Abstract
Hydrophilic loops in the receptor binding domain of the amphotropic murine leukemia virus (MLV) envelope glycoprotein (SU) are predicted and may participate in SU-receptor interactions. We have replaced five segments of 6 to 15 amino acids located in each of these regions with an 11-amino-acid tag from the vesicular stomatitis virus glycoprotein (VSV-G). Substitution was compatible with envelope processing, transport, and incorporation into virions. However, three substitution mutants showed a temperature-dependent phenotype, suggesting structural unstability. Accessibility of the tagging epitope for a monoclonal anti-VSV-G antibody was greater in oligomeric than in monomeric SUs when insertion was done in VRA, a domain essential for receptor recognition. In contrast, accessibility was independent of structural constraints when insertion was done in VRB, a domain playing an accessory role in receptor binding. Interaction with the amphotropic receptor was investigated by interference assay and study of binding and infection of target cells with MLV particles coated with the substituted envelopes. Envelope-receptor interaction was abolished when substitution was performed in a potential loop-forming segment located at the N-terminal half of VRA. Although interaction was affected to variable extents, depending on the substituted segment, other mutants conserved the ability to interact with the amphotropic receptor. These experiments indicate the 14-amino-acid segment between positions 50 and 64 of SU as an essential determinant of amphotropic-receptor recognition. They also show that a foreign linear epitope can be tolerated in several locations of the amphotropic SU receptor binding site, and this result has implications for the design of targeted retroviral vectors.
Retrovirus infection is initiated by the attachment of viral particles to specific receptor proteins present at the target cell surface. Receptor recognition is mediated by the surface subunits (SUs) of viral envelope glycoprotein oligomers, which are bound at the virion surface through interaction with transmembrane subunits (TMs). Five murine leukemia virus (MLV) subgroups which bind different cell surface receptors have been identified (35). The ecotropic and amphotropic MLV subgroups interact with multiple membranes spanning transporters for cationic amino acids (15, 34) and inorganic phosphate (14), respectively. The receptor binding domain of MLV SUs has been located in the first half of the SU (9), and two hypervariable regions, VRA and VRB, have been shown to contribute to receptor recognition (2, 23, 25). Fusion between the viral and the cytoplasmic lipid bilayers is likely to be triggered by conformational changes of the SU-TM heterodimers, which follow receptor binding. A fusogenic peptide most probably located at the N-terminal extremity of the TM subunit (10) and the C-terminal half of the SU are involved in the fusion process (24, 27). The N-terminal receptor binding domain of the SU is connected to the C-terminal moiety by a proline-rich hinge.
The map of disulfide bridges is available for the ecotropic (17) and polytropic (18) MLV SUs. Sequence alignment of SU N-terminal halves indicates that most cysteine residues engaged in disulfide bridge formation are conserved between MLV subgroups (2), suggesting that the maps of amphotropic, xenotropic, and 10A1 N-terminal disulfide bridges must be closely related. According to these findings, the formation of hydrophilic loops in three different locations of the MLV SU receptor binding site can be predicted: the N-terminal half of VRA, the C-terminal half of VRA, and VRB. An additional hydrophilic loop may exist at the N-terminal edge of the amphotropic VRB. These structures are candidates for mediating interaction with cell surface receptors. Point mutations introduced in the ecotropic SU revealed that the loop-forming structure located in the N-terminal half of VRA may be involved in the recognition of the ecotropic receptor (20).
The aim of the present work was to examine the role of each of the potential loop-forming structures located in the amphotropic SU receptor binding site. The method consisted of substitution of an epitope tag for the sequence of interest and assessment of the capacity of modified envelopes to become incorporated into virions and to mediate interaction with the amphotropic receptor.
MATERIALS AND METHODS
Cells and viruses.
Mouse NIH 3T3 and human TE671 and TELCeB6 cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum. Helper-free ecotropic and amphotropic stocks of an LXSN-derived retroviral vector (21) carrying the Escherichia coli nls-lacZ gene were generated from Ψ-CRE and Ψ-CRIP producer clones, respectively. Vector titers were determined by scoring the number of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal)-positive foci 48 h after the infection of subconfluent mouse NIH 3T3 cells and were expressed as β-galactosidase (β-Gal) focus-forming units (FFU). Stocks used in the experiments contained 5 × 105 and 2 × 106 β-Gal FFU/ml for the ecotropic and amphotropic vectors, respectively. Retrovirus particles bearing substituted envelopes were obtained by cotransfection of the mutant envelope and the neo gene into the TELCeB6 cell line (6). After selection of stably transfected cells with G418, supernatants were harvested from bulk populations and filtered (0.45-μm pore size) before use.
Construction of envelope glycoprotein expression vectors.
A wild-type amphotropic envelope expression vector was constructed by isolating a BglII-ClaI fragment from the pCRUCA vector (2), which encompasses the 3′-polymerase gene and the env splice acceptor site and coding sequence from the 4070 A MLV, and inserting this fragment in the MscI/ClaI sites of the pFB3 vector (9). The resulting vector (L29.A) produces a spliced env mRNA from the Friend MLV FB29 long terminal repeat and contains a BamHI site immediately upstream of the env gene ATG. Substitution mutants containing an 11-amino-acid epitope from the cytoplasmic tail of the vesicular stomatitis virus glycoprotein (VSV-G) (16) were constructed by using two PCR-generated fragments and a ClaI site in the VSV-G sequence. The 5′ amplimer encompassed the BamHI site upstream of the ATG, the env gene up to the 5′ substitution point, and the 5′ half of the VSV-G epitope to the ClaI site. The 3′ amplimer contained the 3′ half of the VSV-G sequence from the ClaI site and the env gene from the 3′ substitution point to the XhoI site downstream of VRB. The amplimers were ligated together into L29.A opened at the BamHI and XhoI sites, giving rise to envelope expression vectors in which the targeted sequences were replaced by the VSV-G epitope coding sequence. Vector structures were verified by sequencing.
Monoclonal and polyclonal antibodies.
The rat 83A25 monoclonal antibody (MAb) (7) and the mouse P5D4 MAb (16) were kindly provided by L. H. Evans (Rocky Mountain Laboratories, Hamilton, Mont.) and T. E. Kreis (Université de Genève, Geneva, Switzerland), respectively. Goat anti-Rauscher leukemia virus gp70 and goat anti-Moloney MLV p30 sera were purchased from Quality-Biotech Inc. (Camden, N.J.).
Immunoprecipitation.
Confluent 60-mm-diameter petri dishes were washed with phosphate-buffered saline (PBS) and incubated in 1 ml of methionine- and cysteine-free Dulbecco modified Eagle medium containing 2% dialyzed fetal calf serum for 45 min at 37 or 32°C. The cells were labeled for 30 min with 200 μCi of methionine-cysteine label mix (Amersham) and chased with cold culture medium for the periods indicated below. Culture media were harvested and supplemented with 10× lysis buffer (0.5% Nonidet P-40, 150 mM NaCl, 20 mM HEPES, 1 mM phenylmethylsulfonyl fluoride). The cells were lysed under the same conditions, and cell extracts were clarified by centrifugation. Preclearing was performed with preimmune rabbit serum and protein A-Sepharose (6MN; Sigma) for 16 h at 4°C. After centrifugation, the supernatants were incubated with goat anti-gp70 serum for 2 h at 4°C and then incubated for 30 min with protein A-Sepharose. After a washing in RIPA buffer (20 mM Tris [pH 7.4], 0.1% deoxycholate, 0.1% sodium dodecyl sulfate, 0.1% Triton X-100, 150 mM NaCl), the immunoprecipitates were subjected to electrophoresis on sodium dodecyl sulfate–12% polyacrylamide gels followed by fluorography.
Western blotting.
After electrophoresis, cell extracts were transferred to a nitrocellulose membrane (Hybond C Super; Amersham) with Phast blot B33 (Biometra). The membrane was blocked for 1 h in PBS–5% dry milk–1% Nonidet P-40, incubated overnight at 4°C with goat anti-gp70 serum or mouse P5D4 MAb (dilution, 1:1,000 in PBS)–5% dry milk–0.1% Tween 20, washed three times, and incubated with a peroxidase-conjugated anti-goat or anti-rabbit immunoglobulin G serum, and signal was revealed with an ECL detection kit (Amersham).
Flow cytometry analysis.
Transfected and nontransfected cells were harvested with 1 mM EDTA in PBS, collected by centrifugation, washed with ice-cold PBS, and resuspended for 30 min on ice in filtered supernatant of 83A25 or P5D4 hybridoma. The cells were washed twice, labeled with phycoerythrin-conjugated goat anti-rat immunoglobulin G (Southern Biotechnology Associates) in PBA (1% bovine serum albumin, 0.1% sodium azide in PBS) and fixed in PBA–1% formaldehyde before analysis with a FACScan cytofluorometer (Becton-Dickinson). For binding studies of particles pseudotyped with substituted envelopes, NIH 3T3 and TE671 cells were incubated for 1 h at 37 or 32°C in 1 ml of culture medium from pseudotype-producing TELCeB6 cells supplemented with 8 μg of Polybrene per ml. After being washed with ice-cold PBS, the cells were treated as described above.
Infection and interference assays.
NIH 3T3 cells (105) expressing or not expressing envelope glycoproteins were plated in 35-mm-diameter dishes 1 day before infection. The cell cultures were then incubated with 1 ml of viral supernatant containing 200 to 300 β-Gal FFU and supplemented with 8 μg of Polybrene per ml for 1 h at 37 or 32°C. The cells were stained with X-Gal 48 h later, and β-Gal-positive foci were scored. Data were expressed as percentages of the values for control cells. Each experiment was repeated at least three times.
RESULTS
Envelope substitution mutants.
We considered four potential loop structures in the amphotropic SU (Fig. 1A): segment 1, located in the amino-terminal half of VRA (loop I of the ecotropic SU, according to Linder et al. [17]); segment 3, located in the carboxy-terminal half of VRA (loop II of the ecotropic SU, according to Linder et al. [17]); segment 4, located in the amino-terminal half of VRB (unique to the amphotropic SU); and segment 5, in the carboxy-terminal half of VRB (loop III of the ecotropic SU, according to Linder et al. [17]). We also considered a fifth segment located in the middle of VRA (segment 2), which is a hydrophilic structure not predicted to form a loop. With the exception of segment 3, these segments are among the most hydrophilic sequences of the N-terminal domain of the amphotropic envelope glycoprotein (Fig. 1B). They are therefore candidates for being exposed at the surface of the envelope glycoprotein and for mediating interaction with cell surface receptors. Each of these segments was replaced with an 11-amino-acid epitope from the cytoplasmic tail of VSV-G (16) (mutants AΔ1 to AΔ5 [Fig. 1C]), and we questioned whether the modified envelopes still interact with amphotropic receptors. The predicted hydrophilicity was slightly decreased in VSV-G-substituted segments 1 and 2, slightly increased in substituted segments 3 and 4, and unmodified in substituted segment 5 (Fig. 1B).
FIG. 1.
Schematic representation of the predicted structure of the N-terminal domain of the amphotropic envelope glycoprotein and substitution mutants. (A) Predicted disulfide bridges according to reference 17 and hydrophilic loops and the locations of the various fragments replaced with the VSV-G epitope, MLV-E and MLV-A, ecotropic and amphotropic MLV, respectively. (B) Hydrophobicity profiles of the 208 N-terminal amino acids of the wild-type and substituted SUs. The locations of the potential hydrophilic loops are indicated on the right (1 to 5), and the peaks showing the hydrophilicity of the modified domains have been shaded for each substitution mutant. (C) Location of VRA and VRB in the N-terminal domain of the amphotropic SU and the sequences of the wild type and substituted SU mutants. The potential hydrophilic loops (1 to 5), residues deleted from the wild-type sequence and replaced (underlined and boxed sequences, respectively), and cysteine residues possibly involved in disulfide bridge formation (asterisks) are indicated. A, wild-type amphotropic SU.
Synthesis and transport.
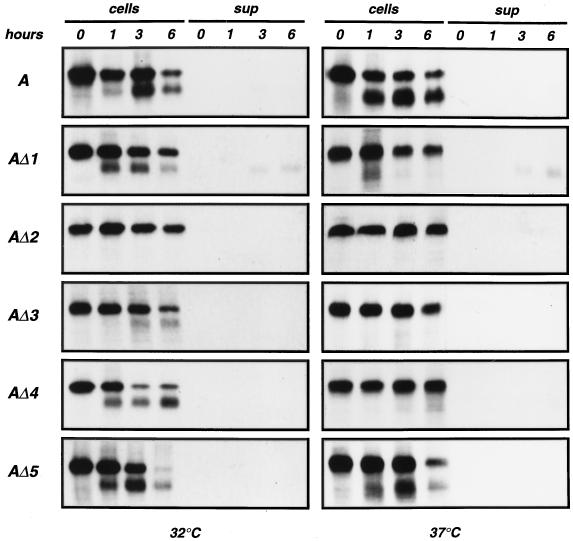
Clones of NIH 3T3 cells transfected with the wild-type envelope gene or modified envelope genes were generated. Western blot analysis of whole-cell extracts was performed with a goat anti-SU serum (Fig. 2). At 37°C, modified envelopes were produced in smaller amounts than the wild type and showed a modified ratio of uncleaved 85-kDa SU-TM precursor to cleaved 70-kDa mature SU product. When the experiment was performed at 32°C, cleavage was improved for all mutants except AΔ2. This indicated a temperature-dependent alteration of the transport and processing of modified envelope precursors. In order to study SU synthesis and processing, cells were labeled for 30 min with [35S]methionine, and both cell extracts and culture supernatants were immunoprecipitated after various times of chase with unlabeled methionine and cysteine (Fig. 3). Experiments performed at 37°C indicated that in comparison with the wild type, cleavage of the 85-kDa envelope precursors was slightly slower for AΔ1 and AΔ5, much slower for AΔ3 and AΔ4, and fully abolished for AΔ2. Cleavage was improved at 32°C for each mutant except AΔ2, with an efficiency of maturation more or less equivalent to that of the wild type. Shedding of cleaved products into the culture medium was detected for AΔ1 only, where it was responsible for a rapid disappearance of mature SU from cell extracts. In summary, at 32°C, AΔ1 cleaved normally but exhibited significant shedding, AΔ2 was not transported correctly, AΔ3 cleaved rather inefficiently, and AΔ4 and AΔ5 were comparable to the wild-type amphotropic envelope.
FIG. 2.
Western blot analysis of NIH 3T3 cells stably transfected with envelope expression vectors. Membranes were incubated with a goat anti-Rauscher leukemia virus gp70 serum (anti-SU) or the P5D4 MAb directed against the VSV-G epitope (anti-VSV Tag). Extracts were prepared from cells grown at 32 or 37°C, as indicated below the gel. A, wild-type amphotropic SU. Lanes -, controls.
FIG. 3.
Pulse-chase analysis of metabolically labeled extracts and supernatants from cells stably transfected with envelope expression vectors. Cells were labeled for 30 min with [35S]methionine and cysteine and chased for the periods indicated at the top in unlabeled culture medium. Labeling and chase were performed at 32 and 37°C. Cell extracts (cells) and culture supernatants (sup) were immunoprecipitated with a goat anti-Rauscher leukemia virus gp70 serum. A, wild-type amphotropic SU.
Western blot analysis of whole-cell extracts was also performed with the anti-VSV MAb P5D4 (Fig. 2). As expected, P5D4 did not recognize the wild-type envelope. Uncleaved SU-TM complexes were recognized equally well whatever the substituted segment. Cleaved SUs were not recognized at 37°C. At 32°C, the ratios of cleaved to uncleaved AΔ4 and AΔ5 molecules with P5D4 and anti-SU serum were equivalent, indicating that the VSV-G epitope was efficiently recognized when inserted in those segments. In contrast, cleaved SUs were poorly recognized with P5D4 in AΔ1 and AΔ3, whereas a strong signal was generated by the anti-SU serum. This suggested that the VSV-G epitope was partially occluded in the context of mature AΔ1 and AΔ3 monomeric SUs.
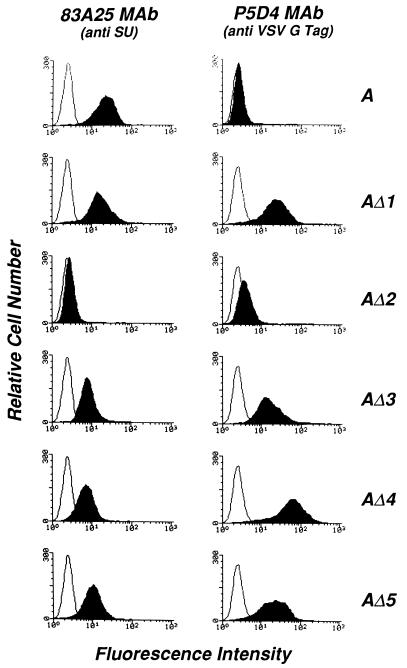
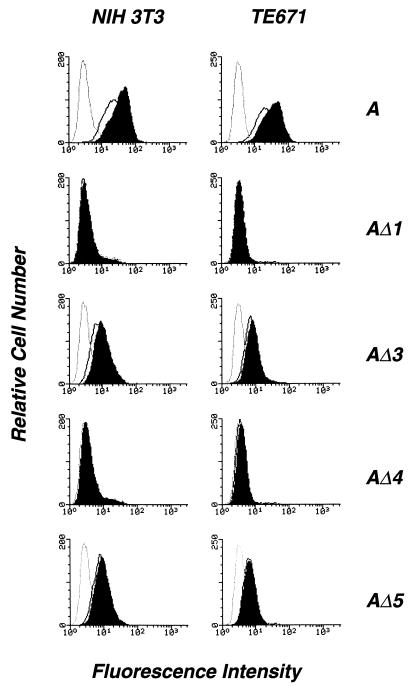
The presence of the mutant proteins at the cell surface was examined by flow cytometry using the anti-SU 83A25 and the anti-VSV-G P5D4 MAbs (Fig. 4). Cell clones expressing the wild-type envelope and fully mature envelope substitution mutants stained positive with 83A25, indicating that the mature protein was transported to the cell surface. Cells expressing AΔ2 were negative, confirming that the maturation and transport of this protein were severely affected. All positive envelopes, including the wild type, showed higher levels of cell surface expression at 32°C than at 37°C (not shown). Substitution mutants detected by 83A25 were also recognized by P5D4, indicating that the VSV-G epitope was accessible to the antibody in the mature protein and therefore presumably exposed at the surface of the molecule. It is noteworthy that AΔ1 and AΔ3, which were poorly detected by P5D4 in Western blots of cell extracts, generated strong signals detected by flow cytometry. AΔ4, which was recognized as well as AΔ5 by Western blotting, produced a stronger signal by flow cytometry. This data suggested that the accessibility of the VSV-G epitope to P5D4 varied depending on the monomeric or oligomeric form of the molecule, as analyzed by Western blotting and flow cytometry, respectively.
FIG. 4.
Flow cytometry analysis of the cell surface expression of wild-type and substituted SUs in transfected NIH 3T3 cells. Cells grown at 32°C were incubated at 4°C with a goat anti-rat immunoglobulin alone (white peaks) or after binding of MAb 83A25 (7), which recognizes a C-terminal epitope of the MLV SUs, or MAb P5D4 (16), which recognizes the VSV-G epitope in substitution mutants (shaded peaks), as indicated at the top. A, wild-type amphotropic SU.
Incorporation into virus particles.
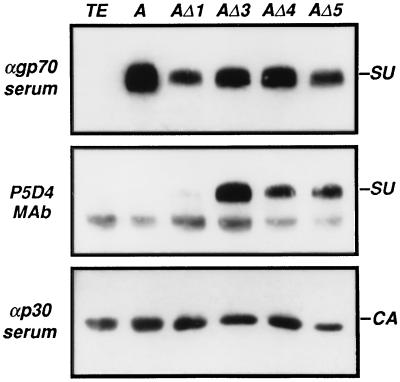
In order to generate virus particles coated with wild-type or AΔ1, AΔ3, AΔ4, or AΔ5 envelopes, the corresponding expression vectors were transfected into TELCeB6 cells, which constitutively express the gag-pol gene of Moloney MLV and a defective retroviral genome encoding the E. coli lacZ gene (6). Bulk populations of envelope-expressing cells were isolated. Viral particles released in culture supernatants at 32°C were pelleted by ultracentrifugation and analyzed by Western blotting for the presence of envelope glycoproteins by using a goat anti-gp70 antiserum or the P5D4 anti-VSV-G MAb (Fig. 5). The detection of capsid proteins by an anti-p30gag antibody allowed quantification of the amount of pelleted viral particles. The anti-SU serum indicated that modified envelopes were efficiently incorporated into virus particles at 32°C. Substituted SUs were also recognized by P5D4. AΔ3, which was poorly recognized by P5D4 in Western blots of cell extracts, generated a strong signal when extracted from virions. In contrast, P5D4 hardly recognized the VSV-G epitope in virion-associated AΔ1.
FIG. 5.
Western blot analysis of virion-associated SUs. Expression vectors encoding the wild-type amphotropic SU (A) or substitution mutant envelope glycoproteins were stably transfected in TELCeB6 cells. Culture supernatants were harvested, pelleted by ultracentrifugation, and analyzed by Western blotting with a goat anti-Moloney MLV p30 serum (αp30 serum), which reveals capsid protein (CA); a goat anti-Rauscher leukemia virus gp70 serum (αgp70 serum), which reveals envelope glycoproteins (SU); or MAb P5D4, which reveals the VSV-G epitope in substitution mutants (SU). TE, control TELCe B6 cells.
Interaction with the amphotropic receptor.
The capacity of substituted envelope glycoprotein to recognize the amphotropic receptor was first examined by interference assay. Resistance to infection with a retrovirus pseudotype of cells transfected with an envelope expression vector indicates that the expressed envelope efficiently binds the receptor and prevents its use by extracellular virions. NIH 3T3 cells stably transfected with the amphotropic wild-type or mutant envelope expression vectors were exposed to 300 β-Gal FFU of either an amphotropic pseudotype or, as a control, an ecotropic pseudotype of a retrovirus vector carrying the E. coli lacZ gene. Data shown in Table 1 indicate that the susceptibility to ecotropic virus infection was not modified in envelope-expressing cells. Cells expressing the wild-type amphotropic envelope or mutants AΔ3 and AΔ5 were resistant to amphotropic-virus infection, indicating that these molecules recognized the amphotropic receptor. Cells expressing the AΔ2 mutant, which is not processed correctly and is therefore presumably unable to bind the receptor, were still susceptible to infection. Cells expressing the AΔ1 and AΔ4 mutants were also susceptible to infection, suggesting that these mutants were altered in their capacity to interact with the amphotropic receptor.
TABLE 1.
Interference assay with NIH 3T3 cells expressing wild-type and substituted amphotropic envelopes
| Pseudotype used for infection | Relative infection (%) with the indicated expressed SU
|
||||||
|---|---|---|---|---|---|---|---|
| None | A | AΔ1 | AΔ2 | AΔ3 | AΔ4 | AΔ5 | |
| Amphotropic | 100a | 0.3 | 80 | 60 | 2 | 60 | 0.2 |
| Ecotropic | 100a | 64 | 84 | 80 | 72 | 140 | 72 |
100% corresponds to 280 and 140 X-Gal-positive foci scored 48 h after exposure of naive NIH 3T3 cells to 300 β-Gal FFU of an amphotropic and an ecotropic pseudotype of a retrovirus carrying the E. coli lacZ gene, respectively.
We next examined whether envelope glycoproteins incorporated into virus particles or released in culture supernatants interact with the amphotropic receptor. For that purpose, mouse NIH 3T3 cells and human TE671 cells were exposed to the supernatant of TELCeB6 cells stably transfected with envelope expression vectors. After a 30-min incubation at 37 or 32°C, the presence of bound SU at the cell surface was revealed by using the 83A25 MAb (Fig. 6) and analyzed by flow cytometry, as described previously (12). Binding was detected on both cell types for the wild-type amphotropic envelope and for the AΔ3 and AΔ5 substitution mutants. Differences in binding efficiencies at 37 and 32°C were minimal. No cell surface binding was detected with the AΔ1 and AΔ4 substitution mutants, confirming that interaction with the amphotropic receptor was affected.
FIG. 6.
Cell surface binding of wild-type amphotropic and substitution mutant SUs. TELCeB6 cells stably transfected with expression vectors encoding the wild-type amphotropic SU (A) or substitution mutant envelope glycoproteins were grown at 37°C (white peaks) or 32°C (black peaks), and the supernatant was incubated at the corresponding temperature with mouse NIH 3T3 or human TE671 cells as indicated at the top. SU molecules bound at the surface of target cells were revealed at 4°C with MAb 83A25 and stained with goat anti-rat immunoglobulins. Results are also shown for a control with goat anti-rat immunoglobulins alone (dotted lines).
We examined the capacity of modified SUs to mediate cell infection. Infection was performed at 37 or 32°C with retrovirus particles bearing the wild-type envelope or substitution mutant envelopes. NIH 3T3 and TE671 cells were exposed to virus particles in the absence or in the presence of a purified N-terminal fragment of the wild-type amphotropic envelope (AS208). This peptide competes with amphotropic particles for receptor binding (3). Because infection through the dog amphotropic receptor can be affected even though interactions with the human or murine receptors are normal (2), dog D17 cells were used to reveal intermediate phenotypes. The results of this experiment are shown in Table 2. The AΔ1 pseudotype was not infectious, confirming that substitution in segment 1 abolished interaction with the amphotropic receptor. Infection with the AΔ3 pseudotype was comparable to that of the wild-type amphotropic pseudotype, albeit slightly less efficient. Inhibition by the AS208 competitor confirmed that AΔ3 recognized the amphotropic receptor. AΔ5 mediated infection of NIH 3T3 and TE671 cells. The efficacy was lower than for the wild-type envelope, and dog cells were not infected. Surprisingly, AΔ4, which did not interfere with incoming virions in NIH 3T3 cells and appeared negative in binding assays, allowed infection of NIH 3T3 and TE671 cells. The efficacy was even lower than for AΔ5, and dog cells were not infected. Inhibition of infection in the presence of AS208 indicated that substitution mutants AΔ4 and AΔ5 actually recognized the amphotropic receptor.
TABLE 2.
Infection of various cell types with virus particles pseudotyped with wild-type or substituted amphotropic envelopes
| Envelope SU | Temp (°C) | Infectious titer (β-Gal FFU/ml) for the indicated target cells
|
||||
|---|---|---|---|---|---|---|
| NIH 3T3
|
TE671
|
D17, without AS208 | ||||
| Without AS208a | With AS208 | Without AS208 | With AS208 | |||
| A | 32 | 106 | <1 | 6 × 105 | <1 | 4 × 105 |
| 37 | 8 × 105 | <1 | 4 × 105 | <1 | 2 × 105 | |
| AΔ1 | 32 | <1 | <1 | <1 | <1 | <1 |
| 37 | <1 | <1 | <1 | <1 | <1 | |
| AΔ3 | 32 | 3 × 105 | <1 | 6 × 105 | <1 | 2 × 105 |
| 37 | 2 × 105 | <1 | 105 | <1 | 2 × 105 | |
| AΔ4 | 32 | 105 | <1 | 105 | <1 | <1 |
| 37 | 104 | <1 | 104 | <1 | <1 | |
| AΔ5 | 32 | 2 × 105 | <1 | 2 × 105 | <1 | <1 |
| 37 | 6 × 104 | <1 | 105 | <1 | <1 | |
AS208, competitor for receptor binding.
DISCUSSION
We have substituted segments of 6 to 15 amino acids located in the amphotropic SU receptor binding site with an 11-amino-acid linear epitope of the VSV-G protein. This peptide, which does not interact with any cell surface receptor (16), was used as a tagging epitope for studies of synthesis, incorporation into virus particles, and receptor interaction of the modified envelope glycoproteins. Substitutions were made at three locations of VRA and two locations of VRB. A summary of the functional properties of the substituted envelopes is given in Table 3.
TABLE 3.
Summary of the functional characteristics of substituted envelopesa
| Envelope SU | SU-TM cleavage | Shedding | Surface expression | Virion incorporation | Receptor binding | Infection interference | Infection of target cells |
|---|---|---|---|---|---|---|---|
| A | + | − | + | + | + | + | + |
| AΔ1 | +, ts | + | + | + | − | − | − |
| AΔ2 | − | − | − | − | − | − | − |
| AΔ3 | +, ts | − | + | + | + | + | + |
| AΔ4 | +, ts | − | + | + | − | − | + |
| AΔ5 | + | − | + | + | + | + | + |
Characteristics are listed as present (+) or absent (−) for each protein.
Substitutions in predicted loop structures (segments 1, 3, 4, and 5) were compatible with envelope processing, transport, and incorporation into virus particles. However, these processes were much less efficient for substituted than for wild-type molecules at 37°C. Significant improvement was observed when the temperature was shifted to 32°C. Temperature-sensitive (ts) mutants altered in the N-terminal extremity (31) or the proline-rich region (8) of the ecotropic SU have been reported. The ts phenotype of substituted SUs suggests that altered processing at 37°C resulted from unstable folding. Folding unstability probably also accounted for spontaneous shedding of the AΔ1 SU. In contrast to those made in potential loop-forming structures, substitution in segment 2 had a severe effect, impairing SU-TM cleavage and consequently transport to the cell surface and incorporation into virions (26, 31). Integrity of segment 2 may therefore be crucial for appropriate folding of the amphotropic SU. Among the point mutations which have been introduced in the corresponding segment of the ecotropic SU (20, 28), some had little consequence, whereas others completely destabilized the molecule.
Despite structural unstability, each loop-substituted mutant gave rise to mature envelope glycoproteins which were stably expressed at the cell surface and incorporated into virus particles, as shown by flow cytometry and Western blot analysis performed with the anti-SU antibodies. Tolerance of substitution supports the prediction of loop structure formation in these segments. Although the VSV-G epitope used here is naturally located at the C-terminal tail of the protein, it can be recognized by the P5D4 antibody when inserted within a polypeptide chain (29). In our experiment, recognition varied greatly, depending on the denatured or native form of the SU and on the location of the substituted segment. Western blot analysis, which revealed monomeric molecules, showed that whatever the location of the epitope, it was equally well recognized in uncleaved SU-TMs. In cleaved SUs, the epitope located in segment 1, and to a lesser extent in segment 3, was less accessible to the P5D4 antibody than that located in segment 4 or 5. Occlusion of the epitope inserted in segment 1 was even more obvious in virus particles. Whether this observation reflects different folding of cell surface-bound and virus-attached monomeric SUs or is a trivial artifact due to the analysis of cell extracts in one case and purified virions in the other is unknown. In contrast, flow cytometry analysis of cell surface proteins detected VSV-G insertion in segment 1 as efficiently as in segments 3 and 5, while insertion in segment 4 gave a more intense signal. The detection of a cell surface signal indicates that substituted segments were exposed on SU oligomers and, consistently with the prediction of hydrophilic loop-forming structures, easily accessible to antibody molecules. Since uncleaved SU-TMs are unlikely to be present at the cell surface (26, 31), the detection of the segment 1 epitope by flow cytometry suggests that, whereas it is poorly exposed in SU monomers, it becomes displayed as a consequence of protein refolding in oligomers. Although preferential binding of MAb to native molecules is usually observed when discontinuous epitopes are recognized, this has also been reported for linear epitopes (22). It is presumable that accessibility to segment 1 depends on stringent structural constraints, whereas segments 4 and 5 of VRB may be more flexible.
Interference assays, binding studies, and infection of target cells indicated that substitutions in potential loop-forming segments of the amphotropic SU receptor binding site do not abolish recognition of the murine and human receptors, except for substitution in segment 1. Therefore, the 14-amino-acid sequence of segment 1 appears to be the main determinant of the interaction. It is very likely that this region of VRA directly contacts receptor molecules. However, substitutions in potential loop-forming segments 3, 4, and 5 also affected binding and infection of murine and human cells and abolished the recognition of the dog amphotropic receptor. The integrity of these segments is therefore important for optimal receptor interaction. Whether these segments mediate accessory contacts with the receptors or participate in the appropriate folding and oligomerization required for optimal exposure of distant epitopes contacting receptors is unknown at this stage.
Sequence homology and disulfide linkage assignment suggest comparable structural organizations of the ecotropic and the amphotropic SU N-terminal domains (Fig. 1A). However, whereas potential loop-forming structures exist in segments 1, 3, and 5 of both SUs, the sizes and sequences of these segments are very different. The most striking differences concern loop-forming segment 1 (see sequence alignment in reference 2), which is 14 amino acids long without an internal cysteine in the amphotropic envelope and 44 amino acids long with two internal disulfide bridges in the ecotropic envelope. The involvement of segment 1 of the ecotropic SU in receptor recognition is strongly suggested by mutagenesis using insertions (8) and point mutations and point deletions (1, 20). Therefore, studies conducted with different strategies lead to the same conclusion that determinants of MLV envelope-receptor interaction are located in segment 1 of VRA (referred to as region B of the ecotropic SU by MacKrell et al. [20]). Whereas loop-forming segment 3 is more conserved than segment 1 between the amphotropic and the ecotropic SUs, mutagenesis studies show more divergent effects. We observed that substitution of segment 3 of the amphotropic SU did not affect maturation, processing, incorporation into virions, or receptor recognition. Point mutation and insertional mutagenesis of the ecotropic SU segment 3 indicates that alteration of the seven N-terminal amino acid residues profoundly affects SU functions (8, 20, 28). Substitutions in amphotropic segments 4 and 5 of VRB did not impair the recognition of the amphotropic receptor. However, they led to suboptimal envelope-receptor interaction. Previous studies with chimeric amphotropic envelopes in which only VRB was replaced by a nonamphotropic homolog led to similar conclusions (2). Point mutation in the ecotropic VRB, which is much smaller than its amphotropic counterpart, had no consequence for receptor recognition (20, 28). It may be assumed that, in addition to specific recognition by epitopes located in segment 1, efficient receptor interaction requires an accessory effect of segment 3 for the ecotropic SU and of VRB for the amphotropic SU.
Modified retroviral envelope glycoproteins have been generated with the aim of retargeting infection through receptors not naturally used by retroviruses (5). Different strategies have been proposed. In one study, the replacement of the whole receptor binding domain of the ecotropic SU by new binding domains extends the virus host range (13), whereas in another study mechanisms supporting this effect are controverted (11). Addition of an N-terminal domain after residue +6 or +19 allowed specific binding to targeted surface proteins (4, 19, 30, 33). However, the efficiency of cell infection was extremely low, probably because fusion was not triggered. The introduction of small modifications in the potential loop-forming segments naturally involved in receptor interaction may allow more-limited reshaping of the SU. The insertion of a 16-amino-acid-long RGD-containing peptide in the subgroup A avian leukosis virus SU allowed infection of mammalian cells but still with very low efficiency (32). Potential advantages of this approach are a better preservation of SU folding and consequently maintenance of the possibility of inducing the conformational changes required for viral fusion in response to receptor binding. With the goal of assessing the validity of this strategy, we are currently screening linear ligand epitopes with a high affinity for cell surface molecules that can be inserted in segment 1 or 5 or both.
ACKNOWLEDGMENTS
We are grateful to L. Evans and T. E. Kreis for providing us with MAbs. We also thank F. L. Cosset for the gift of the TELCeB6 cell line.
This work was supported by grants from Institut Pasteur, Centre National de la Recherche Scientifique, and Ministère de la Recherche et de l’Enseignement Supérieur. J.L.B. was supported by a fellowship from the Fondation Roux.
REFERENCES
- 1.Bae Y, Kingsman S M, Kingsman A J. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J Virol. 1997;71:2092–2099. doi: 10.1128/jvi.71.3.2092-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battini J L, Heard J M, Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battini J L, Rodrigues P, Müller R, Danos O, Heard J M. Receptor-binding properties of a purified fragment of the 4070A amphotropic murine leukemia virus envelope glycoprotein. J Virol. 1996;70:4387–4393. doi: 10.1128/jvi.70.7.4387-4393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosset F L, Morling F J, Takeuchi Y, Weiss R A, Collins M K L, Russell S J. Retroviral retargeting by envelopes expressing an N-terminal binding domain. J Virol. 1995;69:6314–6322. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosset F L, Russell S J. Targeting retrovirus entry. Gene Ther. 1996;3:946–956. [PubMed] [Google Scholar]
- 6.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans L H, Morrison R P, Malik F G, Portis J, Britt W. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990;64:6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray K D, Roth M. Mutational analysis of the envelope gene of Moloney murine leukemia virus. J Virol. 1993;67:3489–3496. doi: 10.1128/jvi.67.6.3489-3496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heard J M, Danos O. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J Virol. 1991;65:4026–4032. doi: 10.1128/jvi.65.8.4026-4032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones J S, Risser R. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J Virol. 1993;67:67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabat D. Targeting retroviral vectors to specific cells. Science. 1995;269:417. doi: 10.1126/science.7618110. [DOI] [PubMed] [Google Scholar]
- 12.Kadan M J, Sturm S, Anderson W F, Eglitis M A. Detection of receptor-specific murine leukemia virus binding to cells by immunofluorescence analysis. J Virol. 1992;66:2281–2287. doi: 10.1128/jvi.66.4.2281-2287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasahara N, Dozy A M, Kan Y W. Tissue-specific targeting of retroviral vectors through ligand-receptor interactions. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 14.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J W, Closs E I, Albritton L M, Cunningham J M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991;352:725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- 16.Kreis T E. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linder M, Linder D, Hahnen J, Schott H H, Stirm S. Localization of the intrachain disulfide bonds of the envelope glycoprotein 71 from Friend murine leukemia virus. Eur J Biochem. 1992;203:65–73. doi: 10.1111/j.1432-1033.1992.tb19828.x. [DOI] [PubMed] [Google Scholar]
- 18.Linder M, Wenzel V, Linder D, Stirm S. Structural elements in glycoprotein 70 from polytropic Friend mink cell focus-inducing virus and glycoprotein 71 from ecotropic Friend murine leukemia virus, as defined by disulfide-bonding pattern and limited proteolysis. J Virol. 1994;68:5133–5141. doi: 10.1128/jvi.68.8.5133-5141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin M, Noël D, Valsesia-Wittman S, Brockly F, Etienne-Julan M, Russell S, Cosset F C, Piechaczyk M. Targeted infection of human cells via major histocompatibility complex class I molecules by Moloney murine leukemia virus-derived viruses displaying single-chain antibody fragment-envelope fusion proteins. J Virol. 1996;70:2957–2962. doi: 10.1128/jvi.70.5.2957-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacKrell A J, Soong N W, Curtis C M, Anderson F. Identification of a subdomain in the Moloney murine leukemia virus envelope protein involved in receptor binding. J Virol. 1996;70:1768–1774. doi: 10.1128/jvi.70.3.1768-1774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller A D, Miller D G, Victor-Garcia J, Lynch C M. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- 22.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan R A, Nussbaum O, Muenchau D D, Shu L, Couture L, Anderson W F. Analysis of the functional and host range-determining regions of the murine ecotropic and amphotropic retrovirus envelope proteins. J Virol. 1993;67:4712–4721. doi: 10.1128/jvi.67.8.4712-4721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nussbaum O, Roop A, Anderson W F. Sequences determining the pH dependence of viral entry are distinct from the host range-determining region of the murine ecotropic and amphotropic retrovirus envelope proteins. J Virol. 1993;67:7402–7405. doi: 10.1128/jvi.67.12.7402-7405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez L G, Hunter E. Mutations within the proteolytic cleavage site of the Rous sarcoma virus glycoprotein that block processing to gp85 and gp37. J Virol. 1987;61:1609–1614. doi: 10.1128/jvi.61.5.1609-1614.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinter A, Chen T E, Lowy A, Cortez N G, Siligari S. Ecotropic murine leukemia virus-induced fusion of murine cells. J Virol. 1986;57:1048–1054. doi: 10.1128/jvi.57.3.1048-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skov H, Andersen K B. Mutational analysis of Moloney murine leukemia virus surface protein gp70. J Gen Virol. 1993;74:707–714. doi: 10.1099/0022-1317-74-4-707. [DOI] [PubMed] [Google Scholar]
- 29.Soldati T, Perriard J C. Intracompartmental sorting of essential myosin light chains: molecular dissection and in vivo monitoring by epitope tagging. Cell. 1991;66:277–289. doi: 10.1016/0092-8674(91)90618-9. [DOI] [PubMed] [Google Scholar]
- 30.Somia N V, Zoppé M, Verma I M. Generation of targeted retroviral vectors by using a single-chain variable fragment: an approach to in vivo gene delivery. Proc Natl Acad Sci USA. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szurek P F, Yuen P H, Ball J K, Wong P K Y. A Val-25-to-Ile substitution in the envelope precursor polyprotein gPr80env is responsible for the temperature sensitivity, inefficient processing of gPr80, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990;64:467–475. doi: 10.1128/jvi.64.2.467-475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valsesia-Wittmann S, Drynda A, Deléage G, Aumailley M, Heard J M, Danos O, Verdier G, Cosset F L. Modifications in the binding domain of avian retrovirus envelope protein to redirect the host range of retroviral vectors. J Virol. 1994;68:4609–4619. doi: 10.1128/jvi.68.7.4609-4619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valsesia-Wittmann S, Morling F J, Russell S J, Cosset F L. Improvement of retroviral retargeting by using amino acid spacers between an additional binding domain and the N terminus of Moloney murine leukemia virus SU. J Virol. 1996;70:2059–2064. doi: 10.1128/jvi.70.3.2059-2064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Kavanaugh M P, North R A, Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991;352:729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- 35.Weiss R A. Cellular receptors and viral glycoproteins involved in retrovirus entry. In: Levy J A, editor. The Retroviridae. New York, N.Y: Plenum Press; 1993. pp. 1–72. [Google Scholar]