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24(1):359–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braunstein SL, Robertson MM, Myers J, Abraham B, Nash D. Increase in CD4+ T cell count at the time of HIV diagnosis and antiretroviral treatment initiation among persons with HIV in New York City. J Infect Dis. 2016;214(11):1682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group TAS, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22. [DOI] [PubMed] [Google Scholar]
- 12.Severe P, Juste MA, Ambroise A, Eliacin L, Marchand C, Apollon S, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363(3):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MS, McCauley M, Sugarman J. Establishing HIV treatment as prevention in the HIV Prevention Trials Network 052 randomized trial: an ethical odyssey. Clin Trials. 2012;9(3):340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection (http://www.who.int/hiv/pub/guidelines/arv2013/download/en/). Geneva: 2013. Accessed 22 April 2019. [Google Scholar]
- 16.WHO. Antiretroviral therapy for HIV infection in adults and adolescents - Recommendations for a public health approach: 2010 revision (https://www.who.int/hiv/pub/arv/adult2010/en/). Geneva: World Health Organization; 2010. Accessed 22 April 2019. [PubMed] [Google Scholar]
- 17.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9. [DOI] [PubMed] [Google Scholar]
- 18.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. [DOI] [PubMed] [Google Scholar]
- 20.Joint United Nations Program on HIV/AIDS (UNAIDS). 90-90-90: an ambitious treatment target to help end the AIDS epidemic 2017. [Google Scholar]
- 21.New York State Department of Health, Ending the Epidemic Task Force. Blueprint to end AIDS 2015 [cited 2015 Oct 1]. https://www.health.ny.gov/diseases/aids/ending_the_epidemic/docs/blueprint.pdf. Accessed 20 April 2019. [Google Scholar]
- 22.New York City Department of Health and Mental Hygiene. Recommendation to expand antiretroviral therapy to all persons living with HIV frequently asked questions (FAQ) for healthcare providers 2011 [updated December 11, 2011. Available from: http://www.nyc.gov/html/doh/downloads/pdf/ah/nyc-hivart-faq-provider.pdf.
- 23.••. Yotebieng M, Brazier E, Addison D, Kimmel AD, Cornell M, Keiser O, et al. Research priorities to inform ‘Treat All’ policy implementation for people living with HIV in sub-Saharan Africa: a consensus statement from the international epidemiology databases to evaluate AIDS (IeDEA). JIAS. 2019;22(1):1–16. 10.1002/jia2.25218. This consensus statement highlights the need to identify effective strategies for earlier diagnosis and ART initiation, and identifies key areas for further research aimed at informing implementation.
- 24.Brazier E, Maruri F, Duda SN, Tymejczyk O, Wester CW, Somi G, et al. Implementation of ‘Treat-all’ at adult HIV care and treatment sites in the Global IeDEA Consortium: results from the Site Assessment Survey. 2018. J Int AIDS Soc (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tymejczyk O, Brazier B, Yiannoutsos CT, Vinikoor M, van Lettow M, Nalugoda F, et al. Rapid HIV treatment initiation after national “treat all” policy adoption in 6 sub-Saharan African countries: Regression discontinuity analysis. PLoS Med 16(6): e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young B, Zuniga JM, Montaner J, Mayer KH. Controlling the HIV epidemic with antiretrovirals: moving from consensus to implementation. Clin Infect Dis. 2014;59(Suppl 1):S1–2. [DOI] [PubMed] [Google Scholar]
- 27.Song R, Hall HI, Green TA, Szwarcwald CL, Pantazis N. Using CD4 data to estimate HIV incidence, prevalence, and percent of undiagnosed infections in the United States. J Acquir Immune Defic Syndr. 2017;74(1):3–9. [DOI] [PubMed] [Google Scholar]
- 28.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV Incidence in the United States, 2006–2009. PLoS One. 6(8):e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC. Diagnoses of HIV Infection in the United States and Dependent Areas, 2017 (http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html; Accessed December 11, 2018). Atlanta: CDC; 2018. [Google Scholar]
- 31.Short Fabic M, Choi Y, Bird S. A systematic review of Demographic and Health Surveys: data availability and utilization for research. Bull World Health Organ. 2012;90(8):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.USAID. The Demographic and Health Survey Program (https://dhsprogram.com/data/;Accessed on December 11, 2018) 2018.
- 33.Kim AA, Parekh BS, Umuro M, Galgalo T, Bunnell R, Makokha E, et al. Identifying Risk Factors for Recent HIV Infection in Kenya Using a Recent Infection Testing Algorithm: Results from a Nationally Representative Population-Based Survey. PLoS One. 2016;11(5):e0155498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Justman J, Reed JB, Bicego G, Donnell D, Li K, Bock N, et al. Swaziland HIV Incidence Measurement Survey (SHIMS): a prospective national cohort study. Lancet HIV. 2017;4(2):e83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.PEFPAR. PEPFAR 2018 Annual Report to Congress (https://www.pepfar.gov/documents/organization/279889.pdf;accessed December 11, 2018). In: State UDo, editor. Washington, DC: 2018. [Google Scholar]
- 36.amfAR. PEFPAR monitoring, evaluation, and reporting database (http://mer.amfar.org; Accessed on December 11, 2018) 2018.
- 37.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perlman DC, Jordan AE, Nash D. Conceptualizing care continua: lessons from HIV, hepatitis C virus, tuberculosis and implications for the development of improved care and prevention continua. Front Public Health. 2016;4:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horn T, Sherwood J, Remien RH, Nash D, Auerbach JD, Treatment Action G, et al. Towards an integrated primary and secondary HIV prevention continuum for the United States: a cyclical process model. J Int AIDS Soc. 2016;19(1):21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [Google Scholar]
- 41.Mukui IN, Ng’ang’a L, Williamson J, Wamicwe JN, Vakil S, Katana A, et al. Rates and predictors of non-adherence to antiretroviral therapy among HIV-positive individuals in Kenya: results from the second Kenya aids indicator survey, 2012. PLoS One. 2016;11(12):e0167465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Justman JE, Mugurungi O, El-Sadr WM. HIV Population surveys - bringing precision to the global response. N Engl J Med. 2018;378(20):1859–61. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Hottz P, Schechter M, Rong L. Modeling the slow CD4+ T cell decline in hiv-infected individuals. PLoS Comput Biol. 2015;11(12):e1004665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okoye AA, Picker LJ. CD4(+) T cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5(1):83–9. [DOI] [PubMed] [Google Scholar]
- 46.Touloumi G, Pantazis N, Pillay D, Paraskevis D, Chaix ML, Bucher HC, et al. Impact of HIV-1 subtype on CD4 count at HIV seroconversion, rate of decline, and viral load set point in European seroconverter cohorts. Clin Infect Dis. 2013;56(6):888–97. [DOI] [PubMed] [Google Scholar]
- 47.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiebaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and < 500 Cells/mm(3): assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011;53(8):817–25. [DOI] [PubMed] [Google Scholar]
- 48.Lodi S, Phillips A, Touloumi G, Pantazis N, Bucher HC, Babiker A, et al. CD4 decline in seroconverter and seroprevalent individuals in the precombination of antiretroviral therapy era. AIDS. 2010;24(17):2697–704. [DOI] [PubMed] [Google Scholar]
- 49.CDC. Diagnoses of HIV Infection in the United States and Dependent Areas, 2012 (https://www.cdc.gov/hiv/pdf/statistics_2012_HIV_Surveillance_Report_vol_24.pdf; Accessed December 11, 2018). Atlanta: CDC; 2012. [Google Scholar]
- 50.Hall HI, Song R, Szwarcwald CL, Green T. Brief report: Time from infection with the human immunodeficiency virus to diagnosis, United States. Journal of Acquired Immune Deficiency Syndromes. 2015;69(2):248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Supervie V, Marty L, Lacombe JM, Dray-Spira R, Costagliola D, Group F-ACs. Looking beyond the cascade of HIV care to end the AIDS epidemic: estimation of the time interval from HIV infection to viral suppression. J Acquir Immune Defic Syndr. 2016. Nov 1;73(3):348–55. [DOI] [PubMed] [Google Scholar]
- 52.Korenromp EL, Williams BG, Schmid GP, Dye C. Clinical prognostic value of RNA viral load and CD4 cell counts during untreated HIV-1 infection–a quantitative review. PLoS One. 2009;4(6):e5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansson J, Kerr CC, Mallitt KA, Wu J, Gray RT, Wilson DP. Inferring HIV incidence from case surveillance with CD4+ cell counts. AIDS. 2015;29(12):1517–25. [DOI] [PubMed] [Google Scholar]
- 54.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4(+) cell count at presentation to HIV care, 1992–2011. Clin Infect Dis. 2013;57(7):1027–37. [DOI] [PubMed] [Google Scholar]
- 55.Robertson MM, Braunstein SL, Hoover DR, Li S, Nash D. Timeliness of HIV diagnosis and antiretroviral treatment initiation in the era of universal test and treat. J Infect Dis 2019. 10.1093/infdis/jiz148. [DOI] [PubMed] [Google Scholar]
- 56.ETEDashboard. Trends in the CD4 count at diagnosis among persons with newly diagnosed HIV infection in New York City, 2006–2017 2018. [Available from: http://etedashboardny.org/data/new-diagnoses-and-linkage/new-diagnoses-trends-nyc/.
- 57.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60(7):1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fordyce J, Forlenza S, Makki M, Mojica B, Nash D, Peters V, et al. 25 years of HIV in New York City: lessons from surveillance. J Urban Health. 2001;78(4):669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nash D, Ramaswamy C, Manning S. Implementation of named HIV reporting–New York City, 2001. MMWR Morb Mortal Wkly Rep. 2004;52(51–52):1248–52. [PubMed] [Google Scholar]
- 60.New York City Department of Health and Mental Hygiene. HIV Surveillance Annual Report, 2016 2017. [Available from: https://www1.nyc.gov/assets/doh/downloads/pdf/dires/hiv-surveillance-annualreport-2016.pdf.
- 61.Hoffman S, Wu Y, Lahuerta M, Kulkarni SG, Nuwagaba-Biribonwoha H, Sadr WE, et al. Advanced disease at enrollment in HIV care in four sub-Saharan African countries: change from 2006 to 2011 and multilevel predictors in 2011. AIDS. 2014;28(16):2429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lahuerta M, Nash D. Reply to Pattern et al. “Advanced HIV disease at ART initiation despite implementation of expanded ART eligibility guidelines during 2007-2012 in Khayelitsha, South Africa”. Clin Infect Dis. 2014. 1;59(3):457–8 [DOI] [PubMed] [Google Scholar]
- 63.Braunstein S, Robertson M, Myers J, Abraham B, Nash D. Reductions in the time from HIV infection to initiation of antiretroviral therapy among persons with HIV in New York City. J Infect Dis. 2016. 10.1093/infdis/jiw438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ransome Y, Terzian A, Addison D, Braunstein S, Myers J, Abraham B, et al. Expanded HIV testing coverage is associated with decreases in late HIV diagnoses. AIDS. 2015;29(11):1369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braunstein SL, Robertson MM, Myers J, Abraham B, Nash D. Increase in CD4+ T cell count at the time of HIV diagnosis and antiretroviral treatment initiation among persons With HIV in New York City. J Infect Dis. 2016; 1;214(11):1682–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Althoff KN, Gange SJ, Klein MB, Brooks JT, Hogg RS, Bosch RJ, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50(11):1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiertiburanakul S, Boettiger D, Lee MP, Omar SF, Tanuma J, Ng OT, et al. Trends of CD4 cell count levels at the initiation of antiretroviral therapy over time and factors associated with late initiation of antiretroviral therapy among Asian HIV-positive patients. J Int AIDS Soc. 2014;17:18804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.IeDEA, Collaborations ARTC, Avila D, Althoff KN, Mugglin C, Wools-Kaloustian K, et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2014;65(1):e8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanna DB, Buchacz K, Gebo KA, Hessol NA, Horberg MA, Jacobson LP, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis. 2013;56(8):1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Auld AF, Shiraishi RW, Oboho I, Ross C, Bateganya M, Pelletier V, et al. Trends in prevalence of advanced HIV disease at antiretroviral therapy enrollment - 10 countries, 2004–2015. MMWR Morb Mortal Wkly Rep. 2017;66(21):558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nash D, Katyal M, Brinkhof MW, Keiser O, May M, Hughes R, et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008;22(17):2291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Makhema KW MJ, Pretorius Holme M, Gaolathe T, Mmalane M, Kadima E, Chakalisa U, Manyake K, Mbikiwa A, Simon S, Letlhogile R, Mukokomani K, van Widenfelt E, Moyo S, Bennett K, Leidner J, Lebelonyane R, Alwano MG, Powis K, Dryden-Peterson S, Kgathi C, Novitsky V, Moore J, Bachanas P, Abrams W, Block L, El-Halabi S, Marukutira T, Mills LA, Bussmann H, Okui L, John O, Shapiro R, DeGruttola V, Lei Q, Wang R, Tchetgen E. Tchetgen,10, Essex M, Lockman S. Impact of prevention and treatment interventions on population HIV incidence: primary results of the community-randomized Ya Tsie Botswana prevention project (http://programme.aids2018.org/Abstract/Abstract/13216). AIDS 2018; Amsterdam: IAS; 2018. Accessed 22 April 2019. [Google Scholar]
- 73.HPTN. HPTN 071 (https://www.hptn.org/research/studies/hptn071; Accessed 12/16/18) 2018.
- 74.Iwuji CC, Orne-Gliemann J, Larmarange J, Balestre E, Thiebaut R, Tanser F, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomized trial. The Lancet HIV, 5(3), e116–e125. 10.1016/S2352-3018(17)30205-9. [DOI] [PubMed] [Google Scholar]
- 75.Havlir EC D, Balzer L, Clark T, Kwarisiima D, Ayieko J, Kabami J, Sang N, Liegler T, Chamie G, Camlin C, Kadede K, Mucunguzi A, Jain V, Ruel T, Shade S, Ssemondo E, Byonanebye D, Mwangwa F, Owaraganise A, Olilo W, Black D, Snyman K, Burger R, Getahun M, Achando J, Awuonda B, Nakato H, Kironde J, Thirumurthy H, Koss C, Brown L, Marquez C, Schwab J, Lavoy G, Plenty A, Mugoma Wafula E, Omanya P, Rooney J, Bacon M, van der Laan M, Cohen C, Bukusi E, Petersen M, Kamya M SEARCH community cluster randomized study of HIV “test and treat” using multi- disease approach and streamlined care in rural Uganda and Kenya (http://programme.aids2018.org/Abstract/Abstract/13469). AIDS 2018; Amsterdam: IAS; 2018. Accessed 22 April 2019. [Google Scholar]
- 76.UNAIDS. Miles to go—closing gaps, breaking barriers, righting injustices (http://www.unaids.org/en/resources/documents/2018/global-aids-update). Geneva; 2018. Accessed 22 April 2019. [Google Scholar]
- 77.Ford N, Meintjes G, Pozniak A, Bygrave H, Hill A, Peter T, et al. The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis. 2015;15(2):241–7. [DOI] [PubMed] [Google Scholar]
- 78.Teasdale CA, Yuengling K, Preko P, Syowai M, Ndagije F, Rabkin M, et al. Persons living with HIV with advanced HIV disease: need for novel care models. J Int AIDS Soc. 2018;21(12):e25210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tymejczyk O, Brazier E, Yiannoutsos C, Wools-Kaloustian K, Althoff K, Crabtree-Ramirez B, et al. HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: a metaregression analysis of programmatic data from 22 countries. PLoS Med. 2018;15(3):e1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zaniewski E, Ostinelli C, Maxwell N, Davies MA, Euvrard J, Van Dijk J, et al. Trends in CD4 and viral load testing in southern Africa: analysis of 6 countries (Abstract number 150). Seattle: CROI; 2019. [Google Scholar]
- 81.Ehrenkranz PD, Baptiste SL, Bygrave H, Ellman T, Doi N, Grimsrud A, et al. The missed potential of CD4 and viral load testing to improve clinical outcomes for people living with HIV in lower-resource settings. PLoS Medicine. 2019. 10.1371/journal.pmed.1002820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hakim J, Musiime V, Szubert AJ, Mallewa J, Siika A, Agutu C, et al. Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. N Engl J Med. 2017;377(3):233–45. 10.1371/journal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. 2017 July 2017. https://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/. Accessed 20 April 2019. [PubMed]
- 84.Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, Ellman T, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528(7580):S68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.PEPFAR. PEPFAR 2018 Country operational plan guidance for standard process countries (https://www.pepfar.gov/documents/organization/276459.pdf). In: State UDo, editor: OGAC; 2018. p. 240. Accessed 22 April 2019. [Google Scholar]
- 86.Ford N BA, Baggaley R, Vitoria M, Low-Beer D, Penazzato M, Vojnov L, Bertagnolio S, Habiyambere V, Doherty M, Hirnschall G. The WHO public health approach to HIV treatment and care: looking back and looking ahead. Lancet Infect Dis. 2018;18(3):e76–e86. 10.1016/S1473-3099(17)30482-6. [DOI] [PubMed] [Google Scholar]
- 87.Carroll L Through the looking glass (https://en.wikipedia.org/wiki/Through_the_Looking-Glass) 1871. Accessed 22 April 2019. [Google Scholar]