Abstract
Effective resolution of inflammation via the heat shock response (HSR) is pivotal in averting the transition to chronic inflammatory states. This transition characterizes a spectrum of debilitating conditions, including insulin resistance, obesity, type 2 diabetes, nonalcoholic fatty liver disease, and cardiovascular ailments. This manuscript explores a range of physiological, pharmacological, and nutraceutical interventions aimed at reinstating the HSR in the context of chronic low-grade inflammation, as well as protocols to assess the HSR. Monitoring the progression or suppression of the HSR in patients and laboratory animals offers predictive insights into the organism’s capacity to combat chronic inflammation, as well as the impact of exercise and hyperthermic treatments (e.g., sauna or hot tub baths) on the HSR. Interestingly, a reciprocal correlation exists between the expression of HSR components in peripheral blood leukocytes (PBL) and the extent of local tissue proinflammatory activity in individuals afflicted by chronic inflammatory disorders. Therefore, the Heck index, contrasting extracellular 70 kDa family of heat shock proteins (HSP70) (proinflammatory) and intracellular HSP70 (anti-inflammatory) in PBL, serves as a valuable metric for HSR assessment. Our laboratory has also developed straightforward protocols for evaluating HSR by subjecting whole blood samples from both rodents and human volunteers to ex vivo heat challenges. Collectively, this discussion underscores the critical role of HSR disruption in the pathogenesis of chronic inflammatory states and emphasizes the significance of simple, cost-effective tools for clinical HSR assessment. This understanding is instrumental in the development of innovative strategies for preventing and managing chronic inflammatory diseases, which continue to exert a substantial global burden on morbidity and mortality.
Keywords: HSP70, Heat shock response, Low-grade inflammation, Heck index of HSP70, Type 2 diabetes mellitus
Graphical abstract
Introduction
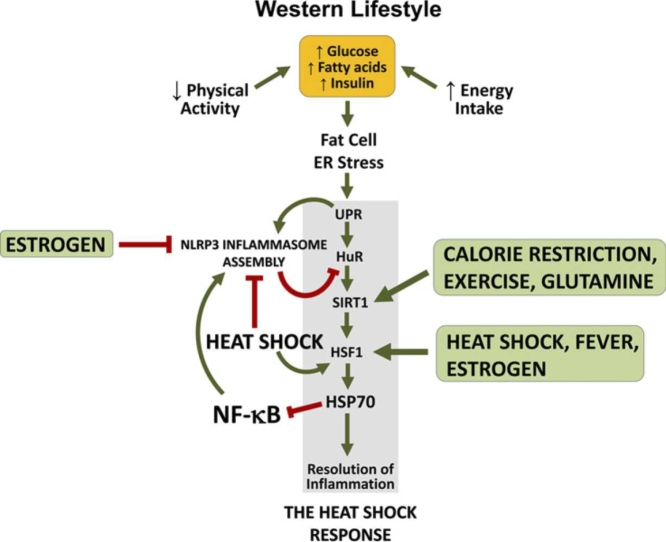
Inflammation is a highly conserved physiological response that evolved to eliminate microorganisms or facilitate tissue recovery during an aseptic injury. While these responses are typically acute, a Western lifestyle based on insufficient physical activity and surplus energy consumption predisposes to progressive suppression of the physiological resolution of inflammation through the heat shock response (HSR). This is mainly because lifestyle-elicited energy imbalances overwhelm the endoplasmic reticulum (ER) of adipose tissue leading to ER stress and eventually to the unfolded protein response (UPR). However, the UPR has an inflammatory branch through the protein kinase-like ER kinase-elF2α-ATF4-CHOP and inositol requiring enzyme-1–c-Jun N-terminal kinase (JNK) pathways that relay inflammatory signals throughout the body.1 As discussed by Schroeder and colleagues,2, 3 continuous activation of UPR downstream pathways culminates in uninterrupted activation of NOD-like receptor pyrin domain-containing protein-3 (NLRP3) inflammasome in adipose tissue, which leads to the production of activated caspase-1. Caspase-1, in turn, progressively degrades human antigen R (also known as embryonic lethal, abnormal vision, Drosophila, homolog-like protein-1), an mRNA-binding protein that is indispensable for the expression and activation of the heat shock transcription factor-1 (HSF1). Therefore, chronic ER stress of adipose tissue gradually impedes the resolution of inflammation via the HSR in several tissues, not just in the adipose tissue. This leads to the establishment of chronic low-grade inflammation that accompanies chronic degenerative conditions such as insulin resistance, obesity, type 2 diabetes mellitus (T2DM), nonalcoholic fatty liver disease (NAFLD), cardiovascular diseases (CVDs), and neurodegenerative diseases (e.g., Alzheimer’s, Parkinson’s, Huntington’s).
Although chronic degenerative diseases of an inflammatory nature take place precisely because the HSR becomes jeopardized as long as noxious stimuli (e.g., Western lifestyle) do not cease to activate NLRP3 inflammasomes, the HSR can be nonetheless re-established through strategies that are known to trigger the activation of HSF1 and 70 kDa family of heat shock proteins (HSP70) expression. If this could appear paradoxical at first glance, heat shock (HS) (i.e., fever, thermotherapy) or even exercise, by different mechanisms, are able to dismantle NLRP3 inflammasome activation, resuming the HSR and thereby relieving the above chronic inflammatory conditions.
In the present manuscript, we review current physiological, pharmacological, and nutraceutical approaches aimed at rearming the HSR in chronic inflammatory conditions. We also discuss simple and cost-effective tools used to clinically evaluate the progression or suppression of the HSR in humans and laboratory animals.
Physiological approaches
The physiological activation of the HSR primarily hinges on two key factors: proteostasis-threatening conditions and metabolic stress. These factors can be mutually exclusive, with the prevalence of one over the other dependent on a delicate metabolic balance referred to as “caloristasis,”4 as elaborated upon in.2 For instance, an increase in the bodily core temperature represents a proteostasis-threatening situation, as heat can alter protein conformation, potentially leading to the formation of toxic protein aggregates that trigger an inflammatory response. Therefore, hyperthermic treatments such as saunas or hot tub baths can robustly activate the HSR throughout the body. Additionally, metabolic signals directed toward energy-conserving pathways, such as 5′-adenosine monophosphate (AMP)-activated protein kinase (AMPK) or NAD+-dependent deacetylase sirtuin-1 (SIRT1), can also induce a genuine HSR. This is evident in the case of physical exercise, where AMPK activation may result from decreased intracellular glycogen levels, directly correlating with elevated mRNA and protein levels of HSP72.5 AMPK activation in such cases inhibits glycogen synthase kinase-3β, which consistently and constitutively represses HSF1 activity,6 thereby mitigating a bona fide HSR. Notably, HSR activation during exercise is not solely reliant on increased body or muscle temperature.7 However, if cellular metabolic sensors perceive that metabolic stress poses a greater threat to overall bodily homeostasis than the activation of the HSR to correct misfolded proteins (an energy-intensive process, as core chaperones like HSP70 or HSP90 utilize ATP to preserve protein function), AMPK may impede HSF1 activation.8, 9 In essence, whether the proteostasis-preserving HSR is triggered or not hinges on the delicate balance governing caloristasis.
With these reservations duly noted, (re)activating the HSR can often be achieved through fever, or rather, by deliberately not suppressing fever in many situations may prove beneficial.2 Remarkably, fever adeptly orchestrates antimicrobial defenses and contributes to the regulation of inflammation and tissue healing, a phenomenon observed even in cold-blooded vertebrates, where there exists a discernible selectivity in the immune responses induced by fever. In essence, fever not only suppresses inflammation but also substantially amplifies the process of wound repair, as detailed in a recent study by Haddad et al.,10 extending its effects across the entire metazoan kingdom.
Fever, in its broad sense, is thought to have evolved in modern animals approximately 600 million years ago.11 It is not limited to homeothermic mammals and birds; many poikilothermic animals, including lower vertebrates, arthropods, and annelids, can also increase their core temperature in response to infection or injury through a variety of behaviors.10, 12 However, generating a fever is a complex response that is very metabolically costly.13 In humans, producing fever may require a six-fold increase in metabolic rate, while maintaining core temperature at febrile levels may demand a 12% increase in metabolic rate per 1 °C increase.11 In essence, despite the significant energy expenditure it demands from an organism, evolution has consistently favored fever as an indispensable mechanism for maintaining homeostasis. Indeed, fever, or the sustained elevation of body temperature, emerges as a last resort and a natural therapeutic tool with the potential to curtail excessive cytokine production in critically septic patients and those afflicted with chronic inflammatory metabolic disorders, as highlighted by Heck et al.13 Consequently, indiscriminately suppressing fever through the use of antipyretic medications seems, to say the least, unwise. This consideration becomes even more crucial when considering the disruption caused by inhibitors of cyclooxygenase-2 (prostaglandin endoperoxide H synthase-2; COXIBs) and traditional nonsteroid anti-inflammatory drugs (NSAIDs) in the physiological resolution of inflammation orchestrated by the HSR, as expounded upon in the works of Schroeder et al.2, 3
Fever works as an integrative response regulating the induction and resolution phases of acute inflammation.10, 11 A rise in core temperature of about 2–3 °C initiates the HSR,11 which provides physiological resolution of inflammation at the same time that it impedes intracellular protein aggregation because the HSR produces anti-aggregative protein chaperones, such as HSP70.14, 15 Structural changes in the plasma membrane during the establishment of fever also participate in HSF1 activation.16
Because HSP70s are stress-inducible proteins activated by both hyperthermia and hypothermia,17, 18 physical approaches that can elevate local or central temperature may be employed to stimulate the HSR. As stated above, exercise is a powerful activator of HSR through mechanisms that include intracellular metabolic challenges (e.g., increase in AMP/ATP and NAD+/NADH ratios), elevated sympathetic tonus to striated muscle, adipose tissue, and pancreatic islets, as well as slight increases in core temperature itself. This explains at least partially the well-known beneficial impacts of exercise.19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Nonetheless, besides exercise, hyperthermic treatment itself has demonstrated innumerable beneficial effects for the body by reducing fat deposition, alleviating insulin resistance, and decreasing total body mass.25
Although hyperthermic treatment was well-known to the ancient Romans and by Hippocrates himself, the first indication for the therapeutic use of thermal therapy occurred, incredible, only in 1999, when Professor Philip L. Hooper investigated the ability of hot-tub therapy (37.8–41.0 °C water temperature with average oral temperature rise of 0.8 °C) to reduce blood glucose in diabetic humans.29 Interestingly, Professor Hooper’s primary curiosity was not the HSR itself. Instead, he and his colleagues conjectured that the effects of partial immersion in a hot tub could mimic the known beneficial effects of exercise by increasing blood flow to skeletal muscle. Actually, most of the beneficial effects of thermotherapy in the muscle are a consequence of sympathetic stimulation of the muscle vasculature (so, vasoconstriction; not vasodilation) in order to prevent a dangerous fall in cardiac output due to cutaneous vasodilation that follows immersion in hot water.25, 30 Thus, thermotherapy could in fact be of value in treating diabetic people, as one of the features of insulin resistance is the decreased expression of HSP72 in the skeletal muscle of T2DM patients.31
Hot tub treatment (40 °C, 30-min sessions) has been shown to improve life quality and hemodynamic function in chronic heart failure patients, including postmenopausal women.32 In our laboratory, for instance, by using a mouse model of atherosclerosis (a typical inflammatory disease), we applied HS treatment with hot water immersion (41.5 °C for 15 min) once a week over 8 weeks and observed an impressive decrease in the animal mortality rate, significant improvement of the established vascular disease, increased blood flow, amelioration of ultrasonographic parameters, along with reversal of the depressed HSR.33 Heat treatment, even in the fever-like range, blocks the NLRP3 inflammasome-dependent senescence-associated secretory phenotype (SASP),34 re-establishing the human antigen R-SIRT1-HSF-1 downstream pathways. Therefore, HSR, through HS may, paradoxically a priori, reactivate its major biochemical pathway, namely, the HSR.
Whatever the mechanisms involved, hyperthermic manipulations have been employed therapeutically in complications related to obesity and T2DM,24, 25, 26, 29, 30 especially for people to whom access to physical exercise is initially difficult.35
While the existing body of research on HSP70 levels in individuals undergoing heat therapy is limited, these studies, as summarized in Table 1, yield promising results. In one study involving young sedentary adults who underwent 4–5 sessions of hot water immersion per week for 8 weeks, an increase in intracellular HSP70 (iHSP70) coincided with a reduction in nuclear transcription factors of the kappa light chain enhancer of activated B cells (κB) family (NF-κB) activation in peripheral blood mononuclear cells (PBMCs).36 Similarly, in a comparable group of volunteers exposed to six consecutive sessions of pulsed short-wave diathermy-induced heat stress, a significant rise in muscular HSP70 levels was observed, which correlated with enhanced mitochondrial function.37 However, sedentary overweight adults undergoing 2 weeks of hot water immersion (a total of 10 sessions) did not experience changes in HSP70 content within monocytes. Nevertheless, they exhibited a decrease in plasma HSP70 levels concurrent with improvements in fasting glucose and insulin.38 This observation holds significance because elevated levels of extracellular HSP70 (eHSP70) are linked to low-grade inflammation and the exacerbation of T2DM.25, 39, 40 Furthermore, a study involving both older and younger vervet monkeys, subjected to biweekly thermal hydrotherapy sessions over 5 weeks, revealed a notable correlation between the elevation of HSP70 levels in skeletal muscle and a reduction in fasting glucose levels.41 These findings raise the possibility that the beneficial effects observed in thermotherapy may extend to individuals with various chronic low-grade inflammatory conditions.
Table 1.
Characteristics of studies in humans employing heat therapy to increase HSP70.
| Study | Sample size | Condition | Treatment | Duration (week) | Frequency (times/week) | HSP70 result | Main findings | |
|---|---|---|---|---|---|---|---|---|
| Ref.36 | 20 | Healthy young | Heat therapy | 8 | 4–5 | ↑ PBMC |
↓ NF-κB in PBMC, superoxide production and MnSOD | |
| Ref.37 | 20 | Healthy young | Heat therapy leg | 1 | 6 | ↑ Muscle |
↑ PGC-1α and mitochondrial respiratory capacity | |
| Ref.38 | 18 | Overweight adults | Hot water immersion | 2 | 5 | ↓ Plasma ↔ monocytes |
↓ FBG and insulin | |
| Ref.42 | 40 | Metabolic syndrome or T2DM middle-aged | MES + HS | 12 | 4 | ↑Expression in monocytes | ↓ Visceral adiposity, wc, BP, FPG, HOMA-IR, TNF-α and CRP. ↑ adiponectin. |
|
| Ref.43 | 60 | Obese with T2DM middle-aged/elderly | MES + HS | 12 | 2, 4, or 7 | ↑Expression in monocytes | ↓ Visceral adiposity, wc, BMI, FPG, HOMA-IR, HbA1c, TNF-α and CRP. ↑ adiponectin. |
Abbreviations used: BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; FPG, fasting plasma glucose; HbA1c, % of glycated hemoglobin A1C; HOMA-IR, homeostasis model assessment-insulin resistance; HS, heat shock; HSP70, 70 kDa family of heat shock proteins; MES, mild electrical stimulation; MnSOD, manganese superoxide dismutase; NF-κB, nuclear transcription factors of the kappa light chain enhancer of activated B cells (κB) family; PBMC, peripheral blood mononuclear cells; T2DM, type 2 diabetes mellitus; TNF-α, tumor necrosis factor-alpha; wc, waist circumference.
Another series of studies examined the effects of mild electrical stimulation (MES) combined with HS (MES + HS) on individuals with chronic low-grade inflammation, including those with metabolic syndrome and T2DM.42, 43 The MES + HS treatment involved placing a device with electrodes on both the front and back of the abdomen, delivering a direct current at 1.4 ± 0.1 V/cm (55 pulses/s) and heat (42 °C), each pulse lasting 0.1 ms. After 4 weeks of MES + HS applied four times a week for 60 min, the studies revealed an increase in HSP70 expression in monocytes. Furthermore, following 12 weeks of treatment, significant improvements were observed in body composition, blood pressure, fasting blood glucose levels, the homeostasis model assessment-insulin resistance (HOMA-IR) index, and inflammatory markers.42 In another study by the same research group, they investigated the effects of MES + HS in obese individuals with T2DM over 12 weeks.43 Participants were divided into three groups, each receiving the treatment at different frequencies: two times a week (group 1), four times a week (group 2), and seven times a week (group 3), all for 60 min per session. Following the treatment, an increase in HSP70 expression in monocytes was observed in all groups. Moreover, when comparing all groups with their baseline measurements, significant reductions were noted in visceral adiposity, waist circumference, body mass index, fasting blood glucose, HOMA-IR, % of glycated hemoglobin A1C (HbA1c), and proinflammatory markers. Conversely, adiponectin levels increased. Notably, higher treatment frequencies were associated with more pronounced reductions in HOMA-IR, HbA1c, and diastolic blood pressure, indicating an improvement in the parasympathetic to sympathetic tone.43 In summary, these studies consistently demonstrated favorable outcomes in terms of body composition, blood glucose regulation, and inflammatory markers when applying HS to specific body regions in conjunction with MES. Additionally, this treatment led to enhanced basal expression of HSP70 in monocytes among individuals with metabolic disorders.
In addition to hot tubs, saunas represent another form of hyperthermic treatment with a proven safety profile across various clinical conditions. Beyond its metabolic benefits, sauna heat therapy has been shown to induce an anti-senescent effect on blood vessels.30 This effect is mediated by the HSR, which leads to the disinhibition of key regulators such as AMPK, SIRT1, and endothelial nitric oxide synthase (eNOS) expressions.44 These findings strongly imply that the decreased risk of sudden cardiac death, fatal coronary heart disease, fatal CVD, and overall mortality associated with increased sauna bathing frequency in humans45 may be attributed to the heightened HSR achieved through heat therapy. Notably, sauna therapy is now recognized as beneficial for CVD patients by improving endothelial function46 and overall cardiovascular health,47, 48 even among those with heart failure.30
While sauna is contraindicated a priori for patients with unstable angina pectoris and recent myocardial infarction, it is generally considered safe for most coronary heart disease patients, particularly those with a history of stable angina pectoris and a remote history of myocardial infarction.49 Furthermore, sauna therapy has been demonstrated as safe during uncomplicated pregnancies among healthy women, as affirmed by numerous studies.49
Table 1 provides an overview of the outcomes from various studies investigating the effects of hyperthermic treatment on the HSR in obese, T2DM, and healthy individuals.
While sympathetic tonus to the musculature may be a principal mechanism for triggering the HSR,25, 30 many of the health-benefiting effects of heat treatment, especially when applied locally, rely on the localized production of the vasodilatory and gaseous free radical nitric oxide (NO). NO, in fact, serves as a potent activator of HSP70 expression50, 51, 52, 53 and the accompanying HSR because it induces mild oxidative stress, ultimately activating HSF1 through disulfide bond formation.15, 30 Consequently, this slight redox imbalance induced by NO acts as a catalyst for the HSR.54 Furthermore, a portion of the cytoprotective and anti-inflammatory effects attributed to estrogen-mediated HSR55, 56, 57, 58 can be linked to estrogen’s capacity to stimulate NO production in various tissues. In alignment with this concept, estrogen’s protective effects against cerebral ischemia59 and various other types of injuries60 are primarily ascribed to its influence on HSP70 expression. An inherent deduction drawn from these observations is that enhancing the production of NO within the vascular wall may furnish a cytoprotective HSR in the context of CVD induced by diabetes, as emphasized by Hooper et al.22
Rearming the HSR through the manipulation of gut microbiota
The HSR is influenced by the gut microbiota, with a direct relationship to the production of NO, a powerful HSR inducer, as stated above. The gut microbiota has the capacity to generate NO from nitrate,61, 62 enhancing the HSR in the intestinal epithelium. However, excessive NO production in the gut can be detrimental, leading to enterocyte apoptosis and hindering epithelial restitution processes.63 While the precise balance of NO remains uncertain, understanding the regulation of HSR at the intestinal level is crucial. This is because the gut microbiota ecosystem impacts low-grade inflammation and related chronic inflammatory diseases,64, 65 and obesity induces alterations in gut microbial metabolism linked to the proinflammatory senescence-associated secretory phenotype (SASP) and tissue senescence.66 Furthermore, the fermentation of carbohydrate prebiotics by the gut microbiota modulates NOS/NO pathways and NO-producing bacteria, simultaneously mitigating systemic endothelial dysfunction.67, 68 Additionally, evidence suggests that the enteric microbiota is a key determinant of immunity through the modulation of HSP production in intestinal epithelial cells.69
Gut microbiota plays a pivotal role in regulating the immune, metabolic, and even eating-behavioral aspects of mammals,70, 71, 72, 73, 74 and the diversity of commensal microbiota is closely tied to an organism’s health.75 For instance, in conditions such as obesity and T2DM, the interaction between gut microbiota and the host plays a pivotal role in the development of metabolic disorders. Excessive dietary fat intake, for example, heightens systemic exposure to potentially proinflammatory free fatty acids and their derivatives, elevating plasma lipopolysaccharide (LPS) levels, a phenomenon termed “metabolic endotoxemia.”64, 65, 76 Imbalances in the distribution of gut microbiota taxa further contribute to the disruption of gut barrier integrity.76 Conversely, adherence to a Mediterranean diet has been linked to various health benefits, including reduced mortality, lower rates of obesity, T2DM, low-grade inflammation, cancer, Alzheimer’s disease, and depression. Additionally, recent studies suggest that following a Mediterranean diet may delay the onset of Crohn’s disease.77
A reduction in gut microbiota species diversity has been observed in obesity and T2DM,78, 79 along with an imbalance between beneficial and harmful species.80 Such alterations can also result from pathogenic infections, as seen in COVID-19.81 Notably, diabetic individuals exhibit an increased presence of Proteobacteria,82, 83, 84 a pattern also associated with conditions like Crohn’s disease and colitis.85 Although Firmicutes and Bacteroidetes represent 90% of gut microbiota, the dominant gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia.86 In addition, the relative proportion of Bacteroidetes genera has long been known to be decreased in obese people in comparison with lean people, while this proportion increases with low-calorie diet-induced weight loss.87
Gut microbiota has a preponderant role in insulin resistance.88 The composition of the microbiota is even proposed as a marker for diseases or disease stages, such as fibrosis in NAFLD patients.89 Furthermore, probiotics, live microorganisms used to promote health,90 have demonstrated potential benefits as adjunctive treatment of chronic inflammatory diseases. For instance, the yeast probiotic Saccharomyces boulardii changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and T2DM db/db mice.91 Probiotics are promising also for the treatment of humans with T2DM.92
Nutritional imbalances and shifts in gut microbiota are key contributors to obesity and insulin resistance. Dysregulation of gut microbiota due to the Western lifestyle results in defects in intestinal tight junctions, leading to chronic endotoxemia and exacerbating systemic inflammation.76 This mechanism is intricately linked to an increase in circulating LPS, as demonstrated in the seminal work by Cani and colleagues, which revealed that a high-fat diet alters gut microbiota and leads to elevated LPS levels and bacterial translocation into the bloodstream in mice.64, 65 In addition to the presence of plasma LPS and bacteremia, this situation is unfortunate as the increased prevalence of bacterial LPS-producing microbes results in LPS-induced metabolic endotoxemia. This condition triggers obesity, insulin resistance, and diabetes by disrupting insulin sensitivity and weight control through the LPS/CD14 system.64 Subsequent studies have consistently reaffirmed these findings.91, 93, 94
Alterations in intestinal barrier permeability and heightened nutrient availability95, 96 are also involved. Dietary fat directly diminishes the distribution and expression levels of tight junction components, including occludin, E-cadherin, claudin, and junctional adherens molecule, leading to the disruption of intestinal permeability.97 Furthermore, high intake of γ-linolenic (ω-6) and docosahexaenoic (ω-3) acids, typical in the Western diet, may also modulate intestinal permeability by stimulating intracellular signaling pathways via protein kinase C pathways.97 Altogether, these factors contribute to low-grade inflammation, fostering insulin resistance independently of weight gain.98 LPS activates toll-like receptor 4 (TLR4), triggering the JNK pathway, which can obstruct insulin activation downstream to insulin receptor phosphorylation.99 Gut microbiota imbalances may also induce insulin receptor S-nitrosation by boosting inducible NOS activity, which is NF-κB-dependent.100, 101
To address this inflammatory condition, the preservation of the intestinal barrier integrity is paramount, and the HSR appears to play a pivotal role. HSF1 directly promotes the expression of the tight junction protein occludin, ensuring a secure seal and integrity of the intestinal epithelial barrier. This occurs through direct binding to its motif in the occludin promoter region.102 Consequently, activation of HSF1 during thermal stress within the fever-like range serves as a protective measure against heat-induced disruption of the intestinal tight junction barrier. Conversely, compromised HSF1 activity in T2DM contributes to gut-derived endotoxemia.
Strategies aimed at elevating intestinal HSR and HSP production have been proposed as therapeutic interventions against gastrointestinal toxicity.103 Alterations in the normal gut microbiota influence mucosal HSP72 expression and may render the organism more susceptible to harmful agents, such as Clostridium difficile toxin A.104 Perturbations in microbiota composition or immune status can contribute to pathogenic processes causing localized intestinal injury.105, 106 Notably, an increase in HSP70 within intestinal barrier cells has demonstrated protective effects in cases of colitis.107 Additionally, HSP70 polymorphisms appear to influence the severity of intestinal barrier disruptions.108 Furthermore, small HSPs, such as HSP27, play a role in the cytoskeletal activity of intestinal epithelial cells, crucial for preserving their integrity.109, 110
Exercise stands as a cost-effective means to combat chronic inflammatory diseases, as extensively documented.24, 25 Notably, exercise also exerts a profound influence on the host-gut microbiota axis.111 A study involving overweight adolescents who underwent a 10-week program of low-calorie intake and increased physical activity demonstrated beneficial effects on gut microbiota composition. This intervention reduced Firmicutes genera while elevating Bacteroidetes.111 Exercise alone can impact gut microbiota composition, although taking into account its influence on gut motility and transit time.112
The intertwined relationship among diet, metabolism, and exercise has become increasingly apparent, with well-controlled studies on elite athletes shedding light on this synergy (see, for instance,113 for review). Within this context, whey protein (WP) supplements have gained prominence. Renowned for their postexercise recovery and muscle hypertrophy benefits, WP and whey protein hydrolysates (WPH) have the potential to influence gut microbiota composition and, by extension, lipid metabolism. This notion is supported by metagenomic analyses in relevant samples.113 Furthermore, WPH is emerging as a novel antidiabetic agent, exhibiting favorable effects on glycemia in animal models and humans. It has been demonstrated to enhance blood glucose clearance, reduce hyperinsulinemia, and restore pancreatic islet insulin secretion in response to glucose in ob/ob mice.114
While there is not a “one-size-fits-all” optimal gut microbiota composition, given its substantial interindividual variation,86 manipulations aimed at promoting a more physiologically balanced bacterial phyla (high diversity of genera) composition in the intestines hold promise for enhancing the HSR capacity in the intestines and, consequently, overall health. As exercise mimics many effects of heat therapy on the body, heat therapy may similarly influence HSR in intestinal barrier cells. Currently, no study has explored the impact of heat therapy on the gut microbiota of individuals with obesity or diabetes. However, we hypothesize that akin to exercise,26, 27 elevating core temperature may induce favorable adaptations in the gut microbiota, potentially enhancing gut barrier function (possibly through increased HSP70 expression in enterocytes), elevating butyrate production, and mitigating chronic LPS/bacterial infiltration into the bloodstream. Our laboratory is actively investigating the effects of heat therapy on gut microbiota to shed light on this intriguing possibility.
Nutraceutical and pharmacological approaches
Various molecules, obtained from both natural sources and synthetic means, have demonstrated their potential as inducers or co-inducers of the HSR, offering benefits in the context of chronic inflammatory diseases. These compounds have been administered as nutraceutical supplements or pharmacological agents, opening promising avenues for therapeutic intervention. Here, we explore some of these approaches.
Commercial WP and WPH represent a notable example of nutraceutical supplementation that has gained popularity, particularly due to their observed positive influence on gut microbiota, as stated in the previous section. WPH, in particular, has demonstrated intriguing anti-inflammatory properties and the ability to augment the HSR. Research on rats subjected to WPH treatment revealed increased HSP70 expression following a single acute treadmill session.115 Therefore, WPH effects depend on a previous HSR arming stress (exercise). Similarly, a study involving healthy older individuals reported an elevation in HSP70 and HSF-1 levels in response to a combination of resistance training and WPH.116
Notably, camel WPH, despite sharing a similar composition with bovine WPH, exhibits superior antioxidant activities due to its higher content of antioxidant amino acids. Studies involving camel WPH have indicated a reduction in NF-κB activity and associated proinflammatory pathways in lymphocytes and hepatocytes in the context of heat stress-induced damage. Importantly, these effects were accompanied by the maintenance of baseline HSP70 levels in both models.117, 118 Indeed, WPH’s beneficial effects can be attributed to its amino acid composition, as the effects observed with WPH supplementation were not replicated when casein or nonhydrolyzed WP were administered. This underscores the potential significance of the amino acid profile in hydrolysates.115
Furthermore, research has highlighted the benefits of supplementing with nonessential amino acids in various conditions, including exercise and diabetes. In the context of the HSR signaling, these amino acids appear to play a crucial role, as evidenced by the reduction in HSF1 activity observed during amino acid deprivation.119 Importantly, studies conducted in vitro and in vivo have demonstrated that the supplementation of l-glutamine or l-alanyl-l-glutamine dipeptide can enhance HSP70 expression in skeletal muscle, liver, and immune cells and tissues of mice. This effect extends to scenarios such as endotoxemia and contributes to improvements in metabolic status and antioxidant profiles.120, 121, 122, 123 Conversely, exercise, a potent trigger of systemic HSR (second only to fever or heat itself), concurrently serves as a significant supplier of glutamine to the circulation.124 Of note, exercise is linked to increased gut permeability and elevated endotoxin levels in human subjects, particularly in hot environments. A single bout of exercise induces gut damage and heightened permeability in healthy individuals, with exacerbated damage observed in hot conditions.125 Conversely, oral glutamine supplementation mitigates this impairment.126 In the same study, it was observed that glutamine enhances the HS-induced expression of HSP70, HSF1, and occludin in cell cultures of the intestinal epithelial line Caco-2.126 These findings align with previous research (Rearming the HSR through the manipulation of gut microbiota) indicating that HSF1 directly promotes occludin expression, and the HSR improves the intestinal epithelial barrier, thereby preventing LPS-mediated metabolic endotoxemia.64, 65, 76, 102
While several amino acids, including glycine, alanine,127 arginine,128 and taurine,129 have demonstrated the capacity to boost HSP70 expression, glutamine stands out as the primary co-inducer within this category. Importantly, in cellular or animal models lacking HSP induction mechanisms, the protective effects of glutamine are not evident,130, 131 thus reinforcing that this amino acid acts by potentiating an existing HSR.
A proposed mechanism for the enhanced HSR elicited by glutamine involves the hexosamine biosynthetic pathway (HBP),132 as outlined by Leite et al.23 Glutamine serves as a substrate for HBP,133, 134 which, in turn, triggers HSF-1 N-acetylglycosylation, ultimately leading to increased HSP70 expression.132, 135 Consequently, glutamine actually operates as a co-inducer of the HSR, necessitating prior activation of HSF1 before the enhancement of HSF1 activity facilitated by glutamine (a concept elaborated upon in the referenced publication).3
Glutamine and its derivatives, known enhancers of the HSR, have demonstrated the capacity to ameliorate metabolic status121, 122 and enhance pancreatic β-cell function both in vivo and in vitro by bolstering the HSR biochemical pathway.123, 136, 137, 138 Studies have also shown that combining glutamine supplementation with exercise provides cytoprotective benefits by augmenting the HSR in animal models.120, 139 However, there are significant unresolved aspects in this context. Notably, plasma glutamine levels have exhibited a positive correlation with coronary artery disease in both male and premenopausal female individuals.140 A meta-analysis of 10,083 women further revealed that menopausal status is associated with elevated serum glutamine levels.141 However, plasma glutamine concentrations may not necessarily reflect the organism’s capacity for glutamine utilization and its associated effects.124, 142 Moreover, the mechanistic and clinical implications of these findings remain to be fully elucidated, especially given that both chronological age and menopausal status are independently linked to CVD risk factors.143 Furthermore, conflicting results have emerged from animal models.144 Currently, there is a dearth of studies specifically investigating the effects of glutamine supplementation, whether alone or in conjunction with HSR inducers like exercise or heat treatment, on menopausal CVD risk factors or hot flushes.30
In addition to the natural regulation of the HSR through methods such as heat treatment, either independently or in conjunction with physical exercise or prebiotics, pharmacological intervention offers an alternative approach. An intriguing example of unexplored and potentially valuable molecules lies in the α,β-unsaturated cyclopentenone prostaglandins (cyPGs) of the A-type (but not other non-α,β-unsaturated PGs), with PGA2 being a prominent representative. cyPGs are highly electrophilic compounds naturally produced during the resolution phase of inflammation and exhibit potent anti-inflammatory and antiproliferative properties. They exert their effects by interrupting the entire NF-κB activation pathway.145 Notably, cyPGs have demonstrated the capability to entirely reverse atherosclerotic lesions both in vivo and in vitro.146 A-type cyPGs also engage in physical interactions with 3-hydroxy-3-methylglutaryl-coenzyme A reductase, the rate-limiting step in cholesterol synthesis.147 This interaction may contribute to the antiproliferative effects of cyPGs, given the indispensable role of cholesterogenesis (not only cholesterol synthesis) in cell proliferation. Consequently, PGA2, an inducer of the HSR, holds promise as a novel nonstatin inhibitor of (3-hydroxy-3-methylglutaryl-coenzyme A) reductase with potential therapeutic applications in CVD. However, PGA2’s efficacy in chronic inflammatory conditions beyond atherosclerosis146, 148 remains unexplored.
Pharmacological interventions utilizing hydroxylamine derivatives such as bimoclomol and BGP-15 have emerged as promising strategies for addressing a spectrum of metabolic disorders characterized by inflammation. These disorders encompass diabetes, obesity, and related conditions such as CVD, NAFLD, as well as neuromuscular and neurodegenerative ailments.149 Much like the observed effects of glutamine, which, upon metabolism through the hexosamine biosynthetic pathway (HBP), extends the activation and transcriptional activity of HSF1,23 bimoclomol and BGP-15 also serve as HSR co-inducers. This implies that these drugs do not instigate a genuine HSR by themselves but rather necessitate a triggering event, such as exercise, heat exposure, or oxidative stress, to potentiate it.
As anticipated, bimoclomol has been demonstrated to accumulate HSP70 in tissues affected by chronic diseases, including diabetes, heart disease, and kidney dysfunction. Importantly, it has exhibited clinical safety in human trials.150 Animal studies have further substantiated the effectiveness of bimoclomol as an insulin sensitizer, ameliorating peripheral neuropathy in diabetic rats151 and affording protection to rat cardiomyocytes from severe heat stress (47 °C for 2 h) ex vivo and myocardial infarction in vivo, with these effects being contingent on HSP70.152
Several natural compounds have demonstrated their ability to induce the HSR, leading to an increase in HSP expression in both in vivo and in vitro settings. Celastrol, a triterpenoid derived from plant root extracts, exhibits anti-inflammatory properties by inhibiting NF-κB and increasing HSP70 levels in cultured cells.153 Leucinostatin, a fungal peptide mycotoxin, has been shown to enhance HSP70 expression in stressed cells.154 Carvacrol, also known as cymophenol, a phenolic monoterpenoid found in plant oils, can co-induce HSP70 expression, both in vitro and in models of arthritis.155, 156 Geranylgeranylacetone is another HSP70 inducer that demonstrates protective effects in rat models of ischemia-reperfusion injury.157 Geranylgeranylacetone has also shown promise in improving glucose tolerance in diabetic monkeys through an increase in HSP70 levels158 and has been considered for use in liver surgery.159 Similarly, various nontoxic hydroxylamine derivatives known to act as HSP inducers have been investigated. For example, arimoclomol160, 161 besides bimoclomol162 has been shown to prolong the activity of HSF1, thereby increasing HSP70 induction.152
Among the above-mentioned HSR co-inducers, BGP-15, apart from other cellular effects that confer cytoprotection,163 stands out as a well-studied compound with excellent tolerability and a promising insulin-sensitizing effect.164, 165, 166, 167 Comparative studies in animal models strongly support its potential application in a range of conditions, including CVD, diabetes, metabolic syndrome, and muscular dystrophies.168, 169, 170, 171 BGP-15 is currently being investigated as a therapeutic option for metabolic diseases.149, 164, 172, 173 BGP-15, which is a small molecule co-inducer of HSP72, mimics the beneficial effects seen with genetic overexpression of HSP72 and exercise training. These effects include an increase in mitochondrial area and enhanced insulin sensitivity.149 The interest in BGP-15 as a potential therapeutic agent for inducing the HSR stemmed from its ability to act as a pharmacological mimic of exercise. During exercise, contracting muscle cells significantly increase the flow of glutamine into the circulation and through the HBP. This heightened HBP flow inhibits glycogen synthase kinase-3β (GSK-3β), which usually hampers HSF1 binding to the HS gene promoters. Additionally, the enhanced HBP flow promotes increased AMPK activity, leading to heightened SIRT1 expression and its binding activity to the HSF-1 promoter of HS genes under circumstances of predominantly proteotoxic stress.2 These combined effects enhance the HSR.23
While exercise remains the most potent physiological inducer of the HSR, comparable only to fever, BGP-15, as an HSR co-inducer, not only reduces the production of reactive oxygen species but also possesses the ability to remodel cholesterol-rich membrane domains. Furthermore, it can block the activity of JNKs through direct binding.173
In summary, a myriad of molecules, ranging from amino acids to dietary compounds and pharmacological agents, have demonstrated their potential to co-induce the HSR, offering promising avenues for therapeutic intervention in chronic inflammatory diseases and metabolic disorders. Further research is needed to unravel the intricacies of these approaches and translate them into effective clinical strategies.
The integrity of the HSR can be assessed by the Heck index and whole blood heat challenge tests
HSP70 members present some peculiarities regarding their site of responses, stressful situations to those each member may be recruited, and compartmentalization of the products.174 Elevated iHSP70 expression is closely related to insulin signaling.31 Insulin itself is a signal for increased expression of iHSP70175 and regulation of its induction.176 The intracellular location of HSP70 is related to the preservation and improvement of insulin sensitivity in both skeletal muscle and vascular endothelium.44, 177 Consequently, HSP70 (HSP72−/−) knockout mice show poor insulin signaling,172, 178 while transgenic mice overexpressing HSP70 (HSP72+/+) present improvement in insulin signal.149, 172 Strikingly, when insulin downstream pathways are turned on, they inhibit the activation of HSF1. There is also a coupling between reduced insulin levels (e.g., restricted nutrient intake) and increased lifespan in a variety of animals, ranging from worms and flies to mice.179 Actually, we presume that, maybe, HSF1 suppression by insulin is also involved in insulin-mediated insulin resistance180 and perpetuation of low-grade inflammation in obesity and T2DM! On the other hand, this metabolic behavior is intertwined with the caloristasis equilibrium.2
The release of the same HSP70 into the plasma (eHSP70), on the other hand, acts as a danger signal, potentially stimulating the innate and adaptive immune system181, 182, 183 and works as an active mediator of inflammatory pathways, including via TLR4 priming.184 Furthermore, eHSP70 (e.g., eHSP72) has been associated with the danger-associated molecular pattern in activating caspase-1185 while it has been suggested that a major part of eHSP70 content in the blood is released by circulating immune cells.186, 187 Acutely, eHSP70 can be an important defense signal for the maintenance of homeostasis in stressful conditions, being also implicated in motoneuron protection21 and even in providing anti-inflammatory resources.188 Chronic exposure to high plasma levels of eHSP70, however, is firmly accepted to be deleterious to an array of tissues (including the pancreas) from obese patients and those bearing T2DM, CVD, NAFLD, and menopause-associated metabolic imbalances.21, 30, 39, 40, 189
The ability to produce and release appropriate amounts of eHSP70 is also associated with the anti-inflammatory HSR,27, 188, 190 while the adequate balance between eHSP70 and iHSP70 is now assumed to be directly correlated to the immunoinflammatory status of individuals.187, 191, 192, 193, 194 Indeed, increased eHSP70-to-iHSP70 ratios are elevated in different acute and chronic challenges to health187 but are virtually always associated with low-grade chronic inflammation thus permitting the use of these ratios to follow up patients and laboratory animals.25 eHSP70 is also linked to arterial hypertension.195 Besides, elevated eHSP70-to-iHSP70 ratios were found to disrupt vascular responses to calcium and to activate the TLR4/MD2 complex in type 1 diabetes mellitus (T1DM), as reported by De Oliveira and colleagues.194 This can be partially explained by the rise of a pro-inflammatory cytokine (eHSP70) that mobilizes Ca2+ in vascular cells and the reduction of a powerful anti-inflammatory (iHSP70), thus leading to vascular cell dysregulation. Additionally, iHSP70 was found to regulate Ca2+ mobilization participating in the contraction of vascular smooth muscle.194 On the other hand, intracellular Ca2+ mobilization within the cytoplasm is a powerful inducer of HSF1 activation and HSR.15 Finally, eHSP70 present in the plasma of T1DM rats increases α-adrenergic-induced contraction of aorta rings in a TLR4/MD2-dependent way, collaborating to disturb vascular reactivity in T1DM.194
Chronically, the elevation of plasma HSP70 (eHSP72) correlates with tumor necrosis factor-α increase, insulin resistance, and pancreatic β-cell failure.40 As expected, there is also a negative correlation of eHSP70 with insulin levels and HOMA-IR in overweight young men.196 Increased eHSP72 levels are associated with sarcopenia197 and duration of T2DM.198 On the other hand, conditions that stimulate the tonus of the sympathetic nervous system (as probably occurring during saunas and hot tubs) can augment HSP70 release from immune cells.24, 25 Additionally, adrenergic sympathetic activation occurs following overfeeding.199 Therefore, in states of continuous surplus energy imbalance,200, 201 such as in obesity, adrenergic stimulus to many tissues may take place. Contrariwise, adipose tissue itself acts as an endocrine organ releasing adipocytokines (e.g. leptin, resistin, adiponectin) that, along with insulin resistance-derived hyperinsulinemia, increase sympathetic activity via signaling at the central level, that is, in the nucleus tractus solitarius.202 Adrenergic stimulus can increase the expression and release of HSP70 by circulating immune cells.203 Together with the fact that immune cells are reputed to be the main exporters of eHSP70 in stressful situations, measuring the release of HSP70 by this “circulating tissue” became a good tool to assess organismal capacity to activate the HSR under stressful conditions.
The antagonistic relation between intra and eHSP70 signals can be used to assess the inflammatory profile. PBMCs are widely used to access HSP70 production in laboratory animals and humans,190, 204, 205, 206 because the expression of HSP70 in PBMC is inversely correlated with the degree of proinflammatory status in tissues from chronic disease patients.187, 190, 207 As PBMC evaluation represents a less invasive method that perfectly matches the muscle content of HSP70,208 we have started to use this approach as a representative of bodily HSP70 intracellular content.
This approach is possible because alterations in proinflammatory parameters are directly correlated with an increased eHSP70‑to‑iHSP70 ratio, so such ratio can be used as a predictor of the proinflammatory or anti-inflammatory response and to the development of insulin resistance, even before alterations in classical parameters, such as HbA1c, can be noticed.24, 25, 209 As previously mentioned, the rationale behind this can be partly explained by the increase in the proinflammatory cytokine eHSP70 and the decrease in the potent anti-inflammatory iHSP70 that correlates with eHSP70‑to‑iHSP70 ratio. Furthermore, our laboratory’s studies have provided clear evidence to support that this postulation is correct.187 Therefore, taking PBMC and plasmas from patients or laboratory animals allows for the assessment of the time course of the evolution of plasma-to-leukocyte ratios with ease. These ratios can be obtained by a simple division between the absolute values of eHSP70 and iHSP70 in different times or situations, irrespective of the method used to assess these HSP70 contents.
Unlike simply estimating the evolution of eHSP70/iHSP70 ratios, which gives a static picture in different situations, the eHSP70-to-iHSP70 ratio index can also be tracked by the Heck index or H-index of organismal HSP70 status. Heck index consists of the comparison between the eHSP70/iHSP70 ratio in a situation with that of the eHSP70/iHSP70 ratio in a different condition, but using the ratio of eHSP70/iHSP70 ratios, instead of merely accompanying the linear progression of the eHSP70/iHSP70 ratio.
Heck index has been recently identified as a novel and comprehensive index of an individual’s immunoinflammatory status. Similarly to that described above for simple eHSP70/iHSP70 ratios, the Heck index can also be taken by evaluating HSP70 contents in PBMC and plasma, but comparing the ratio of ratios. This is possible because eHSP70/iHSP70 ratios between plasma and PBMC reflect eHSP70/iHSP70 ratios between plasma and (metabolic) tissues.24, 25, 181, 187, 191, 207, 209, 210, 211, 212, 213, 214, 215, 216
The reasoning for this is that higher eHSP70 levels signify an increase in inflammatory signals, as eHSP70 is inherently proinflammatory. Conversely, cells that respond to stressful stimuli by enhancing intracellular iHSP70 levels are more likely to be in a state of anti-inflammation or equilibrated HSR. To calculate the Heck index, one initially takes Rc = (eHSP70)c/(iHSP70)c as the HSP70 ratio in a baseline (control) situation and Rj = (eHSP70)j/(iHSP70)j as the HSP70 ratio in any other situation “j,” irrespective of the techniques used to measure each eHSP70 and iHSP70. Heck index can then be calculated as the quotient of any Rj by the control Rc, which is considered to be unity (Rc = 1) and normalizes all other results in “j” situations allowing for easy comparisons between different conditions. Hence, the Heck index = Rj/Rc and can be used to compare any stressful situation “j” with the assumed control situation. This index can be used to estimate the immunoinflammatory status of an individual (or groups of individuals) in a variety of situations, including immune responses, diabetes, and the immunological impacts of exercise.
As previously shown,25, 187 assuming a Heck index of 1 (Rc = 1) for the controls (resting, unstimulated), exercise can cause a shift in Heck indices of up to approximately 5, which is accompanied by an increase in inflammatory markers and cell proliferation. A Heck index value >5 indicates an exacerbated proinflammatory condition. Conversely, a Heck index value between 1 and 5 suggests a predominantly equilibrated HSR while values <1 indicate an anti-inflammatory status (for more details, see supplemental Table S2 in Ref.187).
Heck index allows for the follow-up of the temporal evolution of immunoinflammatory status in patients and laboratory animals, that is, the occurrence of a background inflammation. High basal Heck indices have been associated with high HOMA-IR values,39, 40 visceral obesity, and insulin resistance,211 as well as elevated levels of ultra-sensitive C-reactive protein.217 Heck index is higher in streptozotocin-treated diabetic rats and correlates with vascular abnormalities observed in the animals194 as well. Consequently, changes in the Heck index have emerged as a potentially novel biomarker for low-grade inflammation and a highly sensitive indicator of an individual’s inflammatory status permitting an initial evaluation of the HSR in patients and laboratory animals. As such, maintaining an appropriate balance between extracellular and iHSP70 is now believed to be directly correlated with an individual’s immunoinflammatory status.191, 192, 193, 194
Although the Heck index is especially useful for giving initial clues about the inflammatory status of an individual, it does not furnish the capacity to trigger a pronounced HSR under stress. To supplement the Heck index, we developed a straightforward technique to evaluate HSR status in rodents and humans, using short-term heat challenges of whole-blood samples under different conditions. This method allows the monitoring of HSR integrity capacity,27 particularly because the expression of HSR components in PBMC is inversely correlated with the degree of proinflammatory “tonus” in chronic disease tissues.13, 190, 207 Notably, PBMC incubated at various temperatures for 2 h exhibit an iHSP70 peak at 42 °C, with lymphocytes as the primary producers under this condition.186 However, after just 1 h of incubation at 40 °C or 43 °C, a significant increase in iHSP70 is observed only at the higher thermal stress level.206 Similar results are observed upon incubations of circulating monocytes at 41 °C or 42 °C (Schöler et al. manuscript in preparation).
Basal iHSP70 contents and thermal stress sensitivity in human PBMC and polymorphonuclear leukocytes differ depending on the cell clusters.218 Monocytes exhibit a more robust response between 39 °C and 41 °C after a 2-h incubation period, while lymphocytes respond better at 42 °C within the same duration.219 At temperatures up to 41 °C, lymphocytes and polymorphonuclear leukocytes show only a modest increase in iHSP70, whereas monocytes display a strong induction at 39 °C, with iHSP70 expression at 41 °C being 10-fold higher than in control monocytes at 37 °C.219 In healthy volunteers who had been exposed to a hot water bath to induce whole-body hyperthermia in a fever-like range (39 °C), iHSP70 induction was observed in all leukocytes, with cell type-specific variations comparable to those observed in vitro, albeit less pronounced.219 Although there are disagreements regarding the temperatures and incubation times used for specific cell types in the literature, our current studies indicate that using a 2-h incubation at 42 °C is optimal for testing iHSP70 production.
To assess organismal HSR under different clinical conditions, we employ a whole-blood HS challenge ex vivo. Three approaches are in use in our laboratory, all involving 2-h incubations of whole blood at 42 °C, with control samples kept for the same period at 37 °C. In protocol #1, extracellular eHSP70 is promptly measured after a 2-h thermal (or control) challenge,190 followed by the evaluation of iHSP70 in the PBMC fraction (e.g., Ficoll-Hypaque) after 6 h of rest at 37 °C in 5% CO2 atmosphere.220
In protocol #2, we compare both HSP70 in PBMC (intracellular, iHSP70) and supernatant HSP70 (extracellular, eHSP70), after 6 h from the beginning of the 2-h incubation period.216 We have used the same protocol to compare the HSR in the human ovarian cortex obtained by closed metal container vitrification or the slow-freezing technique of cryopreservation.221
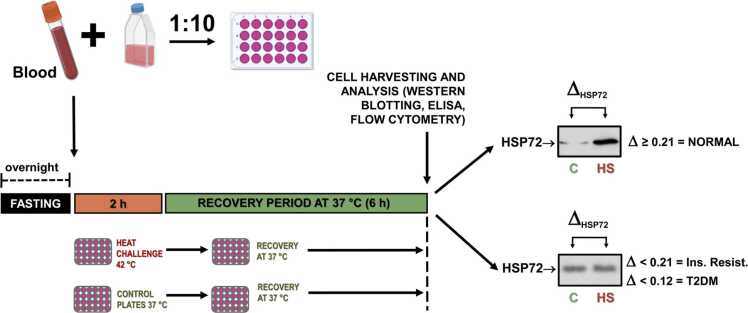
Although we believe that the protocols explained above satisfactorily permit evaluation of organismal HSR (see details in Table 2), they have some limitations under specific conditions. A considerable sample volume (at least 400 µL) is required for the test, which for human samples is perfect but is limiting when studying small animals (e.g., mice). Also, the use of gradient-separating products, such as Histopaque or Ficoll-Hypaque (protocol #1 and protocol #2), makes the method more complex and expensive. This is why we set up a simpler and straightforward technique to assess the status of the HSR in rodents and humans by using the short-term heat challenge of whole-blood samples, without requiring prior or postincubation cell separation. Moreover, changing the culture medium after the heat challenge does not affect the results (Schroeder et al. manuscript in preparation). Therefore, protocol #3 involves diluting whole-blood samples and incubating them at 42 °C (or 37 °C for controls) for 2 h. The samples are then incubated for an additional 6 h at 37 °C, allowing for the accumulation of inducible HSP70 (HSP72), a marker of HSR capacity. By using this technique, it is possible to establish the timeline of HSR suppression in animal models of high-fat diet-induced obesity, based on the differences in iHSP70 between the two test temperatures (ΔHSP70). The ΔHSP70 demonstrates a 5-parameter logistic correlation with fasting glycemia (negative), fast insulinemia (negative), HOMA-IR, negative, and Quantitative Insulin Sensitivity Check Index (positive) (Schroeder et al. manuscript in preparation). The illustration of protocol #3 can be found in Figure 1.
Table 2.
Characteristics of current HSR assessment approaches
| Technique | Method | Characteristics | Interpretation |
|---|---|---|---|
| Basal measurement | eHSP70 by ELISA kit | Direct measurement with absolute values to compare | Reference values are not known |
| iHSP70 by ELISA kit, WB, FCM, IHC | Possible visualization of cellular compartmentalization of the iHSP70 | ||
| Heck index (eHSP70 to iHSP70 ratio index) | Allow quantitation of the ratios between intra and extracellular HSP70 forms as pro/anti-inflammatory markers | R < 1 = anti-inflammatory status; 1 ≤R ≤ 5 equilibrated immunoinflammatory surveillance status; R > 5 chronic proinflammatory status | |
| Heat shock challenge | Protocol #1 Whole Blood (isolation after test) | Provides different combinations and evaluates the overall stimulation of cells | ↑HSP70 = HSR preserved |
| Protocol #2 Whole Blood (isolation before incubation) | Isolation responses from different cell types | ||
| Protocol #3 Whole Blood (without PBMC isolation) | Easy to perform, cost-effective |
Abbreviations used: eHSP70, extracellular HSP70; FCM, flow cytometry; HSP70, 70 kDa family of heat shock proteins; HSR, heat shock response; iHSP70, intracellular HSP70; IHC, immunohistochemistry; PBMC, peripheral blood mononuclear cells; WB, Western blotting.
Fig. 1.
Whole-blood heat shock challenge ex vivo to assess organismal heat shock response. Heparinized blood samples are collected from overnight-fasted individuals and diluted 1:10 with culture medium. Diluted blood samples are, then, incubated in a temperature-controlled water bath at 42.00 ± 0.01 °C for 2 h. Parallel control preparations are to be maintained in another water bath at 37 °C for the same time period. After incubations, cells from both groups are incubated for additional 6 h at 37 °C to allow for a robust accumulation of the inducible forms of HSP70 (HSP72), which serves as a marker of the HSR capacity. Total experimental time, since blood harvesting to the end of incubations, is 8 h. HSP70 accumulation can be assessed by Western blotting, ELISA, and flow cytometry. The difference (Δ) between HSP72 expressed after 42 °C compared with that after 37 °C is consistent with IR and T2DM. This illustration was prepared by using free icons from Biorender.com (available at https://app.biorender.com/). Abbreviations used: HS, heat shock; HSP70, 70 kDa family of heat shock proteins; HSR, heat shock response; T2DM, type 2 diabetes mellitus.
Blood samples collected in heparinized tubes are subjected to dispersion and then diluted in a 1:10 ratio with an appropriate culture medium. The resulting diluted blood samples are incubated in a temperature-controlled water bath at 42.00 ± 0.01 °C for a period of 2 h. Parallel control preparations are maintained in another water bath at 37 °C for the same duration of time. Following the water bath incubations, cells from both the control and heat-treated groups are incubated for an additional 6 h at 37 °C to allow for the significant accumulation of the inducible forms of HSP70 (HSP72), which serve as markers of the HSR capacity. After the incubation period, cells can be processed for iHSP70 analysis using various techniques, including EIA kits, Western blotting, flow cytometry, or immunofluorescence. This technique is particularly useful when there is no significant difference in basal expressions of iHSP70 between control and test groups, such as in cases of diabetes, obesity, or CVD. In our experience, evaluation of the HSR by ex vivo whole-blood HSR protocol #3 is sensitive enough to detect insulin resistance in mice from week to week, even when glucose tolerance tests and basal glycemia are normal.
Concluding remarks
In summary, inflammation, a fundamental physiological response, has evolved as a crucial defense mechanism against microbial threats and tissue damage. However, in our modern Western lifestyle characterized by sedentary habits and excessive calorie consumption, the body’s natural mechanisms for resolving inflammation, notably the HSR, become jeopardized. The relentless strain placed on adipose tissue’s ER leads to chronic ER stress and the subsequent UPR. Unfortunately, the UPR possesses an inflammatory arm that propagates inflammatory signals throughout the body. This persistent activation of UPR pathways eventually triggers the continuous activation of the NLRP3 inflammasome, initially in adipose tissue, and then affecting the resolution of inflammation by the HSR in multiple tissues beyond just adipose tissue.
This domino effect leads to the establishment of chronic low-grade inflammation, a hallmark of various degenerative conditions including insulin resistance, obesity, T2DM, NFLD, CVDs, and neurodegenerative diseases. Remarkably, strategies do exist to re-establish the HSR, even in the face of persistent noxious stimuli of our Western lifestyle. Heat-based interventions, such as fever and thermotherapy, as well as exercise, operate through distinct mechanisms to dismantle NLRP3 inflammasome activation and reactivate the HSR. This offers hope for alleviating chronic inflammatory conditions.
In this manuscript, we have explored current physiological, pharmacological, and nutraceutical approaches designed to reawaken the HSR in chronic inflammatory conditions. Additionally, we have highlighted clinical tools that can evaluate HSP70 status, offering valuable insights into the HSR’s progression or suppression in both patients and experimental animals. Overall, understanding and harnessing the power of the HSR holds promise for helping to combat the widespread burden of chronic inflammatory diseases in our society.
“Quæ medicamenta non sanat æ ferrum sanat. Quæ ferrum non sanat æ ignis sanat. Quæ vero ignis non sanat æ insanabilia existimare oportet.
That which drugs fail to cure the scalpel can cure. That which the scalpel fails to cure heat can cure. If heat cannot cure, it must be determined to be incurable.”
(Aphorisms of Hippocrates, by Elias Marks, from the Latin version of Verhoofd, Collins & Co. New York, 1817).
Funding and support
This work has been supported by the State of Rio Grande do Sul Foundation for Research Support (FAPERGS/Decit/SCTIE/MS/CNPq/SESRS n. 03/2017-PPSUS #17/2551-0001424-3 to MK and #19/2551-0001713-8 to PIHB) and the Brazilian National Council for Scientific and Technological Development (CNPq, process #303853/2017-4 to PIHBJ, process #307926/2022-2 to TGH and process #302959/2020-3 to MK). Financial support from CAPES (Coordination of Superior Level Staff Improvement) is also acknowledged.
Author contribution
HTS and PIHB conceptualized the paper while HTS prepared the first draft. All the authors were involved in co-writing this work. PIHB prepared the figures and supervised the finalization of the manuscript. All the authors have read and agreed to the submitted and published versions of the manuscript.
Declarations of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. On behalf of all the authors, Paulo Ivo Homem de Bittencourt declares that they have no potential conflict of interest related to the present manuscript, and no competing interests such as consultancies, financial involvement, patent ownership, etc. in relation to the work described. CNPq, FAPERGS, and CAPES (the funding organisms) had no involvement in the propositions presented in this manuscript.
Acknowledgments
The authors are indebted to Dr. Maria Inês Lavina Rodrigues for her invaluable technical support during the experiments that support many of the findings described herein. The authors apologize for not including many important primary studies in their text due to space constraints. Their goal was to ensure a smooth flow of information throughout the text.
Data availability statement
Data will be made available on request.
References
- 1.Chen Y., Brandizzi F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013;23:547–555. doi: 10.1016/j.tcb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder HT, De Lemos Muller CH, Heck TG, Krause M, Homem de Bittencourt PI. The dance of proteostasis and metabolism: unveiling the caloristatic controlling switch. Cell Stress Chaperones (CSTRES-D-23–00001_R2); 2024.S1355-8145(24)00050-6. [DOI] [PubMed]
- 3.Schroeder HT, De Lemos Muller CH, Heck TG, Krause M, Homem de Bittencourt PI. Heat shock response during the resolution of inflammation and its progressive suppression in chronic-degenerative inflammatory diseases. Cell Stress Chaperones; 2024. 10.1016/j.cstres.2024.01.002. [DOI] [PubMed]
- 4.Tezgin D., Giardina C., Perdrizet G.A., Hightower L.E. The effect of hyperbaric oxygen on mitochondrial and glycolytic energy metabolism: the caloristasis concept. Cell Stress Chaperones. 2020;25:667–677. doi: 10.1007/s12192-020-01100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Febbraio M.A., Steensberg A., Walsh R., et al. Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle. J Physiol. 2002;538:911–917. doi: 10.1113/jphysiol.2001.013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderwood S.K. Regulatory interfaces between the stress protein response and other gene expression programs in the cell. Methods. 2005;35:139–148. doi: 10.1016/j.ymeth.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Febbraio M.A., Koukoulas I. HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J Appl Physiol. 2000;89:1055–1060. doi: 10.1152/jappl.2000.89.3.1055. [DOI] [PubMed] [Google Scholar]
- 8.Dai S., Tang Z., Cao J., et al. Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J. 2015;34:275–293. doi: 10.15252/embj.201489062. 10.15252/embj.201489062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai C. The heat-shock, or HSF1-mediated proteotoxic stress, response in cancer: from proteomic stability to oncogenesis. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160525. doi: 10.1098/rstb.2016.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddad F., Soliman A.M., Wong M.E., et al. Fever integrates antimicrobial defences, inflammation control, and tissue repair in a cold-blooded vertebrate. Elife. 2023;12 doi: 10.7554/eLife.83644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh I.S., Hasday J.D. Fever, hyperthermia and the heat shock response. Int J Hyperthermia. 2013;29:423–435. doi: 10.3109/02656736.2013.808766. [DOI] [PubMed] [Google Scholar]
- 12.Hasday J.D., Singh I.S. Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones. 2000;5:471–480. doi: 10.1379/1466-1268(2000)005<0471:fathsr>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heck T.G., Ludwig M.S., Frizzo M.N., Rasia-Filho A.A., Homem de Bittencourt P.I. Suppressed anti-inflammatory heat shock response in high-risk COVID-19 patients: lessons from basic research (inclusive bats), light on conceivable therapies. Clin Sci. 2020;134:1991–2017. doi: 10.1042/CS20200596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 15.Anckar J., Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- 16.Török Z., Crul T., Maresca B., et al. Plasma membranes as heat stress sensors: from lipid-controlled molecular switches to therapeutic applications. Biochim Biophys Acta. 2014;1838:1594–1618. doi: 10.1016/j.bbamem.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Li G.C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci USA. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rada A., Tonino P., Anselmi G., Strauss M. Is hypothermia a stress condition in HepG2 cells? Expression and localization of Hsp70 in human hepatoma cell line. Tissue Cell. 2005;37:59–65. doi: 10.1016/j.tice.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Hooper P.L. Insulin signaling, GSK-3, heat shock proteins and the natural history of type 2 diabetes mellitus: a hypothesis. Metab Syndr Relat Disord. 2007;5:220–230. doi: 10.1089/met.2007.0005. [DOI] [PubMed] [Google Scholar]
- 20.Hooper P.L., Hooper P.L. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14:113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause M., Rodrigues-Krause J. da C. Extracellular heat shock proteins (eHSP70) in exercise: possible targets outside the immune system and their role for neurodegenerative disorders treatment. Med Hypotheses. 2011;76:286–290. doi: 10.1016/j.mehy.2010.10.025. https://doi.org/S0306-9877(10)00438-X. [DOI] [PubMed] [Google Scholar]
- 22.Hooper P.L., Balogh G., Rivas E., Kavanagh K., Vígh L. The importance of the cellular stress response in the pathogenesis and treatment of type 2 diabetes. Cell Stress Chaperones. 2014;19:447–464. doi: 10.1007/s12192-014-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leite J.S.M., Cruzat V.F., Krause M.S., Homem de Bittencourt P.I. Physiological regulation of the heat shock response by glutamine: implications for chronic low-grade inflammatory diseases in age-related conditions. Nutrire. 2016;41 doi: 10.1186/s41110-016-0021-y. [DOI] [Google Scholar]
- 24.Krause M., Bock P.M., Takahashi H.K., Homem de Bittencourt P.I., Newsholme P. The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin Sci. 2015;128:789–803. doi: 10.1042/CS20140695. [DOI] [PubMed] [Google Scholar]
- 25.Krause M., Heck T.G., Bittencourt A., et al. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/249205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause M., Ludwig M.S., Heck T.G., Takahashi H.K. Heat shock proteins and heat therapy for type 2 diabetes: pros and cons. Curr Opin Clin Nutr Metab Care. 2015;18:374–380. doi: 10.1097/MCO.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 27.De Lemos Muller C.H., De Matos J.R., Grigolo G.B., Schroeder H.T., Rodrigues-Krause J., Krause M. Exercise training for the elderly: inflammaging and the central role for HSP70. J Sci Sport Exercise. 2019;1:97–115. doi: 10.1007/s42978-019-0015-6. [DOI] [Google Scholar]
- 28.Bittencourt A., Schroeder H.T., Porto R.R., De Lemos Muller C.H., Krause M., Homem de Bittencourt P.I. Heat shock response to exercise in pancreatic islets of obese mice. Biochimie. 2020;168:28–40. doi: 10.1016/j.biochi.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Hooper P.L. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341:924–925. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- 30.Miragem A.A., Homem de Bittencourt P.I. Nitric oxide-heat shock protein axis in menopausal hot flushes: neglected metabolic issues of chronic inflammatory diseases associated with deranged heat shock response. Hum Reprod. 2017;23:600–628. doi: 10.1093/humupd/dmx020. [DOI] [PubMed] [Google Scholar]
- 31.Kurucz I., Morva A., Vaag A., et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. doi: 10.2337/diabetes.51.4.1102. 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- 32.Michalsen A., Lüdtke R., Bühring M., Spahn G., Langhorst J., Dobos G.J. Thermal hydrotherapy improves quality of life and hemodynamic function in patients with chronic heart failure. Am Heart J. 2003;146:728–733. doi: 10.1016/S0002-8703(03)00314-4. [DOI] [PubMed] [Google Scholar]
- 33.Bruxel M.A., Tavares A.M.V., Zavarize Neto L.D., et al. Chronic whole-body heat treatment relieves atherosclerotic lesions, cardiovascular and metabolic abnormalities, and enhances survival time restoring the anti-inflammatory and anti-senescent heat shock response in mice. Biochimie. 2019;156:33–46. doi: 10.1016/j.biochi.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Levin T.C., Wickliffe K.E., Leppla S.H., Moayeri M. Heat shock inhibits caspase-1 activity while also preventing its inflammasome-mediated activation by anthrax lethal toxin. Cell Microbiol. 2008;10:2434–2446. doi: 10.1111/j.1462-5822.2008.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoekstra S.P., Bishop N.C., Leicht C.A. Elevating body temperature to reduce low-grade inflammation: a welcome strategy for those unable to exercise? Exerc Immunol Rev. 2020;26:42–55. [PubMed] [Google Scholar]
- 36.Brunt V.E., Wiedenfeld-Needham K., Comrada L.N., Minson C.T. Passive heat therapy protects against endothelial cell hypoxia-reoxygenation via effects of elevations in temperature and circulating factors. J Physiol. 2018;596:4831–4845. doi: 10.1113/JP276559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hafen P.S., Preece C.N., Sorensen J.R., Hancock C.R., Hyldahl R.D. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J Appl Physiol. 2018;125:1447–1455. doi: 10.1152/japplphysiol.00383.2018. [DOI] [PubMed] [Google Scholar]
- 38.Hoekstra S.P., Bishop N.C., Faulkner S.H., Bailey S.J., Leicht C.A. Acute and chronic effects of hot water immersion on inflammation and metabolism in sedentary, overweight adults. J Appl Physiol. 2018;125:2008–2018. doi: 10.1152/japplphysiol.00407.2018. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues-Krause J., Krause M., O'Hagan C., et al. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17:293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krause M., Keane K., Rodrigues-Krause J., et al. Elevated levels of extracellular heat-shock protein 72 (eHSP72) are positively correlated with insulin resistance in vivo and cause pancreatic β-cell dysfunction and death in vitro. Clin Sci. 2014;126:739–752. doi: 10.1042/CS20130678. [DOI] [PubMed] [Google Scholar]
- 41.Kavanagh K., Davis A.T., Jenkins K.A., Flynn D.M. Effects of heated hydrotherapy on muscle HSP70 and glucose metabolism in old and young vervet monkeys. Cell Stress Chaperones. 2016;21:717–725. doi: 10.1007/s12192-016-0699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondo T., Ono K., Kitano S., et al. Mild electrical stimulation with heat shock reduces visceral adiposity and improves metabolic abnormalities in subjects with metabolic syndrome or type 2 diabetes: randomized crossover trials. EBioMedicine. 2014;1:80–89. doi: 10.1016/j.ebiom.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondo T., Goto R., Ono K., et al. Activation of heat shock response to treat obese subjects with type 2 diabetes: a prospective, frequency-escalating, randomized, open-label, triple-arm trial. Sci Rep. 2016;6 doi: 10.1038/srep35690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karpe P.A., Tikoo K. Heat shock prevents insulin resistance-induced vascular complications by augmenting angiotensin-(1-7) signaling. Diabetes. 2014;63:1124–1139. doi: 10.2337/db13-1267. [DOI] [PubMed] [Google Scholar]
- 45.Laukkanen T., Khan H., Zaccardi F., Laukkanen J.A. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med. 2015;175:542–548. doi: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- 46.Talebipour B., Rodrigues L.O.C., Moreira M.C.V. Effects of sauna on cardiovascular and lifestyle-related diseases. Rev Bras Med Esporte. 2006;12:216–220. doi: 10.1590/S1517-86922006000400010. [DOI] [Google Scholar]
- 47.Blum N., Blum A. Beneficial effects of sauna bathing for heart failure patients. Exp Clin Cardiol. 2007;2:29–32. [PMC free article] [PubMed] [Google Scholar]
- 48.Weber A.A., Silver M.A. Heat therapy in the management of heart failure. Congest Heart Fail. 2007;13:81–83. doi: 10.1111/j.1527-5299.2007.06348.x. [DOI] [PubMed] [Google Scholar]
- 49.Hannuksela M.L., Ellahham S. Benefits and risks of sauna bathing. Am J Med. 2001;110:118–126. doi: 10.1016/s0002-9343(00)00671-9. [DOI] [PubMed] [Google Scholar]
- 50.Malyshev I.Yu, Manukhina E.B., Mikoyan V.D., Kubrina L.N., Vanin A.F. Nitric oxide is involved in heat-induced HSP70 accumulation. FEBS Lett. 1995;370:159–162. doi: 10.1016/0014-5793(95)00801-f. [DOI] [PubMed] [Google Scholar]
- 51.Malyshev I.Yu, Malugin A.V., Golubeva L.Yu, et al. Nitric oxide donor induces HSP70 accumulation in the heart and in cultured cells. FEBS Lett. 1996;391:21–23. doi: 10.1016/0014-5793(96)00691-6. [DOI] [PubMed] [Google Scholar]
- 52.Malyshev I.Yu, Bayda L.A., Trifonov A.I., et al. Cross-talk between nitric oxide and HSP70 in the antihypotensive effect of adaptation to heat. Physiol Res. 2000;49:99–105. [PubMed] [Google Scholar]
- 53.Xu Q., Hu Y., Kleindienst R., Wick G. Nitric oxide induces heat-shock protein 70 expression in vascular smooth muscle cells via activation of heat shock factor 1. J Clin Invest. 1997;100:1089–1097. doi: 10.1172/JCI119619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Stella A.M. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 55.Hamilton K.L., Gupta S., Knowlton A.A. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NF-κB signalling. J Mol Cell Cardiol. 2004;36:577–584. doi: 10.1016/j.yjmcc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton K.L., Mbai F.N., Gupta S., Knowlton A.A. Estrogen, heat shock proteins, and NF-κB in human vascular endothelium. Arterioscler Thromb Vasc Biol. 2004;24:1628–1633. doi: 10.1161/01.ATV.0000137188.76195.fb. [DOI] [PubMed] [Google Scholar]
- 57.Takao T., Kumagai C., Hisakawa N., Matsumoto R., Hashimoto K. Effect of 17β-estradiol on tumor necrosis factor-alpha-induced cytotoxicity in the human peripheral T lymphocytes. J Endocrinol. 2005;184:191–197. doi: 10.1677/joe.1.05914. [DOI] [PubMed] [Google Scholar]
- 58.Stice J.P., Knowlton A.A. Estrogen, NF-κB, and the heat shock response. Mol Med. 2008;14:517–527. doi: 10.2119/2008-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu A., Ran R.Q., Clark J., Reilly M., Nee A., Sharp F.R. 17-beta-estradiol induces heat shock proteins in brain arteries and potentiates ischemic heat shock protein induction in glia and neurons. J Cereb Blood Flow Metab. 2002;22:183–195. doi: 10.1097/00004647-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Mendelsohn M.E., Karas R.H. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 61.Vermeiren J., Van de Wiele T., Verstraete W., Boeckx P., Boon N. Nitric oxide production by the human intestinal microbiota by dissimilatory nitrate reduction to ammonium. J Biomed Biotechnol. 2009;2009 doi: 10.1155/2009/284718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vermeiren J., Van de Wiele T., Van Nieuwenhuyse G., Boeckx P., Verstraete W., Boon N. Sulfide- and nitrite-dependent nitric oxide production in the intestinal tract. Microb Biotechnol. 2012;5:379–387. doi: 10.1111/j.1751-7915.2011.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grishin A., Bowling J., Bell B., Wang J., Ford H.R. Roles of nitric oxide and intestinal microbiota in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 2016;51:13–17. doi: 10.1016/j.jpedsurg.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cani P.D., Amar J., Iglesias M.A., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 65.Cani P.D., Bibiloni R., Knauf C., et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.1016/j.smim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Yoshimoto S., Loo T.M., Atarashi K., et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. Erratum in: Nature. 2014 Feb 20;506:396. Hattori, Masahisa [corrected to Hattori, Masahira. [DOI] [PubMed] [Google Scholar]
- 67.Roberfroid M.B. Introducing inulin-type fructans. Br J Nutr. 2005;93(Suppl 1):S13–S25. doi: 10.1079/bjn20041350. [DOI] [PubMed] [Google Scholar]
- 68.Catry E., Bindels L.B., Tailleux A., et al. Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut. 2018;67:271–283. doi: 10.1136/gutjnl-2016-313316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu H., Dicksved J., Lundh T., Lindberg J.E. Heat shock proteins: intestinal gatekeepers that are influenced by dietary components and the gut microbiota. Pathogens. 2014;3:187–210. doi: 10.3390/pathogens3010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burcelin R., Garidou L., Pomié C. Immuno-microbiota cross and talk: the new paradigm of metabolic diseases. Semin Immunol. 2012;24:67–74. doi: 10.1016/j.smim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 71.Saad M.J., Santos A., Prada P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology. 2016;31:283–293. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 72.Alcock J., Maley C.C., Aktipis C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. Bioessays. 2014;36:940–949. doi: 10.1002/bies.201400071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Homem de Bittencourt P.I., Newsholme P. You, your children, your grandchildren, and their inflammatory responses are what you eat. Curr Opin Clin Nutr Metab Care. 2015;18:325–327. doi: 10.1097/MCO.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 74.Newsholme P., Homem de Bittencourt P.I. Gut associated bacteria are critical to metabolism, inflammation and health. Curr Opin Clin Nutr Metab Care. 2016;19:245–249. doi: 10.1097/MCO.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 75.Thavamani A., Salem I., Sferra T.J., Sankararaman S. Impact of altered gut microbiota and its metabolites in cystic fibrosis. Metabolites. 2021;11:123. doi: 10.3390/metabo11020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geurts L., Neyrinck A.M., Delzenne N.M., Knauf C., Cani P.D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5:3–17. doi: 10.3920/BM2012.0065. [DOI] [PubMed] [Google Scholar]
- 77.Cani P.D., Van Hul M. Mediterranean diet, gut microbiota and health: when age and calories do not add up! Gut. 2020;69:1167–1168. doi: 10.1136/gutjnl-2020-320781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim M.-H., Yun K.E., Kim J., et al. Gut microbiota and metabolic health among overweight and obese individuals. Sci Rep. 2020;10:11. doi: 10.1038/s41598-020-76474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwimmer J.B., Johnson J.S., Angeles J.E., et al. Microbiome signatures associated with steatohepatitis and moderate to severe fibrosis in children with nonalcoholic fatty liver disease. Gastroenterology. 2019;157:1109–1122. doi: 10.1053/j.gastro.2019.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stanislawski M.A., Lozupone C.A., Wagner B.D., et al. Gut microbiota in adolescents and the association with fatty liver: the EPOCH study. Pediatr Res. 2018;84:219–327. doi: 10.1038/pr.2018.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yeoh Y.K., Zuo T., Lui G.C., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Z., Jiang W., Huang W., Lin Y., Chan F.K., Ng S.C. Gut microbiota in patients with obesity and metabolic disorders - a systematic review. Genes Nutr. 2022;17(1):18. doi: 10.1186/s12263-021-00703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gevers D., Kugathasan S., Denson L.A., et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rizzatti G., Lopetuso L., Gibiino G., Binda C., Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed Res Int. 2017;2017 doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rinninella E., Raoul P., Cintoni M., et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 88.Caricilli A.M., Saad M.J. The role of gut microbiota on insulin resistance. Nutrients. 2013;5:829–851. doi: 10.3390/nu5030829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loomba R., Seguritan V., Li W., et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25:1054–1062.e5. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hill C., Guarner F., Reid G., et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 91.Everard A., Matamoros S., Geurts L., Delzenne N.M., Cani P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. mBio. 2014;5 doi: 10.1128/mBio.01011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bock P.M., Telo G.H., Ramalho R., et al. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2021;64:26–41. doi: 10.1007/s00125-020-05295-1. [DOI] [PubMed] [Google Scholar]
- 93.Cani P.D., Osto M., Geurts L., Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–288. doi: 10.4161/gmic.19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lecomte V., Kaakoush N.O., Maloney C.A., et al. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amar J., Chabo C., Waget A., et al. Intestinal mucosal adherence and translocation of com-mensal bacteria at the early onset of Type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brun P., Castagliuolo I., Di Leo V., et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 97.Rohr M.W., Narasimhulu C.A., Rudeski-Rohr T.A., Parthasarathy S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr. 2020;11:77–91. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caricilli A.M., Picardi P.K., de Abreu L.L., Ueno M., Prada P.O., Ropelle E.R., et al. Gut microbiota is a key modulator of insulin resistance in TLR2 knockout mice. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stamler J.S., Simon D.I., Osborne J.A., et al. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stamler J.S., Toone E.J., Lipton S.A., Sucher N.J. (S)NO signals: translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1152/ajpregu.00755.2006. [DOI] [PubMed] [Google Scholar]
- 102.Dokladny K., Ye D., Kennedy J.C., Moseley P.L., Ma T.Y. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: regulatory role of heat shock factor-1. Am J Pathol. 2008;172:659–670. doi: 10.2353/ajpath.2008.070522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sylvester C.L., Anderson P.H., Stringer A.M. New therapeutic strategies for combatting gastrointestinal toxicity. Curr Opin Support Palliat Care. 2020;14:142–152. doi: 10.1097/SPC.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 104.Kojima K., Musch M.W., Ren H., et al. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology. 2003;124:1395–1407. doi: 10.1016/s0016-5085(03)00215-4. [DOI] [PubMed] [Google Scholar]
- 105.Hu S.E., Wang Y.W., Lichtenstein L., et al. Regional differences in colonic mucosa-associated microbiota determine the physiological expression of host heat shock proteins. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1266–G1275. doi: 10.1152/ajpgi.00357.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.David J.C., Grongnet J.F., Lalles J.P. Weaning affects the expression of heat shock proteins in different regions of the gastrointestinal tract of piglets. J Nutr. 2002;132:2551–2561. doi: 10.1093/jn/132.9.2551. [DOI] [PubMed] [Google Scholar]
- 107.Liu H.Y., Gu F., Zhu C., et al. Epithelial heat shock proteins mediate the protective effects of Limosilactobacillus reuteri in dextran sulfate sodium-induced colitis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.865982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nam S.Y., Kim N., Kim J.S., Lim S.H., Jung H.C., Song I.S. Heat shock protein gene 70-2 polymorphism is differentially associated with the clinical phenotypes of ulcerative colitis and Crohn's disease. J Gastroenterol Hepatol. 2007;22:1032–1038. doi: 10.1111/j.1440-1746.2007.04927.x. [DOI] [PubMed] [Google Scholar]
- 109.Mounier N., Arrigo A.P. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ren H.Y., Musch M.W., Kojima K., Boone D., Ma A., Chang E.B. Short-chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC 18 cells. Gastroenterology. 2001;121:631–639. doi: 10.1053/gast.2001.27028. [DOI] [PubMed] [Google Scholar]
- 111.Santacruz A., Marcos A., Wärnberg J., et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity. 2009;17:1906–1915. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 112.Hold G.L. The gut microbiota, dietary extremes and exercise. Gut. 2014;63:1838–1839. doi: 10.1136/gutjnl-2014-307305. [DOI] [PubMed] [Google Scholar]
- 113.Cronin O., Molloy M.G., Shanahan F. Exercise, fitness, and the gut. Curr Opin Gastroenterol. 2016;32:67–73. doi: 10.1097/MOG.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 114.Gaudel C., Nongonierma A.B., Maher S., et al. A whey protein hydrolysate promotes insulinotropic activity in a clonal pancreatic β-cell line and enhances glycemic function in ob/ob mice. J Nutr. 2013;143:1109–1114. doi: 10.3945/jn.113.174912. [DOI] [PubMed] [Google Scholar]
- 115.De Moura C.S., Lollo P.C., Morato P.N., Carneiro E.M., Amaya-Farfan J. Whey protein hydrolysate enhances the exercise-induced heat shock protein (HSP70) response in rats. Food Chem. 2013;136:1350–1357. doi: 10.1016/j.foodchem.2012.09.070. [DOI] [PubMed] [Google Scholar]
- 116.Krause M., Crognale D., Cogan K., et al. The effects of a combined bodyweight-based and elastic bands resistance training, with or without protein supplementation, on muscle mass, signaling and heat shock response in healthy older people. Exp Gerontol. 2019;115:104–113. doi: 10.1016/j.exger.2018.12.004. http://doi.org/10.16/j.exger.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 117.Badr G., Ramadan N., Abdel-Tawab H., Ahmed S.F., Mahmoud M.H. Camel whey protein protects lymphocytes from apoptosis via the PI3K–AKT, NF-κB, ATF-3, and HSP-70 signaling pathways in heat-stressed male mice. Biochem Cell Biol. 2018;96:407–416. doi: 10.1139/bcb-2017-0217. [DOI] [PubMed] [Google Scholar]
- 118.Du D., Lv W., Su R., et al. Hydrolyzed camel whey protein alleviated heat stress-induced hepatocyte damage by activated Nrf2/HO-1 signaling pathway and inhibited NF-κB/NLRP3 axis. Cell Stress Chaperones. 2021;26:387–401. doi: 10.1007/s12192-020-01184-z. (De Moura) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hensen S.M.M., Heldens L., Van Enckevort C.M.W. Heat shock factor 1 is inactivated by amino acid deprivation. Cell Stress Chaperones. 2012;17:743–755. doi: 10.1007/s12192-012-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petry E.R., Cruzat V.F., Heck T.G., Leite J.S.M., Homem de Bittencourt P.I., Tirapegui J. Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: involvement of heat shock protein pathways. Life Sci. 2014;94:130–136. doi: 10.1016/j.lfs.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 121.Cruzat V.F., Bittencourt A., Scomazzon S.P., Leite J.S., Homem de Bittencourt P.I., Tirapegui J. Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia. Nutrition. 2014;30:602–611. doi: 10.1016/j.nut.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 122.Cruzat V.F., Pantaleão L.C., Donato J., Homem de Bittencourt P.I., Tirapegui J. Oral supplementations with free and dipeptide forms of L-glutamine in endotoxemic mice: effects on muscle glutamine-glutathione axis and heat shock proteins. J Nutr Biochem. 2014;25:345–352. doi: 10.1016/j.jnutbio.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 123.Cruzat V.F., Keane K.N., Scheinpflug A.L., Cordeiro R., Soares M.J., Newsholme P. Alanyl-glutamine improves pancreatic β-cell function following ex vivo inflammatory challenge. J Endocrinol. 2015;224:261–271. doi: 10.1530/JOE-14-0677. [DOI] [PubMed] [Google Scholar]
- 124.Curi R., Newsholme P., Marzuca-Nassr G.N., et al. Regulatory principles in metabolism-then and now. Biochem J. 2016;473:1845–1857. doi: 10.1042/BCJ20160103. [DOI] [PubMed] [Google Scholar]
- 125.Chantler S., Griffiths A., Matu J., Davison G., Jones B., Deighton K. The effects of exercise on indirect markers of gut damage and permeability: a systematic review and meta-analysis. Sports Med. 2021;51:113–124. doi: 10.1007/s40279-020-01348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zuhl M.N., Lanphere K.R., Kravitz L., et al. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol. 2014;116(2):183–191. doi: 10.1152/japplphysiol.00646.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nissim I., Hardy M., Pleasure J., Nissim I., States B. A mechanism of glycine and alanine cytoprotective action: stimulation of stress-induced HSP70 mRNA. Kidney Int. 1992;42:775–782. doi: 10.1038/ki.1992.347. Erratum in: Kidney Int 1992 Dec;42(6):1488. [DOI] [PubMed] [Google Scholar]
- 128.Moura C.S., Lollo P.C.B., Morato P.N., Risso E.M., Amaya-Farfan J. Modulatory effects of arginine, glutamine and branched-chain amino acids on heat shock proteins, immunity and antioxidant response in exercised rats. Food Funct. 2017;8:3228–3238. doi: 10.1039/c7fo00465f. [DOI] [PubMed] [Google Scholar]
- 129.Setyarani M., Zinellu A., Carru C., Zulli A. High dietary taurine inhibits myocardial apoptosis during an atherogenic diet: association with increased myocardial HSP70 and HSF-1 but not caspase 3. Eur J Nutr. 2014;53:929–937. doi: 10.1007/s00394-013-0596-5. [DOI] [PubMed] [Google Scholar]
- 130.Peng Z.Y., Serkova N.J., Kominsky D.J., Brown J.L., Wischmeyer P.E. Glutamine-mediated attenuation of cellular metabolic dysfunction and cell death after injury is dependent on heat shock factor-1 expression. JPEN J Parenter Enteral Nutr. 2006;30:373–378. doi: 10.1177/0148607106030005373. [DOI] [PubMed] [Google Scholar]
- 131.Singleton K.D., Wischmeyer P.E. Glutamine’s protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol Regul Integr Comp Physiol. 2007;292:1839–1845. doi: 10.1152/ajpregu.00755.2006. [DOI] [PubMed] [Google Scholar]
- 132.Hamiel C.R., Pinto S., Hau A. Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am J Physiol Cell Physiol. 2009;297:1509–1519. doi: 10.1152/ajpcell.00240.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brasse-Lagnel C., Fairand A., Lavoinne A., Husson A. Glutamine stimulates argininosuccinate synthetase gene expression through cytosolic O-glycosylation of Sp1 in Caco-2 cells. J Biol Chem. 2003;278:52504–52510. doi: 10.1074/jbc.m306752200. [DOI] [PubMed] [Google Scholar]
- 134.Zachara N.E., Hart G.W. O-GlcNAc modification: a nutritional sensor that modulates proteasome function. Trends Cell Biol. 2004;14:218–221. doi: 10.1016/j.tcb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 135.Zachara N.E., O’Donnell N., Cheung W.D., Mercer J.J., Marth J.D., Hart G.W. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–30142. doi: 10.1074/jbc.m403773200. [DOI] [PubMed] [Google Scholar]
- 136.Newsholme P., Procopio J., Lima M.M., Pithon-Curi T.C., Curi R. Glutamine and glutamate - their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. https://doi.org/1010.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 137.Verdile G., Keane K.N., Cruzat V.F., et al. Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer's disease. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Keane K.N., Cruzat V.F., Carlessi R., Homem de Bittencourt P.I., Newsholme P. Molecular events linking oxidative stress and inflammation to insulin resistance and β-Cell dysfunction. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/181643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Petry É.R., Cruzat V.F., Heck T.G., Homem de Bittencourt P.I., Tirapegui J. L-glutamine supplementations enhance liver glutamine-glutathione axis and heat shock factor-1 expression in endurance-exercise trained rats. Int J Sport Nutr Exerc Metab. 2015;25:188–197. doi: 10.1123/ijsnem.2014-0131. [DOI] [PubMed] [Google Scholar]
- 140.Würtz P., Raiko J.R., Magnussen C.G., et al. High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. Eur Heart J. 2012;33:2307–2316. doi: 10.1093/eurheartj/ehs020. [DOI] [PubMed] [Google Scholar]
- 141.Auro K., Joensuu A., Fischer K., et al. A metabolic view on menopause and ageing. Nat Commun. 2014;5 doi: 10.1038/ncomms5708. [DOI] [PubMed] [Google Scholar]
- 142.Curi R., Lagranha C.J., Doi S.Q., et al. Molecular mechanisms of glutamine action. J Cell Physiol. 2005;204:392–401. doi: 10.1002/jcp.20339. [DOI] [PubMed] [Google Scholar]
- 143.De Kat A.C., Dam V., Onland-Moret N.C., Eijkemans M.J., Broekmans F.J., van der Schouw Y.T. Unraveling the associations of age and menopause with cardiovascular risk factors in a large population-based study. BMC Med. 2017;15 doi: 10.1186/s12916-016-0762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bock P.M., Krause M., Schroeder H.T., et al. Oral supplementations with L-glutamine or L-alanyl-L-glutamine do not change metabolic alterations induced by long-term high-fat diet in the B6.129F2/J mouse model of insulin resistance. Mol Cell Biochem. 2016;411:351–362. doi: 10.1007/s11010-015-2597-6. [DOI] [PubMed] [Google Scholar]
- 145.Homem de Bittencourt P.I., Curi R. Antiproliferative prostaglandins and the MRP/GS-X pump role in cancer immunosuppression and insight into new strategies in cancer gene therapy. Biochem Pharmacol. 2001;62:811–819. doi: 10.1016/s0006-2952(01)00738-9. [DOI] [PubMed] [Google Scholar]
- 146.Homem de Bittencourt P.I., Lagranha D.J., Maslinkiewicz A., et al. LipoCardium: endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis. 2007;193:245–258. doi: 10.1016/j.atherosclerosis.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 147.Gutierrez L.L.P., Marques C.V., Scomazzon S.P., et al. A-family anti-inflammatory cyclopentenone prostaglandins: A novel class of non-statin inhibitors of HMG-CoA reductase. Biochimie. 2021;182:37–50. doi: 10.1016/j.biochi.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 148.Gutierrez L.L., Maslinkiewicz A., Curi R., Homem de Bittencourt P.I. Atherosclerosis: a redox-sensitive lipid imbalance suppressible by cyclopentenone prostaglandins. Biochem Pharmacol. 2008;75:2245–2262. doi: 10.1016/j.bcp.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 149.Henstridge D.C., Bruce C.R., Drew B.G., et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63:1881–1894. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vígh L., Literáti P.N., Horváth I., et al. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med. 1997;3:1150–1154. doi: 10.1038/nm1097-1150. [DOI] [PubMed] [Google Scholar]
- 151.Bíró K., Jednákovits A., Kukorelli T., Hegedüs E., Korányi L. Bimoclomol (BRLP-42) ameliorates peripheral neuropathy in streptozotocin-induced diabetic rats. Brain Res Bull. 1997;44:259–263. doi: 10.1016/s0361-9230(97)00118-4. [DOI] [PubMed] [Google Scholar]
- 152.Hargitai J., Lewis H., Boros I., et al. Bimoclomol, a heat shock protein co-inducer, acts by the prolonged activation of heat shock factor-1. Biochem Biophys Res Commun. 2003;307:689–695. doi: 10.1016/s0006-291x(03)01254-3. [DOI] [PubMed] [Google Scholar]
- 153.Paimela T., Hyttinen J.M., Viiri J., et al. Celastrol regulates innate immunity response via NF-κB and Hsp70 in human retinal pigment epithelial cells. Pharmacol Res. 2011;64:501–508. doi: 10.1016/j.phrs.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 154.Lyu Q., Ludwig I.S., Kooten P.J.S., et al. Leucinostatin acts as a co-inducer for heat shock protein 70 in cultured canine retinal pigment epithelial cells. Cell Stress Chaperones. 2020;25:235–243. doi: 10.1007/s12192-019-01066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ohtsuka K., Kawashima D., Gu Y., Saito K. Inducers and co-inducers of molecular chaperones. Int J Hyperthermia. 2005;21:703–711. doi: 10.1080/02656730500384248. [DOI] [PubMed] [Google Scholar]
- 156.Wieten L., van der Zee R., Spiering R., et al. A novel heat-shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum. 2010;62:1026–1035. doi: 10.1002/art.27344. [DOI] [PubMed] [Google Scholar]
- 157.Zhou C., Bai J., Jiang C., Ye L., Pan Y., Zhang H. Geranylgeranylacetone attenuates myocardium ischemic/reperfusion injury through HSP70 and Akt/GSK-3β/eNOS pathway. Am J Transl Res. 2017;9:386–395. [PMC free article] [PubMed] [Google Scholar]
- 158.Kavanagh K., Flynn D.M., Jenkins K.A., Zhang L., Wagner J.D. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300:E894–e901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tashiro S., Miyake H., Rokutan K. Role of geranylgeranylacetone as non-toxic HSP70 inducer in liver surgery: clinical application. J Hepatobiliary Pancreat Sci. 2018;25:269–274. doi: 10.1002/jhbp.549. [DOI] [PubMed] [Google Scholar]
- 160.Xu K., Sun X., Erokwu B.O., Cernak I., Lamanna J.C. A heat-shock protein co-inducer treatment improves behavioral performance in rats exposed to hypoxia. Adv Exp Med Biol. 2011;701:313–318. doi: 10.1007/978-1-4419-7756-4_42. [DOI] [PubMed] [Google Scholar]
- 161.Parfitt D.A., Aguila M., McCulley C.H., et al. The heat-shock response co-inducer arimoclomol protects against retinal degeneration in rhodopsin retinitis pigmentosa. Cell Death Dis. 2014;5(5) doi: 10.1038/cddis.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kalmar B., Greensmith L., Malcangio M., McMahon S.B., Csermely P., Burnstock G. The effect of treatment with BRX-220, a co-inducer of heat shock proteins, on sensory fibers of the rat following peripheral nerve injury. Exp Neurol. 2003;184:636–647. doi: 10.1016/S0014-4886(03)00343-1. [DOI] [PubMed] [Google Scholar]
- 163.Sumegi K., Fekete K., Antus C., et al. BGP-15 protects against oxidative stress- or lipopolysaccharide-induced mitochondrial destabilization and reduces mitochondrial production of reactive oxygen species. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Literáti-Nagy B., Kulcsár E., Literáti-Nagy Z., et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res. 2009;41:374–380. doi: 10.1055/s-0028-1128142. [DOI] [PubMed] [Google Scholar]
- 165.Literáti-Nagy B., Péterfai E., Kulcsár E., et al. Beneficial effect of the insulin sensitizer (HSP inducer) BGP-15 on olanzapine-induced metabolic disorders. Brain Res Bull. 2010;83:340–344. doi: 10.1016/j.brainresbull.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 166.Literáti-Nagy Z., Tory K., Literáti-Nagy B., et al. The HSP co-inducer BGP-15 can prevent the metabolic side effects of the atypical antipsychotics. Cell Stress Chaperones. 2012;17:517–521. doi: 10.1007/s12192-012-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Crul T., Toth N., Piotto S., et al. Hydroximic acid derivatives: pleiotropic HSP co-inducers restoring homeostasis and robustness. Curr Pharm Des. 2013;19:309–346. doi: 10.2174/138161213804143716. [DOI] [PubMed] [Google Scholar]
- 168.Literáti-Nagy Z., Tory K., Literáti-Nagy B., et al. Synergic insulin sensitizing effect of rimonabant and BGP-15 in Zucker-obese rats. Pathol Oncol Res. 2013;19:571–575. doi: 10.1007/s12253-013-9620-6. [DOI] [PubMed] [Google Scholar]
- 169.Literáti-Nagy B., Tory K., Peitl B., et al. Improvement of insulin sensitivity by a novel drug candidate, BGP-15, in different animal studies. Metab Syndr Relat Disord. 2014;12:125–131. doi: 10.1089/met.2013.0098. [DOI] [PubMed] [Google Scholar]
- 170.Sapra G., Tham Y.K., Cemerlang N., et al. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat Commun. 2014;5 doi: 10.1038/ncomms6705. [DOI] [PubMed] [Google Scholar]
- 171.Kennedy T.L., Swiderski K., Murphy K.T., et al. BGP-15 improves aspects of the dystrophic pathology in mdx and dko mice with differing efficacies in heart and skeletal muscle. Am J Pathol. 2016;186:3246–3260. doi: 10.1016/j.ajpath.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 172.Chung J., Nguyen A.K., Henstridge D.C., et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pető Á., Kósa D., Fehér P., et al. Pharmacological overview of the BGP-15 chemical agent as a new drug candidate for the treatment of symptoms of metabolic syndrome. Molecules. 2020;25:429. doi: 10.3390/molecules25020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Noble E.G., Milne K.J., Melling C.W. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab. 2008;33:1050–1065. doi: 10.1139/H08-069. [DOI] [PubMed] [Google Scholar]
- 175.Ting L.P., Tu C.L., Chou C.K. Insulin-induced expression of human heat-shock protein gene hsp70. J Biol Chem. 1989;264:3404–3408. [PubMed] [Google Scholar]
- 176.Shinohara T., Takahashi N., Ooie T., et al. Phosphatidylinositol 3-kinase-dependent activation of akt, an essential signal for hyperthermia-induced heat-shock protein 72, is attenuated in streptozotocin-induced diabetic heart. Diabetes. 2006;55:1307–1315. doi: 10.2337/db05-0266. [DOI] [PubMed] [Google Scholar]
- 177.Geiger P.C., Gupte A.A. Heat shock proteins are important mediators of skeletal muscle insulin sensitivity. Exerc Sport Sci Rev. 2011;39:34–42. doi: 10.1097/JES.0b013e318201f236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kitano S., Kondo T., Matsuyama R., et al. Impact of hepatic HSP72 on insulin signaling. Am J Physiol Endocrinol Metab. 2019;316:E305–E318. doi: 10.1152/ajpendo.00215.2018. [DOI] [PubMed] [Google Scholar]
- 179.Vihervaara A., Sistonen L. HSF1 at a glance. J Cell Sci. 2014;127:261–266. doi: 10.1242/jcs.132605. [DOI] [PubMed] [Google Scholar]
- 180.Liu H.Y., Cao S.Y., Hong T., Han J., Liu Z., Cao W. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM) J Biol Chem. 2009;284:27090–27100. doi: 10.1074/jbc.M109.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Heck T.G., Schöler C.M., Homem de Bittencourt P.I. HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct. 2011;29:215–226. doi: 10.1002/cbf.1739. [DOI] [PubMed] [Google Scholar]
- 182.De Maio A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones. 2011;16:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.De Maio A., Vazquez D. Extracellular heat shock proteins: a new location, a new function. Shock. 2013;40:239–246. doi: 10.1097/SHK.0b013e3182a185ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Asea A. Initiation of the immune response by extracellular Hsp72: chaperokine activity of Hsp72. Curr Immunol Rev. 2006;2:209–215. doi: 10.2174/157339506778018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Maslanik T., Mahaffey L., Tannura K., Beninson L., Greenwood B.N., Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun. 2013;28:54–62. doi: 10.1016/j.bbi.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 186.Hunter-Lavin C., Davies E.L., Bacelar M.M., Marshall M.J., Andrew S.M., Williams J.H. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 187.Heck T.G., Scomazzon S.P., Nunes P.R., et al. Acute exercise boosts cell proliferation and the heat shock response in lymphocytes: correlation with cytokine production and extracellular-to-intracellular HSP70 ratio. Cell Stress Chaperones. 2017;22:271–291. doi: 10.1007/s12192-017-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Borges T.J., Wieten L., van Herwijnen M.J., et al. The anti-inflammatory mechanisms of Hsp70. Front Immunol. 2012;3:95. doi: 10.3389/fimmu.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Newsholme P., Homem de Bittencourt P.I. The fat cell senescence hypothesis: a mechanism responsible for abrogating the resolution of inflammation in chronic disease. Curr Opin Clin Nutr Metab Care. 2014;17:295–305. doi: 10.1097/MCO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 190.De Lemos Muller C.H., Rech A., Botton C.E., et al. Heat-induced extracellular HSP72 release is blunted in elderly diabetic people compared with healthy middle-aged and older adults, but it is partially restored by resistance training. Exp Gerontol. 2018;111:180–187. doi: 10.1016/j.exger.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 191.Costa-Beber L.C., Hirsch G.E., Heck T.G., Ludwig M.S. Chaperone duality: the role of extracellular and intracellular HSP70 as a biomarker of endothelial dysfunction in the development of atherosclerosis. Arch Physiol Biochem. 2022;128:1016–1023. doi: 10.1080/13813455.2020.1745850. [DOI] [PubMed] [Google Scholar]
- 192.Edkins A.L., Price J.T., Pockley A.G., Blatch G.L. Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160521. doi: 10.1098/rstb.2016.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Archer A.E., Von Schulze A.T., Geiger P.C. Exercise, heat shock proteins and insulin resistance. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160529. doi: 10.1098/rstb.2016.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.De Oliveira A.A., Priviero F., Webb R.C., Nunes K.P. Increased eHSP70-to-iHSP70 ratio disrupts vascular responses to calcium and activates the TLR4-MD2 complex in type 1 diabetes. Life Sci. 2022;310 doi: 10.1016/j.lfs.2022.121079. [DOI] [PubMed] [Google Scholar]
- 195.Rodriguez-Iturbe B., Johnson R.J., Sanchez-Lozada L.G., Pons H. HSP70 and primary arterial hypertension. Biomolecules. 2023;13:272. doi: 10.3390/biom13020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lubkowska A., Dudzińska W., Pluta W. Antioxidant enzyme activity and serum HSP70 concentrations in relation to insulin resistance and lipid profile in lean and overweight young men. Antioxidants. 2023;12:655. doi: 10.3390/antiox12030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ogawa K., Kim H.K., Shimizu T., Abe S., Shiga Y., Calderwood S.K. Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones. 2012;17:349–359. doi: 10.1007/s12192-011-0310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nakhjavani M., Morteza A., Khajeali L., et al. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15:959–964. doi: 10.1007/s12192-010-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dulloo A.G., Jacquet J. An adipose-specific control of thermogenesis in body weight regulation. Int J Obes Relat Metab Disord. 2001;25:S22–S29. doi: 10.1038/sj.ijo.0801907. [DOI] [PubMed] [Google Scholar]
- 200.De Lemos Muller C.H., Moritz C.E.J., Schroeder H.T., et al. Influence of body composition and cardiorespiratory fitness on plasma HSP72, norepinephrine, insulin, and glucose responses to an acute aerobic exercise bout performed in the fed state. Cell Stress Chaperones. 2023 doi: 10.1007/s12192-023-01364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Lemos Muller C.H., Moritz C.E.J, Schroeder H.T., Battastini A.M.O, Reischak-Oliveira A., de Bittencourt Júnior P.I.H, De Vito G., Krause M. Influence of body composition and cardiorespiratory fitness on plasma HSP72, norepinephrine, insulin, and glucose responses to an acute aerobic exercise bout performed in the fed state. Cell Stress Chaperones. 2023;28(6):721–729. doi: 10.1007/s12192-023-01364-7. Epub 2023 Jul 18. PMID: 37462825; PMCID: PMC10746641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Smith M.M., Minson C.T. Obesity and adipokines: effects on sympathetic overactivity. J Physiol. 2012;590:1787–1801. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Giraldo E., Multhoff G., Ortega E. Noradrenaline increases the expression and release of Hsp72 by human neutrophils. Brain Behav Immun. 2010;24:672–677. doi: 10.1016/j.bbi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 204.Rao D.V., Watson K., Jones G.L. Age-related attenuation in the expression of the major heat shock proteins in human peripheral lymphocytes. Mech Ageing Dev. 1999;107:105–118. doi: 10.1016/s0047-6374(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 205.Njemini R., Abeele M.V., Demanet C., Lambert M., Vandebosch S., Mets T. Age-related decrease in the inducibility of heat-shock protein 70 in human peripheral blood mononuclear cells. J Clin Immunol. 2002;22:195–205. doi: 10.1023/a:1016036724386. [DOI] [PubMed] [Google Scholar]
- 206.Lancaster G.I., Febbraio M.A. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 207.Schöler C.M., Marques C.V., Da Silva G.S., Heck T.G., De Oliveira Junior L.P., Homem de Bittencourt P.I. Modulation of rat monocyte/macrophage innate functions by increasing intensities of swimming exercise is associated with heat shock protein status. Mol Cell Biochem. 2016;421:111–125. doi: 10.1007/s11010-016-2791-1. [DOI] [PubMed] [Google Scholar]
- 208.Tuttle J.A., Chrismas B.C.R., Gibson O.R., et al. The Hsp72 and Hsp90α mRNA responses to hot downhill running are reduced following a prior bout of hot downhill running and occur concurrently within leukocytes and the vastus lateralis. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Seibert P., Anklam C.F.V., Costa-Beber L.C., et al. Increased eHSP70-to-iHSP70 ratio in prediabetic and diabetic postmenopausal women: a biomarker of cardiometabolic risk. Cell Stress Chaperones. 2022;27:523–534. doi: 10.1007/s12192-022-01288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ludwig M.S., Minguetti-Câmara V.C., Heck T.G., et al. Short-term but not long-term hypoglycaemia enhances plasma levels and hepatic expression of HSP72 in insulin-treated rats: an effect associated with increased IL-6 levels but not with IL-10 or TNFα. Mol Cell Biochem. 2014;397:97–107. doi: 10.1007/s11010-014-2176-2. [DOI] [PubMed] [Google Scholar]
- 211.Goettems-Fiorin P.B., Salamoni B., Baldissera F.G., et al. Fine particulate matter potentiates type 2 diabetes development in high-fat diet treated mice: stress response and extracellular to intracellular HSP70 ratio analysis. J Physiol Biochem. 2016;72:643–656. doi: 10.1007/s13105-016-0503-7. [DOI] [PubMed] [Google Scholar]
- 212.Mai A.S., Dos Santos A.B., Beber L.C.C., et al. Exercise training under exposure to low levels of fine particulate matter: effects on heart oxidative stress and extra-to-intracellular HSP70 ratio. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/9067875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kostrycki I.M., Wildner G., Donato Y.H., et al. Effects of high-fat diet on eHSP72 and extra-to-intracellular HSP70 levels in mice submitted to exercise under exposure to fine particulate matter. J Diabetes Res. 2019;2019 doi: 10.1155/2019/4858740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lissarassa Y.P.S., Vincensi C.F., Costa-Beber L.C., et al. Chronic heat treatment positively impacts metabolic profile of ovariectomized rats: association with heat shock response pathways. Cell Stress Chaperones. 2020;25:467–479. doi: 10.1007/s12192-020-01087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Soares M., Santos A.B.D., Weich T.M., et al. Heat shock response in noise-induced hearing loss: effects of alanyl-glutamine dipeptide supplementation on heat shock proteins status. Braz J Otorhinolaryngol. 2020;86:703–710. doi: 10.1016/j.bjorl.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Costa-Beber L.C., Heck T.G., Fiorin P.B.G., Ludwig M.S. HSP70 as a biomarker of the thin threshold between benefit and injury due to physical exercise when exposed to air pollution. Cell Stress Chaperones. 2021;26:889–915. doi: 10.1007/s12192-021-01241-1. 2021 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Madden J., Coward J.C., Shearman C.P., Grimble R.F., Calder P.C. Hsp70 expression in monocytes from patients with peripheral arterial disease and healthy controls: monocyte Hsp70 in PAD. Cell Biol Toxicol. 2010;26:215–223. doi: 10.1007/s10565-009-9134-x. [DOI] [PubMed] [Google Scholar]
- 218.Boyko A.A., Azhikina T.L., Streltsova M.A., Sapozhnikov A.M., Kovalenko E.I. HSP70 in human polymorphonuclear and mononuclear leukocytes: comparison of the protein content and transcriptional activity of HSPA genes. Cell Stress Chaperones. 2017;22:67–76. doi: 10.1007/s12192-016-0744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Oehler R., Pusch E., Zellner M., et al. Cell type-specific variations in the induction of hsp70 in human leukocytes by feverlike whole-body hyperthermia. Cell Stress Chaperones. 2001;6:306–315. doi: 10.1379/1466-1268(2001)006<0306:ctsvit>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Borges Russo M.K., Kowalewski L.S., da Natividade G.R., et al. Elevated extracellular HSP72 and blunted heat shock response in severe COVID-19 patients. Biomolecules. 2022;12:1374. doi: 10.3390/biom12101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Galbinski S., Kowalewski L.S., Grigolo G.B., et al. Comparison between two cryopreservation techniques of human ovarian cortex: morphological aspects and the heat shock response (HSR) Cell Stress Chaperones. 2022;27:97–106. doi: 10.1007/s12192-022-01252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.