Membrane conjugation of GABARAP sequesters the FLCN-FNIP complex to coordinate activation of MiT/TFE family transcription factors.
Abstract
Adaptive changes in lysosomal capacity are driven by the transcription factors TFEB and TFE3 in response to increased autophagic flux and endolysosomal stress, yet the molecular details of their activation are unclear. LC3 and GABARAP members of the ATG8 protein family are required for selective autophagy and sensing perturbation within the endolysosomal system. Here, we show that during the conjugation of ATG8 to single membranes (CASM), Parkin-dependent mitophagy, and Salmonella-induced xenophagy, the membrane conjugation of GABARAP, but not LC3, is required for activation of TFEB/TFE3 to control lysosomal capacity. GABARAP directly binds to a previously unidentified LC3-interacting motif (LIR) in the FLCN/FNIP tumor suppressor complex and mediates sequestration to GABARAP-conjugated membrane compartments. This disrupts FLCN/FNIP GAP function toward RagC/D, resulting in impaired substrate-specific mTOR-dependent phosphorylation of TFEB. Thus, the GABARAP-FLCN/FNIP-TFEB axis serves as a molecular sensor that coordinates lysosomal homeostasis with perturbations and cargo flux within the autophagy-lysosomal network.
INTRODUCTION
Within the cell, the proteasome and lysosome serve as degradative hubs to maintain proteostasis and cellular fitness (1). The lysosome contains hydrolytic enzymes capable of degrading the contents of autophagosomes, endosomes, and phagosomes as well as cytoplasmic material delivered directly to the lysosome through chaperone-mediated autophagy (2–9). Without a robust lysosomal compartment, adaptation to intracellular stress is limited and will result in disease (4, 10). Lysosomes also store ions, which regulate lysosomal pH, fusion with other organelles, and the host-pathogen response (11). Aside from these intralysosomal functions, the lysosomal surface provides a signaling platform for the activation of the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1), which coordinates anabolic processes in response to changes in nutrient availability (12). mTORC1 exerts effects on the lysosomal network through the regulation of the TFE/MiTF bHLH transcription factor family (13), which are master regulators of lysosomal biogenesis and autophagy (14–16). In the fed state, mTORC1 suppresses autophagosome biogenesis and the nuclear translocation and activation of TFE3/TFEB. Starvation inhibits mTORC1 and allows increased autophagosome biogenesis to be coupled with TFE3/TFEB activation, which expands the lysosomal compartment and facilitates autophagic flux. Not all autophagy-enhancing signals depend on the inhibition of mTORC1, but how lysosomal capacity is regulated in these settings is unknown.
Conjugation of ATG8 homologs (e.g., LC3 and GABARAP proteins) to double-membrane structures during autophagosome biogenesis mediates phagosome maturation and efficient delivery of cargo to the lysosome for degradation and recycling (3). ATG8 proteins can also be conjugated to single-membrane organelles within the endocytic system, but the functional consequence of this is not well understood (17). Conjugation of ATG8 to single membranes, referred to here as CASM, occurs during LC3-associated phagocytosis (LAP) (6), LC3-associated endocytosis (LANDO) (8), and upon perturbation of endolysosomal ion gradients during pathogen infection (18, 19). Recently, it was reported that ATG8 proteins are directly conjugated to the lysosomal membrane upon disruption of lysosomal homeostasis by agents such as L-leucyl-L-leucine O-methyl ester (LLOMe), oxalate crystals, and membrane-permeabilizing pathogen virulence factors (20, 21). This autophagy-independent ATG8 conjugation was required for the activation of TFEB, specifically uncoupling TFEB, but not other substrates, from regulation by mTORC1. This prompts the question of whether the commonality of ATG8 conjugation across lysosomal delivery pathways serves as a mechanism to coordinate TFE3/TFEB activation and, if so, what is the unifying mechanism.
RESULTS
TRPML1 agonists stimulate CASM
Given the complexity of using lysosomal membrane disrupting agents, we chose to acutely alter ion concentrations within the lysosome by harnessing pharmacological agonists of the lysosomal transient receptor potential mucolipin channel 1 (TRPML1). Treatment with the TRPML1 agonists MK6-83 (22), ML-SA1 (23), or a recently published, more potent channel agonist (designated as compound 8 “C8”) (24) resulted in the rapid conversion of LC3 from its cytoplasmic “I” form to the lipidated, punctate “II” form in both wild-type (WT) and autophagy-deficient cells (ATG13_KO). In contrast, the mTOR inhibitor AZD8055, a well-established agent to induce autophagosome biogenesis (25), was able to regulate LC3 lipidation in WT but not ATG13_KO cells (Fig. 1, A and B; fig. S1, A and B; and movies S1 and S2). AZD8055 or EBSS starvation induced conversion of LC3, which was sensitive to the VPS34 inhibitor PIK-III and potentiated with the vacuolater ATPase (V-ATPase) inhibitor bafilomycin A1 (BafA1) (Fig. 1C). Treatment with C8 robustly induced VPS34-independent LC3 lipidation that was inhibited by BafA1 with no impact on mTOR activity (Fig. 1C). This rapid lipidation depends on TRPML1 (fig. S2A) and is not accompanied by lysosomal alkalization or membrane damage (fig. S3, A and B). Together, these features are characteristic of CASM, where ATG8s are conjugated to endolysosomal membranes (26). Consistent with this, TRPML1 agonist treatment also induced strong colocalization of ATG8s (LC3B or GABARAPL1) with the lysosomal marker LAMP1 (Fig. 1, D to F).
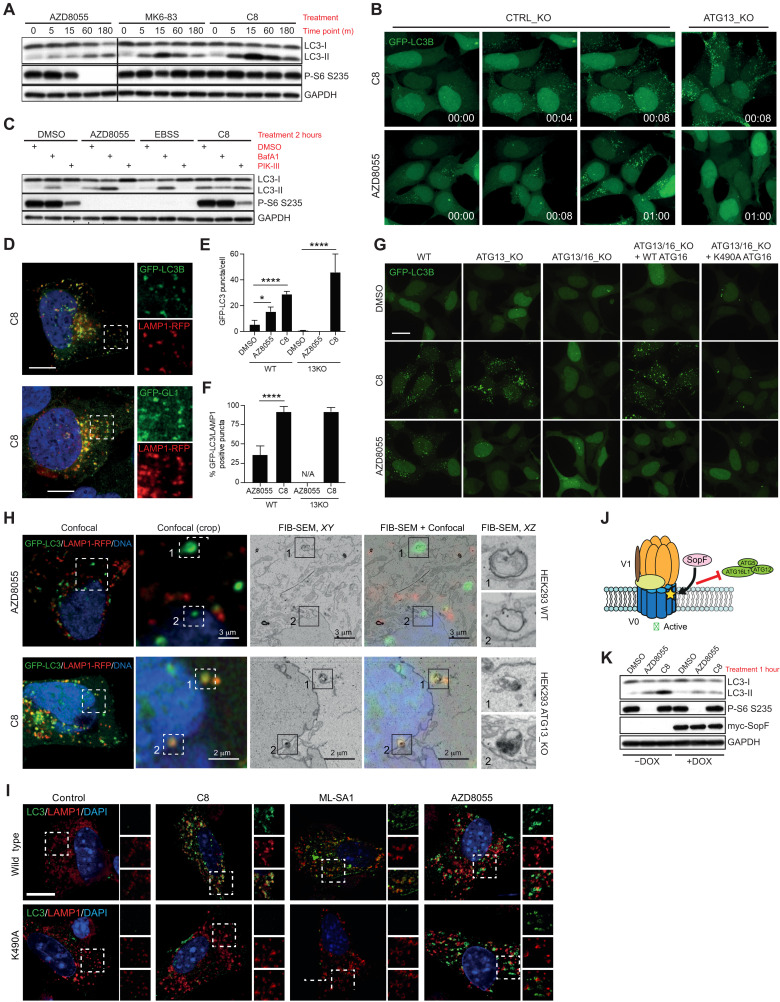
Fig. 1. Activation of the lysosomal ion channel TRPML1 results in ATG8 conjugation to the lysosomal membrane independent of autophagy.
(A) Western blot of HEK293T cells treated with the mTOR inhibitor AZD8055 (1 μM) or the TRPML1 agonists MK6-83 (25 μM) or C8 (2 μM) for the indicated time points. (B) Time-lapse imaging of GFP-LC3B in WT or ATG13_KO HEK2393T cells treated as in (A). (C) Western blot of LC3 lipidation sensitivity to either BafA1 (100 nM) or PIK-III (5 μM). DMSO, dimethyl sulfoxide. (D) Colocalization of GFP-tagged ATG8 homologs with the lysosomal marker LAMP1-RFP in C8-treated HEK293T. (E) GFP-LC3B puncta count in WT or ATG13_KO HEK293T cells. Data represent means ± SD from independent experiments. *P < 0.01 and ****P < 0.001, unpaired t test. (F) GFP-LC3B colocalization with RFP-LAMP1 in WT and ATG13_KO HEK293T cells. Data represent percentage of GFP-LC3B puncta also LAMP1 positive. Means ± SD from independent experiments. ****P < 0.001, unpaired t test. (G) Immunofluorescence analysis of GFP-LC3B puncta formation in HEK293T of the indicated genotype treated as in (A). (H) Ultrastructural CLEM, FIB-SEM analysis of GFP-LC3B HEK293T cells of the indicated genotype. CLEM representative images shown in optimal X/Y resolution. Zoom FIB-SEM images shown in X/Z plane. (I) Immunofluorescence of primary BMDMs treated with the indicated compounds for 1 hour. (J) Diagram of SopF function. (K) Western blot of LC3 lipidation in SopF-inducible (+DOX) HeLa cells upon indicated treatment.
ATG16L1-K490A is a recently found allele with a mutation in the C-terminal WD repeats of ATG16L1 required for CASM but not autophagosome formation (17). Expression of ATG16L1-K490A in autophagy-deficient cells blocked TRPML1 agonist–induced LC3 puncta formation (Fig. 1G and fig. S4). Using focused ion beam–scanning electron microscopy (FIB-SEM) correlative light and electron microscopy (CLEM), TRPML1 agonist–induced green fluorescent protein (GFP)–LC3 positive structures were identified as single-membrane late endosome/lysosomes, as opposed to the double-membrane autophagosomes in AZD8055-treated cells (Fig. 1H and movies S3 and S4).
Next, we asked whether TRPML1 activation could induce CASM in primary immune cells from a knockin ATG16L1-K490A mouse model. Treatment of ATG16L1K490A or ATG16L1WT primary bone marrow–derived macrophages (BMDMs) with AZD8055 showed similar levels of LC3 puncta, but C8 or ML-SA1 was unable to induce LC3 puncta in ATG16L1K490A-expressing cells (Fig. 1K and fig. S5). Given the sensitivity to BafA1 and requirement of the ATG16L1 C-terminal WD repeats, we explored the role of vATPase in CASM. We used the Salmonella Typhimurium SopF effector protein, which has recently been shown to block interaction between ATG16L1 and the vATPase through adenosine diphosphate (ADP)–ribosylation of the V0C subunit (19). Expression of SopF was sufficient to block LC3-II formation upon TRPML1 activation but not AZD8055 treatment (Fig. 1, I and J). Collectively, using genetic, pharmacological, and ultrastructural methodologies, these data provide strong evidence that TRPML1 activation can induce CASM directly on lysosomes.
CASM is required for TFEB activation downstream of TRPML1
The activation of TRPML1 upon nutrient starvation is known to result in the nuclear localization of the transcription factors TFEB and TFE3, in part, due to local calcium-mediated activation of the phosphatase calcineurin (CaN) to dephosphorylate TFEB/TFE3 (27). Treatment of fed cells with TRPML1 agonists resulted in TFEB nuclear accumulation, with no impact on pharmacological or genetic inhibition of CaN (fig. S6), suggesting a regulatory mechanism that differs from nutrient starvation (27). To investigate whether CASM was involved in TFEB activation, we focused first on a requirement for the vATPase. Acute treatment with BafA1 was sufficient to block TFEB activation by TRPML1 agonists; however, it did not affect TFEB activation upon mTOR inhibition (Fig. 2A). In addition, inhibition of CASM through expression of SopF blocked TFEB nuclear localization induced by TRPML1 agonist but not AZD8055 treatment (fig. S7). CRISPR-mediated knockout (KO) of the ATG8 lipidation machinery such as ATG16L1, ATG5, or ATG7 but not FIP200, ATG9A, or VPS34, which are required for autophagosome biogenesis, blocked TRPML1 agonist–induced TFEB activation (Fig. 2B). Lysosomal calcium release stimulated by C8 or the activation of TFEB upon nutrient starvation was insensitive to BafA1 treatment or ATG16L1 KO (Fig. 2C and fig. S8).
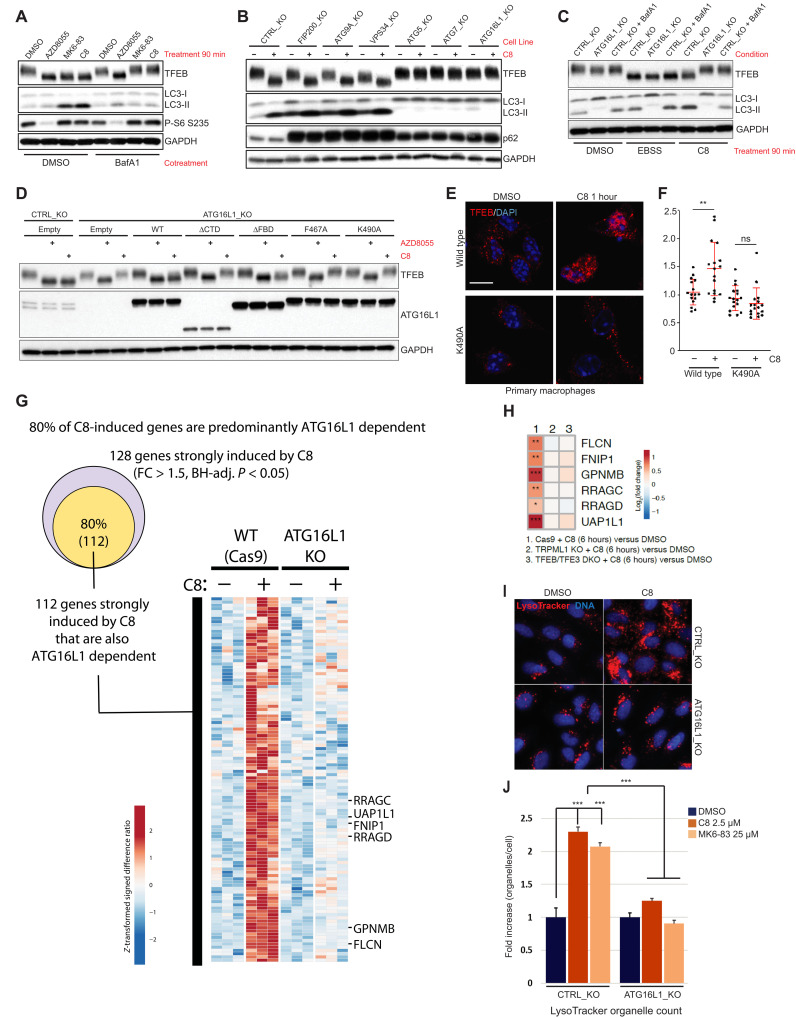
Fig. 2. ATG16L1-dependent ATG8 conjugation to single membranes is required for TFEB activation and lysosomal biogenesis upon TRPML1 activation.
(A) Western blot of TFEB phosphorylation in HeLa cells treated with the indicated compounds. (B) CRISPR KO HeLa panel treated as in (A). (C) Western blot of TFEB activation sensitivity to BafA1 (100 nM) or ATG16L1_KO upon indicated treatments. (D) Western blot of HeLa cells of the indicated genotype expressing ATG16L1 variants treated as in (A). (E) Primary BMDMs of the indicated genotype treated with 2 μM C8 and immunostained for endogenous TFEB. (F) Quantification of nuclear/cytosolic ratio of TFEB in (E). Data represent means ± SD from independent experiments. **P < 0.01, unpaired t test. ns, not significant. (G) RNA-seq profiling of genes induced by C8 compound stimulation for 24 hours in ATG16L1 KO and WT HeLa cells. The Venn diagram shows that 80% of C8-induced genes are also ATG16L1 dependent. Differentially induced genes identified by fold change (FC) > 1.5 and Benjamini and Hochberg’s (BH)–adjusted P < 0.05. (H) TFEB target gene expression upon TRPML1 agonist (C8) treatment of indicated genotype HeLa cells. RNA-seq performed in triplicates. (I) U2OS cells of the indicated genotype treated for 24 hours and stained with LysoTracker dye. Representative images are shown. (J) Quantification of LysoTracker organelle count from (I). Fold change organelles per cell ± SD from independent experiments. ***P < 0.005, unpaired t test.
Consistent with modulation of CASM by SopF expression, ATG16L1_KO cells reexpressing WT or an autophagy-deficient ATG16L1 FIP200 binding mutant (FBD) supported TRPML1-induced TFEB activation, while reexpression of a C-terminal domain truncation (ΔCTD) or F467A or K490A mutations deficient for CASM (17) did not (Fig. 2D and fig. S9). In contrast, AZD8055 activated TFEB irrespective of ATG16L1 allele status. Furthermore, treatment of ATG16L1K490A or ATG16L1WT primary BMDMs revealed that TRPML1-mediated TFEB activation was abolished in ATG16L1K490A-expressing cells (Fig. 2, E and F), further confirming the presence of a novel CASM-regulated pathway that induces TFEB activity in diverse cell types. These data suggest that ATG8 conjugation to single-membrane organelles can promote activation of the TFEB/TFE3 transcription factors through a vATPase-dependent, ATG8-dependent mechanism, which is distinct to the regulation of TFEB in the context of nutrient starvation or mTOR inhibition.
Upon nuclear localization, TFEB serves as the primary transcription factor responsible for lysosomal biogenesis (14). The TRPML1-dependent transcriptomic response was largely dependent on ATG16L1 and included numerous TFEB target genes involved in lysosomal function (Fig. 2, G and H, and fig. S10). Consistent with this profile, we found that TRPML1 activation by treatment with C8 for 24 hours increased the number of LysoTracker-positive organelles in an ATG16L1-dependent manner (Fig. 2, I and J). Together, these observations demonstrate that, following changes in lysosomal ion balance, the WD40 domain in ATG16L1 regulates lysosomal CASM and that this is required for TFEB activation and lysosomal biogenesis.
GABARAPs selectively bind and sequester the FLCN-FNIP tumor suppressor complex to lysosomes
Mammalian ATG8 homologs consist of three members of the MAP1LC3 family (LC3A/B/C) and three members of the GABA type A receptor–associated protein family (GABARAP/L1/L2) (28). Using a combinatorial CRISPR KO approach, the GABARAP subfamily was found to be essential for the TRPML1-mediated activation of TFEB (Fig. 3A). In agreement with protein interaction databases linking GABARAP, but not LC3 proteins, with the TFEB regulators FLCN and FNIP1/2 (29–31), we confirmed that the FLCN-FNIP1 complex interacts with GABARAP through LIR-dependent binding (Fig. 3, B to D, and fig. S11). The connection between GABARAP and the FLCN/FNIP complex was intriguing given the role that the FLCN/FNIP substrate RagC/D plays in regulating TFEB (32).
Fig. 3. GABARAP is required for TFEB activation and FLCN-FNIP sequestration upon acute TRPML1 stimulation.
(A) CRISPR KO of ATG8 homologs in HeLa cells. RAP_TKO = GABARAP, GABARAPL1, and GABARAPL2 KO. LC3_TKO = LC3A, LC3B, and LC3C KO. Western blot of cells treated with TRPML1 agonist. (B) Immunoprecipitation of HEK293T cells transfected with the indicated constructs for 20 hours. (C) Immunoprecipitation of HEK293T cells transfected with the indicated constructs for 20 hours. LIR binding motif (LBM) mutant = K39Q/Y40H/Q74E/F75L. (D) GABARAP_MBP complexes with FLCN/FNIP2 over Superose 6 column. Overlay of individual and complexed chromatograms. (E) GABARAP binds FLCN/FNIP2 with picomolar affinity (300 pM Kd) in single cycle SPR, while LC3B does not bind to FLCN/FNIP2. (F and G) GABARAP and (I and J) LC3B bind to the FIR domain of p62 with equivalent affinity (700 nM Kd) in multicycle SPR. (H) GABARAP binds FLCN/FNIP2 with picomolar affinity in multicycle SPR. (K) Immunofluorescence of U2OS cells of the indicated genotype treated with TRPML1 agonist for 20 min. (L) Western blot analysis of membrane fractions from indicated HeLa cells after treatment with TRPML1 agonist. (M) U2OS cells of the indicated genotype stably expressing 3×HA-TMEM192. LysoIP was performed after treatment with C8 (2 μM, 15 min). (N) Western blot of FLCN-FNIP1 membrane recruitment upon TRPML1 agonist treatment in LAMTOR1_KO cells.
We next determined the binding affinity of the interaction between full-length GABARAP and FLCN/FNIP2 by surface plasmon resonance (SPR). GABARAP bound to immobilized FLCN/FNIP2 with a picomolar affinity in single cycle [300 ± 18 pM dissociation constant (Kd)] and multicycle kinetic SPR formats (71 ± 10 pM Kd), whereas LC3B did not bind to FLCN/FNIP2 under identical assay conditions (Fig. 3, E and F). To confirm the functionality of the full-length LC3B, we used multicycle SPR to measure the affinity of GABARAP and LC3B to immobilized p62 FIR (FIP200-interacting region) domain. Both GABARAP (670 ± 80 nM Kd) and LC3B (820 ± 40 nM Kd) bound p62 with nanomolar affinities in steady-state SPR measurements (Fig. 3, G to J), which is consistent with previous reports (33). Together, the specific binding of GABARAP to FLCN/FNIP2, with an unusually high affinity for an ATG8-binding event, is consistent with the important role GABARAP plays in regulating TFEB activation described above.
We hypothesized that direct conjugation of GABARAPs to the lysosomal membrane could redistribute the FLCN/FNIP complex. Following TRPML1 activation, there was a rapid and robust FLCN colocalization with the lysosomal marker LAMP1 (Fig. 3K). We also observed a time-sensitive increase in membrane-associated FLCN and FNIP1, and this was dependent on the GABARAP proteins and vATPase function (Fig. 3L and fig. S12), which was further confirmed on TMEM192 immuno-isolated lysosomes (Fig. 3M). Lysosomal localization of FLCN also occurs upon nutrient starvation, where FLCN specifically binds to RagAGDP and forms the inhibitory lysosomal folliculin complex (LFC) (34, 35). However, in cells deficient for the Ragulator complex component LAMTOR1, which is part of the LFC, TRPML1 activation promoted FLCN membrane recruitment (Fig. 3N). In addition, using NPRL2_KO cells that have constitutive RagA/BGTP and defective lysosomal localization of FLCN upon starvation (36), we found that the FLCN-FNIP1 complex distributed to membranes following TRPML1 activation (fig. S13). These data indicate that the GABARAP-dependent sequestration of FLCN/FNIP1 is a process distinct from LFC formation.
We reasoned that GABARAP-dependent recruitment of FLCN to the lysosome could inhibit its guanosine triphosphatase (GTPase) activating protein (GAP) activity toward RagC/RagD, the heterodimeric partner of RagA/B, analogous to how lysosomal recruitment inhibits FLCN-FNIP GAP activity during LFC formation (34, 35). In this model, FLCN-FNIP1 would normally exert its GAP function away from the lysosomal surface, thus promoting cytosolic RagC/DGDP and subsequent TFEB cytosolic retention (32, 37). RagGTPase dimers have been shown to interact dynamically with the lysosome under fed conditions (38, 39). Using NPRL2_KO cells mentioned above, additional KO of FLCN resulted in complete nuclear localization of TFEB under nutrient-rich conditions in both WT and LFC-deficient NPRL2_KO cells, supporting a model where FLCN-FNIP1 GAP activity toward RagC/D can occur outside the context of the LFC (fig. S14). In two complementary approaches, we artificially tethered FLCN to either the lysosomal surface (lyso-FLCN, see Materials and Methods) or the plasma membrane (PM-FLCN) and found this sufficient to activate TFEB and TFE3 in full nutrient conditions without affecting mTOR signaling to S6K1 (figs. S15 and S16). In addition, we found that, in contrast to nutrient starvation, TRPML1 agonist treatment retained the ability to activate TFEB in NPRL2_KO cells, suggesting intact FLCN GAP activity toward RagGTPases (fig. S17). However, expression of RagGTPases locked in the active state (RagBQ99L/RagDS77L), which are no longer regulated by FLCN-FNIP1, suppressed the mobility shift of TFEB and its homolog TFE3 following TRPML1 activation but not with AZD8055 (fig. S17). Collectively, these data support a model in which the redistribution of the FLCN-FNIP1 complex to the lysosomal membrane can regulate the nucleotide binding state of RagC/D, thus resulting in a novel mechanism for TFEB activation.
A novel LIR domain in FNIP1/2 mediates high-affinity GABARAP interaction
Carboxyl group footprinting was used as an unbiased approach to identify the molecular interface between GABARAP and the FLCN-FNIP complex (40, 41). This method also leverages the observation that aspartate and/or glutamate residues are commonly observed upstream of LIR motifs (33, 42). Analysis of covalent modification by liquid chromatography–mass spectrometry (LC-MS) revealed three FNIP2 peptides showing the most notable protection (553 to 559, 560 to 573, and 564 to 573), which span a 21-residue segment containing a single LIR motif (YVVI) at positions 567 to 570 (Fig. 4B and table S5). Additional peptides with a lower level of protection were observed on FLCN (283 to 290 and 275 to 290) and FNIP2 (286 to 295 and 331 to 346) (table S4); however, these lacked an obvious LIR motif and may reflect potential conformational rearrangements upon complex formation. The putative FNIP2 LIR motif mapped to a >300-residue disordered loop within FLCN/FNIP2 from the LFC complex that was recently solved by cryo–electron microscopy (cryo-EM) (34, 35). This loop sits within the C-shaped FLCN/FNIP2 cradle at a region distal to the RagA/RagC binding interface (Fig. 4C). Mutation of the orthologous LIR sequence in FNIP1 from YVLV to AVLA completely blocked GABARAP interaction with the FLCN-FNIP1 complex, whereas the FNIP1 LIR mutation did not affect its association with FLCN (Fig. 4D and fig. S18).
Fig. 4. GABARAP binds the FLCN-FNIP complex through a novel LIR motif-driven interface.
(A) Overview of chemical footprinting assay. GEE and EDC label carboxyl groups of Asp/Glu residues. (B) Significant protection observed for three overlapping peptides in FNIP2. (C) Location of putative LIR domain within reported FLCN-FNIP2 cryo-EM structure. GABARAP binds a region located in an unresolved disordered loop, distinct from the RagGTPase binding interface (purple). (D) Identified LIR domain is required for GABARAP-FNIP1 interaction. Immunoprecipitation of HEK293T cells transfected with the indicated constructs for 20 hours. LIR mutation = Y583A/V586A. (E) Crystal structure of FNIP2-GABARAP fusion protein. HP1 pocket shaded in green, and HP2 shaded in purple. FNIP2 LIR motif forms a β-sheet hairpin structure, with added interactions within the context of the hairpin N terminus to the core LIR motif. (F) Representation of similarity between GABARAP and LC3B in the LIR docking site. (G) Competition SPR of immobilized FLCN/FNIP2 and GABARAP in solution with FLCN/FNIP1 protein and FNIP2 peptide competitors highlights the essential role of the LIR motif in driving the initial GABARAP-FLCN/FNIP2 interaction. Regions outside of the LIR result in stabilization and strengthening of the interaction. (H) Molecular interactions within the FNIP2 hairpin outside the core LIR motif. Key residues are underlined. (I) Sequence divergence of key underlined residues in LC3B.
We determined the crystal structure of GABARAP in complex with residues 558 to 576 of FNIP2 at 1.8 Å (fig. S19 and tables S1 to S3). Tyr567 and Ile57 comprise the x0 and x3 positions of the four-residue LIR motif, which occupy the canonical hydrophobic pockets 1 and 2, respectively, on GABARAP (Fig. 4E). Published costructures of ATG8 family members often reveal additional interactions upstream and/or downstream of the LIR motif, which appear to contribute to affinity and selectivity. On the C-terminal side of the LIR, the GABARAP/FNIP2 costructure contains a hydrogen bond between Thr571 (x4) and Arg28GAB, but no further downstream contacts in contrast to other reports showing interactions up to (x10) (33). The lack of interaction on the C-terminal side is not unexpected given the lack of sequence conservation between FNIP1 and FNIP2 between x5 and x10 (fig. S20).
On the N-terminal side of the LIR, FNIP2 forms a β-hairpin loop that contributes a number of side chain–mediated interactions (Fig. 4E and fig. S19). In contrast to the C-terminal side, the N-terminal region is strongly conserved between FNIP1 and FNIP2. Glu558 (x−9) and Glu564 (x−3), which were protected by glycine ethyl ester (GEE) labeling (fig. S21), form hydrogen bonds and salt bridges. Glu558 forms a bidentate engagement with Lys24GAB and Gln25GAB, while Glu564, in combination with Ser565 (x−3), participate in a hydrogen bonding network that includes Tyr5GAB, Glu17GAB, and Lys48GAB (Fig. 4E). Val562 occupies a shallow cleft that involves the hydrophobic portion of a trio of aliphatic residues.
To confirm the importance of the molecular interactions outside the core LIR motif, we developed a competition SPR assay using isolated FNIP2 peptides. A peptide spanning the LIR motif in FNIP2 (amino acids 558 to 576) was able to fully compete interaction of GABARAP with FLCN/FNIP2 (Fig. 4G). However, we noted a 104 lower affinity of our FNIP2 LIR peptide for GABARAP (6.2 ± 3 μM) than full-length FLCN/FNIP2. N-terminal extension of the FNIP2 peptide to incorporate the full stabilized hairpin structure (amino acids 550 to 576) showed markedly increased affinity for GABARAP (29 ± 2 nM Kd) in competition SPR. Mutation of the candidate LIR sequence in the elongated peptide blocked competition with the GABARAP and FLCN/FNIP2, confirming the role of the LIR motif in driving the interaction between GABARAP and FLCN/FNIP2 (Fig. 4G).
It remains unclear how selectivity is established within the GABARAP and LC3B branches of the ATG8 family, as universal rules have not yet been established for interaction partners. A comparison of LC3B and GABARAP shows that four of the five residues that form side chain:side chain–mediated interactions in the x-ray structure are not conserved between LC3B and GABARAP (Fig. 4, H and I). These amino acid differences may contribute to the observed affinity differences of LC3B and GABARAP for the FLCN/FNIP complex (Fig. 3E).
GABARAP sequestration of FLCN-FNIP complex to membranes is required for TFEB activation during CASM and selective autophagy
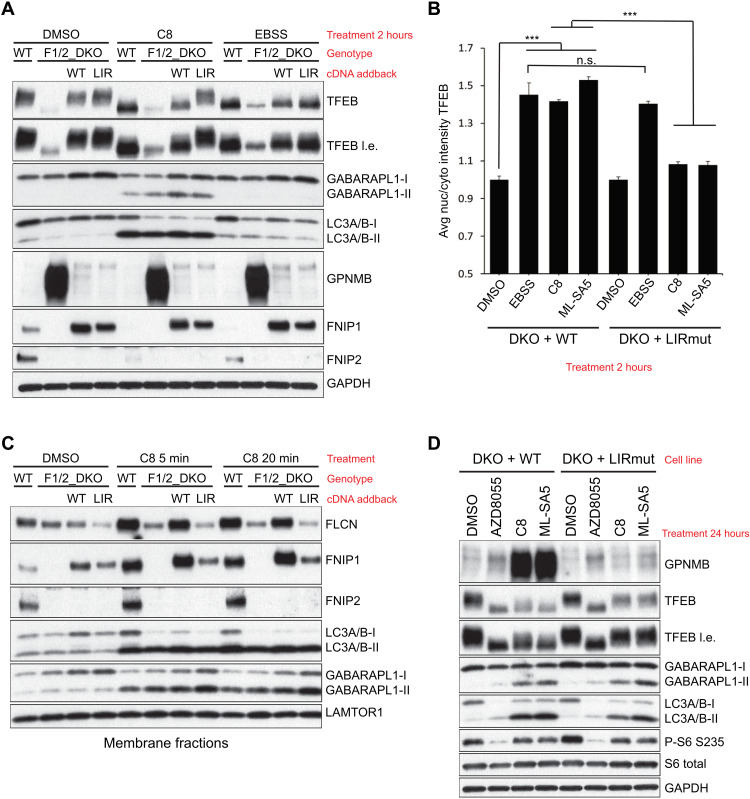
To establish the functional requirement of the FNIP LIR domain, we reconstituted FNIP1/2 double KO cells with WT or LIR-mutant (LIR) FNIP1. Both FNIP1-WT and FNIP1-LIR were able to rescue the constitutive TFEB activation in FNIP1/2_DKO cells, as evidenced by suppression of GPNMB protein levels, a robust transcriptional target of the MiT/TFE family selected from our RNA sequencing (RNA-seq) profiling in Fig. 2G (Fig. 5A). TFEB activation upon acute TRPML1 stimulation was blocked specifically in cells expressing FNIP1-LIR, whereas TFEB activation in response to nutrient starvation was not affected by FNIP1-LIR (Fig. 5, A and B). Upon chronic treatment with the TRPML1 agonist, the functional TFEB transcriptional response can be measured by GPNMB protein levels. GPNMB expression was completely blocked in FNIP1-LIR mutant cells (Fig. 5C). GPNMB protein levels were also largely suppressed upon AZD8055 treatment despite robust TFEB activation. This highlights how concurrent inhibition of protein translation may minimize the effective scope of the TFEB transcriptional activation (Fig. 5C). ATG8 conjugation was not altered by modulation of FNIP1 (Fig. 5, A and C). Membrane sequestration of the FLCN-FNIP complex also required the identified FNIP1-LIR domain, confirming our hypothesis that GABARAP binding to the FLCN-FNIP complex is responsible for its relocalization (Fig. 5D). Other inducers of CASM, such as the ionophore monensin, could induce FLCN-FNIP1 complex sequestration independently of TRPML1 (fig. S22A) and regulated TFEB activation in an FNIP1-LIR domain–dependent manner (fig. S22, B and C). This suggests that perturbation of lysosomal ion homeostasis, rather than TRPML1 activation specifically, serves as a trigger for GABARAP-dependent FLCN-FNIP relocalization.
Fig. 5. GABARAP-dependent sequestration of FLCN-FNIP complex is required to activate TFEB upon disruption of endolysosomal ion balance.
(A) Reconstitution of FNIP1/2 double KO (DKO) cells with either WT or LIR (LIR-mutant Y583A/V586A) FNIP1 reveals functional requirement of GABARAP interaction for TRPML1 agonist, but not EBSS, activation of TFEB. Western blot analysis of FNIP1 allele series treated with the indicated stimuli. l.e., long exposure. (B) Quantification of TFEB nuclear localization in WT or LIR expressing FNIP1/2_DKO HeLa cells treated with the indicated stimuli. Analysis was performed using high-content imaging. Means ± SD representative of independent experiments. Minimum of 1500 cells quantified per condition. C8 = 2 μM. ML-SA5 = 1 μM. ***P < 0.005, unpaired t test. (C) Functional TFEB response upon prolonged TRPML1 activation requires FNIP1 LIR domain. Western blot analysis of WT or LIR expressing FNIP1/2_DKO HeLa cells treated with the indicated stimuli. GPNMB is a validated TFEB/TFE3 transcriptional target. (D) Western blot analysis of membrane fractions from FNIP1 allele series after acute treatment with TRPML1 agonist for the indicated time points.
It is intriguing to consider that any instance where GABARAP proteins are conjugated to subcellular membranes might result in TFEB activation via the high-affinity sequestration of FLCN/FNIP. Thus, we examined distinct forms of selective autophagy, mitophagy, and xenophagy. It has been shown that TFEB activation occurs during parkin-dependent mitophagy (43), and we found that this required GABARAP proteins (Fig. 6, A and B). Furthermore, TFEB activation was defective in cells stably expressing LIR-mutant FNIP1, confirming that GABARAP-dependent relocalization of FLCN mechanistically links TFEB activation to mitophagy (Fig. 6C). An earlier study observed that FLCN and FNIP could localize to mitochondria upon depolarization (44), and the TFEB activation mechanism revealed in the current study explains the relevance of this.
Fig. 6. GABARAP regulates TFEB activation through FLCN relocalization during selective autophagy.
(A) HeLa.Cas9 or HeLa.Cas9 + Parkin cells treated with the indicated compounds for 4 hours and analyzed by immunofluorescence for TFEB. Data represent means ± SD from independent experiments. ***P < 0.005, unpaired t test. (B) Western blot of HeLa cells expressing Parkin and CRISPR KO for the indicated ATG8 family members treated with 0.78 μM valinomycin for 24 hours. (C) Cells of the indicated genotype treated with mitophagy inducers for 24 hours. (D) Western blot of TFEB mobility shift upon challenge with WT or ΔsopF Salmonella. HeLa cells infected for 30 min with the indicated strain. (E) Immunofluorescence of nuclear TFEB upon infection with Salmonella of the indicated genotype for 2 hours. (F) Quantification of TFEB nuclear localization. Minimum of 100 cells quantified per condition. ****P < 0.001, unpaired t test. (G) Cells of the indicated genotype infected with ΔsopF Salmonella and analyzed by immunofluorescence at 2 hours post-infection (h.p.i.). (H) Quantification of TFEB nuclear localization. A minimum of 100 cells were quantified per condition. ****P < 0.001, unpaired t test. (I) Analysis of TFEB transcriptional activity in cells of the indicated genotype at 10 h.p.i. with ΔsopF Salmonella. GPNMB and RRAGD represent TFEB target genes. (J) Immunofluorescence analysis of FLCN recruitment to Salmonella vacuoles.
Last, we used a Salmonella infection model of xenophagy to determine if TFEB activation was regulated by GABARAP-mediated FLCN sequestration. A portion of Salmonella enterica serovar Typhimurium (S. Typhimurium) are rapidly targeted by the autophagy machinery and become decorated with ATG8 homologs (45). It was recently found that S. Typhimurium antagonizes the ATG8 response through the bacterial effector SopF (19). We hypothesized that if ATG8 proteins were involved in TFEB activation, then ΔsopF S. Typhimurium would show a greater TFEB activation than WT due to increased ATG8 conjugation. ΔsopF S. Typhimurium produced a robust activation of TFEB that occurred in a higher percentage of cells for a longer duration of time after infection (Fig. 6, G to I). TFEB activation was blunted by deletion of GABARAP family members (RAP_TKO) but was not affected by KO of LC3 isoforms (LC3_TKO) (Fig. 6, J to L). We next examined the localization of FLCN and found a notable relocalization of FLCN to coat the S. Typhimurium Salmonella-containing vacuole membrane (Fig. 6M). This relocalization required GABARAP proteins, indicating that GABARAP-dependent sequestration of FLCN to the Salmonella vacuoles results in TFEB activation upon infection (Fig. 6M). Together, the mitophagy and xenophagy examples of selective autophagy highlight that GABARAP-dependent sequestration of FLCN to distinct cellular membranes may serve as a universal mechanism to couple activation of TFE3/TFEB transcription factors to the initiation of selective autophagy (Fig. 7).
Fig. 7. Model.
GABARAP-dependent membrane sequestration of the FLCN-FNIP complex represents a TFEB activation paradigm distinct from nutrient starvation. The FLCN-FNIP GAP complex critically regulates the mTOR-dependent phosphorylation and cytosolic retention of the TFEB/TFE3 transcription factors by promoting the GDP-bound state of RagC/D. GDP-bound RagC/D directly binds to and presents TFEB/TFE3 as a substrate to mTOR (center inset), as described previously. During nutrient starvation (A), recruitment of FLCN-FNIP to the lysosomal membrane helps form the LFC, which has reduced GAP activity toward RagC/D. This is coincident with mTORC1 inhibition. Independently of LFC formation, GABARAP proteins bind directly to the FLCN-FNIP complex and sequester it at diverse intracellular membranes (B). This membrane recruitment is required for TFEB activation in response to endolysosomal ion disruption (CASM) and forms of selective autophagy (xenophagy and mitophagy). This suggests that FLCN-FNIP regulates cytosolic RagC-GTP (guanosine triphosphate) and its sequestration on intracellular membranes reduces access to this substrate, allowing nuclear retention of TFEB/TFE3 due to impaired Rag binding. Unlike (A), this novel TFEB activation pathway is permissive with mTORC1 activity. Subcellular redistribution of the FLCN-FNIP complex to both single and double membranes serves to broadly coordinate lysosomal capacity with homeostasis and perturbations within the endolysosomal network.
DISCUSSION
We describe a previously unrecognized molecular mechanism that explains how lysosomal biogenesis is coupled to the onset of different forms of autophagy. Changes in endolysosomal ion levels and induction of CASM helped uncover a novel regulatory input to TFEB nuclear localization that is independent of nutrient status and requires the ATG5-ATG12-ATG16L1 conjugation machinery. GABARAP-dependent membrane sequestration of the FLCN-FNIP complex uncouples its regulation of RagC/D, revealing a new paradigm for TFEB activation distinct from LFC formation during nutrient starvation. Moreover, GABARAP-dependent TFEB activation is permissive with mTORC1 activity, offering new insights into strategies to enhance lysosomal biogenesis. The requirement of the GABARAP-FLCN-FNIP axis for TFEB activation during mitophagy and xenophagy suggests that this newly identified mechanism can broadly serve to coordinate lysosomal capacity (see model, fig. S20). Whether conjugation of GABARAP and sequestration of FLCN-FNIP to the forming autophagosome occurs and functions as a stage gate ensuring tight coordination of macroautophagy with lysosomal capacity requires further investigation.
Germline mutations in FLCN underlie the FLCN-FNIP complex loss-of-function phenotype and TFEB dependency in Birt-Hogg-Dubé syndrome (37, 46), a rare disorder that predisposes patients to kidney tumors. Ras-driven pancreatic adenocarcinoma cells (PDACs) show constitutive nuclear localization of TFEB/TFE3 and notable colocalization of LC3 with LAMP2-positive lysosomes (47, 48). Moreover, elevated activity and permeability of lysosomes has been noted in PDACs (48), and our study suggests a mechanism to explain TFEB nuclear localization despite nutrient-replete and mTOR-active conditions (47). Understanding the involvement of FLCN-FNIP membrane sequestration in this setting and whether oncogenic signals might take advantage of this mechanism to drive TFEB-dependent tumor growth may offer new therapeutic opportunities for lysosome-dependent tumors.
Linking TFEB activation to GABARAP membrane conjugation allows sensitive detection of dysfunction within the endolysosomal pathway, which is relevant for the host-pathogen response. Pathogens have evolved virulence factors to inhibit and evade CASM, for example, SopF of S. Typhimurium (19), CpsA of Mycobacterium tuberculosis (49), and RavZ of Legionella (50). Recently, it has been proposed that disruption of the phagosomal ion gradient triggered ATG8 modification of the ΔsopF S. Typhimurium–containing vacuole and that this precedes vacuole rupture and xenophagy (19). Here, induction of CASM could serve to couple TFEB-dependent transcription of cytoprotective/antimicrobial genes (51) and lysosomal biogenesis to limit pathogen infection. While CASM induction does result in the conjugation of both LC3 and GABARAP homologs to target membranes, our study highlights that different ATG8s serve distinct functions. While LC3 regulates vesicle maturation and fusion with lysosomes (52, 53), the primary role of GABARAP is likely to coordinate lysosomal capacity.
Our data highlight the importance of the RagC nucleotide state in the regulation of TFEB by mTOR (37). mTOR has been shown to mediate the nuclear export of TFEB/TFE3 transcription factors, with phosphorylation promoting cytosolic retention (54). In the absence of FLCN-FNIP activity, the regulation of nuclear export is impaired due to a lack of mTOR access to TFEB/TFE3 as substrates, resulting in nuclear retention rather than active translocation of these transcription factors (37). The GABARAP-dependent sequestration of FLCN-FNIP represents a new paradigm for the control of the RagC/D nucleotide state, previously thought to solely be regulated by nutrient levels. The contribution of this new mechanism to both the basal and induced adaptive TFEB/TFE3 responses will be interesting to test in future work. Mice expressing a C-terminal ATG16L1 truncation, thus defective in CASM, show an Alzheimer’s disease (AD) phenotype (55), and deficiencies in lysosomal biogenesis/homeostasis are well characterized in AD and other neurodegenerative disorders (56). Further understanding the regulation of this GABARAP-dependent sequestration of FLCN-FNIP and whether a homeostatic defect in TFEB/TFE3 activation contributes to neurodegeneration in vivo will provide additional insights into therapeutic opportunities.
MATERIALS AND METHODS
Antibodies
Antibodies used in this study were as follows: ATG16L1 (8089, human), phospho-ATG14 S29 (92340), ATG14 (96752), phospho-Beclin S30 (54101), FIP200 (12436), FLCN (3697), GABARAPL1 (26632), GABARAPL2 (14256), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [5174, 1:10,000 for WB (Western blot)], DYKDDDDK tag (14793), HA tag (3724), myc tag (2278), LC3A/B (12741), LC3B (3868), LAMTOR1 (8975), LAMP1 [15665, 1:1000 for immunofluorescence (IF)], NFAT1 (5861, 1:250 for IF), NPRL2 (37344), phospho-S6K (9234), S6K (2708), phospho-S6 S235/236 (4858, 1:3000 for WB), S6 (2217, 1:5000 for WB), TAX1BP1 (5105), TFEB (4240), TFEB (37785, 1:200 for IF), and phospho-ULK S757 (14202) were from Cell Signaling Technology. Mouse monoclonal anti–S. Typhimurium lipopolysaccharide (clone 1E6, ab8274) and FNIP1 (ab134969) were from Abcam. TFE3 (HPA023881) was from MilliporeSigma. p62 (GP62-C) was from Progen. Galectin-3 (sc-23938) was from Santa Cruz Biotechnology. TFEB (A303-673A, 1:200 for IF in murine cells) was from Bethyl Laboratories. All antibodies were used at a 1:1000 dilution for Western blotting unless otherwise noted.
Generation of KO cell lines with CRISPR-Cas9
HeLa or U2OS cells were made to stably express Cas9 through lentiviral transduction (vector catalog no. SVC9-PS-Hygro, Cellecta). KO cell lines were generated as pooled populations following subsequent lentiviral transduction with guide RNA (gRNA) sequences as indicated (vector catalog no. SVCRU6UP-L, Cellecta). Pooled populations were selected for 3 days with puromycin (2 μg/ml, Life Technologies) and used for experiments 7 to 9 days after transduction with gRNA. Clones were isolated for ATG16L1_KO to use for reconstitution experiments. gRNA sequences were as follows: sgCTRL, 5′-GTAGCGAACGTGTCCGGCGT-3′; sgRB1CC1 (FIP200), 5′-CAGGTGCTGGTGGTCAATGG-3′; sgATG9A, 5′-TCTGGAAACGGAGGATGCGG-3′; sgPIK3C3 (VPS34), 5′-CATACACATCCCATATGGTG-3′; sgATG5, 5′-GCTTCAATTGCATCCTTAGA-3′; sgATG7, 5′-TCCGTGACCGTACCATGCAG-3′; sgATG16L1, 5′-GCCACATCTCGGAGCAACTG-3′; sgLAMTOR1, 5′-GCTGCTGTAGCAGCACCCCA-3′; sgNPRL2, 5′-GATGCGGCAGCCGCTGCCCA-3′; sgMAP1LC3A (LC3A), 5′-GTCAAGCAGCGGCGGAGCTT-3′; sgMAP1LC3B (LC3B), 5′-GCAGCATCCAACCAAAATCC-3′; sgMAP1LC3C (LC3C), 5′-GCTTGAAGGGTCTGACGCTT-3′; sgGABARAP, 5′-GGATCTTCTCGCCCTCAGAG-3′; sgGABARAPL1, 5′-CATGAAGTTCCAGTACAAGG-3′; sgGABARAPL2, 5′-TTCCCGCCGCCGCCATGAAG-3′; sgFLCN, 5′-TCACGCCATTCCTACACCAG-3′; sgFNIP1, 5′-TCTGGCTTACAATGATGTCG-3′; sgFNIP2, 5′-CCAGTTGATATGCCAAGCAG-3′.
cDNA expression constructs
WT and K490A mutant mouse ATG16L1 were cloned into pBabe-Puro-Flag-S-tag plasmids as previously described (17). pBabe-Blast-GFP-LC3A has been previously described (5).
The following constructs were generated for use in this study:
| Insert | Epitope tag | Terminus | Expression vector |
| Human FNIP1 WT | 3× HA | N | pcDNA-DEST40 |
| Human FLCN WT | FLAG | C | pCDNA-DEST40 Tet Lenti |
| Human lyso-FLCN (Nterm 39aa of LAMTOR1 fusion) |
FLAG | C | Tet Lenti |
| Human GABARAP WT | myc | N | pcDNA-DEST40 |
| Human GABARAP LBMmut | myc | N | pcDNA-DEST40 |
| Human ATG16L1 WT | FLAG | N | Tet Lenti |
| Human ATG16L1 ΔCTD | FLAG | N | Tet Lenti |
| Human ATG16L1 ΔFBD | FLAG | N | Tet Lenti |
| Human ATG16L1 F467A | FLAG | N | Tet Lenti |
| Human ATG16L1 K490A | FLAG | N | Tet Lenti |
| S. Typhimurium SopF | myc | N | Tet-Lenti |
| Human LAMP1 | RFP | C | pBabe |
| Human FNIP1 WT | 3×HA | N | Tet Lenti |
| Human FNIP1 Y583A/V586A | 3×HA | N | Tet Lenti |
| Human TMEM192 | 3×HA | C | Tet Lenti |
Complementary DNA (cDNA) constructs with the indicated epitope tags were synthesized (GenScript, USA) and provided as entry clones. Gateway recombination was used to shuttle cassettes into pcDNA-DEST40 (Life Technologies) or a lentiviral vector allowing tetracycline-inducible expression referred to as Tet-Lenti (synthesized by GenScript, USA).
Cell culture
Cell lines used in this study were U2OS, HeLa, and RAW264.7 and were obtained from the American Type Culture Collection (ATCC). Human embryonic kidney (HEK) 293FT cells were obtained from Thermo Fisher Scientific. Cell lines were verified to be mycoplasma-free by routine testing. All cells were cultured in a humidified incubator at 37°C and 5% CO2. Cell culture reagents were obtained from Invitrogen unless otherwise specified. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
Reagents
BafA1, PIK-III, and AZD8055 were purchased from Selleckchem. ML-SA1 and MK6-83 were purchased from Tocris. Monensin, nigericin, salinomycin, valinomycin, and LLoMe were purchased from Sigma-Aldrich. C8 is available for purchase through ChemShuttle (catalog no. 187417).
Viral production and transduction
For lentiviral production of CRISPR gRNA or Cas9 virus and cDNA overexpression virus, 8 × 105 293FT cells were plated in six-well plates. The next day, cells were transfected with lentiviral packaging mix (1 μg of psPAX2 and 0.25 μg of VSV-G) along with 1.5 μg of the lentiviral backbone using Lipofectamine 2000 (Thermo Fisher Scientific). Supernatant was removed from 293FT cells after 48 hours, centrifuged at 2000 rpm for 5 min, and then syringe-filtered using a 0.45-μm filter (Millipore). Polybrene was then added to a final concentration of 8 μg/ml, and target cells were infected overnight. Cells were then allowed to recover for 24 hours in DMEM/10% FBS before being selected with neomycin (1 mg/ml; G418:geneticin, Thermo Fisher Scientific), puromycin (2 μg/ml; Thermo Fisher Scientific), or hygromycin B (500 μg/ml; Thermo Fisher Scientific) for 72 hours.
Retroviral infection was performed using centrifugation. Stable populations were selected with puromycin (2 mg/ml) or blasticidin (10 mg/ml) for 3 to 5 days.
Cell lysis and Western blotting
For preparation of total cell lysates, cells were lysed in radioimmunoprecipitation assay buffer (no. 9806, Cell Signaling Technology) supplemented with SDS (Boston BioProducts) to 1% final concentration and protease inhibitor tablets (cOmplete EDTA-free, Roche). Lysates were homogenized by sequential passaging through QIAshredder columns (Qiagen), and protein levels were quantified by Lowry DC protein assay (Bio-Rad). Proteins were denatured in 6× Laemmli SDS loading buffer (Boston BioProducts) at 100°C for 5 min.
For preparation of membrane fractions, 1.5 × 106 cells were plated the day before in 6-cm tissue culture dishes (BD Falcon). Cellular fractions were prepared using the Mem-PER kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Protein levels were quantified by Lowry DC protein assay (Bio-Rad) and denatured in 6× Laemmli SDS loading buffer (Boston BioProducts).
For Western blotting, equivalent amounts of total proteins were separated on Tris-Glycine TGx SDS–polyacrylamide gel electrophoresis (PAGE) gels (Bio-Rad). Proteins were transferred to nitrocellulose using standard methods, and membranes were blocked in 5% nonfat dry milk (Cell Signaling Technology) in tris-buffered saline (TBS) with 0.2% Tween 20 (Boston BioProducts). Primary antibodies were diluted in 5% bovine serum albumin (BSA; Cell Signaling Technology) in TBS with 0.2% Tween 20 and were incubated with membranes at 4°C overnight. Horseradish peroxidase–conjugated secondary antibodies were diluted in blocking solution (1:20,000, Thermo Fisher Scientific) and incubated with membranes at room temperature for 1 hour. Western blots were developed using West Pico PLUS Super Signal ECL reagents (Pierce) and film (GE Healthcare).
Immunoprecipitation
For immunoprecipitations, cells were lysed in immunoprecipitation (IP) CHAPS lysis buffer: 0.3% CHAPS, 10 mM β-glycerol phosphate, 10 mM pyrophosphate, 40 mM Hepes (pH 7.4), 2.5 mM MgCl2, supplemented with protease inhibitor tablets (Roche) and calyculin A (Cell Signaling Technology). Lysates were clarified by centrifugation and equilibrated as described above. For FLAG IP, lysates were incubated with anti-M2 FLAG-conjugated agarose beads (Sigma-Aldrich) at 10 μl bed volume per 1 mg of protein and incubated for 1 hour at 4°C with gentle rocking. For MYC IP, lysates were incubated with 10-μl bed volume per 1 mg of protein of anti-myc 9E10-conjugated agarose beads (Sigma-Aldrich). Beads were then centrifuged and washed three times with lysis buffer supplemented with 300 mM NaCl. Immunoprecipitate was eluted by addition of 6× Laemmli SDS loading buffer at 100°C for 5 min.
LysoIP was performed as described previously [see reference (57)]. Briefly, U2OS cells stably expressing 3×HA-TMEM192 were washed twice with ice-cold phosphate-buffered saline (PBS) and then scraped into 1 ml of ice-cold LysoIP buffer [136 mM KCl, 10 mM KH2PO4 (pH 7.25) in Optima LC-MS water]. Cell suspension (100 μl) was reserved for input sample. Cells were then homogenized using 35 strokes of a Dounce homogenizer followed by centrifugation for 10 min at 1500g in a 4°C centrifuge. Clarified homogenates were then incubated with 50 μl of prewashed anti-HA magnetic beads for 30 min with constant rotation at 4°C. Beads were then washed four times with LysoIP washing buffer [136 mM KCl, 10 mM KH2PO4 (pH 7.25) in Optima LC-MS water, 300 mM NaCl]. Both input and immunoprecipitated samples were then lysed with LysoIP lysis buffer [40 mM Hepes (pH 7.4), 1% Triton X-100, 10 mM β-glycerol phosphate, 10 mM pyrophosphate, 2.5 mM MgCl2, and cOmplete EDTA-free protease inhibitor cocktail (Roche)]. Laemmli SDS loading buffer (6×) was added, and samples were placed at 100°C for 5 min.
Immunofluorescence and high-content image analysis
Following indicated treatments, GFP-LC3 LAMP1–red fluorescent protein (RFP)–expressing cells were fixed with ice-cold methanol for 3 min at −20°C. Cells were washed in PBS, and image acquisition was performed using a Confocal Zeiss LSM 780 microscope (Carl Zeiss Ltd.) equipped with a 40× oil immersion 1.40 numerical aperture (NA) objective using Zen software (Carl Zeiss Ltd.).
For quantification, the number of GFP-LC3 puncta was counted for >20 cells across multiple fields of view. For colocalization quantification, GFP-LC3 puncta were assessed for LAMP1-RFP.
For LC3 and LAMP1 staining in primary BMDMs, cells were plated on 18-mm coverslips. The next day, cells were treated as indicated and fixed in ice-cold methanol as above. Cells were then blocked in PBS and 5% BSA for 1 hour before addition of primary antibodies (anti-LC3A/B, Cell Signaling Technology, no. 4108, 1:100; anti-LAMP1, BD Biosciences, no. 555798, 1:100) diluted in blocking buffer overnight at 4°C. Cells were washed and incubated with fluorescent secondary antibodies in blocking buffer for 1 hour at room temperature. Cells were washed in PBS incubated with 4′,6-diamidino-2-phenylindole (DAPI) and mounted on glass slides using ProLong antifade reagent (Life Technologies).
For endogenous TFEB staining in mouse macrophage, cells were fixed in 3.7% formaldehyde for 15 min at room temperature, washed in PBS, and permeabilized in 0.2% Triton X-100/PBS for 5 min. Cells were then processed as above for primary (anti-TFEB, Bethyl Laboratories, no. A303-673A, 1:200) and secondary antibodies. Images were acquired using a Confocal Zeiss LSM 780 microscope (Carl Zeiss Ltd.) equipped with a 40× oil immersion 1.40 NA objective using Zen software (Carl Zeiss Ltd.). Analysis was performed using ImageJ. For nuclear cytosol quantification, the ratio of fluorescent intensity of TFEB within the DAPI mask versus the cytosol of 30 cells across two independent experiments was measured.
For high-content image acquisition, cells were plated in 96-well glass bottom, black-wall plates (Greiner, no. 655892) or 384-well polystyrene, black-wall plates (Greiner, no. 781091) and grown overnight to 70% confluency. Treatments were performed as indicated. Cells were fixed for 10 min in either −20°C methanol or 4% paraformaldehyde (Electron Microscopy Sciences). Cells were blocked and permeabilized in a solution containing 1:1 Odyssey blocking buffer (LiCor)/PBS (Invitrogen) with 0.1% Triton X-100 (Sigma-Aldrich) and 1% normal goat serum (Invitrogen) for 1 hour at room temperature. Primary antibodies were added overnight at 4°C in blocking buffer described above. After washing plates with PBS using an EL-406 plate washer (BioTek), secondary Alexa Fluor–conjugated antibodies (Life Technologies) were diluted 1:1000 in blocking solution supplemented with DAPI and applied for 1 hour at room temperature. Cells were then washed again in PBS as described and imaged using an IN Cell 6500 high-content imager (GE Healthcare). Images were analyzed using the GE InCarta software.
For imaging in the context of Salmonella infection, cells were fixed with 4% paraformaldehyde in PBS at 37°C for 10 min. Immunostaining was performed as previously described (58). Cells were imaged using a Quorum spinning disk microscope with a 63-Å oil immersion objective (Leica DMIRE2 inverted fluorescence microscope equipped with a Hamamatsu Back-Thinned EM-CCD camera or Hamamatsu CMOS FL-400 camera, spinning disk confocal scan head) and Volocity 6.3 acquisition software (Improvision). Confocal z stacks of 0.3 μm were acquired, and images were analyzed with Volocity 6.3 software.
Correlative FIB-SEM
Cells were seeded in 35-mm glass-bottom dishes (MatTek Corp., USA, no. P35G-2-14-CGRD). They were fixed with 4% formaldehyde [TAAB F017, 16% (w/v) solution formaldehyde-methanol free] in 0.1 M phosphate buffer (PB; pH 7.4 ) for 30 min at 4°C. They were washed in PB and imaged with a 40×/1.4 NA objective on an inverted confocal microscope (Zeiss LSM780). Further fixation was carried out with 2% formaldehyde and 2.5% glutaraldehyde (TAAB G011/2, 25% solution glutaraldehyde) in PB for 2 hours before further processing.
Samples were embedded using a protocol as described previously (59, 60). The cells were washed in PB five times and postfixed in 1% osmium tetroxide (Agar Scientific, R1023, 4% solution osmium tetroxide) and 1.5% potassium ferrocyanide (v/v) [Sigma-Aldrich, P3289-100G, potassium hexacyanoferrate (II) trihydrate] for 1 hour on ice and then dehydrated and embedded in Hard Plus Resin 812 (Electron Microscopy Sciences, no. 14115). The samples were polymerized for 72 hours at 60°C. The coverslip was removed from the resin by dipping the block into liquid nitrogen. After locating the region of interest (ROI) on the block surface using the imprint of the grid, the block was cut to fit on an aluminum stub using a hacksaw and trimmed with a razorblade. The block/stub was then coated with 20-nm Pt using a Safematic CCU-010 sputter coater (Labtech) to create a conductive surface.
The block/stub was placed in the chamber of a Zeiss 550 CrossBeam FIB-SEM, and the surface was imaged using the electron beam at 10 kV to locate the grid and underlying cells. Once the ROI had been identified, Atlas software (Fibics) was used to operate the system. A trench was cut into the resin to expose the target cell, and serial SEM images were acquired with 7-nm isotropic resolution using a 1.5-kV electron beam. For three-dimensional image analysis, image stacks were processed using Atlas software and viewed using ImageJ.
RNA isolation and RNA-seq analysis
RNA isolation
Total RNA was prepared from cells treated with dimethyl sulfoxide or 2 μM C8 for 24 hours using TRIzol extraction and the RNeasy Mini Kit (Qiagen). A total of 2 μg of RNA with a RNA integrity number (RIN) score of >9.8 was submitted for RNA-seq analysis.
Library preparation, HiSeq sequencing, and analysis
RNA-seq libraries were prepared using the NEBNext Ultra RNA Library Prep Kit for Illumina using the manufacturer’s instructions (New England Biolabs, MA). mRNAs were enriched with oligo(dT) beads, and the enriched mRNAs were fragmented at 94°C (15 min). This was followed by first-strand and second-strand cDNA synthesis. cDNA fragments were end-repaired and adenylated at 3’ ends. Universal adapters were then ligated to cDNA fragments, followed by index addition and library enrichment by polymerase chain reaction (PCR) with limited cycles. The sequencing library and RNA samples for RNA-seq were quantified using the Qubit 2.0 Fluorometer (Life Technologies, CA), and RNA integrity was checked using Agilent TapeStation 4200 (Agilent Technologies, CA).
The sequencing libraries were clustered on a single lane of a flow cell on the Illumina HiSeq 4000 system according to the manufacturer’s instructions. The samples were sequenced using a 2 × 150–base pair paired-end configuration. Image analysis and base calling were conducted by the HiSeq Control Software. Raw sequence data (.bcl files) generated from Illumina HiSeq were converted into fastq files and demultiplexed using Illumina’s bcl2fastq 2.17 software. One mismatch was allowed for index sequence identification. Sequence reads were mapped to the Homo sapiens reference genome version GRCh38 available on ENSEMBL using the STAR aligner v.2.5.2b. Unique gene hit counts were calculated by using featureCounts from the Subread package v.1.5.2. Only unique reads that fell within exon regions were counted.
The count data were normalized by the trimmed mean of M-values normalization (TMM) method, followed by variance estimation and applying generalized linear models (GLMs), using functions from empirical analysis of digital gene expression (61) to identify differentially expressed genes as described previously (62, 63). Factorial designs were incorporated into the analysis by fitting these linear models with the coefficient for each of the factor combinations and then simultaneously extracting contrasts for the respective “differential-of-differential” analysis in the two experimental dimensions (C8 stimulation and genotype status: ATG16L1 KO and WT). The associated P values were adjusted to control the false discovery rate in multiple testing, using the Benjamini and Hochberg’s (BH) method (BH-adjusted P < 0.05). The heatmap visualization shows log2(counts per million)-derived values for these genes, expressed as Z-transformed signed difference ratios (SDRs) relative to their respective unstimulated baseline controls (either ATG16L1 KO or WT), and then scaled by normalizing to the maximum absolute deviation of each gene’s expression level from the unstimulated control.
Pathway and biological process enrichment analysis were performed as previously described (63, 64). Briefly, data were interrogated from KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways and Gene Ontology biological processes. Each module or category was assessed for statistical enrichment or overrepresentation among differentially expressed genes relative to their representation in the global set of genes in the genome. P values were computed using the hypergeometric test.
Quantitative PCR with reverse transcription analysis
RNA extractions were performed using the RNeasy Mini Kit (Qiagen), and cDNA was subsequently generated using the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative PCR analysis was performed on the QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific) using the SsoFast EvaGreen Supermix Kit (Bio-Rad) and the following primer sets: GPNMB human (5′-GCCTTTAAGGATGGCAAACA-3′ and 5′-TGCACGGTTGAGAAAGACAC-3′), RRAGD human (5′-TCCGGTGGATATGCAAACCT-3′ and 5′-ACAAAGCAAACGAGAGCCAG-3′), and GAPDH human (5′-GGAGCGAGATCCCTCCAAAAT-3′ and 5′-GGCTGTTGTCATACTTCTCATGG-3′). Data were normalized to that of the housekeeping gene GAPDH.
LysoTracker staining
U2OS.Cas9 cells expressing a control gRNA or knocked out for ATG16L1 were treated for 24 hours with 2 μM C8. Cells were then washed and incubated live for 20 min with 25 nM LysoTracker Red DND-99 (Thermo Fisher Scientific) and Hoechst 33342 (Thermo Fisher Scientific) diluted in warmed imaging buffer [20 mM Hepes (pH 7.4), 140 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM d-glucose, and 5% (v/v) FBS]. Staining solution was removed, and cells were incubated in imaging buffer for an additional 30 min before image acquisition on IN Cell 6500. Images were analyzed using the GE InCarta software.
Generation of ATG16L1 K490A knockin mouse model
The K490A point mutation was introduced into C57/BL6 mice via direct zygote injection of CRISPR-Cas9 reagents. Briefly, a single-stranded guide sequence was designed and synthesized along with a tracerRNA from Dharmacon. A repair donor single-stranded DNA (ssDNA) sequence was designed to introduce the K490A point mutation and mutate the protospacer adjacent motif (PAM) sequence to stop retargeting of the Cas9 complex to already edited DNA. These reagents, along with recombinant Cas9, were injected into mouse zygotes. Pups born from these injections were genotyped via Transnetyx, and heterozygous founders were bred with WT mice to obtain pure heterozygote animals. Further breeding yielded mice homozygous for the K490A mutation. Mice were housed in the Biological Support Unit at the Babraham Institute under specific pathogen–free conditions. All animal experiments at the Babraham Institute were reviewed and approved by the Animal Welfare and Ethics Review Body and performed under Home Office Project license PPL/PO302B91A.
The following primers were used: K490A guide sequence, GUUAGGGGCCAUCACGGCUCGUUUUAGAGCUAUGCUGUUUUG; repair donor ssDNA, GCTGTCTCCCTTAGGTCAGAGAGAGTGTGGTCCGAGAGATGGAACTGTTAGGGGCGATCACCGCTTTGGACCTAAACCCTGAGAGAACTGAGCTCCTGAGCTGCTCCCGTGATGACCTG.
BMDM isolation
C57/BL6 WT and ATG16L1 K490A mice, aged 13 to 15 weeks, were used to obtain bone marrow-derived dendritic cells (BMDCs). Bone marrow cells were isolated by flushing tibias and femurs with PBS and 2% FBS. Cells were pelleted and resuspended in 1 ml of red blood cell lysis buffer (150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA) for 2 min at room temperature. Cells were pelleted and resuspended in RPMI 1640 (Invitrogen 22409-031), 10% FBS, 1% penicillin/streptomycin, and 2-mercaptoethanol (50 μM) supplemented with macrophage colony-stimulating factor (20 ng/ml; PeproTech, no. AF-315-02) and fungizone (50 ng/ml; amphotericin B) (Gibco, no. 15290018). The medium was refreshed on days 3 and 6, and cells were plated for assays on day 8.
HEK293 GFP-LC3B ATG13/ATG16L1_DKO cells
ATG13_KO HEK293 cells stably expressing GFP-LC3B maintained in DMEM, 10% FBS, and 1% penicillin/streptomycin were used as previously described (7). To generate ATG16L1 KO, gRNA sequence (GTGGATACTCATCCTGGTTC) with overhangs for containing a Bpi I site was annealed and cloned into the pSpCas9(BB)-2A-GFP plasmid (Addgene, 48138; deposited by F. Zhang) digested with the Bpi I restriction enzyme (Thermo Fisher Scientific, ER1011). The recombinant plasmid along with a pBabe-puro construct (Addgene, 1764; deposited by H. Land) expressing mouse ATG16L1 variants was transfected into HEK293 ATG13_KO GFP-LC3B cells via Lipofectamine 2000 (Invitrogen). Cells were selected with puromycin (2.5 μg/ml; P8833, Sigma-Aldrich) for 48 hours, and single-cell clones were obtained by limiting dilution. After clonal expansion, ATG16L1 KO clones were selected on the basis of the absence of ATG16L1 protein as detected by Western blot. RAW264.7 WT and ATG16L1_KO cells were provided by A. Simonsen (65) and maintained in DMEM 10% FBS and 1% penicillin/streptomycin.
Live imaging time-lapse confocal microscopy
HEK293 cells were plated on 35-mm glass-bottom dishes (MatTek, Ashland, MA). Images were acquired every 2 min using a spinning disk confocal microscope, comprising Nikon Ti-E stand, Nikon 60× 1.45 NA oil immersion lens, Yokogawa CSU-X scanhead, Andor iXon 897 EM-CCD camera, and Andor laser combiner. All imaging with live cells was performed within incubation chambers at 37°C and 5% CO2. Image acquisition and analysis were performed with Andor iQ3 (Andor Technology, UK) and ImageJ.
Endogenous calcium imaging
HeLa WT and HeLa ATG16L1KO cells were trypsinized and seeded at 20,000 per well of poly-D-lysine-coated Greiner Bio plates for 2 hours. Cells were loaded with 10 μl of calcium 6 dye solution for 1.5 hours at room temperature. After incubation, the dye was removed from the plates and replaced with 10 μl of low Ca2+ solution containing 145 mM NaCl, 5 mM KCl, 3 mM MgCl2, 10 mM glucose, 1 mM EGTA, and 20 mM Hepes (pH 7.4). With 1 mM EGTA, the free Ca2+ concentration is estimated to be <10 nM based on the Maxchelator software (https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/). Compound plates were prepared with low calcium solution. Cell and compound plates were loaded onto the fluorescence imaging plate reader (FLIPR), and the 15-min protocol was run. The fluorescence intensity at 470 nm was monitored. After an initial 10-s baseline read, compounds were added to the cells. Images were taken for 15 min to monitor effects on Ca2+ fluorescence. Data were exported as max-min relative fluorescence unit (RFU).
Recombinant protein expression
Protein purification
For evaluation of the FLCN/FNIP2/GABARAP complex by size exclusion chromatography, FLCN and FNIP2 were subcloned as twin-strep-FLAG and glutathione S-transferase (GST) fusion proteins, respectively, and purified as described (34). Final purified complexes were snap-frozen in liquid nitrogen in buffer A [25 mM Hepes (pH 7.4), 130 mM NaCl, 2.5 mM MgCl2, 2 mM EGTA, and 0.5 mM tris(2-carboxyethyl)phosphine (TCEP)]. Full-length human GABARAP (1 to 117) was subcloned with a C-terminal maltose-binding protein tag (GABARAP_MBP) separated by a GSSGSS linker in pET21b and expressed in Escherichia coli following induction at 16°C for 16 hours in LB. Cells were lysed in 50 mM tris (pH 7.4), 500 mM NaCl, 0.5 mM TCEP, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and benzamidine (15 μg/ml); sonicated; and clarified by centrifugation. GABARAP_MBP was purified using amylose resin equilibrated in wash buffer [50 mM tris (pH 7.4), 500 mM NaCl, 0.5 mM TCEP] and eluted with wash buffer and 30 mM maltose. The protein was further purified by size exclusion chromatography using a Superdex 75 column equilibrated buffer A and snap-frozen in liquid nitrogen. Purified GABARAP_MBP was mixed with FLCN/FNIP2 at a ratio of 1:0.8 and gently mixed for 2 hours at 4°C. The sample was injected onto a Superose 6 Increase (GE Healthcare) column (1 column volume = 24 ml) that was preequilibrated in buffer A. The retention time of peak fractions was compared to FLCN/FNIP2 and GABARAP_MBP alone followed by evaluation of samples using 12% SDS-PAGE.
For GEE labeling studies, full-length human FLCN-PreScission-MBP and His8-TEV-FNIP2 were subcloned for coexpression in mammalian cells. Cells were lysed in 50 mM tris (pH 8.0), 200 mM NaCl, 10% glycerol, 1% Triton X-100, and 2 mM MgCl2 with protease inhibitor cocktail (Roche) and clarified by centrifugation. Protease inhibitor tablets were included throughout purification to prevent degradation. The FLCN/FNIP2 complex was purified over an amylose column equilibrated in wash buffer [50 mM tris (pH 8.0), 500 mM NaCl, 10% glycerol, 1% Triton X-100, and 2 mM MgCl2], washed, and eluted in wash buffer and 10 mM maltose. The complex was further purified by immobilized metal affinity chromatography (IMAC) and eluted in buffer B [50 mM tris (pH 8.0), 500 mM NaCl, 10% glycerol, 0.11 mM NG311, and 2 mM MgCl2] and 250 mM imidazole followed by overnight cleavage with PreScission Protease at 4°C. The cleaved MBP tag was removed with a second amylose column, and the complex was concentrated before purification by size exclusion chromatography using a Superdex 200 column equilibrated in buffer B. The sample was snap-frozen in liquid nitrogen. GABARAP (1 to 117) was cloned as GST-PreScission-His8-TEV-GABARAP for expression in E. coli and lysis as described for GABARAP_MBP. GABARAP was purified using glutathione resin equilibrated in buffer C [50 mM tris (pH 8.0), 500 mM NaCl, and 0.5 mM TCEP] and eluted using buffer C and 10 mM glutathione. The GST tag was cleaved overnight at 4°C with PreScission Protease and purified by size exclusion chromatography using a Superdex 75 column equilibrated in buffer D [50 mM tris (pH 8.0), 150 mM NaCl, and 0.5 mM TCEP]. His8-TEV-GABARAP was applied to an IMAC column equilibrated in buffer E [25 mM tris (pH 8.0), 150 mM NaCl, and 0.5 mM TCEP], eluted in buffer E and 250 mM imidazole, and dialyzed into buffer E. Final samples were concentrated and snap-frozen in liquid nitrogen.
For x-ray crystallography, a single polypeptide clone was designed using GST-PreScission-His8-TEV followed by residues 558 to 576 of FNIP2, a Gly-Ser spacer, and full-length GABARAP. Protein was expressed and purified using glutathione resin as described for GST-PreScission-His8-TEV-GABARAP. The tag was cleaved using TEV protease and purified by size exclusion chromatography using a Superdex 75 column in buffer D. Residual tag was removed by IMAC, and the final protein was exchanged into buffer E by dialysis, concentrated to 18.7 mg/ml prior, and snap-frozen in liquid nitrogen.
For evaluation of the binding affinity of GABARAP and LC3B for the FLCN/FNIP2 complex via SPR, full-length human FLCN-8xG-AviTag-PreScission-MBP and full-length human His8-TEV-FNIP2 were expressed in mammalian cells and purified above as described for GEE labeling studies. Biotinylation of the AviTag in full-length human FLCN-8xG-AviTag-PreScission-MBP was performed according to the manufacturer’s (Avidity) suggested protocol and following PreScission Protease cleavage and before size exclusion chromatography. Full-length human GABARAP (1 to 117) was purified as described above for GEE studies. Full-length human LC3B (1 to 125) was cloned as GST-PreScission-His8-TEV-LC3B for expression in E. coli and lysis as described for GABARAP_MBP. LC3B was purified using glutathione resin equilibrated in buffer C [50 mM tris (pH 8.0), 500 mM NaCl, and 0.5 mM TCEP] and eluted using buffer C and 10 mM glutathione. The GST tag was cleaved overnight at 4°C with PreScission Protease and purified by size exclusion chromatography using a Superdex 75 column equilibrated in buffer D [50 mM tris (pH 8.0), 150 mM NaCl, and 0.5 mM TCEP]. His8-TEV-LC3B was applied to an IMAC column equilibrated in buffer E [25 mM tris (pH 8.0), 150 mM NaCl, and 0.5 mM TCEP], eluted in buffer E and 250 mM imidazole, and dialyzed into buffer E. Final samples were concentrated and snap-frozen in liquid nitrogen.
For evaluation of the binding affinity of GABARAP and LC3B for the p62 FIR domain via SPR, the FIR region of human p62 (326 to 380) with the 4P mutations (S349E, S365E, S366E, and S370E) (66) was cloned as GST-PreScission-p62 FIR 4P for expression in E. coli following induction at 18°C for 16 hours in TB. Cells were lysed in 50 mM Hepes (pH 7.0), 300 mM NaCl, 5 mM MgCl2, 2 mM CaCl2, 1 mM TCEP, and deoxyribonuclease I (1 U/ml); sonicated; and clarified by centrifugation. GST-PreScission-p62 FIR 4P was purified using glutathione resin equilibrated in 50 mM Hepes (pH 7.0), 300 mM NaCl, and 1 mM TCEP and eluted using the same buffer supplemented with 10 mM glutathione. The GST tag was left on the protein, and GST-PreScission-p62 FIR 4P was further purified by size exclusion chromatography using a Superdex 200 column equilibrated in 50 mM Hepes (pH 7.0), 300 mM NaCl, and 1 mM TCEP. Fractions containing GST-PreScission-p62 FIR 4P were pooled, concentrated, and snap-frozen in liquid nitrogen. GST protein used as a reference for SPR was purchased from Cytiva.
Protein crystallization
FNIP2-GABARAP chimera was crystallized by the vapor diffusion method using equal volumes of protein and 0.1 M magnesium acetate, 0.1 M Mops (pH 7.5), and 12% (v/v) polyethylene glycol 8000 in sitting drops. Crystals were frozen using well solution supplemented with 20% glycerol. Data were collected at beamline BL18U1 of the Shanghai Synchrotron Radiation Facility and processed with XDS (67) and AIMLESS (68). The structure was solved by molecular replacement with Phaser (69) using 6hyo (33) as a search model and refined with REFMAC5 (70) and PHENIX (71). Model building was performed using Coot (72).
Peptide synthesis
WT and mutant peptides corresponding to residues 550 to 576 (long) or 558 to 576 (short) of FNIP2 were ordered from New England Peptide. All peptides were purified to >99% purity, with the exception of the WT long FNIP2 peptide that was purified to 70% purity. Five milligrams of each short peptide was resuspended in water to a concentration of 500 μM, whereas 5 mg of aliquots of long peptides was resuspended in water supplemented with 3 μl of 1 M ammonium bicarbonate to a final concentration of 500 μM.
Measurement of in vitro binding kinetics of GABARAP and LC3B with FLCN/FNIP2 by SPR
Direct binding of GABARAP and LC3B to immobilized FLCN/FNIP2 was assayed on a Biacore S200 (Cytiva) using the Biotin CAPture Kit (Series S Sensor Chip CAP and Biotin CAPture reagent) (Cytiva). Before beginning each run, the S200 system was equilibrated using a 4× Multiprime of running buffer [1× HBS-P+ (Cytiva) supplemented with 1 mM TCEP]. The chip surface was conditioned with three successive 60-s pulses of 6 M guanidine HCl/50 mM NaOH at a flow rate of 30 μl/min. Before kinetic analysis, three startup cycles were performed using standard conditions for the CAPture Kit (Cytiva). For each cycle of kinetic analysis, undiluted Biotin CAPture reagent was injected onto all four flow cells for 150 s at a flow rate of 4 μl/min. Biotinylated FLCN-8xGlycine-AviTag/FNIP2 (40 μg/ml) was injected onto flow cells 2 and 4 at 10 μl/min for 60 s to produce an immobilization level of 100 to 150 response units (RU). GABARAP (0.06 to 31.25 nM) or LC3B protein (0.06 to 4000 nM) was injected using “high-performance multicycle kinetics” or “single cycle kinetics” injections for 60 s at a flow rate of 100 μl/min and was allowed to dissociate from FLCN/FNIP2 for 750 s before regeneration of the chip surface with 30-s pulses of 6 M guanidine HCl/50 mM NaOH followed by 30% acetonitrile/250 mM NaOH.
Sensorgrams were processed with Cytiva Biacore S200 Evaluation Software (version 1.1, Build 28). Double referencing was performed by first subtracting the GABARAP/LC3B responses over the reference flow cells (1 and 3) from the corresponding responses in sample flow cells (2 and 4, respectively), followed by subtraction of averaged sensorgrams for 0 nM GABARAP/LC3B from sensorgrams corresponding to all other concentrations of GABARAP/LC3B. After double referencing kinetic data, injection and pump spikes were manually removed from sensorgrams and the data were fit globally by nonlinear regression to a simple 1:1 Langmuir binding model without a bulk refractive index (RI) term (RI = 0 RU) to determine association/dissociation rate constants (Ka, Kd), analyte binding capacity (Rmax), and the equilibrium Kd. Rmax values obtained during kinetic analysis represented 50 to 80% of the theoretical Rmax value indicating a surface activity of 50 to 80%. Sensorgrams and 1:1 binding model curve fits were exported from the S200 Evaluation Software and replotted in GraphPad Prism (v8.4.3).
Measurement of in vitro binding kinetics of GABARAP and LC3B with the p62 FIR 4P domain by SPR
Direct binding of GABARAP and LC3B to immobilized GST-p62 FIR 4P protein was assayed on a Biacore S200 (Cytiva) using a CM5 chip (Cytiva). Before beginning each run, the S200 system was equilibrated using a 4× Multiprime of 1× HBS-N buffer (Cytiva). The CM5 chip surface was preconditioned using five cycles of successive 60-s injections of 50 mM NaOH, 100 mM HCl, 0.2% SDS, and nuclease-free water at 30 μl/min. GST-p62 FIR 4P (sample cells, 2 and 4) or GST (reference cells, 1 and 3) was covalently coupled to the chip surface using N-hydroxysuccinimide/1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (NHS/EDC) supplied in the Cytiva Amine Coupling Kit. GST-p62 and GST (Cytiva) were diluted to 0.1 μM in 10 mM sodium acetate (pH 4.5) and injected onto flow cells 2 and 4 (GST-p62) or 1 and 3 (GST) of the NHS/EDC activated chip until 250 RU of GST-p62 or 200 RU of GST was immobilized. Ethanolamine was then injected across all four flow cells according to the Amine Coupling Kit protocol to block remaining reactive sites on the chip surface.
The SPR system was then equilibrated using a 4× Multiprime of running buffer [1× HBS-P+ (Cytiva) supplemented with 1 mM TCEP]. Before kinetic analysis, 10 startup cycles were performed using 60-s sample injections of running buffer at 30 μl/min and 30-s dissociation times followed by two 30-s injections of pH 2.0 Glycine (Cytiva) at 30 μl/min and a “carryover control” injection. For each cycle of kinetic analysis, 40 to 10,000 nM GABARAP or LC3B protein was injected across all four flow cells using high-performance multicycle kinetics injections for 60 s at a flow rate of 100 μl/min and allowed to dissociate from GST-p62 FIR 4P and GST surfaces for 1000 s before regeneration of the chip surface with two 30-s injections of pH 2.0 Glycine (Cytiva) at 30 μl/min followed by a 300-s stabilization period and a carryover control injection.
Sensorgrams were processed with Cytiva Biacore S200 Evaluation Software as described above. While sensorgrams for LC3B binding to GST-p62 FIR 4P domain showed high-affinity binding with a very slow off rate (estimated 80 nM Kd with 1200-s half-life), they were more complicated than could be accurately described using a simple 1:1 Langmuir binding model with a bulk RI term. Despite their complexity, the LC3B sensorgrams and sensorgrams for GABARAP binding to GST-p62 reached a steady state during injection onto the chip surface and were fit using a “steady-state affinity model” with the offset parameter set to zero. Rmax values obtained during steady-state affinity analysis represented ~85% of the theoretical Rmax value, indicating a surface activity of ~85%. Sensorgrams and steady-state affinity binding isotherm fits were exported from the S200 Evaluation Software and replotted in GraphPad Prism (v8.4.3).
Measurement of the affinity of p62, FLCN/FNIP1, and FNIP2 peptides for GABARAP by competition in solution/affinity in solution SPR
We used the “competition in solution” (also called “affinity in solution”) method (73–75) to measure the affinity of the GST-p62 FIR 4P domain, full-length FLCN/FNIP1 protein, and FNIP2 peptide (New England Peptide) competitors for GABARAP. Full-length AviTag-FLCN/FNIP2 heterodimers were immobilized on the Series S Sensor Chip CAP surface (as described above for FLCN/FNIP binding kinetics with GABARAP and LC3B) and used to capture and measure the concentration of free GABRAP in preequilibrated mixtures containing a constant amount of GABARAP with varying amounts of soluble p62, FLCN/FNIP1, and FNIP2 peptide competitors.
Calibration curves of 0 to 15.6 nM GABRAP were prepared by twofold serial dilution of 31.2 nM GABARAP into running buffer [1× HBS-P+ buffer (Cytiva) supplemented with 1 mM TCEP]. Calibration curves of GABARAP were injected onto the chip surface for 60 s at 30 μl/min, and the maximum RU for each concentration of GABARAP was recorded. Following sample injections, the chip surface was regenerated by injecting one 30-s pulse of 6 M guanidine hydrochloride with 0.25 M NaOH and a second 30-s pulse of 30% acetonitrile with 0.25 M NaOH, followed by a “carryover control injection.”
Mixtures containing a fixed amount of GABARAP (7.8 nM) and soluble GST-p62 FIR, FLCN/FNIP1 protein, and FNIP2 peptide competitors were prepared by 1:1 dilution of 15.6 nM GABARAP with twofold serial dilutions of competitors in running buffer. Each mixture of GABARAP and competitor was injected to produce a series of sensorgrams that were recorded as described for the GABARAP calibration curve.
Sensorgrams were processed with Cytiva Biacore S200 Evaluation Software (version 1.1, Build 28). Double referencing was performed by first subtracting the GABARAP/LC3B responses over the reference flow cells (1 and 3) from the corresponding responses in sample flow cells (2 and 4, respectively), followed by subtraction of averaged sensorgrams for 0 nM GABARAP/LC3B from sensorgrams corresponding to all other concentrations of GABARAP/LC3B.
Sensorgrams were processed with the Cytiva Biacore S200 Evaluation Software. The y axes were zeroed at the baseline for each cycle, and x axes were aligned at the injection start. Bulk refractive index changes and systematic deviations in sensorgrams were removed by double referencing as described above. The concentration of free GABARAP in each mixture with competitors was determined from the calibration curve, exported into Prism, plotted against the log of competitor concentration, and fit to the equation detailed on page 192 of the Biacore S200 Software Handbook (revision 29-1431-08 AA). The equation assumes the existence of a single binding site between FLCN/FNIP2 competitors and GABARAP.
Bacterial strains and infections
Infections were performed with WT S. Typhimurium SL1344 and isogenic mutant lacking the SopF effector. Mutation in the S. Typhimurium SL1344 background lacking SopF (ΔsopF) was a gift from F. Shao and described previously (19). A previously established approach was used for infection of epithelial cells, using late-log S. Typhimurium cultures as inocula (76). Briefly, subcultured Salmonella strains were pelleted at 10,000g for 2 min, resuspended and diluted 1:50 in PBS (pH 7.2), and added to cells for 10 min at 37°C. Selection for intracellular bacteria was performed at 30 min after infection using gentamicin at 100 μg ml−1, a concentration that was decreased to 10 μg ml−1 at 2 hours after infection for maintenance purposes. If applicable, cells were fixed with 4% paraformaldehyde in PBS at 37°C for 10 min.
Statistics
All quantifications are presented as the means ± SD. Significance was determined by two-tailed t test; *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001. Statistical analysis was performed using GraphPad or Spotfire software. High-content imaging experiments include a minimum of three technical replicate wells and nine fields of view per well for quantification. Information specific to each experiment can be found in figure legends. Statistical analysis of transcriptomic data is described above and in the figure legends.
Acknowledgments
We would like the thank J. Hurley, S. Martens, K. Dionne, F. Gentile, B. Tepper, and the employees of Casma Therapeutics for critical feedback in preparation of this manuscript. We thank all staff in the Biological Support Unit at the Babraham Institute for their outstanding care of our mouse colony and S. Walker in the Imaging Facility for his support. We thank D. Spensberger for his role in generating the K490A mouse and E. Jacquin for early work on vATPase. We thank A. Simonsen for providing the ATG16L1 KO RAW264.7 cell line. Funding: This work was supported by BBSRC grants BB/P013384/1 (BBS/E/B/000C0432 and BBS/E/B/000C0434) and BB/R019258/1, Cancer Research UK Career Development award C47718/A16337, and the Canadian Institutes of Health Research (FDN no. 154329 to J.H.B.). J.H.B. holds the Pitblado Chair in Cell Biology. Author contributions: J.M.G., L.O.M., and O.F. designed all experiments and wrote the manuscript. J.M.G., W.G.W., K.H., T.Z.L., and S.K. performed experiments. K.F. and O.F. generated the ATG16L1 K490A knockin mouse model. A.N. performed data analysis of RNA-seq profiling. A.J., A.A., S.R.B., and S.R.R. performed calcium imaging experiments. C.K.-I. performed ultrastructural analysis. J.K. and M.R.C. performed the chemical footprinting analysis. T.L. and J.H.B. performed the Salmonella experiments and provided valuable scientific support. J.G.-F. and J.S. provided chemistry support. M.W., B.A.A., D.B., Q.T., and Y.F. performed protein purification and complex formation assays. A.B. provided valuable scientific guidance throughout the study. Competing interests: J.M.G., W.G.W., A.N., T.L., S.K., A.J., J.G.-F., D.B., B.A.A., J.S., and L.O.M. are employees and shareholders of Casma Therapeutics. A.B. is a scientific cofounder and shareholder of Casma Therapeutics and serves on the advisory board of Next Generation Diagnostics and Avilar Therapeutics. In addition, J.M.G., O.F., and L.O.M. are authors on a patent related to TFEB modulation. O.F. is a paid consultant for Casma Therapeutics. The authors declare no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. RNA-seq data files are hosted on figshare and can be retrieved using the following link: https://doi.org/10.6084/m9.figshare.14075372. The reagents in this paper generated at Casma Therapeutics can be provided by Casma Therapeutics pending scientific review and a completed material transfer agreement. Requests for the reagents should be submitted to J.M.G. at jgoodwin@casmatx.com.
Supplementary Materials
This PDF file includes:
Figs. S1 to S22
Tables S1 to S5
Other Supplementary Material for this manuscript includes the following:
Movies S1 to S4
REFERENCES AND NOTES
- 1.Dikic I., Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 86, 193–224 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Lawrence R. E., Zoncu R., The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 21, 133–142 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Kaur J., Debnath J., Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 16, 461–472 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Ballabio A., Bonifacino J. S., Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 21, 101–118 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Florey O., Kim S. E., Sandoval C. P., Haynes C. M., Overholtzer M., Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat. Cell Biol. 13, 1335–1343 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez J. S., Almendinger J., Oberst A., Ness R., Dillon C. P., Fitzgerald P., Hengartner M. O., Green D. R., Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. U.S.A. 108, 17396–17401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Jacquin E., Leclerc-Mercier S., Judon C., Blanchard E., Fraitag S., Florey O., Pharmacological modulators of autophagy activate a parallel noncanonical pathway driving unconventional LC3 lipidation. Autophagy 13, 854–867 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heckmann B. L., Teubner B. J. W., Tummers B., Boada-Romero E., Harris L., Yang M., Guy C. S., Zakharenko S. S., Green D. R., LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell 178, 536–551.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaushik S., Cuervo A. M., The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 19, 365–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lie P. P. Y., Nixon R. A., Lysosome trafficking and signaling in health and neurodegenerative diseases. Neurobiol. Dis. 122, 94–105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H., Ren D., Lysosomal physiology. Annu. Rev. Physiol. 77, 57–80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G. Y., Sabatini D. M., mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Settembre C., Fraldi A., Medina D. L., Ballabio A., Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sardiello M., Palmieri M., di Ronza A., Medina D. L., Valenza M., Gennarino V. A., Di Malta C., Donaudy F., Embrione V., Polishchuk R. S., Banfi S., Parenti G., Cattaneo E., Ballabio A., A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Martina J. A., Diab H. I., Lishu L., Jeong-A L., Patange S., Raben N., Puertollano R., The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal. 7, ra9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Settembre C., Di Malta C., Polito V. A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S. U., Huynh T., Medina D., Colella P., Sardiello M., Rubinsztein D. C., Ballabio A., TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher K., Ulferts R., Jacquin E., Veith T., Gammoh N., Arasteh J. M., Mayer U., Carding S. R., Wileman T., Beale R., Florey O., The WD40 domain of ATG16L1 is required for its non-canonical role in lipidation of LC3 at single membranes. EMBO J. 37, e97840 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galluzzi L., Green D. R., Autophagy-independent functions of the autophagy machinery. Cell 177, 1682–1699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y., Zhou P., Cheng S., Lu Q., Nowak K., Hopp A.-K., Li L., Shi X., Zhou Z., Gao W., Li D., He H., Liu X., Ding J., Hottiger M. O., Shao F., A bacterial effector reveals the V-ATPase-ATG16L1 axis that initiates xenophagy. Cell 178, 552–566.20 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Nakamura S., Shigeyama S., Minami S., Shima T., Akayama S., Matsuda T., Esposito A., Napolitano G., Kuma A., Namba-Humano T., Nakamura J., Yamamoto K., Sasai M., Tokumura A., Miyamoto M., Oe Y., Fujita T., Terawaki S., Takahashi A., Hamasaki M., Yamamoto M., Okada Y., Komatsu M., Nagai T., Takabatake Y., Xu H., Isaka Y., Ballabio A., Yoshimori T., LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat. Cell Biol. 22, 1252–1263 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Kumar S., Jain A., Choi S. W., Duarte da Silva G. P., Allers L., Mudd M. H., Peters R. S., Anonsen J. H., Rusten T.-E., Lazarou M., Deretic V., Mammalian Atg8 proteins and the autophagy factor IRGM control mTOR and TFEB at a regulatory node critical for responses to pathogens. Nat. Cell Biol. 22, 973–985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C.-C., Keller M., Hess M., Schiffmann R., Urban N., Wolfgardt A., Schaefer M., Bracher F., Biel M., Wahl-Scott C., Grimm C., A small molecule restores function to TRPML1 mutant isoforms responsible for mucolipidosis type IV. Nat. Commun. 5, 4681 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Shen D., Wang X., Li X., Zhang X., Yao Z., Dibble S., Dong X.-P., Yu T., Lieberman A. P., Showalter H. D., Xu H., Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lyososomal calcium release. Nat. Commun. 3, 731 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.C. Liang, Piperazine derivatives as trpml modulators, WO2018/005713A1 (2018).
- 25.Chresta C. M., Davies B. R., Hickson I., Harding T., Cosulich S., Critchlow S. E., Vincent J. P., Ellston R., Jones D., Sini P., James D., Howard Z., Dudley P., Hughes G., Smith L., Maguire S., Hummersone M., Malagu K., Menear K., Jenkins R., Jacobsen M., Smith G. C. M., Guichard S., Pass M., AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 70, 288–298 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Florey O., Gammoh N., Kim S. E., Jiang X., Overholtzer M., V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation. Autophagy 11, 88–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medina D. L., Di Paola S., Peluso I., Armani A., De Stefani D., Venditti R., Montefusco S., Scotto-Rosato A., Prezioso C., Forrester A., Settembre C., Wang W., Gao Q., Xu H., Sandri M., Rizzuto R., De Matteis M. A., Ballabio A., Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288–299 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansen T., Lamark T., Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 432, 80–103 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Dunlop E. A., Seifan S., Claessens T., Behrends C., Af Kamps M., Rozycka E., Kemp A. J., Nookala R. K., Blenis J., Coull B. J., Murray J. T., van Steensel M. A., Wilkinson S., Tee A. R., FLCN, a novel autophagy component, interacts with GABARAP and is regulated by ULK1 phosphorylation. Autophagy 10, 1749–1760 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S. B., Oh H. B., Valera V. A., Baba M., Schmidt L. S., Linehan W. M., Inactivation of the FLCN tumor suppressor gene induces TFE3 transcriptional activity by increasing its nuclear localization. PLOS ONE 5, e15793 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petit C. S., Roczniak-Ferguson A., Ferguson S. M., Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J. Cell Biol. 202, 1107–1122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martina J. A., Puertollano R., Rag GTPases mediate amino acid–dependent recruitment of TFEB and MITF to lysosomes. J. Cell Biol. 200, 475–491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirth M., Zhang W., Razi M., Nyoni L., Joshi D., O’Reilly N., Johansen T., Tooze S. A., Mouilleron S., Molecular determinants regulating selective binding of autophagy adapters and receptors to ATG8 proteins. Nat. Commun. 10, 2055 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence R. E., Fromm S. A., Fu Y., Yokom A. L., Kim D. J., Thelen A. M., Young L. N., Lim C.-Y., Samelson A. J., Hurley J. H., Zoncu R., Structural mechanism of a Rag GTPase activation checkpoint by the lysosomal folliculin complex. Science 366, 971–977 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen K., Rogala K. B., Chou H.-T., Huang R., Yu Z., Sabatini D. M., Cryo-EM structure of the human FLCN-FNIP2-Rag-Ragulator complex. Cell 179, 1319–1329 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng J., Ferguson S. M., GATOR1-dependent recruitment of FLCN–FNIP to lysosomes coordinates Rag GTPase heterodimer nucleotide status in response to amino acids. J. Cell Biol. 217, 2765–2776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Napolitano G., Di Malta C., Esposito A., de Araujo M. E. G., Pece S., Bertalot G., Matarese M., Benedetti V., Zampelli A., Stasyk T., Siciliano D., Venuta A., Cesana M., Vilardo C., Nusco E., Monfregola J., Calcagnì A., Di Fiore P. P., Huber L. A., Ballabio A., A substrate-specific Mtorc1 pathway underlies Birt–Hogg–Dubé syndrome. Nature 585, 597–602 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manifava M., Smith M., Rotondo S., Walker S., Niewczas I., Zoncu R., Clark J., Ktistakis N. T., Dynamics of Mtorc1 activation in response to amino acids. ELife 5, e19960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence R. E., Cho K. F., Rappold R., Thrun A., Tofaute M., Kim D. J., Moldavski O., Hurley J. H., Zoncu R., A nutrient-induced affinity switch controls Mtorc1 activation by its Rag GTPase–Ragulator lysosomal scaffold. Nat. Cell Biol. 20, 1052–1063 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parminder K., Tomechko S. E., Kiselar J., Shi W., Deperalta G., Wecksler A. T., Gokulrangan G., Ling V., Chance M. R., Characterizing monoclonal antibody structure by carboxyl group footprinting. Mabs 7, 540–552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H., Wen J., Huang R. Y.-C., Blankenship R. E., Gross M. L., Mass spectrometry-based carboxyl footprinting of proteins: Method evaluation. Int. J. Mass Spectrom. 312, 78–86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noda N. N., Ohsumi T., Inagaki F., ATG8-family interacting motif crucial for selective autophagy. FEBS Lett. 584, 1379–1385 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Nezich C. L., Wang C., Fogel A. I., Youle R. J., MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and ATG5. J. Cell Biol. 210, 435–450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heo J.-M., Ordureau A., Swarup S., Paulo J. A., Shen K., Sabatini D. M., Harper J. W., RAB7A phosphorylation by TBK1 promotes mitophagy via the PINK-PARKIN pathway. Sci. Adv. 4, eaav0443 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birmingham C. L., Smith A. C., Bakowski M. A., Yoshimori T., Brumell J. H., Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281, 11374–11383 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Schmidt L. S., Linehan W. M., FLCN: The causative gene for Birt–Hogg–Dubé syndrome. Gene 640, 28–42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perera R. M., Stoykova S., Nicolay B. N., Ross K. N., Fitament J., Boukhali M., Lengrand J., Deshpande V., Selig M. K., Ferrone C. R., Settleman J., Stephanopoulos G., Dyson N. J., Zoncu R., Ramaswamy S., Haas W., Bardeesy N., Transcriptional control of autophagy–lysosome function drives pancreatic cancer metabolism. Nature 524, 361–365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta S., Yano J., Mercier V., Htwe H. H., Shin H. R., Rademaker G., Cakir Z., Ituarte T., Wen K. W., Kim G. E., Zoncu R., Roux A., Dawson D. W., Perera R. M., Lysosomal retargeting of Myoferlin mitigates membrane stress to enable pancreatic cancer growth. Nat. Cell Biol. 23, 232–242 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Köster S., Upadhyay S., Chandra P., Papavinasasundaram K., Yang G., Hassan A., Grigsby S. J., Mittal E., Park H. S., Jones V., Hsu F.-F., Jackson M., Sassetti C. M., Philipps J. A., Mycobacterium tuberculosisis protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc. Natl. Acad. Sci. U.S.A. 114, E8711–E8720 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choy A., Dancourt J., Mugo B., O’Connor T. J., Isberg R. R., Melia T. J., Roy C. R., The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 338, 1072–1076 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visvikis O., Ihuegbu N., Labed S. A., Luhachack L. G., Alves A.-M. F., Wollenberg A. C., Stuart L. M., Stormo G. D., Irazoqui J. E., Innate host defense requires TFEB-mediated transcription of cytoprotective and antimicrobial genes. Immunity 40, 896–909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanjuan M. A., Dillon C. P., Tait S. W. G., Moshiach S., Dorsey F., Connell S., Komatsu M., Tanaka K., Cleveland J. L., Withoff S., Green D. R., Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450, 1253–1257 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Martinez J., Subbarao Malireddi R. K., Lu Q., Dias Cunha L., Pelletier S., Gingras S., Orchard R., Guan J. L., Tan H., Peng J., Kanneganti T.-D., Virgin H. W., Green D. R., Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 17, 893–906 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Napolitano G., Esposito A., Choi H., Matarese M., Benedetti V., Di Malta C., Monfregola J., Medina D. L., Lippincott-Schwartz J., Ballabio A., mTOR-dependent phosphorylation controls TFEB nuclear export. Nat. Commun. 9, 3312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heckmann B. L., Teubner B. J. W., Boada-Romero E., Tummers B., Guy C., Fitzgerald P., Mayer U., Carding S., Zakharenko S. S., Wileman T., Green D. R., Noncanonical function of an autophagy protein prevents spontaneous Alzheimer’s disease. Sci. Adv. 6, eabb9036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonam S. R., Wang F., Muller S., Lysosomes as a therapeutic target. Nat. Rev. Drug Disc. 18, 923–948 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abu-Remaileh M., Wyant G. A., Kim C., Laqtom N. N., Abbasi M., Chan S. H., Freinkman E., Sabatini D. M., Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358, 807–813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brumell J. H., Rosenberger C. M., Gotto G. T., Marcus S. L., Finlay B. B., SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell. Microbiol. 3, 75–84 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Kishi-Itakura C., Koyama-Honda I., Itakura E., Mizushima N., Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J. Cell Sci. 127, 4089–4102 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Kishi-Itakura C., Buss F., The use of correlative light-electron microscopy (CLEM) to study PINK1/Parkin-mediated mitophagy. Methods Mol. Biol. 1759, 29–39 (2018). [DOI] [PubMed] [Google Scholar]
- 61.McCarthy D. J., Chen Y., Smyth G. K., Differential expression analysis of multifactor RNA-seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouziat R., Hinterleitner R., Brown J. J., Stencel-Baerenwald J. E., Ikizler M., Mayassi T., Meisel M., Kim S. M., Discepolo V., Pruijssers A. J., Ernest J. D., Iskarpatyoti J. A., Costes L. M. M., Lawrence I., Palanski B. A., Varma M., Zurenski M. A., Khomandiak S., McAllister N., Aravamudhan P., Boehme K. W., Hu F., Samsom J. N., Reinecker H. C., Kupfer S. S., Guandalini S., Semrad C. E., Abadie V., Khosla C., Barreiro L. B., Xavier R. J., Ng A., Dermody T. S., Jabri B., Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 356, 44–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouziat R., Biering S. B., Kouame E., Sangani K. A., Kang S., Ernest J. D., Varma M., Brown J. J., Urbanek K., Dermody T. S., Ng A., Hinterleitner R., Hwang S., Jabri B., Murine norovirus infection induces TH1 inflammatory responses to dietary antigens. Cell Host Microbe 24, 677–688.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeJesus R., Moretti F., McAllister G., Wang Z., Bergman P., Liu S., Frias E., Alford J., Reece-Hoyes J. S., Lindeman A., Kelliher J., Russ C., Knehr J., Carbone W., Beibel M., Roma G., Ng A., Tallarico J. A., Porter J. A., Xavier R. J., Mickanin C., Murphy L. O., Hoffman G. R., Nyfeler B., Functional CRISPR screening identifies the ufmylation pathway as a regulator of SQSTM1/p62. eLife 5, e17290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lystad A. H., Carlsson S. R., de la Ballina L. R., Kauffman K. J., Nag S., Yoshimori T., Melia T. J., Simonsen A., Distinct functions of ATG16L1 isoforms in membrane binding and LC3B lipidation in autophagy-related processes. Nat. Cell Biol. 21, 372–383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turco E., Witt M., Abert C., Bock-Bierbaum T., Su M. Y., Trapannone R., Sztacho M., Danieli A., Shi X., Zaffagnini G., Gamper A., Schuschnig M., Fracchiolla D., Bernklau D., Romanov J., Hartl M., Hurley J. H., Daumke O., Martens S., FIP200 Claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Mol. Cell 74, 330–346.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kabsch W., XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evans P. R., Murshudov G. N., How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J., Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murshudov G. N., Skubák P., Lebedev A. A., Pannu N. S., Steiner R. A., Nicholls R. A., Winn M. D., Long F., Vagin A. A., REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H., PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nieba L., Krebber A., Plückthun A., Competition BIAcore for measuring true affinities: Large differences from values determined from binding kinetics. Anal. Biochem. 234, 155–165 (1996). [DOI] [PubMed] [Google Scholar]
- 74.Lazar G. A., Dang W., Karki S., Vafa O., Peng J. S., Hyun L., Chan C., Chung H. S., Eivazi A., Yoder S. C., Vielmetter J., Carmichael D. F., Hayes R. J., Dahiyat B. I., Engineered antibody Fc variants with enhanced effector function. Proc. Natl. Acad. Sci. U.S.A. 103, 4005–4010 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walkup IV W. G., Mastro T. L., Schenker L. T., Vielmetter J., Hu R., Iancu A., Reghunathan M., Bannon B. D., Kennedy M. B., A model for regulation by synGAP-α1 of binding of synaptic proteins to PDZ-domain ‘Slots’ in the postsynaptic density. eLife 5, e16813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Szeto J., Namolovan A., Osborne S. E., Coombes B. K., Brumell J. H., Salmonella-containing vacuoles display centrifugal movement associated with cell-to-cell transfer in epithelial cells. Infect. Immun. 77, 996–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S22
Tables S1 to S5
Movies S1 to S4