Abstract
Objective:
The postnatal activation of the hypothalamic-pituitary-gonadal (HPG) axis is usually known as “minipuberty”. There are still open questions about its biological function and significance depending on sex, gestational age (GA) and birth weight (BW) with few available longitudinal data.
Methods:
A single-centre, longitudinal study to quantify urinary follicle stimulating hormone (uFSH), luteinizing hormone (uLH) and testosterone (uTs) in male neonates. Neonates were enrolled and stratified into three subgroups: full-term boys appropriate for GA (FT AGA); FT boys with BW ≤3rd centile [FT small for gestational age (SGA)]; and preterm (PT) boys ≤33 weeks of GA. Urinary hormones were correlated to simultaneous auxological parameters, linear growth and external genitalia at scheduled time-points.
Results:
Forty-six boys were recruited, with subgroup sizes FT AGA n=23, FT SGA n=11 and PT n=12. PT boys display a pulsatile pattern of urinary gonadotropins (uGns) with higher levels of uLH and a gradual increase of uTs. Testicular descent started from 29-32 weeks with the peak of uTs. During the first 12-months post-term age (PTA), FT AGA boys displayed a better linear growth (p<0.05). PT showed higher uGns levels until 3-months PTA. PT babies had higher uLH levels than FT AGA, with a peak at 7 and 30 days, during the first 90 days of life (p<0.001) and higher uTs levels. Correlation analysis between penile growth of all neonates and uTs was significant (p=0.04) but not within subgroups.
Conclusion:
This study investigated postnatal HPG axis activation in term and PT infants. Minipuberty may involve an early window of opportunity to evaluate the functionality of the HPG axis. Further studies with a long-term follow-up are needed with a special focus on possible consequences of GA and BW.
Keywords: Minipuberty, urinary gonadotropins, newborn, infants, prematurity, growth
What is already known on this topic?
Previous studies have shown hypothalamic-pituitary-gonadal axis activation during early life, also referred to as “minipuberty”. This period has sex-related differences in levels and duration of both gonadotropins and sex steroid secretion. The role of minipuberty seems to be significant mainly for development and maturation of reproductive organs, particularly in males.
What this study adds?
This study provides a longitudinal analysis with serial samples and a between-group comparison in term and preterm (PT) infants. Our results suggest that minipuberty is increased and prolonged in PT boys only when the analysis depends on calendar age, suggesting an ontogenetic regulation. Full-term small for gestational age infants had the lowest postnatal testosterone levels with higher urinary gonadotropins levels.
Introduction
Puberty is preceded by two periods marked with a transitional activation of the hypothalamic-pituitary-gonadal (HPG) axis: the first during foetal life, playing a crucial role in sex determination, the second during the first postnatal months, the second period of HPG activation is referred to as “minipuberty”(1), and its biological significance is still not completely understood.
Studies with moderate-sized cohorts of neonates have shown that minipuberty has sex-related differences (2) in levels and duration of both gonadotropin and sex steroid secretion. The gonadotropins start rising at around one week of age, peak (reaching pubertal levels) between one and three months of life, and decline to prepubertal values towards the age of six months (3,4,5,6,7). Male neonates have a luteinizing hormone (LH) peak higher than follicle stimulating hormone (FSH) levels with a gradual decrease to prepubertal levels at around 6-9 months (3,6). Testosterone (T) level starts to increase following the LH rise with a similar pattern (5,6,8). Both LH and T reach higher levels in boys than in girls (5,7,8,9,10). In contrast, female infants have a predominantly FSH level rise that remains elevated for a longer period, whereas estradiol (E) levels display a fluctuating pattern, probably reflecting the ovarian follicular cycle of growth and atrophy (1).
The role of minipuberty seems to be significant mainly for development and maturation of reproductive organs, particularly in males (11). In addition, androgens have also been related to other aspects of infant growth, such as cutaneous manifestations (12,13), linear growth, bone mineralization, psychosexual development and behaviour (14,15,16,17,18,19,20,21,22,23,24,25).
Little is known about the influence of factors such as birthweight or prematurity on the HPG axis. The HPG axis activation in babies born small for gestational age (SGA) is not well defined and its short-term and long-term effects on growth and development are still controversial. Studies on SGA females have found higher postnatal FSH levels compared with neonates born appropriate for gestational age (AGA). Meanwhile, in male SGA term neonates, HPG axis activation has been linked to both lower (26) and higher (27) gonadotropins and androgen (28) levels, with uncertain effects in adult life (29). In addition the impact of prematurity has been investigated mainly using cross-sectional studies based on serum samples (3,4,6) but only a few studies (1,6,7) have adopted a longitudinal approach. Based on recent longitudinal studies using urinary gonadotropins (uGns), preterm (PT) birth does not seem to influence the onset of postnatal HPG axis activation, as gonadotropin levels begin to rise with the same timing in full-term (FT) and PT infants (1). Moreover, minipuberty in PT babies seems to be stronger and prolonged with uncertain significance and effects (30,31,32) but data are still not univocal.
Understanding the physiological trend of hormonal levels during minipuberty and its differences between term, PT and SGA boys will therefore be helpful in understanding possible short-term and long-term effects.
The aims of the present study were to quantify urinary FSH (uFSH), urinary LH (uLH) and urinary testosterone (uTs) in male neonates, and to compare changes in timing and magnitude of uGns (uFSH and uLH) and uTs secretion in three different groups of neonates: FT boys appropriate for gestational age (GA) (FT AGA); FT boys small for GA (FT SGA); and PT boys ≤33 weeks of GA. Secondary aims were to make between-group comparisons of external genitalia clinical examination (penile length, testicular volume and position) and linear growth, with a focus on catch-up growth in FT SGA and PT infants.
Methods
Population
This was a single-centre, longitudinal and prospective study conducted on male neonates.
Neonates enrolled were split into three categories: FT AGA boys, FT SGA boys and PT boys born before 33 weeks of GA. Comparisons were made according to calendar age or post-term age (PTA). Neonates with antenatal genetic diagnosis or severe metabolic, cardiological, endocrine or sex development disorders (33) were excluded. Being classified as AGA or SGA among FT boys was based on birth weight (BW), as previously described (34). SGA was considered as a boy with a BW ≤3rd centile whereas all FT AGA boys had a BW >10th centile. For PT babies, no classification according to BW was performed.
Medical data were collected from clinical records. Clinical assessments for FT neonates were: within the first 72 hours of life, at 1 week, at 1-3 and 6-12 months of life. For PT neonates evaluations were: within first 72 hours of life, once a week since term age, at 1-3 and 6-12 months of PTA. The number of evaluations depended on GA at birth and the child’s clinical condition.
At each scheduled time-point, both clinical evaluation and urinary collection were performed. Clinical evaluation included auxological parameters [weight, length, head circumference (HC)] and external genitalia assessment (penile length, testicular position and volume).
Weight was measured with a digital baby scale to the nearest 5 g. The recumbent length was measured by a portable, high quality infantometer (GIMA, Baby Height Measuring Mat, cod. 27331) to the nearest 0.1 cm. HC was measured using a measuring tape from the most prominent part of the forehead around to the widest part of the back of the head. If the baby’s condition was to poor for measurements among PT babies in the Neonatal Intensive Care Unit, both clinical evaluation and urine collection were postponed.
For PT babies, birth weight and the following growth till term age was monitored using national charts (34). For term babies, being AGA or SGA was assessed according to national charts (34). The international longitudinal charts World Health Organization 2006 (35) were used from 1- to 12-months PTA. National charts at birth and within term corrected age was preferred due to the single centre nature of the study. Furthermore, national charts within the first two years PTA are not available.
Penile length was measured as described by Boas et al. (11) and reported as a numeric value. The penis was slightly straightened and the distance between the lower edge of the pubic bone and the tip of the glans penis (excluding foreskin) was measured using a caliper.
All measurements were assessed by a trainee in Paediatrics and repeated twice to reduce errors. Testicular position was classified according to Boisen et al. (36) as “non-palpable”, “inguinal” or “descended”. Testicular volume was quantified using the Prader orchidometer.
Urine Collection and Assays
Urine samples were collected with a plastic bag (GIMA, cod.28685) made to fit baby’s genital area.
Assays were ideally performed on fresh urine for: uFSH, uLH, uTs and urinary creatinine (uCr). When the analysis on fresh urine was not possible, urine samples were stored in a fridge at 4 °C and sent to the laboratory for the analysis within the next 24 hours. Each urine sample was analysed by the department of laboratory medicine and pathological anatomy of the University Hospital in Modena. uFSH and uLH were quantified with an electrochemiluminescence immunoassay “ECLIA” (Cobas E601, Roche Diagnostics, Rotkreuz, Switzerland). Lower limit of uGns detection was 0.1 mIU/mL, both for FSH and for LH. Intermediate precision was <4.5% and <3.7% at concentrations ranging from 0.65-152 mIU/mL for FSH, and <2.2% and <1.6% at concentrations ranging from 1.3-123 mIU/mL for LH. uTs was quantified with the same ECLIA. Lower and upper limits of uTs detection were 0.025-15 ng/mL, respectively. Intermediate precision was <14.8% and <2.1% at concentrations ranging from 0.063-14 ng/mL. The conversion factor for uTs is ng/mL x 3.47=nmol/L. uCr was quantified using the certified VITROS 5600 Integrated System (Ortho Clinical Diagnostics). Lower and upper limits of uCr detection were 1.2-346.5 mg/dL, respectively. Intermediate precision was <2.3% and <2.1% at concentrations ranging from 61.7-157.5 mg/dL.
Both uGns and uTs values were also corrected for creatinine by dividing the uGns or uTs value for the corresponding creatinine content of each urine sample. However, due to the significant difference in uCr between term and PT infants, results have been interpreted only using non-corrected values.
Statistical Analysis
All data are expressed as median (range) or mean±standard deviation. Due to missing samples, especially in PT babies, comparison of continuous variables was carried out using a mixed model analysis. Time (calendar age or PTA) and category of neonates were included in the model as fixed effects. Subject ID and twinness were included as random effects. Bonferroni adjustment was used for multiple comparisons. All results of hormone levels below or above the detection limits were considered as the lowest or highest values, respectively, to avoid zero or negative values. The average uTs during the first three months of age was calculated using the area under the curve with the trapezoidal rule and divided by the total time. Subsequently, the average hormone level was correlated with the penile and linear growth over the same period using Spearman’s correlation. IBM Statistical Package for the Social Sciences statistics for Windows, version 26.0 (IBM Inc., Armonk, NY, USA) and Jamovi software (contact@jamovi.org) version 1.0.7.0 were used for statistical analysis. A p<0.05 was considered statistically significant.
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The AOU/AUSL Modena Local Ethics Committee approved the protocol (no: 329/17, date: 29.11.2017). Informed consent was obtained from all families. Data supporting these findings are available on request from the corresponding author only, due to privacy and ethical restrictions.
Results
Subjects
Data from 46 neonates were analyzed including: FT AGA n=23 (50%); FT SGA n=11 (24%); and PT neonates ≤33 weeks of GA n=12 (26%). Most were Caucasian (94%) and 10/46 neonates (22%) were twins from diamniotic bichorial pregnancies. Maternal and obstetrical data, together with clinical information of male neonates, are described in Supplementary Tables 1 and 2, respectively.
There were nine (19%) babies lost to follow-up: FT AGA n=4, FT SGA n=4 and PT n=1.
Comparison Between Groups Depending on PTA
Auxological parameters and external genitalia: Auxological parameters and penile length are shown in Table 1. The between-group comparison is shown in Figure 1.
Table 1. Auxological parameters [average (range)] for each category of male neonates: FT AGA (full-term AGA), FT SGA (full-term SGA) and PT (preterm). Data are shown from birth (presumed term of GA for PT boys) to 12 months of PTA. Significant differences in percentiles are marked as follow: °p<0.05; ^p<0.001; *p<0.0001.
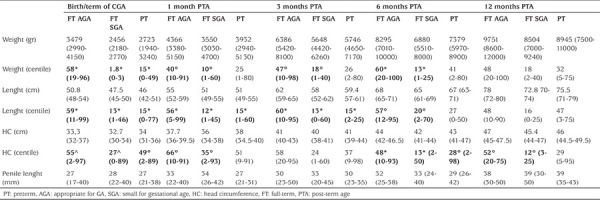
Figure 1.
Linear trend of auxological and external genitalia parameters from birth (corresponding to the presumed term of GA for PT boys) to 12 months of post-term age. Neonates were divided into 3 categories: FT AGA (blue), FT SGA (red), PT (green). Differences statistically significant are indicated with this legend: *FT AGA≠PT, ♦FT AGA≠FT SGA, ◊FT SGA≠PT
GA: gestational age, PT: preterm, PTA: post-term age, AGA: appropriate for GA, SGA: small for gestational age, HC: head circumference
At birth (or at expected term for PT infants) weight was higher in FT AGA neonates compared to FT SGA (p<0.001) and PT (p<0.001). This difference remained significant at 1, 3 and 6 months PTA only between FT AGA and FT SGA babies (respectively p=0.007; p=0.013; and p<0.001). Moreover, no differences between FT SGA and PT boys were observed at any time-point.
Body length percentile was higher in FT AGA boys from birth to 6-months PTA, both when compared to FT SGA (p<0.001) and to PT babies (p<0.001).
No differences in penile length were found apart from a slightly divergence at 1-month PTA in which FT AGA and FT SGA had longer measurements in comparison with PT boys (p=0.03). Testicular volume did not show any difference.
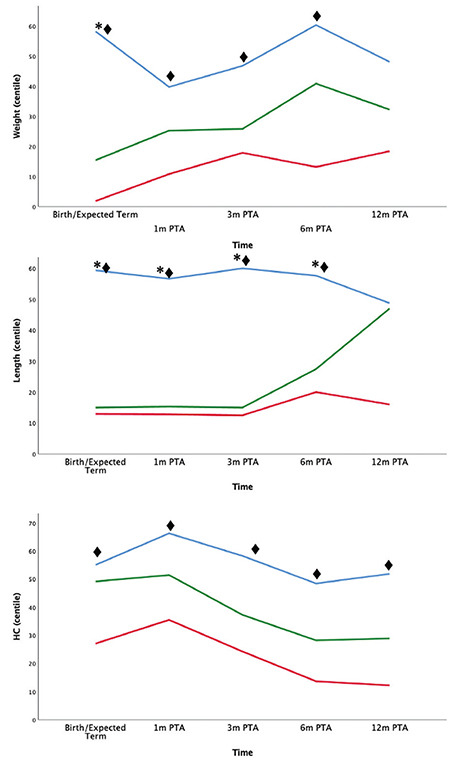
Urinary gonadotropins and urinary testosterone: Longitudinal trend of hormonal levels until 12-months PTA are shown in Figure 2. uFSH and uLH were significantly higher at the expected term in PT neonates in comparison with FT SGA (p=0.054 and p=0.021, respectively). Comparison between PT boys and FT AGA displayed higher level of uLH at expected term and 3-months PTA in the PT group (p<0.001 and p=0.026, respectively). However, uTs was significantly higher in FT AGA neonates both in comparison with FT SGA and PT babies (p<0.001).
Figure 2.
Trend of hormone levels from birth (expected term of GA for PT boys) until 12 months of PTA. Neonates were divided into 3 categories: FT AGA (blue), FT SGA (red), PT (green). Urine samples were assessed for uFSH (A), uLH (B), uTs (C). Differences statistically significant are indicated with this legend: *FT AGA≠PT, ♦FT AGA≠FT SGA, ◊FT SGA≠PT
uFSH: urinary follicle stimulating hormone, GA: gestational age, PT: preterm, PTA: post-term age, AGA: appropriate for GA, SGA: small for gestational age, HC: head circumference, uLH: urinary luteinizing hormone, uTs: urinary testosterone
Linear Growth, External Genitalia Development and Hormone Levels in Preterm Infants
The longitudinal trend of linear growth in PT boys from birth to discharge showed a significant decrease during the hospital stay (p<0.05).
Looking at the individual values of uGns and uTs, there was much variability among PT male neonates. Both uFSH and uLH displayed a pulsatile pattern starting within the first four weeks of life. Subsequently, uTs values also had a rise over this period. However, individual trends of duration and magnitude of this HPG axis activation over the first two months of life was greatly variable between different subjects.
Average levels of uFSH and uLH displayed a pulsatile pattern with higher values of uLH, as expected in male neonates. uTs was high at birth with a subsequent decrease and a second rise just after the uLH peak at 3-4 weeks of life. Thereafter uTs levels remained high and testicular descent of PT babies was parallel to the uTs surge. Individual and mean values of urinary hormone levels are shown in Figure 3.
Figure 3.
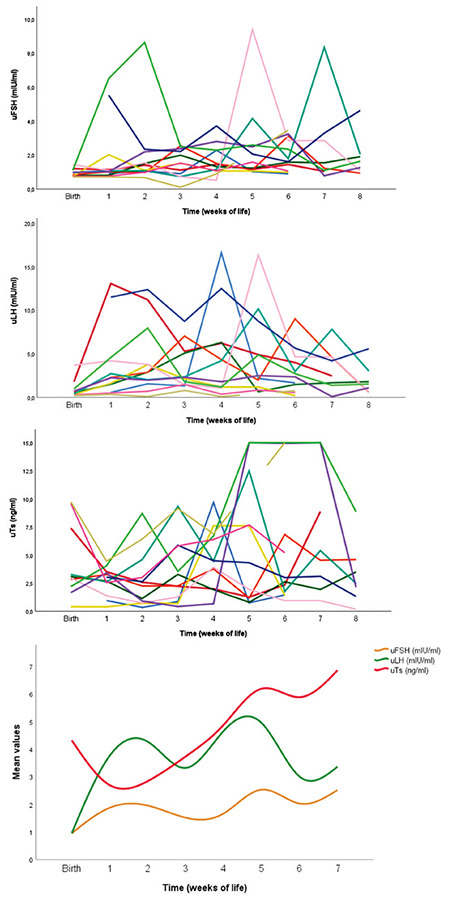
Trends of hormone levels during first weeks of life among PT babies. First three graphs show individual pattern of each premature boy using a different colour for each subject. The graph at the bottom of this figure shows the mean trend of urinary hormone levels of all PT boys
uFSH: urinary follicle stimulating hormone, uLH: urinary luteinizing hormone, uTs: urinary testosterone, PT: preterm
Figure 4 shows that PT babies had a postnatal HPG axis activation that was prolonged in its duration but also in its amplitude, in comparison with both groups of term neonates (AGA and SGA).
Figure 4.
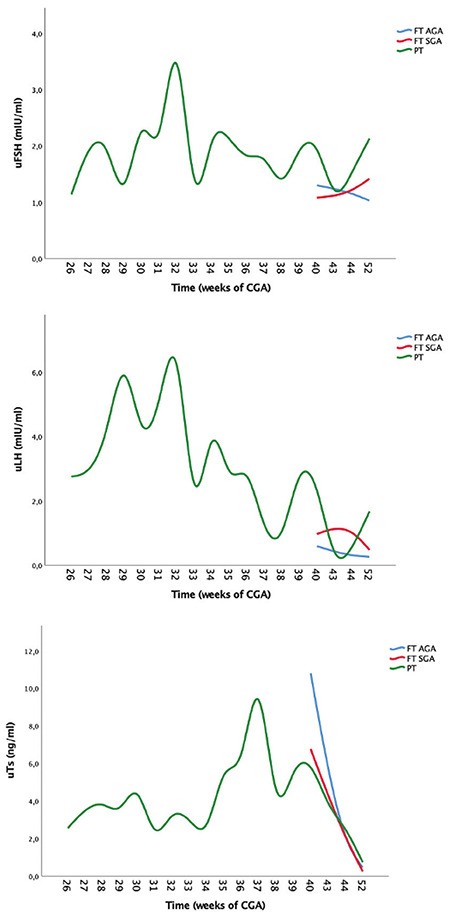
Effects of preterm birth on levels of uFSH, uLH and uTs. Trend of preterm male neonates is compared with full-term neonates until 52 weeks of CGA
uFSH: urinary follicle stimulating hormone, AGA: appropriate for gestational age, SGA: small for gestational age, uLH: urinary luteinizing hormone, uTs: urinary testosterone
Comparison Between Groups Depending on Calendar Age
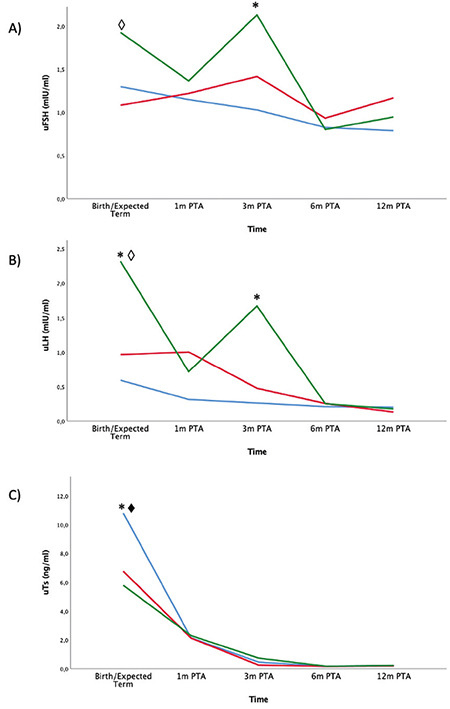
External genitalia: Penile length was higher in FT AGA and FT SGA neonates at birth, at one week and at 30 days of life (p<0.001) compared to PT boys. Penile length was higher in SGA neonates at 90 days (p=0.028).
Urinary gonadotropins and urinary testosterone: The mixed model analysis revealed a very different pattern in longitudinal assessment of urinary hormone levels between groups using calendar age during the first three months of life. At birth, uTs was higher in FT AGA neonates compared with PT and FT SGA babies (p<0.001 and p=0.001, respectively). At seven days of life, uLH had a peak at higher levels in PT boys compared to FT AGA (p<0.001). At 30 days PT male neonates continue to have higher levels of uLH, compared to both FT AGA and FT SGA (p<0.001) and PT babies also had higher uTs levels in comparison with FT AGA boys (p<0.05).
At 90 days of life PT boys still had higher levels of uTs, in comparison with both FT AGA and SGA male babies (p=0.015 and p<0.05, respectively) as shown in Figure 5.
Figure 5.
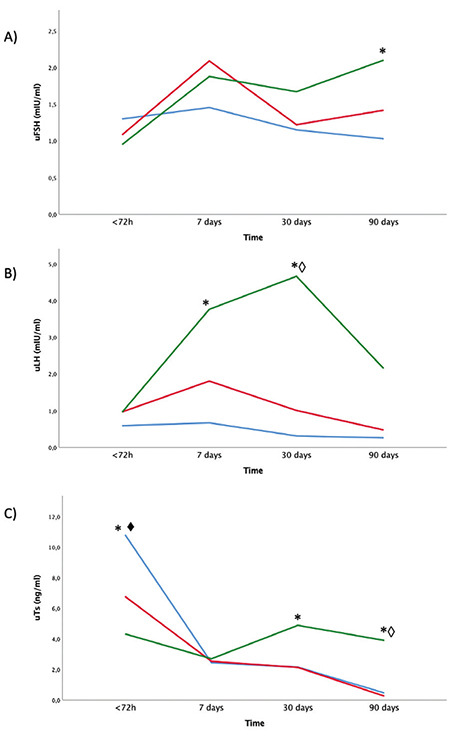
Longitudinal assessment of hormone levels from birth to 3 months of life using calendar age. Neonates were divided into 3 categories: FT AGA (blue), FT SGA (red), PT (green). Urine samples were assessed for uFSH (A), uLH (B), uTs (C). Differences statistically significant are indicated with this legend: *FT AGA≠PT, ♦FT AGA≠FT SGA, ◊FT SGA≠PT
uFSH: urinary follicle stimulating hormone, uLH: urinary luteinizing hormone, uTs: urinary testosterone, PT: preterm, AGA: appropriate for gestational age, SGA: small for gestational age, HC: head circumference, FT: full-term
Correlation Analyses
To test the hypothesis that minipuberty correlates with external genitalia development and linear growth, a correlation analysis was performed between uTs levels and auxological parameters (recumbent length and penile length), using the calendar age.
No significant differences were found between uTs and linear growth within the first three months. However, a significant correlation was found between uTs and penile growth (rho=0.412, p=0.04).
Discussion
The development of highly sensitive immunofluorometric and chemiluminometric assays for measurement of gonadotropin levels in clinical research studies highlighted a strong correlation between serum and urine samples (37,38,39). Despite this, there are still no validated reference values for uGns in children according to sex, age and pubertal stage that allow their use in routine clinical settings. However, all studies using urine samples to investigate the HPG axis agree on their use in clinical research settings (18,40,41,42).
At birth uGns are low with a subsequent gradual increase and a peak between one week and three months, with uLH levels predominant over uFSHs. Our longitudinal study in male infants allowed the identification of temporal relationships in hormonal levels and their effects on catch-up growth and external genitalia development during the first months. Moreover, splitting neonates into three categories allowed us to better elucidate the influence of birthweight and prematurity on this post-natal HPG axis activation.
The influence of prematurity on sexual hormone levels has been investigated by few studies, mainly cross-sectional and with slightly different findings (30,31,32). Only one study with a longitudinal design (7) found higher uLH and uT levels in PT boys, as well as an increase in T levels in all neonates with a peak at one month and a positive correlation with penile growth. Even the impact of being SGA on the postnatal HPG axis activation is still not elucidate (26,27,29,43). Data are not univocal and the definition itself of a SGA neonate embraces multiple possible criteria (44,45).
Our results are in line with the longitudinal study by Kuiri-Hänninen et al (7). PT boys have higher uLH and uTs levels from one week to three months of life, even if this difference is not always significant. uLH levels at seven days and one month were also higher in FT SGA neonates than in FT AGA boys. uTs levels were higher in PT boys with a postnatal rise of T at around one month in all neonates. uFSH has a higher peak at one week in FT SGA neonates but a subsequent decline towards one month of age. In contrast, uFSH in PT babies peaked later but lasted longer with higher levels at three months in comparison with FT AGA boys. Therefore, PT birth seems to have a great influence both on magnitude and duration of this postnatal HPG axis activation.
However, when comparing neonates depending on PTA, it is interesting to note that differences between FT and PT boys gradually reduced with still higher uGns in PT at the expected term of gestation but no differences at 6- and 12-months PTA.
These findings support the suggestion that the postnatal HPG axis activation is developmentally regulated (1). Postnatal pituitary activation begins with a similar timing in PT and FT neonates but persists longer and higher in PT babies. PT boys thus probably experience a maturation of the hypothalamic feedback mechanisms as term approaches.
Postnatal HPG axis activation has been suggested to also play a role in completing the development of external genitalia (18,40,41). We found a significant correlation between uTs levels and penile growth. However, even if PT boys displayed higher levels of uTs, penile growth of PT boys was not significantly different from FT AGA and FT SGA male neonates, in contrast with previous studies (7,11). Penile length of PT boys showed a catch-up growth during the first months of life leading to no significant differences at 3-6 months of age. Moreover, T surge and testicular descent were strongly related in PT infants.
Coming to the effects of T levels on catch-up growth and in contrast with previous studies (18), no significant correlation between uTs and growth velocity was found, but the recumbent length at 6-months PTA showed no differences between groups, suggesting a catch-up growth of FT SGA and PT boys.
The strength of our study is related to the age of our population. First, we performed a longitudinal analysis with serial samples and a between-group comparison that allows a better analysis of the influence of birth weight and prematurity on the HPG axis. As far as we understand, the present study is the only one to investigate the HPG axis in extremely and moderately PT babies. In parallel, we did not include neonates with a weight between the 4th and 9th percentile, so as not to overlap with other studies and to better appreciate the influence of low birth weight on this axis.
Study Limitations
Some limitations need to be highlighted. First, the sample size was limited. Recruitment of PT babies can be challenging because of their clinical conditions and their lower numbers. Concurrently, the enrolment of healthy infants in a prospective study until 12-months of age can be difficult for families’ compliance. We did not perform a correlation analysis with serum samples, but the use of uGns in research studies have been largely developed, making us confident that our results can reflect the pattern of serum gonadotropins and this design also probably improved family compliance. However, it is important to underline how the methodology of this study could have been empowered by the comparison between uGns and serum gonadotrophins and salivary T, especially in PT babies due to the immaturity of the tubular activity.
The need for creatinine correction during the neonatal period is still controversial, especially in neonates (37,38,39,40,46,47). Creatinine clearance is low at birth and the rate is positively correlated with weight, length and post-conceptional age, but negatively correlated with GA (48). This information can explain our lower Ucr values in premature infants and it is probably the reason of the between-groups significant difference. We can hypothesize that the use of uGns not-corrected for creatinine excretion in a comparison between FT and PT neonates was acceptable but this suggestion needs to be interpreted with caution. uTs levels were assessed with a chemiluminescent radioimmunoassay, not with mass spectrometry. This may be relevant, not only for excluding the influence of other androgens, but mainly to detect low levels after the peak of T at 1-3 months. In fact, our results in terms of PTA revealed low to undetectable uTs levels just after three months whereas other studies on serum and urine samples displayed higher levels in male infants until 6-9 months PTA. However the use of an immunoenzymatic method for T detection is in line with other studies that used urine samples (12,18).
Conclusion
Our study provided insight into the postnatal HPG axis activation in FT AGA and SGA boys, as well as in PT infants. The results suggest that minipuberty is increased and prolonged in PT boys in comparison with pairwise FT babies, only when the analysis depends on calendar age, suggesting an ontogenetic regulation. The enhanced activation of the HPG axis translates to a more pronounced androgen secretion with a faster penile growth in PT neonates during the first three months.
However, the results in FT SGA infants are difficult to interpret and need a sample size extension. SGA neonates had the lowest postnatal T levels even if with higher uGns levels and a same trend in penile growth, possibly reflecting a different intrauterine stimulation due to a growth restriction. However, the lack of a postnatal peak of T translated to a slower catch-up growth in FT SGA neonates. In conclusion, minipuberty provides an important window of opportunity for the evaluation of HPG axis functionality before puberty. uGns have been demonstrated to be a valid, practical and non-invasive tool for the purpose; wider acceptance of this method among infants may be clinically beneficial.
The possible short-term and long-term implications of this different postnatal activity in SGA or PT neonates need to be clarified. Further studies with a long-term follow-up are needed with regards to healthy infants but should also take account of birthweight and prematurity.
Acknowledgments
We want to thank all the neonatal nurses and paediatric trainees of the University Hospital of Modena for their help with samples’ collection. Many thanks also to Prof. Faisal Ahmed and Dr. Martina Rodie from the Developmental Endocrinology Research Group of Glasgow University for remote support.
Footnotes
Ethics
Ethics Committee Approval: The AOU/AUSL Modena Local Ethics Committee approved the protocol (no: 329/17, date: 29.11.2017).
Informed Consent: Informed consent was obtained from all families.
Authorship Contributions
Surgical and Medical Practices: Alessandra Boncompagni, Elisa Pietrella, Erica Passini, Licia Lugli, Alberto Berardi, Lorenzo Iughetti, Laura Lucaccioni, Concept: Alessandra Boncompagni, Laura Lucaccioni, Design: Alessandra Boncompagni, Laura Lucaccioni, Data Collection or Processing: Alessandra Boncompagni, Elisa Pietrella, Erica Passini, Chiarina Grisolia, Mara Tagliazucchi, Enrico Tagliafico, Analysis or Interpretation: Alessandra Boncompagni, Chiarina Grisolia, Mara Tagliazucchi, Enrico Tagliafico, Lorenzo Iughetti, Laura Lucaccioni, Literature Search: Alessandra Boncompagni, Licia Lugli, Alberto Berardi, Lorenzo Iughetti, Laura Lucaccioni, Writing: Alessandra Boncompagni, Elisa Pietrella, Licia Lugli, Alberto Berardi, Lorenzo Iughetti, Laura Lucaccioni.
Conflict of interest: None declared.
Financial Disclosure: This research project was supported with a research grant of the University of Modena and Reggio Emilia for the Department of Clinical and Surgical Sciences for Mothers, Children and Adults at the University Hospital of Modena (FAR 2017).
References
- 1.Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82:73–80. doi: 10.1159/000362414. [DOI] [PubMed] [Google Scholar]
- 2.de Zegher F, Devlieger H, Veldhuis JD. Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatr Res. 1992;32:605–607. doi: 10.1203/00006450-199211000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Winter JS, Hughes IA, Reyes FI, Faiman C. Pituitary-gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. J Clin Endocrinol Metab. 1976;42:679–686. doi: 10.1210/jcem-42-4-679. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt H, Schwarz HP. Serum concentrations of LH and FSH in the healthy newborn. Eur J Endocrinol. 2000;143:213–215. doi: 10.1530/eje.0.1430213. [DOI] [PubMed] [Google Scholar]
- 5.Bergadá I, Milani C, Bedecarrás P, Andreone L, Ropelato MG, Gottlieb S, Bergadá C, Campo S, Rey RA. Time course of the serum gonadotropin surge, inhibins, and anti-Müllerian hormone in normal newborn males during the first month of life. J Clin Endocrinol Metab. 2006;91:4092–4098. doi: 10.1210/jc.2006-1079. [DOI] [PubMed] [Google Scholar]
- 6.Andersson AM, Toppari J, Haavisto AM, Petersen JH, Simell T, Simell O, Skakkebaek NE. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab. 1998;83:675–681. doi: 10.1210/jcem.83.2.4603. [DOI] [PubMed] [Google Scholar]
- 7.Kuiri-Hänninen T, Seuri R, Tyrväinen E, Turpeinen U, Hämäläinen E, Stenman UH, Dunkel L, Sankilampi U. Increased activity of the hypothalamic-pituitary-testicular axis in infancy results in increased androgen action in premature boys. J Clin Endocrinol Metab. 2011;96:98–105. doi: 10.1210/jc.2010-1359. [DOI] [PubMed] [Google Scholar]
- 8.Forest MG, Cathiard AM, Bertrand JA. Evidence of testicular activity in early infancy. J Clin Endocrinol Metab. 1973;37:148–151. doi: 10.1210/jcem-37-1-148. [DOI] [PubMed] [Google Scholar]
- 9.Garagorri JM, Rodríguez G, Lario-Elboj AJ, Olivares JL, Lario-Muñoz A, Orden I. Reference levels for 17-hydroxyprogesterone, 11-desoxycortisol, cortisol, testosterone, dehydroepiandrosterone sulfate and androstenedione in infants from birth to six months of age. Eur J Pediatr. 2008;167:647–653. doi: 10.1007/s00431-007-0565-1. [DOI] [PubMed] [Google Scholar]
- 10.Barry JA, Hardiman PJ, Siddiqui MR, Thomas M. Meta-analysis of sex difference in testosterone levels in umbilical cord blood. J Obstet Gynaecol. 2011;31:697–702. doi: 10.3109/01443615.2011.614971. [DOI] [PubMed] [Google Scholar]
- 11.Boas M, Boisen KA, Virtanen HE, Kaleva M, Suomi AM, Schmidt IM, Damgaard IN, Kai CM, Chellakooty M, Skakkebaek NE, Toppari J, Main KM. Postnatal penile length and growth rate correlate to serum testosterone levels: a longitudinal study of 1962 normal boys. Eur J Endocrinol. 2006;154:125–129. doi: 10.1530/eje.1.02066. [DOI] [PubMed] [Google Scholar]
- 12.Kuiri-Hänninen T, Haanpää M, Turpeinen U, Hämäläinen E, Dunkel L, Sankilampi U. Transient postnatal secretion of androgen hormones is associated with acne and sebaceous gland hypertrophy in early infancy. J Clin Endocrinol Metab. 2013;98:199–206. doi: 10.1210/jc.2012-2680. [DOI] [PubMed] [Google Scholar]
- 13.Janus D, Wojcik M, Tyrawa K, Starzyk J. Transient isolated scrotal hair development in infancy. Clin Pediatr (Phila) 2013;52:628–632. doi: 10.1177/0009922813480845. [DOI] [PubMed] [Google Scholar]
- 14.Perry RJ, Farquharson C, Ahmed SF. The role of sex steroids in controlling pubertal growth. Clin Endocrinol (Oxf) 2008;68:4–15. doi: 10.1111/j.1365-2265.2007.02960.x. [DOI] [PubMed] [Google Scholar]
- 15.Roche AF, Guo S, Moore WM. Weight and recumbent length from 1 to 12 mo of age: reference data for 1-mo increments. Am J Clin Nutr. 1989;49:599–607. doi: 10.1093/ajcn/49.4.599. [DOI] [PubMed] [Google Scholar]
- 16.Gasser T, Sheehy A, Molinari L, Largo RH. Sex dimorphism in growth. Ann Hum Biol. 2000;27:187–197. doi: 10.1080/030144600282299. [DOI] [PubMed] [Google Scholar]
- 17.Becker M, Oehler K, Partsch CJ, Ulmen U, Schmutzler R, Cammann H, Hesse V. Hormonal ‘minipuberty’ influences the somatic development of boys but not of girls up to the age of 6 years. Clin Endocrinol (Oxf) 2015;83:694–701. doi: 10.1111/cen.12827. [DOI] [PubMed] [Google Scholar]
- 18.Kiviranta P, Kuiri-Hänninen T, Saari A, Lamidi ML, Dunkel L, Sankilampi U. Transient Postnatal Gonadal Activation and Growth Velocity in Infancy. Pediatrics. 2016;138:e20153561. doi: 10.1542/peds.2015-3561. [DOI] [PubMed] [Google Scholar]
- 19.Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, Hines M. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci. 2009;20:144–148. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamminmäki A, Hines M, Kuiri-Hänninen T, Kilpeläinen L, Dunkel L, Sankilampi U. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm Behav. 2012;61:611–616. doi: 10.1016/j.yhbeh.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Kung KT, Browne WV, Constantinescu M, Noorderhaven RM, Hines M. Early postnatal testosterone predicts sex-related differences in early expressive vocabulary. Psychoneuroendocrinology. 2016;68:111–116. doi: 10.1016/j.psyneuen.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Schaadt G, Hesse V, Friederici AD. Sex hormones in early infancy seem to predict aspects of later language development. Brain Lang. 2015;141:70–76. doi: 10.1016/j.bandl.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Keenan BS, Richards GE, Ponder SW, Dallas JS, Nagamani M, Smith ER. Androgen-stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth hormone and insulin-like growth factor-I in the treatment of short stature and delayed puberty. J Clin Endocrinol Metab. 1993;76:996–1001. doi: 10.1210/jcem.76.4.8473416. [DOI] [PubMed] [Google Scholar]
- 24.Golombok S, Rust J. The measurement of gender role behaviour in pre-school children: a research note. J Child Psychol Psychiatry. 1993;34:805–811. doi: 10.1111/j.1469-7610.1993.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 25.Pasterski V, Zucker KJ, Hindmarsh PC, Hughes IA, Acerini C, Spencer D, Neufeld S, Hines M. Increased Cross-Gender Identification Independent of Gender Role Behavior in Girls with Congenital Adrenal Hyperplasia: Results from a Standardized Assessment of 4-to 11-Year-Old Children. Arch Sex Behav. 2015;44:1363–1375. doi: 10.1007/s10508-014-0385-0. [DOI] [PubMed] [Google Scholar]
- 26.Nagai S, Kawai M, Myowa-Yamakoshi M, Morimoto T, Matsukura T, Heike T. Gonadotropin levels in urine during early postnatal period in small for gestational age preterm male infants with fetal growth restriction. J Perinatol. 2017;37:843–847. doi: 10.1038/jp.2017.55. [DOI] [PubMed] [Google Scholar]
- 27.Ibáñez L, Potau N, Enriquez G, Marcos MV, de Zegher F. Hypergonadotrophinaemia with reduced uterine and ovarian size in women born small-for-gestational-age. Hum Reprod. 2003;18:1565–1569. doi: 10.1093/humrep/deg351. [DOI] [PubMed] [Google Scholar]
- 28.Forest MG, de Peretti E, Bertrand J. Testicular and adrenal androgens and their binding to plasma proteins in the perinatal period: developmental patterns of plasma testosterone, 4-androstenedione, dehydroepiandrosterone and its sulfate in premature and small for date infants as compared with that of full-term infants. J Steroid Biochem. 1980;12:25–36. doi: 10.1016/0022-4731(80)90247-2. [DOI] [PubMed] [Google Scholar]
- 29.Cicognani A, Alessandroni R, Pasini A, Pirazzoli P, Cassio A, Barbieri E, Cacciari E. Low birth weight for gestational age and subsequent male gonadal function. J Pediatr. 2002;141:376–379. doi: 10.1067/mpd.2002.126300. [DOI] [PubMed] [Google Scholar]
- 30.Shinkawa O, Furuhashi N, Fukaya T, Suzuki M, Kono H, Tachibana Y. Changes of serum gonadotropin levels and sex differences in premature and mature infant during neonatal life. J Clin Endocrinol Metab. 1983;56:1327–1331. doi: 10.1210/jcem-56-6-1327. [DOI] [PubMed] [Google Scholar]
- 31.Greaves RF, Hunt RW, Chiriano AS, Zacharin MR. Luteinizing hormone and follicle-stimulating hormone levels in extreme prematurity: development of reference intervals. Pediatrics. 2008;121:574–580. doi: 10.1542/peds.2007-1327. [DOI] [PubMed] [Google Scholar]
- 32.Tapanainen J, Koivisto M, Vihko R, Huhtaniemi I. Enhanced activity of the pituitary-gonadal axis in premature human infants. J Clin Endocrinol Metab. 1981;52:235–238. doi: 10.1210/jcem-52-2-235. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed SF, Achermann JC, Arlt W, Balen A, Conway G, Edwards Z, Elford S, Hughes IA, Izatt L, Krone N, Miles H, O’Toole S, Perry L, Sanders C, Simmonds M, Watt A, Willis D. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015) Clin Endocrinol (Oxf) 2016;84:771–788. doi: 10.1111/cen.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, Gagliardi L, Gilli G, Bona G, Fabris C, De Curtis M, Milani S. Neonatal anthropometric charts: the Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. 2010;51:353–361. doi: 10.1097/MPG.0b013e3181da213e. [DOI] [PubMed] [Google Scholar]
- 35.WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Onis M de, editor. Geneva: WHO Press; 2006. p. 312. Available from: [Internet] https://www.who.int/publications/i/item/924154693X.
- 36.Boisen KA, Kaleva M, Main KM, Virtanen HE, Haavisto AM, Schmidt IM, Chellakooty M, Damgaard IN, Mau C, Reunanen M, Skakkebaek NE, Toppari J. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004;363:1264–1269. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- 37.McNeilly JD, Mason A, Khanna S, Galloway PJ, Ahmed SF. Urinary gonadotrophins: a useful non-invasive marker of activation of the hypothalamic pituitary-gonadal axis. Int J Pediatr Endocrinol. 2012;2012:10. doi: 10.1186/1687-9856-2012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucaccioni L, McNeilly J, Mason A, Giacomozzi C, Kyriakou A, Shaikh MG, Iughetti L, Ahmed SF. The measurement of urinary gonadotropins for assessment and management of pubertal disorder. Hormones (Athens) 2016;15:377–384. doi: 10.14310/horm.2002.1690. [DOI] [PubMed] [Google Scholar]
- 39.Kolby N, Busch AS, Aksglaede L, Sørensen K, Petersen JH, Andersson AM, Juul A. Nocturnal Urinary Excretion of FSH and LH in Children and Adolescents With Normal and Early Puberty. J Clin Endocrinol Metab. 2017;102:3830–3838. doi: 10.1210/jc.2017-01192. [DOI] [PubMed] [Google Scholar]
- 40.Kuiri-Hänninen T, Kallio S, Seuri R, Tyrväinen E, Liakka A, Tapanainen J, Sankilampi U, Dunkel L. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J Clin Endocrinol Metab. 2011;96:3432–3439. doi: 10.1210/jc.2011-1502. [DOI] [PubMed] [Google Scholar]
- 41.Kuiri-Hänninen T, Haanpää M, Turpeinen U, Hämäläinen E, Seuri R, Tyrväinen E, Sankilampi U, Dunkel L. Postnatal ovarian activation has effects in estrogen target tissues in infant girls. J Clin Endocrinol Metab. 2013;98:4709–4716. doi: 10.1210/jc.2013-1677. [DOI] [PubMed] [Google Scholar]
- 42.Boncompagni A, McNeilly J, Murtaza M, Lucaccioni L, Iughetti L, Wong SC, Mason A. Clinical utility of urinary gonadotrophins in hypergonadotrophic states as Turner syndrome. J Pediatr Endocrinol Metab. 2020;33:1373–1381. doi: 10.1515/jpem-2020-0170. [DOI] [PubMed] [Google Scholar]
- 43.Baker TG, Scrimgeour JB. Development of the gonad in normal and anencephalic human fetuses. Reproduction. 1980;60:193–199. doi: 10.1530/jrf.0.0600193. [DOI] [PubMed] [Google Scholar]
- 44.Saenger P, Czernichow P, Hughes I, Reiter EO. Small for gestational age: short stature and beyond. Endocr Rev. 2007;28:219–251. doi: 10.1210/er.2006-0039. [DOI] [PubMed] [Google Scholar]
- 45.Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. 2007;92:804–810. doi: 10.1210/jc.2006-2017. [DOI] [PubMed] [Google Scholar]
- 46.Demir A, Alfthan H, Stenman UH, Voutilainen R. A clinically useful method for detecting gonadotropins in children: assessment of luteinizing hormone and follicle-stimulating hormone from urine as an alternative to serum by ultrasensitive time-resolved immunofluorometric assays. Pediatr Res. 1994;36:221–226. doi: 10.1203/00006450-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Demir A, Dunkel L, Stenman UH, Voutilainen R. Age-related course of urinary gonadotropins in children. J Clin Endocrinol Metab. 1995;80:1457–1460. doi: 10.1210/jcem.80.4.7714124. [DOI] [PubMed] [Google Scholar]
- 48.Mannan MA, Shahidulla M, Salam F, Alam MS, Hossain MA, Hossain M. Postnatal development of renal function in preterm and term neonates. Mymensingh Med J. 2012;21:103–108. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


