Abstract
Purpose:
Infrapopliteal lesions are generally complex to treat due to small vessel diameter, long lesion length, multilevel disease, and severe calcification. Therefore, different vessel preparation devices have been developed to contribute to better peri- and postprocedural outcomes. This systematic review aims to compare different vessel preparation techniques prior to plain old balloon angioplasty (POBA) or drug-coated balloon (DCB) angioplasty with POBA or DCB alone in infrapopliteal arterial disease.
Methods:
Medline, EMBASE, and Cochrane databases were searched for studies published between 2000 and 2022 assessing the value of adjunctive vessel preparation in infrapopliteal arterial disease. The primary outcomes were 12-month primary patency and limb salvage.
Results:
A total of 1685 patients with 1913 lesions were included in 11 POBA studies. Methodological quality was assessed as poor to moderate in these studies. Only 2 studies with 144 patients assessed vessel preparation in conjunction with DCB angioplasty. These randomized trials were assessed as high quality and found no significant benefit of adjunctive atherectomy to DCB angioplasty. The pooled Kaplan-Meier estimates of 12-month primary patency and limb salvage in the POBA studies were 67.8% and 80.9% for POBA, 62.1% and 86.4% for scoring balloons, 67.9% and 79.6% for mechanical atherectomy (MA), and 79.7% and 82.6% for laser atherectomy, respectively. Within the pooled data only scoring balloons and MA demonstrated significantly improved 12-month limb salvage compared to POBA.
Conclusions:
Different forms of adjunctive vessel preparation demonstrate similar 12-month outcomes compared to POBA and DCB angioplasty alone in infrapopliteal disease, with the exception of improved 12-month limb salvage in scoring balloons and MA. However, since the included studies were heterogeneous and assessed as poor to moderate methodological quality, selection bias may have played an important role. Main conclusion is that this systematic review found no additional value of standard use of vessel preparation.
Clinical Impact
Infrapopliteal arterial disease is associated with chronic limb-threatening ischemia (CLTI) and generally complex to treat due to small vessel diameter, long lesion length, multilevel disease and severe calcification. A wide range of vessel preparation devices have been developed to contribute to improved peri- and postprocedural outcomes in these complex lesions. This systematic review aims to compare different vessel preparation techniques prior to plain old balloon angioplasty (POBA) or drug coated balloon (DCB) angioplasty with POBA or DCB angioplasty alone in infrapopliteal arterial disease. Different forms of adjunctive vessel preparation demonstrate similar 12-month outcomes compared to POBA and DCB angioplasty alone in infrapopliteal disease, with the exception of improved 12-month limb salvage in scoring balloons and mechanical atherectomy (MA). However, since the included studies were heterogeneous and assessed as poor to moderate methodological quality, selection bias may have played an important role. Main conclusion is that this systematic review found no additional value of standard use of vessel preparation.
Keywords: vessel preparation, atherectomy, scoring balloons, infrapopliteal, tibial, below the knee
Introduction
Peripheral arterial disease (PAD) is a progressive disease that is caused by arterial stenoses and/or occlusions on different anatomical levels. Infrapopliteal arterial disease is associated with chronic limb-threatening ischemia (CLTI), the end stage of PAD. 1 CLTI comprises ischemic rest pain and tissue loss (ulceration or gangrene) due to atherosclerosis. 2 CLTI is common in the diabetic population, in which infrapopliteal disease is often more extensive with a 10 to 30 fold increase in major amputations. 3 The worldwide prevalence of diabetes mellitus (DM) is estimated to be 463 million people with an expected increase of 51% in the next 25 years, 4 which will subsequently cause an increase in the prevalence of CLTI, infrapopliteal arterial disease, and the need for interventions.
If CLTI is left untreated, both mortality and limb loss rates at 12 months are estimated to be 22%. 5 Above that, infrapopliteal lesions are generally complex to treat due to small vessel diameter, long lesion length, multilevel disease, and severe calcification. 6 In these complex lesions, plain old balloon angioplasty (POBA) is the preferred endovascular treatment option, since there is insufficient evidence to support other techniques, such as drug-coated balloon (DCB) angioplasty or stenting.2,7,8
Vessel preparation could possibly contribute to better peri- and postprocedural outcomes in these complex lesions, especially in the case of severe calcification. 9 Vessel preparation devices include mechanical atherectomy [MA: directional atherectomy (DA), rotational atherectomy (RA), and orbital atherectomy (OA)], laser atherectomy (LA), scoring balloons, and intravascular lithotripsy (IVL). MA devices debulk atheroma by sanding, shaving, drilling, or aspiration, while LA devices remove plaque by vaporizing. Scoring balloons feature atherotomes or wires mounted on the balloons’ surface which function as microsurgical blades to generate a controlled plaque incision. 9 Lastly, IVL utilizes an angioplasty balloon, which generates pulsatile sonic waves causing micro-fractures within the intimal and medial wall calcification. 10
The use of vessel preparation devices is associated with high direct costs, making it especially important to systematically gather evidence about their effectiveness. To our knowledge, 1 systematic review published in 2018 compared atherectomy and POBA with POBA alone in infrapopliteal arterial disease. Herein, 4 studies were included and pooled data showed similar 12-month amputation rates [odds ratio (OR) 1.02, 95% confidence interval (CI), 0.83–1.26]. 11 However, single-armed studies, DCB angioplasty, and other vessel preparation modalities than atherectomy were excluded. Furthermore, new comparative studies have since been performed. Therefore, this meta-analysis was conducted to compare the 12-month outcomes of various vessel preparation techniques prior to POBA or DCB angioplasty with POBA or DCB alone in infrapopliteal arterial disease.
Methods
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 12 and the protocol was made publicly available before the literature search was performed (PROSPERO ID: CRD42021226826).
The Medline, EMBASE, and Cochrane databases were searched for eligible studies published between January 2000 and May 2022. Reference lists of included articles and reviews were manually searched for additional eligible articles. Keywords describing a form of vessel preparation and infrapopliteal disease were combined. The full search strategy can be found as Supplemental Material.
Study Selection and Quality Assessment
After removal of duplicates, 2 authors (M.N., R.W.) independently screened titles and abstracts of all identified studies. Of the remaining relevant studies, the same 2 authors read the full texts to create the final selection of included studies. In controversial cases, a third author (C.H.) was consulted to reach consensus.
Articles were eligible if they investigated a vessel preparation device other than POBA in patients with infrapopliteal arterial disease. Vessel preparation devices include those that remove or modify plaque to optimize the result of the final POBA or DCB treatment and comprise DA, RA, OA, LA, scoring balloons, and IVL. Both single- and double-armed studies were included, as were studies that included both femoropopliteal and infrapopliteal arterial disease, but separated the outcomes in subgroups. Exclusion criteria were articles not in English, no full text available, no relevant Kaplan-Meier survival curves and 12-month outcomes available (primary patency or limb salvage), case reports, articles with less than 10 infrapopliteal lesions, articles that consisted of an infrapopliteal cohort with more than 10% popliteal or suprapopliteal lesions, commentaries, letters, conference abstracts, and reviews. In the case that different studies were suspected to have overlapping cohorts, the study with the most patients and most interesting outcomes was included after agreement by the 3 reviewers. Furthermore, it was preferred to distinguish the CLTI cohort from the claudication cohort if possible.
The methodological index for non-randomized studies (MINORS) score was used by 2 authors (M.N., R.W.) to assess the quality of the included studies. 13 This score provides 8 items for noncomparative studies and 12 items for comparative studies, in which 2 points per item can be obtained. For noncomparative and comparative studies a score of ≤8 and ≤12 is considered poor quality, 9 to 14 and 13 to 18 moderate quality and 15 to 16 and 19 to 24 high quality, respectively.
Data Collection and Outcome Measures
Two authors (M.N., R.W.) collected the following baseline variables: study design, number of patients and lesions, vessel preparation device, mean age, sex, Rutherford stage, DM, hypertension, smoking, dyslipidemia, coronary artery disease, degree of calcification, lesion length, chronic total occlusions (CTO), and bailout stenting.
The primary outcomes were the 12-month limb salvage and primary patency. Limb salvage was defined as freedom from major amputation (above the ankle). Primary patency was defined as freedom from a significant restenosis (>50% or a peak systolic velocity rate ≥2.5 on duplex ultrasound). The outcomes of studies on vessel preparation adjunctive to DCB angioplasty were not pooled with the POBA studies because of the different mechanism of action.
Data Analysis
In studies that provided Kaplan-Meier survival curves of primary patency or limb salvage, original patient data reconstruction was used to make a pooled survival estimate with 95% CI. For this process, graph points of Kaplan-Meier curves were converted to a coordinate system using the DigitizeIt version 2.5 software package. Using RStudio version 1.3 and an algorithm previously presented by Guyot et al 14 original patient data that corresponds with the Kaplan-Meier curve was reconstructed. This algorithm has been independently validated to be the most accurate of reconstruction methods. 15 Using the R “survival” and ‘survminer” packages, new Kaplan-Meier estimates of pooled data—based on device types; percutaneous transluminal angioplasty (PTA), scoring balloon, MA, LA, or combined methods—were made.
Reconstructed 1-year-number-at-risk and event data were supplemented with numbers from included studies that presented these without Kaplan-Meier survival curve. Together, these results were used to construct forest plots for both vessel preparation and POBA results. Events and patients at risk were finally pooled to calculate relative risks (RR) with 95% CI comparing different vessel preparation techniques with POBA. A random effects model was used for pooled data analysis. The threshold for statistical significance was set at p<0.05.
Results
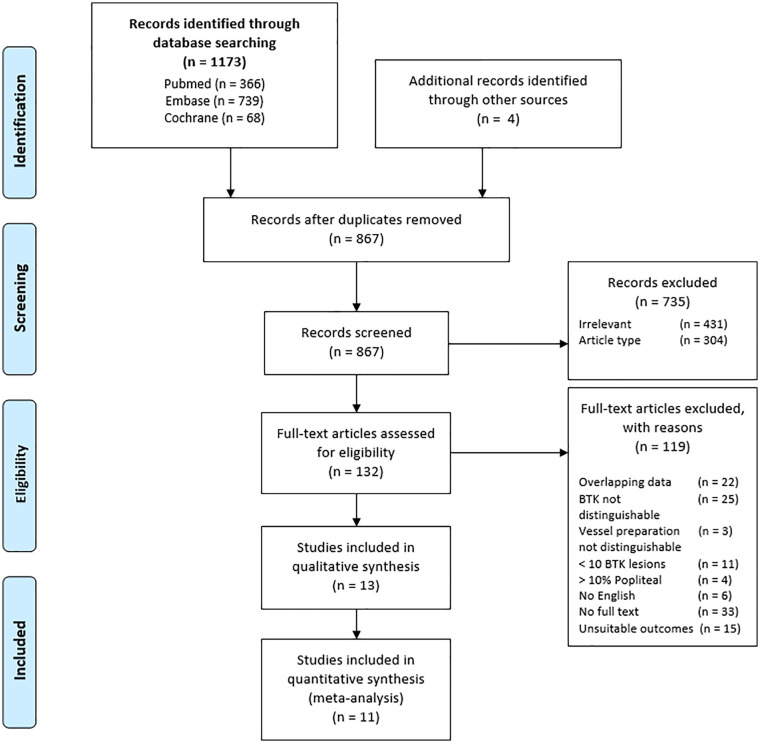
The search resulted in 1173 articles. Another 4 articles were identified by searching through reference lists of reviews and included studies. After removal of duplicates and screening of titles and abstracts, 132 articles were eligible for full text screening. Articles were excluded because of overlapping data, no English full text available, no distinguishable infrapopliteal lesions or vessel preparation cohort, less than 10 infrapopliteal lesions, more than 10% (supra)popliteal lesions or lack of interesting outcomes. After full text screening, 13 articles were eligible for data abstraction (Figure 1).
Figure 1.
PRISMA flow diagram for literature search to identify studies reporting on a form of vessel preparation in infrapopliteal arterial disease.
Study Characteristics
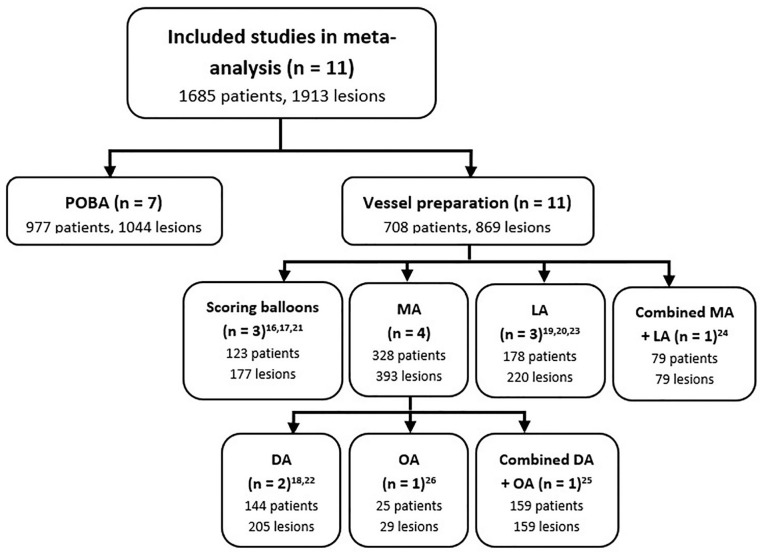
The 13 included studies comprised 5 prospective case series,16 –20 5 retrospective case series,21 –25 and 3 randomized trials.26 –28 Vessel preparation techniques investigated were scoring balloons in 3 studies,16,17,21 MA in 6 studies,18,22,25 –28 LA in 3 studies,19,20,23 and a combination of MA and LA in 1 study. 24 Only 2 studies were found investigating the combination of vessel preparation with DCB angioplasty.27,28 The other 11 studies that investigated vessel preparation devices in conjunction with POBA were included in the meta-analysis (see Figure 2 for a complete patient distribution per endovascular technique).16 –26
Figure 2.
Patient distribution per endovascular technique in this meta-analysis. DA, directional atherectomy; LA, laser atherectomy; MA, mechanical atherectomy; OA, orbital atherectomy; POBA, plain old balloon angioplasty.
Scoring balloon devices included the Boston Scien-tific16,21 and the Angiosculpt balloon (Angioscore, Inc.). 17 DA devices comprised the SilverHawk Plaque Excision Device (Covidien)18,22,24,25,27 and the TurboHawk Peripheral Plaque Excision System (Covidien).18,27 Two studies were identified using the Shockwave Medical Peripheral IVL System (Shockwave Medical)10,29 and 2 studies using the Rotablator Peripheral Rotational System (Boston Scientific),30,31 but were excluded because the follow-up period was limited to 30 days and 6 months, respectively. Other atherectomy devices comprised the Diamondback 360° Orbital Atherectomy PAD System (Cardiovascular Systems, Inc.)24 –26,28 and the Excimer Laser Atherectomy (Spectranetics Corporation).19,20,23 –25
Altogether, the 4 noncomparative and 7 comparative POBA studies were assessed as poor to moderate quality with an average of 10 (range 8–12) and 16 (range 14–18) on the MINORS score, respectively. The DCB studies were assessed as high quality (Supplemental Table S2).
Vessel Preparation in Conjunction With DCB Angioplasty
The first study investigating vessel preparation in conjunction with (paclitaxel coated) DCB angioplasty randomized 80 patients (71% CLTI, 65% DM) with long infrapopliteal lesions between DA plus DCB and DCB alone. 27 At 12 months, the primary patency, secondary patency, freedom from clinically driven target lesion revascularization (CD-TLR), and limb salvage were 45% versus 33% (p=0.426), 74% versus 67% (p=0.574), 70% versus 57% (p=0.308), and 78% versus 68% (p=0.618) in the DA plus DCB and DCB group, respectively.
The second study randomized 66 patients (73% CLTI, 67% DM) between OA plus DCB and DCB alone. 28 The primary patency, freedom from CD-TLR, and limb salvage at 12 months were 88% versus 55% (p=0.076), 86% versus 91% (p=0.626), and 97% versus 100% (p=0.309) in the OA plus DCB and DCB group, respectively. In conclusion, these randomized trials found comparable results between MA plus DCB and DCB alone at 12 months.
Vessel Preparation in Conjunction with POBA
In the included POBA studies, a total of 1685 patients with 1913 lesions were treated, of which 708 patients with 869 lesions with a vessel preparation device and 977 patients with 1044 lesions with POBA alone. CLTI was present in 100% (1616/1616) and DM in 69.5% (631/908) of the patients. Lesions were located in the popliteal artery in 0.5% (9/1913) and were total occlusions in 59.6% (454/762). All baseline and lesion characteristics are summarized in Tables 1 and 2.
Table 1.
Baseline Characteristics of the Included Studies.
| Study | MINORS score a | FU (m) | Patients (n) | Mean age (y) | Male | DM | DL | HT | Smoking | CAD | Rutherford stage 2–3/4/5–6 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Canaud et al 16 | 12 (16) | 24 | 69 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Bosiers et al 17 | 10 (16) | 12 | 31 | 76 | 17 (55) | 14 (45) | 5 (16) | 18 (58) | 5 (16) | N/A | 0/61/39 |
| Iezzi et al 21 | 8 (16) | 12 | 23 | 69.6 | 16 (70) | 21 (91) | 13 (57) | 11 (48) | 14 (61) | N/A | 0/60/40 |
| Gallagher et al 22 | 16 (24) | 24 | 74 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0/100 b |
| Control (PTA) | 72 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0/100 b | ||
| Rastan et al 18 | 8 (16) | 12 | 70 | 74.3 | 39 (56) | 55 (79) | 55 (79) | 64 (91) | 7 (10) | 24 (34) | 0/34/66 |
| Shammas et al 26 | 18 (24) | 12 | 25 | 70.7 | 17 (68) | 18 (72) | 20 (83) | 21 (84) | 15 (60) | 11 (44) | 0/48/52 |
| Control (PTA) | 25 | 71.8 | 15 (60) | 14 (56) | 18 (72) | 21 (84) | 15 (60) | 14 (56) | 0/48/52 | ||
| Bosiers et al 19 | 16 (24) | 12 | 64 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0/100 b |
| Control (PTA) | 79 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0/100 b | ||
| Sultan et al 20 | 18 (24) | 36 | 38 | 68 | 22 (58) | N/A c (77) | N/A c (77) | N/A c (62) | 33 (87) | 14 (37) | 0/18/81 |
| Control (PTA) | 42 | 70 | 25 (60) | N/A c (82) | N/A c (78) | 29 (69) | 38 (90) | N/A c (34) | 0/21/79 | ||
| Kokkinidis et al 23 | 14 (24) | 24 | 76 | 69.5 | 57 (75) | 49 (64) | N/A | 66 (87) | 42 (55) | 25 (33) | 0/100 b |
| Control (PTA) | 237 | 70.1 | 160 (68) | 186 (78) | N/A | 204 (86) | 127 (54) | 122 (52) | 0/100 b | ||
| Todd et al 24 | 14 (24) | 36 | 79 | 70 | 55 (70) | 60 (76) | 53 (67) | 77 (97) | 49 (62) | N/A c (60) | 0/30/70 |
| Control (PTA) | 339 | 71 | N/A c (60) | N/A c (71) | N/A c (64) | N/A c (91) | N/A c (65) | N/A c (53) | 0/33/67 | ||
| Zia et al 25 | 16 (24) | 18 | 159 | 67 | 91 (57) | 97 (61) | 104 (65) | 143 (90) | 74 (47) | 65 (41) | 0/21/79 |
| Control (PTA) | 183 | 67.3 | 116 (63) | 117 (64) | 126 (69) | 168 (92) | 85 (46) | 99 (54) | 0/16/84 | ||
| Rastan et al 27 | 20 (24) | 12 | 40 | 71.5 | 28 (70) | 28 (70) | 37 (93) | 37 (93) | 6 (15) | 19 (48) | 35/10/55 |
| Control (DCB) | 40 | 72.7 | 33 (83) | 24 (60) | 31 (77) | 40 (100) | 4 (11) | 16 (40) | 23/23/55 | ||
| Zeller et al 28 | 20 (24) | 12 | 32 | 73.4 | 26 (81) | 24 (75) | 29 (91) | 31 (97) | 23 (72) | 19 (59) | 31/6/63 |
| Control (DCB) | 34 | 76.5 | 25 (74) | 20 (59) | 24 (71) | 31 (91) | 17 (50) | 15 (44) | 24/12/65 |
Data are given as n (%) unless stated otherwise.
Abbreviations: CAD, coronary artery disease; DCB, drug-coated balloon; DL, dyslipidemia; DM, diabetes mellitus; FU, follow-up; HT, hypertension; PTA, percutaneous transluminal angioplasty.
The MINORS is used to assess the quality of the included studies. Values are given as score (maximum possible score).
Data are given as Rutherford stage 2–3/4–6, that is, intermittent claudication/chronic limb-threatening ischemia (%).
In the demographics table only the exact percentage is given. However, this percentage cannot be converted into exact number of patients.
Table 2.
Lesion and Device Characteristics of the Included Studies.
| Study | Vessel preparation device | Lesions (n) | Popliteal lesions | Calcification | Lesion length (mm) | CTO | Bailout stenting |
|---|---|---|---|---|---|---|---|
| Canaud et al 16 | CB (Boston Scientific) | 116 | 0 (0) | N/A | N/A | N/A | N/A |
| Bosiers et al 17 | CB (Angiosculpt) | 36 | 1 (3) | 17 (55) | 32.4 | N/A | 11 (36) |
| Iezzi et al 21 | CB (Boston Scientific) | 25 | 2 (8) | N/A | 17 | N/A | 0 (0) |
| Gallagher et al 22 | DA (SilverHawk) | 109 | 0 (0) | N/A | N/A | 109 (100) | N/A |
| Control (PTA) | 85 | 0 (0) | N/A | N/A | 85 (100) | ||
| Rastan et al 18 | DA (SilverHawk, TurboHawk) | 96 | 0 (0) | 30 (31) | 60 | 32 (33) | N/A |
| Shammas et al 26 | OA (Diamondback 360 OAS) | 29 | 2 (7) | 29 (100) | 91 | N/A | 2 (7) |
| Control (PTA) | 35 | 4 (11) | 35 (100) | 69 | N/A | 5 (14) | |
| Bosiers et al 19 | LA | 64 | 0 (0) | N/A | N/A | N/A | N/A |
| Control (PTA) | 79 | 0 (0) | N/A | N/A | N/A | N/A | |
| Sultan et al 20 | LA | 80 | 0 (0) | 15 (36)a | 170 | 24 (57) a | 15 (39) |
| Control (PTA) | 86 | 0 (0) | 13 (28%) a | 160 | 27 (57) a | 14 (33) | |
| Kokkinidis et al 23 | LA | 76 | 0 (0) | 25 (34) b | 165.7 | 43 (58) | 11 (14) |
| Control (PTA) | 237 | 0 (0) | 61 (27) b | 94.1 | 99 (43) | 19 (8) | |
| Todd et al 24 | DA, OA, LA (SilverHawk, Diamondback 360 OAS, Excimer) | 79 | 0 (0) | N/A | N/A | 35 (44) | 0 (0) |
| Control (PTA) | 339 | 0 (0) | N/A | N/A | N/A c (44) | 6 (2) | |
| Zia et al 25 | DA, OA (SilverHawk, Diamondback 360 OAS) | 159 | 0 (0) | N/A | 60.2 | N/A | N/A |
| Control (PTA) | 183 | 0 (0) | N/A | 65.5 | N/A | N/A | |
| Rastan et al 27 | DA (SilverHawk, TurboHark) | 40 | 0 (0) | 28 (70) b | 191.6 | 22 (55) | 6 (15) |
| Control (DCB) | 40 | 0 (0) | 30 (76.9) | 160.8 | 18 (45) | 5 (13) | |
| Zeller et al 28 | OA (Diamondback 360 OAS) | 33 | 1 (3) | 29 (100) b | 101.3 | 14 (42) | N/A |
| Control (DCB) | 37 | 0 (0) | 30 (94) b | 78.8 | 12 (32) | N/A |
Data are given as n (%) unless stated otherwise.
Abbreviations:CB, cutting balloon; CTO, chronic total occlusions; DA, directional atherectomy; DCB, drug-coated balloon; LA, laser atherectomy; OA, orbital atherectomy; PTA, percutaneous transluminal angioplasty.
Lesion characteristics appear to be divided by the number of procedures (Laser: 42, PTA: 47) and not the number of lesions.
Moderate or severe calcification.
In the demographics table only the exact percentage is given. However, this percentage cannot be converted into exact number of patients.
Pooled Survival Curves
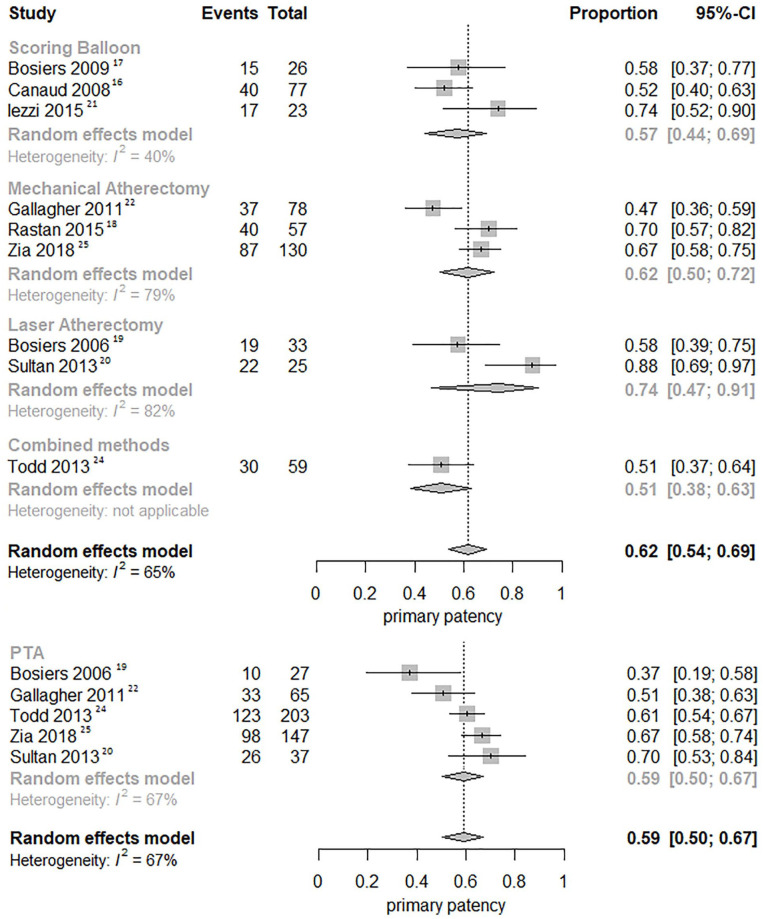
Nine studies (3 scoring balloon, 3 MA, 2 LA, and 1 combined atherectomy)16 –22,24,25 were pooled for primary patency and rates at 12 months were 67.8% for POBA (95% CI, 64.0%–71.8%), 62.1% for scoring balloons (95% CI, 54.4%–70.8%), 67.9% for MA (95% CI, 62.9%–73.4%), 79.7% for LA (95% CI, 71.5%–88.9%), and 62.1% for combined atherectomy (95% CI, 52.1%–74.1%), respectively (Figure 3A).
Figure 3.
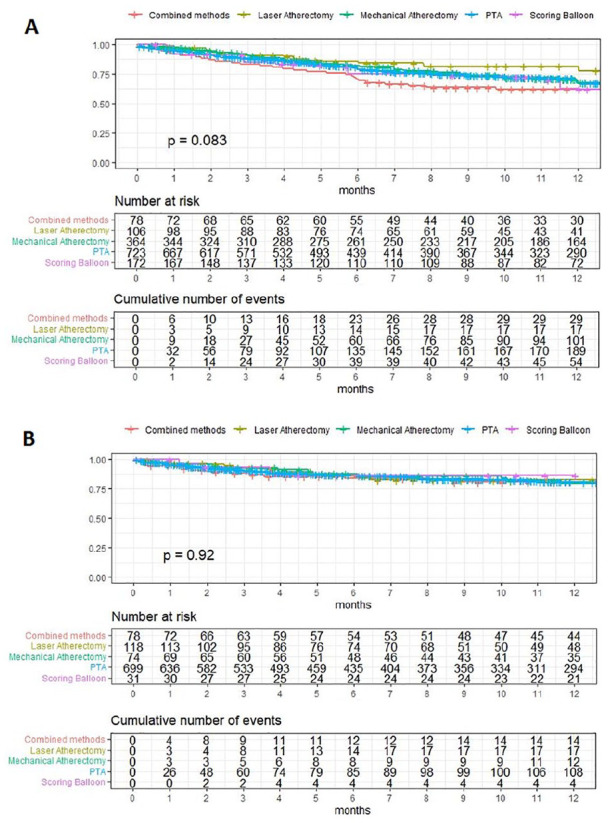
Pooled Kaplan-Meier survival curves of (A) primary patency and (B) limb salvage. In the survival curve of primary patency no group exceeded 10% SE in the observed time period (5.8%, 4.4%, 2.7%, 2.0%, and 4.2% respectively). In the survival curve of limb salvage no group exceeded 10% SE in the observed time period (4.9%, 3.8%, 5.4%, 1.7%, and 6.3% respectively). Combined methods refers to a combination of MA and LA. PTA, percutaneous transluminal angioplasty.
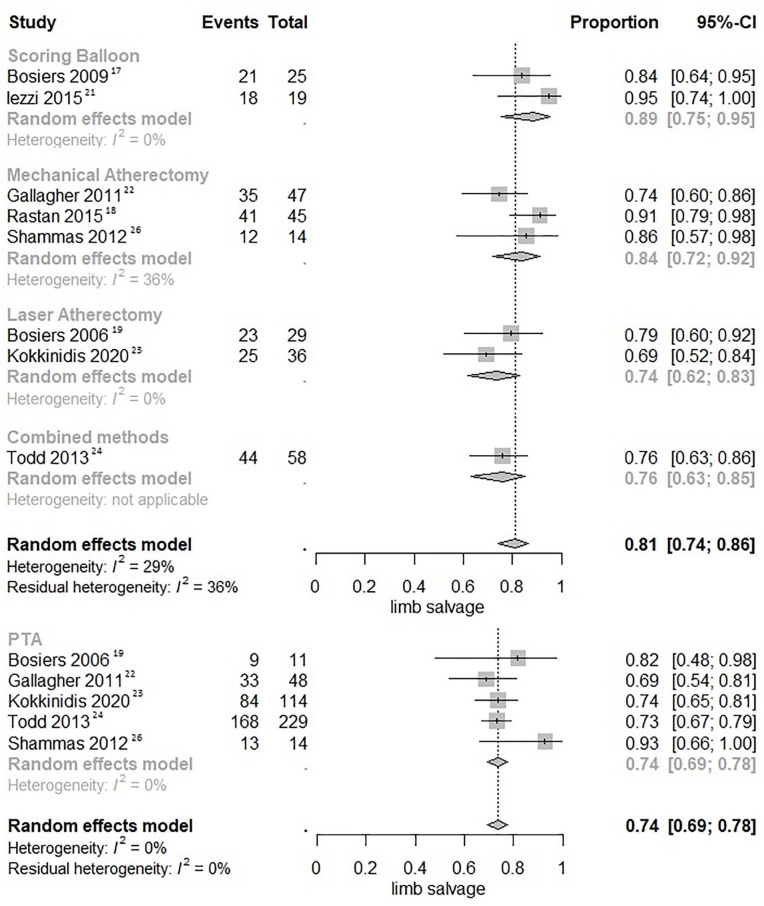
Five studies (1 scoring balloon, 1 MA, 2 LA, and 1 combined atherectomy)17,19,22 –24 were pooled likewise for limb salvage in Kaplan-Meier curves and rates at 12 months were 80.9% for POBA (95% CI, 77.7%–84.3%), 86.4% for scoring balloons (95% CI, 74.9%–99.7%), 79.6% for MA (95% CI, 69.6%–90.9%), 82.6% for LA (95% CI, 75.3%–90.6%), and 80.5% for combined atherectomy (95% CI, 71.8%–90.3%), respectively (Figure 3B).
Other Analyses
Not all included studies featured Kaplan-Meier survival curves of relevant outcomes. Therefore, data from 9 (3 scoring balloon, 3 MA, 2 LA, and 1 combined atherectomy)16 –22,24,25 and 8 studies (2 scoring balloon, 3 MA, 2 LA, and 1 combined atherectomy)17 –19, 21 –24,26 were also pooled in forest plots for 12-month primary patency and limb salvage, respectively (Figures 4 and 5). The 12-month primary patency was not significantly different for scoring balloons versus POBA (RR 0.94; 95% CI, 0.80–1.12; p=0.499), MA versus POBA (RR 1.02; 95% CI, 0.91–1.15; p=0.718), LA versus POBA (RR 1.17; 95% CI, 0.97–1.40; p=0.093), and combined atherectomy versus POBA (RR 0.84; 95% CI, 0.65–1.09; p=0.190). The 12-month limb salvage was significantly higher for scoring balloons versus POBA (RR 1.20; 95% CI, 1.06–1.35; p=0.003) and MA versus POBA (RR 1.12; 95% CI, 1.01–1.25; p=0.026), but not for LA versus POBA (RR 1.00; 95% CI, 0.86–1.17; p=0.994) and combined atherectomy versus POBA (RR 1.03; 95% CI, 0.88–1.20; p=0.729).
Figure 4.
Forest plots of 12-month primary patency. Combined methods refers to a combination of MA and LA. CI, confidence interval; PTA, percutaneous transluminal angioplasty.
Figure 5.
Forest plots of 12-month limb salvage. Combined methods refers to a combination of MA and LA. CI, confidence interval; PTA, percutaneous transluminal angioplasty.
Discussion
This systematic review and meta-analysis was conducted to compare different forms of vessel preparation prior to POBA or DCB angioplasty with POBA or DCB alone in infrapopliteal arterial disease. Scoring balloons and MA significantly improve the 12-month limb salvage compared to POBA alone. No further significant differences were found between any form of vessel preparation and POBA or DCB angioplasty alone in terms of limb salvage and primary patency.
Although the limb salvage at 12 months appears to be significantly higher for scoring balloons and MA compared to POBA, this finding should be interpreted with great caution for several reasons. Firstly, the 2 scoring balloon and 3 MA studies that investigated limb salvage were assessed as poor to moderate quality on the MINORS score (Supplemental Table S2). These small studies only included 54 and 169 patients in the scoring balloon and MA groups, respectively. Both of the scoring balloon and 1 out of 3 MA studies were noncomparative and their patient cohorts were not contemporary with the POBA cohorts. Furthermore, differences in baseline and lesion characteristics may have biased the outcomes. The scoring balloon group featured fewer patients with DM (65% vs 72%), more popliteal lesions (6% vs 0%), and shorter lesion lengths compared to the POBA group (27 mm vs 93 mm). The MA group also featured shorter lesion lengths (60 mm). As a consequence, selection bias is likely to have influenced the outcomes. Lastly and perhaps most importantly, the 12-month primary patency of scoring balloons and MA are similar or even slightly lower than POBA. Furthermore, a pooled survival analysis of secondary patency, including the same 2 scoring balloon and 2 out of 3 MA studies, showed similar rates between scoring balloons and POBA (85.0% vs 83.5%; p=0.849) and significantly lower rates for MA than POBA (76.0% vs 83.5%, p=0.001), which may indicate that limbs were not salvaged for different reasons than loss of patency.
In terms of primary patency no significant differences were found between different vessel preparation modalities and POBA. However, LA shows a tendency toward an improved 12-month primary patency (79.7% vs 67.8%, p=0.093). In this subgroup only 106 patients were treated with LA and for that reason the comparison may have been underpowered to show significant benefit of LA. One retrospective study including 726 patients with popliteal and infrapopliteal lesions was excluded from this review because only procedural and 36-month outcomes were presented and approximately 30% of the lesions were located in the popliteal artery. This double-armed study concluded that despite worse baseline angiographic characteristics in the LA group, LA was associated with higher procedural success and similar limb salvage, repeat revascularization and mortality rates at 36 months as compared with POBA alone. 32 Future studies are warranted to provide more data on the potential benefit of LA in infrapopliteal disease.
In recent practice, vessel preparation, in particular atherectomy, is often combined with DCB angioplasty instead of POBA. 33 To this day, 3 studies were published comparing atherectomy plus (paclitaxel coated) DCB versus DCB alone.27,28,34 Two studies were included in this review and found no significant benefit of adjunctive atherectomy. However, a trend toward superior primary patency 28 and 6-month amputation-free survival was suggested. 27 One retrospective study investigating the efficacy of adjunctive LA to DCB angioplasty was not included in this review, because only 24-month outcomes were available. 34 This study included 79 patients and found improved 24-month primary patency (80% vs 52%, p=0.010), freedom from CD-TLR (86% vs 66%, p=0.044), and limb salvage (94% vs 77%, p=0.036). According to the authors, the first 2 trials were likely underpowered, hence a larger confirmatory study is needed to be conclusive. Future trials, such as Prestige Pilot (NCT ID: 03744572) will provide more data on the safety and efficacy of adjunctive atherectomy compared to DCB angioplasty alone.
In this systematic review, periprocedural outcomes, such as procedural success, dissections, or need for bailout stenting, were not analyzed. Firstly, these outcomes could not be pooled due to major heterogeneity in definitions. For example, some studies only mentioned the dissections in need for stenting, while others mentioned all manifest dissections on angiography. Secondly, periprocedural outcomes, in particular dissections, are significantly underestimated on angiography compared to intravascular ultrasound. 35
As mentioned before, POBA is the current preferred endovascular treatment option for infrapopliteal arterial disease, because there is insufficient evidence to support other, more expensive techniques, such as atherectomy, DCB angioplasty, or stenting. However, these techniques may all be reasonable options in certain lesion morphologies. 2 A systematic review including 8 trials demonstrated short-term (12-month) benefits for drug-eluting stents (DES) in relatively short (<3 cm) tibial lesions, in particular for sirolimus coated stents. 36 Similarly, current systematic review demonstrates no overall benefit of vessel preparation in infrapopliteal disease, although a benefit might be plausible in certain lesion morphologies, for example, in the case of severe calcification. Future studies should focus on patient selection and cost-effectiveness of different endovascular treatment strategies.
IVL is one of the recently developed endovascular techniques for calcified lesions with promising results. Only 2 studies so far investigated IVL in below-the-knee arterial disease.10,29 These observational studies enrolled 20 and 101 patients, respectively, and demonstrated that calcified infrapopliteal lesions can be successfully treated with IVL with 95% to 99% procedural success and no major adverse events at 30 days. In femoropopliteal disease the randomized Disrupt PAD III trial found in 306 patients that IVL prior to DCB resulted in greater procedural success (65.8% vs 50.4%; p=0.01), fewer flow-limiting dissections (1.4% vs 6.8%; p=0.03), and less need for stent placement (4.6% vs 18.3%; p<0.001) compared to POBA prior to DCB. 37 Future studies on femoropopliteal and infrapopliteal disease are warranted to provide more data about the long-term follow-up of IVL.
In this systematic review several limitations must be acknowledged. Firstly, all POBA studies were assessed as poor to moderate quality on the MINORS score. Only 3 randomized trials and 6 comparative cohort studies were identified. Especially in the scoring balloon cohort, in which no comparative studies were identified, this could have resulted in selection bias. In addition, several relevant studies on vessel preparation have been excluded because no full English text was available or the presented results were unsuitable for this systematic review, for example, because only procedural or 36-month outcomes were available. Secondly, heterogeneity in study designs, follow-up periods and endpoint definitions may have influenced the outcomes. For example, some studies included all patients in the follow-up of their Kaplan-Meier survival curves, while other studies only included patients with technical success. Finally, various studies lacked relevant baseline characteristics, for example, only 7 studies reported a degree of calcification in their study cohort, even though calcification is one of the main indications for vessel preparation. 9
Conclusion
Different forms of adjunctive vessel preparation demonstrate similar 12-month outcomes compared to POBA or DCB angioplasty alone in infrapopliteal disease, with the exception of improved 12-month limb salvage in scoring balloons and MA. However, this finding should be interpreted with great caution, since the included studies were heterogeneous and assessed as poor to moderate quality on the MINORS score. Therefore, selection bias may have played an important role. Main conclusion is that this systematic review found no additional value of standard use of vessel preparation.
Supplemental Material
Supplemental material, sj-docx-1-jet-10.1177_15266028221120752 for Vessel Preparation in Infrapopliteal Arterial Disease: A Systematic Review and Meta-Analysis by Michael J. Nugteren, Rutger H. A. Welling, Olaf J. Bakker, Çağdaş Ünlü and Constantijn E. V. B. Hazenberg in Journal of Endovascular Therapy
Supplemental material, sj-docx-2-jet-10.1177_15266028221120752 for Vessel Preparation in Infrapopliteal Arterial Disease: A Systematic Review and Meta-Analysis by Michael J. Nugteren, Rutger H. A. Welling, Olaf J. Bakker, Çağdaş Ünlü and Constantijn E. V. B. Hazenberg in Journal of Endovascular Therapy
Acknowledgments
We thank Prof. Dr. G. J. de Borst for his critical revision of the manuscript.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Michael J. Nugteren  https://orcid.org/0000-0003-2154-4077
https://orcid.org/0000-0003-2154-4077
Rutger H. A. Welling  https://orcid.org/0000-0001-6854-2277
https://orcid.org/0000-0001-6854-2277
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Graziani L, Silvestro A, Bertone V, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007;33:453–460. [DOI] [PubMed] [Google Scholar]
- 2. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 2019;69:3S–125S.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung J. Endovascular devices and revascularization techniques for limb-threatening ischemia in individuals with diabetes. J Diabetes Sci Technol. 2017;11:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 5. Abu Dabrh AM, Steffen MW, Undavalli C, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62:1642–1651.e3. [DOI] [PubMed] [Google Scholar]
- 6. Baumann F, Engelberger RP, Willenberg T, et al. Infrapopliteal lesion morphology in patients with critical limb ischemia: implications for the development of anti-restenosis technologies. J Endovasc Ther. 2013;20:149–156. [DOI] [PubMed] [Google Scholar]
- 7. Hsu CCT, Kwan GNC, Singh D, et al. Angioplasty versus stenting for infrapopliteal arterial lesions in chronic limb-threatening ischemia. Cochrane Database Syst Rev. 2018;12:CD009195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ipema J, Huizing E, Schreve MA, et al. Drug coated balloon angioplasty vs. Standard percutaneous transluminal angioplasty in below the knee peripheral arterial disease: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2020;59:265–275. [DOI] [PubMed] [Google Scholar]
- 9. Tummala S, Amin A, Mehta A. Infrapopliteal artery occlusive disease: an overview of vessel preparation and treatment options. J Clin Med. 2020;9:E3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brodmann M, Holden A, Zeller T. Safety and feasibility of intravascular lithotripsy for treatment of below-the-knee arterial stenoses. J Endovasc Ther. 2018;25:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdullah O, Omran J, Enezate T, et al. Percutaneous angioplasty versus atherectomy for treatment of symptomatic infra-popliteal arterial disease. Cardiovasc Revasc Med. 2018;19:423–428. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Biomed J. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. [DOI] [PubMed] [Google Scholar]
- 14. Guyot P, Ades AE, Ouwens MJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saluja R, Cheng S, Delos Santos KA, et al. Estimating hazard ratios from published Kaplan-Meier survival curves: a methods validation study. Res Synth Methods. 2019;10:465–475. [DOI] [PubMed] [Google Scholar]
- 16. Canaud L, Alric P, Berthet J, et al. Infrainguinal cutting balloon angioplasty in de novo arterial lesions. J Vasc Surg. 2008;48:1182–1188. [DOI] [PubMed] [Google Scholar]
- 17. Bosiers M, Deloose K, Cagiannos C, et al. Use of the AngioSculpt scoring balloon for infrapopliteal lesions in patients with critical limb ischemia: 1-year outcome. Vascular. 2009;17:29–35. [DOI] [PubMed] [Google Scholar]
- 18. Rastan A, McKinsey J, Garcia LA, et al. One-year outcomes following directional atherectomy of infrapopliteal artery lesions: subgroup results of the prospective, multicenter DEFINITIVE LE trial. J Endovasc Ther. 2015;22:839–846. [DOI] [PubMed] [Google Scholar]
- 19. Bosiers M, Hart JP, Deloose K, et al. Endovascular therapy as the primary approach for limb salvage in patients with critical limb ischemia: experience with 443 infrapopliteal procedures. Vascular. 2006;14:63–69. [DOI] [PubMed] [Google Scholar]
- 20. Sultan S, Tawfick W, Hynes N. Cool excimer laser-assisted angioplasty (CELA) and tibial balloon angioplasty (TBA) in management of infragenicular arterial occlusion in critical lower limb ischemia (CLI). Vasc Endovascular Surg. 2013;47:179–191. [DOI] [PubMed] [Google Scholar]
- 21. Iezzi R, Posa A, Santoro M, et al. Cutting balloon angioplasty in the treatment of short infrapopliteal bifurcation disease. J Endovasc Ther. 2015;22:485–492. [DOI] [PubMed] [Google Scholar]
- 22. Gallagher KA, Meltzer AJ, Ravin RA, et al. Endovascular management as first therapy for chronic total occlusion of the lower extremity arteries: comparison of balloon angioplasty, stenting, and directional atherectomy. J Endovasc Ther. 2011;18:624–637. [DOI] [PubMed] [Google Scholar]
- 23. Kokkinidis DG, Giannopoulos S, Jawaid O, et al. Laser atherectomy for infrapopliteal lesions in patients with critical limb ischemia. Cardiovasc Revasc Med. 2021;23:79–83. [DOI] [PubMed] [Google Scholar]
- 24. Todd KE, Jr, Ahanchi SS, Maurer CA, et al. Atherectomy offers no benefits over balloon angioplasty in tibial interventions for critical limb ischemia. J Vasc Surg. 2013;58:941–948. [DOI] [PubMed] [Google Scholar]
- 25. Zia S, Juneja A, Shams S, et al. Contemporary outcomes of infrapopliteal atherectomy with angioplasty versus balloon angioplasty alone for critical limb ischemia. J Vasc Surg. 2020;71:2056–2064. [DOI] [PubMed] [Google Scholar]
- 26. Shammas NW, Lam R, Mustapha J, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. Balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther. 2012;19:480–488. [DOI] [PubMed] [Google Scholar]
- 27. Rastan A, Brodmann M, Böhme T, et al. Atherectomy and drug-coated balloon angioplasty for the treatment of long infrapopliteal lesions: a randomized controlled trial. Circ Cardiovasc Interv. 2021;14:e010280. [DOI] [PubMed] [Google Scholar]
- 28. Zeller T, Giannopoulos S, Brodmann M, et al. Orbital atherectomy prior to drug-coated balloon angioplasty in calcified infrapopliteal lesions: a randomized, multicentre pilot study. J Endovasc Ther. Published online January 27, 2022. doi: 10.1177/15266028211070968 [DOI] [PubMed] [Google Scholar]
- 29. Adams G, Soukas PA, Mehrle A, et al. Intravascular lithotripsy for treatment of calcified infrapopliteal lesions: results from the Disrupt PAD III observational study. J Endovasc Ther. 2022;29:76–83. [DOI] [PubMed] [Google Scholar]
- 30. Jahnke T, Link J, Müller-Hülsbeck S, et al. Treatment of infrapopliteal occlusive disease by high-speed rotational atherectomy: initial and mid-term results. J Vasc Interv Radiol. 2001;12:221–226. [DOI] [PubMed] [Google Scholar]
- 31. Dua A, Rothenberg KA, Lee JJ, et al. Six-month freedom from amputation rates and quality of life following tibial and pedal endovascular revascularization for critical limb ischemia. Vasc Endovascular Surg. 2019;53:212–215. [DOI] [PubMed] [Google Scholar]
- 32. Piyalskulkaew C, Parvateneni K, Ballout H, et al. Laser in infrapopliteal and popliteal stenosis 2 study (LIPS2): long-term outcomes of laser-assisted balloon angioplasty versus balloon angioplasty for below knee peripheral arterial disease. Catheter Cardiovasc Interv. 2015;86:1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin F, Wang H, Ding W, et al. Atherectomy plus drug-coated balloon versus drug-coated balloon only for treatment of femoropopliteal artery lesions: a systematic review and meta-analysis. Vascular. 2021;29:883–896. [DOI] [PubMed] [Google Scholar]
- 34. Yang S, Li S, Hou L, et al. Excimer laser atherectomy combined with drug-coated balloon versus drug-eluting balloon angioplasty for the treatment of infrapopliteal arterial revascularization in ischemic diabetic foot: 24-month outcomes. Lasers Med Sci. 2022;37:1531–1537. [DOI] [PubMed] [Google Scholar]
- 35. Shammas NW, Torey JT, Shammas WJ, et al. Intravascular ultrasound assessment and correlation with angiographic findings demonstrating femoropopliteal arterial dissections post atherectomy: results from the iDissection study. J Invasive Cardiol. 2018;30:240–244. [PubMed] [Google Scholar]
- 36. Liu X, Zheng G, Wen S. Drug-eluting stents versus control therapy in the infrapopliteal disease: a meta-analysis of eight randomized controlled trials and two cohort studies. Int J Surg. 2017;44:166–175. [DOI] [PubMed] [Google Scholar]
- 37. Tepe G, Brodmann M, Werner M, et al. Intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized Disrupt PAD III trial. JACC Cardiovasc Interv. 2021;14:1352–1361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jet-10.1177_15266028221120752 for Vessel Preparation in Infrapopliteal Arterial Disease: A Systematic Review and Meta-Analysis by Michael J. Nugteren, Rutger H. A. Welling, Olaf J. Bakker, Çağdaş Ünlü and Constantijn E. V. B. Hazenberg in Journal of Endovascular Therapy
Supplemental material, sj-docx-2-jet-10.1177_15266028221120752 for Vessel Preparation in Infrapopliteal Arterial Disease: A Systematic Review and Meta-Analysis by Michael J. Nugteren, Rutger H. A. Welling, Olaf J. Bakker, Çağdaş Ünlü and Constantijn E. V. B. Hazenberg in Journal of Endovascular Therapy