Abstract
Tissue-resident macrophages play an important role in the local maintenance of homeostasis and immune surveillance. In the central nervous system (CNS), brain macrophages are anatomically divided into parenchymal microglia and non-parenchymal border-associated macrophages (BAMs). Among these immune cell populations, microglia have been well-studied for their roles during development as well as in health and disease. BAMs, mostly located in the choroid plexus, meningeal and perivascular spaces, are now gaining increased attention due to advancements in multi-omics technologies and genetic methodologies. Research on BAMs over the past decade has focused on their ontogeny, immunophenotypes, involvement in various CNS diseases, and potential as therapeutic targets. Unlike microglia, BAMs display mixed origins and distinct self-renewal capacity. BAMs are believed to regulate neuroimmune responses associated with brain barriers and contribute to immune-mediated neuropathology. Notably, BAMs have been observed to function in diverse cerebral pathologies, including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, ischemic stroke, and gliomas. The elucidation of the heterogeneity and diverse functions of BAMs during homeostasis and neuroinflammation is mesmerizing, since it may shed light on the precision medicine that emphasizes deep insights into programming cues in the unique brain immune microenvironment. In this review, we delve into the latest findings on BAMs, covering aspects like their origins, self-renewal capacity, adaptability, and implications in different brain disorders.
Keywords: Brain, Central nervous system, Border-associated macrophages, Microglia, Neurodegeneration, Cancer
Introduction
Macrophages are known to be phagocytes of the innate immune system. They not only perform a defensive function against invading pathogens such as bacteria and viruses, but also help maintain immune homeostasis through the removal of apoptotic or necrotic cells in the body [1]. Macrophages take part in both innate and adaptive immune responses involved in development, inflammation, tissue repair, and immunological memory [2, 3]. Macrophages express a variety of sensors such as scavenger receptors, integrins, and Toll-like receptors, enabling them to detect and respond to a wide range of environmental stimuli [1, 2]. Macrophages utilize multifaceted mechanisms to fulfill their immunomodulatory functions. These include phagocytosis; the release of diverse inflammatory mediators such as cytokines, chemokines, bioactive lipids, enzymes, reactive oxygen and nitrogen species, membrane-enclosed vesicles, and certain metabolites; the expression of co-stimulatory molecules such as CD40 and PD-L1; and triggering a T cell response through antigen processing and presentation [4, 5].
Macrophages are found in every tissue and exhibit tissue-specific functions. Therefore, tissue-resident macrophages are extremely heterogeneous and phenotypically distinct. They are also dynamic and adaptive. Work from research over the last decade has revealed that macrophages can be epigenetically reprogrammed to react to both physiological and danger signals from the tissue microenvironment [3, 4, 6]. As a result, macrophages demonstrate both tissue and disease state-associated characteristics, contributing to tissue remodeling, host defense, wound healing, and immune modulation. As the developmental and disease-relevant heterogeneity of tissue macrophages unravels, macrophages residing in the central nervous system (CNS) have become a focus of attention. The CNS has been considered unique for being immune-privileged, as the blood–brain barrier (BBB) acts as a roadblock, preventing microorganism entry and the influx of circulating immune cells [7]. CNS homeostasis is principally maintained by brain-resident macrophages. Notably, in physiological conditions, brain macrophages are anatomically classified as microglia in the brain parenchyma, and non-parenchymal border-associated macrophages (BAMs) which are located at the blood–brain and blood-CSF (cerebrospinal fluid) barriers as well as in the meninges [8, 9]. Microglia serve as the most abundant phagocytes in the adult brain, accounting for approximately 10% of total cells [10, 11]. Furthermore, microglia have been the subject of study since their discovery in 1919 [12]. Until now, historic breakthroughs in investigations of microglia have included their ontogeny and self-maintenance during homeostasis [8, 13], physiological regulations such as synapse pruning and myelin turnover [14, 15], and functions in the context of neurodegenerative and neuropsychiatric diseases, as well as traumatic brain injury [8, 13, 16–18]. Research on BAMs began much later than on microglia but has progressed rapidly owing to new technologies, such as mass cytometry, fate-mapping, single-cell RNA sequencing (scRNA-seq), in vivo imaging, and Cre recombinase-mediated mutagenesis [19]. Emerging evidence particularly indicates that BAMs, compared to microglia, function differently in neuropathology [20]. In this review, we aim to summarize recent advances in BAMs, focusing on their ontogeny, self-maintenance, and roles in normal neurodevelopment and different CNS disorders.
The macrophage system
Macrophages were first identified by Metchnikoff as a type of responding cells with phagocytic activity [21]. Later, it was presumed that they originated from the reticuloendothelial system, which supports the generation and differentiation of vascular endothelial cells [22]. This early concept, which posited that macrophages originated from tissue, was then challenged, as advancing experimental methods provided evidence that a large group of macrophages were derived from circulating monocytes in the blood [23, 24]. Subsequently, this led to the establishment of a broader definition describing distinct macrophage subsets in all tissues: the mononuclear phagocytic system (MPS) [24]. MPS encompasses all terminal-differentiated and intermediate phagocytic cells, along with their progenitors. It proposes a linear pattern where bone marrow-resident precursors evolve into blood monocytes as an intermediate state, which then migrate and differentiate into specialized macrophages in various organs [24].
In adult mammals, macrophages display remarkable morphological and functional diversity in multiple organs, such as the brain, liver, lung, spleen, kidney, skin, and adipose tissues. It was believed that in these organs, definitive organizational structures dictated the differentiation process of specialized macrophages, and that hematopoiesis in bone marrow supported the generation of their common progenitors. However, this evolutionary trajectory of tissue-resident macrophages came into question when several studies discovered that some tissue-resident macrophages had the capacity of self-renewal and exhibited local proliferation under certain circumstances [25–27]. In addition, it was observed that the resident peritoneal macrophages in mice could survive for a long period in a steady state without replacement by recruited blood monocytes, which raised the possibility of a dual origin for some tissue-resident macrophages [28]. Subsequently, developed techniques, including immunohistochemistry, flow cytometry, DNA microarray, and fate-mapping/lineage tracing with genetically modified mice, gradually revealed that during ontogeny, primitive macrophage populations derived from the embryonic yolk sac or fetal liver spread into entire peripheral tissues, colonize, and maintain themselves in tissues by self-renewal. These populations are the sources of some tissue-resident macrophages in adulthood, such as microglia and Langerhans cells [29–36]. Finally, all these discoveries led to an improved notion of the in vivo macrophage system. In fetal development, macrophage precursors from the yolk sac and fetal liver migrate and settle down in all tissue rudiments, constituting the original tissue macrophages with self-proliferative capacities. Into adulthood, macrophages derived from hematopoietic stem cells in bone marrow, with blood monocytes as the intermediate cell-type, replenish most of the tissue-resident macrophage pools within the body [37]. However, there are some exceptions. For instance, brain-resident microglia are derived solely from macrophage precursors in the yolk sac and repopulate in the CNS throughout life [33, 34]; Langerhans cells in the epidermal layer of the skin, originating embryonically, remain independent of bone marrow-derived precursors in the steady state [38–42].
Brain macrophages
To solve the mystery of the CNS immune system, extensive studies on brain macrophages have been undertaken for many years. Brain macrophages are highly heterogeneous, comprising resident populations including microglia and BAMs, and infiltrating monocyte-derived macrophages (MDMs) under physiological and disease conditions. Until recently, research utilizing transgenic mouse models and high-throughput sequencing technologies has revealed that these distinct macrophage subsets (Fig. 1), corresponding to their origins, exert differential functions in both brain homeostasis and pathogenesis [43–48].
Fig. 1.
Distinct macrophage subsets in the central nervous system. Parenchyma: microglia; Brain-circulation interface: macrophages located in the meninges, choroid plexus, and perivascular spaces; Migrated from blood: monocytes that migrate into the brain from a dysregulated brain–blood barrier and differentiate into macrophages. BAMs border-associated macrophages, CSF cerebrospinal fluid, MФ macrophages
Ontogenetically, microglia exclusively arise from early myeloid progenitors in the embryonic yolk sac, which move to the CNS and differentiate into microglia [33, 34]. Microglia maintain their population in the brain by self-renewal and show different functional states through regulating their proliferation and phenotypes [8, 10, 11, 13–18]. Compared to microglia, BAMs exhibit heterogeneous ontogenies and are essentially comprised of different macrophage subsets, including meningeal macrophages, perivascular macrophages (PVMs), and choroid plexus macrophages [9, 48–50]. During embryonic development, both yolk sac- and fetal liver-derived progenitors contribute to the BAM pool, and they sustain via the clonal expansion at brain border structures throughout life [37, 48–50]. BAMs can be divided into more detailed subpopulations based on their anatomical sites: subdural/leptomeningeal macrophages (sdΜΦ), dural macrophages (dmΜΦ), stromal choroid plexus macrophages (cpΜΦ), choroid epiplexus macrophages (cpepiΜΦ), and PVMs [49, 50]. The sdΜΦ populate the pia mater and the dmΜΦ are located at the dura mater of the meninges. Choroid plexus macrophages reside in the stromal space between the epithelial and endothelial layers (cpΜΦ) and along the apical epithelial surface (cpepiΜΦ, “Kolmer cells”) [9, 49–51]. PVMs are primarily found surrounding cerebral vessels. Of note, the maintenance of PVMs around the BBB and sdΜΦ in the leptomeninges does not depend on circulating monocytes in the blood. They can subsist over a long period and are thereby known as long-lived macrophages [48, 50]. Intriguingly, the dmΜΦ have shown a dual origin: one subpopulation expressing major histocompatibility complex class II (MHCII) emerges from childhood, revealing its recruitment from bone marrow; the other subset which lacks MHCII expression presents the long-lived characteristic, originating from embryonic progenitors [48, 49]. A recent investigation revealed that, under homeostasis, a group of monocytes in the brain and spinal dural meninges originates not from the blood, but directly from the adjacent skull and vertebral bone marrow [52]. In the choroid plexus, both cpΜΦ and cpepiΜΦ, which are located around the blood-CSF barrier (BCB),derive from primitive macrophages during embryogenesis. However, the cpΜΦ are constantly replenished by CCR2+Ly6Chigh monocytes from the bloodstream throughout adult life, rather than through self-renewal [48–51]. Thus, in comparison to PVMs and sdΜΦ, which have minimal turnover, the cpΜΦ in the choroid plexus demonstrate a relatively short lifespan due to their steady turnover by blood monocytes [49–51]. Similar to microglia, these subsets of BAMs display distinct transcriptional profiles in both healthy and diseased conditions [53–55]. Their tissue-specific functions warrant thorough exploration in future research.
In the CNS, recruited monocytes and MDMs stem from the hematopoietic system in the bone marrow. After birth, in a steady state, hematopoietic stem cells (HSCs) continue to differentiate into Ly6C+ monocytes and exit the bone marrow niche [56, 57]. Ly6C+ monocytes, expressing CCR2, can rapidly traffic into tissues and lymph nodes or transform into blood-resident Ly6C− monocytes [36, 58]. Postnatal Ly6C+ monocytes can mature into tissue-resident macrophages in certain organs, such as brain (choroid plexus), skin, lung, intestine, and heart, where they are capable of self-proliferation [41, 42, 48, 50, 51, 59–61]. During inflammation or injury, local tissue debris or pro-inflammatory mediators can continuously attract circulating monocytes to migrate into the tissue. Once in the inflamed tissue, monocytes are cued to secrete more inflammatory mediators, accelerating their accumulation and maturation into macrophages [57]. Apparently, the phenotypes of MDMs in different body positions are largely determined by the local environment and the stage in the inflammatory process. During aging, there is an increase in the proportion of MDMs in the CNS, a trend that is more evident in animals with neurodegenerative diseases [45]. Yet, macrophages in aged organisms exhibit more pro-inflammatory signatures compared to those in healthy, young adults. This includes reduced autophagy and phagocytosis, and increased secretion of IL-6 and TNFα [62, 63]. Furthermore, studies have shown evidence that aging impacts the BBB integrity, characterized by increased leakage and susceptibility to breakdown [64–67]. The damaged BBB offers opportunities for patrolling monocytes in the cerebrovascular system to infiltrate into the brain parenchyma. Accordingly, among brain-resident macrophage populations, the density of MDMs is at least partially regulated by the organism’s age. In addition, during aging, blood-borne macrophages tend to induce inflammation in the brain.
To date, microglia and BAMs are considered key players in controlling brain development, homeostasis, and neurodegenerative disorders such as Alzheimer's disease (AD) and Parkinson’s disease (PD) [19, 43–45]. However, blood-borne macrophages have been shown to migrate and vastly accumulate in the brain parenchyma in certain neuroinflammatory diseases. These include multiple sclerosis (MS) [68], ischemic stroke [69], traumatic brain injury [70], gliomas [71–73] and certain brain infections [74, 75]. Thus, it has been proposed that in CNS diseases, brain-resident macrophages contribute to the clearance of various debris and the resolution of inflammation, whereas blood-borne phagocytes play a pivotal role in driving cerebral immunopathology [20]. Although empirically determining which specialized macrophage population is driving or suppressing immune effector function in different disease contexts remains challenging, relevant studies are increasingly emerging. Notably, using a mouse model of heterotopic transplantation of bone marrow into brain parenchyma, a study has revealed that graft-derived macrophages in the brain display distinct responses to peripheral endotoxin challenge compared to microglia, although they have exhibited significant microglial characteristics, such as ramified morphology, longevity, clonal expansion, and radio-resistance [76]. Furthermore, over time in the CNS niche, the transcriptomes and chromatin accessibility landscapes of bone marrow-derived macrophages remain distinct from those of host microglia [76]. These results suggest that different brain macrophage subsets inherently impact disease progression in distinct ways. In a neuroinflammatory mouse model infected by Trypanosoma brucei, a species of parasites that invades the brain through its borders, researchers have revealed that brain-resident macrophages, including microglia and BAMs, initiate the initial immune defense and subsequent migration of blood monocytes across disrupted brain barriers. However, as the disease progresses, MDMs expand greatly and eventually outnumber the resident macrophages [20, 77]. These MDMs exhibit greater transcriptional plasticity and antimicrobial features, which lead to exacerbated inflammation as well as effective parasite killing [77]. Upon disease resolution, microglia progressively revert to a homeostatic state, while the recruited macrophages are rapidly cleared from the brain parenchyma [77]. Notably, BAMs, in contrast to disease-associated microglia (DAM), exhibit long-term transcriptional alterations [77], highlighting their unique functions in CNS disorders. Likewise, to systematically delineate brain macrophages, a study utilized scRNA-seq to interrogate the heterogeneity of myeloid cells in aging brains and murine models of AD [45]. Interestingly, the study has shown that the previously identified DAM in AD brains actually consist of two ontogenetically and functionally distinct cell populations. These include the triggering receptor expressed on myeloid cells 2 (TREM2)-dependent DAM, which exhibit a neuroprotective signature, and monocyte-derived disease inflammatory macrophages (DIMs) that typically accumulate in the brain during aging [45, 62]. Compared to healthy brains, the number of DIMs increases in AD brains and their function is independent of TREM2 [45]. In conclusion, a better understanding of the differential roles of brain macrophage subpopulations, such as BAMs, will aid in developing targeted therapeutic strategies and precision medicine for neurodegenerative and neuroinflammatory diseases.
Border-associated macrophages
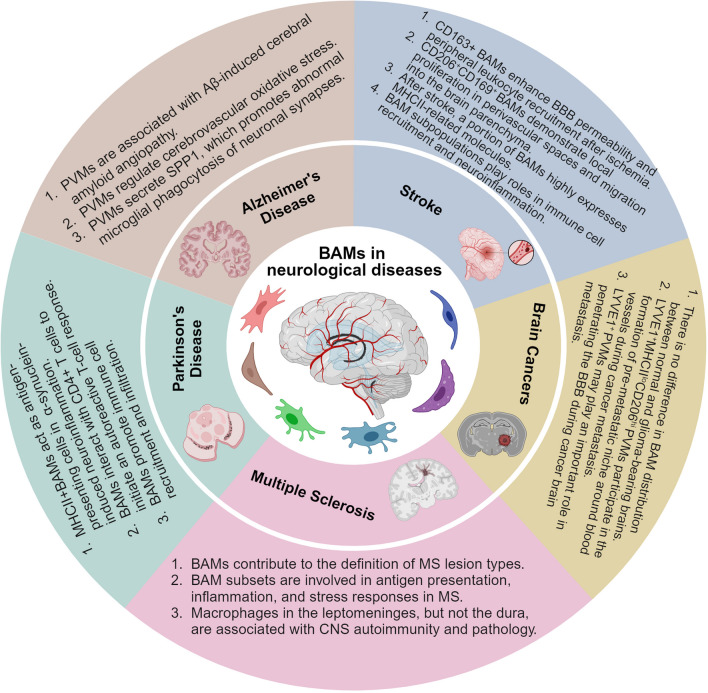
BAMs are receiving more attention since they have been observed to function in diverse cerebral pathologies [19], including AD [78], PD [79], MS [80], ischemic stroke [46], and gliomas [44]. Unlike microglia, BAMs show mixed ontogenies and distinct self-renewal capacity following experimental depletion and repopulation [49]. We now understand that BAMs comprise several anatomical subpopulations, including sdΜΦ, dmΜΦ, cpΜΦ, cpepiΜΦ, and PVMs. Their phenotypes and potential involvement in CNS diseases are gradually being revealed through high-dimensional resolution techniques such as mass cytometry and scRNA-seq, bulk RNA-sequencing, fate-mapping, and microscopy [49]. Herein, we have summarized current data on the characteristics of BAMs (Table 1) and their roles in various neurological diseases (Fig. 2 and Table 2).
Table 1.
Characteristics of brain-resident microglia and BAMs
| Subset | Origin | Turnover | Morphology | Motility | Cell markers | Transcriptional profiles | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Microglia |
Embryonic yolk sac |
Self-renewal |
Homeostasis: ramified; Inflammation: amoeboid; Brain injury: bipolar rod |
Cell bodies: stationary; Processes: actively mobile | SIGLEC-H, SALL1, TMEM119, P2RY12 | Tmem119, Siglech, Slc2a5, P2ry12, Sparc, Fcrls, Olfml3, Sall1, Hexb, Trem2 | [33, 34, 50, 53, 81–83, 91, 96–99, 106] | ||
| BAMs | sdΜΦ | Yolk sac; fetal liver; | Self-renewal | Homeostasis: elongated; Inflammation: extended protrusions | Cell bodies: partial motility; Processes: highly dynamic | CD206, CD38, CD169, CD163, CD36, LYVE1 | Lyve1, P2rx7, Ccr1, Egfl7 | Mrc1, Cd163, Cd169, Cd36, Apoe, Ms4a7, Ms4a6c, Stab1, Lyz2, Pf4, Cbr2, Tgfbi | [45, 48–50, 52, 84, 85, 91] |
| dmΜΦ | Yolk sac; fetal liver | Self-renewal; skull and vertebral bone marrow | Homeostasis: pleomorphic, bipolar with many dendrites; | ||||||
| PVMs | Yolk sac; fetal liver | Self-renewal | Homeostasis: elongated; Inflammation: extended dendritic processes | Cell bodies: stationary; Processes: constantly extending and retracting their protrusions | Mrc1, Cd163, Lyz2, Pf4, Lyve1 | [46–48, 50, 88, 89, 91, 104, 106, 110] | |||
| cpΜΦ | Yolk sac; fetal liver | Self-renewal; blood monocyte | Embryonic: amoeboid with small processes; Adult: cpΜΦ, stellate with long, thin processes; cpepiΜΦ, an amoeboid shape | Cell bodies: non-motile; Processes: highly dynamic | Ccnd2, Ttr, Lilra5 | [48–51, 85–87, 90, 91, 105] | |||
| cpepiΜΦ | Yolk sac; fetal liver | Self-renewal | Cell bodies: high motility; Processes: highly dynamic | Significantly similar to microglia | |||||
Fig. 2.
BAMs are involved in diverse cerebral pathologies. BAMs border-associated macrophages, PVMs perivascular macrophages, MS multiple sclerosis, CNS central nervous system
Table 2.
BAMs in distinct neurological diseases
| Disease | Material | Potential roles of BAMs | References |
|---|---|---|---|
| Alzheimer’s Disease |
1. TgCRND8 transgenic mice 2. Tg2576 transgenic mice |
1. PVM turnover reduce cerebral amyloid angiopathy 2. PVMs express both NOX2 and CD36, which exacerbate the Aβ-induced oxidative stress 3. PVMs promote the expression of C1QA, GRN, and CTSB in microglia, leading to aberrant phagocytosis of neuronal synapses 4. PVMs may express APOE4, which is associated with neurovascular alterations and BBB breakdown |
[78, 114–117, 121, 123–125] |
| Parkinson’s Disease |
1. α-Synuclein overexpression in transgenic mice 2. Human postmortem brain tissues |
1. MHCII+ BAMs present antigens to CD4+ T cells to initiate the anti-α-synuclein CD4+ T cell response 2. The depletion of BAMs reduces microglia activation and the recruitment of Ly6Chi monocytes and CD4+ Th cells 3. BAMs in the meninges may contribute to the clearance of brain α-synuclein via the glymphatic system and meningeal lymphatic vessels 4. CD68+ BAMs have been observed to interact with CD3+ T cells in human brain tissues |
[79, 132, 133] |
| Ischemic Stroke |
1. A rat model of ischemia–reperfusion 2. MCAO mouse model 3. Post-mortem brain tissues of patients |
1. CD163+ BAMs enhance leukocyte recruitment and BBB permeability via the production of VEGF after ischemia 2. Proliferated CD163+ BAMs can migrate to the brain parenchyma and induce inflammation 3. After ischemia, CD206+CD169+ BAMs show proliferation in perivascular spaces and subsequently accumulate in the brain parenchyma 4. MHCII+ BAM subsets may promote neuroinflammation by regulating adaptive immune responses, cellular oxidative phosphorylation, endocytosis, and immune cell recruitment |
[104, 141–143] |
| Multiple Sclerosis |
1. Human samples 2. EAE mouse model |
1. BAMs are associated with MS lesion types and correlate with the pathology of MS 2. A subset of BAMs expressing CD163, F13A1, and LYVE1 is involved in lesion inflammation and antigen presentation 3. Both sdΜΦ and PVMs in the leptomeninges participate in autoantigen presentation and the activation of autoreactive effector T cells 4. PVMs continue to proliferate during the chronic phase of MS |
[80, 150, 151, 154, 157] |
| Brain Cancers |
1. Mouse glioma model of GL261 2. Transgenic mouse with 4T1 mammary adenocarcinoma 3. Human brain metastasis tissues 4. Transgenic mouse models of lung and breast adenocarcinoma brain metastases |
1. BAMs are evenly distributed in both naïve and glioma-bearing brains 2. LYVE1+MHCIIloCD206hi PVMs are involved in the formation of a pre-metastatic niche around blood vessels during cancer metastasis 3. LYVE1+ PVMs may contribute to the breakdown of BBB during tumor metastasis to the brain 4. BAMs located in the meninges may play a role in facilitating cancer metastasis to brain-border structures |
[44, 61, 171, 174, 176, 177] |
Biological features
As is widely known, in the steady state, microglia are highly ramified cells with multiple branches and processes extending from a small soma, actively moving to scan the entire brain parenchyma for detecting any homeostatic or pathological changes [81]. Upon sensing foreign or damage-associated factors, microglia transform from a ramified to an amoeboid shape, embodied in enlarged cell bodies and shortened cell processes. Amoeboid morphology reflects an activated or reactive state associated with phagocytosis and inflammatory functions regardless of whether these are protective or detrimental [13, 82]. Additionally, bipolar rod-shaped microglia have been observed during brain injury, probably related to aberrant neuronal circuits [83]. However, BAM subsets exhibit considerable variation in morphology. During homeostasis, the sdΜΦ and PVM subsets appear in a more elongated shape than microglia, and reside around blood vessels [9, 50, 84]. In contrast, the dmΜΦ are pleomorphic, mostly displaying a bipolar shape with many dendrites [50, 85]. Embryonic choroid plexus macrophages exhibit amoeboid morphologies with small processes [86, 87]. In adults, choroid plexus macrophages are characterized by their stellate shape and long, thin processes [50, 51, 85]. In inflammatory conditions, PVMs extend dendritic processes along the perivascular space in response to environmental chemotactic cues, and meningeal macrophages elongate their existing protrusions [88, 89]. Technical challenges, primarily due to their location in the ventricular system, have limited the observation of choroid plexus macrophages in the inflammatory status. The BAM pool also shows differences in motility, indicating differential adaptive functions among its subsets. PVMs, resembling microglia, exhibit limited motility, constantly extending and retracting their protrusions at the periphery of blood vessels [50, 88]. Similarly, meningeal macrophages display partial motility, using their processes to monitor the meningeal space [50, 84]. Of interest, the cpΜΦ show relatively stationary cell bodies but high motility in their processes which supports their role in immune surveillance. The cpepiΜΦ, on the other hand, demonstrate substantial mobility in their cell bodies, allowing them to patrol the surface of the choroid plexus and closely survey their surroundings [90].
Several studies have outlined the molecular and genetic signatures of BAMs to distinguish them from other tissue macrophages [45, 48–50, 91, 92]. BAMs have been identified to express several pan-macrophage markers similar to those found in microglia, including CD45, CD11b, CSF1R, CD64, F4/80, MERTK, MHCII, CX3CR1, and IBA1 [8, 46, 50, 91, 93], making it challenging to discriminate between these populations. Immunological analyses, such as flow cytometry, have indicated that varying expression levels of certain markers could be used to differentiate between cell populations in studies [50]. For example, BAMs typically express higher levels of CD45 and MHCII compared to microglia [8, 50]. However, the use of CD45 levels as a reference is not always reliable, as some BAM subsets have been shown to express low levels of CD45 [91]. Moreover, under disease conditions, activated microglia exhibit an upregulation of CD45 and infiltrated monocyte-derived cells express high levels of both CD45 and CD11b, which complicate the characterization of BAMs [8, 94, 95]. Conversely, distinguishing microglia from other brain macrophage subsets can be more straightforward, as certain proteins, such as TMEM119 and P2RY12, are exclusively expressed by microglia [96, 97]. SIGLEC-H and SALL1 have also been identified as microglia-specific markers [91, 98, 99]. However, it is notable that the cpepiΜΦ, a subset of choroid plexus macrophages originating from embryonic precursors, also express these markers and display a microglial transcriptome signature [49]. Additionally, while markers such as CX3CR1, TREM2, and CD33 are universally expressed in tissue macrophages, they have been extensively used in studies exploring microglial functions in both the steady-state and perturbed CNS [100–102]. Apparently, these investigations assessed a mixture of CNS macrophages rather than pure microglial populations. Now, both the anatomical compartment and morphology of BAMs have been included in their discrimination from microglia through immunohistochemistry methods [103]. BAMs can also be distinguished from microglia based on CD206 expression, which is extremely low in microglia under the steady-state condition [48, 50, 91, 104]. To further differentiate between BAM subsets, several surface proteins have been suggested, including CD38, MHCII, CCR2, and LYVE1 [91]. Most BAMs, approximately 75% of total cells, are CD38+ (or LYVE1+) MHCII− subset [91]. The CD38+ (or LYVE1+) MHCII+ BAMs mainly make up subpopulations of sdΜΦ and PVMs [91]. The dmΜΦ predominantly contain single-positive MHCII+ BAMs and fewer CD38+ (or LYVE1+) MHCII+ subset [91]. Choroid plexus macrophages consist of three BAM subsets, each with a similar frequency: MHCII−, LYVE1+MHCII+, single-positive MHCII+. Furthermore, MHCII+ BAMs, located in the choroid plexus and dura mater, uniquely express CCR2, suggesting a monocytic origin [91, 105].
In terms of transcriptional profiles, previous reports described microglial signature genes including Tmem119, Siglech, Slc2a5, P2ry12, Sparc, Fcrls, Olfml3, Sall1, Hexb, and Trem2 [49, 50, 54, 91, 106]. However, genes such as Fcrls, Hexb, and Trem2 show comparable expression in certain BAM subsets [46, 49]. As previously mentioned, the cpepiΜΦ subset exhibits significant transcriptional similarities with microglia [49]. Therefore, genes like Sall1, Sparc, Siglech, P2ry12, and Tmem119 are more reliably considered microglial core signatures [49, 54, 107]. Yet, in mouse brains, Sall1 expression has been observed in certain neurons and other glial cells, including astrocytes and oligodendrocytes [108]. During pathological conditions such as MS and AD, none of the aforementioned signature genes, except Hexb, have been found to be consistently expressed in DAM, nevertheless [107, 109]. The Hexb locus has been suggested for use in genetic manipulation and fate mapping of microglia, but not for BAMs, in the CNS [109]. Several sets of genes have been identified as comparatively specific to BAMs over microglia, including those expressed universally across different subsets or uniquely in a specific subpopulation. Common core genes of BAMs, apart from cpepiΜΦ, include Cd206 (Mrc1), Cd163, Cd169, Cd36, Apoe, Ms4a7, Ms4a6c, Stab1, Lyz2, Pf4, Cbr2, and Tgfbi [46, 49, 54]. The transcriptome of PVMs includes genes such as Mrc1, Cd163, Lyz2, Pf4, and Lyve1 [47, 50, 106, 110]. Signature genes enriched in the sdΜΦ include Lyve1, P2rx7, Ccr1, and Egfl7, while those in choroid plexus macrophages include Ccnd2, Ttr, and Lilra5 [49]. Furthermore, in the aging and inflamed CNS, the phenotype and transcriptional profiles of BAMs are adaptively altered but remain distinguishable from other myeloid cell populations through the use of high-dimensional mapping techniques, such as mass cytometry [91, 92, 104]. The utilization of different combinations of myeloid cell makers, including CD45, Cx3CR1, MHCII, CD11c, CD38, CD44, CD206, CD169, CD43, Ly6C, SIGLEC-H, and CD14, allows for more specific definition of cellular subsets [91, 92, 104].
BAMs in Alzheimer’s disease
The anatomical position of BAMs at CNS borders, along with their biological features, confers upon them fundamental functions such as waste clearance, nutrient uptake, antigen recognition and presentation, and regulation of BBB permeability [110, 111]. In AD research, the focus primarily centers on microglia-mediated phagocytic clearance of amyloid β (Aβ) aggregates, yet the impact of BAM subsets on AD progression is less understood. AD, the most common brain disorder causing senile dementia, is characterized by permanent neuronal damage due to excessive extracellular masses of Aβ peptides and intracellular bundles of fibrillar Tau protein [8, 13]. Abnormal deposition of Aβ peptides in cerebral blood vessels can lead to cerebral amyloid angiopathy (CAA), a typical pathological feature of AD [112, 113]. Interestingly, early research using the TgCRND8 mouse model of AD revealed that stimulating PVM turnover, rather than microglial or astrocytic responses, could reduce CAA load, implying a pivotal role for PVMs in CAA progression [114]. Subsequently, it was discovered that PVMs contribute to Aβ-induced cerebrovascular oxidative stress by highly expressing the reactive oxygen species (ROS)-producing enzyme NOX2 and CD36, an Aβ-binding scavenger receptor [115, 116]. In Tg2576 transgenic mice, a model of AD, the deletion of CD36 through PVM repopulation reduced ROS induction and ameliorated neurovascular damage induced by Aβ deposition [117]. Additionally, depleting PVMs with clodronate, a type of chemicals known as bisphosphonates, suppressed ROS production and alleviated Aβ-induced cerebrovascular dysfunction [118]. A recent study indicated that the interaction between PVMs and microglia regulates microglial ability to engulf neuronal synapses during the early onset of AD [78]. PVMs secrete large amounts of SPP1, which promotes microglial expression of phagocytic markers such as C1QA, GRN, and CTSB, leading to aberrant engulfment of synapses [78]. In AD mouse models, the deletion of Spp1 contributed to a reduction in synaptic loss [78]. In addition, ApoE4, the strongest genetic risk factor for late-onset and sporadic forms of AD, has been found to be associated with neurovascular alterations and BBB breakdown [119–121]. ApoE4 carriers show more severe dysregulated cerebral blood flow and cognitive impairment compared to non-carriers [122]. Accordingly, while ApoE4 has been proven to be functionally related to microglia, astrocytes, and oligodendrocytes, its sources and targets may also be linked to PVMs in the brain [123–125]. Future studies investigating the conditional deletion of Apoe4 in PVMs may provide insights into the neurovascular pathologies associated with AD. Importantly, the development of new Cre transgenic mice, capable of differentially targeting parenchymal microglia and Lyve1+ PVMs, will be instrumental in elucidating the role of PVMs in AD progression [47].
BAMs in Parkinson’s disease
PD is a well-known neurodegenerative disease characterized by movement deficits and autonomic dysfunction [126]. The neuropathological hallmarks of PD include the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the formation of intraneuronal protein aggregates, known as Lewy bodies and Lewy neurites, primarily composed of insoluble alpha-synuclein (α-synuclein) [126]. α-Synuclein is mainly expressed in neurons and, to a lesser extent, in astrocytes, microglia, and macrophages [127]. Normally, α-synuclein exists as a soluble monomer, but under cellular stress, it can aggregate into insoluble forms such as oligomers, protofibrils, or fibrils [128]. Misfolded α-synuclein not only directly causes neurotoxicity resulting in neuronal death, but also activates immune cells that beget neuroinflammatory lesions. Microglia have been observed to exacerbate α-synuclein-mediated cerebral pathology by facilitating cell-to-cell transmission of α-synuclein through their exosomes [129]. A recent study has indicated that BAMs, not microglia, play a crucial role in α-synuclein-related neuroinflammation, through acting as antigen-presenting cells to initiate a CD4+ T cell response [79]. MHCII has been proven pivotal in mediating the communication between antigen-presenting cells and α-synuclein-specific CD4+ T cells associated with PD [130, 131]. In an α-synuclein overexpression mouse model of PD, the conditional deletion of MHCII in microglia has shown no effects on α-synuclein-induced neuroinflammation, such as the infiltration of monocytes and T cells [79]. In contrast, the depletion of BAM subsets significantly reduced inflammatory processes, including microglial activation and the recruitment of Ly6Chi monocytes and CD4+ T helper cells [79, 132]. Additionally, an in-depth analysis of the transcriptional profiles of PD-associated BAMs has revealed that only a few BAM subsets undergo proliferation, respond to IFN-γ, and exhibit early stages of activation [79]. Meanwhile, the majority of BAMs remain quiescent, reflecting the proportion of MHCII+ BAMs within the total BAM population [79]. Most BAMs express phagocytosis-related genes, such as Cd68. The “disease-activated BAMs”, however, express multiple genes involved in inflammation, antigen presentation, and immune cell recruitment and infiltration, including Il1b, Itgax, H2-Aa, Cd80, Cd74, Cd274, Ccl5, Cxcl10, and Mmp14 [79, 132]. In human PD postmortem brain tissues, CD3+ T cells have been observed to be closely adjacent to CD68+ BAMs in the perivascular spaces [79]. Overall, these results highlight that, in the pathogenesis of PD, BAMs are indispensable for antigen presentation, T-cell activation, and the infiltration of inflammatory cells. Furthermore, a study employing a transgenic mouse model of PD indicates that meningeal macrophages might participate in the clearance of brain α-synuclein via the glymphatic system and meningeal lymphatic vessels [43, 133]. Taken together, the mechanisms by which distinct subsets of BAM regulate PD progression are worth further exploration and may offer new directions for PD therapy in the future.
BAMs in Stroke
Nowadays, ischemic stroke is a leading cause of mortality and long-term disability worldwide [134]. Both clinical features and brain imaging are used in the diagnosis of ischemic stroke versus intracerebral hemorrhage [134]. Generally, occlusion of a cerebral artery leads to an ischemic stroke, manifested by a severely insufficient blood and oxygen supply to the brain parenchyma, which induces widespread neuroinflammation and neuronal death [135]. After the onset of ischemia, the structure and function of the BBB are progressively disrupted, followed by the influx of hematogenous fluid into the extravascular space, leading to the development of vasogenic edema [136]. During the acute phase of a stroke, pathological changes in endothelial cells and the production of ROS further exacerbate the permeability of the BBB [137]. Microglia are quickly activated within the first few hours and release a considerable amount of pro-inflammatory cytokines, which subsequently recruit peripheral immune cells into brain parenchyma [138]. Infiltrated immune cells, such as monocytes, macrophages, and neutrophils, further aggravate the dysfunction of the BBB [138, 139]. BAMs, especially perivascular macrophages, may contribute to the pathological progression following ischemia due to their location. It has been revealed that BAM subsets are involved in early immune responses and persist into the late chronic phase of stroke [140]. However, the similarity in phenotypes and transcriptional signatures among microglia, BAMs, and blood-borne macrophages has increased the difficulty of accurately identifying pure BAM populations. Nevertheless, several previous studies investigating the role of BAMs in ischemic stroke might provide valuable references for future research.
Using CD163 as a marker for BAMs, a study analyzed the transcriptome of sorted CD163+ brain macrophages 16 h after ischemia–reperfusion in a rat model. It was found that, post-ischemia, these CD163+ BAMs underwent a functional shift towards enhancing leukocyte recruitment and increasing BBB permeability through the induction of vascular endothelial growth factor (VEGF) [141]. Depletion of BAMs using clodronate liposomes led to reduced granulocyte recruitment and decreased permeability of leptomeningeal and cortical vessels 24 h after ischemia [141]. Similarly, in post-mortem brain tissues of ischemic stroke patients, CD163+ PVMs were observed to express VEGF strongly [141]. Another study demonstrated that in both human and rat stroke samples, CD163+ BAMs exhibited both local proliferation and migration to the brain parenchyma three days after ischemic injury [104, 142]. Additionally, RNA-sequencing results indicated that CD163+ BAMs in the brain parenchyma displayed pro-inflammatory phenotypes, with many pro-inflammatory genes, such as Nos2, being highly induced [104]. Furthermore, in a mouse model of ischemic stroke, CD206+CD169+ BAMs demonstrated pronounced proliferation in perivascular spaces after ischemia, followed by their accumulation in the brain parenchyma [104]. Notably, in mice four days post-stroke, as a subset of CD169+ BAMs translocated into the brain parenchyma, blood-borne MDMs began to occupy the perivascular area [104]. Recently, using a mouse model of middle cerebral artery occlusion (MCAO), a study examined the transcriptional profiles of brain cell subsets 24 h after stroke. Six distinct subsets of BAMs, based on core signature genes such as Lyve1, Cd163, Mrc1, and Cbr2, were identified [143]. Moreover, the proportion of BAMs among all cells was found to significantly increase post-ischemia in the mouse brain [143]. Of note, one particular BAM subset, mainly found in the MCAO group, expressed high levels of MHCII-related antigen presentation molecules (such as H2-Aa, H2-Ab1 and Cd74), suggesting that BAMs may play important roles in modulating the adaptive immune response after ischemic stroke [143]. In addition, certain BAM subpopulations expressed genes associated with cellular oxidative phosphorylation, endocytosis, and immune cell recruitment, potentially contributing to aggravated neuroinflammation [143]. It has been proposed that peripheral administration of IL-13 in ischemic stroke could induce anti-inflammatory responses in both microglia and macrophages, leading to neuroprotection [144]. However, in the later chronic phases of stroke, the massive infiltration of myeloid cells from the bone marrow and the phenotypic adaptation of BAMs [46], present challenges in identifying BAM-specific regulations in disease progression. Therefore, more refined genetic models and tools will help resolve many unanswered questions about the role of BAMs in the resolution stage of stroke.
BAMs in multiple sclerosis
MS is a chronic autoimmune disease that causes inflammatory demyelination in the CNS, leading to progressive and irreversible neurodegeneration [68]. It stands as the most common immune-mediated disorder, featuring extensive infiltration and activation of peripheral immune cells in the CNS [68, 145]. Pathologically, MS is marked by demyelination, gliosis, and the loss of axons and neurons [145, 146]. MS typically manifests in young adults and often follows a relapsing–remitting pattern in patients, leading to progressive physical and cognitive disability with aging [147]. Neuroinflammation is recognized as a key mediator of lesion formation in the CNS during both the acute and chronic phases of MS [68, 145, 148]. A large body of evidence suggests that activated microglia and T cells, which accumulate at MS lesion sites, are major contributors to the disease [148, 149]. Sustained inflammation, driven by aberrant activities of microglia and T cells, results in a multitude of molecular stresses that cause damage in BBB, neurons, and oligodendrocytes [150]. Nevertheless, the potential role of BAMs in MS has not been as widely recognized.
So far, a few studies have demonstrated that BAMs may be involved in each stage of MS and contribute to defining lesion types in the CNS [77, 150–156]. An early study found that in normal human brains, CD163+ macrophages were confined to CNS-border areas. However, in MS brains, these macrophages were primarily observed in acute active lesions and at the rims of chronic active lesions, whereas they were rare in chronic inactive lesions and the centers of chronic active lesions [150]. Recent single-cell analyses of human MS identified the immune cell landscape, revealing that several BAM subsets correlate with MS pathology [151]. A subset of BAMs, expressing signature genes such as CD163, F13A1, and LYVE1, was found to be abundantly enriched in active MS lesions, where they are closely associated with inflammation and antigen presentation [80]. This subset was notably scarce in non-active lesions and predominantly located near blood vessels [80]. Conversely, a cluster of BAMs, characterized by genes regulating stress response and oxygen levels, such as heat shock proteins (HSPs), was specially observed in perilesional areas. These areas appeared normal in axonal myelin but showed macrophage infiltration [80].
Furthermore, the assessment of immunological mechanisms in human MS samples is challenging, leading to a reliance on studies conducted in the animal model of experimental autoimmune encephalomyelitis (EAE) [152]. In EAE mice, depending on the in vivo imaging technique, it has been revealed that the functional phenotypes of both microglia and macrophages evolve and adapt to the local microenvironment during the formation and resolution of neuroinflammatory lesions [153]. Importantly, the leptomeninges are now recognized as a crucial factor in MS, as considerable immune cells have been found to infiltrate the leptomeninges in both MS patients and EAE mice, and lesions commonly form in cortical areas adjacent to these locations [154, 155]. Of interest, compared to the leptomeninges, the dural layer has shown significantly less immune cell infiltration during chronic EAE and MS [155]. In addition, macrophages in the dura mater have been found to be less efficient than those in the leptomeninges at autoantigen presentation and T cell activation, leading to a defective inflammatory process in the dural meninges [155]. Biopsies from patients in the early phases of MS have shown that leptomeningeal inflammation often co-occurs with cortical demyelination [156]. Additionally, in most human MS cases, the severity of cortical demyelination has been found to positively correlate with the extent of leptomeningeal inflammation [156]. In the EAE model, the sdΜΦ and PVMs in the leptomeninges, proliferate during disease onset, and participate in antigen presentation and the activation of autoreactive effector T cells, which then migrate into the brain parenchyma and trigger lesion formation [157]. Moreover, during the chronic phase of MS, while the population of sdΜΦ decreases, PVMs continue to proliferate [154]. Thus, distinct BAM subsets exert differential functions in MS, influencing the progression of neuroinflammation.
Currently, there are limited drugs that target both microglia and BAMs for treating progressive MS, characterized by a predominance of proinflammatory myeloid cells [158]. Lately, an investigation showed that inhibiting the insulin-like growth factor-1 (IGF-1) signaling pathway in both microglia and BAMs significantly reduced the severity of CNS inflammation in EAE mice [159]. The absence of IGF-1 signaling resulted in minor changes in microglia but remarkably altered the transcriptional profile of BAMs, underscoring their role in progressive MS [159]. Another study used PLX5622, a CSF1R inhibitor, to deplete both microglia and BAMs. It was found that PLX5622 treatment significantly delayed the onset of EAE, although it had no effect on the chronic progression of the disease [160]. In summary, therapeutic agents targeting brain-resident macrophages offer promising avenues for the treatment of MS and for neuroprotection.
BAMs in brain cancers
Malignant brain tumors can generally be divided into primary tumors that arise in the brain, such as gliomas, and brain metastases (BrMs) from cancers such as non-small-cell lung carcinoma (NSCLC), breast cancer, and melanoma [73]. Brain cancers are typically lethal, with most patients having a very poor prognosis, exemplified by a median survival of less than two years for patients with glioblastoma (GBM), the most malignant primary brain tumor in adults [71]. Studies on gliomas and CNS metastases have unveiled that macrophages represent the most abundant stromal cell-type and comprise up to 30–50% of the tumor mass [71, 161]. In the CNS, tumor-associated macrophages (TAMs) are a heterogeneous population that includes brain-resident microglia, BAMs and MDMs, which together create an immunosuppressive tumor microenvironment (TME) [73, 162]. TAMs contribute to most hallmarks of brain cancers, including tumor growth, invasion, metastasis, angiogenesis, and immune evasion [71, 73, 162]. Moreover, TAMs affect therapeutic responses in patients and limit the clinical efficacy of most immunotherapies such as immune checkpoint inhibitor (ICB), by fostering a symbiotic interaction between tumor cells and the TME [163]. Although TAMs have been extensively studied during past decades, research specifying the functional features of different TAMs subtypes within tumor lesions has been inadequate. Until lately, advances in single-cell technologies have enabled the characterization of TAMs at the single-cell level, identifying subpopulations with distinct tumor-modulatory functions.
In gliomas and BrMs, TAMs outnumber other immune cell populations such as dendritic cells, T cells, natural killer (NK) cells and neutrophils [164, 165]. Remarkably, TAMs are more abundant in primary brain tumors than in BrMs [164, 165]. Microglia and MDMs predominate among TAMs, leading most research to focus on understanding the functions of these two populations [71, 73, 161, 162]. It has been revealed that tumor-associated microglia and MDMs display distinct spatial distributions and have incompletely overlapping functions in brain tumors [166–168]. However, despite their potential importance in tumor angiogenesis, invasion, and metastasis through the modulation of vascular integrity and function [169], the role of BAMs in brain tumors has been less frequently reported.
A recent study revealed that BAMs are evenly distributed in both naïve and glioma-bearing mouse brains, with a significant cluster of cells highly and specifically expressing BAM markers such as Pf4, Dab2, and F13a1 [44]. However, no distinct cluster was identified exclusively in glioma-bearing brains. It appears that metastatic cancer cells, which must breach the CNS interface to colonize the brain, are likely to interact with BAMs. PVMs, situated around arteries and veins in the brain parenchyma, may facilitate the extravasation of cancer cells across the BBB and aid in the formation of pre-metastatic niches. In specimens of human brain metastases from breast cancer, NSCLC, and melanoma, significant intra- and peri-tumoral infiltration of brain microglia and macrophages has been observed [170]. These cells display phenotypes associated with enhanced phagocytic and pro-tumorigenic functions [170]. A subset of PVMs, characterized as LYVE1+MHCIIloCD206hi macrophages, is found in various healthy tissues, including the brain [61]. Of late, this macrophage subpopulation has been shown to coordinate the formation of multi-cellular “nest” structures near blood vessels, which leads to reduced effectiveness of the chemotherapy, in a murine model of breast cancer [171]. The inhibition of Tie2 activity in a PVM subpopulation that expresses Tie2 and VEGFA has been shown to prevent breast cancer metastasis and improve the overall survival in animal models [172]. In addition, there is evidence suggesting that PVM activation via tenascin C signaling is critical for co-opting endothelial cells to create a pro-metastatic vascular niche, facilitating breast cancer colonization in the lung [173]. LYVE1+ PVMs in the lung have been shown to maintain vascular tone by interacting with vascular smooth muscle cells, thereby contributing to the metastasis of cancer cells through the bloodstream [174]. Furthermore, penetrating the BBB poses a major challenge for melanoma cells in establishing melanoma brain metastases (MBM). Proteases such as cathepsin-S, have been reported to be essential for BBB breakdown [175], with brain-resident PVMs potentially being an important source of these proteases [176]. Consequently, functional remodeling of PVMs located in the brain may be one of the requisites for the formation of brain metastases. In addition, brain metastasis to the dura and leptomeninges has also been observed in patients with melanoma, lung, and breast cancers [177]. Although currently the data on the definitive role of BAMs in these tumors is lacking, it is probable that meningeal macrophages and PVMs, due to their anatomical locations, are involved in the formation of metastatic niches and the modulation of the local TME.
Conclusion
Over the years, the potential role of BAMs in CNS-associated diseases has often been overlooked. Indeed, the number of BAMs is much lower than that of microglial cells, which play critical roles in brain development and neuronal excitability. In the past, techniques to fully assess these myeloid cell populations were underdeveloped. Recent advances in lineage tracing and the utilization of genetic models have largely unraveled the puzzle of BAM ontogenesis, setting the stage for further exploration of their functions. Particularly, the use of multi-omics technology in studying diverse CNS diseases has unveiled that BAM subpopulations are intricately linked with the pathogenesis and disease progression. However, most current data addressing the specific functions of BAMs and microglia under disease conditions, struggle with the technical challenge of differentiating and exclusively interrogating these cells, partly due to their phenotypic overlap and similarities. Nonetheless, understanding the precise role of different BAM subsets in both neurodegenerative and neuroinflammatory diseases is crucial, as the development of novel targeted therapies depends on an in-depth understanding of how distinct immune cell populations contribute to the CNS neuropathology. Future research on the genetic or epigenetic manipulation of specific BAM subpopulations will be pivotal in pinpointing their roles in various diseases.
Abbreviations
- AD
Alzheimer's disease
- Aβ
Amyloid β
- α-synuclein
Alpha-synuclein
- BAMs
Border-associated macrophages
- BBB
Blood–brain barrier
- BCB
Blood-cerebrospinal fluid barrier
- BrMs
Brain metastases
- CAA
Cerebral amyloid angiopathy
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- cpMФ
Stromal choroid plexus macrophages
- cpepiMФ
Choroid epiplexus macrophages
- DAM
Disease-associated microglia
- DIMs
Disease inflammatory macrophages
- dmMФ
Dural macrophages
- EAE
Experimental autoimmune encephalomyelitis
- GBM
Glioblastoma
- HSCs
Hematopoietic stem cells
- HSPs
Heat shock proteins
- ICB
Immune checkpoint inhibitor
- IGF-1
Insulin-like growth factor-1
- MBM
Melanoma brain metastases
- MCAO
Middle cerebral artery occlusion
- MDMs
Monocyte-derived macrophages
- MHCII
Major histocompatibility complex class II
- MPS
Mononuclear phagocytic system
- MS
Multiple sclerosis
- NK
Natural killer
- NSCLC
Non-small-cell lung carcinoma
- PD
Parkinson’s disease
- PVMs
Perivascular macrophages
- ROS
Reactive oxygen species
- scRNA-seq
Single-cell RNA sequencing
- sdMФ
Subdural/leptomeningeal macrophages
- SNpc
Substantia nigra pars compacta
- TAMs
Tumor-associated macrophages
- TME
Tumor microenvironment
- TREM2
Triggering receptor expressed on myeloid cells 2
- VEGF
Vascular endothelial growth factor
Author contributions
Conceptualization: R. S., H. J.; literature documentation, manuscript writing: R. S.; editing: R. S., H. J.; supervision: R. S., H. J.
Funding
This work was supported by (Fund NC0006597 to H. J.) granted by Department of Anesthesiology, Washington University School of Medicine in St. Louis.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rui Sun, Email: rsun4186@gmail.com.
Haowu Jiang, Email: haowu.jiang@wustl.edu.
References
- 1.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Logie C, Stunnenberg HG. Epigenetic memory: a macrophage perspective. Semin Immunol. 2016;28(4):359–367. doi: 10.1016/j.smim.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 6.Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastorakos P, McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci Immunol. 2019;4(37):eaav0492. doi: 10.1126/sciimmunol.aav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–242. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- 9.Kierdorf K, Masuda T, Jordão MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019;20(9):547–562. doi: 10.1038/s41583-019-0201-x. [DOI] [PubMed] [Google Scholar]
- 10.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48(2):405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 11.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 12.Sierra A, de Castro F, Del Río-Hortega J, Rafael Iglesias-Rozas J, Garrosa M, Kettenmann H. The "Big-Bang" for modern glial biology: translation and comments on Pío del Río-Hortega 1919 series of papers on microglia. Glia. 2016;64(11):1801–1840. doi: 10.1002/glia.23046. [DOI] [PubMed] [Google Scholar]
- 13.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar E, Barak B. Microglia roles in synaptic plasticity and myelination in homeostatic conditions and neurodevelopmental disorders. Glia. 2019;67(11):2125–2141. doi: 10.1002/glia.23637. [DOI] [PubMed] [Google Scholar]
- 15.Safaiyan S, Kannaiyan N, Snaidero N, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19(8):995–998. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018;173(5):1073–1081. doi: 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Blank T, Prinz M. Microglia as modulators of cognition and neuropsychiatric disorders. Glia. 2013;61(1):62–70. doi: 10.1002/glia.22372. [DOI] [PubMed] [Google Scholar]
- 18.Loane DJ, Kumar A. Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp Neurol. 2016;275(3):316–327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silvin A, Qian J, Ginhoux F. Brain macrophage development, diversity and dysregulation in health and disease. Cell Mol Immunol. 2023;20(11):1277–1289. doi: 10.1038/s41423-023-01053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mundt S, Greter M, Becher B. The CNS mononuclear phagocyte system in health and disease. Neuron. 2022;110(21):3497–3512. doi: 10.1016/j.neuron.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Steven JL. Metchnikoff on the comparative pathology of inflammation. Glasgow Med J. 1892;38(3):195–205. [PMC free article] [PubMed] [Google Scholar]
- 22.Baas J, Senninger N, Elser H. The reticuloendothelial system. An overview of function, pathology and recent methods of measurement. Z Gastroenterol. 1994;32(2):117–23. [PubMed] [Google Scholar]
- 23.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]
- 25.Sawyer RT, Strausbauch PH, Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest. 1982;46(2):165–170. [PubMed] [Google Scholar]
- 26.Parwaresch MR, Wacker HH. Origin and kinetics of resident tissue macrophages. Parabiosis studies with radiolabelled leucocytes. Cell Tissue Kinet. 1984;17(1):25–39. doi: 10.1111/j.1365-2184.1984.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 27.Czernielewski JM, Demarchez M. Further evidence for the self-reproducing capacity of Langerhans cells in human skin. J Invest Dermatol. 1987;88(1):17–20. doi: 10.1111/1523-1747.ep12464659. [DOI] [PubMed] [Google Scholar]
- 28.Melnicoff MJ, Horan PK, Breslin EW, Morahan PS. Maintenance of peritoneal macrophages in the steady state. J Leukoc Biol. 1998;44(5):367–375. doi: 10.1002/jlb.44.5.367. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Yamamura F, Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol. 1989;45(2):87–96. doi: 10.1002/jlb.45.2.87. [DOI] [PubMed] [Google Scholar]
- 30.Naito M, Umeda S, Yamamoto T, et al. Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J Leukoc Biol. 1996;59(2):133–138. doi: 10.1002/jlb.59.2.133. [DOI] [PubMed] [Google Scholar]
- 31.Lichanska AM, Hume DA. Origins and functions of phagocytes in the embryo. Exp Hematol. 2000;28(6):601–611. doi: 10.1016/s0301-472x(00)00157-0. [DOI] [PubMed] [Google Scholar]
- 32.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 33.Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 35.Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guilliams M, Ginhoux F, Jakubzick C, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Greter M, Lelios I, Pelczar P, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37(6):1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collin M, Milne P. Langerhans cell origin and regulation. Curr Opin Hematol. 2016;23(1):28–35. doi: 10.1097/MOH.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doebel T, Voisin B, Nagao K. Langerhans cells—the macrophage in dendritic cell clothing. Trends Immunol. 2017;38(11):817–828. doi: 10.1016/j.it.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Bogale TA, Faustini G, Longhena F, Mitola S, Pizzi M, Bellucci A. Alpha-synuclein in the regulation of brain endothelial and perivascular cells: gaps and future perspectives. Front Immunol. 2021;12:611761. doi: 10.3389/fimmu.2021.611761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochocka N, Segit P, Walentynowicz KA, et al. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat Commun. 2021;12(1):1151. doi: 10.1038/s41467-021-21407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silvin A, Uderhardt S, Piot C, et al. Dual ontogeny of disease-associated microglia and disease inflammatory macrophages in aging and neurodegeneration. Immunity. 2022;55(8):1448–1465.e6. doi: 10.1016/j.immuni.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Gerganova G, Riddell A, Miller AA. CNS border-associated macrophages in the homeostatic and ischaemic brain. Pharmacol Ther. 2022;240:108220. doi: 10.1016/j.pharmthera.2022.108220. [DOI] [PubMed] [Google Scholar]
- 47.Kim JS, Kolesnikov M, Peled-Hajaj S, et al. A binary cre transgenic approach dissects microglia and CNS border-associated macrophages. Immunity. 2021;54(1):176–190.e7. doi: 10.1016/j.immuni.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Utz SG, See P, Mildenberger W, et al. Early fate defines microglia and non-parenchymal brain macrophage development. Cell. 2020;181(3):557–573.e18. doi: 10.1016/j.cell.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 49.Van Hove H, Martens L, Scheyltjens I, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22(6):1021–1035. doi: 10.1038/s41593-019-0393-4. [DOI] [PubMed] [Google Scholar]
- 50.Goldmann T, Wieghofer P, Jordão MJ, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17(7):797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cui J, Xu H, Lehtinen MK. Macrophages on the margin: choroid plexus immune responses. Trends Neurosci. 2021;44(11):864–875. doi: 10.1016/j.tins.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cugurra A, Mamuladze T, Rustenhoven J, et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021;373(6553):eabf7844. doi: 10.1126/science.abf7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Füger P, Hefendehl JK, Veeraraghavalu K, et al. Microglia turnover with aging and in an Alzheimer's model via long-term in vivo single-cell imaging. Nat Neurosci. 2017;20(10):1371–1376. doi: 10.1038/nn.4631. [DOI] [PubMed] [Google Scholar]
- 54.Jordão MJC, Sankowski R, Brendecke SM, et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363(6425):eaat7554. doi: 10.1126/science.aat7554. [DOI] [PubMed] [Google Scholar]
- 55.Mildenberger W, Stifter SA, Greter M. Diversity and function of brain-associated macrophages. Curr Opin Immunol. 2022;76:102181. doi: 10.1016/j.coi.2022.102181. [DOI] [PubMed] [Google Scholar]
- 56.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17(6):349–362. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 58.Jakubzick C, Gautier EL, Gibbings SL, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39(3):599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamoutounour S, Guilliams M, Montanana Sanchis F, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39(5):925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Gibbings SL, Goyal R, Desch AN, et al. Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood. 2015;126(11):1357–1366. doi: 10.1182/blood-2015-01-624809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ensan S, Li A, Besla R, et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol. 2016;17(2):159–168. doi: 10.1038/ni.3343. [DOI] [PubMed] [Google Scholar]
- 62.De Maeyer RPH, Chambers ES. The impact of ageing on monocytes and macrophages. Immunol Lett. 2021;230:1–10. doi: 10.1016/j.imlet.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain. 2016;139(Pt3):653–661. doi: 10.1093/brain/awv395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montagne A, Barnes SR, Sweeney MD, et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verheggen ICM, de Jong JJA, van Boxtel MPJ, et al. Increase in blood–brain barrier leakage in healthy, older adults. Geroscience. 2020;42(4):1183–1193. doi: 10.1007/s11357-020-00211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banks WA, Reed MJ, Logsdon AF, Rhea EM, Erickson MA. Healthy aging and the blood–brain barrier. Nat Aging. 2021;1(3):243–254. doi: 10.1038/s43587-021-00043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andjelkovic AV, Situ M, Citalan-Madrid AF, Stamatovic SM, Xiang J, Keep RF. Blood–brain barrier dysfunction in normal aging and neurodegeneration: mechanisms, impact, and treatments. Stroke. 2023;54(3):661–672. doi: 10.1161/STROKEAHA.122.040578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramaglia V, Rojas O, Naouar I, Gommerman JL. The ins and outs of central nervous system inflammation-lessons learned from multiple sclerosis. Annu Rev Immunol. 2021;39:199–226. doi: 10.1146/annurev-immunol-093019-124155. [DOI] [PubMed] [Google Scholar]
- 69.Zhang W, Zhao J, Wang R, et al. Macrophages reprogram after ischemic stroke and promote efferocytosis and inflammation resolution in the mouse brain. CNS Neurosci Ther. 2019;25(12):1329–1342. doi: 10.1111/cns.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95(6):1246–1265. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun R, Kim AH. The multifaceted mechanisms of malignant glioblastoma progression and clinical implications. Cancer Metastasis Rev. 2022;41(4):871–898. doi: 10.1007/s10555-022-10051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun R, Han R, McCornack C, et al. TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma. Sci Adv. 2023;9(19):eade3559. doi: 10.1126/sciadv.ade3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winkler B, Funke D, Benmimoun B, et al. Brain inflammation triggers macrophage invasion across the blood–brain barrier in. Sci Adv. 2021;7(44):eabh0050. doi: 10.1126/sciadv.abh0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rebejac J, Eme-Scolan E, Arnaud Paroutaud L, et al. Meningeal macrophages protect against viral neuroinfection. Immunity. 2022;55(11):2103–2117.e10. doi: 10.1016/j.immuni.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Shemer A, Grozovski J, Tay TL, et al. Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat Commun. 2018;9(1):5206. doi: 10.1038/s41467-018-07548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Vlaminck K, Van Hove H, Kancheva D, et al. Differential plasticity and fate of brain-resident and recruited macrophages during the onset and resolution of neuroinflammation. Immunity. 2022;55(11):2085–2102.e9. doi: 10.1016/j.immuni.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 78.De Schepper S, Ge JZ, Crowley G, et al. Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer's disease. Nat Neurosci. 2023;26(3):406–415. doi: 10.1038/s41593-023-01257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schonhoff AM, Figge DA, Williams GP, et al. Border-associated macrophages mediate the neuroinflammatory response in an alpha-synuclein model of Parkinson disease. Nat Commun. 2023;14(1):3754. doi: 10.1038/s41467-023-39060-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miedema A, Gerrits E, Brouwer N, et al. Brain macrophages acquire distinct transcriptomes in multiple sclerosis lesions and normal appearing white matter. Acta Neuropathol Commun. 2022;10(1):8. doi: 10.1186/s40478-021-01306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 82.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 83.Ziebell JM, Taylor SE, Cao T, Harrison JL, Lifshitz J. Rod microglia: elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J Neuroinflammation. 2012;9:247. doi: 10.1186/1742-2094-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nayak D, Zinselmeyer BH, Corps KN, McGavern DB. In vivo dynamics of innate immune sentinels in the CNS. Intravital. 2012;1(2):95–106. doi: 10.4161/intv.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McMenamin PG, Wealthall RJ, Deverall M, Cooper SJ, Griffin B. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res. 2003;313(3):259–269. doi: 10.1007/s00441-003-0779-0. [DOI] [PubMed] [Google Scholar]
- 86.Cui J, Shipley FB, Shannon ML, et al. Inflammation of the embryonic choroid plexus barrier following maternal immune Activation. Dev Cell. 2020;55(5):617–628.e6. doi: 10.1016/j.devcel.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dani N, Herbst RH, McCabe C, et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184(11):3056–3074.e21. doi: 10.1016/j.cell.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barkauskas DS, Evans TA, Myers J, Petrosiute A, Silver J, Huang AY. Extravascular CX3CR1+ cells extend intravascular dendritic processes into intact central nervous system vessel lumen. Microsc Microanal. 2013;19(4):778–790. doi: 10.1017/S1431927613000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Russo MV, Latour LL, McGavern DB. Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat Immunol. 2018;19(5):442–452. doi: 10.1038/s41590-018-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shipley FB, Dani N, Xu H, et al. Tracking calcium dynamics and immune surveillance at the choroid plexus blood-cerebrospinal fluid interface. Neuron. 2020;108(4):623–639.e10. doi: 10.1016/j.neuron.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mrdjen D, Pavlovic A, Hartmann FJ, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48(2):380–395.e6. doi: 10.1016/j.immuni.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Ajami B, Samusik N, Wieghofer P, et al. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat Neurosci. 2018;21(4):541–551. doi: 10.1038/s41593-018-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14(10):1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 94.Ju H, Park KW, Kim ID, Cave JW, Cho S. Phagocytosis converts infiltrated monocytes to microglia-like phenotype in experimental brain ischemia. J Neuroinflammation. 2022;19(1):190. doi: 10.1186/s12974-022-02552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci. 2018;19(10):622–635. doi: 10.1038/s41583-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Butovsky O, Jedrychowski MP, Moore CS, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA. 2016;113(12):E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Konishi H, Kobayashi M, Kunisawa T, et al. Siglec-H is a microglia-specific marker that discriminates microglia from CNS-associated macrophages and CNS-infiltrating monocytes. Glia. 2017;65(12):1927–1943. doi: 10.1002/glia.23204. [DOI] [PubMed] [Google Scholar]
- 99.Buttgereit A, Lelios I, Yu X, et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol. 2016;17(12):1397–1406. doi: 10.1038/ni.3585. [DOI] [PubMed] [Google Scholar]
- 100.Zhan Y, Paolicelli RC, Sforazzini F, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17(3):400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 101.Colonna M, Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci. 2016;17(4):201–207. doi: 10.1038/nrn.2016.7. [DOI] [PubMed] [Google Scholar]
- 102.Griciuc A, Serrano-Pozo A, Parrado AR, et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78(4):631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Swanson MEV, Murray HC, Ryan B, Faull RLM, Dragunow M, Curtis MA. Quantitative immunohistochemical analysis of myeloid cell marker expression in human cortex captures microglia heterogeneity with anatomical context. Sci Rep. 2020;10(1):11693. doi: 10.1038/s41598-020-68086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rajan WD, Wojtas B, Gielniewski B, et al. Defining molecular identity and fates of CNS-border associated macrophages after ischemic stroke in rodents and humans. Neurobiol Dis. 2020;137:104722. doi: 10.1016/j.nbd.2019.104722. [DOI] [PubMed] [Google Scholar]
- 105.Brioschi S, Belk JA, Peng V, et al. A Cre-deleter specific for embryo-derived brain macrophages reveals distinct features of microglia and border macrophages. Immunity. 2023;56(5):1027–1045.e8. doi: 10.1016/j.immuni.2023.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeisel A, Muñoz-Manchado AB, Codeluppi S, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347(6226):1138–42. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 107.Masuda T, Sankowski R, Staszewski O, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566(7744):388–392. doi: 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 108.Chappell-Maor L, Kolesnikov M, Kim JS, et al. Comparative analysis of CreER transgenic mice for the study of brain macrophages: a case study. Eur J Immunol. 2020;50(3):353–362. doi: 10.1002/eji.201948342. [DOI] [PubMed] [Google Scholar]
- 109.Masuda T, Amann L, Sankowski R, et al. Novel Hexb-based tools for studying microglia in the CNS. Nat Immunol. 2020;21(7):802–815. doi: 10.1038/s41590-020-0707-4. [DOI] [PubMed] [Google Scholar]
- 110.Fabriek BO, Van Haastert ES, Galea I, et al. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia. 2005;51(4):297–305. doi: 10.1002/glia.20208. [DOI] [PubMed] [Google Scholar]
- 111.Lim HY, Lim SY, Tan CK, et al. Hyaluronan receptor LYVE-1-expressing macrophages maintain arterial tone through hyaluronan-mediated regulation of smooth muscle cell collagen. Immunity. 2018;49(2):326–341.e7. doi: 10.1016/j.immuni.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 112.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nat Rev Neurol. 2020;16(1):30–42. doi: 10.1038/s41582-019-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jucker M, Walker LC. Alzheimer's disease: from immunotherapy to immunoprevention. Cell. 2023;186(20):4260–4270. doi: 10.1016/j.cell.2023.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci USA. 2009;106(4):1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Santisteban MM, Ahn SJ, Lane D, et al. Endothelium-macrophage crosstalk mediates blood–brain barrier dysfunction in hypertension. Hypertension. 2020;76(3):795–807. doi: 10.1161/HYPERTENSIONAHA.120.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park L, Wang G, Zhou P, et al. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci USA. 2011;108(12):5063–5068. doi: 10.1073/pnas.1015413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Uekawa K, Hattori Y, Ahn SJ, et al. Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress. Mol Neurodegener. 2023;18(1):73. doi: 10.1186/s13024-023-00660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park L, Uekawa K, Garcia-Bonilla L, et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ Res. 2017;121(3):258–269. doi: 10.1161/CIRCRESAHA.117.311054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol. 2019;15(9):501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Montagne A, Nikolakopoulou AM, Huuskonen MT, et al. APOE4 accelerates advanced-stage vascular and neurodegenerative disorder in old Alzheimer's mice via cyclophilin A independently of amyloid-β. Nat Aging. 2021;1(7):624. doi: 10.1038/s43587-021-00090-y. [DOI] [PubMed] [Google Scholar]
- 122.Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581(7806):71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yin Z, Rosenzweig N, Kleemann KL, et al. APOE4 impairs the microglial response in Alzheimer's disease by inducing TGFβ-mediated checkpoints. Nat Immunol. 2023;24(11):1839–1853. doi: 10.1038/s41590-023-01627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tcw J, Qian L, Pipalia NH, et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell. 2022;185(13):2213–2233.e25. doi: 10.1016/j.cell.2022.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blanchard JW, Akay LA, Davila-Velderrain J, et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature. 2022;611(7937):769–779. doi: 10.1038/s41586-022-05439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 127.Pei Y, Maitta RW. Alpha synuclein in hematopoiesis and immunity. Heliyon. 2019;5(10):e02590. doi: 10.1016/j.heliyon.2019.e02590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4(11):1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 129.Guo M, Wang J, Zhao Y, et al. Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease. Brain. 2020;143(5):1476–1497. doi: 10.1093/brain/awaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Williams GP, Schonhoff AM, Jurkuvenaite A, Gallups NJ, Standaert DG, Harms AS. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson's disease. Brain. 2021;144(7):2047–2059. doi: 10.1093/brain/awab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harms AS, Cao S, Rowse AL, et al. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33(23):9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frosch M, Amann L, Prinz M. CNS-associated macrophages shape the inflammatory response in a mouse model of Parkinson's disease. Nat Commun. 2023;14(1):3753. doi: 10.1038/s41467-023-39061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zou W, Pu T, Feng W, et al. Blocking meningeal lymphatic drainage aggravates Parkinson's disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener. 2019;8:7. doi: 10.1186/s40035-019-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Campbell BCV, Khatri P. Stroke. Lancet. 2020;396(10244):129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- 135.Zhu H, Hu S, Li Y, et al. Interleukins and ischemic stroke. Front Immunol. 2022;13:828447. doi: 10.3389/fimmu.2022.828447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khatri R, McKinney AM, Swenson B, Janardhan V. Blood–brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13 Suppl 1):S52–S57. doi: 10.1212/WNL.0b013e3182697e70. [DOI] [PubMed] [Google Scholar]
- 137.Candelario-Jalil E, Dijkhuizen RM, Magnus T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. 2022;53(5):1473–1486. doi: 10.1161/STROKEAHA.122.036946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Planas AM. Role of immune cells migrating to the ischemic brain. Stroke. 2018;49(9):2261–2267. doi: 10.1161/STROKEAHA.118.021474. [DOI] [PubMed] [Google Scholar]
- 139.Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 140.Blank-Stein N, Mass E. Macrophage and monocyte subsets in response to ischemic stroke. Eur J Immunol. 2023;53(10):e2250233. doi: 10.1002/eji.202250233. [DOI] [PubMed] [Google Scholar]
- 141.Pedragosa J, Salas-Perdomo A, Gallizioli M, et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol Commun. 2018;6(1):76. doi: 10.1186/s40478-018-0581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Holfelder K, Schittenhelm J, Trautmann K, Haybaeck J, Meyermann R, Beschorner R. De novo expression of the hemoglobin scavenger receptor CD163 by activated microglia is not associated with hemorrhages in human brain lesions. Histol Histopathol. 2011;26(8):1007–17. doi: 10.14670/HH-26.1007. [DOI] [PubMed] [Google Scholar]
- 143.Zheng K, Lin L, Jiang W, et al. Single-cell RNA-seq reveals the transcriptional landscape in ischemic stroke. J Cereb Blood Flow Metab. 2022;42(1):56–73. doi: 10.1177/0271678X211026770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kolosowska N, Keuters MH, Wojciechowski S, et al. Peripheral administration of IL-13 induces anti-inflammatory microglial/macrophage responses and provides neuroprotection in ischemic stroke. Neurotherapeutics. 2019;16(4):1304–1319. doi: 10.1007/s13311-019-00761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 146.Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380–1390.e2. doi: 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lünemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16(1):56–62. doi: 10.1038/s41582-019-0268-z. [DOI] [PubMed] [Google Scholar]
- 148.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 149.Dong Y, Yong VW. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat Rev Neurol. 2019;15(12):704–717. doi: 10.1038/s41582-019-0253-6. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Z, Zhang ZY, Schittenhelm J, Wu Y, Meyermann R, Schluesener HJ. Parenchymal accumulation of CD163+ macrophages/microglia in multiple sclerosis brains. J Neuroimmunol. 2011;237(1–2):73–79. doi: 10.1016/j.jneuroim.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 151.Ostkamp P, Deffner M, Schulte-Mecklenbeck A, et al. A single-cell analysis framework allows for characterization of CSF leukocytes and their tissue of origin in multiple sclerosis. Sci Transl Med. 2022;14(673):eadc9778. doi: 10.1126/scitranslmed.adc9778. [DOI] [PubMed] [Google Scholar]
- 152.t’Hart BA, Gran B, Weissert R. EAE: imperfect but useful models of multiple sclerosis. Trends Mol Med. 2011;17(3):119–25. doi: 10.1016/j.molmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 153.Locatelli G, Theodorou D, Kendirli A, et al. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci. 2018;21(9):1196–1208. doi: 10.1038/s41593-018-0212-3. [DOI] [PubMed] [Google Scholar]
- 154.Derk J, Jones HE, Como C, Pawlikowski B, Siegenthaler JA. Living on the edge of the CNS: meninges cell diversity in health and disease. Front Cell Neurosci. 2021;15:703944. doi: 10.3389/fncel.2021.703944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Merlini A, Haberl M, Strauß J, et al. Distinct roles of the meningeal layers in CNS autoimmunity. Nat Neurosci. 2022;25(7):887–899. doi: 10.1038/s41593-022-01108-3. [DOI] [PubMed] [Google Scholar]
- 156.Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365(23):2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schläger C, Körner H, Krueger M, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530(7590):349–353. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 158.Kamma E, Lasisi W, Libner C, Ng HS, Plemel JR. Central nervous system macrophages in progressive multiple sclerosis: relationship to neurodegeneration and therapeutics. J Neuroinflammation. 2022;19(1):45. doi: 10.1186/s12974-022-02408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ivan DC, Berve KC, Walthert S, et al. Insulin-like growth factor-1 receptor controls the function of CNS-resident macrophages and their contribution to neuroinflammation. Acta Neuropathol Commun. 2023;11(1):35. doi: 10.1186/s40478-023-01535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Montilla A, Zabala A, Er-Lukowiak M, et al. Microglia and meningeal macrophages depletion delays the onset of experimental autoimmune encephalomyelitis. Cell Death Dis. 2023;14(1):16. doi: 10.1038/s41419-023-05551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xuan W, Lesniak MS, James CD, Heimberger AB, Chen P. Context-dependent glioblastoma-macrophage/microglia symbiosis and associated mechanisms. Trends Immunol. 2021;42(4):280–292. doi: 10.1016/j.it.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pang L, Khan F, Heimberger AB, Chen P. Mechanism and therapeutic potential of tumor-immune symbiosis in glioblastoma. Trends Cancer. 2022;8(10):839–854. doi: 10.1016/j.trecan.2022.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Klemm F, Maas RR, Bowman RL, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181(7):1643–1660.e17. doi: 10.1016/j.cell.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Friebel E, Kapolou K, Unger S, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell. 2020;181(7):1626–1642.e20. doi: 10.1016/j.cell.2020.04.055. [DOI] [PubMed] [Google Scholar]
- 166.Darmanis S, Sloan SA, Croote D, et al. Single-cell RNA-Seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 2017;21(5):1399–1410. doi: 10.1016/j.celrep.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yu K, Hu Y, Wu F, et al. Surveying brain tumor heterogeneity by single-cell RNA-sequencing of multi-sector biopsies. Natl Sci Rev. 2020;7(8):1306–1318. doi: 10.1093/nsr/nwaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guldner IH, Wang Q, Yang L, et al. CNS-native myeloid cells drive immune suppression in the brain metastatic niche through Cxcl10. Cell. 2020;183(5):1234–1248.e25. doi: 10.1016/j.cell.2020.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reymond N, d'Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 170.Berghoff AS, Lassmann H, Preusser M, Höftberger R. Characterization of the inflammatory response to solid cancer metastases in the human brain. Clin Exp Metastasis. 2013;30(1):69–81. doi: 10.1007/s10585-012-9510-4. [DOI] [PubMed] [Google Scholar]
- 171.Anstee JE, Feehan KT, Opzoomer JW, et al. LYVE-1+ macrophages form a collaborative CCR5-dependent perivascular niche that influences chemotherapy responses in murine breast cancer. Dev Cell. 2023;58(17):1548–1561.e10. doi: 10.1016/j.devcel.2023.06.006. [DOI] [PubMed] [Google Scholar]
- 172.Harney AS, Karagiannis GS, Pignatelli J, et al. The selective Tie2 inhibitor Rebastinib blocks recruitment and function of Tie2. Mol Cancer Ther. 2017;16(11):2486–2501. doi: 10.1158/1535-7163.MCT-17-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hongu T, Pein M, Insua-Rodríguez J, et al. Perivascular tenascin C triggers sequential activation of macrophages and endothelial cells to generate a pro-metastatic vascular niche in the lungs. Nat Cancer. 2022;3(4):486–504. doi: 10.1038/s43018-022-00353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aegerter H, Lambrecht BN, Jakubzick CV. Biology of lung macrophages in health and disease. Immunity. 2022;55(9):1564–1580. doi: 10.1016/j.immuni.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Phadke M, Ozgun A, Eroglu Z, Smalley KSM. Melanoma brain metastases: biological basis and novel therapeutic strategies. Exp Dermatol. 2022;31(1):31–42. doi: 10.1111/exd.14286. [DOI] [PubMed] [Google Scholar]
- 176.Valiente M, Obenauf AC, Jin X, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156(5):1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.You H, Baluszek S, Kaminska B. Supportive roles of brain macrophages in CNS metastases and assessment of new approaches targeting their functions. Theranostics. 2020;10(7):2949–2964. doi: 10.7150/thno.40783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.