Abstract
The coronavirus disease 2019 (COVID-19) pandemic substantially impacted different age groups, with children and young people not exempted. Many have experienced enduring health consequences. Presently, there is no consensus on the health outcomes to assess in children and young people with post-COVID-19 condition. Furthermore, it is unclear which measurement instruments are appropriate for use in research and clinical management of children and young people with post-COVID-19. To address these unmet needs, we conducted a consensus study, aiming to develop a core outcome set (COS) and an associated core outcome measurement set (COMS) for evaluating post-COVID-19 condition in children and young people. Our methodology comprised of two phases. In phase 1 (to create a COS), we performed an extensive literature review and categorisation of outcomes, and prioritised those outcomes in a two-round online modified Delphi process followed by a consensus meeting. In phase 2 (to create the COMS), we performed another modified Delphi consensus process to evaluate measurement instruments for previously defined core outcomes from phase 1, followed by an online consensus workshop to finalise recommendations regarding the most appropriate instruments for each core outcome. In phase 1, 214 participants from 37 countries participated, with 154 (72%) contributing to both Delphi rounds. The subsequent online consensus meeting resulted in a final COS which encompassed seven critical outcomes: fatigue; post-exertion symptoms; work/occupational and study changes; as well as functional changes, symptoms, and conditions relating to cardiovascular, neuro-cognitive, gastrointestinal and physical outcomes. In phase 2, 11 international experts were involved in a modified Delphi process, selecting measurement instruments for a subsequent online consensus workshop where 30 voting participants discussed and independently scored the selected instruments. As a result of this consensus process, four instruments met a priori consensus criteria for inclusion: PedsQL multidimensional fatigue scale for “fatigue”; PedsQL gastrointestinal symptom scales for “gastrointestinal”; PedsQL cognitive functioning scale for “neurocognitive” and EQ-5D for “physical functioning”. Despite proposing outcome measurement instruments for the remaining three core outcomes (“cardiovascular”, “post-exertional malaise”, “work/occupational and study changes”), a consensus was not achieved. Our international, consensus-based initiative presents a robust framework for evaluating post-COVID-19 condition in children and young people in research and clinical practice via a rigorously defined COS and associated COMS. It will aid in the uniform measurement and reporting of relevant health outcomes worldwide.
Shareable abstract
Agreed measurement instruments should be considered in future work and insights from this study should guide policymakers in creating initiatives that address the effects of long COVID on children and young people in healthcare and research environments https://bit.ly/3NdHLFB
Introduction
While the majority of people infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) recover quickly, a significant number experience ongoing or relapsing symptoms for a prolonged period of time. Most research on the post-coronavirus disease 2019 (COVID-19) condition has focused on adults, with a much smaller number of paediatric studies. The prevalence of signs/symptoms after COVID-19 in children and young people remains largely unknown due to heterogeneity in terminology used and methodology applied [1], but a recent systematic review estimated prevalence of symptoms 1 month after infection to be up to 25% [2, 3]. A large multinational study estimated that ∼3% of individuals aged <20 years with symptomatic SARS-CoV-2 infections had persistent fatigue, cognitive and respiratory symptom clusters upon recovery from the acute infection [1, 4], while reassuring data from the recent United Kingdom Office for National Statistics suggests that the incidence of post-COVID-19 condition is now <1% [5]. Some studies estimated cumulative incidence of persistent symptoms following SARS-CoV-2 infection between 24% and 58% of children and young people [6].
A diversity of outcomes is being evaluated in research on post-COVID-19 condition in children and young people. This heterogeneity hinders the ability to compare findings and conduct meta-analyses to inform evidence-based decisions. There is also a risk that ongoing or future interventional trials will not address some critically important outcomes, as some outcomes important in one group may not be important in another, or vice versa. These issues highlight the need for core outcome set (COS) development, to ensure that important outcomes are not missed in research or clinical practice on post-COVID-19 condition in children and young people [7]. COS are useful in various medical fields and can improve data quality, harmonisation and comparability between different studies and clinical practices [8, 9]. A COS is a universally agreed-upon, harmonised set of outcomes that, at a minimum, should be measured and reported in every clinical trial within a specific medical area. These sets are also developed in other types of research and clinical practice. They represent a consensus on the most critical outcomes for people with lived experience, their families, researchers, health professionals and other key stakeholders. The “gold standard” approach to COS development has been outlined by the Core Outcome Measures in Effectiveness Trials (COMET) framework and consists of two steps: 1) what to measure and 2) how to measure. Once the COS is developed, the most appropriate outcome measurement instruments for assessing the “core outcomes” should be defined to provide practical measurement instruments for researchers and practitioners [9].
In 2021, an international group of experts defined the COS domains recommended to be used in all future research and clinical care for adults with post-COVID-19 condition [10] and the second phase of this project defined the Core Outcome Measurement Set (COMS) in 2022 [11]. However, adults and children and young people have distinct physiological and developmental characteristics, which may result in different presentations and long-term implications of post-COVID-19 condition. Hence, it is crucial to have a tailored COS and COMS specifically designed for children and young people to accurately capture and address these nuances as COS/COMS potentially may be required for different groups of paediatric population. To this end, we conducted an international study to develop a COS and COMS for post-COVID-19 condition in children and young people for use in clinical research and practice.
Methods
First phase (COS development)
The development of the COS involved three stages: 1) reviewing the outcomes reported in studies on post-COVID-19 condition in CYP to develop a list of outcomes for stakeholder consideration; 2) a two-round online modified Delphi consensus process to rate the importance of the outcomes for the COS; and 3) an online interactive consensus meeting to review and agree upon the final COS. The study protocol was developed a priori, and the project was registered (www.comet-initiative.org/Studies/Details/1847). Ethical approval for the study was obtained from the Sechenov University ethics committee on 20 January 2022 (protocol number 01–22).
The intended COS was developed for children and young people aged <18 years, to be applied to post-COVID-19 condition in clinical research and practice settings. The terms post-COVID-19 condition and long COVID were used interchangeably throughout the process.
Study group and participants
An international and multidisciplinary group of experts, including children and young people with post-COVID-19 experience and their caregivers, conducted a project under the International Severe Acute Respiratory and Emerging Infection Consortium umbrella. The COMET Initiative and the World Health Organization (WHO) collaborated with this project.
Participants were categorised into three distinct stakeholder groups: 1) children and young people with post-COVID-19 condition and their carers; 2) health professionals working with children and young people with post-COVID-19 condition; and 3) researchers studying post-COVID-19 condition in children and young people. For health professionals and researchers, prerequisites for participation included experience in treating children and young people with post-COVID-19 condition and conducting research in children and young people with post-COVID-19 condition, respectively. More details can be found in the supplementary material (appendix 5).
Developing a list of outcomes
The COS consensus process was informed by a comprehensive search of MEDLINE, Embase and the WHO COVID-19 Research Database (from inception until 29 December 2021). An additional search was performed on 1 June 2023, prior to consensus meeting, to screen for more recent evidence. The search was limited to English-language publications and protocols. The detailed search strategy can be found in the supplementary material (appendix 1).
Data from research protocols were extracted from two clinical trials registries, Clinical Trials.gov and the International Clinical Trials Registry Platform, and reviewed (by N. Seylanova, A. Chernyavskaya, A. Mursalova, N. Degtyareva, A. Ajam, L. Xiao, P. Bobkova, P. Roshchin and K. Aktulaeva), with two reviewers extracting the data from each record independently. We classified unique outcomes from the list into domains (supplementary material, appendix 1) using an existing taxonomy by Dodd et al. [12].
Delphi process and definitions
We conducted a two-round online modified Delphi consensus process [9]. In the first round, survey participants anonymously rated each outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) scale [13], which is a nine-point scale commonly divided into three categories for COS projects: not important (1–3); important, but not critical (4–6); and critically important (7–9). Each outcome had an “unable to rate” option and an option to add text-based comments. More details can be found in the supplementary material (appendix 5).
In the second round of the Delphi process, participants were shown their original rating from the first round alongside overall ratings of each of the three stakeholder groups for each outcome. They were then asked to rate each outcome again using the same scale.
Consensus for inclusion of an outcome in the COS was defined a priori as ≥80% of participants in each stakeholder group rating the outcome as critically important. Consensus for exclusion of an outcome from the COS was defined as ≤50% of respondents in each stakeholder group rating the outcome as critically important. Outcomes that did not meet these criteria were discussed at the consensus meeting.
The Delphi materials and all participant information were available in English, Chinese, Russian, French and Spanish. The Delphi survey was delivered using DelphiManager software (www.comet-initiative.org/delphimanager). Further details of the Delphi consensus process are included in the supplementary material (appendix 1).
Consensus meeting
We conducted an interactive online consensus meeting via Zoom, extending invitations to individuals with first-hand experience and their caregivers. The consensus meeting was conducted in English under the guidance of an experienced independent facilitator. The meeting was organised around the results from the second round of the Delphi.
The agenda prioritised outcomes that met the inclusion consensus by at least one stakeholder group, despite not being agreed upon by all. Additionally, outcomes deemed “critically important” by ≥50% (but not more than 80%) of the participants in each stakeholder group were also selected for discussion.
Each of three stakeholder groups assessed outcomes independently, utilising the aforementioned threshold for defining inclusion, i.e. an outcome rated as critically important by ≥80% participants in all stakeholder groups. For further details regarding the consensus meeting process, please refer to the supplementary material (appendix 2).
Data analysis
Descriptive statistics were used to show the overall scores of each stakeholder group for the three GRADE categories for all outcomes considered at each stage, to determine whether they met the pre-defined criteria for inclusion or exclusion.
Similarly to the PC-COS adult project [10], we agreed a priori that only responses from Delphi participants who rated ≥50% of outcomes would be included in the analysis. Free-text comments were translated into English from the French, Russian, Spanish and Chinese surveys and collated and reviewed by the core group. Bar plots displaying the distribution of ratings for each outcome, faceted by stakeholder group, were produced using R (version 4.2.1) and shown to participants in the second Delphi round.
Second phase (outcome measurement instruments consensus)
Literature review of outcome measurement instruments
The core group reviewed all measurement instruments that emerged from our literature search. More details can be found in the supplementary material (appendix 5).
Given that the measurement properties of non-COVID-specific instruments had not been assessed in a post-COVID-19 population, assessment of the measurement properties of these instruments was not undertaken [11].
For all instruments, feasibility-related data (e.g. time, cost, language/translations) were considered by the experts and presented at consensus meeting to the participants. It was decided a priori that instruments requiring trained personnel, additional software, clinical facilities or not pertaining to core outcomes would be excluded to ensure applicability of COMS across different settings. The instruments needed to be available for use even in “low-resource areas” and not require in-person assessment or medical equipment.
Expert Delphi consensus
The core group refined a comprehensive list of instruments derived from systematic literature and clinical trials review. Instruments requiring trained personnel, additional software, clinical facilities or not pertaining to core outcomes were excluded.
A group of independent international experts, with extensive experience in post-COVID-19 condition research and/or clinical practice, anonymously reviewed these instruments over two rounds. They provided feedback in Excel spreadsheets on each instrument and suggested potential additions, which were assessed for feasibility and applicability by the core group. Approved new instruments were presented in the second round for further review.
In the second round, each expert received a list of instruments accompanied by anonymised expert feedback from the first round. After reviewing the comments from the first round, they had the opportunity to modify their initial selection or retain it. Each expert indicated their preference for each instrument's inclusion in the consensus workshop.
Instruments that garnered “include” or “maybe” responses from >50% of the experts were forwarded to the online consensus meeting. We prepared “instrument cards”, modified for the purposes of the project from the previous studies (www.improvelto.com/instruments/), for each outcome, collating a summary table of instruments selected for discussion. These were shared with the consensus workshop participants beforehand.
Consensus workshop
Upon obtaining expert review results, we convened at an online consensus workshop to discuss the shortlisted instruments. The consensus meeting was conducted in English and the study lead (D. Munblit) acted as a facilitator without voting rights.
Instruments selected as a result of “expert review” as per criteria outlined earlier were discussed at the meeting. Consensus for an instrument to be included was defined as ≥70% participants from a total number of voting participants. If participants did not cast a vote on a given instrument, not less than 70% of voting participants were required to consider the vote valid.
Results
Literature review
We conducted a review of available studies and trial protocols on post-COVID-19 condition in children and young people. This review found 212 studies and protocols that met the inclusion criteria, as detailed in the supplementary material (appendix 1). These studies and protocols reported a total of 1097 outcomes, as detailed in appendix 1.
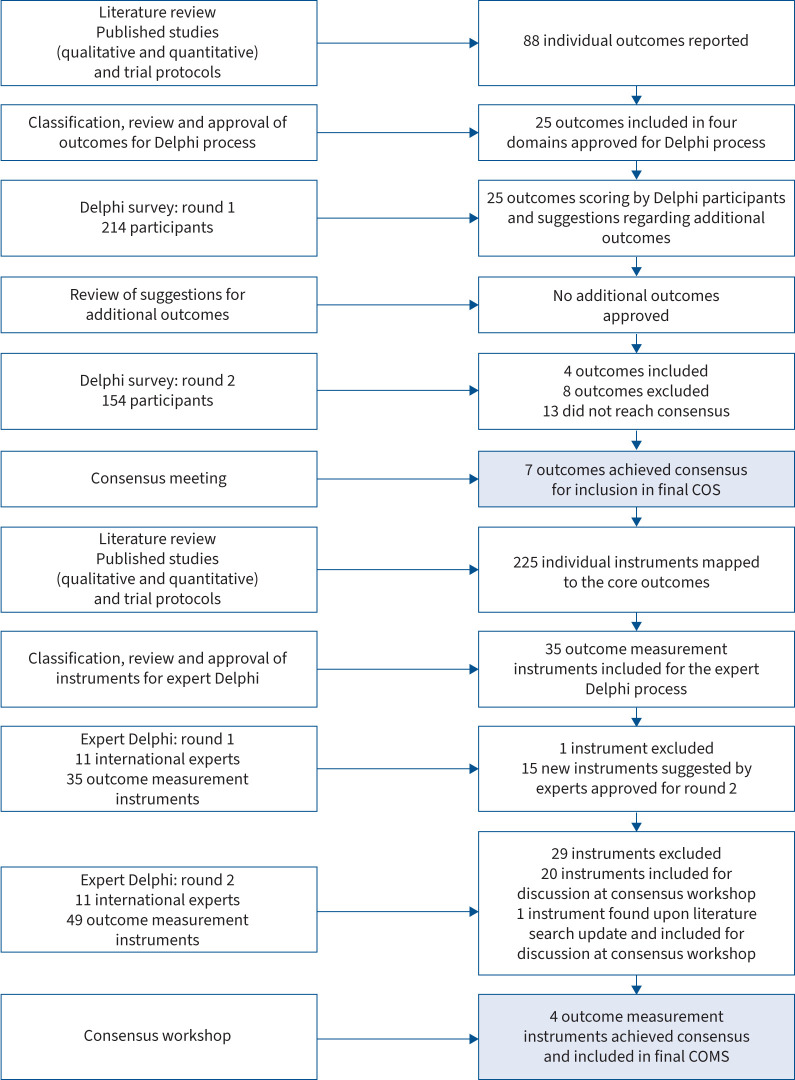
The outcomes were classified and reviewed iteratively by the core group and project steering committee. After discussion, the steering committee approved 25 outcomes (supplementary material, appendix 1) for consideration in the first round of the Delphi process. These 25 outcomes were categorised into four domains: survival (one outcome); physiological or clinical (17 outcomes); life impact (five outcomes); and resource use (two outcomes). Figure 1 summarises the steps taken in the development of the COS and COMS.
FIGURE 1.
Overview of the core outcome set (COS) and core outcome measurement set (COMS) development process.
First phase (COS development)
Delphi process
The first round of the online Delphi process was conducted from 23 November to 24 December 2022. A total of 228 individuals registered to participate in the study, and 214 (94%) participants from 37 countries completed the first round, which required them to rate ≥50% of the 25 outcomes. Of these participants, 154 (72%) from 31 countries participated in the second round of the Delphi process and rated ≥50% of the outcomes in this subsequent round. Demographic characteristics of the participants for each Delphi round are presented in table 1. Further details about the Delphi participants can be found in the supplementary material (appendix 1).
TABLE 1.
Demographics of the core outcome set (COS) Delphi participants
| Delphi round 1 | Delphi round 2 | |
| Participants | 214 | 154 |
| Stakeholder group | ||
| Children and young people (aged ≤18 years) who have experience of living with post-COVID-19 condition# | 26 (12) | 21 (14) |
| Family and carers of children and young people (aged ≤18 years) with post-COVID-19 condition# | 115 (54) | 76 (49) |
| Health professionals who have experience treating children and young people (age ≤18 years) with post-COVID-19 condition# | 37 (17) | 32 (21) |
| Researchers studying post-COVID-19 condition# in children and young people (aged ≤18 years) | 36 (17) | 25 (16) |
| Other | Participants reclassified after round 1 review and analysed within appropriate groups | |
| Gender | ||
| Male | 47 (22) | 34 (22) |
| Female | 166 (78) | 119 (77) |
| Nonbinary | 1 (<1) | 1 (<1) |
| Other | 0 (0) | 0 (0) |
| Prefer not to answer | 0 (0) | 0 (0) |
| Age group years | ||
| 2–11 | 6 (3) | 3 (2) |
| 12– <18 | 21 (10) | 19 (12) |
| 18–39 | 40 (19) | 33 (21) |
| 40–59 | 139 (65) | 94 (61) |
| 60–79 | 8 (4) | 5 (3) |
| Geographical area | ||
| Asia | 8 (4) | 6 (4) |
| Africa | 1 (<1) | 1 (<1) |
| Australasia | 11 (5) | 8 (5) |
| Europe | 163 (76) | 120 (78) |
| North America | 24 (11) | 13 (8) |
| Central America | 1 (<1) | 0 (0) |
| South America | 6 (3) | 6 (4) |
| Ethnicity | ||
| White | 180 (84) | 130 (84) |
| South Asian | 5 (2) | 4 (3) |
| Hispanic/Latino/Spanish | 8 (4) | 6 (4) |
| East Asian/Pacific Islander | 4 (2) | 1 (<1) |
| Indigenous peoples | 0 (0) | 0 (0) |
| Black | 1 (<1) | 1 (<1) |
| Middle Eastern/North African | 6 (3) | 5 (3) |
| Other | 10 (5) | 7 (5) |
Data are presented as n or n (%). Not all percentages add up to 100% owing to rounding. COVID-19: coronavirus disease 2019. #: also known as long COVID.
Upon completion of the first round of the Delphi process, the participant ratings indicated that the COS should include three of the 25 outcomes, while four outcomes should be excluded, and consensus criteria for 18 outcomes were not met. Table 2 and the supplementary material (appendix 1) provide further details.
TABLE 2.
Summary of Delphi and consensus meeting voting on outcomes stratified by domains
| Delphi round 1 | Delphi round 2 | Consensus meeting | |
| Mortality/survival | |||
| Survival | No consensus | No consensus: for discussion | Exclude |
| Physiological/clinical | |||
| Cardiovascular functioning, symptoms and conditions# | No consensus | Include in the COS | NA |
| Endocrine and metabolic functioning, symptoms and conditions | No consensus | Exclude | NA |
| Hearing-related functioning, symptoms and conditions | Exclude | Exclude | NA |
| Gastrointestinal functioning, symptoms and conditions# | No consensus | No consensus: for discussion | Include in the COS |
| Pain | No consensus | No consensus: for discussion | Exclude |
| Fatigue or exhaustion# | Include | Include in the COS | NA |
| Sleep-related functioning, symptoms and conditions | No consensus | No consensus: for discussion | Exclude |
| Muscle and joint symptoms and conditions | No consensus | No consensus: for discussion | Exclude |
| Taste- and/or smell-related functioning, symptoms and conditions | Exclude | Exclude | NA |
| Neurocognitive system functioning, symptoms and conditions# | Include | Include in the COS | NA |
| Mental/psychological functioning, symptoms and conditions | No consensus | No consensus: for discussion | Exclude |
| Kidney and urinary-related functioning, symptoms and conditions | No consensus | Exclude | NA |
| Respiratory functioning, symptoms and conditions | No consensus | No consensus: for discussion | Exclude |
| Skin, hair, dental and/or nail-related functioning, symptoms and conditions | Exclude | Exclude | NA |
| Post-exertion symptoms# | No consensus | No consensus: for discussion | Include in the COS |
| Vision-related functioning, symptoms and conditions | No consensus | Exclude | NA |
| Fever/body temperature changes | No consensus | Exclude | NA |
| Life impact | |||
| Satisfaction with life or personal enjoyment | No consensus | No consensus: for discussion | Exclude |
| Physical functioning, symptoms and conditions# | Include | Include in the COS | NA |
| Social role functioning and relationships problems | No consensus | No consensus: for discussion | Exclude |
| Work/occupational and study changes# | No consensus | No consensus: for discussion | Include in the COS |
| Stigma | Exclude | Exclude | NA |
| Resource use | |||
| Healthcare resource utilisation | No consensus | No consensus: for discussion | Exclude |
| Family/carer burden | No consensus | No consensus: for discussion | Exclude |
All outcomes from Delphi round 1 were included in round 2, regardless of ratings in round 1. COS: core outcomes set; NA: not applicable (outcomes were included in the COS after 2 rounds of Delphi). #: included in the COS.
The core group reviewed 72 submitted free-text responses related to additional outcomes, with no new outcomes added in the second Delphi round. Four participants suggested adding “recurrent infections” as a new outcome. This suggestion was discussed within the core group with a decision made for not including it due to the lack of evidence for post-COVID immune deficiency in children, the complexity of the outcome, and the difficulty in differentiating it from infections stemming from other aetiologies. In addition, there was overlap with some of the outcomes already present as a part of the Delphi process, and the core group highlighted practical challenges in monitoring and documenting such infections.
The second Delphi round occurred between 19 February and 31 March 2023, during which 154 participants assessed the 25 outcomes. Subsequently, four outcomes met criteria for inclusion, with three in the physiological or clinical domain and one in the life impact domain. Eight outcomes were excluded. 13 other outcomes received mixed ratings across the stakeholder groups, which led to their discussion at a subsequent consensus meeting.
Consensus meeting
The consensus meeting was conducted online on 28 April 2023. For feasibility purposes, voting participants were divided into two stakeholder groups: 1) children and young people with post-COVID-19 condition and their carers (n=11); and 2) health professionals working with children and young people with post-COVID-19 condition and researchers studying post-COVID-19 condition in children and young people (n=12). Detailed descriptions of the participants who attended the consensus meeting can be found in the supplementary material (appendix 2).
Upon ratification of outcomes that were voted “in” and “out” upon the Delphi process, the 13 outcomes were discussed in the following order: survival; post-exertion symptoms; mental/psychological functioning, symptoms and conditions; respiratory functioning, symptoms and conditions; pain; sleep-related functioning, symptoms and conditions; gastrointestinal functioning, symptoms and conditions; muscle and joint symptoms and conditions; work/occupational and study changes; satisfaction with life or personal enjoyment; social role functioning and relationships problems; healthcare resource utilisation; family/carer burden.
After discussions and subsequent voting, three additional outcomes met the pre-defined consensus definition for inclusion. These included “post-exertion symptoms” with 100% (11 out of 11) of the children and young people with post-COVID-19 condition and their carers and 84% (10 out of 12) of the healthcare professionals and researchers rated it as critically important, based on the GRADE rating of 7–9; “gastrointestinal functioning, symptoms and conditions” with 100% (11 out of 11) and 84% (10 out of 12); as well as “work/occupational and study changes” rated as critical by 100% (11 out of 11) and 91% (11 out of 12) participants. Consequently, three outcomes were incorporated into the COS, joining the four previously agreed-upon outcomes. This brought the total number of outcomes in the COS to seven. The results derived from both the Delphi process and the consensus meeting can be accessed in the supplementary material (appendix 1). A report of the consensus meeting is available in the supplementary material (appendix 2).
Second phase (COMS development)
Literature review of outcome measurement instruments
A comprehensive literature review found 1762 instruments used across post-COVID-19 condition studies and trial protocols. Following removal of duplicates and mapping of identified instruments to the core outcomes, the number was reduced to 225. An independent assessment of these instruments by the core group, taking into account a priori defined criteria, further reduced the list to 30. In addition to these, the study group identified five relevant Patient-Reported Outcomes Measurement Information System (PROMIS) instruments, bringing the total to 35 outcome measurement instruments. These instruments, detailed in the supplementary material (appendix 3), were mapped to seven core outcomes described earlier. The COS development steps are summarised in figure 1.
Expert Delphi
A group of 11 international experts anonymously reviewed instruments provided by the study team over two Delphi rounds. Round 1 ran from 8 June to 21 June 2023, with all the experts completing this round. All the experts were invited to participate in round two. Round 2 ran from 3 July to 13 July 2023, with all the experts providing their feedback and scoring. Further details of experts involved in the Delphi process are presented in the supplementary material (appendix 3).
18 out of 35 instruments reviewed in round 1 met pre-specified criteria for inclusion for discussion at the consensus workshop. A single instrument (stomach reflux symptom by visual analogue score) was excluded by the core group due to the nonspecific nature of this visual analogue scale. All other instruments from round 1 were taken forward to round 2. Additional potential instruments were assessed for feasibility and applicability by the core group. 15 approved new instruments were presented in the second round for further review, including one instrument that was specific to the post-COVID-19 condition in adults and which is currently in the process of validation for children and young people. A total of 49 instruments were reviewed in round 2 and 20 of them met pre-specified criteria for inclusion for discussion at consensus workshop. The WHO Disability Assessment Schedule (WHODAS 2.0) Children and Youth 36-Item Version instrument was found upon the pre-meeting literature search update and included for discussion at the consensus workshop.
Consensus workshop
Ahead of the consensus workshop, materials were circulated to all individuals invited to the meeting. The online consensus workshop was held on 31 July 2023, with 46 individuals participating in this 3.5-h session. In attendance were six study team members, nine observers and 30 voting participants (eight carers of children and young people with post-COVID-19 condition and 22 health professionals and researchers with expertise in post-COVID-19 condition in children and young people, mirroring the approach taken for the first phase of the project and previous process of COS development for the adult population [10, 11]). Details of those who participated in the consensus workshop can be found in the supplementary material (appendix 4).
At the start of the online workshop, participants were briefed about the process and a priori defined criteria for consensus. Participants were reminded that multiple instruments could be chosen or voted “in” within a domain. Voting on each instrument was independent. The subsequent outcomes and measurement instruments discussed were cardiovascular functioning, symptoms and conditions (PedsQL Cardiac Module; Symptom Burden Questionnaire for Long COVID (circulation scale) and Malmo POTS (postural orthostatic tachycardia syndrome) score); gastrointestinal functioning, symptoms and conditions (PedsQL Gastrointestinal Symptoms Scales; Questionnaire on Pediatric Gastrointestinal Symptoms and Symptom Burden Questionnaire for Long COVID (stomach and digestion scale)); neurocognitive functioning, symptoms and conditions (PROMIS Pediatric Cognitive Function – Short Form 7a; PedsQL Cognitive Functioning Scale and Symptom Burden Questionnaire for Long COVID (memory, thinking and communication scale, movement scale, muscles and joints, pain scales)); Fatigue (Chalder fatigue questionnaire; PROMIS Paediatric Fatigue; PedsQL Multidimensional Fatigue Scale and Symptom Burden Questionnaire for Long COVID (fatigue scale)); post-exertion symptoms (Centers for Disease Control and Prevention symptom inventory for chronic fatigue syndrome; post-exertional malaise items from the DePaul Symptom Questionnaire and Symptom Burden Questionnaire for Long COVID (fatigue scale)); and physical functioning, symptoms and conditions (EQ-5DY instrument; PROMIS Physical Activity and Symptom Burden Questionnaire for Long COVID (impact on daily life scale)); work, occupational and study changes (Symptom Burden Questionnaire for Long COVID (impact on daily life scale) and WHODAS 2.0 Children and Youth 36-Item Version).
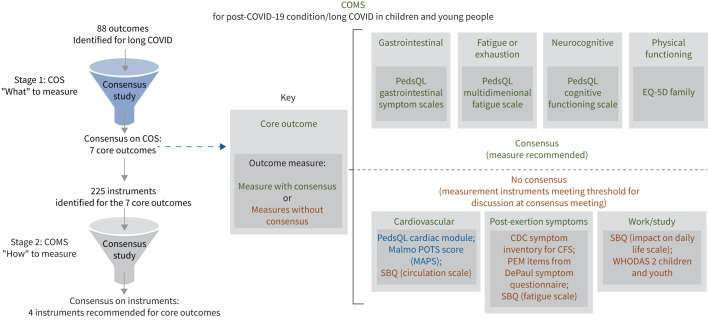
Following discussion and voting, 26 (100%) out of 26 consensus meeting participants voted “yes” for inclusion the PedsQL Multidimensional Fatigue Scale instrument for fatigue, so it was added to the COMS; PedsQL Gastrointestinal Symptom Scales for gastrointestinal (23 out of 26, 88%); PedsQL Cognitive Functioning Scale for neurocognitive (21 out of 25, 84%); and EQ-5D family questionnaires for physical functioning (24 out of 25, 96%) were also added. Overall, four measurement instruments were selected for inclusion into COMS (table 3, figure 2).
TABLE 3.
Consensus workshop voting results for outcome measurement instruments
| Outcome measure | Participants voting to include in consensus meeting n/N (%) | Result | |
| Cardiovascular functioning, symptoms and conditions | PedsQL Cardiac Module | 16/28 (57) | Not included in the COMS |
| Symptom Burden Questionnaire for Long COVID (circulation scale) | 7/27 (25) | Not included in the COMS | |
| Malmo POTS score | 18/27 (64) | Not included in the COMS | |
| Gastrointestinal functioning, symptoms and conditions # | PedsQL Gastrointestinal Symptoms Scales# | 23/26 (88) | Included in the COMS |
| Questionnaire on Pediatric Gastrointestinal Symptoms | 2/26 (8) | Not included in the COMS | |
| Symptom Burden Questionnaire for Long COVID (Stomach and Digestion Scale) | 6/26 (23) | Not included in the COMS | |
| Fatigue or exhaustion# | Chalder fatigue questionnaire | 3/26 (12) | Not included in the COMS |
| PROMIS Paediatric Fatigue | 3/26 (12) | Not included in the COMS | |
| PedsQL Multidimensional Fatigue Scale# | 26/26 (100) | Included in the COMS | |
| Symptom Burden Questionnaire for Long COVID (fatigue scale) | 3/26 (12) | Not included in the COMS | |
| Post-exertion symptoms | CDC symptom inventory for CFS | 5/26 (19) | Not included in the COMS |
| PEM items from DePaul Symptom Questionnaire | 10/26 (38) | Not included in the COMS | |
| Symptom Burden Questionnaire for Long COVID (fatigue scale) | 6/26 (23) | Not included in the COMS | |
| Neurocognitive functioning, symptoms and conditions # | PROMIS Pediatric Cognitive Function – Short Form 7a | 9/24 (36) | Not included in the COMS |
| PedsQL Cognitive Functioning Scale# | 21/25 (84) | Included in the COMS | |
| Symptom Burden Questionnaire for Long COVID (memory, thinking and communication scale, movement scale, muscles and joints, pain scales) | 4/24 (16) | Not included in the COMS | |
| Physical functioning, symptoms and conditions# | EQ-5DY instrument# | 24/25 (96) | Included in the COMS |
| PROMIS Physical Activity | 2/25 (8) | Not included in the COMS | |
| Symptom Burden Questionnaire for Long COVID (impact on daily life scale) | 3/25 (12) | Not included in the COMS | |
| Work/occupational and study changes | Symptom Burden Questionnaire for Long COVID (impact on daily life scale) | 5/22 (23) | Not included in the COMS |
| WHODAS 2.0 Children and Youth 36-Item Version | 7/23 (30) | Not included in the COMS |
COMS: core outcome measures set; COVID: coronavirus disease 2019; POTS: postural orthostatic tachycardia syndrome; PROMIS: Patient-Reported Outcomes Measurement Information System; CDC: Centers for Disease Control and Prevention; CFS: chronic fatigue syndrome; PEM: post-exertional malaise; WHODAS: World Health Organization Disability Assessment Schedule. #: included in the COMS.
FIGURE 2.
Core outcome measurement set (COMS) for post-coronavirus disease 2019 (COVID-19) condition in children and young people. Green indicates core outcomes and instruments reaching consensus for use in relation to a particular outcome; blue indicates instruments not reaching consensus, with more than a half of consensus meeting participants voting for this instrument prioritisation; brown indicates instruments not reaching consensus, with less than a half of consensus meeting participants voting for this instrument prioritisation. COS: core outcome set; SBQ: Symptom Burden Questionnaire; CDC: Centers for Disease Control and Prevention; CFS: chronic fatigue syndrome; PEM: post-exertional malaise; WHODAS: World Health Organization Disability Assessment Schedule.
Consensus was not achieved for recommending measurement instruments for the remaining three core outcomes. Table 3 indicates the voting results and reasons for exclusion for the instruments discussed at the meeting but not reaching consensus. A detailed consensus workshop report is available in the supplementary material (appendix 4).
Discussion
This article presents the findings of a large, rigorous international consensus study aimed at developing a COS and a COMS for post-COVID-19 condition that are intended for use in children and young people in research and clinical practice settings. Seven outcomes achieved the pre-defined consensus definition for inclusion in the COS: fatigue; post-exertion symptoms; work, occupational and study changes; as well as functional changes, symptoms and conditions relating to cardiovascular, neurocognitive, gastrointestinal and physical outcomes. Agreement regarding the most appropriate instruments to be used was reached for four outcomes: these were the EQ-5D family (for physical functioning) and the fatigue, gastrointestinal symptoms and cognitive functioning scales of the PedsQL. The consensus process reduced the number of potential instruments for measuring the seven core outcomes from >200, despite no single measurement instrument reaching consensus for the remaining three outcomes.
Through our consensus process, we identified seven critical outcomes to be incorporated in both research and clinical practice, ensuring that the most salient aspects of the condition are consistently and effectively addressed. Five of the seven consensus-based outcomes in this COS are in the physiological or clinical outcomes domain and cover many of the frequently reported symptoms in children and young people. While the WHO clinical case definition of post-COVID-19 condition in children and young people [14] offers a consistent clinical terminology, the COS delineates the essential outcomes that ought to be assessed in every study and clinical setting.
Across stakeholder groups, there was a broad consensus on the significance of most outcomes. Two outcomes, namely “sleep-related functioning, symptoms and conditions” and “pain”, narrowly missed the pre-defined threshold. A notable divergence in perspectives emerged regarding the “family/carer burden” outcome. Children and young people with post-COVID-19 condition and their carers deemed this outcome as critically important. In contrast, only 34% of healthcare professionals and researchers viewed it with the same level of importance. Despite not meeting the criteria for inclusion in the COS, the significance of this outcome was recognised by both groups, with 100% of children and young people and caregivers and 84% of healthcare professionals and researchers rating it as either important or critically important (supplementary material, appendix 2). The emphasis placed on these outcomes suggests that they warrant consideration in research and clinical settings. It is important to note that the COS is a necessary minimum that should always be measured, but does not preclude measuring other outcomes.
It is also worth noting that a small number of children and young people with long COVID and their family and carers acknowledged the critical importance of “mental” outcome assessment, with concerns of stigmatisation being raised. Many parents shared their experience of being troubled and hesitant to discuss mental problems of their child with healthcare providers, as the symptoms in a child are often attributed to mental health challenges/issues. This is in contrast to the COS for post-COVID-19 condition in adults, which includes this outcome [10]. All health professionals/researchers considered this outcome important, with seven (59%) out of 12 feeling that it is critical. Mental health related symptoms are common, and it is understandable to suffer effects on emotional wellbeing due to having an illness such as post-COVID-19 condition as it has a direct effect on an individual's life. Concerns of stigmatisation should not stand in the way of being able to assess the child or young person holistically and hence provide necessary support. Health professionals and researchers need to approach this delicate topic with care, while carers of children and young people with post-COVID-19 condition should not see an attempt to assess mental health as lack of trust regarding their concerns about their child.
Overall, the paediatric COS seems to focus more on functional and symptomatic outcomes directly relevant to the daily lives of children and young people, such as school and physical activities, while the adult COS encompasses a broader range of health aspects, including respiratory, mental health and survival, which are important for all age groups, but more pertinent to the adult population. These differences underscore the unique health impacts and assessment needs of these two age groups in post-COVID-19 condition research.
The PedsQL and EQ-5D families of instruments offer multiple age-specific versions [15, 16]. These versions contain questions pertinent to a child's development, and they have been translated into various languages and are used across different medical disciplines.
Сonsensus regarding measurement instruments was not achieved for three outcomes. There were several potential reasons for this. Firstly, post-COVID-19 condition is a recently discovered condition and the mechanistic understanding in children and young people is still in its infancy. This heterogeneity can influence instrument preference, and the unique considerations of the paediatric population, such as specific needs for different age groups or inability to appropriately articulate their complaints in younger children introduce added complexity. Secondly, past experiences with various instruments may have introduced implicit bias, thereby influencing participant scoring [17]. At least one of these measurement instruments can be potentially considered for each core outcome, although they should be used with caution taking into account feedback from workshop participants (supplementary material, appendix 4).
Our study has some limitations. Firstly, while the Delphi consensus process for the COS incorporated individuals from diverse geographical locations, the majority were white, and were resident in the United Kingdom and the United States. The Delphi process also saw an underrepresentation of male participants, which is a common problem in survey/Delphi research, and particularly related to children and young people, and has been acknowledged previously [18, 19]. Both imbalances could potentially result in a lack of external validity or generalisability. Although the Delphi has been conducted in multiple languages, some widely used languages (e.g. Hindi and Arabic) were missing. These demographic imbalances might challenge the external validity of our findings. Long COVID disproportionately impacts underprivileged groups, with potential rural versus urban disparities in healthcare access and quality. This might influence the utilisation rating among family and carers, who form a significant portion of participants. Treatment for long COVID can be costlier, hitting lower-income individuals and populations of low- and middle-income countries harder [20]. Secondly, a consensus meeting during the first phase of the project included only a limited subset of Delphi participants, whose perspectives might not encompass the full spectrum of views on the subject. However, this limitation is an inherent component in the Delphi methodology. It is also important to note that the meeting did not overturn the “in/out” results from the Delphi, and it allowed discussion of those not reaching consensus previously. Thirdly, given the pressing public health implications of COS development, we expedited our study. Consequently, we did not gather data on chronicity, time since diagnosis, and participants’ socioeconomic status. A similar approach was previously employed for the adult COS development. Yet, it is worth noting that comprehensive data collection on Delphi participants is not standard practice. In line with the WHO definition, our study included individuals with both confirmed and probable SARS-CoV-2 infections. However, it is possible that some with a “probable” diagnosis might not have had the infection. Lastly, in the second phase of the project, aiming at outcome measurement instrument selection, the Delphi process has been conducted without involvement of children and young people with post-COVID-19 condition and their carers. Instead, an international panel of experts conducted a Delphi process. This approach aimed to expedite the consensus process and reduce the potential burden on participants, drawing insights from a similar process conducted for adults. This has been mitigated in part by involvement of carers of children and young people with post-COVID-19 condition at the final consensus workshop. Another limitation is absence of Consensus-Based Standards for the Selection of Health Measurement Instruments methodology for selecting instruments implementation in the COMS development, as measurement properties of non-COVID-19-specific instruments had not been assessed in a post-COVID-19 population.
While the incidence of new acute SARS-CoV-2 cases has seen a decline, it is imperative to address the lingering legacy of post-COVID-19 condition, particularly due to its prolonged persistence. With the acute cases becoming less frequent, there is a potential risk of the broader community adopting an “out of sight, out of mind” perspective. However, it is crucial to highlight the substantial absolute number of children and young people globally who are grappling with long COVID. The long-term implications of this condition on their growth, maturation and overall development underscore the need to recognise post-COVID-19 condition, not merely as a transient concern, but rather as a chronic health issue. This rigorous international consensus study has successfully delineated a COS and a COMS tailored for post-COVID-19 condition in children and young people. While the consensus provides clarity in a nascent and multifaceted field, it also underscores the need for continued exploration, especially for outcomes where consensus remains elusive. As we navigate the complexities of post-COVID-19 conditions in children and young people, this consensus serves as a guidance for both research endeavours and clinical practices towards a more unified and informed approach (box 1). The outcomes of this study may also be useful not only within its immediate context, but also as a model for future pandemic situations. We believe that the generalisable knowledge derived from this COMS exercise can significantly benefit the broader academic and medical communities in the future challenges.
BOX 1 Key messages
| Rationale and approach |
| • In children and young people, the post-coronavirus disease 2019 (COVID-19) condition, also known as long COVID, is associated with a range of persistent symptoms following infection with severe acute respiratory syndrome coronavirus 2. |
| • Research on post-COVID-19 condition varies in outcomes studied. A consensus on a minimum set of essential outcomes, referred to as a core outcome set (COS) is needed for better data comparison in children and young people. |
| • There is also an urgent need for decisions to be made on which measurement instruments are the most appropriate for assessing these core outcomes, in order to develop a core outcome measurement set (COMS), to optimise data comparability and synthesis. |
| • To develop the COS, we conducted a study that included a literature review, a two-round online Delphi process with >214 participants from 37 countries, with over half of them being parents of children with post-COVID-19 condition and children and young people, and an online consensus meeting. The Delphi process included rating 25 different outcomes. |
| • For the development of COMS, we then performed an expert online modified Delphi process and an online consensus workshop to discuss and then vote anonymously on measurement instruments. |
| Findings |
| • In the field of paediatric care, it is recommended that the following outcomes to be consistently measured in research and clinical practice when assessing post-COVID-19 condition: fatigue; post-exertion symptoms; alterations in studies, work or occupational activities; as well as functional changes, symptoms and conditions relating to cardiovascular, neurocognitive, gastrointestinal and physical health. |
| • Instruments for measurement of fatigue, gastrointestinal, neurocognitive outcomes and physical functioning were recommended for use in research and clinical practice for children and young people with post-COVID-19 condition. For the three other core outcomes, the most favoured measurement instruments identified from this consensus procedure have been documented, even though no individual measurement instrument met a priori criteria for consensus. |
| Future directions and implications |
| • To enhance our understanding of post-COVID-19 condition in children, there is a need for further standardisation of clinical and research practices using the identified core outcomes and associated measurement instruments. |
| • Future research should focus on refining and validating the measurement instruments that were favoured but did not achieve consensus among participants. |
| • Incorporating the lived experiences and perspectives of children and young people affected by post-COVID-19 condition as well as their carers is crucial for future research, including instrument development and improvements to patient care. |
| • Agreed measurement instruments should be considered in future work and insights from this research should guide policymakers in creating initiatives that address the effects of post-COVID-19 condition on children and young people in both healthcare and research environments. |
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Appendix 1 ERJ-01761-2023.Appendix_1 (2MB, pdf)
Appendix 2 ERJ-01761-2023.Appendix_2 (402.6KB, pdf)
Appendix 3 ERJ-01761-2023.Appendix_3 (757.2KB, pdf)
Appendix 4 ERJ-01761-2023.Appendix_4 (398.2KB, pdf)
Appendix 5 ERJ-01761-2023.Appendix_5 (154.9KB, pdf)
Shareable PDF
Acknowledgements
We thank all participants of the consensus development process, and particularly the people with lived experience of post-COVID-19 condition and their carers; WHO for their collaboration; the translators who provided translation of all English materials to additional languages; Mike Clarke for his kind agreement to act as an independent facilitator of the online interactive consensus meeting and wonderful conduct of this meeting. Declaration of AI and AI-assisted technologies in the writing process: During the preparation of this work the authors used ChatGPT in order to improve the grammatical structure and readability. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
The collaborating members of the PC-COS Children Project Steering Committee Group are as follows. Asiah Kassim (Women and Children Hospital, Kuala Lumpur, Malaysia); Jennifer R. Chevinsky (CDC COVID-19 Emergency Response, USA); Karen Choong (McMaster University, Hamilton, ON, Canada); Rae Duncan (Newcastle Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK); Susanna Esposito (University of Parma, Parma, Italy); Rebecca Foster (Royal Surrey Hospital, Guildford, UK); Alla Guekht (Moscow Research and Clinical Centre for Neuropsychiatry, Moscow, Russia); Allison Jaure (The University of Sydney, Sydney, Australia); Carrie Mackenzie (Sheffield Children's NHS Foundation Trust, Sheffield, UK); Vivienne Matthies-Bonn (Long Covid Kinderen, the Netherlands); Karen Matvienko-Sikar (University College Cork, Cork, Ireland); Lorraine McCloud (Long Covid Kids, Salisbury, UK); Donna C. McParland (Long Covid Kids, Salisbury, UK); Victor Daniel Miron (Carol Davila University of Medicine and Pharmacy, Bucharest, Romania); Srinivas Murthy (University of British Columbia, Vancouver, BC, Canada); Sarah O Connel (Long Covid Kids, Salisbury, UK); Margaret O'Hara (Long Covid Support, London, UK); Anna Ogonowska-Slodownik (Faculty of Rehabilitation, Jozef Pilsudski University of Physical Education in Warsaw, Warsaw, Poland); Jana Pavare (Riga Stradins University, Riga, Latvia); Paula Robertson (Paediatric Emergency Department, Eric Williams Medical Complex, Trinidad and Tobago); Jeremy Rossman (University of Kent, Canterbury, UK); Oana Săndulescu (Carol Davila University of Medicine and Pharmacy, Bucharest, Romania and National Institute for Infectious Diseases “Prof. Dr Matei Bal”, Bucharest, Romania); Sharon H. Saydah (CDC/DDID/NCIRD/CORVD; CDC COVID-19 Response, USA); Jochen Schmitt (Center for Evidence-based Healthcare, Medical Faculty Carl Gustav Carus, TU Dresden, Dresden, Germany); Malcolm “Calum” G. Semple (University of Liverpool and Alder Hey Children's Hospital, Liverpool, UK); Roz Shafran (UCL Great Ormond Street Institute of Child Health, London, UK); Ausra Snipaitiene (Lithuanian University of Health Sciences Pediatric Department, Kaunas, Lithuania); Arne Søraas (Oslo University Hospital, Oslo, Norway); Terence Stephenson (University College London, London, UK); Lola Tulen (National Institute for Public Health and the Environment, Bilthoven, the Netherlands); Gonzalo Valenzuela Galaz (Pontifical Catholic University of Chile, Santiago, Chile); David Vickers (Cambridgeshire Community Services NHS Trust, Cambridgeshire, UK); Daniel Christian Vilser (Hospital for Pediatrics and Adolescent Medicine, Jena University Hospital, Jena, Germany); Michaela Waak (The University of Queensland, Brisbane, Australia); Julie Wells (Long COVID Kids, Salisbury, UK); Elizabeth Whittaker (Section of Paediatric Infectious Diseases, Imperial College London and Department of Paediatric Infectious Diseases, Imperial College Healthcare NHS Trust, London, UK); Michelle Winer (Long COVID Kids, Salisbury, UK); Alan Asmanov (The Research and Clinical Institute for Pediatrics named after Academician Yuri Veltischev of the Pirogov Russian National Research Medical University, Moscow, Russia); Pasquale Comberiati (Department of Clinical and Experimental Medicine, Section of Pediatrics, University of Pisa, Italy).
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC), the National Institutes of Health (NIH), and the World Health Organization (WHO). The project has received input from WHO technical teams during the study design, data collection and analysis.
Ethics statement: Ethical approval for the study was obtained from the Sechenov University Ethics Committee on 20 January 2022 (protocol number 01-22).
Author contributions: D. Munblit conceived the idea for the study. D. Munblit led the methodological team and supervised the research team work throughout the project. D. Munblit, T.R. Nicholson, P.R. Williamson, D.M. Needham and N. Seylanova designed the study protocol. D. Munblit, T.R. Nicholson, P.R. Williamson and D.M. Needham carried out the methodological discussions at the start of the project. D. Munblit, N. Seylanova and A. Chernyavskaya were responsible for the day-to-day running of the project. A. Mursalova, N. Degtyareva, A. Ajam, L. Xiao, P. Bobkova, P. Roshchin and K. Aktulaeva undertook the literature review, identified outcome measures and outcome measurement instruments and categorised them for inclusion in the online Delphi survey and expert Delphi survey. N. Seylanova and A. Chernyavskaya coordinated the data revision process. N. Seylanova and A. Chernyavskaya developed the online Delphi surveys and contributed to the day-to-day management of the project. N. Seylanova, A. Chernyavskaya, A. Mursalova and N. Degtyareva were responsible for setting up the Delphi Manager. D. Munblit, N. Seylanova, A. Chernyavskaya, A. Mursalova and N. Degtyareva were responsible for communication with stakeholders. N. Seylanova, A. Chernyavskaya, A. Mursalova and N. Degtyareva prepared the instructions and materials for Delphi process participants. N. Seylanova, A. Mursalova and N. Degtyareva were involved in the process of setting up and updating the website. D. Munblit, N. Seylanova, A. Chernyavskaya, A. Mursalova, N. Degtyareva, A. Ajam and L. Xiao organised the “what to measure” consensus meeting. D. Munblit, N. Seylanova, A. Chernyavskaya and A. Mursalova were responsible for instrument cards design and contents. D. Munblit, A. Chernyavskaya, N. Seylanova, A. Mursalova, A. Ajam and L. Xiao organised the “how to measure” consensus meeting. D. Munblit, A. Chernyavskaya, N. Seylanova, D. Buonsenso, C. Brackel and S. Vijverberg participated in the project methodology discussions throughout the duration of the project. N. Nekliudov undertook the data analysis. N. Seylanova and A. Chernyavskaya organised the consensus meeting and consensus workshop. K. Kuppalli, N. Schiess and J.V. Diaz led the WHO administrative aspects of the study. S. McFarland provided and coordinated invaluable perspectives of people with lived experience throughout the study into its design and implementation. D. Munblit, N. Seylanova and A. Chernyavskaya drafted the manuscript; all authors reviewed and approved the final manuscript.
Conflict of interest: D. Munblit is a Co-Chair of International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Global Paediatric Long COVID Working Group, member of ISARIC working group on long-term follow-up in adults. C. Apfelbacher reports grants or contracts from Dr Wolff Group, Bionorica and The European Cooperation in Science and Technology (COST); he also acknowledges consulting fees from the Dr Wolff Group, Bionorica, Sanofi and LEO Pharma; he serves as a Co-Chair Harmonising Outcome Measures for Eczema (HOME) initiative and is Co-Chair of the Hand Eczema Core Outcome Set (HECOS) initiative and is core principal investigator of the KUNOKids Health Study (Regensburg, Germany). J.V. Diaz is the lead of the clinical management response pillar for COVID-19 and in that capacity convenes the WHO Clinical Characterization and Management Research working group; the Post COVID-19 COS steering committee was a sub-working group of this bigger group. O.L. Aiyegbusi has received research grants from UCB, Kidney Research UK, Gilead Sciences Ltd, The Health Foundation, NIHR Birmingham BRC, NIHR ARC, NIHR BTRU, Innovate UK, Merck, GSK, Anthony Nolan and Sarcoma UK; he has also received personal fees from GSK, Gilead Sciences, Innovate UK and Merck. C.R. Oliveira receives grant support from the National Institutes of Health (NIH), grant numbers OT2HL161847 and K23AI159518. P.R. Williamson is chair of the Core Outcome Measures in Effectiveness Trials (COMET) Management Group. Other authors declare that they have no competing interests.
Contributor Information
Collaborators: Asiah Kassim, Jennifer R. Chevinsky, Karen Choong, Rae Duncan, Susanna Esposito, Rebecca Foster, Alla Guekht, Allison Jaure, Carrie Mackenzie, Vivienne Matthies-Bonn, Karen Matvienko-Sikar, Lorraine McCloud, Donna C. McParland, Victor Daniel Miron, Srinivas Murthy, Sarah O Connel, Margaret O’Hara, Anna Ogonowska-Slodownik, Jana Pavare, Paula Robertson, Jeremy Rossman, Oana Săndulescu, Sharon H. Saydah, Jochen Schmitt, Malcolm “Calum” G. Semple, Roz Shafran, Ausra Snipaitiene, Arne Søraas, Terence Stephenson, Lola Tulen, Gonzalo Valenzuela Galaz, David Vickers, Daniel Christian Vilser, Michaela Waak, Julie Wells, Elizabeth Whittaker, Michelle Winer, Alan Asmanov, and Pasquale Comberiati
References
- 1.Munblit D, O'Hara ME, Akrami A, et al. Long COVID: aiming for a consensus. Lancet Respir Med 2022; 10: 632–634. 10.1016/S2213-2600(22)00135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Leon S, Wegman-Ostrosky T, Ayuzo Del Valle NC, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep 2022; 12: 9950. doi: 10.1038/s41598-022-13495-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J 2021; 40: e482–e487. doi: 10.1097/INF.0000000000003328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Burden of Disease Long COVID Collaborators , Wulf Hanson S, Abbafati C, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022; 328: 1604–1615. doi: 10.1001/jama.2022.18931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rea M, Pawelek P, Ayoubkhani D. Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK. 2023. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/30march2023#prevalence-of-ongoing-symptoms-following-coronavirus-infection-in-the-uk-data. Date last accessed: 17 September 2023. Date last updated: 30 March 2023.
- 6.Trapani G, Verlato G, Bertino E, et al. Long COVID-19 in children: an Italian cohort study. Ital J Pediatr 2022; 48: 83. doi: 10.1186/s13052-022-01282-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munblit D, Buonsenso D, Sigfrid L, et al. Post-COVID-19 condition in children: a COS is urgently needed. Lancet Respir Med 2022; 10: 628–629. doi: 10.1016/S2213-2600(22)00211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkham JJ, Williamson P. Core outcome sets in medical research. BMJ Med 2022; 1: e000284. doi: 10.1136/bmjmed-2022-000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson PR, Altman DG, Bagley H, et al. The COMET Handbook: version 1.0. Trials 2017; 18: Suppl. 3, 280. doi: 10.1186/s13063-017-1978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munblit D, Nicholson T, Akrami A, et al. A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: an international Delphi consensus study. Lancet Respir Med 2022; 10: 715–724. doi: 10.1016/S2213-2600(22)00169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorst S, Seylanova N, Harman N, et al. A Core Outcome Measurement Set (COMS) for Research and Clinical Practice in Post COVID-19 Condition (Long COVID) in Adults: an International Delphi Consensus Study. 2023. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4317875. Date last accessed: 17 September 2023.
- 12.Dodd S, Clarke M, Becker L, et al. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol 2018; 96: 84–92. doi: 10.1016/j.jclinepi.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011; 64: 395–400. doi: 10.1016/j.jclinepi.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) . A Clinical Case Definition for Post COVID-19 Condition in Children and Adolescents by Expert Consensus. 2023. www.who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1. Date last accessed: 20 September 2023. Date last updated: 16 February 2023.
- 15.Verstraete J, Scott D. Comparison of the EQ-5D-Y-5L, EQ-5D-Y-3L and PedsQL in children and adolescents. J Patient Rep Outcomes 2022; 6: 67. doi: 10.1186/s41687-022-00480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devlin N, Pan T, Kreimeier S, et al. Valuing EQ-5D-Y: the current state of play. Health Qual Life Outcomes 2022; 20: 105. doi: 10.1186/s12955-022-01998-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorst SL, Prinsen CAC, Salcher-Konrad M, et al. Methods used in the selection of instruments for outcomes included in core outcome sets have improved since the publication of the COSMIN/COMET guideline. J Clin Epidemiol 2020; 125: 64–75. doi: 10.1016/j.jclinepi.2020.05.021 [DOI] [PubMed] [Google Scholar]
- 18.Hansen RA, Henley AC, Brouwer ES, et al. Geographic Information System mapping as a tool to assess nonresponse bias in survey research. Res Social Adm Pharm 2007; 3: 249–264. doi: 10.1016/j.sapharm.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 19.Smith W. Does Gender Influence Online Survey Participation? A Record-Linkage Analysis of University Faculty Online Survey Response Behavior. 2008. https://files.eric.ed.gov/fulltext/ED501717.pdf. Date last accessed: 17 September 2023.
- 20.Jassat W, Reyes LF, Munblit D, et al. Long COVID in low-income and middle-income countries: the hidden public health crisis. Lancet 2023: 402: 1115–1117. doi: 10.1016/S0140-6736(23)01685-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Appendix 1 ERJ-01761-2023.Appendix_1 (2MB, pdf)
Appendix 2 ERJ-01761-2023.Appendix_2 (402.6KB, pdf)
Appendix 3 ERJ-01761-2023.Appendix_3 (757.2KB, pdf)
Appendix 4 ERJ-01761-2023.Appendix_4 (398.2KB, pdf)
Appendix 5 ERJ-01761-2023.Appendix_5 (154.9KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01761-2023.Shareable (642.3KB, pdf)