Abstract
Background
Female genital schistosomiasis (FGS) is a chronic gynaecological disease affecting girls and women in sub-Saharan Africa (SSA), caused by the parasite Schistosoma (S.) haematobium. FGS is associated with sexual dysfunction, reproductive tract morbidity and increased prevalence of HIV and cervical precancer lesions.
Source of data
Key peer-reviewed published literature.
Areas of agreement
FGS screening and diagnosis require costly equipment and specialized training, seldom available in resource-limited settings. FGS surveillance is not included in wider schistosomiasis control strategies. The interplay of FGS with other SRH infections is not fully understood. Integration of FGS within sexual and reproductive health (SRH) control programmes needs to be explored.
Areas of controversy
There are no standardized methods for individual or population-based FGS screening and diagnosis, hindering accurate disease burden estimates and targeted resource allocation. Treatment recommendations rely on public health guidelines, without rigorous clinical evidence on efficacy.
Growing points
Integrating FGS screening with SRH programmes offers an opportunity to reach at-risk women with limited access to healthcare services. Home-based self-sampling coupled with handheld colposcopes operated by primary healthcare workers show promise for FGS diagnosis and surveillance at scale.
Areas timely for developing research
There is growing interest in decentralizing strategies for FGS screening and diagnosis. The accurate predictions on the ‘cost-effectiveness’ of these approaches will determine their affordability and feasibility within the overburdened health systems in SSA. Clinical trials are needed to optimize FGS treatment. Longitudinal studies can expand on the epidemiological knowledge on co-morbidities and integration within other SRH interventions.
Keywords: female genital schistosomiasis, FGS screening, diagnosis, cost-effectiveness, programmes integration
Introduction
Female genital schistosomiasis (FGS) is a chronic gynaecological condition caused by the waterborne parasite Schistosoma (S.) haematobium.1,2 It affects an estimated 30–56 million girls and women globally, mostly in sub-Saharan African (SSA) countries.1 FGS is characterized by the presence of S. haematobium eggs or DNA in genital tissues or fluids, causing chronic inflammation and granulomas.1,2 This can lead to debilitating morbidity, organ dysfunction and poor reproductive outcomes including infertility, abortion and ectopic pregnancy.1 Emerging cross-sectional evidence suggests that women with FGS have increased prevalence of HIV and high-risk (HR-) human papillomavirus (HPV), the causative agent of cervical cancer.1,3,4 Current schistosomiasis control strategies do not include FGS.5 Treatment recommendations for FGS rely on schistosomiasis public health guidelines, which promote mass drug administration (MDA) of praziquantel, as preventive chemotherapy.1,5 However, there is very limited evidence on the effectiveness of praziquantel for FGS treatment and lesion prevention and reversibility, resulting in suboptimal control of FGS in endemic settings.1
Clinical manifestations of FGS include vaginal discharge, vaginal itching, post-coital bleeding and abdominal pain.6 Due to the similarity of symptoms, FGS is often misdiagnosed as a sexually transmitted infection (STI) in primary healthcare clinics, resulting in unnecessary STI treatment and overlooking FGS.1 An added hurdle is the lack of awareness of FGS amongst women and healthcare workers in endemic communities, making self-recognition problematic.1,6 Education and information dissemination on FGS remain grossly insufficient.1
There is currently no standardized method for individual or population-based FGS screening and diagnosis, which hinders accurate assessments of disease burden.1,6,7 To date, approximately only 15 000 girls and women across endemic settings have been properly assessed for the condition.8 The debilitating sexual and reproductive health (SRH) complications associated with FGS are not yet included in the global burden of disease estimates for schistosomiasis.1 Conventional diagnosis relies on expensive colposcopes to visually identify FGS-associated lesions.1,6 This requires high-level specialized training and advanced clinical infrastructure, which are often unavailable in S. haematobium endemic settings.1,6 Community-based sampling and testing using home-based self-sampling and point-of-care (POC) diagnostics offers a promising, accurate and cost-saving opportunity for surveillance at scale.6 Before implementing a new screening and diagnostic strategy, it is essential to evaluate their uptake, ‘cost-effectiveness’ and available implementation capacity within the overburdened healthcare systems in SSA.9
Women living in SSA face a triple burden of disease suffering from FGS, HIV and cervical cancer.1 The S. haematobium eggs deposited in the genital tract are suspected to contribute to chronic genital inflammation, friable blood vessels and contact bleeding, increasing the risk of HIV and HR-HPV transmission.1,3,4 Considering the synergies between FGS, HIV and HR-HPV, integrating FGS control within the existing SRH services could improve screening and treatment coverage for all clinical entities.1
This review explores, for the first time, the ‘cost-effectiveness’, ‘scalability’ and ‘feasibility’ of implementing new FGS screening and diagnostic strategies within the overburdened health systems in SSA. We draw together recent findings from the FGS literature and advancements in controlling other SRH conditions in the region to identify potential cost-effective and scalable strategies for FGS screening and diagnosis.
Epidemiology and pathology of FGS
The epidemiology of FGS is closely linked to the distribution and transmission patterns of the waterborne parasite S. haematobium.2,3 Exposure to S. haematobium occurs through skin contact with larvae (cercariae) in contaminated freshwater sources commonly used for daily chores and activities, particularly in rural communities.2,3 Inside the human host, the parasites mature into adults and live in the blood vessels, where the female worms produce eggs.2,3 These eggs travel through the pelvic plexus to the urogenital organs.2,3,6 Some S. haematobium eggs get trapped in genital tissues where they induce an inflammatory response, formation of granulomas and pathological mucosal changes. These alterations in the genital tract can be observed using a colposcope.2,3
Girls and women living in S. haematobium-endemic areas are susceptible to schistosomiasis from a young age, resulting in FGS affecting women of all age groups.1,10 In endemic settings, schistosomiasis is a recurrent infection that can occur throughout life after repeated exposures to infected freshwater sources.1 Between 30 and 75% of girls and women with urinary S. haematobium are estimated to develop FGS.4,11 Genital signs and symptoms associated with FGS are likely to start developing in early childhood after schistosomiasis infection, but the precise timing of cervicovaginal lesion appearance is not known.1 Previous studies have shown that older women have a higher prevalence of visually diagnosed genital lesions.10,12 This is likely due to the gradual development of chronic and long-standing S. haematobium egg deposition in the genital tract from accumulated schistosome infection.10,12 In contrast, younger women often present with a higher intensity of egg-patent urinary S. haematobium infection and higher rates of schistosome DNA retrieval from the genital tract.6,10,12 Importantly, chronic and fibrotic FGS can persist beyond active urinary S. haematobium infection.4,11
FGS and other genital co-infections and co-morbidities
FGS and HIV share a substantial geographical overlap, with 67% of people living with HIV residing in SSA, where FGS is highly prevalent (Fig. 1).1 Several studies have shown that the cervicovaginal inflammation and vascularization in women with FGS contribute to increased HIV transmission.1 Two systematic reviews found that women with urinary S. haematobium infection are more likely to have prevalent HIV compared to S. haematobium-negative women (OR = 1.85, 95% CI 1.17–2.92 and OR = 2.31, 95% CI 1.23–4.33).13,14 A smaller simple size in a retrospective cohort study in Zambia limited the ability to provide a robust estimate of the risk to seroconvert associated with FGS (adjusted rate ratio (RR) = 2.16, 95% CI 0.21–12.30).15 Another study in Lusaka, Zambia retrospectively tested sera for detection of schistosome-specific antibody levels from heterosexual HIV-discordant couples.16 They found that schistosome-specific antibodies were associated with increased transmission of HIV from both sexes (adjusted hazard ratio (aHR) =1.8, P < 0.05 for women and aHR = 1.4, P < 0.05 for men), HIV acquisition in women (aHR = 1.4, P < 0.05) and increased progression to death in HIV-positive women (aHR = 2.2, P < 0.001).16
Fig. 1.
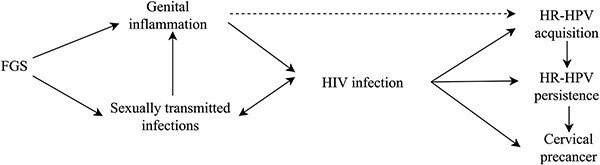
Conceptual pathway adapted from Sturt et al.3 and Rafferty et al.4 describing the potential association and synergies between FGS and other sexual and reproductive health conditions including HIV infection, cervical precancer and sexually transmitted infections.
Women living with HIV are six-times more likely to develop cervical cancer, compared to HIV-negative women.17 This increased risk is due to several factors, including higher susceptibility to HR-HPV acquisition, faster progression to precancerous and cancerous lesions and higher recurrence rates after treatment of precancerous lesions (Fig. 1).17 The immune suppression associated with HIV also indirectly enhances the oncogenic effects of HR-HPV, as shown by the correlation between cervical cancer and lower CD4 cell count in HIV-positive women.17
The genital chronic inflammation caused by S. haematobium egg deposition is suspected to be on the casual pathway leading to cervical cancer (Fig. 1).4 Studies have found co-existence of FGS and cervical tissue changes, with a positive association between FGS diagnosed by polymerase chain reaction (PCR) and cervical dysplasia by visual inspection with acetic acid (VIA) (OR = 6.08, P = 0.016).4 The chronic genital inflammation in women with FGS is also hypothesized to facilitate the transmission of different STIs, further contributing to the substantial morbidity associated with SRH amongst women in SSA.18 A study in Madagascar found that 35% of women with egg-patent urinary S. haematobium infection had concurrent STIs including Neisseria gonorrhoea, Chlamydia trachomatis, Mycoplasma genitalium and/or Trichomonas (T.) vaginalis, detected using molecular testing of cervical swabs.18 Another study in South Africa amongst 933 young women did not find a statistically significant difference in prevalence of T. vaginalis and herpes simplex virus (HSV) stratified by FGS status, although T. vaginalis was associated with the clinical finding of ‘homogeneous sandy patches’ (Fig. 2B) (adjusted odds ratio (OR): 1.74, 95% CI 1.10–2.75, P-value = 0.019).19
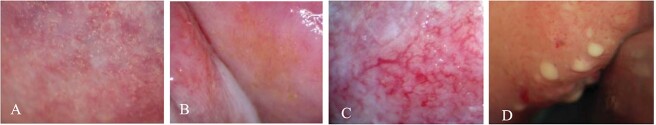
Fig. 2.
Genital lesions associated with FGS. (A) Grainy sandy patches, (B) homogeneous sandy patches, (C) abnormal blood vessels and (D) rubbery papules.22
Several studies in SSA have investigated the role of STIs as risk factors for HIV transmission (Fig. 1).20 Similarly to FGS, the presence of STIs triggers inflammation in the genital tract, increasing the number of HIV target cells transmission.20 A meta-analysis of studies across SSA countries revealed that women infected with T. vaginalis have a 50% higher risk of HIV acquisition (HR 1.5; 95% CI 1.3–1.7), suggesting a substantial contribution of T. vaginalis to the HIV pandemic.21 This association is likely attributed to pathogenic mechanisms, such as inflammation, resulting in an increase in HIV target cells and a higher prevalence of bacterial vaginosis, a known risk factor for HIV acquisition.21
Despite the potential overlap between FGS, HIV, HR-HPV and other STIs, current SRH control programmes have largely overlooked FGS.1 Large longitudinal studies should be conducted to further understand the temporal association of FGS in the development of HIV, cervical cancer and different STIs and to identify other shared risk factors. However, integration of FGS into existing SRH programmes requires immediate attention to timely address the low FGS screening coverage and neglected treatment needs.1
FGS diagnostic methods
Conventional FGS diagnosis involves visual inspection of the cervicovaginal mucosa using a colposcope.1,6 Trained medical experts analyze the resulting images and classify them as suggestive of visual FGS if homogeneous yellow sandy patches, grainy sandy patches, abnormal blood vessels or rubbery papules are observed (Fig. 2).22,23 A visual diagnostic pocket atlas has been developed by the World Health Organization (WHO) to aid clinical healthcare providers in endemic settings with a guidance and description of the characteristic clinical lesions of FGS.23 Yet, colposcopy requires expensive equipment and specialized training, hindering its scalability for visual diagnosis of FGS in S. haematobium endemic settings (Table 1).1,6,22 Handheld colposcopy has been proposed as a more scalable and decentralized diagnostic strategy that can be operated by primary healthcare workers.24 Despite these visual aids, lesions may still be overlooked if the ova are deposited deeply in the submucosa.1,6,22 Importantly, recent findings have highlighted the limited diagnostic accuracy and specificity of colposcopy.22 The mucosal changes observed in FGS-positive women have been associated with a range of STIs and cervical cancer, leading to false- positive results and the unnecessary treatment.22 New approaches to FGS image reading through computer analysis are currently being explored with the final aim to create artificial intelligence algorithms to aid in the visual diagnosis of FGS.25
Table 1.
Overview of the strengths and limitation of the different diagnostic methods for FGS
| Diagnostic strategy | Strength | Limitation | Estimated cost of equipment |
|---|---|---|---|
| Histopathology | - Gold standard, indisputable evidence of FGS if eggs are detected in genital tissue. | - Needs trained histopathologist and adequate histopathology - Collection of biopsy samples could miss eggs in the genital tract. - Require infrastructure and clinic visit. - Hypothetically increases the risk of STI acquisition in high endemic settings if sex is not avoided before healing after the biopsy |
Between USD$50 and USD$100 per biopsy |
| Traditional colposcopy | - Conventional method for FGS visual detection of lesions. - Diagnosis is supported by the pocket atlas and poster, both freely available resources from WHO developed to aid healthcare workers to identify FGS lesions. |
- Low specificity. - Expensive equipment - Requires infrastructure, electricity and clinic visit. - Requires extensive training for operation and image interpretation. |
Between USD$8000 and USD$20 000 USD depending on colposcope |
| Handheld colposcopy | - Requires less training than colposcopy and can be operated by primary health workers and nursing staff. - Cheaper and easier to use than traditional colposcopes. |
- Low specificity. - Limited sensitivity - Still requires some training to operate. - Requires infrastructure and clinic visit. |
Between USD$2000 and USD$4000 USD depending on device |
| PCR testing of genital samples | - High sensitivity and specificity (almost 100%). - Can be performed on self-collected genital samples. |
- Requires advanced laboratory set-up and expensive equipment - Requires specialized personnel. - Not suitable for point-of-care diagnosis. |
Cost of thermocycler: between USD$10 000 and USD$30 000 depending on the additional equipment needed. Cost per sample processed: USD$1.5-$15 |
| RPA testing of genital samples | - Moderate to high sensitivity and specificity. - Can be performed on self-collected genital samples. - Deployable at the point-of care: minimal equipment, portable, user-friendly and cheaper devices - Rapid test results (<1 hour from sample preparation to results), enabling direct follow-up and targeted treatment. |
- Lower sensitivity than PCR. - More studies needed to evaluate diagnostic performance for FGS. It has not yet been field-evaluated in health facilities or mobile laboratories |
Cost of DNA isolation by DNA extraction method: Pure extraction—USD$ 6.0 Crude extraction—between USD$0.5 and USD$1.5 Cost of Sh-RPA—USD$4-$20 Cost of RPA device—USD5000–10 000 based on device |
Molecular testing using PCR to detect S. haematobium DNA from genital samples is a highly sensitive and specific diagnostic method for FGS.6 Previous studies found that PCR testing from stored cervicovaginal lavage (CVL) had high specificity for FGS diagnosis (between 78 and 83%), but its sensitivity was lower (ranging from 15 to 53%) compared to visual methods of FGS detection.6,10 A recent study in Zambia validated PCR testing on genital self-collected samples as an alternative, closer-to-the-user screening strategy for FGS.6 Compared to a composite definition of FGS using genital PCR specimen (CVL, vaginal or cervical swabs), PCR of genital self-samples demonstrated a sensitivity of 80% (95% CI 61.4–92.3%). The sensitivity increased to 89% (95% CI 51.8–99.7) when restricting to women with ‘active’ schistosomiasis identified by positive circulating anodic antigen in urine (CAA).6
Isothermal molecular diagnostic methods, such as the recombinase polymerase amplification (RPA) and loop-mediated isothermal amplification (LAMP) are field-appropriate alternatives to PCR.1 These assays do not require the use of thermal cycling; they are rapid and can be performed with minimal equipment, making them suitable for resource limited settings and point-of-care applications.26 The RPA assay (Sh-RPA) has shown promise for detecting Schistosoma DNA for FGS diagnosis from CVL and vaginal self-swabs.26 Compared to real-time PCR, the Sh-RPA assay on self-collected vaginal swabs showed a sensitivity of 93.3% and specificity of 96.6%.25 For CVL samples, the Sh-RPA assay demonstrated sensitivity ranging between 71.4 and 85.7% depending on the DNA extraction method used.26 A LAMP assay has not yet been evaluated for FGS diagnosis.1 Further research studies are needed to evaluate the performance and application of the Sh-RPA assay in field settings.1,26
Treatment of FGS
FGS treatment and control currently rely on schistosomiasis public health guidelines, which promote preventive chemotherapy with praziquantel through mass drug administration (MDA) programmes.1 Praziquantel significantly reduces urinary egg excretion, but the evidence on its effectiveness in reversing FGS lesions is scarce.3 Treatment programmes in endemic settings are based on schistosomiasis prevalence.5 The WHO recommends annual preventive chemotherapy with praziquantel for all individuals over 2 years old living in communities with egg-patent Schistosoma prevalence ≥10%.5 In contrast, communities with schistosomiasis prevalence, <10% are suggested to use a clinical approach of test-and-treat.5 This strategy could prevent the development of patent FGS in some girls and women included in treatment programmes. However, a recent study across two S. haematobium endemic areas in Malawi found a high prevalence of FGS diagnosed by PCR of cervicovaginal swab (18%) in low-prevalence communities (below 10%).12 This highlights the significant neglect in providing FGS treatment amongst girls and women in lower schistosomiasis prevalence settings. Thus, it is imperative to expand FGS control based on specific FGS screening and treatment strategies that include communities with medium and low S. haematobium prevalence (Table 2).
Table 2.
Summary of key knowledge gaps in FGS and research needs to bring FGS out of neglect
| Knowledge gaps on FGS | Research needs to bring FGS out of neglect |
|---|---|
| Measure the global burden of FGS | ➔ Conduct burden of disease studies across S. haematobium endemic settings ➔ Develop a framework to accurately estimate the disability weights associated with FGS including associated complications and morbidity. |
| Explore the pathophysiological and epidemiological associations of FGS with other SRH conditions | ➔ Conduct larger longitudinal cohort studies to understand the temporal association between FGS and other SRH conditions, as well as the shared risk factors |
| Further validate scalable, affordable, accurate and user-friendly diagnostic strategies against a validated gold standard. | ➔ Define a reference standard of care for FGS diagnosis that can be used across studies and settings for comparison of diagnostic test results |
| Understand the impact of praziquantel treatment on FGS clinical outcomes and genital and sexual signs and symptoms | ➔ Conduct randomized controlled trials to understand the effect of praziquantel for treating FGS across age groups and endemic settings |
| Strategies to implement FGS control strategies outside research settings | ➔ Conduct further costing studies to understand the affordability of different control strategies. ➔ Evaluate the cost-effectiveness of different FGS control strategies to inform effective implementation by the health system |
| Evaluate an integrated approach for FGS control within the SRH agenda | ➔ Conduct further studies to understand the affordability, cost-effectiveness, coverage and acceptability of integrated control strategies for FGS and SRH conditions. |
Economic evaluation of new FGS screening interventions
Economic evaluations of new health interventions systematically compare alternatives to optimize health gains under a budget constraint.9 Incremental costs and health outcomes of different interventions are compared to provide information about which intervention delivers the maximum health benefits with minimum costs.9 Cost analyses estimate the costs of a health intervention in a specific population, time period and location.9 These cost estimates are then combined with specific health outcomes to calculate cost-effectiveness ratios, which can inform decision-making on which intervention to adopt.9
To ensure the effective implementation of new FGS screening strategies in S. haematobium endemic countries, there is a need to evaluate the available capacity within the health system. Given that there is currently no standard of care for FGS screening, introducing a new screening strategy is associated with incremental costs. Moreover, health systems in FGS endemic countries face strong pressures to meet the increasing population’s needs with constrained resources, making it challenging to accommodate an additional service without potentially displacing resources from other activities.9 To achieve universal health coverage, it is paramount to assess the cost effectiveness of new interventions by evaluating trade-offs between costs and benefits, fairness and social values.27 Consequently, when allocating health resources, policy makers need to consider whether the benefits of introducing FGS screening and diagnosis for one group of patients outweighs gains from other health interventions.9 This can be achieved by comparing the incremental costs per case detected for different screening pathways to each other and to a ‘no intervention’ scenario, to identify the most ‘cost-effective’ strategy (the strategy that affords the most value for money).9
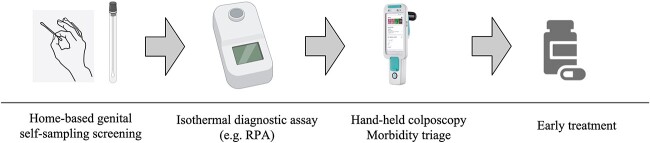
Economic evaluations of cervical cancer screening have shown that community-based genital self-sampling followed by molecular testing is a feasible and cost-effective option, either as an addition to existing screening programmes or as a primary screening strategy.28,29 For FGS screening and diagnosis, home-based genital self-sampling coupled with isothermal diagnostic methods and handheld colposcopes, operated by primary healthcare workers, is a promising approach (Fig. 3).1,6,26 This strategy has been shown to be feasible and well accepted by the community and is potentially associated with better health outcomes at lower incremental costs compared to clinic-based screening.6 By bringing screening closer to the user, this strategy could enable the decentralization of FGS screening and diagnosis for community-based surveillance at scale.6
Fig. 3.
FGS screening and diagnosis algorithm determined by home-based genital self-sampling coupled with isothermal diagnostic methods and handheld colposcopy.
Integration of FGS screening services in reproductive health programmes
The integration of FGS services into existing healthcare programmes presents a significant opportunity to improve the control of FGS and address the neglect of this condition.1 Integration involves managing and delivering health services in a comprehensive and coordinated way, tailored to patients’ needs whilst ensuring continuity of care across disease programmes and at different levels of the healthcare system.30 Researchers and policymakers recognize the importance of integrating health services to optimize resource utilization and improve healthcare outcomes.30 By integrating FGS surveillance and management into existing SRH programmes, resources may be allocated more efficiently by leveraging existing healthcare services.
The current guidelines for schistosomiasis control and elimination lack specific recommendations for FGS surveillance.5 It is crucial to establish specific healthcare programmes for FGS to improve effective detection, management and control of disease. These should be integrated into existing schistosomiasis guidelines and target girls and women in communities with different S. haematobium prevalence levels.1
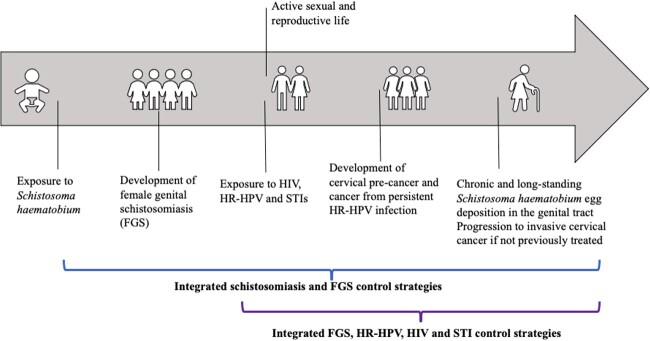
There is consensus amongst experts to integrate FGS control services into the SRH agenda given that FGS complications predominantly affects women of reproductive age (Fig. 4).1 Given the plausible associations between FGS, HIV and cervical precancer, integrating FGS screening and treatment within existing SRH control programmes could provide an opportunity to reduce the burden of all these diseases and improve the long-term health and well-being of women and girls in the African region.4,31 Global initiatives such as ‘UNAIDS 90-90-90’ and the ‘WHO cervical cancer elimination guidelines’ offer robust entry points for integration of FGS into the SRH agenda.31,32 In addition, existing healthcare delivery systems for HIV and cervical cancer prevention and control can be used to increase FGS prevention, screening and treatment.31 The current shift from clinic-based to community-based screening and testing for cervical cancer and HIV across SSA provides a further opportunity to deliver an integrated home-based screening and testing package that includes FGS testing.1,30 This could represent a cost-effective strategy to detect multiple pathogens and increase surveillance for different SRH conditions.32
Fig. 4.
Natural exposure and disease progression of different sexual and reproductive health conditions, including FGS, HIV, HR-HPV and STIs at different stages of a woman’s life—adapted from Bustinduy et al.1 The different brackets represent potential opportunities for the integration of control strategies for these conditions.
Integrating FGS and SRH services is potentially associated with ‘lower costs’ and increased efficiency compared to delivering screening separately.33,34 From a patient’s perspective, integrating services, delivered either at home or in clinic, can reduce the time and inconvenience of utilizing healthcare for multiple conditions, thus improving patient’s access to care and health outcomes.35 From a provider perspective, integration improves processes and resource allocation by capitalizing on commonalities in care delivery and distribution systems and by sharing capital resources.30,35 This approach improves efficient health production through economies of scale and scope.34,35 Economies of scale occur when integration results in increased demand and provision of services, leading to lower unit cost of production.34 For example, the integration of home-based self-sampling for different SRH conditions within a single screening package could spread fixed laboratory costs and personnel costs over multiple strategies. Similarly, clinic-based screening programmes can result in economies of scale by distributing fixed facility costs across different SRH services.34 Economies of scope arise when the unit costs of different services are lower when these are provided together, either through cost complementarity or by spreading fixed costs over multiple outputs.34 An integrated screening approach for FGS and other SRH conditions, either through home-based or clinic-based screening, could reduce excess capital capacity and spread the fixed costs.34 In addition, integration of FGS and SRH screening could reduce the marginal or average incremental cost per delivered service, resulting in cost complementarities.34
From theory to practice: challenges in implementing integrated screening programmes
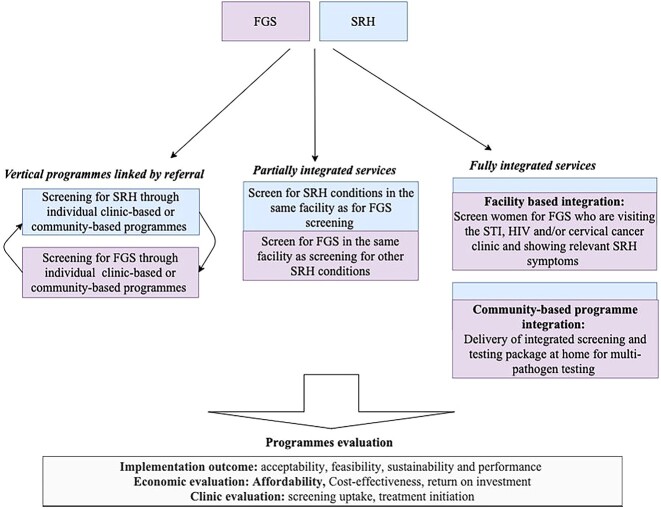
There are currently no models providing evidence on the integration of FGS services within SRH programmes. As shown in Fig. 5, integration can occur either between disease-specific programmes (‘vertical integration’) or across system-wide structures and policies (‘horizontal integration’).36 In disease-specific (vertical) models, FGS and SRH services are provided at different service delivery points in the same location and linked through a referral system.36 An individual would enter the health system by using the SRH services through clinic-based or community-based programmes and then get referred to the FGS services or vice versa.36 ‘Vertical integration’ models are considered an initial step toward providing integrated services as they can be implemented at the lowest level of the healthcare system, requiring minimal additional training for staff and fewer additional resources.36 However, for this model to be effective, there must be a strong referral system in place to ensure access to patient’s services. In addition, vertical models are not convenient from a patient’s perspective due to the need for multiple visits and increased direct and indirect costs.36
Fig. 5.
Three possible models of FGS and SRH service integration.
‘Horizontal integration’ models are divided into ‘partially’ or ‘fully integrated’ models.37 In ‘partially integrated’ models, FGS and SRH services are provided in the same health facility, with some FGS services provided in different SRH departments and vice versa, whilst the main services are still provided at separate clinics served by different staff.36 ‘Partially integrated’ models, compared to standalone clinics, provide more comprehensive services, reducing patient’s time for follow-up treatment and potentially decreasing direct and indirect patients cost associated with attending different health facilities.36 However, implementing this model requires effective coordination and organization of services, initial investments for training and expansion of clinic space.36 In ‘fully integrated’ models, both FGS and SRH screening services are provided by a single healthcare provider.35 This one-stop approach can take place in clinic or through home visits.36 ‘Fully integrated’ models can use existing home-based and clinic-based screening pathway of other SRH conditions to incorporate FGS screening. For example, women with regular HIV-related appointments could conveniently access combined FGS, cervical cancer and HIV services.17 In community-based fully integrated models, an FGS and HPV screening package could be included in HIV self-testing kits delivered at home by a community-health worker.36 This would empower women with a one-stop screening and testing approach at home. Overall, ‘fully integrated’ models prioritize patient-centred care, delivering comprehensive services whilst minimizing costs and inconvenience. This approach is likely to reduce barriers to services, increase access to screening and treatment and improve patients’ outcomes. However, successful implementation of ‘fully integrated’ models requires additional resources and capacity building within the health system. This makes the implementation of these models rare in practice due to excessive costs and healthcare budget constraints.36
Moreover, in practice, integrating screening and treatment services may not be cost-effective in resource-limited settings, where combining services could divert scarce resources from existing interventions, resulting in inefficiencies. A recent econometric study analyzing data from Kenya and Swaziland on the integration of HIV and SRH services found that integration may increase the total cost of service delivery and is associated with modest efficiency gains compared to standalone programmes.38 Key barriers to effective integration of services were poor facility management, staff shortage, high turnover, inadequate staff training and infrastructures limitations.39 Notably, the study revealed that efficiency gains from integration are more likely to be achieved when services are delivered at a low scale with high levels of fixed costs shared across combined services.40 Yet, the cost-effectiveness and efficiency gains of integrated models have generally been evaluated from a clinical perspective using decision analytical models. Further empirical studies are required to demonstrate the cost-effectiveness and efficiency at scale of integrated services at the point-of-care across different settings (Table 2).34
Conclusion
FGS is a significant public health concern affecting the lives of millions of women in SSA who have limited access to effective screening, diagnostics and treatment. Urgent action is needed to establish FGS control guidelines for early disease diagnosis and prevention of sexual dysfunction and reproductive tract morbidity. Given the association between FGS, HIV and cervical pre-cancer, screening and treatment for FGS could provide an opportunity to reduce the burden and improve the global SRH of affected girls and women. Bringing FGS out of neglect and improving screening strategies involves a sequential strategy, starting with vertical integration of FGS closer-to-the-user screening and diagnostic strategies and gradually moving to an horizontal integration into established SRH programmes. Affordability and ‘cost-effectiveness’ are crucial elements to consider before implementing new health interventions within the overburdened SSA health systems. ‘Scaling-up’ effective control strategies demand strong advocacy and multisectoral approaches, country level and international strong commitment. The time has come to bring FGS out of neglect.
Author contributions
Olimpia Lamberti (Investigation, Writing—original draft), Fiammetta Bozzani (Supervision, Validation, Writing—review & editing), Kiyoshi Kita (Supervision, Validation, Writing—review & editing) and Amaya Bustinduy (Conceptualization, Methodology, Writing—review & editing)
Conflict of interest statement
The authors have no potential conflicts of interest.
Data availability
No new data were generated or analyzed in support of this review.
Contributor Information
Olimpia Lamberti, Department of Clinical Research, London School of Hygiene and Tropical Medicine, London, UK.
Fiammetta Bozzani, Department of Global Health and Development, London School of Hygiene and Tropical Medicine, London, UK.
Kita Kiyoshi, School of Tropical Medicine and Global Health, Nagasaki University, Nagasaki, Japan; Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan.
Amaya L Bustinduy, Department of Clinical Research, London School of Hygiene and Tropical Medicine, London, UK.
References
- 1. Bustinduy AL, Randriansolo B, Sturt AS, et al. An update on female and male genital schistosomiasis and a call to integrate efforts to escalate diagnosis, treatment and awareness in endemic and non-endemic settings: the time is now. Adv Parasitol 2022;115:1–44. 10.1016/bs.apar.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 2. Kjetland EF, Leutscher PDC, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol 2012;28:58–65. 10.1016/j.pt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 3. Sturt A, Webb E, Francis S, et al. Beyond the barrier: female genital schistosomiasis as a potential risk factor for HIV-1 acquisition. Acta Trop 2020;209:105524. 10.1016/j.actatropica.2020.105524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rafferty H, Sturt AS, Phiri CR, et al. Association between cervical dysplasia and female genital schistosomiasis diagnosed by genital PCR in Zambian women. BMC Infect Dis 2021;21:691. 10.1186/s12879-021-06380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO Guideline on Control and Elimination of Human Schistosomiasis. Geneva: World Health Organization, 2022, Licence: CC BY-NC-SA 3.0 IGO [PubMed] [Google Scholar]
- 6. Sturt AS, Webb EL, Phiri CR, et al. Genital self-sampling compared with cervicovaginal lavage for the diagnosis of female genital schistosomiasis in Zambian women: the BILHIV study. PLoS Negl Trop Dis 2020;14:e0008337. 10.1371/journal.pntd.0008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pillay P, Downs JA, Changalucha JM, et al. Detection of Schistosoma DNA in genital specimens and urine: a comparison between five female African study populations originating from S. haematobium and/or S. mansoni endemic areas. Acta Trop 2020;204:105363. 10.1016/j.actatropica.2020.105363. [DOI] [PubMed] [Google Scholar]
- 8. Engels D, Hotez PJ, Ducker C, et al. Integration of prevention and control measures for female genital schistosomiasis, HIV and cervical cancer. Bull World Health Organ 2020;98:615–24. 10.2471/BLT.20.252270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drummond M. Methods for the Economic Evaluation of Health Care Programmes, Fourth edn. Oxford; New York, NY: Oxford University Press, 2015,445 (Oxford medical publications) [Google Scholar]
- 10. Friis H, Midzi N, Ndhlovu PD, et al. Schistosomiasis PCR in vaginal lavage as an indicator of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg 2009;81:1050–5. 10.4269/ajtmh.2009.09-0081. [DOI] [PubMed] [Google Scholar]
- 11. Kjetland EF, Ndhlovu PD, Mduluza T, et al. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg 2005;72:311–9. 10.4269/ajtmh.2005.72.311. [DOI] [PubMed] [Google Scholar]
- 12. Olimpia L, Kayuni S, Kumwenda D, et al. Female genital schistosomiasis burden and risk factors in two endemic areas in Malawi nested in the morbidity operational research for Bilharziasis implementation decisions (MORBID) study. 2023; Manuscript under review. [DOI] [PMC free article] [PubMed]
- 13. Patel P, Rose CE, Kjetland EF, et al. Association of schistosomiasis and HIV infections: a systematic review and meta-analysis. Int J Infect Dis 2021;102:544–53. 10.1016/j.ijid.2020.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zirimenya L, Mahmud-Ajeigbe F, McQuillan R, et al. A systematic review and meta-analysis to assess the association between urogenital schistosomiasis and HIV/AIDS infection. PLoS Negl Trop Dis 2020;14:e0008383. 10.1371/journal.pntd.0008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sturt AS, Webb EL, Phiri CR, et al. Female genital schistosomiasis and HIV-1 incidence in Zambian women: a retrospective cohort study. Open Forum Infect Dis 2021;8(7). 10.1093/ofid/ofab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wall KM, Kilembe W, Vwalika B, et al. Schistosomiasis is associated with incident HIV transmission and death in Zambia. PLoS Negl Trop Dis 2018;12:e0006902. 10.1371/journal.pntd.0006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stelzle D, Tanaka LF, Lee KK, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health 2021;9:e161–9. 10.1016/S2214-109X(20)30459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leutscher PDC, Ramarokoto C, Hoffmann S, et al. Coexistence of urogenital schistosomiasis and sexually transmitted infection in women and men living in an area where Schistosoma haematobium is endemic. Clin Infect Dis 2008;47:775–82. 10.1086/591127. [DOI] [PubMed] [Google Scholar]
- 19. Shukla JD, Kleppa E, Holmen S, et al. Female genital schistosomiasis and reproductive tract infections. A cross-sectional study in rural adolescents in South Africa. Sex Reprod Health 2019;27(3):291–96. 10.1101/19009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2:33–42. 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 21. Masha SC, Cools P, Sanders EJ, et al. Trichomonas vaginalis and HIV infection acquisition: a systematic review and meta-analysis. Sex Transm Infect 2019;95:36–42. 10.1136/sextrans-2018-053713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sturt A, Bristowe H, Webb E, et al. Visual diagnosis of female genital schistosomiasis in Zambian women from hand-held colposcopy: agreement of expert image review. Wellcome Open Res 2023;8:14. 10.12688/wellcomeopenres.18737.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . Female Genital Schistosomiasis, A Pocket Atlas for Clinical Health-Care Professionals. Geneva, Switzerland: WHO.
- 24. Søfteland S, Sebitloane MH, Taylor M, et al. A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int J Gynaecol Obstet 2021;153:190–9. 10.1002/ijgo.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holmen SD, Kjetland EF, Taylor M, et al. Colourimetric image analysis as a diagnostic tool in female genital schistosomiasis. Med Eng Phys 2015;37:309–14. 10.1016/j.medengphy.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 26. Archer J, Patwary FK, Sturt AS, et al. Validation of the isothermal Schistosoma haematobium recombinase polymerase amplification (RPA) assay, coupled with simplified sample preparation, for diagnosing female genital schistosomiasis using cervicovaginal lavage and vaginal self-swab samples. PLoS Negl Trop Dis 2022;16:e0010276. 10.1371/journal.pntd.0010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pichon-Riviere A, Drummond M, Palacios A, et al. Determining the efficiency path to universal health coverage: cost-effectiveness thresholds for 174 countries based on growth in life expectancy and health expenditures. Lancet Glob Health 2023;11:e833–42. 10.1016/S2214-109X(23)00162-6. [DOI] [PubMed] [Google Scholar]
- 28. Mezei AK, Armstrong HL, Pedersen HN, et al. Cost-effectiveness of cervical cancer screening methods in low- and middle-income countries: a systematic review. Int J Cancer 2017;141:437–46. 10.1002/ijc.30695. [DOI] [PubMed] [Google Scholar]
- 29. Malone C, Barnabas RV, Buist DSM, et al. Cost-effectiveness studies of HPV self-sampling: a systematic review. Prev Med 2020;132:105953. 10.1016/j.ypmed.2019.105953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watt N, Sigfrid L, Legido-Quigley H, et al. Health systems facilitators and barriers to the integration of HIV and chronic disease services: a systematic review. Health Policy Plan 2017;32:iv13–26. 10.1093/heapol/czw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. UNAIDS . No More Neglect: Female Genital Schistosomiasis and HIV. Geneva, Switzerland: UNAIDS, 2019.
- 32. World Health Organization . Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Geneva: World Health Organization; 2020. 52 Available from: https://apps.who.int/iris/handle/10665/336583 [Google Scholar]
- 33. Sweeney S, Obure CD, Maier CB, et al. Costs and efficiency of integrating HIV/AIDS services with other health services: a systematic review of evidence and experience. Sex Transm Infect 2012;88:85–99. 10.1136/sextrans-2011-050199. [DOI] [PubMed] [Google Scholar]
- 34. Nugent R, Barnabas RV, Golovaty I, et al. Costs and cost-effectiveness of HIV/noncommunicable disease integration in Africa: from theory to practice. AIDS 2018;32:S83–92. 10.1097/QAD.0000000000001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bulstra CA, Hontelez JAC, Otto M, et al. Integrating HIV services and other health services: a systematic review and meta-analysis. PLoS Med 2021;18:e1003836. 10.1371/journal.pmed.1003836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wandwalo E, Moodie C, Haile Y, et al. Best Practices in the Integration of TB and HIV/AIDS Services Experience from Five Countries: Benin Cambodia Kenya Malawi and Rwanda. Kenya, Nairobi: The Tuberculosis Coallition for Technical Assistance, 2010 [Google Scholar]
- 37. Kalonji D, Mahomed OH. Health system challenges affecting HIV and tuberculosis integration at primary healthcare clinics in Durban, South Africa. Afr J Prim Health Care Fam Med 2019;11:1831. https://phcfm.org/index.php/phcfm/article/view/1831 (cited 21 April 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Obure CD, Sweeney S, Darsamo V, et al. The costs of delivering integrated HIV and sexual reproductive health Services in Limited Resource Settings. PLoS One 2015;10:e0124476. 10.1371/journal.pone.0124476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Obure CD, Jacobs R, Guinness L, et al. Does integration of HIV and sexual and reproductive health services improve technical efficiency in Kenya and Swaziland? An application of a two-stage semi parametric approach incorporating quality measures. Soc Sci Med 2016;151:147–56. 10.1016/j.socscimed.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Obure CD, Guinness L, Sweeney S, et al. Does integration of HIV and SRH services achieve economies of scale and scope in practice? A cost function analysis of the Integra initiative. Sex Transm Infect 2016;92:130–4. 10.1136/sextrans-2015-052039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this review.