Abstract
This study aimed to synthesize evidence from studies that addressed the influence of bias domains in randomized controlled trials on rehabilitation intervention effect estimates and discuss how these findings can maximize the trustworthiness of an RCT in rehabilitation. We screened studies about the influence of bias on rehabilitation intervention effect estimates published until June 2023. The characteristics and results of the included studies were categorized based on methodological characteristics and summarized narratively. We included seven studies with data on 227,806 RCT participants. Our findings showed that rehabilitation intervention effect estimates are likely exaggerated in trials with inadequate/unclear sequence generation and allocation concealment when using continuous outcomes. The influence of blinding was inconsistent and different from the rest of medical science, as meta-epidemiological studies showed overestimation, underestimation, or neutral associations for different types of blinding on rehabilitation treatment effect estimates. Still, it showed a more consistent pattern when looking at patient-reported outcomes. The impact of attrition bias and intention to treat has been analyzed only in two studies with inconsistent results. The risk of reporting bias seems to be associated with overestimation of treatment effects. Bias domains can influence rehabilitation treatment effects in different directions. The evidence is mixed and inconclusive due to the poor methodological quality of RCTs and the limited number and quality of studies looking at the influence of bias and treatment effects in rehabilitation. Further studies about the influence of bias in RCTs on rehabilitation intervention effect estimates are needed.
Key words: Rehabilitation, Bias, Randomized controlled trials as topic
Randomized controlled trials (RCTs) are prospective studies that measure the efficacy, effectiveness and safety of interventions. Although no study design is likely to prove absolute causality, randomization reduces bias and facilitates the examination of cause-effect relationships between an intervention and an outcome. This is because randomization and allocation concealment help balance participant characteristics (both known and unknown) between the groups allowing unbiased conclusions about the effect of an intervention under study. This is very unlikely with any other study design.1
The RCT design requires that researchers carefully select the population, the interventions to be compared, and the outcomes of interest.2 Once these are defined, the sample size needs to be calculated to estimate the appropriate number of participants to detect, with reasonable probability, an expected and/or clinically relevant difference between groups for a given study. To achieve comparability between groups, the participant characteristics must be homogeneous, cointerventions should be avoided or similar between groups, participants, intervention providers, and outcome assessors should be ideally blinded to the intervention allocations if possible, and dropout from the study should be minimal.3 If the intervention is the only difference between the trial groups, any differences in the outcomes could reliably be attributed to the intervention. These ideal experimental conditions allow high internal validity of RCTs. Still, the generalizability of their results to patients outside the studied population (external validity) is inherently limited, and clinicians must employ their judgment when applying results from RCTs to their clinical practice, which is potentially problematic.4, 5 During the last two decades, we have seen significant expansion in the use of RCT’s model to evaluate interventions in real-world circumstances, referred to as “effectiveness” research rather than traditional RCT “efficacy” research.6-8 In this particular “effectiveness” context, the number of RCTs published in the rehabilitation field is increasing more than in other fields.7 In 2022, rehabilitation has been defined by Cochrane Rehabilitation as “a multimodal, person-centered, collaborative process, including interventions targeting a person’s capacity (by addressing body structures, functions, and activities/participation) and/or contextual factors related to performance, with the goal of optimizing the functioning of persons with health conditions currently experiencing disability or likely to experience disability, or persons with disability in interaction with the environment”9. This new definition proposes that rehabilitation interventions are “complex interventions” because they are a process (changing in time), multimodal (more than one intervention), collaborative (interaction of behaviors) and person-centered (aims and means can accordingly change). It is suggested that “the greater the difficulty in defining precisely what exactly are the `active ingredients’ of an intervention and how they relate to each other, the greater the likelihood that you are dealing with a complex intervention”10. Therefore, there are challenges when applying methodological standards of high-quality clinical research to rehabilitation interventions.11
The “effectiveness RCT”, also considered as pragmatic trials8 can be a trade-off between rehabilitation characteristics and the ideal RCT model because they are typically more inclusive, reflecting the heterogeneity of patients in clinical practice, allowing for flexible delivery of the interventions, measuring functional outcomes, and often lack of blinding of participants and those providing treatment.5 However, by maintaining randomization while relaxing the other experimental conditions (e.g., strict fidelity to rehabilitation interventions, measurement of functional outcomes, and blinding), it is argued that the trial results could be rigorous enough, generalizable to real-world settings and probably applicable in the rehabilitation context.6
In the literature, many studies have developed specific frameworks for developing and evaluating complex interventions and advocate for methodological standards that should be respected when planning an effectiveness study using complex interventions.12, 13 In addition, many manuscripts have been published on improving the quality of the evidence in rehabilitation.6, 7, 14, 15 These studies have highlighted the need to identify whether biases resulting from methodological issues related to rehabilitation characteristics, such as lack or difficulty of blinding, lack or difficulty of intervention standardization or fidelity and adherence to the intervention, can lead to an overestimation or underestimation of the true rehabilitation intervention effect estimates. This can be achieved using empirical evidence, by using meta-epidemiological studies, which collate numerous meta-analyses and, within each of them, contrast the estimates of intervention effects of trials with and without a methodological characteristic of interest.16 Indeed, effect size estimates may be related to the conduct (i.e., methodological variables) of the studies, beyond the study design per se (RCTs vs. observational studies).17 Therefore, it is important to determine what important methodological features of studies conducted in the rehabilitation field could potentially bias treatment effects results.
In the last few years, several meta-epidemiological studies about the potential influence of bias on treatment effects have been conducted in the area of rehabilitation; however, to our knowledge, no one has attempted to synthesize this evidence to date. To respond to this need, we decided to conduct a meta-research study synthesizing this information. This manuscript is part of a series of papers developed by the Cochrane Rehabilitation field and discussed extensively during the 5th Cochrane Rehabilitation Methodological Meeting: “The Rehabilitation evidence ecosystem: useful study designs”, that took place in Milan on 7th and 8th September 2023. This series of papers has attempted to answer the following questions: 1) Which study design can and should be used for which kind of research question to provide useful evidence for rehabilitation? 2) How do we maximize the trustworthiness of the results of these study designs to produce useful evidence on rehabilitation? 3) How can we conduct studies of different designs to make them useful for rehabilitation end-users (clinicians, policymakers and patients)? 4) When and how can these designs contribute to building the evidence in rehabilitation if RCTs are not yet available?
To provide data to address question 2, we summarized all relevant studies that addressed the influence of bias domains in RCTs, examining rehabilitation intervention effect estimates and discussing how these findings can help to maximize the trustworthiness of RCTs in the field of rehabilitation.
Methods
We systematically identified and summarized studies which investigated the association between reported bias domains (selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias) and rehabilitation intervention effect estimates in RCTs included in Agency for Healthcare Research and Quality – Evidence-based Practice Center (AHRQ-EPC) review, published in 2014,18 and in Page’s systematic review, published in 2016.19 We focused on bias domains, as suggested by Cochrane3 because it is argued that these are the main methodological characteristics that can change the direction of effect regardless of the study design used to evaluate the effectiveness of the intervention.
Evidence collection and eligibility criteria
We screened eligible studies included in the AHRQ-EPC review (latest search September 2012)18 and in Page’s systematic review (latest search May 2015).19 To identify more recent meta-epidemiological studies in rehabilitation, we performed a narrow quick search in PubMed from June 2015 to June 2023 using the keywords “meta-epidemiological studies” AND “rehabilitation” without the development of a specific search strategy. No language limitations were applied in the database searches nor as inclusion criteria. Two authors (CA and SM) independently reviewed the reference lists of all the included meta-epidemiological studies to identify additional meta-epidemiological studies in rehabilitation that the electronic search could have missed.
The eligibility criteria were: 1) type of studies: meta-epidemiological studies or studies that evaluate the effect of methodological characteristics on effect estimate20 of rehabilitation interventions, and 2) types of methodological characteristics: studies had to evaluate biases using the conceptual framework of the Cochrane risk of bias tool for RCTs as described above.3
Two independent reviewers (EP and CA) screened the studies using the predefined eligibility criteria. The studies were analyzed using the available information and a senior author (SM) resolved disagreements between reviewers through discussion.
Data extraction
We extracted the following data: study characteristics (author, year, design, setting and type of rehabilitation intervention), number of RCTs and participants included in the meta-epidemiological synthesis, and type of bias domains (i.e., selection bias, performance bias, detection bias, attrition bias and reporting bias). We also extracted all estimates of the relationship between different types of methodological characteristics and treatment effects. One reviewer extracted the information (CA), and a second reviewer independently checked all data extracted (SM).
Data analysis and synthesis
The information obtained from the included studies was organized by type of methodological characteristics and summarized using a narrative approach, including the effect size estimation (ES) and 95% confidence interval (CI). Evidence tables and figures were used to present data where appropriate.
Results
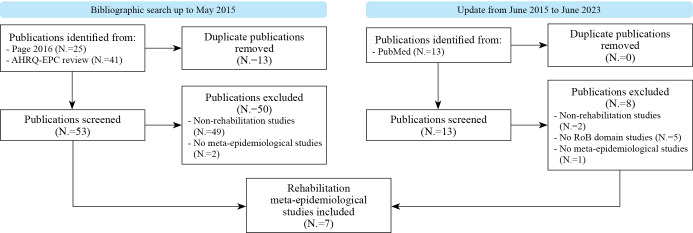
We retrieved 53 full-text articles from the two previous systematic reviews18, 19 and 13 full-text articles from the electronic search. We found only studies published in English. Seven meta-epidemiological studies met the inclusion criteria.21-27 See Figure 1 for the screening process.
Figure 1.
—Flowchart of the studies.
Characteristics of the included studies
We included seven eligible meta-epidemiological studies (included data on 227,806 participants)21-27 in a rehabilitation context. Armijo-Olivo and De Almeida23, 24 addressed the influence of sequence generation and allocation concealment on treatment effect, Armijo-Olivo and Liu21, 27 on blinding of participants, clinicians and assessors, Armijo-Olivo22 on attrition bias, Hayden 26 on reporting bias and Fuentes25 on sponsorship bias. The rehabilitation interventions considered were physical therapy (PT). Still, in four studies,21-23, 25 there was no definition of which interventions they focused on, and De Almeida24 included the following interventions as physical therapy: exercise therapy, electrophysical agents, manual therapy, acupuncture, behavioral therapy, advice or education, multidisciplinary treatment, taping, and traction. Liu27 included only progressive resistance strength intervention, and Hayden26 only exercise therapy.
All studies used a meta-analytic approach20 to analyze the influence of certain methodological characteristics (rated as inadequate/adequate/unclear) on treatment effect estimates. The average bias associated with methodological characteristics was reported in all meta-epidemiological studies. Full details are reported in Table I.21-27
Table I. —Characteristics of included studies.21-27.
| Author year | Design | Setting | Rehabilitation intervention | Outcome | Sample size | Number of included RCTs | Methodological characterstics |
|---|---|---|---|---|---|---|---|
| Armijo-Olivo 23 | Meta-epidemiological study | Rehabilitation | Physical Therapy | NA | 44622 | 393 | Sequence generation and allocation concealment |
| Armijo-Olivo 21 | Meta-epidemiological study | Rehabilitation | Physical therapy | NA | 44622 | 393 | Blinding |
| Armijo-Olivo 22 | Meta-epidemiological study | Rehabilitation | Physical therapy | NA | 44622 | 393 | Bias related to attrition, missing data and the use of ITT |
| De Almeida 24 | Meta-epidemiological study | Rehabilitation | Exercise therapy, electrophysical agents, manual therapy, acupuncture, multidisciplinary treatment, taping, traction, educa- tion, behavioral therapy | Low back pain | 20555 | 128 | Allocation Concealment and ITT analysis |
| Fuentes 25 | Meta-epidemiological study | Rehabilitation | Physical therapy | NA | 44622 | 393 | Sponsorhip bias |
| Hayden 26 | Cross-sectional meta-epidemiological study | Rehabilitation | Exercise therapy for chronic low back pain | Low back pain | 25704 | 279 | Publication integrity, quality of conduct and reporting |
| Liu 27 | Meta-epidemiological study | Rehabilitation | Progressive resistance strength training | Lower limb muscle strength | 3059 | 73 | Blinding |
NA: not applicable; RCTs: randomized control trials; ITT: intention to treat.
Selection bias – random sequence generation and allocation concealment
Armijo-Olivo and De Almeida23, 24 evaluated the association between random sequence generation and allocation concealment on physical therapy effect estimates.
Armijo-Olivo23 included 43 meta-analyses, 393 RCTs with 44,622 participants. It found no difference between adequate and inadequate random sequence generation (ES difference: 0.02; 95% CI -0.12 to 0.15), while trials with inadequate allocation concealment tended to have an overestimated effect estimate when compared with those with adequate allocation concealment, but the mean difference was non-statistically significant (ES difference: 0.12; 95% CI -0.06 to 0.30).
De Almeida24 included 128 RCTs with 20,555 participants, who were treated using exercise therapy, electrophysical agents, manual therapy, acupuncture, behavioral therapy, advice or education, multidisciplinary treatment, taping, and traction. It showed that, although trials that had inadequate allocation concealment had larger treatment effect estimates than those trials with adequate allocation concealment, the difference between the estimates was not statistically significant for pain (ES difference: 0.31 95% CI -0.04 to 0.66) and disability (ES difference: 0.19; 95% CI -0.16 to 0.54) outcomes.
Performance bias and detection bias – blinding
Armijo-Olivo and Liu21, 27 evaluated the association between blinding of participants, assessors, and/or health providers (overall) on treatment effect estimates when analyzing physical therapy interventions or progressive resistance strength using continuous outcomes. Armijo-Olivo21 included 393 RCTs with 44,622 participants. It suggested that inappropriate overall blinding (whether the appropriate component of blinding [participants, assessors, therapist or irrelevant] based on the main outcome of interest for the trial was used) underestimated treatment effect estimates, but the mean difference between trials with inappropriate vs. appropriate overall blinding was not statistically significant (ES -0.08; 95% CI -0.28 to 0.12). In addition, although trials with inappropriate blinding of assessors and participants tended to underestimate treatment effects when compared with trials with appropriate blinding of assessors and participants, the difference was not statistically significant (ES: -0.07; 95% CI -0.22, 0.08; ES -0.12; 95% CI -0.30, 0.06, respectively).
Liu,27 which includes 73 RCTs with 3,059 participants within a meta-analysis, showed that trials using blinded assessors tended to report smaller effect sizes than those using unblinded assessors on muscle strength outcomes, with a difference of -0.80 (95% CI -1.35 to -0.25). The reported effects were exaggerated in trials that used unblinded assessors.
Attrition bias and intention-to-treat analysis
Two studies21, 23 evaluated the association between attrition bias and/or intention-to-treat (ITT) analysis. Armijo-Olivo22 including 43 meta-analysis, 393 RCTs with 44,622 participants, evaluated the association between attrition bias and treatment effect estimates when evaluating physical therapy interventions using continuous outcomes. This study22 showed that trials which did not use the ITT principle, or which were assessed as having inappropriate control of incomplete outcome data, tended to underestimate the effect estimates when compared with trials with adequate use of ITT (ES difference: -0.13; 95% CI -0.26 to 0.01) and control of incomplete outcome data (ES difference: -0.18; 95% CI -0.29 to -0.08).
Almeida,24 stated that, overall, ITT had no significant influence on treatment effects in trials for low back pain. However, the findings demonstrated that trials using ITT had a significantly lower effect estimate than those without ITT. The difference between trials with and without ITT on pain outcomes was ES: 0.49; 95%CI 0.12 to 0.87. In contrast, for disability outcomes, the difference between trials with and without ITT was not statistically significant (ES: 0.16; 95%CI -0.21 to 0.53).
Reporting bias
Hayden,26 including 279 RCTs with 25,704 participants within a Cochrane review, evaluating the association between reporting bias and exercise therapy estimation’s effect on pain and functional outcomes. This study reported that RCTs that did not report trial registration or had no published protocol available reported larger mean differences in pain outcomes (MD: -5.0; 95% CI -8.8 to -1.3 unadjusted model; MD: -2.3; 95% CI -6.7 to 2.1 adjusted model). This was true also for studies that did not report core outcome measures (MD, -8.1; 95% CI -11.9 to -4.4 unadjusted model; MD: -7.3; 95% CI -11.3 to -3.3 for the adjusted model), that did not include follow-up outcome measurement of at least 12 weeks (MD: -3.9; 95% CI -7.5 to -0.3 unadjusted model; MD: -2.9, 95% CI -7.1 to 1.4 for the adjusted model), or that did not report complete participant flow information (i.e., CONSORT flow chart; MD: -3.9; 95% CI -7.5 to -0.3 unadjusted model; MD: -1.8; 95% CI: -5.8 to 2.2 for the adjusted model). Only missing core outcome measures remained significantly associated with increased mean effect estimates on pain outcome after adjustment for all the rest of variables (12 in total) (MD: -7.3; 95% CI -11.3 to -3.3 for the adjusted model). Inadequate sample size was found to be statistically significantly associated with increased functional limitations outcomes reported in the included trials (MD: -7.1; 95% CI -12.2 to -1.9) in unadjusted and adjusted analyses (MD: -6.4; 95% CI -12.0 to -0.8).
Other bias - sponsorship bias
Fuentes,25 including 43 meta-analyses, 377 RCTs and 43,651 participants, evaluated the association between sponsorship bias and treatment effect estimates for physical therapy intervention using continuous outcomes. This study reported that funding was not declared in many trials (N.=85, 22%). When it was declared, the influence of the trial sponsor was assessed as being appropriate (i.e., statement of no sponsor involvement in the trial) in 246 trials (63%), and considered inappropriate/unclear (i.e., statement of sponsor involvement in the trial/not enough information) in the remaining 147 (37%) trials. The authors concluded that trials with inappropriate/unclear influence of funders tended to have, on average, a larger effect size than those with the appropriate influence of funding (ES difference: 0.15; 95% CI −0.03 to 0.33). Although the difference was not statistically significant, trials with sponsor involvement tended to overestimate the effect estimates. See Table II for full details.
Table II. —Data synthesis.
| Bias | N. studies | Rehabilitation intervention | Outcomes | Effect estimates | Findings |
|---|---|---|---|---|---|
| Selection bias (random sequence generation and allocation concealment) | 2 | Physical Therapy and exercise therapy | Pain and disability outcomes (only one study) | ES difference: 0.12; 95% CI -0.06 to 0.30 Pain: ES difference: 0.31 95% CI -0.04 to 0.66 Disability: ES difference: 0.19; 95%CI -0.16 to 0.54 |
Inadequate allocation concealment tended to have an overestimated effect estimate when compared with those with adequate allocation concealment |
| Performance bias and detection bias (blinding) | 2 | Physical therapy and progressive resistance strength | Continuous outcomes and muscle strength outcomes | ES: -0.08; 95% CI -0.28 to 0.12 | 1) Inappropriate overall blinding underestimated treatment effect estimates; inappropriate blinding of assessors and participants tended to underestimate treatment effects 2) Blinded assessors underestimated the treatment effect estimates |
| Attrition bias and intention-to-treat analysis | 1 | Physical intervention | Pain and disability (continuous data) | ES difference (attrition): -0.13; 95% CI -0.26 to 0.01; ES (ITT): 0.49; 95% CI 0.12 to 0.87 | Inappropriate control of incomplete outcome data, tended to underestimate the effect estimates. ITT tended to underestimate estimate than those trials without ITT |
| Reporting bias | 1 | Exercise therapy | Pain and functional outcomes | MD: −5.0; 95% CI −8.8 to −1.3 unadjusted model; MD: −2.3; 95% CI −6.7 to 2.1 adjusted model | No trial registration or no published protocol available tended to overestimate the treatment effect |
| Other bias (sponsorship) | 1 | Physical therapy | Continuous outcomes | ES: 0.15; 95% CI −0.03 to 0.33 | Inappropriate/unclear influence of funders tended to overestimate the treatment effect estimates |
ES: effect size estimation; CI: confidence interval; MD: mean difference; ITT: intention-to-treat.
Discussion
We found only seven studies that investigated the influence of bias in treatment estimates in the rehabilitation field. This limited evidence is contrasted with the higher number of meta-epidemiological studies conducted in other medical fields identified by Page’s systematic review and AHRQ-EPC review (N.=49). Our findings align with the findings of Page’s systematic review,19 agreeing that intervention effect estimates are most likely exaggerated in trials with inadequate/unclear sequence generation and allocation concealment (selection bias) when using continuous outcomes. The influence of blinding (performance and detection bias) on the results of trials in rehabilitation seems to be inconsistent. It differs from other medical research, which shows a more consistent pattern when looking at patient-reported outcome measures (PROMs). In other medical fields, it seems that failure to blind patients probably overestimates treatment effects for PROMs. At the same time, there is high uncertainty about its influence on other outcomes. Also, blinding of outcome assessors has been found to influence treatment effect estimates in medical trials, and thus, blinding of outcome assessors has been found important for subjective outcomes. We found that studies looking at rehabilitation studies and blinding (N.=2) showed overestimation, underestimation, or neutral associations for different types of blinding (i.e., participants, assessors, therapists, statisticians) on rehabilitation treatment effect estimates. Consequently, no clear conclusions can be drawn on the influence of blinding on trial estimates based on this literature.28 This means that blinding, as currently evaluated, probably could be less important than often believed29 or that studies may have used other ways to decrease performance or detection biases (but not necessarily have used blinding of allocation). For example, a way of decreasing these biases could be by providing limited information about the hypothesis of the study to participants and assessors, using expertise-based RCTs to avoid biases at the therapist level, or providing neutral expectations for treatments offered, among others.30 These alternative ways of conducting RCTs in the rehabilitation field arguably highlight that – despite the challenges associated with blinding within RCTs in rehabilitation – there are potential adaptations which may help overcome these biases.
The influence of attrition bias on treatment effects on continuous outcomes is also unclear. This could be related to the variation of the definition of attrition and associated biases across meta-epidemiological studies. Also, the definition of the concept of ITT has been misused and misinterpreted by several studies31, 32 (e.g., trials reported the phrase “intention to treat” with no apparent deviation in the description or trials that correctly described the ITT principle).33 In our study, from the two included studies that looked at the concept of ITT, one found an overestimation for trials with inappropriate ITT, and the other found an underestimation regardless of the type of outcome. This difference could depend on the definition of attrition bias used,34 as well as the outcomes investigated since, according to some,35 the influence of biases on treatment effect estimates depends on the outcome investigated.
The results of this study suggest also that the lack of core outcome reporting (reporting bias) seems to be associated with higher treatment effect sizes reported for pain outcomes and probably in functional limitations outcomes. The influence of other characteristics on treatment effect estimates in rehabilitation is uncertain because, as far as our knowledge, they have not been yet examined.
Given the scarce number of studies looking at the influence of bias and treatment effects in the rehabilitation field and their heterogeneity regarding the definition of the biases and types of rehabilitation interventions and outcomes, we cannot draw any definitive conclusion regarding the influence of bias on rehabilitation treatment effects. In addition, these included studies have been exploratory since they did not perform sample size calculations36 and were underpowered due to the small magnitude of the differences between trials with and without the bias domain, small sample sizes, and the high heterogeneity of the datasets. It has been recommended18, 37 that larger sample sizes (≥600 trials and/or 50 meta-analyses or even more38) are required to have adequate power to investigate these associations between bias and treatment effects. None of the included studies accomplished this standard. Also, it has been suggested28 to assemble a homogenous set of meta-analyses and trials in a specific area of research to decrease the heterogeneity of the datasets and increase the likelihood of finding a difference when it really exists.
It is also important to highlight that most existing meta-epidemiological studies have analysed the isolated effect of biases on treatment effect estimates. However, it has been highlighted that the influence of certain biases on treatment effect estimates may vary across different areas of research and be influenced by interaction with other biases.18 Therefore, future meta-epidemiological studies should look at this interaction, to address this important gap in the literature.
Empirical evidence from meta-epidemiological studies is of value to guiding risk of bias assessment when conducting systematic reviews to guide healthcare decision-making. Although these studies have been recognized as a good method to investigate biases, they are not free from limitations, as highlighted by Moustgaard et al.,16 and Page,39 who have emphasized several steps when interpreting the results of these studies; especially confounding. Thus, results from these studies should be interpreted with caution. Future meta-epidemiological studies should follow this guidance to provide accurate estimates of the influence of biases in treatment effect estimates in health research and the rehabilitation literature. Further, the conclusions of these included studies report that often, the description of the methodological characteristics of RCTs was very poor in primary studies, relevant information was commonly missing, and most of the RCTs included in meta-epidemiological studies were underpowered.
Once again, we highlight the urgent need to improve the quality of the reporting and conduct of RCTs by following the CONSORT statement, because if we do not have enough information on how an RCT has been conducted, we cannot know whether the study is biased and whether the bias can influence the treatment effect estimates and consequently the trustworthiness and value of the evidence provided by them.40 This might be supported by investing time, energy, and funding in training future generations on methodological principles and epidemiology. It has been shown that researchers who are inexperienced and untrained in the steps and procedures of the scientific method may not know how to develop a research question and how to design a well-conducted RCT in an efficient manner. Training future rehabilitation scientists would eventually improve the quality of conduct of trials in our field.41 We also need to produce more high-quality RCTs to have more reliable data and consistent findings that allow us to study the relationship between bias and effect estimates and to be more confident regarding the effectiveness of the rehabilitation interventions.
Limitations of the study
This study had several limitations. Firstly, a limited search strategy in PubMed was used for searching meta-epidemiological studies published between 2015 and 2023. However, it should be acknowledged that PubMed has been recognized as one of the most complete and updated databases in the health literature. This was a meta-research study with a narrow scope. Therefore, some studies could have been missed. However, the aim was to bring together and discuss how the findings from meta-epidemiological studies can support the trustworthiness of RCTs in rehabilitation. Our study included only published reports, meaning that there is a risk that we have failed to incorporate unpublished studies. We did not consider whether the studies adhered to the actual definition of meta-epidemiological studies as described by Moustgaard et al16, 42 As highlighted by the literature, the definition of meta-epidemiological studies is unclear, and researchers used it inconsistently. Secondly, meta-epidemiological studies did not assess the risk of bias; therefore, they included RCTs of any methodological quality, indirectly creating bias across the included meta-epidemiological studies.43, 44 For this reason, we only considered the bias domains used in the Cochrane Risk of Bias tool, excluding other significant biases such as publication45 or bias in measuring the outcome.43 Thirdly, most interventions included in meta-epidemiological studies were physical therapy and exercise without any information regarding controls or the outcome measurements. This might lead to limited generalizability of the findings to other rehabilitation interventions, participants, and outcomes.
Conclusions
Our findings show that bias domains can influence the treatment effect in different directions. The evidence is mixed and inconclusive due to the poor methodological quality of RCTs as well as the limited number and quality of studies looking at the influence of bias and treatment effects in rehabilitation. We can only confirm the need to improve the reporting and the conduction of randomized studies. Further studies about the influence of bias in RCTs on rehabilitation intervention effect estimates are needed.
Acknowledgments
This work was supported by the International Society of Physical and Rehabilitation Medicine (ISPRM).
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding: This study was supported and funded by the Italian Ministry of Health - Ricerca Corrente. The funders had no role in study design, data collection and analysis, publication decisions, or manuscript preparation.
Contributor Information
Participants in the 5th Cochrane Rehabilitation Methodological Meeting:
Irene BATTEL, Maria G. CERAVOLO, Christopher COLVIN, Claudio CORDANI, Pierre CÔTÉ, Anne CUSICK, Bernard DAN, Wouter De GROOTE, Matteo J. DEL FURIA, Susanna EVERY-PALMER, Francesca GIMIGLIANO, Christoph GUTENBRUNNER, Tiziano INNOCENTI, Carsten B. JUHL, William M. M. LEVACK, Sara LIGUORI, Wendy MACHALICEK, Rachelle MARTIN, Federico MERLO, Thorsten MEYER-FEIL, Luca MIRANDA, Bianca MOSCONI, Cecilie RØE, Heather SHEARER, and Jessica WONG
References
- 1.Guyatt GH, Rennie D. Users’ guides to the medical literature. JAMA 1993;270:2096–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8411578&dopt=Abstract 10.1001/jama.1993.03510170086037 [DOI] [PubMed] [Google Scholar]
- 2.Hoogeboom TJ, Kousemaker MC, van Meeteren NL, Howe T, Bo K, Tugwell P, et al. i-CONTENT tool for assessing therapeutic quality of exercise programs employed in randomised clinical trials. Br J Sports Med 2021;55:1153–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33144350&dopt=Abstract 10.1136/bjsports-2019-101630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4; 2023. [Google Scholar]
- 4.Paci M, Prestera C, Ferrarello F. Generalizability of Results from Randomized Controlled Trials in Post-Stroke Physiotherapy. Physiother Can 2020;72:382–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35110812&dopt=Abstract 10.3138/ptc-2018-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutron I, Dutton S, Ravaud P, Altman DG. Reporting and interpretation of randomized controlled trials with statistically nonsignificant results for primary outcomes. JAMA 2010;303:2058–64. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20501928&dopt=Abstract 10.1001/jama.2010.651 [DOI] [PubMed] [Google Scholar]
- 6.Negrini S, Arienti C, Pollet J, Engkasan JP, Francisco GE, Frontera WR, et al. ; REREP study participants. Clinical replicability of rehabilitation interventions in randomized controlled trials reported in main journals is inadequate. J Clin Epidemiol 2019;114:108–17. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31220570&dopt=Abstract 10.1016/j.jclinepi.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 7.Negrini S, Levack W, Gimigliano F, Arienti C, Villafañe JH, Kiekens C. The Struggle for Evidence in Physical and Rehabilitation Medicine: Publication Rate of Randomized Controlled Trials and Systematic Reviews Is Growing More Than in Other Therapeutic Fields. Am J Phys Med Rehabil 2019;98:258–65. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30277918&dopt=Abstract 10.1097/PHM.0000000000001058 [DOI] [PubMed] [Google Scholar]
- 8.Ford I, Norrie J. Pragmatic Trials. N Engl J Med 2016;375:454–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27518663&dopt=Abstract 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- 9.Negrini S, Selb M, Kiekens C, Todhunter-Brown A, Arienti C, Stucki G, et al. ; 3rd Cochrane Rehabilitation Methodology Meeting participants. Rehabilitation definition for research purposes. A global stakeholders’ initiative by Cochrane Rehabilitation. Eur J Phys Rehabil Med 2022;58:333–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35306803&dopt=Abstract 10.23736/S1973-9087.22.07509-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10987780&dopt=Abstract 10.1136/bmj.321.7262.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armijo-Olivo S, Cummings GG, Fuentes J, Saltaji H, Ha C, Chisholm A, et al. Identifying items to assess methodological quality in physical therapy trials: a factor analysis. Phys Ther 2014;94:1272–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24786942&dopt=Abstract 10.2522/ptj.20130464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esmail LC, Barasky R, Mittman BS, Hickam DH. Improving Comparative Effectiveness Research of Complex Health Interventions: Standards from the Patient-Centered Outcomes Research Institute (PCORI). J Gen Intern Med 2020;35(Suppl 2):875–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33107006&dopt=Abstract 10.1007/s11606-020-06093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374:n2061. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34593508&dopt=Abstract 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arienti C, Armijo-Olivo S, Minozzi S, Tjosvold L, Lazzarini SG, Patrini M, et al. Methodological Issues in Rehabilitation Research: A Scoping Review. Arch Phys Med Rehabil 2021;102:1614–1622.e14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33989598&dopt=Abstract 10.1016/j.apmr.2021.04.006 [DOI] [PubMed] [Google Scholar]
- 15.Negrini S, Armijo-Olivo S, Patrini M, Frontera WR, Heinemann AW, Machalicek W, et al. ; RCTRACK Promoters. The Randomized Controlled Trials Rehabilitation Checklist: Methodology of Development of a Reporting Guideline Specific to Rehabilitation. Am J Phys Med Rehabil 2020;99:210–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31851008&dopt=Abstract 10.1097/PHM.0000000000001370 [DOI] [PubMed] [Google Scholar]
- 16.Moustgaard H, Jones HE, Savović J, Clayton GL, Sterne JA, Higgins JP, et al. Ten questions to consider when interpreting results of a meta-epidemiological study-the MetaBLIND study as a case. Res Synth Methods 2020;11:260–74. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31851427&dopt=Abstract 10.1002/jrsm.1392 [DOI] [PubMed] [Google Scholar]
- 17.Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ 1998;317:1185–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9794851&dopt=Abstract 10.1136/bmj.317.7167.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berkman ND, Santaguida PL, Viswanathan M, Morton SC. The Empirical Evidence of Bias in Trials Measuring Treatment Differences. Methods Research Report. (Prepared by the RTI-UNC Evidence-based Practice Center under Contract No. 290-2007- 10056-I.) AHRQ Publication No. 14-EHC050-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed]
- 19.Page MJ, Higgins JP, Clayton G, Sterne JA, Hróbjartsson A, Savović J. Empirical Evidence of Study Design Biases in Randomized Trials: Systematic Review of Meta-Epidemiological Studies. PLoS One 2016;11:e0159267. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27398997&dopt=Abstract 10.1371/journal.pone.0159267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Jüni P, Schulz KF, Altman DG, Bartlett C, Egger M. Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’ research. Stat Med 2002;21:1513–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12111917&dopt=Abstract 10.1002/sim.1184 [DOI] [PubMed] [Google Scholar]
- 21.Armijo-Olivo S, Fuentes J, da Costa BR, Saltaji H, Ha C, Cummings GG. Blinding in Physical Therapy Trials and Its Association with Treatment Effects: A Meta-epidemiological Study. Am J Phys Med Rehabil 2017;96:34–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27149591&dopt=Abstract 10.1097/PHM.0000000000000521 [DOI] [PubMed] [Google Scholar]
- 22.Armijo-Olivo S, R da Costa B, Ha C, Saltaji H, Cummings GG, Fuentes J. Are Biases Related to Attrition, Missing Data, and the Use of Intention to Treat Related to the Magnitude of Treatment Effects in Physical Therapy Trials?: A Meta-Epidemiological Study. Am J Phys Med Rehabil 2022;101:520–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34225281&dopt=Abstract 10.1097/PHM.0000000000001837 [DOI] [PubMed] [Google Scholar]
- 23.Armijo-Olivo S, Saltaji H, da Costa BR, Fuentes J, Ha C, Cummings GG. What is the influence of randomisation sequence generation and allocation concealment on treatment effects of physical therapy trials? A meta-epidemiological study. BMJ Open 2015;5:e008562. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26338841&dopt=Abstract 10.1136/bmjopen-2015-008562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Almeida MO, Saragiotto BT, Maher C, Costa LO. Allocation Concealment and Intention-To-Treat Analysis Do Not Influence the Treatment Effects of Physical Therapy Interventions in Low Back Pain Trials: a Meta-epidemiologic Study. Arch Phys Med Rehabil 2019;100:1359–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30710510&dopt=Abstract 10.1016/j.apmr.2018.12.036 [DOI] [PubMed] [Google Scholar]
- 25.Fuentes J, Armijo-Olivo S, da Costa BR, Ha C, Saltaji H, Arenti C, et al. Does Type of Sponsorship of Randomized Controlled Trials Influence Treatment Effect Size Estimates in Rehabilitation: A Meta-Epidemiological Study. Am J Phys Med Rehabil 2020;99:909–16. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32960528&dopt=Abstract 10.1097/PHM.0000000000001444 [DOI] [PubMed] [Google Scholar]
- 26.Hayden JA, Ellis J, Ogilvie R, Boulos L, Stanojevic S. Meta-epidemiological study of publication integrity, and quality of conduct and reporting of randomized trials included in a systematic review of low back pain. J Clin Epidemiol 2021;134:65–78. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33545270&dopt=Abstract 10.1016/j.jclinepi.2021.01.020 [DOI] [PubMed] [Google Scholar]
- 27.Liu CJ, LaValley M, Latham NK. Do unblinded assessors bias muscle strength outcomes in randomized controlled trials of progressive resistance strength training in older adults? Am J Phys Med Rehabil 2011;90:190–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21173683&dopt=Abstract 10.1097/PHM.0b013e31820174b3 [DOI] [PubMed] [Google Scholar]
- 28.Armijo-Olivo S, Dennett L, Arienti C, Dahchi M, Arokoski J, Heinemann AW, et al. Blinding in Rehabilitation Research: Empirical Evidence on the Association Between Blinding and Treatment Effect Estimates. Am J Phys Med Rehabil 2020;99:198–209. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31913147&dopt=Abstract 10.1097/PHM.0000000000001377 [DOI] [PubMed] [Google Scholar]
- 29.Moustgaard H, Clayton GL, Jones HE, Boutron I, Jørgensen L, Laursen DR, et al. Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study. BMJ 2020;368:l6802. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31964641&dopt=Abstract 10.1136/bmj.l6802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armijo-Olivo S, Mohamad N, Sobral de Oliveira-Souza AI, de Castro-Carletti EM, Ballenberger N, Fuentes J. Performance, Detection, Contamination, Compliance, and Cointervention Biases in Rehabilitation Research: What Are They and How Can They Affect the Results of Randomized Controlled Trials? Basic Information for Junior Researchers and Clinicians. Am J Phys Med Rehabil 2022;101:864–78. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35978455&dopt=Abstract 10.1097/PHM.0000000000001893 [DOI] [PubMed] [Google Scholar]
- 31.Abraha I, Cherubini A, Cozzolino F, De Florio R, Luchetta ML, Rimland JM, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study. BMJ 2015;350:h2445. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26016488&dopt=Abstract 10.1136/bmj.h2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alshurafa M, Briel M, Akl EA, Haines T, Moayyedi P, Gentles SJ, et al. Inconsistent definitions for intention-to-treat in relation to missing outcome data: systematic review of the methods literature. PLoS One 2012;7:e49163. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23166608&dopt=Abstract 10.1371/journal.pone.0049163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babic A, Tokalic R, Amílcar Silva Cunha J, Novak I, Suto J, Vidak M, et al. Assessments of attrition bias in Cochrane systematic reviews are highly inconsistent and thus hindering trial comparability. BMC Med Res Methodol 2019;19:76. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30953448&dopt=Abstract 10.1186/s12874-019-0717-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10480822&dopt=Abstract 10.1136/bmj.319.7211.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hempel S, Suttorp MJ, Miles JN, Wang Z, Maglione M, Morton S, et al Empirical Evidence of Associations Between Trial Quality and Effect Size. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. [PubMed] [Google Scholar]
- 36.Hempel S, Miles J, Suttorp M, Wang Z, Johnsen B, Morton S, et al. Detection of Associations between Trial Quality and Effect Sizes. Methods Research Report. Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2007-10062-I. AHRQ Publication No. 12-EHC010-EF. Rockville, MD: Agency for Healthcare Research and Quality; January 2012. [PubMed]
- 37.Hempel S, Suttorp MJ, Miles JN, Wang Z, Maglione M, Morton S, et al. Empirical Evidence of Associations Between Trial Quality and Effect Sizes. Methods Research Report (Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2007-10062-I). AHRQ Publication No. 11-EHC045-EF. Rockville, MD: Agency for Healthcare Research and Quality. June 2011. [PubMed]
- 38.Giraudeau B, Higgins JP, Tavernier E, Trinquart L. Sample size calculation for meta-epidemiological studies. Stat Med 2016;35:239–50. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26286683&dopt=Abstract 10.1002/sim.6627 [DOI] [PubMed] [Google Scholar]
- 39.Page MJ. Controversy and Debate on Meta-epidemiology. Paper 4: confounding and other concerns in meta-epidemiological studies of bias. J Clin Epidemiol 2020;123:133–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32251682&dopt=Abstract 10.1016/j.jclinepi.2020.03.022 [DOI] [PubMed] [Google Scholar]
- 40.Innocenti T, Giagio S, Salvioli S, Feller D, Minnucci S, Brindisino F, et al. Completeness of Reporting Is Suboptimal in Randomized Controlled Trials Published in Rehabilitation Journals, With Trials With Low Risk of Bias Displaying Better Reporting: A Meta-research Study. Arch Phys Med Rehabil 2022;103:1839–47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35192799&dopt=Abstract 10.1016/j.apmr.2022.01.156 [DOI] [PubMed] [Google Scholar]
- 41.Ubeda SR. How to Build and Assess the Quality of Healthcare-Related Research Questions. Glob J Qual Saf Healthc 2022;5:39–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37260836&dopt=Abstract 10.36401/JQSH-21-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puljak L. Caution is needed when describing a study design as meta-epidemiological. J Clin Epidemiol 2022;152:326–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36309145&dopt=Abstract 10.1016/j.jclinepi.2022.10.017 [DOI] [PubMed] [Google Scholar]
- 43.Innocenti T, Hayden JA, Salvioli S, Giagio S, Piano L, Cosentino C, et al. Bias in the measurement of the outcome is associated with effect sizes in randomized clinical trials on exercise therapy for chronic low back pain: a meta-epidemiological study. J Clin Epidemiol 2023;162:145–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37704114&dopt=Abstract 10.1016/j.jclinepi.2023.09.001 [DOI] [PubMed] [Google Scholar]
- 44.Herbert RD. Controversy and Debate on Meta-epidemiology. Paper 2: meta-epidemiological studies of bias may themselves be biased. J Clin Epidemiol 2020;123:127–30. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32247656&dopt=Abstract 10.1016/j.jclinepi.2020.03.024 [DOI] [PubMed] [Google Scholar]
- 45.Every-Palmer S, Howick J. How evidence-based medicine is failing due to biased trials and selective publication. J Eval Clin Pract 2014;20:908–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24819404&dopt=Abstract 10.1111/jep.12147 [DOI] [PubMed] [Google Scholar]