Abstract
Manipulating matter at the nanometer scale to create desired plasmonic nanostructures holds great promise in the field of biomedical photoacoustic (PA) imaging. We demonstrate a strategy for regulating PA signal generation from anisotropic nano-sized assemblies of gold nanospheres (Au NSs) by adjusting the inter-particle connectivity between neighboring Au NSs. The inter-particle connectivity is controlled by modulating the diameter and inter-particle spacing of Au NSs in the nanoassemblies. The results indicate that nanoassemblies with semi-connectivity, i.e., assemblies with a finite inter-particle spacing shorter than the theoretical limit of repulsion between nearby Au NSs, exhibit 3.4-fold and 2.4-fold higher PA signals compared to nanoassemblies with no connectivity and full connectivity, respectively. Furthermore, due to the reduced diffusion of Au atoms, the semi-connectivity Au nanoassemblies demonstrate high photodamage threshold and, therefore, excellent photostability at fluences above the current American National Standards Institute limits. The exceptional photostability of the semi-connectivity nanoassemblies highlights their potential to surpass conventional plasmonic contrast agents for continuing PA imaging. Collectively, our findings indicate that semi-connected nanostructures are a promising option for reliable, high-contrast PA imaging applications over multiple imaging sessions due to their strong PA signals and enhanced photostability.
Keywords: Photoacoustics, exogenous imaging contrast agents, gold nanoparticles, inter-particle connectivity, nanoscale engineering
Graphical abstract

Gold nanosphere assemblies with tunable inter-particle connectivity are developed for photoacoustic imaging. Semi-connectivity between proximate gold nanospheres in the assemblies generates high photoacoustic signals via more efficient optical absorption than assemblies with no connectivity and full connectivity. Moreover, assemblies with semi-connectivity demonstrate superior photostability compared to conventional gold nanorods. Overall, gold nanoassemblies with semi-connectivity enable enhanced photoacoustic imaging in vivo.
1. Introduction
Photoacoustic (PA) imaging is a non-invasive and non-ionizing modality that leverages the conversion of light to sound using nanosecond laser pulses[1,2]. The absorption of incident laser pulses by molecules results in rapid and localized thermoelastic expansion, which then produces acoustic waves that are detected by ultrasound (US) transducers[3–5]. While endogenous molecules, such as hemoglobin, produce PA signals, the use of exogenous contrast agents can greatly increase PA imaging contrast, allowing for better identification of specific cellular and molecular events[6–10]. The near-infrared (NIR) optical window (650 nm to 1300 nm) offers relatively low background PA signals from endogenous molecules in tissue and allows for deeper penetration due to less scattering[1–3]. Therefore, the development of exogenous contrast agents with strong optical absorption within the NIR window is critical for high-contrast PA imaging [8–10].
Gold nanoparticles (Au NPs) have been widely utilized as exogenous PA contrast agents due to their size- and shape-dependent tunable optical properties and biocompatibility[11–13]. The conventional approach for designing Au NPs as contrast agents for PA imaging is to maximize the optical absorption cross-section in the NIR spectral region by altering their aspect ratio[14–16]. As the aspect ratio of Au NPs increases, the optical absorption peak is shifted towards longer wavelengths. Thus, anisotropic Au NPs with a high aspect ratio, such as nanorods (NRs) and nanoprisms, exhibit a strong and sharp optical absorption peak within the NIR window[7,8,11,14–18]. However, the thermal stability of Au NPs is inversely proportional to their aspect ratio[19–21]. As a result, anisotropic Au NPs with high aspect ratios are prone to photodamage from high-intensity laser pulses used in PA imaging, often exceeding 10 mJ cm−2, which causes rapid structural changes into spheres via surface diffusion of Au atoms to minimize surface energy[21–23]. This structural deformation results in decreased optical absorption and decay of PA signals in the NIR window[8,15,22], making anisotropic Au NPs unsuitable for reliable PA imaging under repetitive laser pulses and over multiple imaging sessions.
Tailoring inter-particle plasmon coupling in colloidal Au NPs is an efficient way to increase optical absorption in the NIR window[14,24–27]. When the inter-particle distance between neighboring particles decreases to a few nanometers, the near-field coupling and hybridization of plasmon modes between adjacent particles leads to an increased optical absorption cross-section with the redshift of the absorption peak[24,25]. The intensity of the plasmon coupling depends on the number of particles involved and the inter-particle spacing[23–25]. Therefore, the formation of an anisotropic assembly of Au nanospheres (Au NSs) that resembles a porous, anisotropic Au nanoconstruct can result in strong NIR-light absorption for high PA signals and contrast. Moreover, since atomic diffusion in a porous structure is slower than the diffusion in a non-porous one[28,29], the porous structure of the anisotropic Au assembly could prevent the diffusion of Au atoms induced by laser pulses. This reduced atomic diffusivity will hinder shape transition, resulting in a higher photodamage threshold and, therefore, improved photostability. Although some previous studies have demonstrated various designs of Au assemblies for PA imaging, these contrast agents lack effective control of size uniformity and inter-particle spacing[30–34].
Herein, we introduce an anisotropic Au nanoassembly (Au NA) consisting of closely packed Au NSs within a nanoscale anisotropic space. These Au NAs allow precise control of Au NS size and inter-particle spacing to manipulate PA signal generation. To explore the effect of the inter-particle spacing between neighboring Au NSs in a single NA on PA signal generation, we tuned the inter-particle connectivity from no connectivity to semi-connectivity and full connectivity. Here, semi-connectivity refers to a state in which the finite spacing between adjacent Au NSs in Au NAs is shorter than the theoretical limit imposed by inter-particle repulsion. Our results showed that semi-connectivity between Au NSs in the anisotropic Au NA significantly enhances PA signal amplitude through more efficient optical absorption compared to no connectivity and full connectivity (Scheme 1). Furthermore, our Au NAs with semi-connectivity exhibited superior thermal stability under laser pulses with fluences higher than American National Standards Institute (ANSI) limits, thus allowing for repetitive multi-session PA imaging in vivo[35]. Our approach of regulating inter-particle connectivity in an anisotropic Au NA provides a roadmap for designing exogenous contrast agents for PA imaging with high imaging contrast.
Scheme 1.
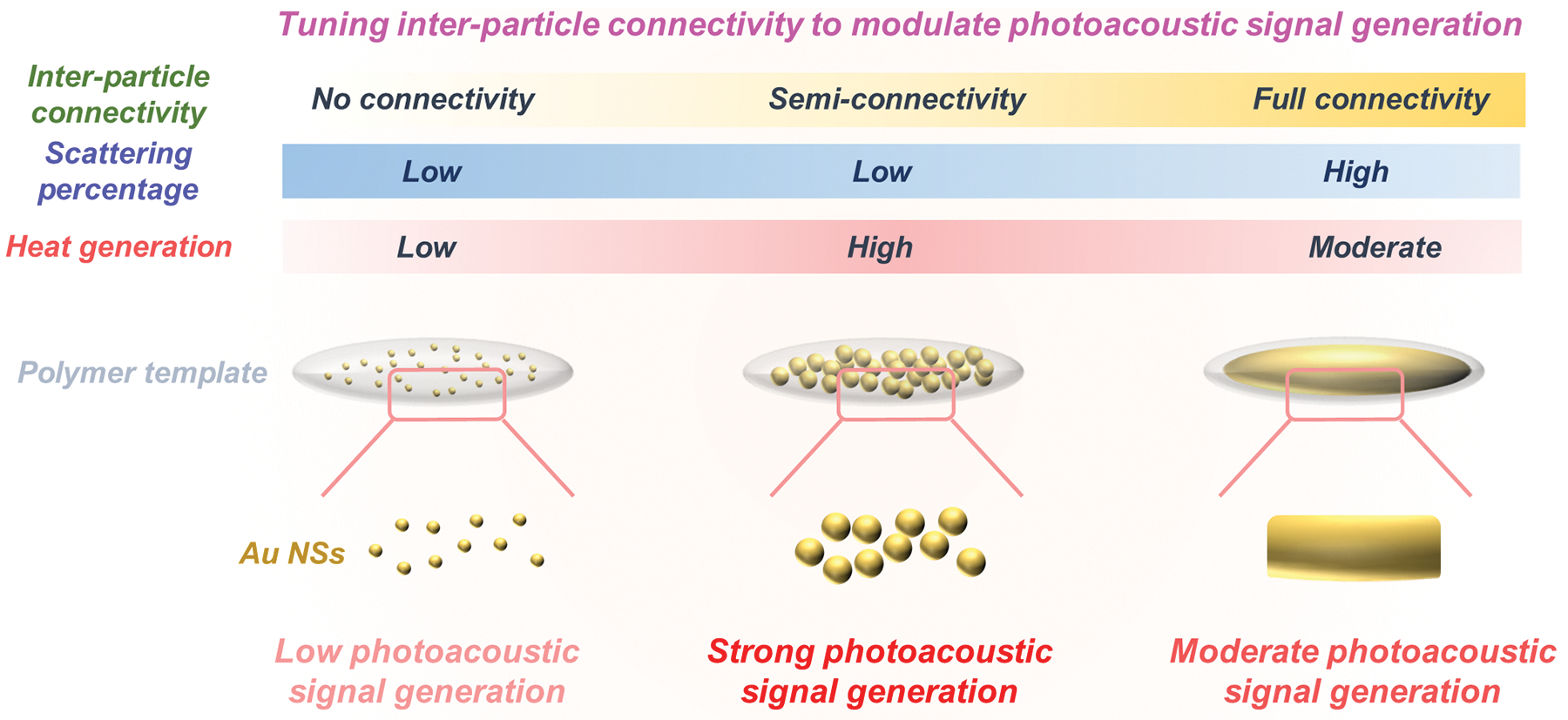
Tuning inter-particle connectivity between proximate Au NSs in an anisotropic nanoscale space for PA signal enhancement.
2. Results and Discussion
2.1. Theoretical analysis of the optical characteristics of linearly spaced Au NSs
In PA imaging, the optical absorption of exogenous contrast agents directly correlates with PA signal amplitude, while the optical scattering reduces the local laser fluence and results in a low PA signal amplitude[1–4]. Thus, to produce high PA signals from Au nanoconstructs, it is important to design Au constructs that have a high absorption cross-section with low scattering. Since Au nanoconstructs smaller than 15 nm theoretically have a negligible scattering percentage (i.e., the contribution of scattering to the overall extinction) [8,14], we pondered that an anisotropic NA of 10 nm-sized Au NSs could increase the optical absorption cross-section via inter-particle plasmon coupling, while minimizing the contribution of scattering cross-section[36,37].
To investigate the optical properties of anisotropically assembled Au NSs, we performed a finite-different time-domain (FDTD) analysis by adopting a simplified Au chain construct consisting of 10 nm-sized Au NSs in water as our simulation model. Specifically, we analyzed the effects of the number of Au NSs per chain and inter-particle spacing between Au NSs on the optical absorption cross-sections (Figure 1a–e). As the particle number was increased from 1 to 11 while keeping the inter-particle gap at 1 nm, the optical absorption peak redshifted, and the scattering percentage remained negligible, below 5% (Figure 1b–c). Furthermore, the absorption cross-section dramatically increased as the number of Au NSs per chain was increased (Figure 1c and Figure S1, Supporting Information). As the diameters of Au NSs comprising the chain were increased from 6 nm to 10 nm with decreasing the inter-particle gap from 5 nm to 1 nm, the absorption cross-section exponentially increased with a redshift of the absorption peak, while maintaining a very low scattering percentage (Figure 1d–f and Figure S2, Supporting Information). The near electric-field enhancement strongly occurred at the inter-particle gap regions at 640 nm and 700 nm parallel to the long axis, showing that the near-field coupling and hybridization of plasmon modes between Au NSs along the long axis of the chain could shift the optical absorption towards the NIR window (Figure S3, Supporting Information).
Figure 1.

Theoretical analysis of the optical properties of linearly spaced Au NSs. a) Schematic of Au chain model with different number of particles. b) Simulated optical absorption peak and scattering percentage profiles of the chain depending on the number of particles in the chain. c) Calculated absorption cross-section at the absorption peak of the chain depending on the number of particles in the chain. d) Schematic of Au chain model with particles of different diameters. e) Simulated optical absorption peak and scattering percentage profiles of the chain depending on the particle diameter. f) Calculated absorption cross-section at the absorption peak of the chain depending on the particle diameter in the chain.
These simulation results highlight several promising aspects of the anisotropic assembly of Au NSs with short inter-particle spacing for PA imaging. First, the redshift of the optical absorption spectrum towards the NIR window will allow PA imaging with high imaging contrast. Second, the dramatically increased absorption cross-section from the plasmon coupling between proximate Au NSs will greatly increase PA signals and contrast. Lastly, the minimal scattering cross-section of the assembled Au NSs will minimize the reduction of local laser fluence, generating high PA signals.
2.2. Design of anisotropic assembly of Au NSs with tunable inter-particle connectivity.
While simulation results suggest promising features of anisotropically assembled Au NSs for PA imaging, it is still challenging to fabricate homogenous anisotropic Au assemblies with tunable inter-particle spacing at the nanometer scale for two main reasons: 1) strong repulsive forces between proximate particles[38,39] and 2) uncontrolled assembly of Au NSs with uneven inter-particle distances and heterogeneous sizes[30,31,34]. In this study, we introduced an in situ seeded growth process of Au seeds pre-fixed within a rod-shaped void (40 nm by 200 nm) covered by a resorcinol-formaldehyde polymer layer to create anisotropic Au NAs with tunable inter-particle connectivity for PA imaging (Supplementary Figure 4, Supporting Information). For the seeded growth of Au seeds, chloroauric acid (HAuCl4) and L-ascorbic acid were employed as an Au precursor and reducing agent, respectively. Furthermore, to prevent self-nucleation of Au NPs outside the polymer layer during the seeded growth, the reduction potential of Au ions was reduced by using ligands, such as hexadecyl methylammonium chloride (CTAC) and potassium iodide (KI), that form coordination complexes with Au ions[16,40,41] (Supplementary Figure 5, Supporting Information).
Since the seed-mediated growth of Au seeds was performed within the anisotropic void that was covered by the polymer layer, we investigated the effect of polymer thicknesses on Au seed growth while maintaining the concentration of added Au precursors and Au seeds within the anisotropic void constant (Figure 2a). The growth of Au seeds in the thin polymer thicknesses of 10 nm and 20 nm led to the formation of highly packed Au NAs within the anisotropic void, while the growth in the 40-nm-thick polymer layer formed less packed Au NAs due to the formation of Au NSs both inside and outside the void space (Figure 2a, b). Therefore, our results suggested that the thick polymer layer inhibited Au ion reduction for the growth of the Au seeds inside the polymer layer due to the reduced transport of Au ions through the thick polymer layer, which ultimately caused free nucleation of Au NSs within the polymer layer and outside the void space[42,43]. Otherwise, thin polymer layers enabled faster transport of Au ions toward the Au seeds inside the void space to ensure direct Au deposition and seeded growth, while minimizing free nucleation of Au NSs within the polymer layer[42,43]. The highly packed Au NAs exhibited strong optical absorption in the NIR window, as compared to the lesser packed Au NAs, due to the more efficient inter-particle plasmon coupling that occurs between particles in close proximity (Figure 2c, d).
Figure 2.

Tuning inter-particle connectivity in Au NAs via the seed-mediated growth inside the anisotropic nano-sized space. a) TEM images of Au NAs with different polymer thicknesses. b) Corresponding quantifications of Au NS diameter and polymer thickness. c) Schematic of the seed-mediated growth mechanism inside different polymer thicknesses. d) UV-vis-NIR spectra of Au NAs with different polymer thicknesses. e) Strategy to fabricate Au NAs with tunable inter-particle connectivity from no connectivity to semi-connectivity and full connectivity. f-i) TEM images of tunable Au NAs fabricated by regulating the added Au ions in the seed-mediated growth. j, k) Corresponding quantifications of the particle diameter and inter-particle distance of Au NSs in Au NAs. l) UV-vis-NIR spectra of Au NAs with tunable inter-particle connectivity. The scale bars are 200 nm. Data are presented as the mean ± standard deviation (n=30).
Next, to create highly packed Au NAs with tunable inter-particle connectivity, we modulated the diameter and inter-particle spacing of Au NSs by regulating Au ion concentration in the growth process, i.e., the amounts of added Au precursors. A 20-nm-thick polymer layer was selected based on results shown in Figure 2a–d. An increase of the size of Au NSs in the void space was observed with increasing the amounts of added Au precursors in the growth process due to the increased number of deposited Au atoms onto Au seeds for the subsequent growth in the void space[40,44] (Figure 2e–i). Four types of Au NAs with varying diameter of Au NSs and inter-particle distances were obtained and denoted as Au NA-1, Au NA-2, Au NA-3, and Au NA-4, respectively (Figure 2e–i). All aforementioned Au NAs have the same overall construct dimensions (80 nm by 240 nm). By increasing the Au ions added in the growth process, the diameter of Au NSs increased from 6.8 nm to 10.2 nm (Figure 2j). This increase in diameter was accompanied by a gradual decrease in inter-particle spacing from 14.0 nm to 10.5 nm. Due to the decrease in inter-particle spacing, i.e., core-to-core distances, down to 10.5 nm (Au NA-3), the edge-to-edge distances between adjacent Au NSs (~ 0.3 nm) decreased below the theoretical limit imposed by steric repulsion caused by CTAC ligands on Au NSs (~ 1.6 nm)[38], leading to semi-connectivity between neighboring Au NSs (Figure 2k and Figure S6, Supporting Information). UV-vis-NIR spectroscopy showed that the extinction peak of Au NA-3 broadened and redshifted compared to Au NA-1 and Au NA-2 due to more efficient inter-particle plasmon coupling (Figure 2l). In contrast, Au NA-4 with full connectivity exhibited shoulder peaks in its UV-vis-NIR spectrum due to plasmon excitation based on its rod-like morphology (Figure 2l). These results collectively demonstrate that our seed-mediated growth approach enables the synthesis of homogenous, anisotropic Au NAs with tunable inter-particle connectivity, and the optical properties of these nanostructures can be controlled by modulating inter-particle plasmon coupling.
2.3. Effect of the inter-particle connectivity in Au NAs on PA signal generation
Given the distinct optical properties of Au NA-1, Au NA-2, Au NA-3, and Au NA-4 due to their different inter-particle connectivity, we sought to demonstrate that controlling the inter-particle connectivity can regulate PA signal generation[4,11,12,14]. To experimentally test our hypothesis, we prepared samples of each Au NA with the same solvent (water), Au mass concentration (adjusted to the added Au ions in the seed-mediated growth process), and NP solution volume, which were placed in individual polyethylene tube phantoms. PA signals were then analyzed from 700–900 nm wavelengths at a constant laser fluence of 10.5 mJ cm−2 (Figure 3a). Our result showed that Au NA-3 produced the highest PA signals within the 700–900 nm spectral region (Figure 3b–e).
Figure 3.

Effect of inter-particle connectivity in Au NAs on PA signal generation. a) Schematic of PA signal generation from Au NAs with tunable inter-particle connectivity. b) PA signals from different Au NA solutions with the identical Au mass concentration within 700–900 nm spectral region and c-e) quantitative analysis for PA signal amplitude of the Au NAs at 700 nm, 800 nm, and 900 nm. Data are presented as the mean ± standard error (n=10). f-i) Calculated absorption, scattering, and extinction cross-sections of the Au NAs within the 700–900 nm spectral region. j) Estimated absorption cross-section per unit Au mass of the tunable Au NAs. k) Mechanism of PA signal generation from Au NAs with different inter-particle connectivity.
PA signal from an NP solution is dependent on the Grüneisen parameter, laser fluence, and optical absorption coefficient, which are determined by the local environment, laser source, and optical density of the NP solution, respectively[1,2,4]. Since the NP solvent (water) and laser fluence for our PA experiments were kept constant, the only difference between the Au NAs is their optical absorption coefficient. We numerically estimated the optical absorption, scattering, and extinction cross-sections of Au NA-1, Au NA-2, Au NA-3, and Au NA-4 in water at 700–900 nm wavelengths (Figure 3f–i). Simulation results indicated that close inter-particle connectivity increased optical absorption cross-sections within the 700–900 nm spectral region through inter-particle plasmon coupling. In addition, while Au NA-4 exhibited a high scattering percentage in the total optical extinction, Au NA-1, Au NA-2, and Au NA-3 showed absorption dominance with a negligible scattering percentage. These results suggest that incorporating small Au NSs with close proximity in an anisotropic nano-sized dimension can result in high optical absorption cross-sections at the NIR wavelengths while minimizing the optical scattering cross-sections.
The optical absorption coefficient of the Au NAs is directly proportional to their absorption cross-section per unit Au mass (Au mass in a single Au NA), as we maintained a constant Au mass concentration in all solutions (See details in Supplementary Note 1, Supplementary Information). We therefore calculated the absorption cross-section per unit Au mass for each Au NA. The highest absorption cross-section per unit Au mass among all Au NAs was observed for Au NA-3 (Figure 3j). In other words, when the mass concentration of Au in the Au NA solutions is kept constant, the semi-connectivity between proximate Au NSs in Au NAs (Au NA-3) leads to a significant increase in optical absorption and subsequent heat pulse generation due to strong inter-particle coupling. This contrasts with Au NAs with no connectivity (Au NA-1) and full connectivity (Au NA-4), which aligns with our findings from the PA experimental results. Furthermore, Au NA-3 with semi-connectivity exhibited stronger PA signals compared to conventional plasmonic PA contrast agents, including Au NRs and Au nanostars (Figure S7, Supplementary Information). Taken together, our simulation and experimental results suggest that Au NAs with semi-connectivity are the ideal candidate to facilitate in vivo PA imaging at lower doses of contrast agents (Figure 3k).
2.4. Improved photostability of Au NAs
The use of high-fluence laser pulses in PA imaging can photodamage conventional anisotropic Au nanoconstructs, such as Au NRs, causing structural changes to Au spheres through the diffusion of Au atoms[19–21,23]. This structural deformation decreases the optical absorption and PA signal ampitudes in the NIR window[22]. Our semi-connectivity Au NAs, however, possess two advantageous features that make them more resistant to high-energy pulsed laser illumination: 1) an assembly consisting of small-sized Au NSs, which have shown to be less susceptible to photodamage[45]; and 2) a porous structure that has a smaller diffusion coefficient than a non-porous one, which prevents the diffusion of Au atoms and shape transition[28,29] under pulsed laser illumination (Figure 4a).
Figure 4.

Photostability test of Au NA-3 under 800 nm wavelength pulsed laser illumination at different laser fluences. a) Mechanism of the improved photostability of Au NA-3. b) PA signal amplitude of Au NA-3 and conventional Au NRs under 100 pulses at 10 mJ cm−2. c) PA signal amplitude of the Au NAs under 1,200 laser pulses at 4 mJ cm−2, 16 mJ cm−2, and 40 mJ cm−2. d) UV-vis-NIR spectra of the Au NAs before and after 1,000 laser pulses at 4 mJ cm−2, 16 mJ cm−2, and 40 mJ cm−2. e) TEM images of Au NA-3 before and after 1,000 laser pulses at 16 mJ cm−2. The scale bars are 200 nm. Data are presented as the mean ± standard deviation (n=3).
To verify the higher photodamage threshold of our Au NA-3 compared to conventional Au nanoconstructs, we studied photostability of Au NA-3 and a conventional Au NR (35 nm by 135 nm) irradiated by 800 nm wavelength laser pulses with a laser fluence of 10 mJ cm−2 (Figure 4b). While the Au NRs exhibited a significant decay of PA signals, approximately 60% within 10 laser pulses, Au NA-3 maintained stable PA signals without any signal decay, confirming that Au NA-3 was more photostable than non-porous rod-shaped Au construct (Figure 4b). Next, to measure the photodamage threshold, Au NA-3 was placed under 800 nm pulsed laser irradiation for 1,000 pulses at multiple laser fluences from 4 mJ cm−2 to 40 mJ cm−2. Up to the laser fluence of 16 mJ cm−2, Au NA-3 maintained its optical and structural characteristics, resulting in consistent PA signal amplitude without any signal decay (Figure 4c–e). Even under a very strong laser fluence of 40 mJ cm−2, Au NA-3 exhibited a slight decrease of PA signal amplitude by only 15% with a slight change in its optical spectrum (Figure 4c, d). It is worth noting that, according to ANSI limit[35], the maximum laser exposure for PA-guided clinical applications is 31.7 mJ cm−2 at 800 nm. The fact that Au NA-3 maintained PA signal amplitude after 1,200 pulses with a laser fluence higher than the ANSI limit[35] (40 mJ cm−2) indicates desired photostability of Au NAs for PA imaging in the NIR window in comparison to existing anisotropic Au NPs, such as Au NR[15], nanoprism[7], nanostars[46] and silica-coated Au NRs[22] (Supplementary Table S1, Supporting Information).
2.5. Assessment of the capability of Au NAs for PA imaging in vitro and in vivo
We tested the potential of Au NAs for longitudinal PA imaging with high imaging contrast both in vitro and in vivo. The spatial resolutions of our PA imaging system, including axial and lateral resolutions, were measured to be approximately 90 μm and 280 μm, respectively (Figure S8, Supporting Information). We performed in vitro PA imaging of human cervical adenocarcinoma (HeLa) cells using Au NA-3 (Figure 5a). For this application, we exchanged the surface ligand of the Au NA-3 from hexadecyl methylammonium chloride (CTAC) to the more cytocompatible polyacrylic acid (PAA)[47]. In the future, carboxyl groups in PAA on the AuNA-3 could be utilized for functionalization of other ligand moieties, such as polyethylene glycol and antibodies, for molecular PA imaging applications requiring prolonged blood circulation[48,49]. The CTAC-coated Au NA-3 had a positive surface charge of 37 mV, while PAA-coated Au NAs displayed a negative surface charge of −32 mV, confirming successful ligand exchange. We then evaluated the cytocompatibility of PAA-coated Au NA-3 using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay. Results demonstrated that our Au NA-3 does not induce significant cell death up to a concentration of 50 μg mL−1 for 24 hours (Figure S9, Supporting Information). Given the result that Au NA-3 at a concentration of 50 μg mL−1 showed no effect on the viability of HeLa cells, HeLa cells were labeled with Au NAs at a concentration of 50 μg mL−1 for 24 hours. The labeled cells were then embedded in a tissue-mimicking gelatin phantom for PA imaging. The labeled cells displayed stable, high PA signal amplitude compared to background PA signal from unlabeled cells at 800 nm wavelength for 1,500 pulses (Figure 5b–d). Furthermore, the quantitative PA signal amplitude of the labeled cells was significantly higher than that of the unlabeled cells within 700–900 nm spectral region (Figure 5e). These results demonstrate the utility of our PA contrast agent for live cell imaging in the NIR window with minimal toxicity.
Figure 5.

In vitro and in vivo PA imaging using Au NAs. a) Schematic of labeling HeLa cells with Au NAs and PA imaging of labeled cells in vitro. b) In vitro US and 800 nm wavelength PA imaging of dome-shaped gelatin phantom containing unlabeled and labeled HeLa cells. c) Three-dimensional US and 800 nm wavelength PA image of dome phantoms containing unlabeled and labeled HeLa cells. d) PA signal amplitude (800 nm wavelength) of the unlabeled and labeled HeLa cells with an identical number of the cells. e) PA signal amplitude of the unlabeled and labeled HeLa cells with an identical number of the cells within the 700–900 nm spectral region. f) Schematic of in vivo US/PA imaging protocol to verify the imaging performance of Au NAs with different inter-particle connectivity. g) In vivo US/PA imaging of Au NA-3 and Au NA-4 at 800 nm. h) Three-dimensional in vivo US/PA imaging of Au NAs with semi-connectivity or full connectivity at 800 nm. i) PA signal amplitude of Au NAs with semi-connectivity or full connectivity at 800 nm. The scale bars are 5 mm. Yellow dotted lines indicate the dome phantoms (Figure 5c) and the mouse regions where Au NAs were injected subcutaneously (Figure 5g–h). Data are presented as the mean ± standard deviation (n=3). The imaging experiments were repeated independently three times and similar results were obtained.
Next, to validate Au NA-3 as an efficient and photostable contrast agent for in vivo PA imaging, we subcutaneously injected approximately 20 μg of Au NA-3 and Au NA-4 suspended in 50% Matrigel and subsequently acquired US/PA images (Figure 5f). We did not observe any noticeable PA signals from pure Matrigel in vivo (Figure S10, Supporting Information). At 800 nm wavelength, Au NA-3 with semi-connectivity exhibited approximately 3-fold higher PA signal compared to Au NA-4 with full connectivity (Figure 5g–i). Furthermore, our Au NA-3 with semi-connectivity displayed 2.5-fold higher PA signal in vivo compared to conventional Au NRs, one of the most common PA contrast agents (Figure S11, Supporting Information). Together, these results verify that our Au NAs with tailored inter-particle connectivity generate higher PA signals both in vitro and in vivo with a potential to outperform traditional PA contrast agents for longitudinal imaging in the future. Previous studies demonstrated that self-assembled Au nanoconstructs (~ 200 nm) composed of small Au NSs, which have a similar construct dimension with our Au NAs, did not cause any severe systemic toxicity for at least 7 days, following intravenous injection[34,50]. Together with our in vitro results showing minimal cytotoxicity (Figure S9, Supporting Information), our Au NAs should not induce significant systemic toxicity. In the future, we plan to investigate the long-term toxicity of the current Au NAs described herein and additional Au NA formulations, where assembly size, shape, and composition of the polymer layer are manipulated, to create a suite of cytocompatible nano-sized assemblies with desired physicochemical characteristics for a variety of biomedical applications.
3. Conclusion
We designed a highly efficient and photostable PA imaging contrast agent by tuning the inter-particle connectivity of Au NSs in a single anisotropic NA and validated its potential for biomedical imaging applications in vivo. First, we developed homogeneously assembled Au NSs within an anisotropic nano-sized dimension with fine-tuned inter-particle connectivity. Then, we demonstrated the effect of inter-particle connectivity on PA signal generation. Specifically, we observed that semi-connectivity between adjacent Au NSs in a single Au NA increases PA signals via enhanced light-to-heat conversion versus no connectivity and full connectivity in the assemblies. Further, we verified the excellent photostability of Au NAs with semi-connectivity for longitudinal PA imaging applications. Finally, due to the high and stable PA signals from Au NAs with semi-connectivity, we accomplished contrast-enhanced PA imaging both in vitro and in vivo. In the future, Au NA features can be further tuned to create a broader class of PA contrast agents based on nano-sized assembly with optimal physicochemical, optical, and thermal characteristics by tuning the combinations of assembly size, shape, polymer, and composition.
4. Experimental Section/Methods
Synthesis of Au NAs with tunable inter-particle connectivity.
β-FeOOH NRs (200 nm) were synthesized via hydrolysis of iron chloride in an aqueous solution[51]. In brief, 100 mL of 0.1 M iron chloride hexahydrate (Sigma Aldrich) aqueous solution was placed in a single neck round bottom flask. The solution was heated up to 80 °C and left undisturbed for 2 hours. The FeOOH NRs were collected via centrifugation and subsequently functionalized with amine groups by conjugating polyethyleneimine (PEI, Sigma Aldrich, molecular weight 25,000). The products were dispersed in 10 mL of deionized water.
Au NSs (2 nm) were fabricated as reported previously[52]. To decorate Au NSs (2 nm) on the FeOOH NRs, 6 mL of the amine-functionalized NRs were mixed with 20 mL of Au NSs, followed by sonication for 10 min. To remove free Au NSs, the products were collected via centrifugation and subsequently functionalized with polyacrylic acid (PAA, Sigma Aldrich, molecular weight 1,800). Finally, the stabilized product was suspended in 8 mL of deionized water.
The resorcinol-formaldehyde (RF) polymer layer was established via a sol-gel process as reported previously[53]. To establish a 20 nm RF layer on the Au NS-decorated FeOOH NRs, 2 mL of the Au NS-decorated FeOOH NRs were re-dispersed in 10 mL of deionized water, followed by adding 11 mg of resorcinol (Sigma Aldrich) and 16 μL of formaldehyde solution (Sigma Aldrich). The thickness of the RF layer is tunable by adjusting the amounts of resorcinol and formaldehyde solution, where 6 mg of resorcinol and 10 μL of formaldehyde solution produce a 10-nm-thick RF layer, and 28 mg of resorcinol and 40 μL of formaldehyde solution produce a 40-nm-thick RF layer. The solution was magnetically stirred for 10 min and heated up to 50 °C. The solution was kept for 1 h at 50 °C and then mixed with 50 μL of 30 wt% ammonium hydroxide solution (Sigma Aldrich). The solution was subsequently heated up to 80 °C and kept for 3 h under vigorous stirring. The RF layer-coated NRs were washed with deionized water via centrifugation three times and suspended in 10 mL of deionized water.
To selectively remove the FeOOH NRs inside the RF layer, 6 mL of an oxalic acid (Sigma Aldrich) aqueous solution (33.3 mg mL−1) was mixed with 4 mL of Au NS-decorated FeOOH NRs within the RF layer. The solution was placed for 12 h at 80 °C to facilitate the etching process. The obtained Au NSs inside the RF layer were washed with deionized water via centrifugation three times and suspended in 8 mL of deionized water.
To fabricate Au NAs with tunable inter-particle connectivity from no connectivity to semi-connectivity, we carried out seeded growth of Au NSs in the RF polymer space. We initially prepared 2 mL of 100 mM hexadecyl methylammonium chloride (CTAC, TCI) aqueous solution. To this solution, we added different volumes of 5 mM HAuCl4·3H2O aqueous solution to modulate the Au NS diameter. Specifically, 100 μL, 200 μL, and 400 μL of 5 mM HAuCl4·3H2O solution was added to the CTAC solution for 4.6 nm, 6.8 nm, 8.4 nm, and 10.2 nm Au NSs, respectively. The solution was magnetically stirred for 3 min, followed by adding 400 μL of 100 mM L-ascorbic acid (Sigma Aldrich) aqueous solution under magnetic stirring. To this solution, we added 200 μL of the prepared Au NSs inside the RF layer. The reaction was kept for 1 h to induce uniform Au growth. Finally, the obtained Au NAs were washed with deionized water via centrifugation (6,000 rpm, 10 min) three times and subsequently dispersed in 400 μL of deionized water. To establish full connectivity between neighboring Au NSs within a single Au NA, we initially prepared 4 mL of 1 wt% polyvinylpyrrolidone (Sigma Aldrich, molecular weight 55,000) aqueous solution. To this solution, we added 0.6 mL of 5 mM HAuCl4·3H2O aqueous solution and 0.6 mL of 20 mM KI aqueous solution. The solution was magnetically stirred for 3 min, followed by injecting 400 μL of 100 mM L-ascorbic acid aqueous solution under magnetic stirring. To this solution, we added 200 μL of the as-prepared Au NSs inside the RF layer. The reaction was kept for 30 min to induce the Au growth. Finally, the obtained Au NAs with full connectivity were washed with deionized water via centrifugation (6,000 rpm, 10 min) three times and subsequently dispersed in 600 μL of deionized water. Note that Au ions in the seed-mediated growth process could be almost reduced into Au atoms due to the excessive amount of reducing agents used for the seed-mediated growth compared to the added Au precursors[40].
Characterization of Au NAs.
Synthesis process was confirmed by TEM (HT7700, Hitachi). The optical absorption properties of Au NAs with tunable inter-particle connectivity of Au NSs and RF thicknesses were characterized by using a UV-vis spectrophotometer (Evolution 220, Thermoscientific). The ligand exchange from CTAC to PAA on Au NAs was confirmed by measuring zeta potential of Au NAs before and after the ligand exchange process using a dynamic light scattering instrument (Zetasizer Nano ZS, Malvern Instruments Ltd.).
Photoacoustic measurements of Au NAs.
To characterize PA signals from Au NAs with different inter-particle connectivity, we used polyethylene tubes (BD Intramedic). Each tube was filled with 60 μl of the Au NA solutions (15 μg) and placed horizontally for US/PA imaging (Vevo2100/LAZR imaging system, FujiFilm VisualSonics Inc.). To compare PA signal generation between Au NA-3 conventional plasmonic contrast agents, such as Au NRs and Au nanostars, we conducted measurements of PA signal amplitude for each type of Au NPs at their corresponding maximum absorption wavelengths, while all other experimental conditions were held constant: optical density (OD=3), laser fluence (10.5 mJ cm−2), the surrounding medium (water), and the volume of NP solutions (60 μl). The synthesis of Au NRs and Au nanostars followed previously reported seed-mediated growth methods[16,54]. US/PA images were obtained by utilizing an LZ250 ultrasound transducer. The laser source was a Q-switched Nd:YAG-pumped optical parametric oscillator (OPO) laser (pulse duration = 7 ns; frame rate = 20 Hz). For PA imaging, the tubes were lased in the spectral range from 700 nm to 900 nm with 2 nm intervals and a persistence of 3. We selected the PA gain and B-mode gain as 40 dB and 18 dB, respectively. 3D US/PA imaging of Au NAs with different inter-particle connectivity was carried out using a 3D translation motor, followed by 3D image reconstruction using the Vevo LAB 5.7.0 software.
Measurements of photostability of Au NAs.
To assess the photostability of Au NAs, their PA signals were measured using an experimental set-up consisting of the Vevo2100 imaging system and a nanosecond Nd:YAG laser (OPOTek). A polyethylene tube containing a suspension of Au NAs was positioned horizontally and perpendicular to the US/PA imaging plane. The tube containing Au NAs was irradiated using a collimated beam of uniform fluence. Orientation of the beam’s propagation axis was orthogonal to both the tube axis and the depth dimension of the PA imaging plane (i.e., the direction of the beam was along the lateral direction in the US/PA imaging plane). Laser fluence, controlled by Q-switch delay timing and neutral density filters, was verified using an energy meter. All PA images were collected using 800 nm wavelength. Datasets were collected at several different levels of optical fluence. Each dataset consisted of a set of PA images collected with the laser running at 10 Hz PRF. For each dataset, PA signal amplitude for all frames was normalized relative to the dataset`s first PA frame.
Cell culture.
HeLa cells were cultured in Dulecco’s Modified Eagle’s Medium (DMEM, Corning) containing 10% fetal bovine serum (FBS, corning), and 1% penicillin-streptomycin. Cells were incubated at 37 °C in a 5% CO2 humidified incubator.
In vitro cytotoxicity test of Au NAs.
HeLa cells were plated in a 96-well plate at a density of 8,000 cells per well and incubated at 37 °C in a 5% CO2 humidified incubator. The next day, the PAA-capped Au NAs were injected into each well at the final mass concentration of 0 μg mL−1, 25 μg mL−1, 50 μg mL−1, 100 μg mL−1, and 200 μg mL−1, respectively. The PAA-coated Au NAs were incubated with HeLa cells for 24 h, followed by washing with 1x phosphate-buffered saline (PBS) twice to remove the free Au NAs out before the cell viability test. Culture medium containing 3-(4,5-Dimethylthiazol-2yl)-2,5-Diphenyltetrazolium Bromide (MTT, 0.5 mg mL−1) was added into each well, followed by incubation for 4 h at 37 °C in a 5% CO2 humidified incubator. The solution was carefully extracted not to remove the precipitated formazan salt on the bottom of the well. The formazan salt in each well was dissolved in 200 μL of dimethyl sulfoxide (DMSO, Sigma Aldrich). To verify the cytotoxicity of Au NAs, the absorbance at 590 nm was subsequently measured by using a multi-well plate reader (Synergy HT, BioTek). Readings were normalized to the 0 μg mL−1 group.
PA imaging of Au NA-labeled cancer cell.
HeLa cells were plated in a 6-well plate at a density of 100,000 cells per well and incubated at 37 °C in a 5% CO2 humidified incubator. The next day, the PAA-coated Au NAs were added to each well at the final mass concentration of 0 μg mL−1 (control group) and 50 μg mL−1, respectively, for obtaining unlabeled cells and labeled cells. The PAA-coated Au NAs were then incubated with HeLa cells for 24 h, followed by washing with PBS three times to remove the free Au NAs. The unlabeled and labeled HeLa cells were then collected, washed with PBS twice, and then dispersed in PBS with the final concentration of 1,300 cells μL−1. For the gelatin-based dome phantom filled with cells, a 16% gelatin solution was prepared and heated it up to approximately 80 °C. Next, the heated gelatin solution was mixed with the cells with a volume ratio of 1:1 (20 μL of the unlabeled or labeled cells and 20 μL of the gelatin solution). The mixture was carefully pipetted onto the phantom base to establish a dome shape. The final concentration of cells in the dome was 650 cells μL−1. In this step, we prepared two domes with an inter-dome spacing of about 8 mm to visualize the difference of their PA signal amplitude directly; one is for the unlabeled cells, and another is for the labeled cells. Before imaging, the created domes were stored at 4 °C before imaging them.
To characterize the PA signals from the cells, the phantom base with the domes was imaged using the Vevo2100/LAZR imaging system. US/PA images were acquired by using an LZ250 ultrasound transducer. For PA imaging, the domes (filled with unlabeled and labeled cells, respectively) were imaged in the spectral range from 700 nm to 900 nm with 3 persistence and 2 nm intervals. We selected the PA gain and B-mode gain as 40 dB and 18 dB, respectively (same as the PA measurements of Au NAs). The PA image merged with the US image was acquired at 800 nm, followed by image reconstruction using the Vevo LAB 5.7.0 software.
In vivo PA imaging by using Au NAs with different inter-particle connectivity and conventional Au NRs.
All in vivo experiments were carried out according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Georgia Institute of Technology (Protocol number A100281). Au NAs or conventional Au NRs (approximately 80 μg in 0.1 mL of PBS) were mixed with 0.1 mL of Matrigel. The NP solution containing 50% of Matrigel (50 μL) was transplanted in mice (6–8 weeks old, female, Balb/c) via subcutaneous injection. The mice were anesthetized and placed on a heated translation motor stage in the Vevo2100/LAZR imaging system. US/PA images were acquired by using an LZ250 ultrasound transducer. For PA imaging, the Au NPs embedded within Matrigel were imaged in the spectral range from 700 nm to 900 nm in 2 nm increments at a persistence of 3. We selected the PA gain and B-mode gain as 40 dB and 18 dB, respectively (same as the in vitro PA measurements of Au NAs within dome phantoms). The PA image merged with the US image was acquired at 800 nm with a laser fluence of 10.5 mJ cm−2, followed by image reconstruction using the Vevo LAB 5.7.0 software.
Statistical Analysis.
All imaging experiments reported in this manuscript were independently repeated three times. The statistical treatment used in the cell viability test was a multiple group comparison through one-way analysis of variance along with Turkey`s post hoc test using GraphPad Prism software (ver. 8.0.2.). N.S. indicates no significant differences.
Supplementary Material
Acknowledgements
This work was supported in part by the National Institute of Health (NIH) under grants R01NS117613 and R01EY030071 as well as the Breast Cancer Research Foundation Grant, BCRF-22-043. J.K. acknowledges K99CA263016 Career Development Award from the NIH.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Myeongsoo Kim, Petit Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, 30332, US; Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University School of Medicine, Atlanta, GA 30332, USA.
Jinhwan Kim, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University School of Medicine, Atlanta, GA 30332, USA; School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA, 30332, USA.
Don VanderLaan, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University School of Medicine, Atlanta, GA 30332, USA; School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA, 30332, USA.
Kelsey P. Kubelick, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University School of Medicine, Atlanta, GA 30332, USA School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA, 30332, USA.
Anamik Jhunjhunwala, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University School of Medicine, Atlanta, GA 30332, USA.
Ayoung Choe, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University School of Medicine, Atlanta, GA 30332, USA; School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA, 30332, USA.
Stanislav Y. Emelianov, Petit Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, 30332, US Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University School of Medicine, Atlanta, GA 30332, USA; School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA, 30332, USA.
Data Availability Statement
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
- [1].Sigrist MW, J. Appl. Phys 1986, 60, R83. [Google Scholar]
- [2].Xu M, Wang LV, Rev. Sci. Instrum 2006, 77, 041101. [Google Scholar]
- [3].Cox BT, Beard PC, J. Acoust. Soc. Am 2005, 117, 3616. [DOI] [PubMed] [Google Scholar]
- [4].Chen Y-S, Frey W, Aglyamov S, Emelianov S, Small 2012, 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Emelianov SY, Li P-C, O’Donnell M, Physics Today 2009, 62, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Donnelly EM, Kubelick KP, Dumani DS, Emelianov SY, Nano Lett 2018, 18, 6625. [DOI] [PubMed] [Google Scholar]
- [7].Luke GP, Bashyam A, Homan KA, Makhija S, Chen Y-S, Emelianov SY, Nanotechnology 2013, 24, 455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen Y-S, Zhao Y, Yoon SJ, Gambhir SS, Emelianov S, Nat. Nanotechnol 2019, 14, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Luke GP, Yeager D, Emelianov SY, Ann. Biomed. Eng 2012, 40, 422. [DOI] [PubMed] [Google Scholar]
- [10].Pu K, Shuhendler AJ, Jokerst JV, Mei J, Gambhir SS, Bao Z, Rao J, Nat. Nanotechnol 2014, 9, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mantri Y, Jokerst JV, ACS Nano 2020, 14, 9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Repenko T, Rix A, Nedilko A, Rose J, Hermann A, Vinokur R, Moli S, Cao-Milàn R, Mayer M, Von Plessen G, Fery A, De Laporte L, Lederle W, Chigrin DN, Kuehne AJC, Adv. Funct. Mater 2018, 28, 1705607. [Google Scholar]
- [13].Han S, Zal T, Sokolov KV, ACS Nano 2021, 15, 9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim M, Lee J, Nam J, Adv. Sci 2019, 6, 1900471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Knights OB, Ye S, Ingram N, Freear S, McLaughlan JR, Nanoscale Adv 2019, 1, 1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ye X, Gao Y, Chen J, Reifsnyder DC, Zheng C, Murray CB, Nano Lett 2013, 13, 2163. [DOI] [PubMed] [Google Scholar]
- [17].Li W, Brown PK, Wang LV, Xia Y, Contrast Media Mol. Imaging 2011, 6, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Snider EJ, Kubelick KP, Tweed K, Kim RK, Li Y, Gao K, Read AT, Emelianov S, Ethier CR, Sci. Rep 2018, 8, 12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Taylor AB, Siddiquee AM, Chon JWM, ACS Nano 2014, 8, 12071. [DOI] [PubMed] [Google Scholar]
- [20].Attia YA, Altalhi TA, Gobouri AA, Advanced in Nanoparticles 2015, 04, 85–97. [Google Scholar]
- [21].Zijlstra P, Chon JWM, Gu M, Phys. Chem. Chem. Phys 2009, 11, 5915. [DOI] [PubMed] [Google Scholar]
- [22].Chen Y-S, Frey W, Kim S, Homan K, Kruizinga P, Sokolov K, Emelianov S, Opt. Express 2010, 18, 8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chang S-S, Shih C-W, Chen C-D, Lai W-C, Wang CRC, Langmuir 1999, 15, 701. [Google Scholar]
- [24].Jain PK, El-Sayed MA, Chem. Phys. Lett 2010, 487, 153. [Google Scholar]
- [25].Ghosh SK, Pal T, Chem. Rev 2007, 107, 4797. [DOI] [PubMed] [Google Scholar]
- [26].Su K-H, Wei Q-H, Zhang X, Mock JJ, Smith DR, Schultz S, Nano Lett 2003, 3, 1087. [Google Scholar]
- [27].Nam SY, Ricles LM, Suggs LJ, Emelianov SY, Opt. Lett 2012, 37, 4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Medveď I, Černý R, Micropor. Mesopos. Mater 2011, 142, 405. [Google Scholar]
- [29].Shen L, Chen Z, Chem. Eng. Sci 2007, 62, 3748. [Google Scholar]
- [30].Nguyen VP, Qian W, Li Y, Liu B, Aaberg M, Henry J, Zhang W, Wang X, Paulus YM, Nat. Commun 2021, 12, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim J, Yu AM, Kubelick KP, Emelianov SY, Photoacoustics 2022, 25, 100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen Y-S, Yoon SJ, Frey W, Dockery M, Emelianov S, Nat. Commun 2017, 8, 15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cheng X, Sun R, Yin L, Chai Z, Shi H, Gao M, Adv. Mater 2017, 29, 1604894. [DOI] [PubMed] [Google Scholar]
- [34].Zhou C, Zhang L, Sun T, Zhang Y, Liu Y, Gong M, Xu Z, Du M, Liu Y, Liu G, Zhang D, Adv. Mater 2021, 33, 2006532. [DOI] [PubMed] [Google Scholar]
- [35].ANSI, American National Standard for Safe Use of Lasers; ANSI Z136.1–2014, 2014. [Google Scholar]
- [36].Khlebtsov B, Zharov V, Melnikov A, Tuchin V, Khlebtsov N, Nanotechnology 2006, 17, 5167. [Google Scholar]
- [37].Baffou G, Quidant R, García De Abajo FJ, ACS Nano 2010, 4, 709. [DOI] [PubMed] [Google Scholar]
- [38].Reichhelm A, Haubold D, Eychmüller A, Adv. Funct. Mater 2017, 27, 1700361. [Google Scholar]
- [39].Pfeiffer C, Rehbock C, Hühn D, Carrillo-Carrion C, De Aberasturi DJ, Merk V, Barcikowski S, Parak WJ, J. R. Soc. Interface 2014, 11, 20130931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yoon S, Kim C, Lee B, Lee JH, Nanoscale Adv 2019, 1, 2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen L, Ji F, Xu Y, He L, Mi Y, Bao F, Sun B, Zhang X, Zhang Q, Nano Lett 2014, 14, 7201. [DOI] [PubMed] [Google Scholar]
- [42].Schmuck M, Bazant MZ, SIAM J Appl. Math 2015, 75, 1369. [Google Scholar]
- [43].McDowell-Boyer L, Hunt J, Sitar N, Water Resour. Res 1986, 22, 1901. [Google Scholar]
- [44].Zheng Y, Zhong X, Li Z, Xia Y, Part. Part. Syst 2014, 31, 266. [Google Scholar]
- [45].Fales AM, Vogt WC, Pfefer TJ, Ilev IK, Sci. Rep 2017, 7, 15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Khanadeev VA, Kushneruk SA, Simonenko AV, Akchurin GG, Akchurin GG, Tuchin VV, Khlebtsov NG, In Saratov Fall Meeting 2019: Laser Physics, Photonic Technologies, and Molecular Modeling (Ed.: Derbov VL), SPIE, Saratov, Russian Federation, 2020, p. 21. [Google Scholar]
- [47].Zhou S, Huo D, Goines S, Yang T-H, Lyu Z, Zhao M, Gilroy KD, Wu Y, Hood ZD, Xie M, Xia Y, J. Am. Chem. Soc 2018, 140, 11898. [DOI] [PubMed] [Google Scholar]
- [48].Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, Adv. Drug Deliv. Rev 2016, 99, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Guerrini L, Alvarez-Puebla R, Pazos-Perez N, Materials 2018, 11, 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yu JH, Jeong MS, Cruz EO, Alam IS, Tumbale SK, Zlitni A, Lee SY, Park YI, Ferrara K, Kwon S-H, Gambhir SS, Rao J, ACS Nano 2023, 17, 2554. [DOI] [PubMed] [Google Scholar]
- [51].Piao Y, Kim J, Na HB, Kim D, Baek JS, Ko MK, Lee JH, Shokouhimehr M, Hyeon T, Nat. Mater 2008, 7, 242. [DOI] [PubMed] [Google Scholar]
- [52].Kim J, Seo D, Lee J, Southard KM, Lim Y, Kim D, Gartner ZJ, Jun Y, Cheon J, Nat. Protoc 2017, 12, 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li N, Zhang Q, Liu J, Joo J, Lee A, Gan Y, Yin Y, Chem. Commun 2013, 49, 5135. [DOI] [PubMed] [Google Scholar]
- [54].Yuan H, Khoury CG, Hwang H, Wilson CM, Grant GA, Vo-Dinh T, Nanotechnology 2012, 23, 075102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.


