Abstract
Complementary DNA clones encoding the murine homolog (mCAR) of the human coxsackievirus and adenovirus receptor (CAR) were isolated. Nonpermissive CHO cells transfected with mCAR cDNA became susceptible to infection by coxsackieviruses B3 and B4 and showed increased susceptibility to adenovirus-mediated gene transfer. These results indicate that the same receptor is responsible for virus interactions with both murine and human cells. Analysis of receptor expression in human and murine tissues should be useful in defining factors governing virus tropism in vivo.
Coxsackieviruses are human picornaviruses belonging to the enterovirus group (22). They are responsible for nonspecific febrile illnesses as well as myocarditis (8, 27), meningoencephalitis (22), and inflammation of the pancreas (15, 34). Coxsackieviruses were initially distinguished from other human enteroviruses—including most poliovirus and echovirus strains—because of their capacity to infect suckling mice (4, 22).
Early experiments suggested that the expression of specific viral receptors on susceptible cells was a major determinant of enterovirus host range and tissue tropism (12, 17), and the particular susceptibility of newborn mice to encephalitis caused by coxsackie B viruses (9, 10) was related to the abundant expression of receptors in the newborn—but not the adult—brain (18, 19). More recent work indicates that coxsackievirus B3 (CB3) forms a detergent-stable complex with a 46-kDa putative receptor protein on the surface of murine YAC-1 cells (13). Similarly, when used to probe proteins blotted onto nitrocellulose, CB3 binds to a 46-kDa putative receptor partially purified from the brains of newborn mice (32). Consistent with the relative resistance of adult mice to encephalitis (9, 10), expression of this receptor in the brain decreases sharply with age (31).
We recently identified a 46-kDa protein, coxsackievirus and adenovirus receptor (CAR), as a receptor responsible for coxsackie B virus infection of human cells and showed that this protein also functions in adenovirus attachment and adenovirus-mediated gene delivery (2). We have now identified a murine CAR homolog (mCAR), and find that it also functions as a receptor for coxsackie B viruses and adenoviruses.
MATERIALS AND METHODS
Isolation of mCAR cDNA and mCAR expression on transfected cells.
A C57BL/6 mouse liver cDNA library (Gibco BRL) in the pCMV-Sport 2 mammalian expression vector was screened with an expressed sequence tag cDNA (GenBank accession no. W70374) found to encode a peptide sequence homologous to the C terminus of human CAR. Two cDNA clones were obtained, and partial nucleotide sequences of the inserts were determined (clone m1, GenBank accession no. Y10320; clone m2, GenBank accession no. Y11929).
Transfection of CHO dhfr cells with mCAR cDNA and selection in nucleoside-free medium were performed as described for transfection with the integrin α2 subunit (3). Flow cytometry and fluorescence-activated cell sorting were performed with rat antiserum raised against the p46 putative murine brain receptor for CB3 (anti-p46) (32) or control serum from an unimmunized rat, followed by fluorescein isothiocyanate-conjugated goat antibody to rat immunoglobulin (Sigma). Sera were preadsorbed against untransfected CHO cells overnight before use. CHO cells expressing human CAR (2) and control CHO cells expressing the human integrin α2 subunit (3) have been described previously.
For immunoblots, cells were extracted in buffer containing 1% Triton X-100. After electrophoresis in a sodium dodecyl sulfate–10% polyacrylamide gel, extracted proteins were electrotransferred to nitrocellulose membranes. Membranes were blocked with Tris-buffered saline containing 5% nonfat dry milk and 0.1% Tween 20 and then incubated with anti-p46 rat serum (1/1,000 dilution) followed by horseradish peroxidase-conjugated sheep antibody to rat immunoglobulin (diluted 1/3,000; Amersham). Proteins were visualized by chemiluminescence using reagents supplied by Amersham.
Radiolabeled virus binding assays, plaque assays, and assays of adenovirus-mediated gene transfer.
CB3 (Nancy) (maintained in the laboratory of R.L.C.) and CB4 (strain JVB) were radiolabeled and purified, and virus binding and plaque assays were performed as described for echovirus 1 (3), except that cell monolayers were incubated with radiolabeled virus for 4 h. 35S-labeled adenovirus 2 was prepared and adenovirus binding assays were performed as described previously (2). To measure susceptibility to adenovirus-mediated gene transfer, cell monolayers were exposed to Ad.CMV-β-gal (21), and β-galactosidase expression was detected with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) as described previously (2). Adenovirus 2 fibers were prepared as described previously (2). Recombinant adenovirus 5 knob domains produced in Escherichia coli (2) were provided by Jeong Hong (University of Alabama at Birmingham); recombinant adenovirus 5 knob domains produced in insect cells were prepared as described previously (26).
Analysis of CAR mRNA expression.
For detection of human CAR mRNA, an 1,137-bp cDNA fragment encoding the human CAR protein (GenBank accession no. Y07593), excised with endonucleases PstI and NdeI, was labeled with 32P and used to probe a multiple-tissue Northern blot (Clontech) containing 2 μg of poly(A)+ RNA from each of eight adult human tissues. A similar blot with RNA from 20-week-old BALB/c mice (Clontech) was probed with a 313-bp fragment, excised from the expressed sequence tag cDNA clone with XhoI and BamHI, that matched extracellular, transmembrane, and cytoplasmic domain sequences identical in both mCAR cDNA clones. Final washes were in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecylsulfate at 50°C.
RESULTS
Identification and expression of mCAR.
We searched the dbEST database to identify a murine cDNA clone that could encode a protein homologous to human CAR and then used it to screen a mouse liver cDNA library. Two cDNA clones were isolated that potentially encoded proteins with >90% amino acid identity to the extracellular domain of human CAR (Fig. 1) and up to 95% identity within the cytoplasmic domain. The predicted mCAR peptide sequences were identical in the extracellular and transmembrane domains but diverged at the C terminus: in clone m2, the C-terminal 26 amino acids of clone m1 were replaced by 13 different amino acids.
FIG. 1.
mCAR and human CAR amino acid sequences. The sequence of human CAR (h), the sequence of a murine homolog (clone m1), and the sequence of a murine homolog with an altered C terminus (clone m2) are shown. Predicted hydrophobic leader (determined as described in reference 24) and transmembrane domains are underlined. Potential sites for N-linked glycosylation are marked with an asterisk.
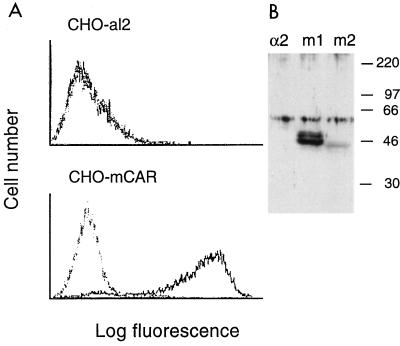
CHO cells transfected with each of the cDNAs expressed antigen detectable by rat antiserum raised against the 46-kDa putative murine CB3 receptor (32) but were not stained by control rat serum (Fig. 2A and data not shown). No staining of control cells transfected with the human integrin α2 subunit (CHO-al2) was detected. On immunoblots, the anti-murine receptor serum specifically detected proteins of approximately 46 kDa in lysates of CHO-mCAR transfectants but not in lysates of control transfectants (Fig. 2B). A protein doublet was detected in CHO cells transfected with clone m1; a similar doublet was previously observed on blots of murine tissues (31, 32). These results suggest that mCAR is the CB3-binding protein previously identified in newborn mouse brain (32).
FIG. 2.
Expression of mCAR on transfected CHO cells. (A) Immunofluorescence analysis. Control CHO cells transfected with the human integrin α2 subunit (CHO-al2) or CHO cells transfected with mCAR cDNA (CHO-mCAR) were incubated first with normal rat serum (dotted line) or with serum from rats immunized with the 46-kDa mouse brain receptor (anti-p46) (solid line) and then with fluorescein isothiocyanate-conjugated goat antibody to rat immunoglobulin. Results with clone m2 are shown; similar results were obtained with clone m1. (B) Immunoblot analysis. Proteins blotted onto nitrocellulose were probed with anti-p46 serum as described in Materials and Methods. Results are shown for extracts of CHO cells transfected with the human integrin α2 subunit and for CHO cells transfected with mCAR clones m1 and m2. Positions of molecular mass markers are shown at the right (in kilodaltons). Specific proteins of 46 to 48 kDa were detected in CHO-mCAR transfectants. A nonspecific band (approximately 60 kDa) was seen in all lanes.
Coxsackie B virus interaction with mCAR.
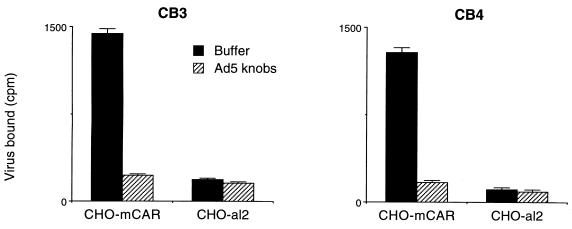
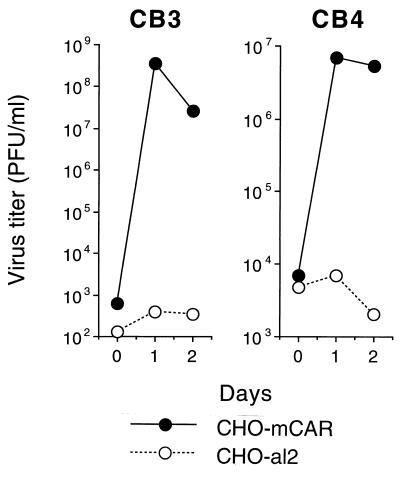
CHO cells transfected with mCAR cDNA (CHO-mCAR), but not control CHO-al2 cells, bound radiolabeled CB3 and CB4 (Fig. 3 and data not shown). When exposed to CB3 and CB4, CHO-mCAR cells became infected, as demonstrated by viral cytopathic effects (not shown) and increase in virus titer (Fig. 4). These results indicate that mCAR is a receptor mediating coxsackie B virus attachment and infection.
FIG. 3.
Coxsackie B virus attachment to mCAR on transfected CHO cells. Confluent monolayers of CHO-mCAR or control CHO-al2 cells were incubated with radiolabeled CB3 or CB4 (29,000 cpm) for 4 h at room temperature and then washed and dissolved for scintillation counting. Results with clone m2 are shown; similar results were obtained with clone m1. Some monolayers were preincubated with recombinant adenovirus 5 knob domains (produced in E. coli [0.7 μg]) before exposure to radiolabeled virus. Results for triplicate samples (mean virus bound [counts per minute] + 1 standard deviation [error bar]) are shown.
FIG. 4.
Coxsackievirus production by transfected CHO cells. CHO-mCAR and CHO-al2 monolayers were exposed to CB3 or CB4 (10 PFU/cell) for 1 h at room temperature and then monolayers were washed and incubated at 37°C for 1 h (0 days), 1 day, or 2 days. Monolayers were frozen and thawed to release virus, and then plaque assays were performed. The figure shows the mean virus titers for triplicate cultures.
Adenovirus interaction with mCAR.
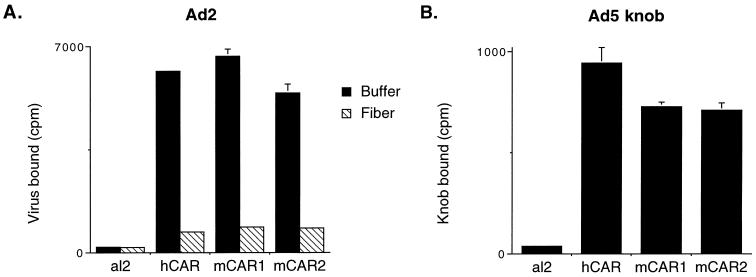
Adenovirus attachment to cells is mediated by globular knobs, located at the tips of fibers that project from the capsid surface (6, 11, 20, 25). Coxsackievirus attachment to mCAR-transfected CHO cells was inhibited by recombinant adenovirus knob domains (Fig. 3), suggesting that mCAR, like human CAR (2), interacts with adenoviruses as well as with coxsackieviruses. Radiolabeled adenovirus 2 bound specifically to CHO cells transfected with mCAR (Fig. 5A), and virus attachment was blocked by isolated adenovirus 2 fibers. In addition, recombinant adenovirus 5 knob domains bound specifically to CHO-mCAR cells (Fig. 5B). These results indicate that mCAR mediates fiber-dependent adenovirus attachment to transfected cells.
FIG. 5.
Adenovirus interaction with mCAR on transfected CHO cells. (A) Virus attachment. Cell monolayers were incubated with radiolabeled adenovirus 2 (20,000 cpm) for 1 h at room temperature and then washed and dissolved for scintillation counting. Some monolayers were preincubated with adenovirus 2 fiber (5 μg) for 1 h and then washed before exposure to radiolabeled virus. (B) Knob attachment. Monolayers were incubated with 125I-labeled adenovirus 5 knob domains (produced in insect cells) for 1 h at room temperature and then washed and dissolved for scintillation counting. Results for triplicate samples (mean virus or knob protein bound [in counts per minute] + 1 standard deviation [error bar]) are shown.
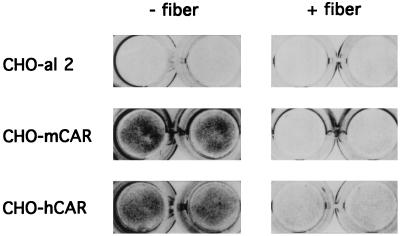
We also measured the efficiency of gene delivery to transfected cells, using adenovirus 5 engineered to encode β-galactosidase (Ad.CMV-βgal [21]) (Fig. 6). As determined by in situ staining with X-Gal, expression of mCAR on transfected cells markedly enhanced adenovirus-mediated gene delivery. Similar results were obtained when CHO cells were transfected with either clone m1 or clone m2. Delivery of the β-galactosidase gene to CHO-mCAR cells was inhibited by adenovirus 2 fibers (Fig. 6). These results indicate that attachment to mCAR promotes adenovirus uptake into cells.
FIG. 6.
Adenovirus-mediated gene transfer. Duplicate monolayers of CHO-al2, CHO-mCAR, or CHO cells transfected with human CAR cDNA (CHO-hCAR) were exposed to Ad.CMV-βgal for 1 h at room temperature, and then monolayers were washed. After incubation at 37°C for 40 h, β-galactosidase activity was detected by in situ staining with X-Gal. Some monolayers were incubated with 1.5 μg of purified adenovirus 2 fibers (+) before exposure to virus. Results with clone m2 are shown; similar results were obtained with clone m1.
Tissue-specific expression of CAR and mCAR RNA.
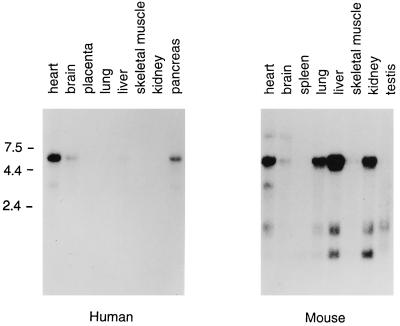
Murine and human CAR cDNA was used to probe Northern blots containing RNA from adult murine and human tissues (Fig. 7). Hybridization with a 6.5-kb RNA species was most prominent in both human and murine tissues, but minor species of other sizes were also observed. The strongest expression of human CAR mRNA was noted in heart, pancreas, and brain, although expression at lower levels could be detected in liver and lung on the original autoradiograph. Murine CAR mRNA was most highly expressed in the murine liver, and relatively high levels of RNA expression were also detected in heart, lung, and kidney. These results suggest that the pattern of tissue-specific CAR expression in humans may differ from the pattern of expression in mice.
FIG. 7.
CAR mRNA expression in human and murine tissues. Multiple-tissue Northern blots (Clontech) containing 2 μg of poly(A)+ RNA from each of the indicated tissues were probed with human CAR and mCAR cDNA as described in Materials and Methods. Positions of marker RNAs are indicated in kilobases. Hybridization with a human actin probe confirmed the presence of equivalent amounts of RNA in each lane.
DISCUSSION
These results demonstrate that mCAR, like the human receptor for coxsackieviruses and adenoviruses, mediates interactions with two genetically and structurally distinct viral pathogens. Expression of mCAR on transfected cells promoted attachment by coxsackie B viruses and adenoviruses. In addition, when transfected with mCAR cDNA, nonpermissive CHO cells became susceptible to infection by CB3 and CB4 and showed increased susceptibility to adenovirus-mediated gene delivery. Specific antibody staining of mCAR-transfected cells and protein detection by immunoblotting indicated that mCAR protein is the 46-kDa coxsackievirus binding protein previously demonstrated in newborn mouse brain (32).
Similar results were obtained when CHO cells were transfected with either of two mCAR cDNA clones, encoding proteins with divergent C termini. It thus appears that the 26 C-terminal amino acids of clone m1, which are nearly identical to those of human CAR, are not essential for mCAR’s receptor function. Using specific primers in nonquantitative reverse transcription-PCR, we have detected RNAs corresponding to both clones m1 and m2 in several murine tissues (unpublished results); both RNA forms were present in each of the tissues examined so far. The protein encoded by clone m2, with a C terminus that differs from that of human CAR, is identical to the mCAR reported by other investigators (28) while the present work was under review.
Pathogenicity in newborn mice was a characteristic originally used to distinguish coxsackieviruses from other human enteroviruses (22), and it is likely that coxsackievirus host range depends on virus interactions with human CAR and mCAR. Age-specific expression of a receptor protein—now identified as mCAR—in the brains of newborn mice (31) has been related to the unique susceptibility of infant mice to CB3 encephalitis (9, 10), suggesting that mCAR may be an important determinant of virus tropism for the murine brain.
In a survey of human tissues, CAR mRNA was most highly expressed in the heart, brain, and pancreas, consistent with the pattern of illness caused by coxsackie B viruses (22). In adult mice, high levels of mCAR RNA were detected in the heart and liver, in which significant lesions are evident during CB3 infection (22). Abundant mRNA was also present in murine kidney and lung, although lesions in these organs are not commonly reported in CB3-infected mice. Little CAR mRNA was detected in the spleen, although two recent reports have indicated that CB3 infects cells—predominantly B lymphocytes—within the spleen (1, 16). Because we have not examined expression of CAR protein itself and because there may be significant age- and strain-dependent variations in receptor expression in murine tissues, further studies will be required to determine how mCAR expression affects CB3 tropism for specific murine tissues.
Human adenoviruses do not replicate in most rodent cells, yet murine as well as human tissues (5, 23, 33) can be transduced with adenovirus vectors. Gene delivery may involve—in addition to the fiber receptor now identified as CAR—virus interaction with αv integrins (7), which have been shown to facilitate virus entry (29). Adenoviruses may also enter cells by fiber-independent pathways (14). However, efficient transduction has been shown to correlate with expression of the fiber receptor (30), and further definition of the receptor’s tissue distribution will be important for efforts to target gene delivery to particular sites. Because murine models are used in preclinical studies of adenovirus-mediated gene delivery, it is important to consider that, as suggested by differences in mRNA expression, the CAR fiber receptor may be more highly expressed in certain murine tissues—including the liver and lung—than it is in human tissues.
ACKNOWLEDGMENTS
We thank Ruliang Xu for the anti-p46 rat serum, Jeong Hong for recombinant adenovirus 5 knob domains produced in E. coli, Toshi Tanaka for Ad.CMV-βgal, and Alejandro Necochea for technical assistance.
This work was supported by grants from the National Institutes of Health (RO1 AI35667 and RO1 CA69703), the American Heart Association (95012650), and the Juvenile Diabetes Foundation. J.M.B. is an Established Investigator of the American Heart Association.
ADDENDUM IN PROOF
Hong et al. (S. S. Hong, L. Karayan, J. T. Tournier, D. T. Curiel, and P. A. Boulanger, EMBO J. 16:2294–2306, 1997) recently presented evidence that there may be another receptor for adenovirus fiber.
REFERENCES
- 1.Anderson D R, Wilson J E, Carthy C M, Yang D, Kandolf R, McManus B M. Direct interactions of coxsackievirus B3 with immune cells in the splenic compartment of mice susceptible or resistant to myocarditis. J Virol. 1996;70:4632–4645. doi: 10.1128/jvi.70.7.4632-4645.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, St. John N, Kawaguchi S, Chan M, Stubdal H, Modlin J, Finberg R W. Infection by echoviruses 1 and 8 depends on the α2 subunit of human VLA-2. J Virol. 1993;67:6847–6852. doi: 10.1128/jvi.67.11.6847-6852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalldorf G. The coxsackie viruses. Bull N Y Acad Med. 1950;26:329–335. [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson B L, Allen E D, Kozarsky K F, Wilson J M, Roessler B J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- 6.Defer C, Belin M-T, Caillet-Boudin M L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman M J, Wilson J M. Expression of αvβ5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J Virol. 1995;69:5951–5958. doi: 10.1128/jvi.69.10.5951-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grist N R, Reid D. Epidemiology of viral infections of the heart. In: Banatvala J, editor. Viral infections of the heart. London, United Kingdom: Edward Arnold; 1993. pp. 23–31. [Google Scholar]
- 9.Grodums E I, Dempster G. The age factor in experimental coxsackie B-3 infection. Can J Microbiol. 1959;5:595–604. doi: 10.1139/m59-073. [DOI] [PubMed] [Google Scholar]
- 10.Grodums E I, Dempster G. Encephalitis in experimental coxsackie B-3 infection. Can J Microbiol. 1961;7:175–184. doi: 10.1139/m61-023. [DOI] [PubMed] [Google Scholar]
- 11.Henry L J, Xia D, Wilke M E, Deisenhofer J, Gerard R D. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994;68:5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland J J. Receptor affinities as major determinants of enterovirus tissue tropism in humans. Virology. 1961;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- 13.Hsu K-H L, Crowell R L. Characterization of a YAC-1 mouse cell receptor for group B coxsackieviruses. J Virol. 1989;63:3105–3108. doi: 10.1128/jvi.63.7.3105-3108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, Kamata T, Yakada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imrie C W, Ferguson J C, Sommerville R G. Coxsackie and mumps virus infection in a prospective study of acute pancreatitis. Gut. 1977;18:53–56. doi: 10.1136/gut.18.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klingel K, Stephan S, Sauter M, Zell R, McManus B, Bültman B, Kandolf R. Pathogenesis of murine enterovirus myocarditis: virus dissemination and immune cell targets. J Virol. 1996;70:8888–8895. doi: 10.1128/jvi.70.12.8888-8895.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunin C L. Cellular susceptibility to enteroviruses. Bacteriol Rev. 1964;28:382–390. doi: 10.1128/br.28.4.382-390.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunin C M. Virus-tissue union and the pathogenesis of enterovirus infections. J Immunol. 1962;88:556–589. [PubMed] [Google Scholar]
- 19.Kunin C M, Halmagyi N E. The relative abundance of viral receptors: an explanation of the differential susceptibility of suckling and adult mice to coxsackie B1 infection. J Clin Invest. 1961;40:1055–1056. [Google Scholar]
- 20.Louis N, Fender P, Barge A, Kitts P, Chroboczek J. Cell-binding domains of the adenovirus serotype 2 fiber. J Virol. 1994;68:4104–4106. doi: 10.1128/jvi.68.6.4104-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manome Y, Wen P Y, Dong Y, Tanaka T, Mitchell B S, Kufe D W, Fine H A. Viral vector transduction of the human deoxycytidine kinase cDNA sensitizes glioma cells to the cytotoxic effects of cytosine arabinoside in vitro and in vivo. Nat Med. 1996;2:567–573. doi: 10.1038/nm0596-567. [DOI] [PubMed] [Google Scholar]
- 22.Melnick J L. Polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 655–712. [Google Scholar]
- 23.Morsy M A, Alford E L, Bett A, Graham F L, Caskey C T. Efficient adenoviral-mediated ornithine transcarbamylase expression in deficient mouse and human hepatocytes. J Clin Invest. 1993;92:1580–1586. doi: 10.1172/JCI116739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielson H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Philipson L, Lonberg-Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roelvink P W, Kovesdi I, Wickham T J. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savoia M C, Oxman M N. Myocarditis and pericarditis. In: Mandell G M, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 799–813. [Google Scholar]
- 28.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 30.Wickham T J, Roelvink P W, Brough D E, Kovesde I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to different cell types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 31.Xu R, Crowell R L. Expression and distribution of the receptors for coxsackievirus B3 during fetal development of the Balb/c mouse and of their brain cells in culture. Virus Res. 1996;46:157–170. doi: 10.1016/S0168-1702(96)01398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu R, Mohanty J G, Crowell R L. Receptor proteins on newborn Balb/c mouse brain cells for coxsackievirus B3 are immunologically distinct from those on HeLa cells. Virus Res. 1995;35:323–340. doi: 10.1016/0168-1702(94)00100-q. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Nunes F A, Berencsi K, Gönczöl E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 34.Yoon J W, Austin M, Onodera T, Notkins A L. Virus-induced diabetes mellitus. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]