Abstract
We report herein on the solid-state structures of three closely related triphenylamine derivatives endowed with tricyanovinyl (TCV) and dicyanovinyl (DCV) groups. The molecules described contain structural features commonly found in the design of functional organic materials, especially donor–acceptor molecular and polymeric architectures. The common feature noticeable in these structures is the impact of these exceptionally strong electron-accepting groups in forcing partial planarity of the portion of the molecule carrying these groups and directing the molecular packing in the solid state, resulting in the formation of π-stacks of dimers within the unit cell of each. Stacks are formed between phenyl groups bearing electron-accepting groups on two adjacent molecules. Short π–π stack distances ranging from 3.283 to 3.671 Å were observed. Such motif patterns are thought to be conducive for better charge transport in organic semiconductors and enhanced device performance. Intramolecular charge transfer is evident from the shortening of the observed experimental bond lengths in all three compounds. The nitrogen atoms (of the cyano groups) have been shown to be extensively involved in short contacts in all three structures, primarily through C–H···NC interactions with distances as short as 2.462 Å. The compounds reported here are (3,3-dicyano-2-(4-(diphenylamino)phenyl)-1λ3-allylidene)amide or tricyanovinyltriphenylamine, Ph3NTCV (1); 2-(4-(diphenylamino)benzylidene)-malononitrile or dicyanovinyltriphenylamine, Ph3NDCV (2); and (3,3-dicyano-2-(4-(di-p-tolylamino)phenyl)-1λ3-allylidene)amide or dimethyltricyanovinyltriphenylamine, Me2Ph3NTCV (3). Results of density functional theory calculations using DFT-B3LYP/6-31G(d,p) indicate the lowering of LUMO levels as a result of the introduction of these groups with band gaps of 3.13, 2.61, and 2.55 eV for compounds 1–3, respectively, compared with 4.65 eV calculated for triphenylamine. This is supported by the electronic and fluorescence spectra of these molecules with absorption λmax of 483, 515, and 545 nm for compounds 1, 2, and 3, respectively.
Introduction
Triphenyl- and triarylamine-based materials constitute an important category of compounds in organic materials chemistry with many uses in organic optoelectronics, solar cells, and electroluminescence and electrochromic applications.1−4 Donor/acceptor molecules incorporating this building block have received considerable attention. Synthetically, many creative and interesting molecular architectures incorporating triphenylamines have been reported.5 However, much less attention has been paid to the solid-state structures of this important class of organic functional materials and how one can predict or attempt to control the way they aggregate to form crystals or thin films suitable for device fabrication. This is probably due to the relative difficulties one encounters in growing single crystals suitable for X-ray studies compared with oligothiophenes, for instance. Recently, enhancement of efficiencies of solar cells up to 24% was reported by manipulating inter- and intramolecular interactions. This achievement was attributed to favorable solid-state packing using S···O and Se···O interactions to induce planarity and π–π stack formation.6 This further demonstrates the importance of studies directed at achieving planarity and π-stack formation in improving device performance by a better understanding of competing inter- and intramolecular interactions in organic functional materials, where conjugated organic molecules and polymers are used.
Therefore, studies directed at understanding the solid-state structures of substituted triphenylamine are highly desirable. Such studies would hopefully provide some insights for further development of solid structure–property relationships and yield better correlation with device performance. In general, our ability to make and selectively control noncovalent interactions and syntheses is much less developed compared with synthetic organic chemistry. Various strategies and methods have been employed in attempting to control the solid-state structures of molecular materials. These methods have included the use of intermolecular interactions such as hydrogen bonding, electrostatic interactions, surface modification, and size confinement.7−9
We reported earlier on structural features of some tricyanovinyl or (TCV)-substituted10 and dicyanovinyl- (DCV) oligothiophenes.11 We demonstrated that the introduction of these strong electron acceptors provides a rather simple approach to modify their electrical properties (as evident from the electrochemical data), enforces molecular planarity, and promotes π-stack formation, In addition, these strong electron-accepting groups appear to significantly improve the thermal stability and solubility, both of which are sought-after characteristics for the fabrication of thin film and single crystal devices from solution and vapor phase.
We set out to examine how these strong electron-accepting groups might impact triphenylamines. We report herein our results on the structural features of three triphenylamine derivatives endowed with tricyanovinyl or TCV and dicyanovinyl or DCV groups. The compounds reported here are (3,3-dicyano-2-(4-(diphenylamino)phenyl)-1λ3-allylidene)amide or tricyanovinyltriphenylamine, Ph3NTCV (1); 2-(4-(diphenyl-amino)benzylidene)-malononitrile or dicyanovinyltriphenylamine, Ph3NDCV (2); and (3,3-dicyano-2-(4-(di-p-tolylamino)phenyl)-1λ3-allylidene)amide or dimethyltricyanovinyltriphenylamine, Me2Ph3NTCV (3).
The structures of these molecules are listed in Figure 1.
Figure 1.
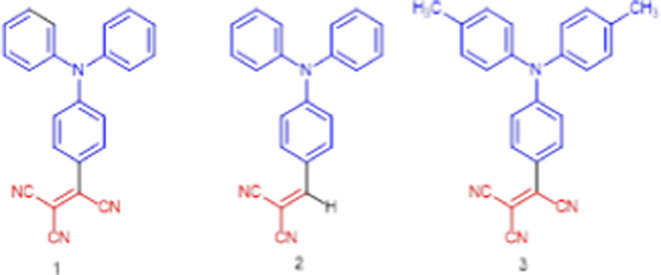
Structures of molecules in this study.
Results and Discussion
Compounds 1–3 were synthesized following modified published procedures as follows.12,13
Synthesis of Compound 1
Triphenylamine (Aldrich) 125 mg (0.5 mmol) was reacted with tetracyanoethylene (TCNE), (Aldrich) 196 mg (0.75 mmol) in DMF (5 mL) in 25 mL of RBF at room temperature. After 2 h, the reaction was worked out either by addition of 6 M HCl or extraction by methylene chloride. The product, Ph3N-TCV (1), is isolated as a purple solid, mp 181–182 C. Typical yields for the synthesis are 100 mg (57%). ν(KBr)/cm–1 2220 (CN); 1H NMR spectroscopy. δH (360 MHz; CDCl3) 7.98 (2H, m), 7.27 (10 H, m), 6.94 (m, 2H); 13C NMR (360 MHz; CDCl3) 154.6, 144.3, 138.2, 132.2, 130.2, 127.1, 127.0, 120.2, 118.0, 114.2, 113.2, 113.0, 81.9.
Synthesis of Compound 2
This compound was prepared employing Knoevenagel condensation reaction by reacting the corresponding aldehyde with malonitrile in dichloromethane or ethanol.11,12
Synthesis of Formyl Triphenylamine
POCl3 (2.4 mL, 25.0 mmol) was added dropwise to a solution of the corresponding triphenylamine derivative (3.0 g, 12.2 mmol) in DMF (30 mL). After stirring at 90 C for 24 h. under nitrogen, the reaction mixture was allowed to cool to room temperature and then poured into ice water. The resulting solid was purified by column chromatography using ethyl acetate/hexanes (1:4). The aldehyde was obtained as an off-white solid, mp 130–132 °C, 3.1 g, 91%. H NMR: 7.01 (d, 2H); 7.15 (m, 6H); 7.32 (t, 4H); 7.70 (d, 2H); 9.81 (s, 1H).
Formyl triphenylamine (300 mg, 1.1 mmol) was dissolved in dry dichloromethane (30 mL). Five drops of triethylamine were added, followed by 100 mg of malonitrile (1.5 mmol). The reaction mixture was stirred at room temperature for 6 h. The solvent was removed under reduced pressure. The residue was then purified by column chromatography using ethyl acetate/hexanes (1:9) to give compound 2 as an orange solid, mp 138–139 C. 2220 (CN); 1H NMR spectroscopy. δH (360 MHz; CDCl3) 7.79, (1H, s); 7.98 (2H, m), 7.27 (10 H, m), 6.94 (m, 2H); 13C NMR (360 MHz; CDCl3) 154.6, 144.3, 138.2, 132.2, 130.2, 127.1, 127.0, 120.2, 118.0, 114.2, 113.2, 113.0, 81.9.
Synthesis of Compound 3
Compound 3 was prepared using a similar procedure as for 1 above, using the proper starting triphenylamine (commercially available, Sigma-Aldrich). Compound 3: mp 189–190 °C, H NMR: 7.13 (d, 6H); 7.15 (d, 4H); 7.30 (d, 2H); 2.32 (s, 6H). We note that heating the reaction mixture up to 80 °C gave better yields in the syntheses of compounds 1 and 2.
Computational Methods
Calculations were carried out at the DFT level of theory with the hybrid functional B3LYP using the 6-311+G** basis set in the gas phase on isolated molecules.14
Upon extraction with dichloromethane and recrystallization from acetonitrile, suitable crystals from these three compounds were selected for X-ray structural analysis. A summary of crystallographic data for compounds 1–3 is shown in Table 1.
Table 1. Summary of Crystallographic Data for Compounds 1–3.
| Ph3NTCV (1) | Ph3NDCV (2) | Me2Ph3NTCV (3) | |
|---|---|---|---|
| formula | C23H14N4 | C22 H15 N3 | C25 H18 N4 |
| formula weight | 346.38 | 321.37 | 374.43 |
| space group, Z | Pbca, 8 | P21/c, 4 | Pbca, 8 |
| a, Å | 11.154(4) | 6.9352(13) | 16.8662(15) |
| b, Å | 14.604(5) | 15.731(3) Å | 12.8555(11) |
| c, Å | 21.685(7) | 16.098(3) | 18.7561(16) |
| α, deg | 90 | 90 | 90 |
| β, deg | 90 | 95.190(3)° | 90 |
| γ, deg | 90 | 90 | 90 |
| V, Å3 | 3532 | 1749.1(6) | 4066.8(6) |
| T, K | 173(2) | 173(2) | 173(2) |
| dcalcd, g cm–3 | 1.303 | 1.220 | 1.223 |
| reflections collected | 4200 | 19646 | 24792 |
| observed reflections | 1633 | 3136 | 2619 |
| goodness-of-fit on F2 | 0.821 | 1.035 | 1.023 |
| final R indices [I > 2σ(I)] | R1 = 0.0490, wR2 = 0.1052 | R1 = 0.0409, wR2 = 0.0916 | R1 = 0.0490, wR2 = 0.1052 |
| R indices (all data) | R1 = 0.0901, wR2 = 0.1268 | R1 = 0.0581, wR2 = 0.0996 | R1 = 0.0901, wR2 = 0.1268 |
| short C–H···N contacts, Å | 2.462; 2.682 | 2.542; 2.645 | 2.637; 2.640 |
| C–N bond length*, Å | 1.380 Å | 1.375 | 1.366 |
| shortest π–π distances, Å | 3.614 | 3.283, 3.470 | 3.444 |
| CCDC Number | 186368 | 2202335 | 2202337 |
The crystal structure of triphenylamine is known and has been examined several times.15 We chose to use the latest report, ref (14), in our discussion. There are no significant close interactions within the unit cell of triphenylamine except for C–H···π with a relatively long distance (2.817 Å). We also note that there have been several recent structural reports on triphenylamine derivatives,16−19 with various structural features including multicyanoderivatives.12
Although the compounds herein are known, to the best of our knowledge, none of the structures in the current work have been reported except for compound 1 whose structure was reported in a conference paper and a brief report in 1975 and 1979, respectively, with rather limited details.20 The authors described two polymorphs.21 However, despite many crystallization experiments from different solvents and by sublimation, we were able to obtain only one polymorph (the orthorhombic form). We point out the slightly different values for the unit cell axes we obtained compared to those reported earlier in [square brackets], a = 11.154; [11.335]; b = 14.604; [14.603]; c = 21.685; [21.731] Å (CYVTPA; CYVTPA01). It is worth mentioning that compounds 1–3 form shiny metallic crystals with large, smooth surfaces (see Supporting Information).
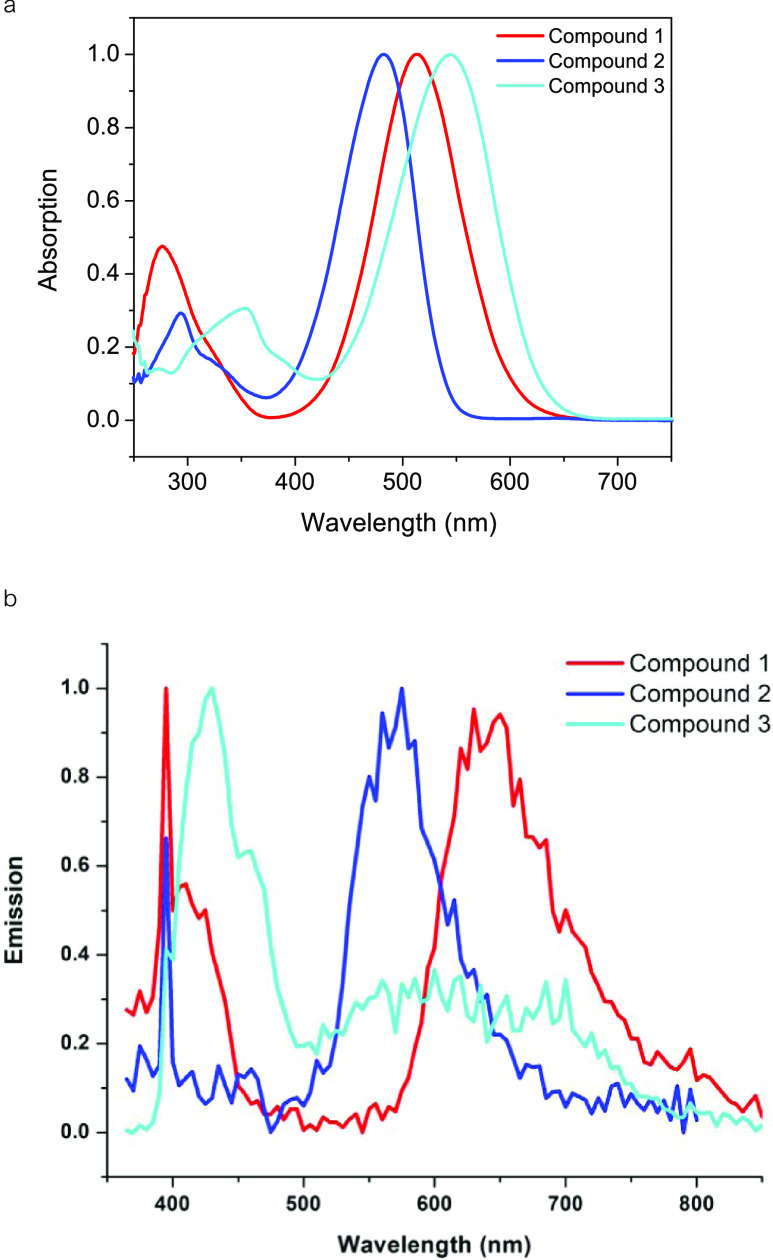
We first consider the description of the structural features of these compounds. We note that all three compounds adopt a propeller molecular shape with (1) and (3) crystallizing in the orthorhombic space group Pbca and (2) and in the monoclinic space group P21/c (Table 1). The angles around the central nitrogen atom were all nearly the same showing similar trends with the smallest angle between the phenyl groups without the electron-accepting group: 116, 121, and 123° in 1; 118, 121, and 121° in 2; and 116, 120, and 123° in 3. Whereas C–N bond lengths clearly were significantly shorter for the ring bearing the electron acceptor: They were as follows: 1.38, 1.44, and 1,44 in 1; 1.37, 1.44, and 1.43 in 2 and 1.36, 1.44, and 1.44 Å in 3. The shortest lengths (italic) are for the N–C bond on the phenyl group carrying the DCV or the TCV groups, suggesting, as expected, intramolecular charge transfer. This is probably the cause of the dramatic shift observed in these materials’ maximum absorption. For instance, λmax for triphenylamine, (a white solid) is 230 nm (methanol) when compared with these compounds which are highly colored with λmax of 483, 515, and 545 nm for 1–3 respectively (Figure 2). The three compounds also exhibited fluorescence with λmax of 650, 575, and 430 nm for 1–3, respectively. Both the absorption and emission spectra were measured in dichloromethane. In addition, a solvatochromic behavior was observed. For example, in compound 1, a bathochromic shift (Δλmax = 20 nm) was observed with increasing polarity of the solvent from hexane to methanol. More optical studies are currently underway in our group on these and closely related structures.
Figure 2.
UV–vis (a) and fluorescence (b) spectra of compounds 1–3. The emission spectra are excited at 355 nm. Both absorption and emission spectra were measured in dichloromethane.
All three molecules form π-stack dimers involving the acceptor-carrying phenyl rings on two adjacent molecules with the shortest distance of 3.616 Å in 1, 3.283 in 2, and 3.497 Å in 3. The dimers are further held by C–H···NC interactions on both ends with distances of 2.462, 2.645, and 2.637 Å in 1, 2, and 3, respectively (Figure 3). Unlike 1 and 3, with identical C–H···N and π–π stack distances, compound 2 showed two distinct stacks and C–H···NC interactions. The additional C–H···NC interaction in 2 resulted in two different distances (2.542, 2.645 Å).
Figure 3.
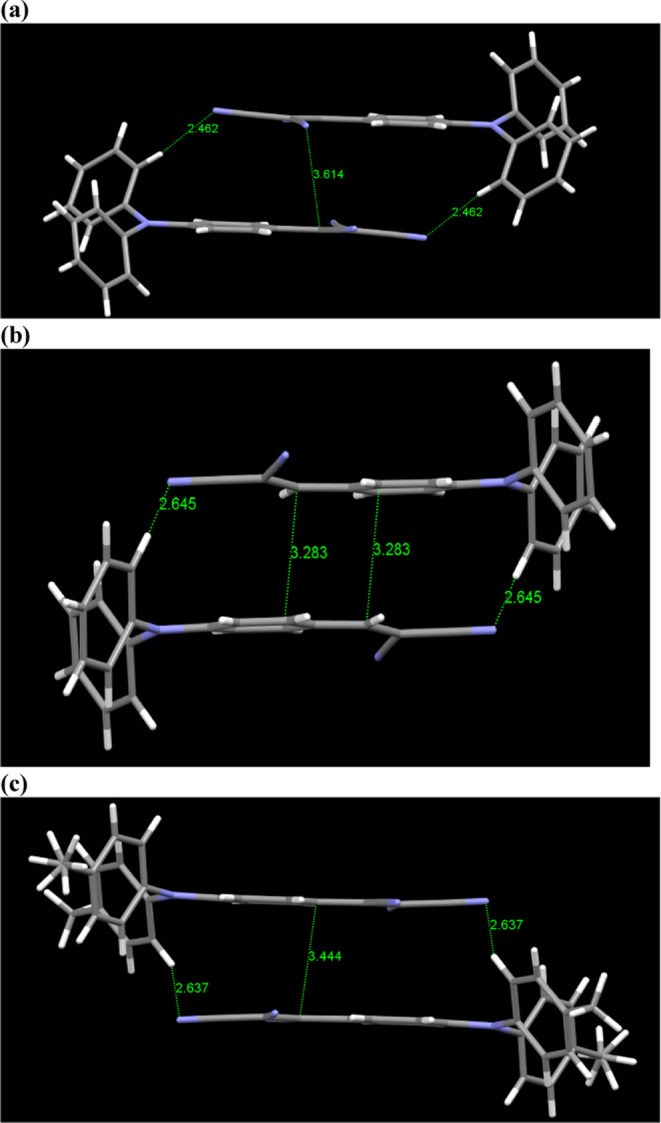
π-Stacking and C–H···N interactions in compounds 1 (a), 2 (b), and 3 (c).
Upon examining bond lengths revealed from the crystal structures, intramolecular charge transfer is evident in all three compounds. This is illustrated for 1 by considering the bond lengths along the conjugation path (Figure 4) and the C–N bond lengths around the central nitrogen as pointed out earlier. For example, in 1, the C–C bonds in the aromatic rings range from 1.365 to 1.411 Å, significantly shorter than the usual (1.440 Å), which indicates conjugated double bonds in a molecule. C–C bond lengths in the phenyl rings without the accepting groups were almost all identical (1.380 Å) in all three structures, possibly indicating nearly equal contribution in donor behavior of these two phenyl rings. Furthermore, these bond length patterns are indicative of intramolecular charge transfer along the path shown for 1 (phenyl groups toward the electron-accepting groups).
Figure 4.
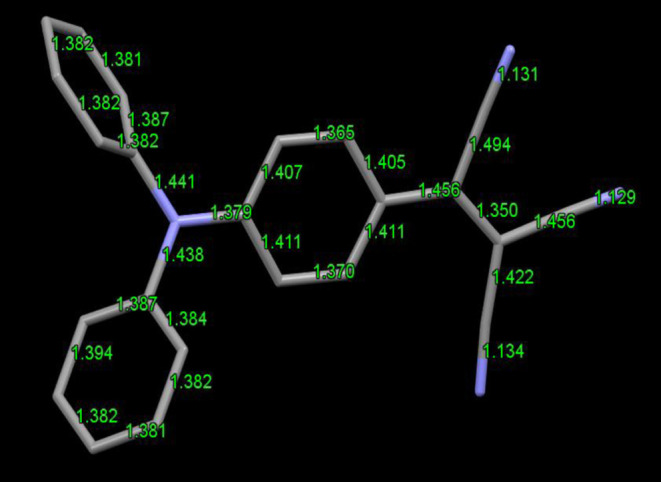
Bond lengths highlight the intramolecular charge transfer path in 1.
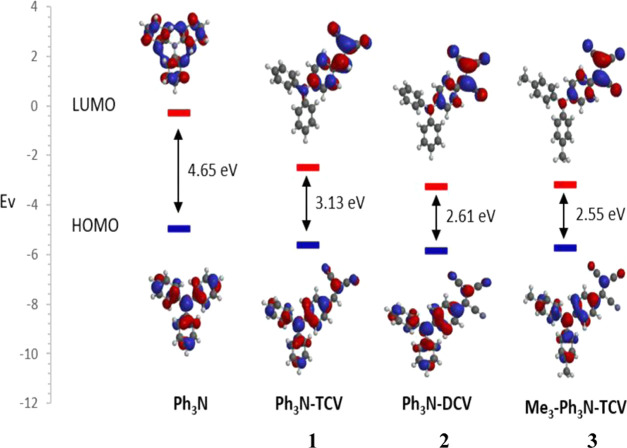
Computational and theoretical studies have proven to be very useful in organic materials chemistry research. These investigations provide insights to guide synthetic work and help interpret experimental results especially when carried out on structurally related series of molecules.22 A recent theoretical investigation on the effects of substitution of the triphenylamine (TPA) on the overall properties of materials based on small push–pull molecules designed as donors for organic photovoltaics (OPV) has been reported.23 Therefore, to augment our work, we carried out density functional theory (DFT) calculations on molecules 1–3 in addition to triphenylamine as a reference point. All calculations reported herein were performed at the density functional theory level using the hybrid functional B3LYP as implemented in the software package Spartan 20.14 The choice of this method is based on earlier studies in which the DFT-B3LYP method was proven to produce geometries and energetics comparable to experimental results.24 We first note the narrowing of the calculated band gap of all three molecules, primarily due to the lowering of the LUMO levels upon the introduction of the TCV and DCV groups (Figure 5), whereas the introduction of the methyl groups slightly raised the HOMO level.
Figure 5.
Calculated molecular orbitals for triphenylamine and compounds 1–3, DFT-B3LYP/6-31G(d,p).
The main points these calculations indicate are as follows: (1) Calculated molecular geometries and shapes were in good agreement with the X-ray experimental data, lending further confidence in this level of computational work. (2) The evolution of LUMO–HOMO levels is as one might expect, with calculated band gaps ranging from 2.55 eV for compound 3 [compared with a band gap of 4.65 eV for triphenylamine] to 3.13 eV for compound 1. This is probably due to 3 having a stronger donor nature due to the presence of the methyl groups and hence the raising of the HOMO levels. (3) The reduction in band gap is mainly due to the lowering of the LUMO levels in all three compounds. (4) Electrostatic potential map (EPM) shows a high negative electrostatic potential (red) located on the TCV group (Figure 6).
Figure 6.
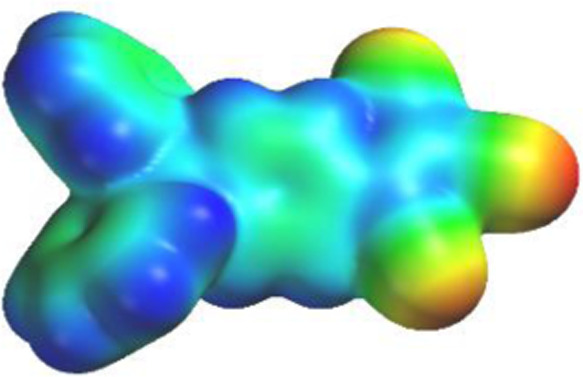
Electrostatic potential map (EPM) as calculated at the DFT-B3LYP level of compound 1 shows a highly negative electrostatic potential (red) located on the TCV group.
Finally, we also note that none of these molecules, despite forming nice large crystals with good quality smooth surfaces suitable for single crystal device fabrications, showed field effects in the thin film or in single crystal device configurations.
Conclusions
We have successfully synthesized and structurally characterized three triphenylamine derivatives endowed with strong electron-accepting groups. They all had a shared feature of forming π-stacks involving phenyl rings carrying the electron acceptor groups between adjacent molecules with π···π distances ranging between 3.28 and 3.61 Å. We also reported on their electronic and fluorescence properties, which support the presence of intramolecular charge transfer as evidenced from experimental crystal structural data. DFT calculations showed the evolution of the HOMO–LUMO levels upon the introduction of these strong electron-accepting groups. In summary, we have shown that the introduction of tricyanovinyl (TCV) and dicyanovinyl (DCV) groups into triphenylamine molecules provides an efficient way to induce partial planarity of the molecule and π-stack formation between the planar parts of adjacent molecules and results in drastic shifts in the electronic and fluorescence behavior of triphenylamine. These electron-accepting groups can provide a new tool for the molecular design and crystal engineering of triphenyl- and possibly triarylamine-based materials. One might use them in other conjugated organic molecules. The optical data suggest strong intramolecular charge transfer, as seen from the significant differences in their electronic spectra with the parent unsubstituted triphenylamine.
Acknowledgments
Research Development Grants and Professional Development Grants from Penn State Scranton (PTP) and internal research grant from Alfaisal University Office of Research (IRG-18421) are highly appreciated. The authors also acknowledge Dr. Radu Custelcean (for his help with solving structure 1), Dr. W. Brennessel and Dr. Victor Young Jr. of the X-ray Crystallographic Laboratory, Department of Chemistry at the University of Minnesota and Dr. Maysoon Saleh and Dr. W. Sun for help with the optical data. We also acknowledge Lamya Al-Fuhaid, Tarfah Al-Rawaf, and numerous organic chemistry lab (II) students both at Alfaisal University and Penn State Scranton in optimizing reaction conditions for the synthesis of compound 1, which was introduced as an experiment on electrophilic aromatic substitution reactions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c05312.
Details of X-ray analysis methods and pictures of single crystals of compound 1. (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Khasbaatar A.; Xu Z.; Lee J.-H.; Campillo-Alvarado G.; Hwang C.; Onusaitis B. N.; Diao Y. From Solution to Thin Film: Molecular Assembly of π-Conjugated Systems and Impact on (Opto)electronic Properties. Chem. Rev. 2023, 123, 8395–8487. 10.1021/acs.chemrev.2c00905. [DOI] [PubMed] [Google Scholar]
- Itoo A. M.; Paul M.; Padaga S. G.; Ghosh B.; Biswas S. Nanotherapeutic Intervention in Photodynamic Therapy for Cancer. ACS Omega 2022, 7, 45882–45909. 10.1021/acsomega.2c05852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Y.; Liu Y.; Guo Y. Intrinsically Stretchable Organic Optoelectronic Devices and Arrays: Progress and Perspective. Sci. Bull. 2023, 68 (10), 975–980. 10.1016/j.scib.2023.04.035. [DOI] [PubMed] [Google Scholar]
- Kong Q.; Qian H.; Zhou Y.; Li J.; Xiao H. Synthesis and Optoelectronic Properties of a Monodispersed Macrocycle Oligomer Consisting of Three Triarylamine Units. Mater. Chem. Phys. 2012, 135 (2), 1048–1056. 10.1016/j.matchemphys.2012.06.012. [DOI] [Google Scholar]
- See for example:; a Ogunyemi B. T.; Oyeneyin O. E.; Esan O. T.; Adejoro I. A. Computational Modelling and Characterization of Phosphole Adopted in Triphenyl Amine Photosensitizers for Solar Cell Applications. Results Chem. 2020, 2, 100069 10.1016/j.rechem.2020.100069. [DOI] [Google Scholar]; b El-Nahass M. M.; Zeyada H. M.; Abd-El-Rahman K. F.; Darwish A. A. A. Structural Characterization and Electrical Properties of Nanostructured 4-Tricyanovinyl-N,N-Diethylaniline Thin Films. Eur. Phys. J. – Appl. Phys. 2013, 62, 10202. 10.1051/epjap/2013120061. [DOI] [Google Scholar]
- Huang H.; Yang L.; Facchetti A.; Marks T. J. Organic and Polymeric Semiconductors Enhanced by Noncovalent Conformational Locks. Chem. Rev. 2017, 117, 10291–10318. 10.1021/acs.chemrev.7b00084. [DOI] [PubMed] [Google Scholar]
- Hobza P.; Řezáč J. Noncovalent Interactions Editorial Introduction. Chem. Rev. 2016, 116 (9), 4911–4912. 10.1021/acs.chemrev.6b00247. [DOI] [PubMed] [Google Scholar]
- Whitesides G. M.; Simanek E. E.; Mathias J. P.; Seto C. T.; Chin D.; Mammen M.; Gordon D. M. Noncovalent Synthesis: Using Physical-Organic Chemistry to Make Aggregates. Acc. Chem. Res. 1995, 28, 37–44. 10.1021/ar00049a006. [DOI] [Google Scholar]
- Zhang K.; Fellah N.; López-Mejías V.; Ward M. D. Polymorphic Phase Transformation Pathways under Nanoconfinement: Flufenamic Acid. Cryst. Growth. Des. 2020, 20, 7098–7103. 10.1021/acs.cgd.0c01207. [DOI] [Google Scholar]
- Pham P-T T.; Bader M. M. The Impact of Vinylene Bridges and Side Chain Alkyl Groups on Solid State Structures of Tricyanovinyl-Substituted Thiophenes. CrystEngComm 2018, 20, 128–132. 10.1039/C7CE01574G. [DOI] [Google Scholar]
- Bader M. M.; Pham P.-T. T.; Elandaloussi E. Dicyanovinyl-Substituted Oligothiophenes. Cryst. Growth Des. 2010, 10, 5027–5030. 10.1021/cg100948m. [DOI] [Google Scholar]
- Tang X.; Liu W.; Wu J.; Lee C.-S.; You J.; Wang P. Synthesis, Crystal Structures, and Photophysical Properties of Triphenylamine-Based Multicyano Derivatives. J. Org. Chem. 2010, 75, 7273–7278. 10.1021/jo101456v. [DOI] [PubMed] [Google Scholar]
- Lambert C. L.; Gaschler W.; Schmalzlin E.; Meerholz K.; Brauchle C. Subchromophore interactions in tricyanovinyl-substituted triarylamines—a combined experimental and computational study. J. Chem. Soc., Perkin Trans. 2 1999, 577–588. 10.1039/a808009g. [DOI] [Google Scholar]
- Spartan 20; Wavefunction, Inc., Irvine, CA: 92612, United States. https://www.wavefun.com/spartan-documentation.
- a Martin E.; Pulham C. R.; Parsons S.. CSD Communication. CCDC Number: 660790. 2007.; b Sobolev A. N.; Belsky V. K.; Romm I. P.; Chernikova N. Y.; Guryanova E. N. Structural investigation of the triaryl derivatives of the Group V elements. IX. Structure of triphenylamine, C18H15N. Acta Crystallogr., Sect. C 1985, 41, 967–971. 10.1107/S0108270185006217. [DOI] [Google Scholar]; c Howells E. R.; Lovell F. M.; Rogers D.; Wilson A. J. C. The space groups of nitrogen triphenyl and phosphorus triphenyl. Acta Crystallogr. 1954, 7, 298–299. 10.1107/S0365110X54000825. [DOI] [Google Scholar]
- Ishi-i T.; Tanaka H.; Youfu R.; Aizawa N.; Yasuda T.; Kato S.; Matsumoto T. Mechanochromic fluorescence based on a combination of acceptor and bulky donor moieties: tuning emission color and regulating emission change direction. New J. Chem. 2019, 43, 4998–5010. 10.1039/C8NJ06050A. [DOI] [Google Scholar]
- Akahane S.; Takeda T.; Hoshino N.; Akutagawa T. Molecular Assemblies of Tetrahedral Triphenylmethanol and Triphenylamine Derivatives Bearing -NHCOCnH2n+1 Chains. Cryst. Growth Des. 2018, 18, 6284–6292. 10.1021/acs.cgd.8b01152. [DOI] [Google Scholar]
- Hariharan P. S.; Prasad V. K.; Nandi S.; Anoop A.; Moon D.; Anthony S. P. Molecular Engineering of Triphenylamine Based Aggregation Enhanced Emissive Fluorophore: Structure-Dependent Mechanochromism and Self-Reversible Fluorescence Switching. Cryst. Growth Des. 2017, 17, 146–155. 10.1021/acs.cgd.6b01363. [DOI] [Google Scholar]
- Song Y.; Di C.; Yang X.; Li S.; Xu W.; Liu Y.; Yang L.; Shuai Z.; Zhang D.; Zhu D. A Cyclic Triphenylamine Dimer for Organic Field-Effect Transistors with High Performance. J. Am. Chem. Soc. 2006, 128, 15940–15941. 10.1021/ja064726s. [DOI] [PubMed] [Google Scholar]
- Fu Q.; Tang X.; Liu H.; Wang R.; Liu T.; Wu Z.; Woo H.; Zhou T.; Wan X.; Chen Y.; Liu Y. Ionic Dopant-Free Polymer Alloy Hole Transport Materials for High-Performance Perovskite Solar Cells. J. Am. Chem. Soc. 2022, 144, 9500–9509. 10.1021/jacs.2c04029. [DOI] [PubMed] [Google Scholar]
- a Vozzhennikov V. M.; Materikin V. L.; Kotov B. V. Photoconductivity of Tricyanovinylarylimine mono crystals at low temperatures. Zh. Fiz. Khim. 1979, 53, 1580. [Google Scholar]; b Popova E. G.; Chetkina L. A.; Kotov B. V. X-ray structural study of p-tricyanovinyl-N,N-dimethylaniline. Zh. Strukt. Khim. 1976, 17, 510. [Google Scholar]
- Malagoli M.; Bredas J. L. Density functional theory study of the geometric structure and energetics of triphenylamine-based hole-transporting molecules. Chem. Phys. Lett. 2000, 327, 13–17. 10.1016/S0009-2614(00)00757-0. [DOI] [Google Scholar]
- Alberga D.; Ciofini I.; Mangiatordi G. F.; Pedone A.; Lattanzi A. P. G.; Lattanzi G.; Roncali J.; Roncali J.; Adamo C. Effects of Substituents on Transport Properties of Molecular Materials for Organic Solar Cells: A Theoretical Investigation. Chem. Mater. 2017, 29, 673–681. 10.1021/acs.chemmater.6b04277. [DOI] [Google Scholar]
- a Bongini A.; Bottoni A. A Theoretical Investigation of the Torsional Potential in 3,3′-Dimethyl-2,2′-bithiophene and 3,4′-Dimethyl-2,2′-bithiophene: A Comparison between HF, MP2, and DFT Theory. J. Phys. Chem. A 1999, 103, 6800–6804. 10.1021/jp990319+. [DOI] [Google Scholar]; b Bader M. M.; Hamada T.; Kakuta A. Theoretical investigation of the geometric structures and the second-order nonlinear optical properties of 8-hydroxyquinoline derivatives. J. Am. Chem. Soc. 1992, 114, 6475–6479. 10.1021/ja00042a028. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.