Abstract
Introduction:
The aim of study is to describe factors associated with non-completion of LTBI therapy.
Methods:
We conducted a retrospective cohort study of adults who initiated LTBI treatment with isoniazid, rifampin, or isoniazid-rifapentine at five clinics. Demographic, treatment and monitoring characteristics were abstracted. We estimated descriptive statistics and compared differences in completers and non-completers using t-tests and chi-square tests.
Results:
The rate of completion across LTBI regimens was 66% (n=393). A greater proportion of non-completers were unmarried, used tobacco and/or alcohol and had more medical problems than completers (all p<0.05). A larger proportion of non-completers had charity care compared to completers (p<0.001). The most common reason for treatment discontinuation was being lost to follow-up, with the majority of these participants being treated with the longest isoniazid only regimen.
Discussion:
Patients at risk of progression to active TB with factors associated with non-completion may benefit from interventions that enhance adherence to LTBI therapy. These interventions could include enhanced outreach, incentive programs, or home visits.
Keywords: Latent Tuberculosis Infection, Rifampin, Isoniazid, Rifapentine, Treatment adherence
Introduction
An estimated 11 million people have latent tuberculosis infection (LTBI) in the United States [1]. Despite studies demonstrating prevention of progression to active tuberculosis (TB) [2–4], cost savings [5, 6], and quality adjusted life years saved with treatment of LTBI [5], the proportion of patients who complete LTBI treatment remains low. Many of the treatment challenges stem from low adherence in “actual use” scenarios in the community often with patients with many medical problems, potential substance use issues and social upheaval. The standard regimen, isoniazid daily for nine months, has less than 60% completion rates [7–9].
Currently, the CDC recommends three regimens: daily isoniazid for nine months [10], daily rifampin for four months [10] or weekly isoniazid and rifapentine with directly observed therapy (DOT) for three months [10, 11]. While a shorter course of treatment improves completion rates [12], understanding which patients may be at risk of non-completion, can help providers identify patients, especially those at risk of progression to active TB, who may need enhanced support or case management to ensure completion.
Prior research identified factors associated with non-compliance related to choice of regimen. Both length of treatment regimen [8, 13, 14] with shorter regimens demonstrating better rates of completion and side effects [15] are associated with non-completion of LTBI therapy. Further research efforts have focused on trying to identify patient-level factors associated with non-completion. Being married appears to be a positive predictor of completion [13]. Several studies have demonstrated patient transportation or clinic access issues as a barrier to completion [16–20]. This is particularly important to take into consideration with the newer DOT therapies available. Homelessness [13, 20], alcohol use [13], and intravenous drug use [21] also all appear to be associated with non-completion. While some studies demonstrate an impact of age on completion, with younger patients less likely to complete therapy [13], others failed to replicate this finding [22]. Similarly mixed findings exist for variables such as level of education [22], number of years spent living in the United States [22], and being foreign born [13]. One study demonstrated that males are more likely to complete therapy than females [20]. However, most of these studies were completed prior to the approval and wide usage of the three-month isoniazid and rifapentine regimen as well as increasing use of the four-month daily rifampin regimen and thus do not provide information on the challenges with additional treatment options.
This study aims to add to the literature of non-completion factors in these newer, shorter LTBI treatment regimens. The purpose of this study is to describe factors associated with non-completion of therapy for three LTBI regimens. Insights from this study can be used to identify modifiable factors and design interventions to improve adherence.
Methods
We conducted a retrospective cohort study of patients who initiated treatment for LTBI with nine months of isoniazid, four months of rifampin, or three months of isoniazid and rifapentine. We enrolled patients 18 years and older who were HIV negative and not pregnant. HIV positive patients were excluded because rifamycin-based regimens have potential drug-drug interactions with many antiretrovirals that this population may be taking. Medications were dispensed at a hospital-based pharmacy for patients that were treated at one of four different hospital-affiliated clinics associated with an academic medical center in Seattle, Washington. These four clinics included a primary care clinic for homeless patients, an employee health clinic, an infectious diseases clinic, and an international/refugee clinic. A fifth site was a county TB clinic.
Data were extracted by three of the authors from the electronic medical record (EMR) and paper charts (county TB clinic) and data entered using a REDCap-project database [23]. We abstracted age, sex, marital status, and insurance status from the EMR. Charity care is defined as someone who met poverty guidelines but could not afford insurance and was provided free care through the medical center. If information on marital status or insurance was unable to be obtained from the chart or was unclear, it was marked as unknown. Patients were classified as using alcohol only if alcohol abuse or dependence was listed in a problem list or they were noted to drink more than three alcohol-containing drinks per day in the social history documentation. Otherwise, the person was classified as not being an alcohol user. Homelessness was defined as living on the street, in shelters, abandoned buildings, cars, tents or staying in housing the patient identified as temporary which is a more inclusive definition than the federal definition of homelessness [24]. Per review of social documentation, those that met this definition were classified as homeless; all others were classified as not homeless. During the second half of the study period, a special incentive program was in place for homeless patients. Homeless patients seen in the TB clinic and the homeless clinic were given special incentives including bus fare or a $10 meal voucher, so that medicine could be taken with food. Patients in the TB clinic were provided medications free of charge as they were either recent contacts of an infectious TB case or high-risk immigrants/refugees. Side effects, such as nausea, vomiting, abdominal pain, neuropathic pain, rash, liver function test (LFT) abnormalities or other laboratory abnormalities were evaluated. Patient side effects were classified as “other” if the patient reported a subjective side effect which was not a known side effect of the medication regimens. Among patients who did not complete therapy, the reasons for discontinuation included being lost to follow-up, side effects, “other” reasons, patient or provider concern, LFT abnormalities, finances or other lab abnormalities. Pharmacy records were used to define treatment completion. Patients were deemed to have completed therapy if they filled prescriptions consecutively that corresponded to 270 doses of isoniazid within 12 months [10], 120 doses of rifampin within 6 months [10], and 12 doses of isoniazid and rifapentine within 4 months [11]. A large proportion of the data abstraction was done by consensus amongst co-authors. Additionally, a random 10% selection of charts were also reviewed amongst co-authors for accuracy.
We estimated the proportion who completed treatment across all regimens combined. We calculated counts, proportions, and means for demographic, treatment, and health characteristics for all participants combined and by treatment completion status. We used Chi-squared tests to compare completers to non-completers on demographic, social and behavioral factors. To better understand adverse effects of treatment, we calculated the distribution of side effects across type of treatment among those reporting side effects. Among those who discontinued treatment, we estimated the proportion reporting various reasons for non-completion across type of treatment. All analyses were conducted using Stata 13.1 [25]. The Human Subjects Division of the University of Washington reviewed and approved this research.
Results
A total of three hundred and ninety-three participants were included in this analysis (n=393). A total of 259 (66%) participants completed therapy (completers) while 132 (34%) did not complete therapy (non-completers) (Table 1) with missing data on two participants. A total of 87 patients were treated with isoniazid and rifapentine, 82 with rifampin alone and 224 with isoniazid alone. Participants treated with the isoniazid and rifapentine regimen had similar completion rates as the rifampin alone group which was statistically higher than those treated with isoniazid alone (Table 1). These associations remained similar in direction and statistically significant after adjusting for clinic location and type of monitoring (data not shown).
Table 1.
Study participant demographics and characteristics.
| Non-completers (n=132) | Completers (n=259) | p-value | |||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | ||
|
| |||||
| Age in years (mean) | 41.6 | 44.6 | 0.06 | ||
| Gender | |||||
| Female | 53 | 40.2 | 122 | 47.1 | 0.19 |
| Male | 79 | 59.9 | 137 | 52.9 | |
| Treatment regimen | |||||
| Isoniazid and rifapentine | 13 | 9.9 | 74 | 28.6 | <0.001 |
| Rifampin alone | 12 | 9.1 | 70 | 27.0 | |
| Isoniazid alone | 107 | 81.1 | 115 | 44.4 | |
| Type of insurance* | |||||
| Private | 10 | 8.7 | 49 | 23.3 | <0.001 |
| Medicare/Medicaid/government-funded | 30 | 26.1 | 45 | 21.4 | |
| Charity/no insurance | 55 | 47.8 | 107 | 51.0 | |
| Other | 20 | 17.4 | 9 | 4.3 | |
| Race | |||||
| White | 11 | 9.1 | 18 | 7.1 | 0.10 |
| Black | 39 | 32.2 | 55 | 21.6 | |
| Asian | 46 | 38.0 | 115 | 45.1 | |
| Mexican/Other Hispanic | 16 | 13.2 | 32 | 12.6 | |
| Pacific Islander | 5 | 4.1 | 27 | 10.6 | |
| Other | 4 | 3.3 | 8 | 3.1 | |
| Foreign-born | 110 | 90.2 | 230 | 91.3 | 0.73 |
| English-speaking | 66 | 50.8 | 136 | 53.3 | 0.63 |
| Marital status† | |||||
| Married | 48 | 60.8 | 115 | 73.7 | 0.04 |
| Unmarried/separated | 31 | 39.2 | 41 | 26.3 | |
| Homeless†† | 18 | 15.0 | 26 | 10.7 | 0.24 |
| Experienced Side effects | 37 | 28.0 | 94 | 36.3 | 0.10 |
| Number of medical problems | |||||
| 0 | 47 | 35.6 | 130 | 50.2 | 0.01 |
| 1–2 | 42 | 31.8 | 77 | 29.7 | |
| 3+ | 43 | 32.6 | 52 | 20.1 | |
| Number of medications | |||||
| 0–1 | 45 | 34.1 | 93 | 35.9 | 0.86 |
| 2–3 | 49 | 37.1 | 89 | 34.4 | |
| 3+ | 38 | 28.8 | 77 | 29.7 | |
| Tobacco use | 23 | 17.4 | 26 | 10.0 | 0.04 |
| Alcohol use (>3 drinks, abuse, or dependence) | 25 | 18.9 | 27 | 10.4 | 0.02 |
Unless indicated, less than 5% of data was missing
16.8% missing data
40.0% missing data
7.1% missing data
Almost half of patients who completed (51.0%) and did not complete therapy (47.8%) received charity care as coverage for their medical care (Table 1). Ninety-one percent of patients who completed therapy were foreign-born which was similar to 90.2% of non-completers who were foreign-born. Among those with data on marital status (60%), almost two-thirds of non-completers were married (60.8%) as compared to 73.7% of completers who were married. A minority of patients were homeless (15.0% in non-completers and 10.7% in completers).
A greater proportion of non-completers were unmarried, used tobacco and/or alcohol and had more medical problems compared to completers (all p-values <0.05) (Table 1). A larger proportion of non-completers had charity care compared to completers (p<0.001). The proportion of completers and non-completers did not differ by those who spoke English, were homeless, were experiencing side effects, or were taking more medications (Table 1). The number of patients using intravenous drugs was too small (n=2) for statistical analyses.
Loss to follow-up was the most common reason for non-completion (Figure 1), and other laboratory abnormalities was the least common reason. Those patients receiving the longest regimen of isoniazid alone had the most patients who were lost to follow-up (53 participants of the 64 total lost to follow-up). Only patients in the isoniazid and rifapentine treatment group reported finances as a reason for non-completion. LFT abnormalities were a cause of discontinuation in the isoniazid only group, and to a lesser extent in the combination isoniazid and rifapentine group. LFT abnormalities were not a cause of discontinuation for anyone in the rifampin only group (Figure 1).
Figure 1.
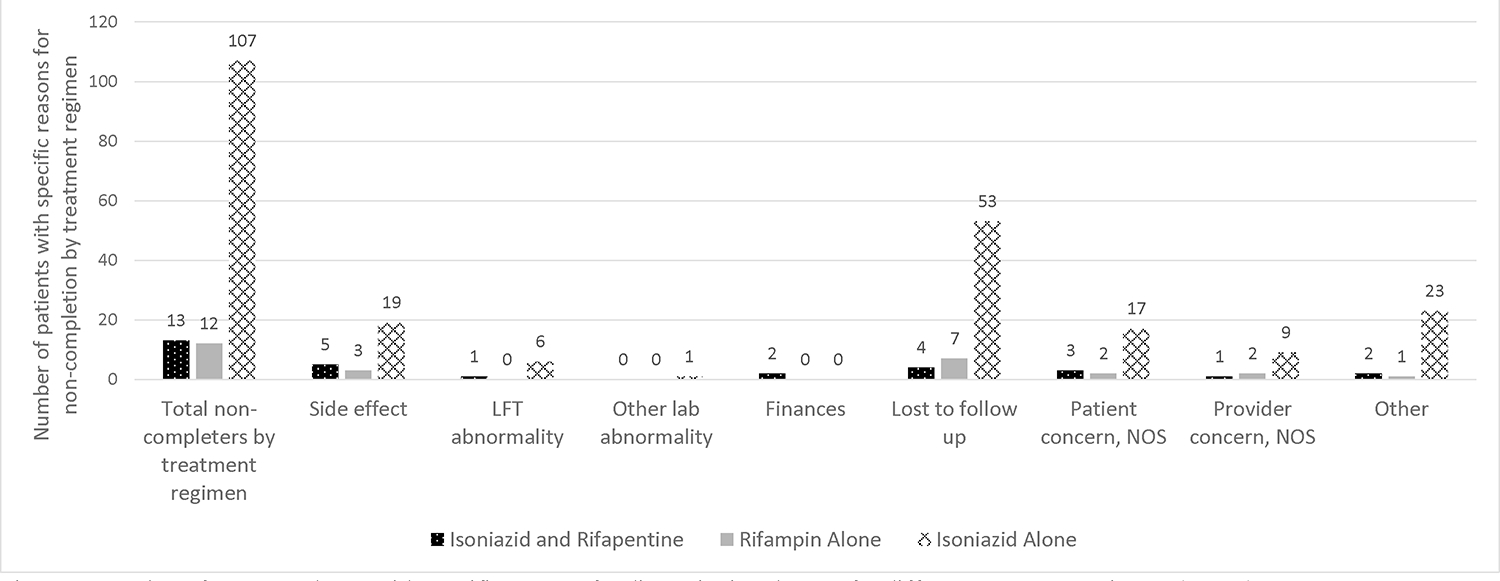
Number of non-completers with specific reasons for discontinuing therapy for different treatment regimens (n=132).
Approximately one-third of all patients reported at least one side effect (Figure 2). Small numbers of patients did report nausea, vomiting, abdominal pain, neuropathic pain or rash but the most commonly reported side effects were ‘other’ (i.e. not falling under the previously defined categories) and not typically associated with the study medications (ie. facial pain). (Figure 2).
Figure 2.
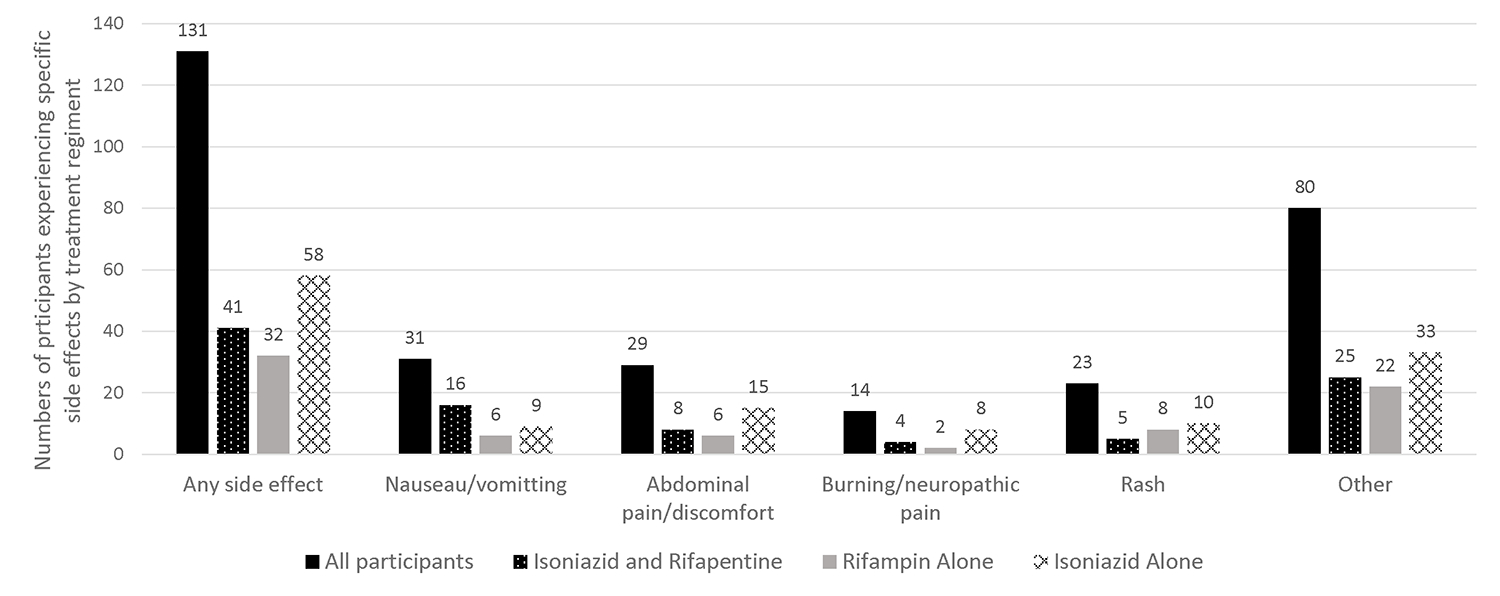
Number of patients experiencing specific side effect by treatment regimen (n=393).
Discussion
This paper characterizes factors associated with completion and non-completion of the three most common regimens for LTBI treatment in five academic clinics. Notably, a lack of insurance was associated with lower treatment completion and being married and abstaining from tobacco and alcohol were associated with successfully completing treatment. In prior studies [9, 14], type and length of treatment is likely one of the most significant factors in predicting treatment completion for latent tuberculosis. In a separate article, we found that the use of DOT was not associated with treatment completion when adjusted for type of treatment [14]. However, there are many additional factors that predict treatment completion such as patient’s understanding and motivation for LTBI treatment, interaction between the patient and the prescribing medical provider and clinical staff, perception and true incidence of side effects, and the response to these events by the patient and the medical provider.
Our study demonstrates that people receiving charity care are less likely than those on private insurance to complete treatment, which is consistent with the findings of other studies [20, 26]. In the subjects who initiated treatment in 2013 or later, the Affordable Care Act had made insurance more broadly available to all but undocumented immigrants. Washington state, where this study was done, adopted Medicaid Expansion and so the majority of patients were insured by 2013. It is notable that this difference persists even though people without insurance or with charity care receive their LTBI treatment for free at all the clinic sites in our study. Lack of resources for even basic insurance is likely a marker for poverty or possibly undocumented status, which may mean that patients had fewer resources and/or support to come to clinic and pick up medicines, even though these medicines and clinic visits were free. These individuals would likely benefit most from early initiation of enhanced adherence models and support for completion that may include more intensive outreach or home visits. Additionally, training of medical providers who care for LTBI patients may improve engagement of patients in treatment, and provide skills to handle minor side effects without medication discontinuation, particularly for patients at risk for progression to active TB. Lastly, cost was listed as a reason for treatment non-completion only in those patients on isoniazid and rifapentine. In general, medications were free or insurance was covering medications and so it is possible that the barrier to this regimen is the cost associated with participating in DOT. Providers will also need to take into consideration patients’ ability to participate in DOT as this may be a major barrier to completion.
Consistent with other studies [13, 21], we found that individuals who were using alcohol or tobacco at the start of therapy completed therapy statistically significantly less often than those not misusing alcohol or tobacco at the start of therapy. We did not find a statistically significant association between drug use and treatment failure, but the numbers for drug use in this study were quite small. This is likely because, at the clinic sites, most providers do not initiate LTBI treatment for individuals with active intravenous drug use (IVDU) due to historically poor adherence.
Positive social networks and a supportive relationship have been found in a number of studies to enhance health and adherence to medication [13, 21]. We also found this to be the case with married patients. This may aid in alerting prescribing providers of whom may be more likely to complete therapy and who may benefit from enhanced adherence support.
An increased number of medical problems was associated with non-completion. A high number of medical and/or social problems may delay or prevent initiation of treatment for LTBI in the first place because there are so many other more pressing issues that need to be covered during a brief clinic visit. The number of medications was not associated with treatment failure, suggesting that people on multiple medications may develop mechanisms for remembering their medicines that make up for the complexity. Poor adherence associated with medical complexity is poorly described to-date in the LTBI treatment adherence literature and is an important factor when deciding who merits enhanced adherence practices.
We found that side effects suggest increasing rates of non-completion though this was not statistically significant. Other studies have reported higher rates of treatment failure associated with side effects [8, 26]. This could be a limitation of our sample size or a result of comparing the three LTBI regimens, one of which is relatively new. This does highlight that providers may want to consider more than just side effect profile when deciding on an LTBI regimen for patients. Given our results about who completes and who does not complete therapy, providers may want to consider duration of regimen and need for DOT.
We were surprised to find that country of birth, English proficiency, and homelessness did not affect completion rates as has been noted in other studies [13]. At our clinic sites that primarily serve homeless and foreign-born populations, there are pre-existing models for enhancing adherence such as incentive programs [27] and cultural mediators/case managers for certain groups of newly-arrived refugees [28]. These cultural mediators/case managers aim to enhance health literacy in a culturally appropriate way for patients new to the United States who may not be familiar with medical/clinic practices. This could explain the lack of difference in completion rates for these populations.
Being “lost to follow-up” was a frequent reason for failure to complete therapy and this has also been demonstrated in other studies [8]. The most participants were lost to follow-up in the isoniazid only regimen which is the longest regimen of the three included in this analysis. This finding argues for careful screening and education prior to initiating therapy and during treatment to enhance the likelihood of follow up. The reasons for being lost to follow-up are unknown and should be investigated in future studies.
This study is helpful in characterizing factors associated with non-completion of all three of the most common LTBI treatment regimens in the US. There are several limitations. This study included five clinics in one city which could impact the generalizability of these findings to other populations. Retrospective chart review has inherent limitations due to the inability to capture all information, and occasionally information is missing or poorly documented in charts, which may limit the accuracy of our findings. Side effects are by patient report which could lead to under-reporting if patients are lost to follow-up. Our method of determining completion by pharmacy records did not include pill counts or other methods of direct testing of adherence. Lastly, this was a convenience sample of patients being treated for LTBI, and not randomly selected patients, which may not be representative of the entire population.
Overall, we found that a lack of insurance is significantly associated with lower likelihood of treatment completion despite free medicines and no-cost visits. We also found that marital status and abstinence from tobacco and alcohol are more closely associated with successfully completing treatment. We hope this informs practitioners about which patients may have barriers to adherence and who may benefit from enhanced adherence efforts such as enhanced outreach, incentive programs and home visits and those described in other studies [21, 27, 29, 30].
Acknowledgements
The authors would like to acknowledge: Caroline L. Pitney PharmD for in-depth data mining in the early stages of this project; David R. Park MD, Shireesha Dhanireddy MD, and John Lynch MD MPH for participation in formulation of this project; Monica Pecha MPH and the public health TB clinic staff for their kind assistance with data sets and charts at Tuberculosis Control Program, Public Health - Seattle & King County.
Funding
We gratefully acknowledge funding support from the Division of General Internal Medicine and data support from the Institute of Translational Health Sciences (UL1 RR025014 from NCRR/NIH), both at the University of Washington.
Abbreviations
- TB
Tuberculosis
- LTBI
Latent Tuberculosis infectio
- DOT
Directly observed therapy
Footnotes
Conflicts of interest: There are no conflicts of interest to disclose.
References
- 1.Bennett DE, et al. , Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999–2000. Am J Respir Crit Care Med, 2008. 177(3): p. 348–55. [DOI] [PubMed] [Google Scholar]
- 2.Ferebee SH, Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc, 1970. 26: p. 28–106. [PubMed] [Google Scholar]
- 3.Smieja MJ, et al. , Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev, 2000(2): p. CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churchyard GJ, et al. , A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med, 2014. 370(4): p. 301–10. [DOI] [PubMed] [Google Scholar]
- 5.Porco TC, et al. , Cost-effectiveness of tuberculosis evaluation and treatment of newly-arrived immigrants. BMC Public Health, 2006. 6: p. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland DP, et al. , Costs and cost-effectiveness of four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med, 2009. 179(11): p. 1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goswami ND, et al. , Predictors of latent tuberculosis treatment initiation and completion at a U.S. public health clinic: a prospective cohort study. BMC Public Health, 2012. 12: p. 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess K, et al. , Isoniazid completion rates for latent tuberculosis infection among college students managed by a community pharmacist. J Am Coll Health, 2009. 57(5): p. 553–5. [DOI] [PubMed] [Google Scholar]
- 9.Rivest P, Street MC, and Allard R, Completion rates of treatment for latent tuberculosis infection in Quebec, Canada from 2006 to 2010. Can J Public Health, 2013. 104(3): p. e235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease C, Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morb Mortal Wkly Rep, 2000. 49(RR-6). [PubMed] [Google Scholar]
- 11.Centers for Disease C, Recommendations for Use of an Isoniazid–Rifapentine Regimen with Direct Observation to Treat Latent Mycobacterium tuberculosis Infection. MMWR Morb Mortal Wkly Rep, 2011. 60: p. 1650–1653. [PubMed] [Google Scholar]
- 12.Sharma SK, et al. , Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev, 2013. 7: p. CD007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch-Moverman Y, et al. , Predictors of latent tuberculosis infection treatment completion in the United States: an inner city experience. Int J Tuberc Lung Dis, 2010. 14(9): p. 1104–11. [PMC free article] [PubMed] [Google Scholar]
- 14.McClintock AH, et al. , Treatment completion for latent tuberculosis infection: a retrospective cohort study comparing 9 months of isoniazid, 4 months of rifampin and 3 months of isoniazid and rifapentine. BMC Infect Dis, 2017. 17(1): p. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machado A Jr., et al. , Risk factors for failure to complete a course of latent tuberculosis infection treatment in Salvador, Brazil. Int J Tuberc Lung Dis, 2009. 13(6): p. 719–25. [PubMed] [Google Scholar]
- 16.Shieh FK, et al. , Predicting non-completion of treatment for latent tuberculous infection: a prospective survey. Am J Respir Crit Care Med, 2006. 174(6): p. 717–21. [DOI] [PubMed] [Google Scholar]
- 17.Wyss LL and Alderman MK, Using theory to interpret beliefs in migrants diagnosed with latent TB. Online J Issues Nurs, 2007. 12(1): p. 7. [PubMed] [Google Scholar]
- 18.West EL, et al. , Tuberculosis knowledge, attitudes, and beliefs among North Carolinians at increased risk of infection. N C Med J, 2008. 69(1): p. 14–20. [PubMed] [Google Scholar]
- 19.McEwen MM and Boyle J, Resistance, health, and latent tuberculosis infection: Mexican immigrants at the U.S.-Mexico border. Res Theory Nurs Pract, 2007. 21(3): p. 185–97. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch-Moverman Y, et al. , Latent tuberculous infection in the United States and Canada: who completes treatment and why? International Journal of Tuberculosis and Lung Disease, 2015. 19(1): p. 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyamathi AM, et al. , A randomized controlled trial of two treatment programs for homeless adults with latent tuberculosis infection. Int J Tuberc Lung Dis, 2006. 10(7): p. 775–82. [PubMed] [Google Scholar]
- 22.Ailinger RL, et al. , Predictors of adherence to latent tuberculosis infection therapy in Latino immigrants. J Community Health Nurs, 2007. 24(3): p. 191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris R.T Paul A.,., Thielke Robert, Payne Jonathon, Gonzalez Nathaniel, Conde Jose G, Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 2009. April 42(2): p. 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Title 42- The Public Health and Welfare, in 42 UCS 254b.
- 25.StataCorp, Stata. 2013, Stata Corp LLC: College Station, TX. [Google Scholar]
- 26.Kwara A, et al. , Factors associated with failure to complete isoniazid treatment for latent tuberculosis infection in Rhode Island. Chest, 2008. 133(4): p. 862–8. [DOI] [PubMed] [Google Scholar]
- 27.Gupta V, et al. , Tuberculosis among the homeless--preventing another outbreak through community action. N Engl J Med, 2015. 372(16): p. 1483–5. [DOI] [PubMed] [Google Scholar]
- 28.Jackson-Carroll L, Graham E, Jackson JC, Beyond Medical Interpretation: The role of Interpreter Cultural Mediators (ICMs) in building bridges between ethnic communities and health institutions. Selecting, training and supporting key outreach staff, H.M.C. University of Washington, Editor. 1998: http://ethnomed.org/about/related-programs/community-house-calls-program/icm-manual98.pdf. [Google Scholar]
- 29.Ailinger RL, et al. , The effect of a cultural intervention on adherence to latent tuberculosis infection therapy in Latino immigrants. Public Health Nurs, 2010. 27(2): p. 115–20. [DOI] [PubMed] [Google Scholar]
- 30.Chang AH, Polesky A, and Bhatia G, House calls by community health workers and public health nurses to improve adherence to isoniazid monotherapy for latent tuberculosis infection: a retrospective study. BMC Public Health, 2013. 13: p. 894. [DOI] [PMC free article] [PubMed] [Google Scholar]


