Abstract
Background:
The need for non-invasive methods in treatment of cutaneous disease has continued to evolve exponentially. Amidst the search for technologies, radiofrequency (RF) has proven efficacious in numerous skin disease processes. Though RF is well known for its cosmetic utility, its mechanism is valued in the treatment of many non-cosmetic cutaneous conditions of various etiologies.
Objective:
To identify and describe studies in which RF was used to treat non-cosmetic skin conditions and to explore the potential of this modality for further application in dermatologic diseases.
Methods & Materials:
The PubMed database was utilized to find relevant articles.
Results:
This search strategy yielded 53 articles that met the eligibility criteria. Non-cosmetic indications discussed in these articles include varicose veins (n=10,550), lymphangioma circumscriptum (n=72), cutaneous neoplasms (n=42), cutaneous leishmaniasis (n=743), acne and acne scarring (n=158), non-acne scarring (n=43), primary axillary hyperhidrosis (n=76), and acute and chronic wounds (n=94).
Conclusion:
Treatment with RF is an effective, generally non-invasive modality with a relatively short post-procedure recovery time and little potential for severe adverse effects in the treatment of several cutaneous conditions. Further clinical studies would prove useful to assess the efficacy and cost-effectiveness of this treatment.
Introduction
Radiofrequency (RF) is a relatively non-invasive method of targeted tissue destruction and rejuvenation that was introduced to the field of dermatologic electrosurgery in 1950.1 Since then it has continued to set the standard for tissue remodeling with minimal damage to surrounding healthy tissue.
The technology delivers an electrical energy derived from collisions of charged molecules that heat water in-situ. As the heat travels to predetermined tissue depths it meets resistance at all levels, particularly in adipose tissue. As heat resistance builds thermal cellular damage is sustained. An interplay of inherent electrical tissue properties, penetration depth, and frequency are individualized to match each patients’ needs. The resultant cellular damage initiates neocollagenesis and remodeling of existing collagen and elastin in a discretely demarcated zone of tissue necrosis.2,3 Heated fibroblasts upregulate cytokines, heat shock proteins, and growth factors to promote collagen formation and targeted transient inflammation that remodels collagen and elastin with the deposition of de novo hyaluronic acid. This remodeling results in a thickened subcutaneous tissue layer while avoiding necrosis, fibrosis, and damage to vasculature and adnexal structures.4 Over the course of weeks to months, these changes provide both cosmetic and clinically desired tissue remodeling.5,6
The device technology contains both cutting and coagulation abilities and is approved for percutaneous, laparoscopic, and intraoperative coagulation and ablation of soft tissue.3 Although all RF technologies rely on the same fundamental principles of intra-tissue heating, three main types, monopolar, bipolar and unipolar, allow for flexible application. Monopolar RF requires a grounding electrode in contact with the patient’s skin, bipolar RF channels energy between a positive and a negative pole usually housed within the same probe, while unipolar RF uses only one electrode.1,3 Monopolar RF provides the deepest penetration potential (up to 20mm); however bipolar RF, usually reaching 1–4 mm depending on the distance between electrodes, can be easily combined with other device technologies. Combination devices commonly include fractionation, a diode laser, or multiple sets of electrodes that reach varying depths simultaneously, to match the efficacy of monopolar RF. Unipolar RF’s utility lies in its ability to reach large areas of subcutaneous tissue typically15–20mm below the skin surface.1
Another differentiation of RF treatments is whether it is continuous or pulsed. Continuous RF is delivered with probe temperatures in an uninterrupted fashion to achieve target tissue destruction. Whereas pulse RF delivers current in 20 ms bursts to keep tissues below 42°C. Neurophysiologic studies show that pulsed RF can relieve pain by altering the transmission of neuronal electrical pain signals without heat-induced tissue destruction. As a result, pulsed RF has become a preferred therapeutic option used for several chronic pain conditions, such as cervical radiculopathy.7
The purpose of this review is to identify and describe studies in which RF is used to treat non-cosmetic skin conditions and to explore the potential of this modality for further application in dermatologic diseases. The authors have defined non-cosmetic dermatologic conditions as clinically apparent primary or secondary cutaneous abnormalities that are either presently symptomatic or have the potential to be symptomatic. Treatment of the sequalae of such conditions is also considered non-cosmetic as the purpose of treatment is to return to the pre-disease, or pre-injury, state of the skin. To that effect, Food and Drug Administration (FDA) approved non-cosmetic dermatologic indications include cutaneous leishmaniasis, acne, acne scarring, varicose veins, non-acne scarring, primary axillary hyperhidrosis, benign cutaneous neoplasms, lymphangioma circumscriptum (LC), and wound healing. Indications that have been excluded on a cosmetic basis are cellulite, skin laxity, wrinkles, rosacea, pigmented lesions, and telangiectasias as these types of effected skin occur over time with natural exposures rather than finite periods of endogenous or exogenous injury.
Methods
A systematic review was conducted following the PRISMA guidelines. A search for peer reviewed articles was performed in August 2018 on the PubMed database using search terms “radiofrequency ablation” or “radio-frequency” or “RF” and “skin” or “dermatology” entered in sperate pairs. The resulting documents were screened for eligibility based on title, abstract, and full-text as necessary. Review of relevant article bibliographies was also conducted. Articles that examined human subjects, reported clinical outcomes of RF use for skin conditions, and were written in English language were included. Articles that justified their research on cosmetic basis alone, did not report clinical outcomes of RF treatment, reported on animal studies, or were written in languages other than English were excluded.
Results
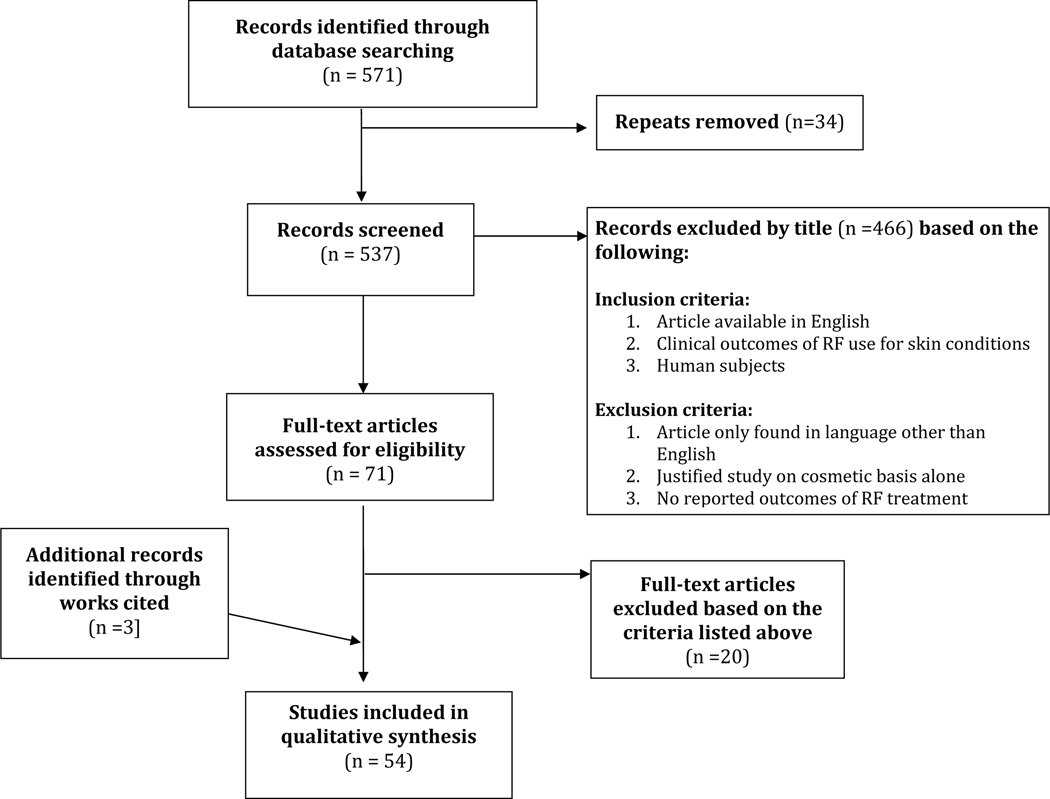
A total of 571 articles published from 1971 until present were retrieved using the search methods described above. A title and abstract screen resulted in 71 articles, with a subsequent full text screen resulting in 53 articles that met the eligibility criteria. These search results are illustrated in the PRISMA diagram in Figure 1.
Figure 1.
Summary of article selection for clinically relevant reports of radiofrequency treatment for non-cosmetic dermatologic conditions
Reviewed studies consist primarily of case series and reports, but also include randomized control trials (RCT) and non-randomized prospective trials. Total number of reports and subjects involved in relevant indications are as follows: four reports on RF use in acne and acne scarring (n=158), four reports on axillary hyperhidrosis (n=76), 13 reports on varicose veins (n=10,550), four reports on wound healing (n=94), five reports on benign neoplasms or cutaneous lesions (n=42), 10 reports of lymphangioma circumscriptum (n=72), five reports on cutaneous leishmaniasis (n=743), and six reports on non-acne scars (n=43). Study design, levels of evidence, and outcome data were extracted for each article and are summarized in Tables 1–8, while significant findings from indication category are summarized below.
Table 1.
Reports of radiofrequency treatment of acne and acne scarring
| Author Publication Year | Evidence | Study Design (Sample Size) | Condition | Intervention | Number of Treatments/Intervals | Outcome Measures | Clinical Outcomes | Patient Reported Outcomes | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Lan et al. 2018 (11) | 3 | Prospective nonrandomized (n = 86) | Acne scarring | Micro-plasma radiofrequency | 1 session q2 months x3 | Echelle d’Evaluation Clinique des Cicatrices d’Acne (ECCA grading scale) |
Avg ECCA score at 6 months: 107.2 at baseline, decreased to 42.27 (p<0.05) |
Patient satisfaction: 100% were “satisfied” or “very satisfied” with treatment at 6 months |
Transient pain, erythema, edema, effusion, and scabs in all pts |
| Min et al. 2015 (9) | 2 | Prospective single-blind randomized split-face trial (n = 20) | Acne and acne scarring | Bipolar radiofrequency (BR) Fractional microneedling radiofrequency (FMR) |
2 sessions q4 weeks x3 | Inflammatory lesion count |
Lesion count
FMR Baseline 100 3 months 19.6 (p<0.001) BR Baseline: 100 3 months: 84.5 (p>0.001) |
Patient satisfaction: BR > FMR on day 1 FMR > BR on day 84 |
Mild pain, erythema, and edema FMR > BR (% not reported) |
| Prieto et al. 2005 (10) | 4 | Case Series (n = 32) | Acne | RF and pulsed light combination | 2 sessions q-week x4 | Lesion count Histology Patient-reported improvement |
Avg reduction in lesion count: 47% (p<0.05) Perifollicular lymphocytic infiltrates: Baseline: 58% 1-month: 33% Avg area of sebaceous glands: Baseline: 0.092 mm2 1-month: 0.070 mm2 |
Patient-reported improvements: Excellent 4.5% Good 59% Very good 32% None to mild 4.5% |
Mild erythema 84% 1° facial burns 12% |
| Shin et al. 2012 (8) | 2 | Prospective single-blind randomized split-face trial (n = 20) | Acne | FMR Fractional carbon dioxide laser therapy (CO2 FS) |
1 – 2 sessions | Global Improvement Scale (GIS) (0 = worsening; 4 = 76 – 100% improvement) Inflammatory lesion count |
GIS at 3 months: FMR: 2.3 CO2 FS: 1.9 (p>0.05) Lesion count 3 months: FMR: 36% reduction CO2 FS: 41% reduction (p>0.005) |
Patient Satisfaction at 3 months: Very satisfied 15% Satisfied 55% Slightly satisfied 10% Unsatisfied 5% |
Erythema FMR: 2.35 ± 1.04 CO2 FS: 1.75 ± 12.67 (p<0.001) Transient hyperpigmentation in 2 CO2 FS pts |
Table 8.
Reports of radiofrequency treatment of varicose veins
| Author Publication Year | Evidence | Study Design (Sample Size) | Condition | Intervention | Number of Treatments/Intervals | Outcome Measures | Clinical Outcomes | Patient Reported Outcomes | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Dunn et al. 2006 (45) | 2 | Prospective nonrandomized (n = 68; 85 extremities) | Incompetent GSV | Endovenous radiofrequency ablation (ERA) w/tumescent anesthesia and sedation | 1 session | Duplex ultrasound (DUS) Occlusion |
Occlusion rate: 96% at 3 days 90% at 6 months Overall success rate: 88% at 6 months |
Not reported | At 3 days (n=83): Ecchymosis 13% Erythema 13% Hematoma 2% Hyperpigmentation 1 pt At 6 months (n=73): Hyperpigmentation 3% Paresthesia 4% |
| Garcia-Madrid et al. 2013 (46) | 3 | Prospective nonrandomized (n = 59; 67 extremities) | Incompetent GSV | ERA w/ tumescent anesthesia | 1 session | DUS Occlusion | Occlusion rate: 100% | Not reported | Erythema 1 pt Class 1 heat-induced thrombosis 1 pt |
| Gibson et al. 2017 (47) | 1 | Randomized control trial (n = 222) | Incompetent GSV | Cyanoacrylate closure (CAC) w/o anesthesia Radiofrequency ablation (RFA) w/ tumescent anesthesia |
1 session | DUS Occlusion Symptoms |
Occlusion rate
At 1 year: RFA 97.0% CAC 97.2% At 2 years: RFA 94.0% CAC 95.3% |
Patient satisfaction at 2 years: RFA 75% CAC 79.1% |
At 12–24 months: Pain (RFA 21%; CAC 30%) Aching (RFA 34%; CAC 29%) Heaviness (RFA 14%; CAC 13%) Swelling (RFA 16%; CAC 16%) |
| Mallick et al. 2016 (48) | 4 | Retrospective cohort (RF n = 7,355; total n = 144,098) | Incompetent GSV | RFA Sclerotherpy Laser ablation |
1 session | Rates of new venous ulcers Rates of additional interventional treatment Disease progression |
New venous ulcers at 1 year:
RF 2.4% Sclerotherapy 1.2% Laser ablation 2.2% Additional treatment sought at 1 year: RF 53.3% Sclerotherapy 63.6% Laser ablation 66.8% Disease progression at 2 years: RF 42.4% Sclerotherapy 28.6% Laser ablation 37.8% |
None collected | Not reported |
| Mendes de Almeida et al. 2016 (49) | 1 | Randomized, controlled trial (n = 18) | Incompetent GSV | ERA in one leg and conventional surgery in the other; both w/ spinal anesthesia | 1 session | DUS occlusion Reflux sapheno-femoral junction (SFJ) and GSV |
DUS Occlusion: 8/10 (80%) at 12 months Reflux SFJ: 0/10 (0%) at 12 months Reflux GSV: 0/15 (0%) at 12 months |
Patient satisfaction at 6 months (1–10): CS 6.93±2.70 RFA 6.86±2.71 Pain at 6 months (1–10): CS 5.64±3.80 RFA 3.71±3.27 |
Not reported |
| Proebstle et al. 2011 and 2015 (57–58) | 3 | Prospective nonrandomized (n = 225; 295 extremities) |
Incompetent great saphenous vein (GSV) | Radiofrequency segmental thermal ablation (RSTA) |
1 session | DUS Occlusion Venous Clinical Severity Score (VCSS) Pain (0–10) |
DUS Occlusion: 92.6% at 3 years 91.9 % 5 years Avg VCSS: Baseline: 3.9 ±2.1 3 years: 0.9 (p=0.05) 5 years: 1.3 Pain: Baseline 17.3% w/ daily pain 3 years: 1.4% w/ daily pain 5 years: 2% w/ daily pain |
None collected | 1 Week: Ecchymosis 5.8% Paresthesia 3.4% Pigmentation 2.4% Erythema 2% 3 years: Leg hyperpigmentation 1 pt Persisting paresthesia 1 pt 5 years: Not reported |
| Schuller-Petrovic et al. 2016 (50) | 4 | Retrospective cohort (n = 258; 389 extremities) | Incompetent GSV and superficial saphenous vein (SSV) | RSTA | 1 session | DUS Occlusion |
DUS at 5 years: 78.5% GSV 58% SSV |
Not Reported | Hypesthesia 1% Hyperesthesia 2% Ecchymoses 4% Phlebitis 4% Deep vein thrombosis 6% |
| Spiliopoulos et al. 2015 (51) | 4 | Prospective case series (n = 60; 74 extremities) | Incompetent GSV and SSV | ERA w/ tumescent anesthesia | 1 session | CEAP Classification (C0 clinical best - C6 clinical worst) VCSS Revascularization rate |
Clinical success: 94.6% improvement Baseline: 72.9% C2-C3 At 1 year: 12.1% C2-C3 VCSS: baseline: 6.2±2.6 1 month: 1.3±1.2 1 year: 0.9±1.4 P<0.0001 Revascularization rate 12.1% |
Not reported | Puncture site infection 1 pt Transient paresthesia 2 pts Puncture site scar 2 pts Skin pigmentation 1 pt |
| Tolva et al. 2012 (52) | 4 | Case series (n = 407) | Incompetence GSV | ERA w/ spinal anesthesia | 1 session | DUS Occlusion |
Occlusion rate: 100% at 1 week and 6 month follow ups |
Not reported | Thrombophlebitis 3 pt Skin pigmentation 1 pt Paresthesia 1 pt Nonocclusive thrombus 1 pt |
| Tzilinis et al. 2005 (53) | 4 | (n = 421; 490 extremities) | Incompetent GSV and SSV | ERA w/ regional or general anesthesia or tumescent anesthesia | 1 session | DUS Occlusion |
Occlusion rate (1 year): 87% |
Not reported | Not reported, except no hospitalizations for procedure-related complications |
| Vasquez et al. 2007 (54) | 3 | Prospective cohort (n = 499; 682 extremities) | Incompetent GSV | ERA w/ tumescent anesthesia | 1 session | VCSS Occlusion rate Symptoms |
VCSS:
Baseline 8.8 At 1 year 3.6 Occlusion rate at 1 year: 87.1% Symptoms: Pain reduced from 95.7% to 15.2% (p < 0.0001) Edema reduced from 92.4% to 17.0% (p < 0.0001) Stasis ulcers healed at rate of 86% |
Patient satisfaction: 98% | Superficial thrombophlebitis (12%) Ecchymosis (13.1%) Erythema (2.5%) Infection (0.5%) Paresthesia (0.3%) |
| Wagner et al. 2004 (55) | 4 | Retrospective cohort (n = 24; 28 extremities) | Incompetent GSV | ERA w/ general anesthesia | 1 session | DUS Occlusion |
Occlusion rate: 100% at 1 year |
Patient satisfaction at 2 years: 94.5% |
Worsening of leg swelling: 1 pt Localized thrombophlebitis: 2 pts. Acute nonocclusive thrombus extending to the common femoral vein from GSV: 1 pt |
| Weiss et al. 2015 (56) | 4 | Retrospective cohort (n = 934 extremities; ERA n = 398) |
Incompetent GSV and SSV | ERA w/ tumescent anesthesia 1,320nm ND:YAG w/ tumescent anesthesia 810nm Diode (n = 34) |
1 session | DUS Occlusion |
Occlusion at 5 years: RFA 61.7% ND:YAG 84.7% Diode laser 65.7% (P<0.0001) |
Not reported | Not reported |
w/ = with; pt = patient; q = every (i.e. q2 weeks = every 2 weeks); x = times to repeat (i.e. x3 = repeat 3 times)
Acne vulgaris and acne scarring
Of the four studies that reported the use of RF as treatment of acne, two studied active acne vulgaris, one studied acne scarring alone, and one studied acne and resultant scarring simultaneously. These reports include two head-to-head split-face clinical trials, comparing RF treatment to fractional carbon dioxide laser therapy8 and fractional microneedling RF.9 Patients received a range of 2–4 four treatment sessions. Varying outcome assessments consisted of acne lesion count, sebum production, pore size, acne scar severity, and patient satisfaction.
RF treatment led to moderate improvements in all acne scarring with superior results in rolling scars compared to boxcar or ice-pick scars. Statistically significant improvements were also noted with decreased pore size, lesion counts, and sebum production.8,9 The majority of subjects (65–100%) expressed satisfaction with treatment tolerability and outcomes and up to 95% reported subjective improvement in their acne.8–11 The split-face study results favored fractional microneedling RF over fractional carbon dioxide laser and bipolar RF for lesion amount, scar size, and sebum reduction.8 Adverse events included minor and self-resolving edema, erythema, scabbing, and hyperpigmentation. Further detail of these findings can be found in Table 1.
Primary Axillary Hyperhidrosis
The four reports on the use of RF for hyperhidrosis include case series, a case report, and a non-randomized control study. Patients received a range of 1–4 treatment sessions with follow up every four weeks for six months. Outcome measures included the hyperhidrosis disease severity index (HDSS), visual analogue score (VAS), dermatology quality of life index (DLQI), starch-iodine sweat testing, transepidermal water loss, sweat gland density, and patient satisfaction.12–15
Radiofrequency improved axillary hyperhidrosis symptoms in most studied patients, with as high as 95% experiencing decreased size of affected area on the starch-iodine test,12 an average of HDSS score decrease from 3.4 to 2.1 in 2–5 months, DLQI improvement, and significant VAS decrease from 9 to 4 at five months post-treatment.12,14 Histological evaluation revealed atrophy and necrosis immediately post-treatment and a decrease in total sweat glands one month post-treatment.12 Patients satisfaction ranged from 50–90%. Reported adverse events included tingling, erythema, hyperpigmentation, swelling, erosions, compensatory hyperhidrosis and numbness; all of which resolved within six-months.12,13 These findings are detailed in Table 2.
Table 2.
Reports of radiofrequency treatment of primary axillary hyperhidrosis
| Author Publication Year | Evidence | Study Design (Sample Size) | Condition | Intervention | Number of Treatments/Intervals | Outcome Measures | Clinical Outcomes | Patient Reported Outcomes | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Fatemi Naeini et al. 2015 (13) | 3 | Single blind sham control (n = 25) | Primary axillary hyperhidrosis | FMR w/ topical anesthesia | 1 session q3 weeks x3 | HDSS Sweating visual analogue scale (VAS) Pre and post histology |
HDSS:
Baseline: treated 3.46; control 3.46 5 months: treated 1.87; control 3.38 (p=<0.001) VAS: Baseline: treated 9; control 9 5 months: treated 3.92; control 8.44 Histology changes: Sweat gland necrosis Mild inflammatory cell infiltrate |
Patient satisfaction: 80% of pts reported more than 50% satisfaction |
Erythema 68% Pinpoint bleeding 56% Hyperpigmentation 44% Tingling 1 pt |
| Fatemi Naeini et al. 2015 (15) | 5 | Case report (n = 1) | Primary axillary hyperhidrosis | FMR | 1 session q2 weeks x4 | Starch-iodine test |
Starch-iodine: Pretreatment positive Post treatment negative |
No hyperhidrosis at 6-months | Skin irritation, erythema, and pin-point bleeding |
| Kim et al. 2013 (12) | 4 | Case series (n = 20) | Primary axillary hyperhidrosis | FMR w/tumescent anesthesia | 1 session q4 weeks x2 | Hyperhidrosis Disease Severity Scale (HDSS): 4-point scale (1–4) Starch-iodine test Histologic analysis |
HDSS: Baseline: 3.3 4 weeks after 1st treatment: 1.5 8 weeks after 2nd treatment: 2.3 (p<0.001) Starch-iodine test: Decreased area in 95% of subjects Histology: Decreased density and size of sweat glands |
Patient satisfaction: (0 “no improvement”- 4 “>75% improvement”): 4 weeks after 1st treatment: 3.3 4 weeks after 2nd treatment: 3.0 8 weeks after 2nd treatment: 2.5 |
Temporary tingling, swelling and erythema in all pts Compensatory hyperhidrosis in 2 pts and transient numbness in 1pt |
| Schick et al. 2016 (14) | 4 | Case series (n = 30) | Grade III axillary hyperhidrosis | FMR w/ local anesthesia | 1 session q6 weeks x3 | HDSS Dermatology Life Quality Index (DLQI) |
HDSS: Baseline: 3.5 5 months 2.1 (p<0.05) DLQI: Baseline: 16 5 months: 7 (p<0.05) |
Patient satisfaction: Satisfied 53% Very satisfied 37% Average reduction 72% |
Petechial bleeding 50% Arm twitching during treatment 27% Erythema 100% Ulceration 7% Post anesthesia pain 70% |
Wound Healing
Four studies describing the efficacy of RF in wound healing show the most potential for RF as an adjunct therapy (Table 3). The various cases demonstrate the utility of treatment with pulsed RF energy (Provant® Regenesis, Scottsdale, AZ) in combination with negative pressure devices, lower limb off-loading, or dermal replacement therapy.5,16–18 These regimens achieved healing in 24 weeks for chronic or surgical diabetic wounds, as well as for venous insufficiency ulcers. Pulsed RF treatments were most effective when applied for 30 seconds twice daily for four weeks or until wounds are healed. All wounds decreased in size at varying rates, one reporting 0.13 cm2 per day.16 Pulsed RF was also shown to reduce chronic wound pain or exacerbated pain with application of compressive therapy.5,7 With decreased pain, patients were able to tolerate other necessary treatments, such as debriding, bandage changing, and compressive wraps.5
Table 3.
Reports of radiofrequency treatment of wounds
| Author Publication Year | Evidence | Study Design (Sample Size) | Condition | Intervention | Number of Treatments/Intervals | Outcome Measures | Clinical Outcomes | Patient Reported Outcomes | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Conner-Kerr and Isenberg et al. 2012 (16) | 4 | Case series (n = 89) | Chronic pressure ulcers (majority on sacrum and ischium) | Pulsed RF Energy (PRFE) | 30-minute treatments BID x 4 weeks | Percent wound surface area reduction (PWAR) Wound healing |
PWAR: 51% median reduction (p<0.0001) Wound healing: Baseline median size 9.8cm2 At 4 weeks median size 4.5cm2 |
Not reported | Not reported |
| Frykberg et al. 2011 (17) | 5 | Case report (n = 1) | Transmetatarsal amputation wound due to necrotizing fasciitis | RRFE w/ negative pressure and dermal replacement | BID x 14 weeks | Wound healing |
Wound healing:
Complete closure at 39 weeks |
Not reported | Fixed equinus deformity |
| Larsen et al. 2008 (18) | 4 | Case series (n = 2) | Case 1: 1.75cm2 diabetic foot ulcer Case 2: 7cm2 transmetatarsal amputation dehiscence |
RRFE w/ Case 1: splint, cane, debridement, silver dressings, petroleum jelly Case 2: silver dressings, debridement, and rook boot. |
Not reported | Wound healing |
Wound healing:
Case 1 and 2 complete closure at 16 weeks; closed at 9 and 7 month follow up, respectively |
Not reported | Not reported |
| Maier et al. 2011 (5) | 4 | Case series (n = 2) | Case 1: Lower extremity ulcers due to thromboses Case 2: Lower extremity ulcer due to scleroderma |
RRFE placed over compression wrapping | 30-minute treatments BID x 2 weeks | Pain level Wound healing |
Pain level:
Both able to tolerate compression dressings again Wound healing: Case 1 complete closure in 3–4 weeks Case 2 80% closure in 11 weeks |
Immediate pain reduction | Not reported |
Cutaneous neoplasms
RF has also proven to be an effective modality for removal of multiple cutaneous neoplasms in disease processes such as neurofibromatosis (Table 4). Reports of patients with Brooke-Spiegler Syndrome, neurofibromatosis I, tuberous sclerosis, and a host of other more common growths, such as seborrheic keratosis, demonstrate a wide application of RF treatment.6,19–23 The primary outcome measures in these studies were decreased lesion numbers, size, and patient satisfaction. All patients received one treatment session only, except a patient with Brooke-Spiegler Syndrome who received 15 treatment sessions and achieved 100% resolution of his cylindromas and 70% resolution of his trichoepitheliomas.20 RF was also successfully used in conjunction with dermabrasion for resection of tuberous sclerosis related angiofibromas in a 30-year-old female. This subject had satisfactory results without scarring or depigmentation.6
Table 4.
Reports of radiofrequency treatment of cutaneous neoplasms
| Author Publication Year | Evidence | Study Design (Sample Size) | Condition | Intervention | Number of Treatments/Intervals | Outcome Measures | Clinical Outcomes | Patient Reported Outcomes | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Chaudhary et al. 2012 (20) | 4 | Case report (n = 1) | Brooke-Spiegler Syndrome | RFA | 15 sessions | Efficacy of treatment VAS Recurrence |
VAS at 6 months: 70% for trichoepitheliomas 100% for cylindromas Recurrence: none at 6 months |
None reported | None reported |
| Gomes et al. 2011 (6) | 4 | Case report (n = 1) | Facial angiofibromas due to tuberous sclerosis | RFA followed by dermal abrasion | 1 session | Recurrence |
Recurrence: Small lesions at 3 months |
Not reported | Pt did not have scarring or pigment changes |
| Imai et al. 2004 (23) | 4 | Case Report (n = 1) | Dermatofibrosarcoma protuberans (DF) (20×10cm) | RFA | 1 session | Lesion size |
Lesion size: Decreased by 50% |
Not reported | Not reported |
| Khunger et al, 2010 (19) | 4 | Case series (n = 3) | Angiolymphoid hyperplasia w/ eosinophilia | RF w/ 3% polidocanol sclerotehrapy | 1 session monthly x1–4 | Lesion size Recurrence |
Lesion size:
Total clearance in all cases Recurrence: None at 6 months or 2–3 years |
Not reported | Not reported |
| Kim et al. 2013 (21) | 4 | Case series (n = 16) | Neurofibromatosis type I | RFA followed by excision w/ general anesthesia | 1 session | Duration of surgical procedure Re-epithelialization time |
Duration of surgical procedure Avg 2 hours Re-epithelialization time: Face: avg 1.5 weeks Trunk/Extremities: avg 2.5 weeks |
Patient satisfaction:
100% |
1 pt with erythema, hyperpigmentation, and scarring |
| Kim et al. 2016 (22) | 4 | Case Series (n = 20) | Telangiectasias (n = 22) Angioma (n = 15) Seborrheic Keratosis (n = 8) Skin Tags (n = 13) Lentigo (n = 1) Neurofibroma (n = 1) Piercing hole (n = 1) Acne (n = 2) Dilate pore (n = 2) Milium (n = 2) |
27.12 MHz RF w/o anesthesia | 1 session | Clinical outcomes: 1) Excellent: Complete resolution 2) Good: > 75% reduction 3) Moderate: >50% reduction 4) Poor: < 50% reduction |
Clinical outcomes: Vascular Lesions: Excellent: 33.3% Good: 44.4% Moderate: 11.1% Poor: 11.1% Non-Vascular Lesions: Excellent: 48.3% Good: 45.2% Poor: 3.2% (lentigo) |
Patient satisfaction:
Vascular lesions: 94.4% very satisfied 5.6% satisfied Nonvascular lesions: 83.9% very satisfied 16.1% satisfied |
Mean pain score during procedure (1–10): Vascular lesions: 3.11 Non-vascular lesions: 3.95 |
RF was shown to be a superior method to surgical excision for cutaneous neurofibromas in 16 neurofibromatosis type I patients as it led to less bleeding and fewer postoperative complications. Except for minimal scarring, all subjects were satisfied with the treatment outcomes.21 One case report demonstrated how RF treatment made surgical removal of a 20cm wide dermatofibroma protuberans possible by reducing its size to 50% pre-operatively.23 Twenty-one additional subjects were treated with RF energy for a variety of telangiectasia, cherry and spider angiomas, acrochordons, seborrheic keratosis, dermatofibrosarcoma protuberans, and milium.22 Among these subjects 78% of vascular and 93% of non-vascular lesions had “excellent” or “good” decrease in lesion size as well as 94% and 84% patient satisfaction, respectively. The only adverse events reported were pain ratings of 3–4 during the procedure.22
Lymphangioma circumscriptum
Ten case series and reports describe the experiences of 72 patients with symptomatic microcytic lymphatic malformation in various cutaneous and oral locations, including the tongue (Table 5). Patients received 1–8 treatments to achieve outcome objectives, which included smaller lesion size, decreased frequency of symptoms, such as bleeding, and infection, as well as lower recurrence rates. All cases reported over 50% and as high as 88% reduction in lesion size with comparable efficacy in cutaneous and mucosal lesions.24–33 Patient satisfaction was high, but appeared to wane with longer follow up of five years, with recurrence of lesions being up to 66% in one study.24 Adverse events included swelling, hypopigmentation, scarring, and intermittent bleeding or infection in the cases of oral LC.
Table 5.
Reports of radiofrequency treatment of lymphangioma circumscriptum (microcytic lymphatic malformation)
| Author Publication Year | Evidence | Study Design (Sample Size) | Condition | Intervention | Number of Treatments/Intervals | Outcome Measures | Clinical Outcomes | Patient Reported Outcomes | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Niti et al. 2010 (24) | 4 | Case series (n = 14) | LC | Group A: RFA w/ sclerotherapy Group B: Sclerotherapy |
Group A: 1– 12 sessions, monthly Group B: 1 treatment |
Lesion surface area Recurrence rate |
Lesion surface area:
RFA: 88.5% reduced in 90% of pts Sclerotherapy: 56% Recurrence rate: RFA at 6–60 months: 60% Sclerotherapy at 2 years: 25% |
Patient-reported efficacy: RFA: Average of 2.2 months for resolution of all symptoms Sclerotherapy: 50% of patients with symptom resolution |
Focal superficial scars and transient hypopigmentation 100% Persistent induration 30% RFA pts Ulceration 40% RFA pts |
| Khurana et al. 2018 (25) | 4 | Case series (n = 1) | LC | RFA w/ bleomycin sclerotherapy and local anesthesia | 1 session monthly x3 | Lesion recurrence |
Lesion recurrence at 6 months: None, except a few vesico-papules near periphery |
Not reported | Hypopigmentation of scar |
| Lapidoth et al. 2006 (26) | 4 | Case series (n = 6) | LC | RF w/ 900nm diode laser and local or general anesthesia | 1 – 3 sessions | Lesion clearance (5-point scale; excellent (75–100%), poor (<25%), worse) |
Lesion clearance at 2 months:
Excellent: 66.6% Good: 33.3% Fair, poor, or worse: 0% |
Not reported | Transient swelling, pain, erythema all pts Ulcers and scarring 1 pt Hypopigmentation 1 pt |
| Hm et al. 2008 (27) | 4 | Case series (n = 2) | LC | RF | 1 session | Lesion remission |
Lesion remission: Pt 1: resolved w/ atrophic scar Pt 2: partial resolution w/ no scarring |
Not reported | Atrophic scarring 1 pt |
| Sachdeva et al. 2011 (28) | 4 | Case report (n = 1) | LC | RF w/ local anesthesia and topical antibiotic | 1 session weekly x3 | Lesion reduction Lesion recurrence |
Lesion reduction at 1 month: Complete resolution Lesion recurrence at 1 year: None |
Not reported | Depigmentation (resolved at 3 months) |
| Subhadarshani et al. 2018 (29) | 4 | Retrospective cohort (n = 9) | LC | RFA w/ local anesthesia (2% lidocaine) | 1– 8 sessions | Global Assessment Scores (GSA) (0–10) |
Physician GSA at 2–36 months: Avg 7.33 (2.49 SD) |
Patient GSA at 2–36 months: Avg 7.55 (2.71 SD) |
Hypertrophic scar at cannula entry 22% Transient edema 100% |
| Oral LC | |||||||||
| Civelek et al. 2012 (30) | 4 | Case report (n = 1) | Lymphangioma circumscriptum (LC) | BR w/ surgical excision | 1 session q4–8 weeks x2 | Lesion size |
Lesion size:
Reduced by 50% |
Patient satisfaction:
Reduction with RFA alone was not satisfactory |
Not reported |
| Grimmer et al. 2006 (31) | 4 | Case report (n = 11) | Oral LC | RF | 1 session | Antibiotics Symptom reduction Postoperative oral intake |
Antibiotics: Reduced Symptom reduction: 54% w/ significant reduction 9% w/ complete resolution Recovery room oral intake: 64% |
Patient satisfaction: 62% of parents reported treatment being better than previous treatments |
Not reported |
| Kim et al. 2011 (32) | 4 | Case series (n = 26) | Oral LC | RFA | 1 – 7 sessions | Symptom control |
Symptom at 3 months: 18/26 pts controlled 13/26 pts w/ resolved symptoms after 1 session |
Patient satisfaction: 21/26 satisfied with outcome of treatment |
Intermittent bleeding 3 pts Persistent infections 1 pt Tongue edema 1 pt |
| Ryu et al. 2008 (33) | 4 | Case report (n = 1) | LC of the tongue | Lower power RFA | 1 session monthly x2 | Symptom control |
Symptom control:
Complete symptomatic relief at 2 months; no recurrence at 1 year |
Patient satisfaction: Complete symptomatic relief at 2 months post treatment |
Pt reported to have none |
Cutaneous Leishmaniasis
Radiofrequency therapy was reported in four RCTs and one case series for the treatment of cutaneous leishmaniasis (Table 6). Subjects received 1–4 treatment sessions and were followed for up to a year post-procedure. Outcome measures included infection resolution, or cure rate, recurrence, and patient satisfaction. Various control groups received a variety of treatments including antimony, placebo,34 intralesional or intramuscular sodium stibogluconate (SSG),35,36 and intralesional meglumine antimoniate.37 Complete clinical response was achieved in 69.4– 98% of the subjects treated with RF.34–36,38 RF was shown to be non-inferior to antimony as 73% of both groups achieved complete clinical response, however, those treated with RF experienced less relapse (6.3% compared to 12.5% of antimony patients). RF was also shown to be comparable to intralesional SSG treatment in 359 subjects who experienced an 83% average cure rate.35,36 Adverse events included secondary infections, keloid formation, and local cellulitis.
Table 6.
Reports of radiofrequency treatment of cutaneous leishmaniasis
| Author Publication Year | Evidence | Study Design (Sample Size) | Condition | Intervention | Number of Treatments/Intervals | Outcome Measures | Clinical Outcomes | Patient Reported Outcomes | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Bumb et al. 2013 (36) | 2 | RTC (n = 100) | Cutaneous Leishmaniasis | Group A: RF w/ 1% lidocaine HCl Group B: Intralesional sodium stibogluconate (ILSSG) |
Group A: 1 session Group B: 1 injection twice weekly x7 |
Cure rate Recurrence |
Cure rate at 6 months:
RF Group: 98% SSG Group: 94% (P > 0.05) Recurrence: At 12 months: 0% At 18 months: 56% RF group; 54% SSG group |
Not reported | Not reported |
| Navin et al. 1990 (34) | 2 | RTC (n = 66; RF n = 22) | Cutaneous Leishmaniasis | Group A: RF Group B: IM injection of pentavalent antimony Group C: Placebo |
Group A: 30 second session q7 days x3 Group B: 30 sec session daily x15 Group C: 30 second session q7 days x3 |
Re-epithelialization Recurrence |
Re-epithelialization at 13 weeks:
RF :73% Antimony: 73% Placebo: 27% Recurrence at 9 months: RF: 6.3% Antimony: 12.5% Placebo: 0% |
Not reported | Antimony: Keloid 1 pt Abnormal amino transferase 1 pt RF: Keloid 2 pts Cellulitis 4 pts |
| Reithinger et al. 2005 (35) | 2 | RTC (n = 259) | Cutaneous Leishmaniasis | 6.78 mHz RF for 30 seconds w/ 1% lidocaine HCl ILSSG Intramuscular SSG (IMSSG) |
1 treatment | Cure rate Median time to cure |
Cure rate:
RF: 69.4% ILSSG: 75.3% IMSSG: 44.8% Median time to cure: RF: 53 days ILSSG: 75 days IMSSG: >100 days |
Not reported | Secondary infection 8 RF pts 5 ILSSG pts 2 IMSSG pts |
| Sadeghian et al. 2006 (37) | 1 | RTC (n = 117) | Cutaneous Leishmaniasis | Group A: RF Group B: Intralesional meglumine antimoniate |
Group A: 1 session weekly x4 Group B: weekly x4 |
Cure rate Scar diameter |
Cure rate at 6 months:
RF: 80.7% Meglumine: 55.3% Mean diameter of scar: RF baseline: 15 mm; 6 months: 14.8 mm (p=0.83) Meglumine baseline: 15.9mm; 6 months: 11mm (p<0.05) |
Not reported | Group A Satellite lesions in 1 case Group B Erythema, edema and pruritus in 4 patients Sporotrichoid lesions in 4 cases Satellite lesions in 3 cases |
| Velasco-Castrejon et al. 1997 (38) | 4 | Case series (n = 201) | Cutaneous Leishmaniasis | Localized current field radio frequency (LCF-RF) w/ 1% lidocaine HCl | 1 session (n = 190) 2 sessions (n = 11) |
Cure rate |
Cure rate at >8 weeks: 90% had completely cured |
Not reported | Not reported |
Non-acne scarring
RF treatment for hypertrophic non-acne and burn scars in 43 patients are discussed in one prospective study, three case series, and two case reports (Table 7). Two of the studies utilized RF in combination with acoustic pressure ultrasound to deliver either triamcinolone for hypertrophic scar or retinoic acid for atrophic scars.39,40 Another case series reported use of non-insulated microneedle fractional RF.41 Outcome measures included scar size and histologic psuedonodule density. Subjects received 1–4 treatment sessions every 3–4 weeks.
Table 7.
Reports of radiofrequency treatment of non-acne scarring
| Author Publication Year | Evidence | Study Design (Sample Size) | Condition | Intervention | Number of Treatments/Intervals | Outcome Measures | Clinical Outcomes | Patient Reported Outcomes | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Ding et al. 2015 (42) | 4 | Case series (n = 2) | Skin graft contraction | Micro-plasma RF w/ 10% lidocaine cream | 1–2 sessions | Scar contraction and dyspigmentation |
Scar contraction at 6 months Scarred skin matched surrounding skin |
Not reported | Not reported |
| Issa et al. 2013 (39) | 4 | Prospective case series (n = 4) | Hypertrophic scars | FMR w/ acoustic pressure ultrasound | 1 session q3–4 weeks x1–4 | Complete resolution |
Complete resolution
75% |
Not reported | Burning, erythema, and edema in all pts Fine scale and mild atrophy in 1 pt |
| Naouri et al 2016 (41) | 4 | Case series (n = 20) | Scars (various etiology) | FMR w/ topical anesthetic | 1–4 sessions | Clinical efficacy (1–10 scale) |
Clinical efficacy
Avg 6.25/10 (± 1.5) |
Patient-rated efficacy Avg 5.8/10 (± 1.8) Patient satisfaction Avg 7/10 (±1.6) |
Erythema in 60% Scabs in 45% Edema in 45% |
| Pinheiro et al. 2015 (43) | 4 | Case report (n = 1) | Hypertrophic burn scars | Monopolar radiofrequency (target temp above then below 40°C) | 5 sessions | Histology (% collagen) |
Histology (% collagen) Normal skin: 30.0 5% Hypertrophic scar: 30.62 % (p>0.05) w/ RF≤ 40°C: 29.26 % w/ RF ≥ 40°C: 49.44 % (p>0.05) |
Not reported | Not reported |
| Seok et al. 2016 (44) | 4 | Case report (n = 1) | Nasolabial necrosis scar | RF w/ pneumatic needleless injector | 5–10 minute session monthly x6 | Physician reported improvement |
Clinical improvement: Significant |
Not reported | Not reported |
| Trelles et al. 2016 (40) | 4 | Case series (n = 14) | Hypertrophic and atrophic scars | FMR w/ acoustic pressure ultrasound and topical lidocaine | 1 session q3 weeks x6 | Scar severity (1–6 scale) Scar attenuation Histology |
Severity: Baseline: 4.12 At 6 months: 2.55 (P<0.0001) Scar attenuation: Avg 67% Histology Atrophic scars: increased collagen Hypertrophic scars: resolved pseudonodules |
Patient satisfaction: Very satisfied: 7 Satisfied: 4 Somewhat satisfied: 1 Dissatisfied:2 |
Erythema, edema, and fine scabs (% not reported) |
All subjects showed scar improvement. The most significant results included hypertrophic scar size reduction from 4.2 to 2.55cm2 width and decreased attenuation by 67%.40 On post-treatment histology atrophic scars showed increased collagen density and hypertrophic scars showed resolution of pseudonodules. Average patient satisfaction was 68%. Adverse events included burning sensation during the procedure, mild erythema, edema, fine scale, and mild atrophy.39–44
Incompetent great saphenous vein
The most studied indication for RF is treatment of incompetent great saphenous veins, leading to lower leg varicosities. A summary of the findings from fourteen articles about non-cosmetic varicosity treatment with RF can be found in Table 8.45–58 The treatments used include endovenous thermal ablation (ETA)45,49,51,52,55 and segmental thermal ablation (STA).50,57,58 Subjects all received one treatment session per limb and were followed for up to five years post-procedure. Outcome measures include distal venous filling on duplex ultrasound and recanalization.
Duplex ultrasound guided evaluation shows that 94.4% of RF treated limbs remain completely occluded at 36 months post-procedure. One study demonstrated 91.9% occlusion at up to five years post procedure.57 A retrospective study comparing RF to 1,320 ND:YAG and 810 diode laser treatments showed that ND:YAG had significantly higher rates of occlusion at 5 years post-treatment compared to RF or diode.56 The highest recanalization rates were 12%.51 Adverse events included transient erythema, endovenous heat induced thrombus, hyperpigmentation, paresthesia, nonocclusive thrombus, worsening leg swelling, superficial thrombophlebitis, ecchymosis, hematoma, and infection.
Discussion
The purpose of this review was to describe findings of RF treatment in non-cosmetic dermatologic indications. RF treatment of acne and resultant acne scarring, primary axillary hyperhidrosis, acute and chronic wounds, cutaneous lesions such as neurofibromas, lymphangioma circumscriptum, cutaneous leishmaniasis, hypertrophic non-acne scarring, and varicose veins were included for review. RF literature demonstrates that this modality has been shown to successfully decrease size, expedite healing, or eliminate disease-associated symptoms in all of the above conditions.
RF has potential to treat recalcitrant moderate to severe acne cystica and associated scarring simultaneously to benefit patients suffering from facial pain, lesion drainage, and protracted self-esteem. The ability of radiofrequency to treat inflammatory conditions such as cystic acne is likely multifactorial. Histopathologic analysis of pre- and post- lesions shows decreased follicular density as well as decreased perifollicular lymphocytic infiltrate and sebaceous gland number.10 Some results also show thickened and compacted collagen type I and III fibers in the upper and lower layers of the dermis post treatment,59 while others demonstrate decreased expression of transforming growth factor beta, interleukin 8, and NF kappa B.9
RF aids in reduction of primary axillary hyperhidrosis symptoms via destruction of sweat glands. Evidence shows RF superiority compared to treatment mainstays that range from topical aluminum salts to axillary curettage, which may carry risks of scarring and only temporary improvement.12
RF treatment of neoplastic conditions, such as cutaneous neurofibromas, LC, and hypertrophic scars is largely successful due to directly targeted dermal depth heating, potentiating both superficial and root cause treatment. RF has shown to be a safe and economical pre-treatment for lesion size reduction, adjunct therapy to deliver medication more precisely, or an alternative to traditional surgical approaches as it is minimally invasive, decreases blood loss, and minimizes scarring.
A similar method of microtissue destruction via collagen contraction is fundamental to RF treatment of incompetent veins. RF generated heat causes segmental venous spasms and collagen shrinkage with minimal thrombus formation.52 Evidence for this therapy shows that RF is comparable to surgical interventions in efficacy, but superior in reducing surgical risk factors, such as infection, post-operative pain, and bruising. Though RF is a well-studied minimally invasive alternative to surgery, head-to-head comparison with 1,320nm ND:YAG shows shortcomings in complete occlusion associated with RF at 5 years post-treatment.56 Future studies may show that laser ablative modalities may have superior long term efficacy compared to RF.
RF was also shown to successfully treat cutaneous leishmaniasis, in which one week of daily RF thermotherapy is lethal to the assaulting parasite (maximum heat tolerance of 39°C). This offers an alternative to lengthy courses of SSG inoculations and potentially higher patient treatment compliance.35 Unfortunately, access to electricity and technology support in resource poor regions with endemic cutaneous leishmaniasis is a significant practical limitation.
A limitation of this review is that the majority of reports are case series. In addition, only a small number of studies are available for some of the discussed indications, such as wound healing and cutaneous leishmaniasis. Furthermore, because RF is a novel therapeutic modality, our analysis of the current literature shows that there is a lack of uniform treatment guidelines or protocols, specifically regarding use of varying energy levels, the number of treatments needed, and intervals at which treatments are given. From all of the studied indications, most variability is seen with the treatment of acne and scarring. Greater consistency was seen in venous ablation where only one session was needed in all studies. Long term follow-up beyond five years would bolster the promising safety and efficacy profile of RF demonstrated so far. Additional studies with larger sample sizes are needed to better correlate clinical findings with molecular and tissue level changes, appropriately expand non-invasive indications, and streamline delivery method for RF.
Conclusion
Treatment with RF is an effective minimally invasive modality. Its use in non-cosmetic cutaneous conditions is beneficial for its short post-procedure recovery time, infrequent severe adverse events, and non-inferior rates of relapse for up to five years post-treatment in certain conditions. This review furthers an ongoing discussion of the utility of this technology in dermatology. Although these studies provide valuable information, better designed studies with increased sample sizes and longer follow up protocols are needed to make definitive recommendations about RF treatment.
Footnotes
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Lolis MS & Goldberg DJ Radiofrequency in cosmetic dermatology: a review. Dermatol Surg 38, 1765–1776, doi: 10.1111/j.1524-4725.2012.02547.x (2012). [DOI] [PubMed] [Google Scholar]
- 2.Kaplan H. & Gat A. Clinical and histopathological results following TriPollar radiofrequency skin treatments. J Cosmet Laser Ther 11, 78–84, doi: 10.1080/14764170902846227 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Beasley KL & Weiss RA Radiofrequency in cosmetic dermatology. Dermatol Clin 32, 79–90, doi: 10.1016/j.det.2013.09.010 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Hantash BM, Renton B, Berkowitz RL, Stridde BC & Newman J. Pilot clinical study of a novel minimally invasive bipolar microneedle radiofrequency device. Lasers Surg Med 41, 87–95, doi: 10.1002/lsm.20687 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Maier M. Pulsed radio frequency energy in the treatment of painful chronic cutaneous wounds: a report of two cases. Pain Med 12, 829–832, doi: 10.1111/j.1526-4637.2011.01081.x (2011). [DOI] [PubMed] [Google Scholar]
- 6.Gomes AA, Gomes YV, Lima FB & Pessoa SG Multiple facial angiofibromas treated with high-frequency equipment. An Bras Dermatol 86, S186–189 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Byrd D. & Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep 12, 37–41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin JU, Lee SH, Jung JY & Lee JH A split-face comparison of a fractional microneedle radiofrequency device and fractional carbon dioxide laser therapy in acne patients. J Cosmet Laser Ther 14, 212–217, doi: 10.3109/14764172.2012.720023 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Min S, Park SY, Yoon JY & Suh DH Comparison of fractional microneedling radiofrequency and bipolar radiofrequency on acne and acne scar and investigation of mechanism: comparative randomized controlled clinical trial. Arch Dermatol Res 307, 897–904, doi: 10.1007/s00403-015-1601-z (2015). [DOI] [PubMed] [Google Scholar]
- 10.Prieto VG, Zhang PS & Sadick NS Evaluation of pulsed light and radiofrequency combined for the treatment of acne vulgaris with histologic analysis of facial skin biopsies. J Cosmet Laser Ther 7, 63–68, doi: 10.1080/14764170500231848 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Lan T, Xiao Y, Tang L, Hamblin MR & Yin R. Treatment of atrophic acne scarring with fractional micro-plasma radio-frequency in Chinese patients: A prospective study. Lasers Surg Med, doi: 10.1002/lsm.22825 (2018). [DOI] [PMC free article] [PubMed]
- 12.Kim M, Shin JY, Lee J, Kim JY & Oh SH Efficacy of fractional microneedle radiofrequency device in the treatment of primary axillary hyperhidrosis: a pilot study. Dermatology 227, 243–249, doi: 10.1159/000354602 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Fatemi Naeini F, Abtahi-Naeini B, Pourazizi M, Nilforoushzadeh MA & Mirmohammadkhani M. Fractionated microneedle radiofrequency for treatment of primary axillary hyperhidrosis: A sham control study. Australas J Dermatol 56, 279–284, doi: 10.1111/ajd.12260 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Schick CH, Grallath T, Schick KS & Hashmonai M. Radiofrequency Thermotherapy for Treating Axillary Hyperhidrosis. Dermatol Surg 42, 624–630, doi: 10.1097/dss.0000000000000703 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Fatemi Naeini F, Pourazizi M, Abtahi-Naeini B, Nilforoushzadeh MA & Najafian J. A novel option for treatment of primary axillary hyperhidrosis: fractionated microneedle radiofrequency. J Postgrad Med 61, 141–143, doi: 10.4103/0022-3859.153111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conner-Kerr T. & Isenberg RA Retrospective analysis of pulsed radiofrequency energy therapy use in the treatment of chronic pressure ulcers. Adv Skin Wound Care 25, 253–260, doi: 10.1097/01.ASW.0000415342.37554.ed (2012). [DOI] [PubMed] [Google Scholar]
- 17.Frykberg R, Martin E, Tallis A. & Tierney E. A case history of multimodal therapy in healing a complicated diabetic foot wound: negative pressure, dermal replacement and pulsed radio frequency energy therapies. Int Wound J 8, 132–139, doi: 10.1111/j.1742-481X.2010.00759.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen JA & Overstreet J. Pulsed radio frequency energy in the treatment of complex diabetic foot wounds: two cases. J Wound Ostomy Continence Nurs 35, 523–527, doi: 10.1097/01.WON.0000335966.98607.1c (2008). [DOI] [PubMed] [Google Scholar]
- 19.Khunger N, Pahwa M. & Jain RK Angiolymphoid hyperplasia with eosinophilia treated with a novel combination technique of radiofrequency ablation and sclerotherapy. Dermatol Surg 36, 422–425, doi: 10.1111/j.1524-4725.2009.01460.x (2010). [DOI] [PubMed] [Google Scholar]
- 20.Chaudhary S. & Dayal S. Radiofrequency ablation: a safe and economical modality in treatment of Brooke-Spiegler syndrome. Dermatol Online J 18, 7 (2012). [PubMed] [Google Scholar]
- 21.Kim SH, Roh SG, Lee NH & Yang KM Radiofrequency ablation and excision of multiple cutaneous lesions in neurofibromatosis type 1. Arch Plast Surg 40, 57–61, doi: 10.5999/aps.2013.40.1.57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH et al. 27.12 MHz Radiofrequency Ablation for Benign Cutaneous Lesions. Biomed Res Int 2016, 6016943, doi: 10.1155/2016/6016943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai Y. et al. A case of a large dermatofibrosarcoma protuberans successfully treated with radiofrequency ablation and transcatheter arterial embolization. J Dermatol 31, 42–46 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Niti K. & Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg 36, 1711–1717, doi: 10.1111/j.1524-4725.2010.01723.x (2010). [DOI] [PubMed] [Google Scholar]
- 25.Khurana A, Gupta A, Ahuja A, Sardana K. & Malhotra P. Lymphangioma circumscriptum treated with combination of Bleomycin sclerotherapy and Radiofrequency ablation. J Cosmet Laser Ther, 1–4, doi: 10.1080/14764172.2018.1493510 (2018). [DOI] [PubMed]
- 26.Lapidoth M. et al. Treatment of lymphangioma circumscriptum with combined radiofrequency current and 900 nm diode laser. Dermatol Surg 32, 790–794, doi: 10.1111/j.1524-4725.2006.32162.x (2006). [DOI] [PubMed] [Google Scholar]
- 27.Hm O. & Sc R. Lymphangioma circumscriptum (microcystic lymphatic malformation): palliative coagulation using radiofrequency current. J Cutan Aesthet Surg 1, 85–88, doi: 10.4103/0974-2077.44165 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachdeva S. Lymphangioma circumscriptum treated with radiofrequency ablation. Indian J Dermatol 56, 77–78, doi: 10.4103/0019-5154.77558 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subhadarshani S, Gupta V, Taneja N, Yadav S. & Gupta S. Efficacy and Safety of a Novel Method of Insulated Intralesional Radiofrequency Ablation for Deep Dermal and Subcutaneous Lesions: A 3-Year Institutional Experience. Dermatol Surg 44, 714–720, doi: 10.1097/dss.0000000000001437 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Civelek S, Sayın I, Ercan I, Cakır BO & Turgut S. Bipolar radiofrequency-induced interstitial thermoablation for oral cavity vascular malformations: Preliminary results in a series of 5 children. Ear Nose Throat J 91, 488–492 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Grimmer JF, Mulliken JB, Burrows PE & Rahbar R. Radiofrequency ablation of microcystic lymphatic malformation in the oral cavity. Arch Otolaryngol Head Neck Surg 132, 1251–1256, doi: 10.1001/archotol.132.11.1251 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Kim SW et al. Long-term outcome of radiofrequency ablation for intraoral microcystic lymphatic malformation. Arch Otolaryngol Head Neck Surg 137, 1247–1250, doi: 10.1001/archoto.2011.199 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Ryu NG, Park SK & Jeong HS Low power radiofrequency ablation for symptomatic microcystic lymphatic malformation of the tongue. Int J Pediatr Otorhinolaryngol 72, 1731–1734, doi: 10.1016/j.ijporl.2008.08.003 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Navin TR, Arana BA, Arana FE, Berman JD & Chajón JF Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J Infect Dis 165, 528–534 (1992). [DOI] [PubMed] [Google Scholar]
- 35.Reithinger R. et al. Efficacy of thermotherapy to treat cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: a randomized, controlled trial. Clin Infect Dis 40, 1148–1155, doi: 10.1086/428736 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Bumb RA et al. Long-term efficacy of single-dose radiofrequency-induced heat therapy vs. intralesional antimonials for cutaneous leishmaniasis in India. Br J Dermatol 168, 1114–1119, doi: 10.1111/bjd.12205 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Sadeghian G, Nilfroushzadeh MA & Iraji F. Efficacy of local heat therapy by radiofrequency in the treatment of cutaneous leishmaniasis, compared with intralesional injection of meglumine antimoniate. Clin Exp Dermatol 32, 371–374, doi: 10.1111/j.1365-2230.2007.02405.x (2007). [DOI] [PubMed] [Google Scholar]
- 38.Velasco-Castrejon O. et al. Treatment of cutaneous leishmaniasis with localized current field (radio frequency) in Tabasco, Mexico. Am J Trop Med Hyg 57, 309–312 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Issa MC, Kassuga LE, Chevrand NS & Pires MT Topical delivery of triamcinolone via skin pretreated with ablative radiofrequency: a new method in hypertrophic scar treatment. Int J Dermatol 52, 367–370, doi: 10.1111/j.1365-4632.2012.05704.x (2013). [DOI] [PubMed] [Google Scholar]
- 40.Trelles MA & Martínez-Carpio PA Clinical and histological results in the treatment of atrophic and hypertrophic scars using a combined method of radiofrequency, ultrasound, and transepidermal drug delivery. Int J Dermatol 55, 926–933, doi: 10.1111/ijd.13253 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Naouri M. & Mazer JM Non-insulated microneedle fractional radiofrequency for the treatment of scars and photoaging. J Eur Acad Dermatol Venereol 30, 499–502, doi: 10.1111/jdv.12890 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Ding JP, Fang L. & Wang LZ The use of micro-plasma radiofrequency technology in secondary skin graft contraction: 2 case reports. J Cosmet Laser Ther 17, 301–303, doi: 10.3109/14764172.2015.1027230 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Pinheiro NM, Melo PR, Crema VO & Mendonça AC Effects of radiofrequency procedure on hypertrophic scar due to burns. J Eur Acad Dermatol Venereol 29, 187–189, doi: 10.1111/jdv.12388 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Seok J. et al. Depressed scar after filler injection successfully treated with pneumatic needleless injector and radiofrequency device. Dermatol Ther 29, 45–47, doi: 10.1111/dth.12280 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Dunn CW, Kabnick LS, Merchant RF, Owens R. & Weiss RA Endovascular radiofrequency obliteration using 90 degrees C for treatment of great saphenous vein. Ann Vasc Surg 20, 625–629, doi: 10.1007/s10016-006-9099-7 (2006). [DOI] [PubMed] [Google Scholar]
- 46.García-Madrid C, Pastor Manrique JO, Sánchez VA & Sala-Planell E. Endovenous radiofrequency ablation (venefit procedure): impact of different energy rates on great saphenous vein shrinkage. Ann Vasc Surg 27, 314–321, doi: 10.1016/j.avsg.2012.06.015 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Gibson K. et al. Twenty-four month results from a randomized trial of cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord, doi: 10.1016/j.jvsv.2018.04.009 (2018). [DOI] [PubMed]
- 48.Mallick R. et al. Treatment Patterns and Outcomes in Patients with Varicose Veins. Am Health Drug Benefits 9, 455–465 (2016). [PMC free article] [PubMed] [Google Scholar]
- 49.Mendes CA et al. Randomized trial of radiofrequency ablation versus conventional surgery for superficial venous insufficiency: if you don’t tell, they won’t know. Clinics (Sao Paulo) 71, 650–656, doi: 10.6061/clinics/2016(11)06 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuller-Petrović S, Pavlović MD, Schuller-Lukić B. & Schuller S. Retrospective analysis of routine use of a double heat cycle (DHC) during radiofrequency segmental ablation (ClosureFAST(™) ) of saphenous veins. J Eur Acad Dermatol Venereol 30, 1009–1012, doi: 10.1111/jdv.13178 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Spiliopoulos S, Theodosiadou V, Sotiriadi A. & Karnabatidis D. Endovenous ablation of incompetent truncal veins and their perforators with a new radiofrequency system. Mid-term outcomes. Vascular 23, 592–598, doi: 10.1177/1708538114564462 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Tolva VS et al. Radiofrequency ablation of the great saphenous vein with the ClosureFAST™ procedure: mid-term experience on 400 patients from a single centre. Surg Today 43, 741–744, doi: 10.1007/s00595-012-0296-4 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Tzilinis A. et al. Chronic venous insufficiency due to great saphenous vein incompetence treated with radiofrequency ablation: an effective and safe procedure in the elderly. Vasc Endovascular Surg 39, 341–345, doi: 10.1177/153857440503900406 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Vasquez MA et al. The utility of the Venous Clinical Severity Score in 682 limbs treated by radiofrequency saphenous vein ablation. J Vasc Surg 45, 1008–1014; discussion 1015, doi: 10.1016/j.jvs.2006.12.061 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Wagner WH et al. Early experience with radiofrequency ablation of the greater saphenous vein. Ann Vasc Surg 18, 42–47, doi: 10.1007/s10016-003-0095-x (2004). [DOI] [PubMed] [Google Scholar]
- 56.Weiss RA et al. Comparative outcomes of different endovenous thermal ablation systems on great and small saphenous vein insufficiency: Long-term results. Lasers Surg Med 47, 156–160, doi: 10.1002/lsm.22335 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Proebstle TM et al. Five-year results from the prospective European multicentre cohort study on radiofrequency segmental thermal ablation for incompetent great saphenous veins. Br J Surg 102, 212–218, doi: 10.1002/bjs.9679 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proebstle TM et al. Three-year European follow-up of endovenous radiofrequency-powered segmental thermal ablation of the great saphenous vein with or without treatment of calf varicosities. J Vasc Surg 54, 146–152, doi: 10.1016/j.jvs.2010.12.051 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Kim JE et al. Objective evaluation of the clinical efficacy of fractional radiofrequency treatment for acne scars and enlarged pores in Asian skin. Dermatol Surg 40, 988–995, doi: 10.1097/01.DSS.0000452625.01889.c3 (2014). [DOI] [PubMed] [Google Scholar]