Abstract
The complexity of the classical inverted U-shaped relationship between cortisol levels and responses transposable to stress reactivity has led to an incomplete understanding of the mechanisms enabling healthy and toxic effects of stress on brain and behavior. A clearer, more detailed, picture of those relationships can be obtained by integrating cortisol effects on large-scale brain networks, in particular, by focusing on neural network configurations from the perspective of inhibition and excitation. A unifying view of Semon and Hebb’s theories of cellular memory links the biophysical and metabolic changes in neuronal ensembles to the strengthening of collective synapses. In that sense, the neuronal capacity to record, store, and retrieve information directly relates to the adaptive capacity of its connectivity and metabolic reserves. Here, we use task-activated cell ensembles or simply engram cells as an example to demonstrate that the adaptive behavioral responses to stress result from collective synapse strength within and across networks of interneurons and excitatory ones.
Keywords: Functional connectivity, Large-scale brain networks, Synapse, Inhibition, Excitation, Retrograde signaling
Introduction
Norepinephrine and cortisol are released by salient experiences to consolidate emotional memories while impairing the retrieval of remote memories [1]. Stress-induced secretion of cortisol aligns with that of the arousal hormone norepinephrine when the environment requires vigilance and superimposes with ultradian/circadian oscillations to constrain bulk effects during wake and lesser during sleep [2]. Together stress hormones drive a conditioned response to promote vigilance, learning and adaptation. Functional coupling of the emotional center in the braind—the amygdalad—with connected limbic regions predicts anxiety and stress hormone regulation serving as working model for assessing the performance of memory systems in health and diseases [3]. However, the relationship between stress hormones and behavioral performances is extremely complex, and the effect of the former on the latter depends on timing and dose at time of exposure [1,4].
The timing between hormones releases and the memory phase predicts the conditioned response because both hormones compound their effects depending on receptors expression and the activity state in the neurons allocated to the task. Studies have clarified the timing of norepinephrine and cortisol on the nervous system, blood flow, and neurovascular system [5,6], and also the extent to which chronic stress impairs the excitability not only of neurons but also the vascular smooth muscle that change local functional coupling with connected regions [7].
The magnitude of stress hormones effect follows an inverted U-shaped dose–response characterized by moderate levels of cortisol as optimal for brain and behavior compared to too low or too high levels [8]. However, this model has yet to be defined to explain biological functions across large-scale brain networks. So far, we rely on correlations between the range of cortisol effects, memory retention and functional connectivity.
Information about salient stimuli present at the time of learning is uploaded and downloaded through the joint activity of cellular ensembles in the brain. A memory trace must be present at distant time-points after learning, be specific of the circuits activated by learning and be contingent of the behavioral experience. Alterations within and across those neural ensembles are consistent with the strengthening of relevant collective synapses and the weakening of irrelevant ones. Engrams (see box 1 for definition) must encode both the fast neuronal input and the slower hormonal contextual information. Related cues occurring concurrently are likely encoded in overlapping engrams, whereas more distal cues even though related are more likely to engage non-overlapping neuronal ensembles. Related experience conveyed by afferent axons from synchronized presynaptic neurons converge at neighboring synapses which proximity and functional clustering allocates an associative memory trace [9] that is sensitive to stress [10]. The state of neuronal excitability at the time of learning impacts the neurons that are most likely to be allocated into engrams, while the size of neuronal memory ensembles is modulated by local inhibition [11]. Question remains whether glucocorticoids use these mechanisms to change the cells allocated to engrams.
Box 1: Definition of important terms.
Inverted U-shape model of stress is based on Yerkes-Dodson law presenting the relationship between stress and task performance as an upside-down U-shaped curve. In this model, the left and right sides of the curve represent low and high arousal/stress, respectively. The center part represents a medium level of arousal/stress, which is conductive of optimal task performance.
Memory engram corresponds to an ensemble of neurons across multiple brain regions that manifest experience-induced changes in anatomical connections and physiological processes. Those persistent cellular and molecular traces experienced collectively are recognized to be substrates for memories. An engram network is initially excitatory then a mixture with inhibitory components as homeostatic scaling develops [69].
Default network assembles large distributed regions of the association cortex that show reduced activity during attention-demanding tasks relative to steady state for integrating new information with existing knowledge [70].
Salience network integrates internal and external sensory inputs via connections between cortical regions (cingulate, prefrontal, insular, temporal), the thalamus, amygdala, ventral striatum, thalamus, and specific brainstem nuclei for motivating reactive responses [71].
Executive network is a fronto-parietal system playing a critical role in actively maintaining and manipulating information in working memory that are necessary for rule-based problem solving and for decision making in the context of goal-directed behavior [72].
Depending on the memory phase, cortisol and its adjuvants enforce or weaken engram formation and maintenance. Opposite effects have been explained by the inverted U-shaped model [8] (see box 1 for definition), but it fails to explain the specificity of stress hormones throughout time domains and brain states. Integrating information about excitation and inhibition at the collective synapses of the engram network at play might provide new insights, whereby specific responses depend on whether the engram network is online or offline [12]. Conditioned responses to stress and cortisol should thus be distinguishable between engram and non-engram cells [13]. Direct proofs are scarce due to experimental limitations for identifying cells that store specific memories. Advances in neuroimaging and engram capture technologies will help characterize either the direct hormonal regulation of glutamatergic engram neuron excitability/firing or the indirect effects via interneuron populations which converge on the glutamatergic engram neurons for output transformation.
Modulating brain connectivity in humans and animals with stress hormones
Functional magnetic resonance imaging of the human brain during emotionally charged memory tasks implicated norepinephrine and cortisol in the stress-induced shift of memory systems from cognitive to habit by acting on large-scale neural correlates [14,15]. Notably, acute stress promotes the utilization of a salience network (see box 1 for definition) linking the hypothalamus, amygdala, cingulate cortex, striatum, and inferotemporal regions for threat processing over the utilization of an executive network (see box 1 for definition) consisting of the prefrontal and parietal cortices for goal-directed decision-making [16]. A shift of responses from cognitive goal-directed toward more compulsive habit-directed behaviors could overtime trigger stress coping responses unfit with the experience and even become pathologic in vulnerable individuals due to a loss of self-control when making emotional choices under duress.
Contrary to task-related imaging studies that focus on neural correlates involved in specific paradigms, resting state studies reveal the alterations of the brain in its default-mode (see box 1 for definition) [16]. Task-independent resting state imaging studies also report effects of stress and cortisol on functional connectivity patterns [17,18], where rising cortisol levels is associated with increased functional coupling in sensory salience detection systems and promotes performance improvement in subsequent attention tasks [19,20]. Importantly, GABA content in the medial prefrontal cortex predicts the strength of functional connectivity with the amygdala in healthy participants [21], and the link between GABA and BOLD signals across the hippocampus and striatum during specific tasks depends on stress levels [22] and memory phases [23]. However, interpretation of data based on human subjects is often limited, and the exact nature of the link between neural correlates and the script, storage, retrieval and erasure of memories remains to be fully elucidated.
Animal studies support human-based findings and offer additional information on those open questions. Neuroimaging in rodents provide a framework for when the changes of functional brain connectivity predict stress-coping trajectories in resilient and susceptible animals [24]. The critical period for progressive changes of brain function depends on glucocorticoid sensitivity—a product of detection and response—that evolves with age, experience and environmental factors [12]. Fast effects alter memory-related network oscillations in the hippocampus, while the slow effects program cell metabolism and architecture to the new connectivity environment [25]. Both temporal components promote adaptation within and across cellular ensembles allocated to the task. Examples of how glucocorticoids rapidly disrupt functional connectivity across large-scale brain networks as a function of ongoing activity have been described in mice [26] where allocation of cells into an engram depends on immediate excitability and neural activation [27,28]. The use of an activity-trapping system to sort hippocampal engram cells revealed that glucocorticoid levels affect the specificity of contextual-fear-memory through changes in the size and the excitability of the engram cell population [13].
Research on how low-affinity and high-affinity glucocorticoid receptors contribute to stress-induced changes within large-scale brain networks is at its infancy. The role of low-affinity glucocorticoid receptors in fear expression and memory has been demonstrated in several studies where those receptors were deleted from specific brain regions (amygdala, cortex, hippocampus) and cell-types (glutamatergic, GABAergic, astrocytes) [29–31]. Deletion of activity-dependent glucocorticoid receptor signaling pathway impairs synapse maintenance and memory retention [32]. High-affinity mineralocorticoid receptors are more restricted than glucocorticoid receptors, with a significant distribution observed in some limbic regions. In vivo recording of hippocampal neurons showed that glucocorticoids affect their excitability and decrease the ratio of theta-to-delta power [33], reflecting changes in inhibition-excitation ratio and neurovascular coupling [6]. It is to be noted that the vast majority of studies published to date focuses on glucocorticoid receptors modulation of glutamatergic transmission. More recent works have however started describing corticosteroid effects on GABAergic interneurons, mostly through intermediate mediators like endocannabinoids [34]. The ratio of both receptors, and their interactions with others systems, influences the diversity of responses to glucocorticoids and stress in physiology and pathology, but the effects on large-scale brain networks remain largely unexplored [35]. However, recent evidence suggests differential reconfiguration of large-scale neural networks in response to acute stress, with recruitment of the salience network over executive control and default-mode networks rapidly after stress exposure, and a reversal of this network shift during the late phase of the stress response [34]. Together, those animal studies examining causal relationships between hormones, functional connectivity and memory performance confirm that glucocorticoids enhance the consolidation of emotional memories through hyperactivity of the amygdala, at the expense of detailed accuracy for the related contextual learning experience resulting in memory generalization out-of-context, through decreased hippocampal activity [36,37].
Building engrams with excitatory and inhibitory components
Glucocorticoids change the balance between inhibition and excitation depending on dose and timing. Acute exposure increases the excitability of principal neurons (PN) in several brain regions including the amygdala, hippocampus, and cortex [38]. These effects are rapid-independent of transcription regulation-employing retrograde synaptic signaling with endocannabinoids and nitric oxide to modify neurotransmitter release and neuronal firing [35,39], which was further confirmed in humans [40]. Chronic exposure uses glucocorticoid receptors to upregulate GABA synthesis-enzyme, GABA neuron-spiking, GABA release and decrease glutamate release in the amygdala [41] and prefrontal cortex [42]. However, while suppression of glutamatergic neurotransmission is supported by both human and animal studies, the exact nature of chronic stress-induced changes in the inhibitory transmission is still debated, and discordance between studies is likely due to variation across brain regions and inhibitory cell types [43]. A single-cell transcriptomic approach following chronic stress suggests a reorganization of functional connectivity in micro-circuits of the prefrontal cortex consistent with increased regulation of excitatory neurons by GABA cells (specifically somatostatin (SST) and parvalbumin (PV) cells) [25] that depends on gender [44]. The effects of chronic stress on GABA and glutamate transmissions are slow—dependent on new gene products-reflected by a loss of glucocorticoid receptor expression specifically in the PV interneurons. Changes within inhibitory transmission have the potential to modify functional connectivity in large-scale brain networks [25]. In humans, stress modulates the relationship between striatal GABA and hippocampal activation during motor sequence memory acquisition [22], emphasizing how changes in inhibitory transmission by stress in one brain region affects activity of connected regions. In rodents, excess inhibition in the infralimbic cortex enhances the glucocorticoid response to chronic stress by reducing glutamate outflow in hypothalamic nuclei [45,46] while chemogenetic activation of PV interneurons within the sensory cortex prevents the loss of functional connectivity onto PN and stress-induced behavioral impairment [47]. The complexity of stress and glucocorticoids effects on inhibitory transmission due to regional and cell type diversity remains the subject of deep investigations and is further evidenced by preclinical and clinical studies on stress-related psychiatric disorders revealing that both negative [48] and positive [49] allosteric modulators of GABAA receptors counteract the behavioral effects of chronic stress.
Morphological analyzes in distinct brain regions after chronic stress support the idea that neuronal excitability is associated with stress-induced changes within PV-interneurons. In the prefrontal cortex, chronic stress increases PV-dependent synaptic inhibition onto PN, consistent with the attrition of dendrites and spine number [42]. In the sensory cortex, PN lose dendritic arbor and spine density as a result of excessive disinhibition driven by stress-induced inability to activate local PV-interneurons [47]. Regional differences are marked by ambivalent changes of responsiveness to neurotrophic factors and glucocorticoid receptors with evidence suggesting that chronic exposure to high glucocorticoid levels opposes the excitability of the sensory-executive-cognitive system to the emotional-reward-habit system [50]. Stress-induced spine loss by glucocorticoid signaling is often restricted to the apical tuft of PN in the cortex and hippocampus preserving the basal dendrites respectively innervated by SST and PV interneurons. Stress and glucocorticoids influence the functional connectivity of the three major subtypes of interneurons—vasoactive intestinal peptide (VIP), SST, and PV—characterized by non-overlapping targets [51]. The proportion of inhibitory neurons among all neurons in various brain structures remains fixed throughout the lifetime of an individual (15%–30%). Question remains whether neural circuits with a different ratio of excitatory:inhibitory neurons still function normally.
Framework
To clarify the inverted U-shaped model, one must distinguish glucocorticoids impact on interneuron subtypes and PN—dindividually and collectively—in various circuit configurations and along a time continuum, as inhibitory engrams are formed as a homeostatic response to excitation [52].
Glutamatergic synapses—dendritic spines—are viewed as the minimal memory storage unit enabling signal outflow. They form clusters holding functional connectivity from proximal inputs signaling the associative components of the learning experience. Maintenance of these clusters depends on the adaptive capacity of the local metabolic reserve [10]. In principle, the energy budget could be neutralized and oxidative damage limited if the metabolic response remained local and the balance of glycolysis to oxidative phosphorylation increased. Such shift of metabolism in keeping with synaptic activity is programmable in the genome of interneurons and PN [25,53]. The persistence of dendritic spine clusters in PN formed at the time of learning in the prefrontal cortex-to-amygdala neurocircuit controls motivated escape behavior that is impaired by chronic stress but restored with successful antidepressant therapy [54]. Restoration of lost clusters has also been reported in the halo of amyloid plaques containing soluble oligomers in animal models of Alzheimer’s disease [73]. Future studies should explore how to control the metabolic reserve sustaining the synaptic engram.
Inhibitory synapses are permissive to the diffusion of the excitatory signal because their positioning with respect to the dendritic spine clusters can alter the storage and retrieval of associative learning [55]. The geometry of interneurons innervation at dendritic territories of PN enables network specificity. PV cells innervating basal dendrites are permissive to signal integration from the apical inputs, while SSTcells would enable the opposite. Recruitment of the cholecystokinin (CCK) cells innervating the primary apical dendrite should override the SSTeffects, while the PV cells should override them all. If one-order of inhibition—via PV, CCK, or SST cells-filters incoming signals, two-orders of inhibition—through VIP cells-enable raw excitation. This model is supported by the morphological data on stress-induced dendritic spine loss at the apical tufts of PN in the cortex, which is override by chemogenetic activation of PV cells [47].
Question remains whether there are as many types of glucocorticoid sensitivities and response outcomes as there are types of GABAergic synapses. Part of the answer resides in the fast modulatory effects of cortisol on neurotransmitters releases (Figure 1a), which dissociates mechanistically from the slow adaptive effects mediated through the genome. Trans-synaptic retrograde signaling systems made of enzymes (e.g. synthesis of nitric oxide, endocannabinoids or protease clipping target-derived peptides …) and receptors (e.g. soluble guanylate cyclase, G-protein coupled receptors CB1, and receptor tyrosine kinase TrkB and P75) are well-established targets of the fast acting cortisol signaling. The Allen Institute for Brain Science atlas available from mouse.brain-map.org has been instrumental at guiding research from the perspective of cellular and molecular anatomy. In particular, there are discrepancies between all 4 types of GABAergic and the glutamatergic synapses concerning the expression of the various components of trans-synaptic retrograde signaling systems (e.g. Brain-derived neurotrophic factor [BDNF], nitric oxide [NO], arachidonic acid derivatives, N-arachidonoylethanolamine [AEA] and 2-arachidonoylglycerol [2-AG], Figure 1b). Cell type specific equipment raises questions about the sensitivity and timing of cortisol responses in shaping the balance of inhibition and excitation locally within the network of task-allocated neurons (Figure 1c). Bell-shaped responses of cortisol on brain and behavior could be attributed to default connectivity of neurons prior allocation by a task. Cell type specific expression of actuators (e.g. optogenes or chemogenes) or dominant active and inactive mutations/variations targeting components of the trans-synaptic retrograde systems (e.g. FAAH, MAGL, DAGL, CB1, TRKB, nNOS, sGC …) will help deconstruct the fast acting effects of cortisol on brain and behavior (Figure 1d). Combining exposure therapy with focal transcranial stimulation techniques (magnetic [56] or current [57]) targeted onto select cortical nodes of the executive network or the default mode network could help revert neural system configuration to a default cognitive state from the stress-induced habit state.
Figure 1.
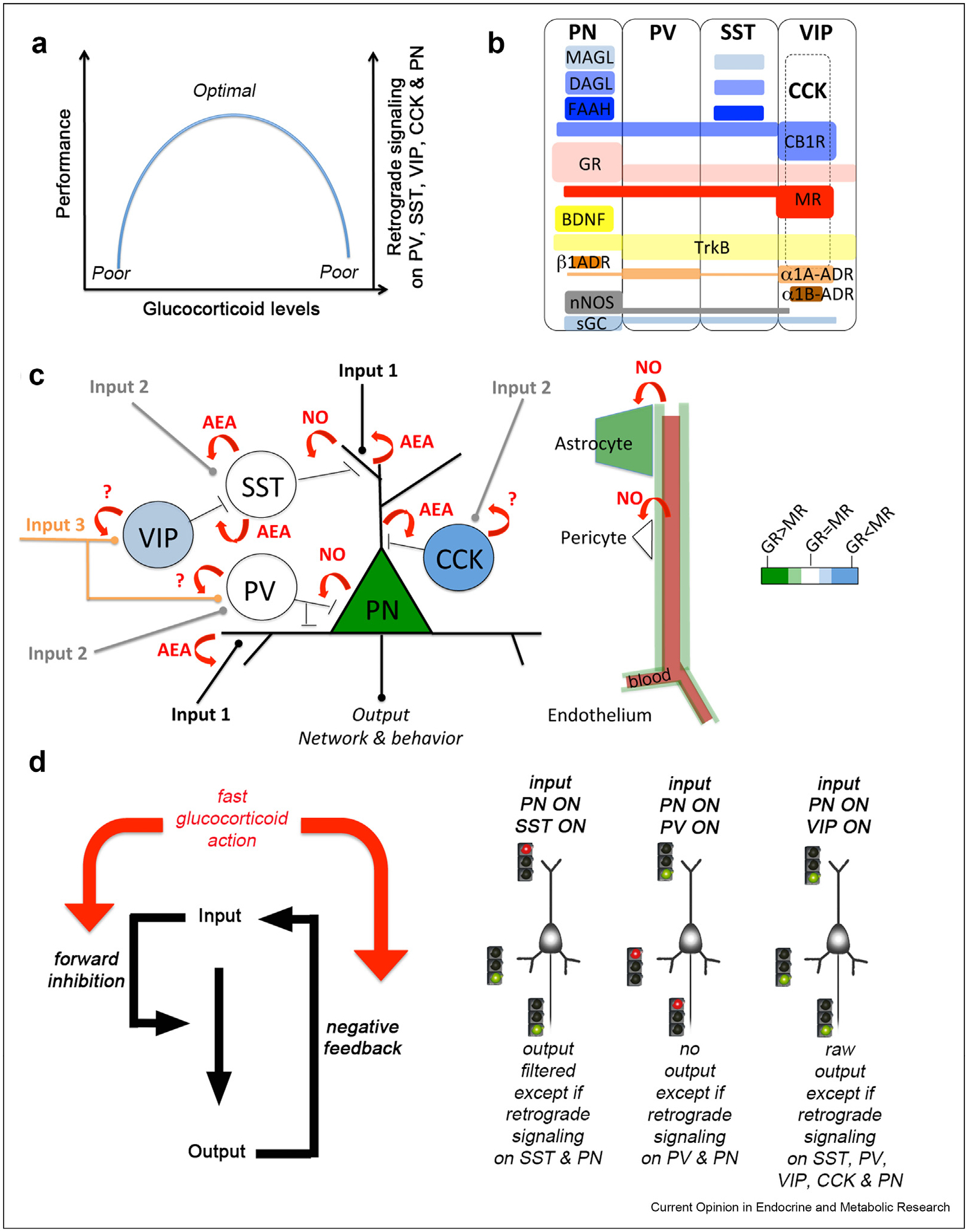
Fast retrograde synaptic signaling as feedback or feedforward regulator of task-activated neural network configuration and behavioral performance. (a) The inverted U-shaped model of glucocorticoid effects on brain and behavior viewed through the prism of the excitatory-to-inhibitory balance. (b) Attributes relevant for the glucocorticoid system in each neuronal subclass of the mouse and human cortex based on the Transcriptomic Explorer Brain Map at the Allen Institute for Brain Science. Resources based on in situ molecular expression atlas across development, virus injection-assisted connectivity in rodent brains, single-cell transcriptomic databases in the human and rodent brains are available at online brain-map.org. (i) Glucocorticoid receptors are most abundant in PN cells and low in PV, SST and VIP interneurons. (ii) Mineralocorticoid receptors are high in VIP and CCK cells but it can be found in PN restricted to some limbic regions. (iii) Components of retrograde synaptic signaling exploited by the fast glucocorticoid actions are abundant in all cell types with neuronal nitric oxide synthase (nNOS) isoforms and its receptor, the soluble guanilate cyclase (sGC) while endocannabinoids synthesis enzymes (monoacylglycerol lipase (MAGL), diacylglycerol lipase (DAGL), fatty acid amide hydrolase (FAAH) are high in PN and SST cells with its receptor (CB1R) found in VIP, CCK, and PN cells. (iv) Adjuvants of glucocorticoids like adrenergic receptors are present in PN (α1a/β1-ADR) and inhibitory cells (α1a-ADR in all and α1b-ADR in VIP); BDNF is exclusive of PN while its high-affinity receptor TrkB is in all types. (c) Fast glucocorticoid signaling controls functional connectivity as a function of the GR/MR ratio and the availability of the synthesis enzymes and receptors for the retrograde trans-synaptic messengers: EAE, endocannabinoids and NO, nitric oxide. Retrograde signaling is shown at each synapse in a hypothetical network of neurons and supporting accessory cells (astrocytes, pericytes, smooth muscle, endothelium) from the prefrontal cortex activated by a task. Neurovascular coupling is reactive to the allocation of neurons by a behavioral task, and therefore likely delayed from neurotransmission in keeping with cortisol modulatory effects. Glutamatergic inputs to the PN defined as 1, 2, and 3 according to their order of innervation can hypothetically transmit dependently or independently. Inputs 1 innervating the basal or the apical dendrites usually come from distinct projection areas; so one or several coincident inputs 1 would integrate multiple contents associated with the behavioral experience. Coincident stimulation of inputs 2 and/or inputs 3 would in theory modify the excitability of the innervated parts of the PN thereby transforming the flow of information through the allocated neuronal network. (d) Geometry of network configuration determines output transmission. We envisioned scenarios in which glucocorticoids would enforce or weaken functional connectivity based on the network configuration. To be consistent with the morphologic studies in chronic stress, direct default of excitation at targeted PN cells would justify the attrition of dendritic territories, low BDNF levels and low glucocorticoid sensitivity. However, indirect default of excitation at targeted PN cells relayed by one-order of inhibitory neurons would drive the opposite response. Finally, two-orders of inhibitory neurons would engage a feedforward inhibitory relay with multiple possible outcomes on the excitatory output given the preference of VIP interneurons for the SST subtype over the PV. These scenarios based on very schematic feedback and feedforward regulation will have to be validated or invalidated. For example, synapse-specific effects of cortisol would be lumped together in the case of VIP interneurons.
Conclusion and implication for policy and practice
The ability of cortisol to boost memory consolidation and impair retrieval in various tasks have tremendous translational values. For instance, recent clinical trials combine contextual manipulations with glucocorticoids and adjuvants (compounds targeting the noradrenergic, glutamatergic receptors and antidepressant medications) to prevent context-dependent relapse of anxiety disorders [58,59]; alterations of circadian fluctuations of cortisol paired with memory retrieval seem to influence memory reconsolidation [60]. However, efforts should be made to reframe current therapeutic strategies for stress-related psychopathologies in the context of glucocorticoids effects on the excitatory:inhibitory balance.
Unfortunately, animal- and human-based studies are largely biased toward studying adult males whereas women are more susceptible to anxiety and stress-related disorders. Sex differences in resilience and vulnerability to stress have been associated with gender-specific activation of various subtypes of cells, including the SST [61,62] and PV [63]. Yet, women taking hormonal contraceptives have elevated blood concentration of cortisol-binding globulin that might interfere with the cortisol effects on memory engrams [64]. Future studies should therefore include both biological sexes, which will eventually promote the development of more efficacious personalized therapeutic interventions.
Similarly, age is an important factor to consider. Frequent or sustained activation of brain systems that respond to stress can be damaging across lifespan. Awareness should be directed to infancy, as neural circuits dealing with stress coping are plastic during the fetal and early childhood periods, and to adolescence, a period of prefrontal inhibitory maturation. Changing the construction of neural networks early on could have lasting consequences on future rhythmic secretion patterns of stress hormones and functional connectivity. Experimental evidence based on prenatal, postnatal, and adolescent stress exposure supports long-lasting consequences of stress on network architecture and PV functional connectivity [65–68]. It remains to be explored whether the modification of the inhibitory:excitatory balance early in life would correct developmental trajectory to psychopathologies later in life.
Novel techniques in neuroscience basic research allowing for investigation of stress effects at different levels of analysis (from cell type to local- and long-range connections) will continue to provide insights on the mechanisms by which glucocorticoids modulate memories. Direct manipulations of cell populations and neural circuits to circumvent stress effects will confirm the causal role of excitatory and inhibitory neurons in the inverted U-shape response to stress. This information will serve as a steppingstone to design innovative therapeutic strategies to treat stress-induced psychiatric disorders.
Acknowledgments
Authors apologize to the many investigators whose work could not be cited. Supporting grants are from the FRM [DEQ20180339191] and NIH [MH115281, MH119090].
Footnotes
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* * of outstanding interest
- 1.de Quervain D, Schwabe L, Roozendaal B: Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci 2017, 18:7–19. [DOI] [PubMed] [Google Scholar]
- 2.Kalafatakis K, Russell GM, Harmer CJ, Munafo MR, Marchant N, Wilson A, Brooks JC, Durant C, Thakrar J, Murphy P, et al. : Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proc Natl Acad Sci U S A 2018, 115:E4091–E4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakamata Y, Komi S, Moriguchi Y, Izawa S, Motomura Y, Sato E, Mizukami S, Kim Y, Hanakawa T, Inoue Y, et al. : Amygdala-centred functional connectivity affects daily cortisol concentrations: a putative link with anxiety. Sci Rep 2017, 7:8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meir Drexler S, Merz CJ, Jentsch VL, Wolf OT: How stress and glucocorticoids timing-dependently affect extinction and relapse. Neurosci Biobehav Rev 2019, 98:145–153. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt S, Hillmer AT, Rusowicz A, Nabulsi N, Matuskey D, Angarita GA, Najafzadeh S, Kapinos M, Southwick SM, Krystal JH, et al. : Imaging brain cortisol regulation in PTSD with a target for 11b-hydroxysteroid dehydrogenase type 1. J Clin Invest 2021, 131, e150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han K, Lee M, Lim HK, Jang MW, Kwon J, Lee CJ, Kim SG, Suh M: Excitation-inhibition imbalance leads to alteration of neuronal coherence and neurovascular coupling under acute stress. J Neurosci 2020, 40:9148–9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT: Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A 2014, 111:7462–7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS, Akil H: Revisiting the stress concept: implications for affective disorders. J Neurosci 2020, 40:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank AC, Huang S, Zhou M, Gdalyahu A, Kastellakis G, Silva TK, Lu E, Wen X, Poirazi P, Trachtenberg JT, et al. : Hotspots of dendritic spine turnover facilitate clustered spine addition and learning and memory. Nat Commun 2018, 9:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dromard Y, Arango-Lievano M, Fontanaud P, Tricaud N, Jeanneteau F: Dual imaging of dendritic spines and mitochondria in vivo reveals hotspots of plasticity and metabolic adaptation to stress. Neurobiol Stress 2021, 15, 100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison DJ, Rashid AJ, Yiu AP, Yan C, Frankland PW, Josselyn SA: Parvalbumin interneurons constrain the size of the lateral amygdala engram. Neurobiol Learn Mem 2016, 135: 91–99. [DOI] [PubMed] [Google Scholar]
- 12.Huzard D, Rappeneau V, Meijer OC, Touma C, Arango-Lievano M, Garabedian MJ, Jeanneteau F: Experience and activity-dependent control of glucocorticoid receptors during the stress response in large-scale brain networks. Stress 2020:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.* *.Lesuis SL, Brosens N, Immerzeel N, van der Loo RJ, Mitrić M, Bielefeld P, Fitzsimons CP, Lucassen PJ, Kushner SA, van den Oever MC, et al. : Glucocorticoids promote fear generalization by increasing the size of a dentate gyrus engram cell population. Biol Psychiatr 2021, 90:494–504. [DOI] [PubMed] [Google Scholar]; Glucocorticoid actions vary if target cells belong or don’t belong to the engram network.
- 14.Soravia LM, Schwab S, Weber N, Nakataki M, Wiest R, Strik W, Heinrichs M, de Quervain D, Federspiel A: Glucocorticoid administration restores salience network activity in patients with spider phobia. Depress Anxiety 2018, 35:925–934. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham TJ, Mattingly SM, Tlatenchi A, Wirth MM, Alger SE, Kensinger EA, Payne JD: Higher post-encoding cortisol benefits the selective consolidation of emotional aspects of memory. Neurobiol Learn Mem 2021, 180, 107411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Llera A, Hashemi MM, Kaldewaij R, Koch SBJ, Beckmann CF, Klumpers F, Roelofs K: Discriminating stress from rest based on resting-state connectivity of the human brain: a supervised machine learning study. Hum Brain Mapp 2020, 41:3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muehlhan M, Alexander N, Trautmann S, Weckesser LJ, Vogel S, Kirschbaum C, Miller R: Cortisol secretion predicts functional macro-scale connectivity of the visual cortex: a data-driven Multivoxel Pattern Analysis (MVPA). Psychoneuroendocrinology 2020, 117, 104695. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Hashemi MM, Kaldewaij R, K SBJ, Beckmann C, Klumpers F, Roelofs K: Acute stress alters the ‘default’ brain processing. Neuroimage 2019, 189:870–877. [DOI] [PubMed] [Google Scholar]
- 19.Weckesser LJ, Alexander NC, Kirschbaum C, Mennigen E, Miller R: Hydrocortisone counteracts adverse stress effects on dual-task performance by improving visual sensory processes. J Cognit Neurosci 2016, 28:1784–1803. [DOI] [PubMed] [Google Scholar]
- 20.Shields GS, Rivers AM, Ramey MM, Trainor BC, Yonelinas AP: Mild acute stress improves response speed without impairing accuracy or interference control in two selective attention tasks: implications for theories of stress and cognition. Psychoneuroendocrinology 2019, 108:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delli Pizzi S, Chiacchiaretta P, Mantini D, Bubbico G, Edden RA, Onofrj M, Ferretti A, Bonanni L: GABA content within medial prefrontal cortex predicts the variability of fronto-limbic effective connectivity. Brain Struct Funct 2017, 222:3217–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolfen N, Veldman MP, Gann MA, von Leupoldt A, Puts NAJ, Edden RAE, Mikkelsen M, Swinnen S, Schwabe L, Albouy G, et al. : A role for GABA in the modulation of striatal and hippocampal systems under stress. Commun Biol 2021, 4:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spurny B, Seiger R, Moser P, Vanicek T, Reed MB, Heckova E, Michenthaler P, Basaran A, Gryglewski G, Klöbl M, et al. : Hippocampal GABA levels correlate with retrieval performance in an associative learning paradigm. Neuroimage 2020, 204, 116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magalhães R, Barrière DA, Novais A, Marques F, Marques P, Cerqueira J, Sousa JC, Cachia A, Boumezbeur F, Bottlaender M, et al. : The dynamics of stress: a longitudinal MRI study of rat brain structure and connectome. Mol Psychiatr 2018, 23: 1998–2006. [DOI] [PubMed] [Google Scholar]
- 25.* *.Newton DF, Oh H, Shukla R, Misquitta K, Fee C, Banasr M, Sibille E: Chronic stress induces coordinated cortical micro-circuit cell-type transcriptomic changes consistent with altered information processing. Biol Psychiatr 2022, 91(9): 798–809, 10.1016/j.biopsych.2021.10.015. [DOI] [PubMed] [Google Scholar]; Single-cell transcriptomic approach to explain the excitation-inhibition ratio changed by chronic stress.
- 26.* *.Barsegyan A, Mirone G, Ronzoni G, Guo C, Song Q, van Kuppeveld D, Schut EHS, Atsak P, Teurlings S, McGaugh JL, et al. : Glucocorticoid enhancement of recognition memory via basolateral amygdala-driven facilitation of prelimbic cortex interactions. Proc Natl Acad Sci U S A 2019, 116: 7077–7082. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adjuvant effect of noradrenaline on glucocorticoid mnemonic effects explained at the network level.
- 27.Rao-Ruiz P, Couey JJ, Marcelo IM, Bouwkamp CG, Slump DE, Matos MR, van der Loo RJ, Martins GJ, van den Hout M, van IWF, et al. : Engram-specific transcriptome profiling of contextual memory consolidation. Nat Commun 2019, 10:2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S, Kramer EE, Mercaldo V, Rashid AJ, Insel N, Frankland PW, Josselyn SA: Neuronal allocation to a hippocampal engram. Neuropsychopharmacology 2016, 1:2987–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagedorn B, Wolf OT, Merz CJ: Cortisol before extinction generalization alters its neural correlates during retrieval. Psychoneuroendocrinology 2021, 136, 105607. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann J, Dedic N, Pohlmann ML, Hausl A, Karst H, Engelhardt C, Westerholz S, Wagner KV, Labermaier C, Hoeijmakers L, et al. : Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol Psychiatr 2017, 22:466–475. [DOI] [PubMed] [Google Scholar]
- 31.Tertil M, Skupi U, Barut J, Dubovyk V, Wawrzczak-Bargiela A, Soltys Z, Golda S, Kudla L, Wiktorowska L, Szklarczyk K, et al. : Glucocorticoid receptor signaling in astrocytes is required for aversive memory formation. Transl Psychiatry 2018, 8:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.* *.Arango-Lievano M, Borie AM, Dromard Y, Murat M, Desarmenien MG, Garabedian MJ, Jeanneteau F: Persistence of learning-induced synapses depends on neurotrophic priming of glucocorticoid receptors. Proc Natl Acad Sci U S A 2019, 116:13097–13106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Activity-dependent glucocorticoid action enables the retention of synaptic memory engrams.
- 33.Tomar A, Polygalov D, Chattarji S, McHugh TJ: Stress enhances hippocampal neuronal synchrony and alters ripple-spike interaction. Neurobiol Stress 2021, 14, 100327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwabe L, Hermans EJ, Joëls M, Roozendaal B: Mechanisms of memory under stress. Neuron 2022, 110:1450–1467. [DOI] [PubMed] [Google Scholar]
- 35.de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH, Joëls M: Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol 2018, 49:124–145. [DOI] [PubMed] [Google Scholar]
- 36.Al Abed AS, Ducourneau EG, Bouarab C, Sellami A, Marighetto A, Desmedt A: Preventing and treating PTSD-like memory by trauma contextualization. Nat Commun 2020, 11:4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roozendaal B, Mirone G: Opposite effects of noradrenergic and glucocorticoid activation on accuracy of an episodic-like memory. Psychoneuroendocrinology 2020, 104588. [DOI] [PubMed] [Google Scholar]
- 38.Karst H, Joëls M: Severe stress hormone conditions cause an extended window of excitability in the mouse basolateral amygdala. Neuropharmacology 2016, 110:175–180. [DOI] [PubMed] [Google Scholar]
- 39.Di S, Itoga CA, Fisher MO, Solomonow J, Roltsch EA, Gilpin NW, Tasker JG: Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J Neurosci 2016, 36:8461–8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayo LM, Asratian A, Lindé J, Morena M, Haataja R, Hammar V, Augier G, Hill MN, Heilig M: Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid Amide Hydrolase: a randomized, controlled experimental medicine trial. Biol Psychiatr 2020, 87:538–547. [DOI] [PubMed] [Google Scholar]
- 41.Wang GY, Zhu ZM, Cui S, Wang JH: Glucocorticoid induces incoordination between glutamatergic and GABAergic neurons in the amygdala. PLoS One 2016, 11, e0166535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, Ghosal S, Mahbod P, Packard BA, Myers B, et al. : Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol Psychiatr 2016, 80:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page CE, Coutellier L: Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci Biobehav Rev 2019, 105:39–51. [DOI] [PubMed] [Google Scholar]
- 44.Girgenti MJ, Wohleb ES, Mehta S, Ghosal S, Fogaca MV, Duman RS: Prefrontal cortex interneurons display dynamic sex-specific stress-induced transcriptomes. Transl Psychiatry 2019, 9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers B, McKlveen JM, Morano R, Ulrich-Lai YM, Solomon MB, Wilson SP, Herman JP: Vesicular glutamate transporter 1 knockdown in infralimbic prefrontal cortex augments neuroendocrine responses to chronic stress in male rats. Endocrinology 2017, 158:3579–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myers B, Carvalho-Netto E, Wick-Carlson D, Wu C, Naser S, Solomon MB, Ulrich-Lai YM, Herman JP: GABAergic signaling within a limbic-hypothalamic circuit integrates social and anxiety-like behavior with stress reactivity. Neuropsychopharmacology 2016, 41:1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen CC, Lu J, Yang R, Ding JB, Zuo Y: Selective activation of parvalbumin interneurons prevents stress-induced synapse loss and perceptual defects. Mol Psychiatr 2018, 23: 1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Troppoli TA, Zanos P, Georgiou P, Gould TD, Rudolph U, Thompson SM: Negative allosteric modulation of GABAARs at a5 subunit-containing benzodiazepine sites reverses stress-induced anhedonia and weakened synaptic function in mice. Biol Psychiatr 2021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lüscher B, Möhler H: Brexanolone, a neurosteroid antidepressant, vindicates the GABAergic deficit hypothesis of depression and may foster resilience. F1000Res 2019, 8: 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeanneteau F, Borie A, Chao M, Garabedian M: Bridging the gap between BDNF and glucocorticoid effects on brain networks. Neuroendocrinology 2019, 109(3):277–284, 10.1159/000496392. [DOI] [PubMed] [Google Scholar]
- 51.McKlveen JM, Moloney RD, Scheimann JR, Myers B, Herman JP: Braking” the prefrontal cortex: the role of glucocorticoids and interneurons in stress adaptation and pathology. Biol Psychiatr 2019, 86:669–681. [DOI] [PubMed] [Google Scholar]
- 52.Barron HC, Vogels TP, Behrens TE, Ramaswami M: Inhibitory engrams in perception and memory. Proc Natl Acad Sci U S A 2017, 114:6666–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeanneteau F, Barrere C, Vos M, De Vries CJ, Rouillard C, Levesque D, Dromard Y, Moisan MP, Duric V, Franklin TC, et al. : The stress-induced transcription factor NR4A1 adjusts mitochondrial function and synapse number in prefrontal cortex. J Neurosci 2018, 38:1335–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.* *.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, et al. : Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019:364. [DOI] [PMC free article] [PubMed] [Google Scholar]; Destruction of a synaptic engram of motivated behavior prevents antidepressant action.
- 55.Boivin JR, Nedivi E: Functional implications of inhibitory synapse placement on signal processing in pyramidal neuron dendrites. Curr Opin Neurobiol 2018:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bogdanov M, Schwabe L: Transcranial stimulation of the dorsolateral PrefrontalCortex prevents stress-induced working memory deficits. J Neurosci 2016, 36:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koolschijn RS, Emir UE, Pantelides AC, Nili H, Behrens TEJ, Barron HC: The Hippocampus and neocortical inhibitory engrams protect against memory interference. Neuron 2019, 101:528–541. e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michopoulos V, Norrholm SD, Stevens JS, Glover EM, Rothbaum BO, Gillespie CF, Schwartz AC, Ressler KJ, Jovanovic T: Dexamethasone facilitates fear extinction and safety discrimination in PTSD: a placebo-controlled, double-blind study. Psychoneuroendocrinology 2017, 83:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inslicht SS, Niles AN, Metzler TJ, Lipshitz SL, Otte C, Milad MR, Orr SP, Marmar CR, Neylan TC: Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology 2021, 10.1038/s41386-021-01222-z. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antypa D, Perrault AA, Vuilleumier P, Schwartz S, Rimmele U: Suppressing the morning cortisol rise after memory reactivation at 4 A.M. enhances episodic memory reconsolidation in humans. J Neurosci 2021, 41:7259–7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jefferson SJ, Feng M, Chon U, Guo Y, Kim Y, Luscher B: Disinhibition of somatostatin interneurons confers resilience to stress in male but not female mice. Neurobiol Stress 2020, 13, 100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen K, Yang G, So KF, Zhang L: Activation of cortical somatostatin interneurons rescues synapse loss and motor deficits after acute MPTP infusion. iScience 2019, 17:230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Page CE, Shepard R, Heslin K, Coutellier L: Prefrontal parvalbumin cells are sensitive to stress and mediate anxiety-related behaviors in female mice. Sci Rep 2019, 9, 19772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merz CJ, Wolf OT: Sex differences in stress effects on emotional learning. J Neurosci Res 2017, 95:93–105. [DOI] [PubMed] [Google Scholar]
- 65.Kim DJ, Davis EP, Sandman CA, Sporns O, O’Donnell BF, Buss C, Hetrick WP: Prenatal maternal cortisol has sex-specific associations with child brain network properties. Cerebr Cortex 2017, 27:5230–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manzano Nieves G, Bravo M, Baskoylu S, Bath KG: Early life adversity decreases pre-adolescent fear expression by accelerating amygdala PV cell development. Elife 2020, 9, e55263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodwill HL, Manzano-Nieves G, LaChance P, Teramoto S, Lin S, Lopez C, Stevenson RJ, Theyel BB, Moore CI, Connors BW, et al. : Early life stress drives sex-selective impairment in reversal learning by affecting parvalbumin interneurons in orbitofrontal cortex of mice. Cell Rep 2018, 25: 2299–2307. e2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Page CE, Coutellier L: Adolescent stress disrupts the maturation of anxiety-related behaviors and alters the developmental trajectory of the prefrontal cortex in a sex- and age-specific manner. Neuroscience 2018, 390:265–277. [DOI] [PubMed] [Google Scholar]
- 69.Josselyn SA, Tonegawa S: Memory engrams: recalling the past and imagining the future. Science 2020, 367:eaaw4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buckner RL, DiNicola LM: The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci 2019, 20:593–608. [DOI] [PubMed] [Google Scholar]
- 71.Seeley WW: The salience network: a neural system for perceiving and responding to homeostatic demands. J Neurosci 2019, 39:9878–9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raffone A, Marzetti L, Del Gratta C, Perrucci MG, Romani GL, Pizzella V: Toward a brain theory of meditation. Prog Brain Res 2019, 244:207–232. [DOI] [PubMed] [Google Scholar]
- 73.Dromard Yann, Arango-Lievano Margarita, Borie Amelie, Dedin Maheva, Fontanaud Pierre, Torrent Joan, Garabedian Michael J, Ginsberg Stephen D, Jeanneteau Freddy: Loss of glucocorticoid receptor phosphorylation contributes to cognitive and neurocentric damages of the amyloid-β pathway. Acta Neuropathol Commun. 2022, 10(1):91, 10.1186/s40478-022-01396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


