Abstract
Nitrogen (N) and potassium (K) are two important mineral nutrients in regulating leaf photosynthesis. However, the influence of N and K interaction on photosynthesis is still not fully understood. Using a hydroponics approach, we studied the effects of different N and K conditions on the physiological characteristics, N allocation and photosynthetic capacity of apple rootstock M9T337. The results showed that high N and low K conditions significantly reduced K content in roots and leaves, resulting in N/K imbalance, and allocated more N in leaves to non-photosynthetic N. Low K conditions increased biochemical limitation (BL), mesophyll limitation (MCL), and stomatal limitation (SL). By setting different N supplies, lowering N levels under low K conditions increased the proportion of water-soluble protein N (Nw) and sodium dodecyl sulfate-soluble proteins (Ns) by balancing N/K and increased the proportion of carboxylation N and electron transfer N. This increased the maximum carboxylation rate and mesophyll conductance, which reduced MCL and BL and alleviated the low K limitation of photosynthesis in apple rootstocks. In general, our results provide new insights into the regulation of photosynthetic capacity by N/K balance, which is conducive to the coordinated supply of N and K nutrients.
Introduction
Potassium (K) plays significant roles in membrane potential regulation, stress adaption, enzyme activation, and other important physiological processes [1–3]. K also participates in photosynthesis phosphorylation, stomatal closing and opening, and transportation of photosynthetic products, thus regulating quality and crop yield [4, 5]. Therefore, appropriate K supply is an important prerequisite for ensuring normal plant growth and improving crop yield and quality. However, the availability of the natural K in the soils will depend on the intensity of processes like weathering and leaching. The available K level in most soils is gradually decreasing. The lack of K in the soil has become a major limiting factor for sustainable agricultural production [6]. An important reason for soil K deficiency is the high cost of K fertilizer. In recent years, the cost of K fertilizer has increased due to increased input costs, supply chain disruption, and the export restrictions of producers. This will further increase the pressure on small-scale growers in developing countries.
Low K stress has received a great deal of attention as a common abiotic stress that limits crop yield and quality. One of the hazards of low K stress is the severe inhibition of C and N metabolism. Photosynthesis is a process in which plants convert carbon dioxide into carbohydrates, which accounts for 90% of plant biomass. Therefore, maintaining good photosynthesis is conducive to plant growth [7]. As the raw material for photosynthesis, atmospheric CO2 needs to diffuse from the leaf surface to the stomata, and then finally reach the carboxylation site of the Rubisco enzyme through the mesophyll cells for photosynthesis. The diffusion process of CO2 must overcome the resistance of the leaf surface boundary layer, the resistance of stomata to CO2 transport, and the resistance of CO2 from the stomatal cavity to the chloroplast Rubisco carboxylation site, in turn. The reduction in photosynthesis due to K deficiency is the result of the combined action of stomatal conductance limitation (SL), leaf conductance limitation (MCL), and biochemical limitation (BL) [8]. In addition, K deficiency inhibits photosynthesis by reducing the number of leaves, decreasing leaf area, decreasing C-metabolizing enzyme activities, and reducing the rate of photosynthetic product assimilation and export, resulting in a severe limitation of C metabolism [9–11]. Recent studies have found that inappropriate N/K would hinder protein synthesis, destroy the stability of mesophyll cells [12], increase the incidence rate and severity of apple canker disease [13], also hinder N assimilation of apples, and reduce photosynthetic nitrogen utilization efficiency [14]. Therefore, maintaining appropriate N/K is of great significance for maintaining plant growth and crop quality.
N is another macronutrient essential for plant growth. It is a key structural component of proteins, amino acids, and chlorophyll, a basic essential element involved in photosynthesis and metabolism, and a major limiting factor for crop yield [3, 15]. N supply levels play significant regulatory roles in C and N metabolism. Furthermore, N supply levels have a direct regulatory effect on the expression of nitrate transporter proteins, with low N (<1 mM) inducing the expression of low-affinity transporter systems and high N inducing the expression of high-affinity transporter systems [16]. N levels also affected N allocation within plants. In maize, Mu et al. [17] found that low N increased the proportion of N allocated to electron transport and photosynthetic phosphorylation components. Hou et al. [18] reported that high N reduced the proportion of leaf N allocated to the electron transport and carboxylation system components and decreased photosynthetic N utilization efficiency (PNUE) in rice. C assimilation is also influenced by leaf N content, because the enzymatic reactions of C metabolism, the capture of light energy, and electron transport processes require the investment of large amounts of N. Studies have shown that photosynthetic capacity is mainly determined by relative N allocation in leaves. In certain adverse environments, plants usually increase N allocation to the cell wall to improve the plant’s tolerance to adversity [19]. Small changes in N distribution also strongly affect PNUE and photosynthesis.
Recent studies have shown that plant growth balance can be maintained by regulating N supply under stress conditions. Sun et al. [20] found that reducing N supply increased the expression of MdAHA2, promoted rhizosphere acidification, increased Fe uptake efficiency, and alleviated Fe deficiency in apples. Wen et al. [21] found that reducing N supply enhanced the citric acid cycle, increased the content of glucose and sucrose, and thus improved the low light tolerance of tall fescue. Dziedek et al. [22] found that N application increased the negative impact of drought on beech. Peng et al. [23] found that increasing nitrate supply partially restored the growth retardation and leaf atrophy caused by magnesium deficiency in soybean. These results show that the regulation of N levels under stress conditions is species-specific, and the biological mechanism of N supply in stress tolerance remains controversial. N is one of the elements most closely related to K, but there are few reports on the mechanisms of regulation of low K by N levels in apples.
Apples have a huge planting area and extremely high production in China. However, due to the low price of N fertilizer and its significant effect on yield, fruit farmers tend to overuse N fertilizer in production [24], ignoring the role of K, and the imbalance of N and K nutrition has become an important factor limiting apple production. Mitigating the adverse effects of N and K imbalance on photosynthesis by optimizing N levels may be a feasible option, but the relevant physiological mechanisms have not been specifically analysed. M9T337 rootstock is currently the most common rootstock used in dense dwarf apple production. Therefore, we investigated the effect of different nitrate levels on the growth and C and N metabolism of M9T337 rootstock under different K levels. In this study, we hypothesized that: N/K imbalance would alter leaf N allocation; optimization of N allocation in leaves would improve photosynthesis. Our results provide new clues to the rational application between N and K in apples and other species.
Result
Plant growth and photosynthesis
Low K (LK) treatments significantly inhibited the root and aboveground growth of plant (Fig. 1). Compared with medium K (MK) treatments, root dry weight was reduced by 21.85, 21.26, and 20.83% and leaf dry weight by 19.85, 35.14, and 40.99% under the LKLN, LKMN, and LKHN treatments, respectively. Under the MK treatments, the biomass of M9T337 rootstock increased and then decreased as the level of nitrate supply increased, and the plant grew best under the MN treatment. Under the LK treatments, apple rootstock leaf dry weight and root dry weight were the highest under the LN treatment.
Figure 1.
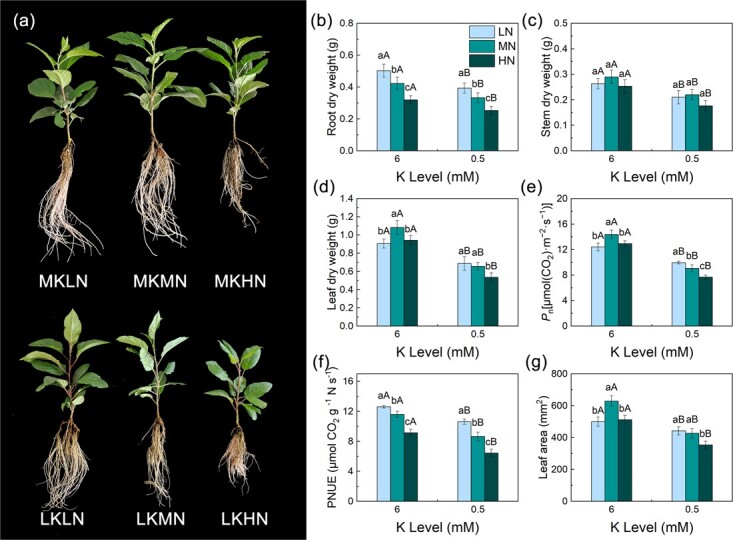
Effects of different N and K levels on M9T337 rootstock growth and photosynthesis. Growth phenotypes of rootstock (a), root dry weight (b), stem dry weight (c), leaf dry weight (d), net photosynthetic rate (e), PNUE (f), and leaf area (g). Data are means ± standard deviation (n = 3). Different capital (lower case) letters indicate statistical differences between K (N) levels under the same N (K) level (P < 0.05) according to Duncan’s test.
K deficiency significantly reduced the leaf area, Pn, and PNUE. Under MK treatments, the leaf area and Pn were highest under MN treatment, while there was no significant difference between the HN and LN treatments in the leaf area and Pn. Under the LK treatments, the leaf area and Pn under the LN treatment were the highest, and the leaf area is increased by 3.51 and 24.90%, respectively, compared with the MN and HN treatments, and the Pn increased by 9.78 and 29.73%, respectively. The results of PNUE showed that with the increase in the N level and the decrease in the K level, PNUE decreased gradually.
N and K uptake
As shown in Fig. 2a, the LK treatments significantly reduced the N and K content of rootstock leaves but increased the N/K ratio. Under the MK treatment, the highest K content in the roots and leaves was in the MN treatment, and the highest N content was in the HN treatment. However, under the LK treatment, the highest K content and lowest N content were found in rootstock leaves under the LN treatment. The N/K ratio decreased with increasing N levels, and the lowest leaf N/K ratio was found under the HNLK treatment. The patterns of N and K content and N/K ratio in the roots were similar to those in the leaves (Fig. 2b).
Figure 2.
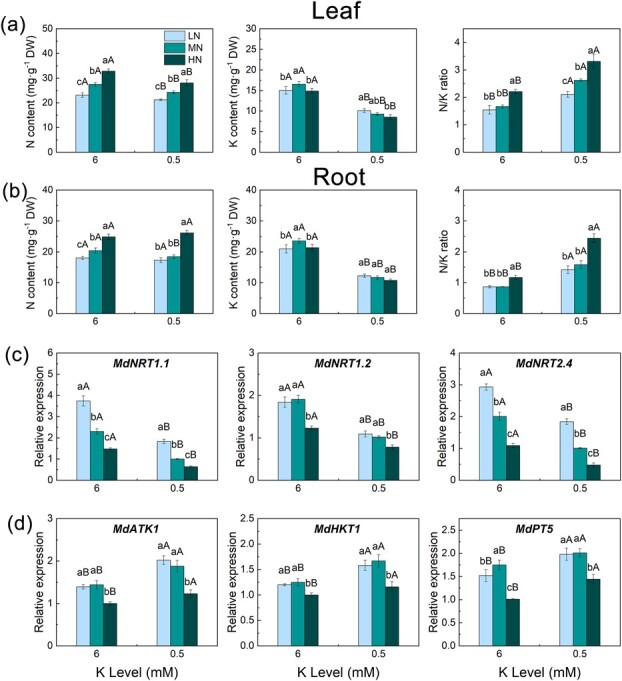
Element content and the relative expression of N and K uptake genes in rootstock roots treated with different N and K levels. N content, K content, and N/K ratio in the leaves (a), N content, K content, and N/K ratio in the roots (b), expression of N uptake genes in roots (c), and expression of K uptake genes in roots (d). Data are means ± standard deviation (n = 3). Different capital (lower case) letters indicate statistical differences between K (N) levels under the same N (K) level (P < 0.05) according to Duncan’s test.
We determined the expression of MdNRT1.1, MdNRT1.2, and MdNRT2.4 in the roots (Fig. 2c). The results showed that the LN treatments inhibited the transcription levels of nitrate transporters (NRTs) and increased the transcription levels of MdAKT1, MdHKT1, and MdPT5. Compared with the MN and HN treatments, the LN treatments increased the expression levels of MdNRT1.1 and MdNRT1.2. The HN treatments decreased the expression levels of MdAKT1, MdHKT1, MdPT5, MdNRT1.1, MdNRT1.2, and MdNRT2.4.
We used 15N to mark the N distribution in the rootstocks. The LK treatments significantly reduced the 15N distribution rate of leaves and increased the 15N distribution rate of roots (Fig. S1a, see online supplementary material). Under the LK treatments, compared with the LKMN and LKHN treatments, the 15N distribution rate of leaves in the LKLN treatment increased by 9.28 and 33.95%, respectively, while that of roots decreased by 4.30 and 12.29%, respectively. We also determined the expression of MdNRT1.5 in roots (Fig. S1, see online supplementary material), which is related to the transport of NO3− to the aboveground parts. The LKLN treatment showed higher MdNRT1.5 expression in the roots than the LKHN and LKMN treatments (Fig. S1b, see online supplementary material).
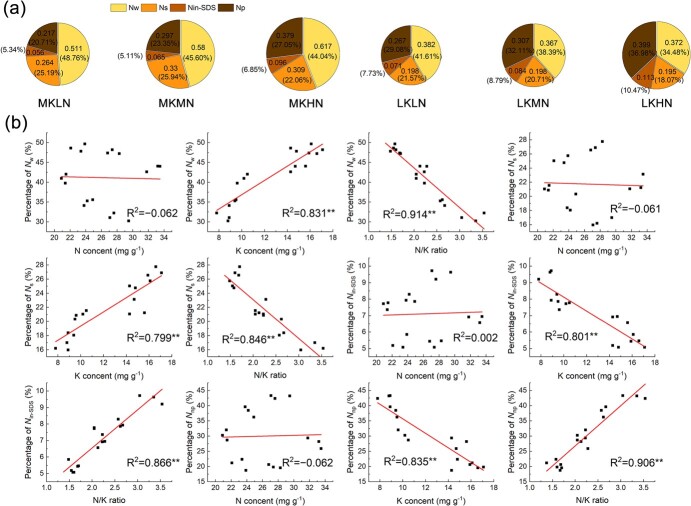
N allocation by form in leaves
We analysed the allocation of N forms in leaves under different N and K treatments (Fig. 3b). The LK treatments significantly reduced the content and proportion of water-soluble proteins (Nw) and SDS-soluble proteins (Ns), but they increased the content and composition of SDS-insoluble proteins (Nin-SDS) and non-protein N (Np). Under the LK treatments, the HN treatment further reduced the content and composition of Nw and Ns, and increased the content and composition of Nin-SDS and Np. Compared with the LKMN and LKHN treatments, the LKLN treatment had the highest content and composition of Nw and Ns. We conducted a correlation analysis between the allocation proportion of different N forms and N content, K content, and the N/K ratio in the leaves (Fig. 3c). The allocation proportion of different N forms had no significant correlation with the N content in the leaves. The allocation proportion of Nw and Ns was positively correlated with K content and negatively correlated with the N/K ratio, while the allocation proportion of Nin-SDS and Nnp was significantly positively correlated with K content and the N/K ratio.
Figure 3.
Effects of different N and K levels on N distribution of M9T337 rootstock leaves. N allocation by morphology (a) and relationship between N allocation and N content, K content, and N/K in leaves (b) of M9T337 rootstock treated with different N and K levels. Data out of the bracket represents the absolute N content (g m−2). Data in the bracket represents the relative content (%). The size of pie chart indicates N content.
Photosynthetic N allocation in leaves
The allocation of photosynthetic N and non-photosynthetic N in leaves was further analysed (Fig. 4). The LK treatments significantly reduced the content and composition of electron transfer N (Net), light capture N (Nlc), and carboxylation N (Ncb) and increased the content and composition of non-photosynthetic N (Nnon-psn). Under the LK treatments, the photosynthetic N allocation in leaves under the LN treatment was the highest, reaching 46.34%, followed by the LKMN treatment at 41.18%, and the LKHN treatment was the lowest, at only 31.05%. Compared with the LKLN and LNMN treatments, the absolute content of Ncb under the LKHN treatment decreased by 15.23 and 11.82%, respectively, and the absolute content of Nlc decreased by 16.16 and 17.93%, respectively, while the absolute content of Nnon-psn increased by 63.92 and 33.33%, respectively. These results showed that LN conditions optimized N allocation in plant organs and leaves under LK conditions.
Figure 4.
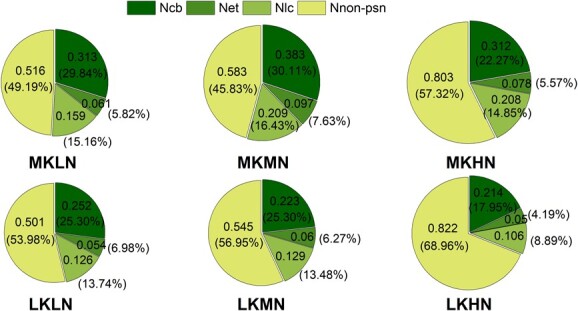
Photosynthetic N allocation of M9T337 rootstock leaves treated with different N and K levels. Data out of the bracket represents the absolute N content (g m−2). Data in the bracket represents the relative content (%). The size of pie chart indicates N content.
Correlation analysis
We analysed the relationship between the absolute and relative content of different N forms and Pn, and the results showed that Pn was positively correlated with the absolute and relative content of Nw, Ns, and Npsn (Fig. 5), and negatively correlated with the relative content of Nin-SDS, Nnp, and Nnon-psn, as well as the absolute content of Nin-SDS and Nnp (Fig. S2, see online supplementary material). The correlation between the relative content of different N and Pn was higher than the absolute content of different N forms. We also analysed the relationship between N and K content, N/K and Pn in leaves. The results showed that Pn was positively correlated with K content, negatively correlated with N content and N/K under LK treatments. Under MK treatments, with the increase of N content and N/K, the Pn first increased and then decreased.
Figure 5.
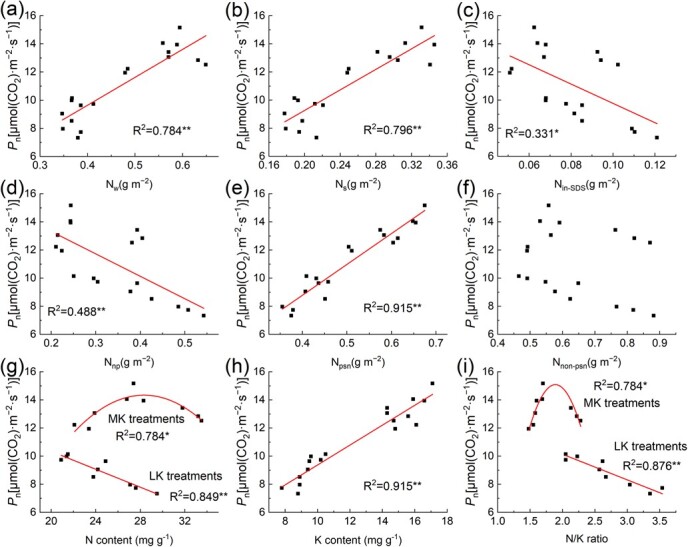
Relationship between the net photosynthetic rate (Pn) and absolute and relative content of the different N forms, N and K content, and N/K.
Leaf anatomical and structural traits
The cell wall thickness and stomatal conductance were significantly influenced by different N and K treatments, but there was no significant difference in leaf thickness (Fig. 6). Under the MK and LK treatments, the cell wall thickness of the HN treatment was significantly higher than those of the LN and MN treatments. Compared with the MKLN and MKMN treatments, the thickness of the cell wall under the MKHN treatment increased by 5.80 and 4.79%, respectively. There was no significant difference in cell wall thickness between the MN and LN treatments. We measured stomatal conductance. Under the MK treatments, the gs of the MN treatment was the largest. Compared with the LKMN and LKHN treatments, the gs of leaves under the LKLN treatment increased by 16.22 and 29.43%, respectively. N and K also affected the cell arrangement. Fig. 6b shows that the palisade tissue was more closely arranged under the LK and HN treatment.
Figure 6.
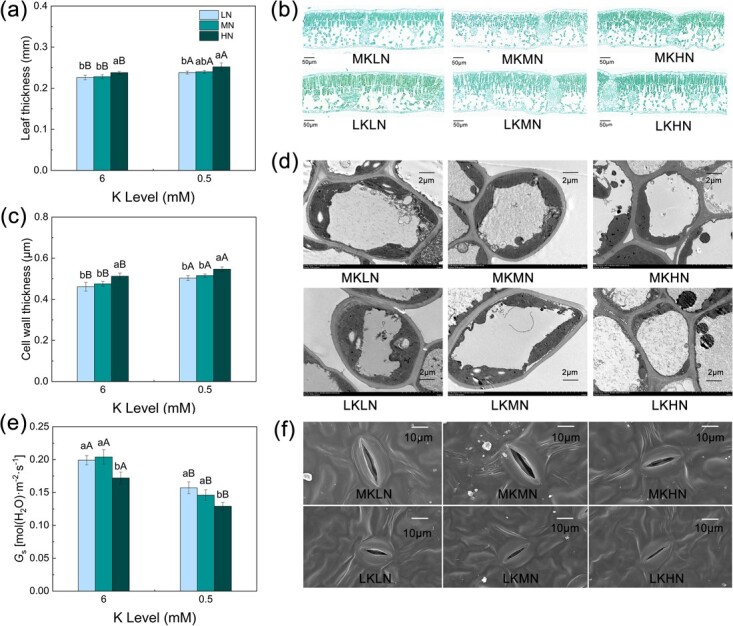
Effects of different N and K levels on leaf structure parameters of M9T337 rootstock. Leaf thickness (a and b), cell wall thickness (c and d), and stomatal conductance (e and f). Data are means ± standard deviation (n = 6). Different capital (lower case) letters indicate statistical differences between K (N) levels under the same N (K) level (P < 0.05) according to Duncan’s test.
Photosynthetic limitation
Under the MK treatments, gsc, gm, and Vmax were highest under the MN treatment, and gm and Vmax were not significantly different between the LN and HN treatments. Leaf gsc, gm, and Vmax increased by 16.22, 9.62, and 4.02%, respectively, under the LKLN treatment compared to the LKMN treatment and by 29.43, 23.32, and 17.96%, respectively, compared to the LKHN treatment. Thus, SL, MCL and BL were minimal under the LKLN treatment.
Under the LN treatments, BL was the highest limitation of photosynthetic decline in leaves, followed by MCL, and SL was the lowest (Fig. 7). Under the MK treatments, MCL was the highest limitation of photosynthetic decline in the leaves.
Figure 7.
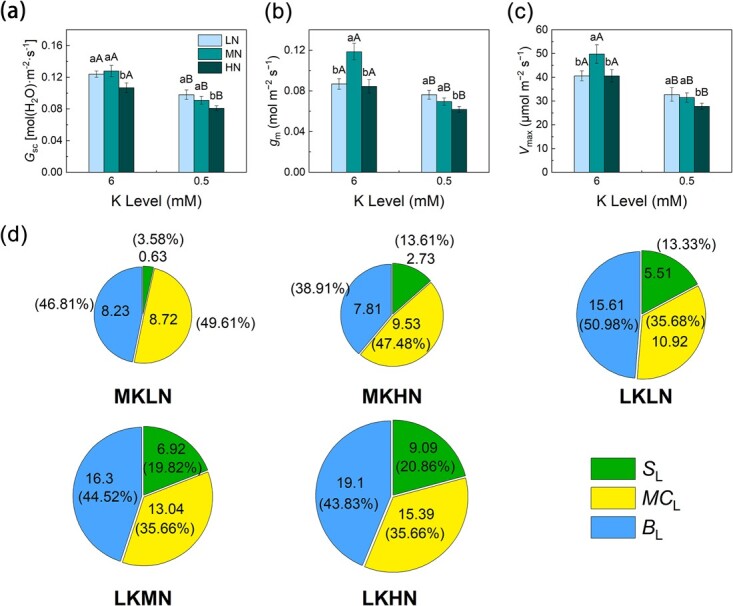
Photosynthesis limitations of M9T337 rootstock treated with different N and K levels. Stomatal conductance to CO2 (a), mesophyll conductance (b), the maximum carboxylation rate of Rubisco (c), photosynthesis limitations and components (d). Data out of the bracket represents the measured values. Data in the bracket represents the relative values. Data are means ± standard deviation (n = 3). Different capital (lower case) letters indicate statistical differences between K (N) levels under the same N level (P < 0.05) according to Duncan’s test. The size of pie chart indicates photosynthetic limitation.
Discussion
N and K are essential nutrients for plants and are crucial for their growth and development. K deficiency limits plant growth, which may be related to an imbalance of N and K nutrients [25]. The interactions between N and K are complex and occur at multiple levels [26]. Previous studies have generally concluded that K+ and NO3− are mutually reinforcing relationships because the charge balance improves [27]. However, the K content in the leaves and the K+ influx rate in the roots decreased under the HN treatments, indicating that high nitrate inhibited the K uptake. The same results were reported by Li et al. [25], who found that increased N fertilization exacerbated K deficiency, but they attributed this to a mutual dilution effect between N and K. However, in our experiments, the HN treatments did not show an increase in biomass. The HN treatment downregulated the expression of MdATK1 and MdPT5, which explains the inhibition of K uptake by high N. The expression of MdATK1, MdHKT1, and MdPT5 was significantly increased under the LK treatments, which is a stress response due to plant K deficiency [28]. Plants actively absorb NO3− through NRTs in roots and then synthesize amino acids and proteins under the action of a series of N metabolism enzymes, such as NR and GS [29]. We found that the LK treatments reduced the expression levels of MdNRT1.1 and MdNRT2.4 in roots, thereby inhibiting nitrate uptake. These results are similar to previous findings. The inhibition of N uptake by the LK treatments was exacerbated by high nitrate levels. Compared with the LKLN and LKHN treatments, the NRT expression in roots was reduced significantly under the LKHN treatment, which is consistent with the results of Sun et al. [30] on apples. Although low K inhibited N uptake, the N/K ratio in the roots and leaves remained significantly elevated.
N and K levels also affect the N distribution in plants and their leaves. Increasing the aboveground N distribution of the plant and the proportion of photosynthetic N in the leaves can increase PNUE, which has been confirmed in rice and oilseed rape [18, 31]. We labeled N allocation in plants with 15N and found that the LK treatments reduced the percentage of aboveground N allocation, which may be related to the fact that low K reduced the expression of MdNRT1.5, a key transporter protein involved in N loading in the xylem [32]. Under low K conditions, high N treatment further inhibited MdNRT1.5 expression, leading to a further reduction in 15N distribution in the leaves, similar to the results found by Wu et al. [33]. Leaf N can be divided into Nw, Ns, Nin-SDS, and Np [34]. Nin-SDS consists mainly of cell wall protein N and nuclear protein N. Plants react to a stressful environment by increasing the amount of cell wall protein N, which has been confirmed in rice [35]. We found that low K conditions reduced the partitioning of Nw and Ns and increased the partitioning of Nin-SDS and Nnp, which is similar to the results of Hou et al. [18] in rice. We analysed the correlation between N content, K content, and the N/K ratio and the allocation proportions of Nw, Ns, Nin-SDS, and Np. The correlation coefficient between the N/K ratio and the distribution proportion of different forms of N was the highest, and the N/K ratio was significantly positively correlated with the distribution proportion of Nin-SDS and Np. Therefore, under low K conditions, high N further increased the proportion of Nin-SDS and Nnp, which may be related to the increase in the N/K ratio due to high N. The more N allocated to Nin-SDS and Nnp fractions, the smaller the proportion allocated to Nw and Ns. Photosynthetic N mainly includes Ncb, Nlc, and Net, of which Ncb belongs to Nw, while Nlc and Net belong to Ns. We further analysed the distribution of photosynthetic N in leaves, with the same results regarding the N form; low K conditions reduced the absolute and relative content of Ncb and Nlc, leading to a reduction in the proportion of Npsn. We also analysed the relationship between the absolute and relative content of N and Pn. The relationship between the relative content of N and Pn was closer than the absolute content. Therefore, different N and K treatments regulated photosynthesis by adjusting the N allocation.
As the main osmoregulatory substance of guard cells, the abundance of K+ affects the function of stomata; thus, low K conditions usually lead to stomatal closure [36, 37]. We confirmed this result by observing the stomatal structure using a scanning electron microscope. High N further reduced gs, which may be related to the inhibition of K uptake under high N conditions. In addition, the gm from the sub-stomatal cavities to the sites of carboxylation inside the chloroplasts is one of the main factors affecting photosynthesis [36]. Previous studies have shown that in stressful environments, plants tend to have smaller leaves and greater leaf pulp cell density and leaf thickness to resist adversity and prolong leaf life [38]. We also observed that low K conditions reduced the leaf area. The results of paraffin sections also showed that the arrangement of palisade tissues was more compact under K deficiency conditions, which is detrimental to the capture of light energy by the leaves and CO2 conduction in the leaves. Hou et al. [18] reported that high N treatment led to more leaf N being allocated to non-photosynthetic N. Our results found that the HN treatment aggravated the N/K imbalance, causing plants to allocate more N to Nin-SDS, which are mainly cell wall proteins and nucleoproteins. Therefore, the increase in Nin-SDS content may indicate an increase in cell wall thickness. We measured the cell wall thickness and found that the LK and HN treatments increased the cell wall thickness. This is similar to the findings of Xie et al. [39] on rice. They also found that under the condition of severe K deficiency, the cell wall thickness increased significantly with an increase in N application, thereby reducing the relative content of Npsn. This phenomena can potentially be explained as nutrient stress promotes more N into cell wall structure to reduce the damage to plants from nutrient imbalance [40]. The thickening of the cell wall further limited the diffusion of CO2 into the chloroplast, as leaves with strong cell wall structures tended to exhibit a lower gm [7, 41]. Hu et al. [12] found that suitable N and K nutrients could coordinate CO2 diffusion and carboxylation in rice, thereby enhancing photosynthetic capacity. We found that under LK conditions, the LN treatment balanced N/K by reducing N content and the proportion of Nin-SDS and Nnp, and more N was distributed to Npsn, such as Ncb. Generally, the higher N allocation to Rubisco, the higher Vmax and subsequently higher Pn [42]. Our results also showed that nitrogen and potassium balance reduces cell wall thickness and increases Vmax, ultimately leading to significant reductions in MCL and BL.
In conclusion, the regulation of N and K balance on photosynthesis is complex. The LK treatments leading to an imbalance of N/K, which led to an increase in leaf Nin-SDS and Nnp allocation and a decrease in Npsn allocation. The change in leaf N distribution led to the decrease in gm and Vmax, leading to increased photosynthetic limitation. Under low K conditions, by adjusting the N supply level to coordinate N/K, the relative content of Nin-SDS was reduced, and plants allocated more N to Ns and Npsn, thus increasing PNUE and Vmax and alleviating the limitation of photosynthesis by low K. In conclusion, maintaining the proper ratio of N and K concentrations in leaves by optimizing the N/K ratio could coordinate the distribution of N, reduce photosynthetic restrictions, and improve leaf photosynthetic capacity, thus promoting apple rootstock growth (Fig. 8). Our findings are expected to provide new insights into the regulation of photosynthetic capacity by N/K balance.
Figure 8.
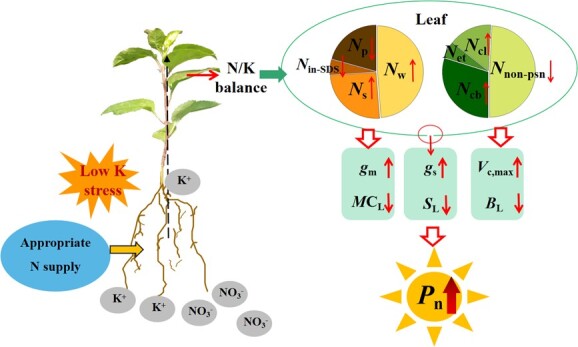
A hypothetical model for regulating photosynthesis of apple rootstock leaves by N/K balance.
Materials and methods
Growth conditions and treatments
Apple seedlings (M9T337 dwarf rootstock, n = 240) were used in the current study. M9T337 rootstocks were planted in a growth chamber under natural light and temperature conditions. We used a hydroponic system designed as described by Xu et al. [43], there were 40 rootstocks in each treatment, and one rootstock was used as a biological repeat. Briefly, consistently grown rootstocks (approximately 10 cm high) were selected and transferred to black plastic containers containing 6 L of 1/2 Hoagland’s nutrient solution [44].
In the pre-experiment, two K supply levels (0.5 and 6 mM) and four N supply levels (0.1, 1, 10, and 20 mM) were set. Apple rootstocks under the 0.1 mM N level showed highly significant N deficiency symptoms, and the growth of aboveground parts was significantly inhibited regardless of the K supply level, while the rootstocks grew best at 1 mM N supply under low K conditions (Fig. S3, see online supplementary material). Thus, we selected three N levels of 1, 10, and 20 mM for treatment in the formal experiment.
The formal trial began after 10 days of growth. Ca(NO3)2 was used as the only N source and K2SO4 as the only K source. Two K supply levels (6 and 0.5 mM) and three N supply levels (1, 10, and 20 mM) were used in this study. Six treatments were included: medium K low L (6 mM K+ + 1 mM NO3−, MKLN), medium K medium N (6 mM K+ + 10 mM NO3−, MKMN), medium K high N (6 mM K+ + 20 mM NO3−, MKHN), low K low L (0.5 mM K+ + 1 mM NO3−, LKLN), low K medium N (0.5 mM K+ + 10 mM NO3−, LKMN), and low K high N (0.5 mM K+ + 20 mM NO3−, LKHN). The other compositions of nutrient solution are equal between groups: 0.1 mM EDTA-Fe, 0.76 μM ZnSO4·7H2O, 37 μM H3BO4, 0.3 μM CuSO4·5H2O, 9 μM MnCl2·4H2O, 1 mM NaH2PO4, and 2 mM MgSO4. CaCl2 was supplemented in the LN and MN treatments at the same Ca2+ level as in the HN treatment. We adjusted the nutrient solution PH to 6.0 ± 0.1 and replaced the solution every 3 days. An air pump was used to maintain the oxygen content of the nutrient solution (12 h per day). The samples were taken to determine the various indices after 28 days of treatment.
15N isotope analysis
Ten rootstocks were selected for 15N labeling in each treatment. Ca(NO3)2 was replaced with Ca(15NO3)2 (with an abundance of 10.14%), and other nutrient contents and management were the same as those described above. The rootstocks were destructively sampled and divided into leaves, stems, and roots. After drying at 80°C to constant weight, they were ground and filtered with a mesh screen (0.25 mm). The abundance of 15N was analysed using a MAT-251-Stable Isotope Ratio Mass Spectrometer. The formula was calculated following the method of Xu et al. [43].
Analysis of growth parameter and mineral element
After 28 days of treatment, rootstocks were collected to analyse dry weight and root morphology. The leaves, roots, and stems of rootstocks were dried to constant weight at 80°C, and each organ was weighed with a 1/1000 electronic balance. The root morphology was analyzed by WinRhizo software (WinRHIZO version2012b, Regent Instruments Canada, Montreal, QC, Canada).
The dried samples were ground into powder and digested with H2SO4–H2O2. The N content was determined using the Kjeldahl apparatus (JK9870). The K content was determined using a flame photometer (M-410; Cole-Parmer, Chicago, IL, USA).
Determination of leaf characteristics and quantitative limitation analyses of Pn
Chlorophyll was extracted from the leaves and measured according to the method of Porra et al. [45]. The leaf area meter (Yaxin-1241, Beijing, China) was used to measure the leaf area.
After 28 days of treatment, gas exchange and Pn − Ci curves of the fourth main-stem leaf were determined using a portable photosynthesis system (LI-6400, LI-COR, Lincoln, Nebraska, USA) between 9:00 and 11:30 a.m. The maximum carboxylation efficiency (Vmax) and maximum electron transfer rate (Jmax) were calculated according to Long and Bernacchi [46]. Mesophyll conductance (gm) was calculated following Harley et al. [47]. Gsc is stomatal conductance to CO2 (gs/1.6).
The limitations to Pn mainly consists of the stomatal limitations (SL), mesophyll conductance limitations (MCL), and biochemical limitations (BL) according to the analyses presented by Grassi and Magnai [48]. The formula is calculated following Lu et al. [49].
N allocation by form and function
A punch (10 mm diameter) was used to collect leaf disks (1 cm2 area) from the leaves, avoiding the main vein, with 20 pieces for each treatment. The leaf disks were used to determine the different forms of N (Fig. S4, see online supplementary material). Different forms of N (water-soluble protein, Nw; SDS-soluble proteins, Ns; SDS-insoluble proteins, Nin-SDS) in leaves were measured according to Liu et al. [50]. The three N components were dried and digested with H2SO4–H2O2 [51]. The N content in the digestion solution was determined by Kjeldahl apparatus (JK9870).
According to the results of Niinemets and Tenhunen [52], leaf N consisting of photosynthetic N (Npsn) was divided into three major parts: proteins for carboxylation in the Calvin cycle (Ncb); light-harvesting components (Nlc); and electron transport components (Net). The calculation formula of the N allocation proportion was as follows:
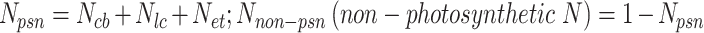 |
(1) |
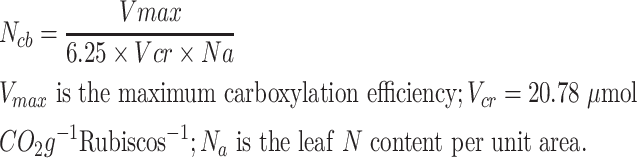 |
(2) |
 |
(3) |
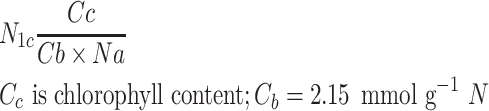 |
(4) |
 |
(5) |
Leaf structure analysis
The structure of the leaves and the thickness of the leaves were studied using tissue paraffin sectioning. The tissue paraffin sectioning and scanning electron microscopy (SEM) slides of the leaves were prepared according to Xu et al. [44]. The transmission electron microscope (TEM) slides of the leaves were prepared according to Xie et al. [39]. The leaf thickness and cell wall thickness were analysed with Image-PRO plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). The measurements were repeated six times for each treatment.
Gene expression analysis by qRT-PCR
Total RNA was extracted from root samples using an RNAprep Pure Plant Kit (Tiangen, Beijing, China). The reaction system contained 2 μL of primers (1 μL of upstream and 1 μL of downstream primers), 1 μL of cDNA, 10 μL of Green qPCR SuperMix, and 7 μL of ddH2O. Data were calculated using the 2–ΔΔCT method [53]. The primers used in the assays are listed in Table S1 (see online supplementary material).
Data analysis
All statistical analyses were analysed using SPSS (Statistics software, version 17.0, IBM, USA). The post hoc test (Duncan's) were used to test for statistical significance. Differences were deemed significant at P ≤ 0.05.
Acknowledgements
This work was supported bythe National Key R & D Program of China (2023YFD2301000), the Special Fund for the Natural Science Foundation of Shandong Province (ZR2021MC093), the earmarked fund for CARS (CARS-27), the Taishan Scholar Assistance Program from Shandong Provincial Government (TSPD20181206), and Xinlianxin Innovation Center for Efficient Use of Nitrogen Fertilizer (2020-apple).
Author contributions
S.G., Y.J., and X.X. conceived and designed the experiments. X.X., W.N., Y.G., C.L., Y.X., X.Z., J.L., Z.F., and G.T. performed all experiments. X.X, X.Z., and S.G. analysed the data and wrote the manuscript.
Data availability
The authors confirm that all experimental data are available and accessible via the main text and/or the supplemental data.
Conflict of interest statement
The authors declare they do not have any conflict of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
Supplementary Material
Contributor Information
Xinxiang Xu, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China; Yantai Academy of Agricultural Sciences, Institute of Pomology, Yan’tai 265500, Shandong, China.
Xu Zhang, Yantai Academy of Agricultural Sciences, Institute of Pomology, Yan’tai 265500, Shandong, China.
Wei Ni, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Chunling Liu, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Hanhan Qin, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Yafei Guan, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Jingquan Liu, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Ziquan Feng, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Yue Xing, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Ge Tian, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Zhanling Zhu, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Shunfeng Ge, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
Yuanmao Jiang, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong, China; Apple Technology Innovation Center of Shandong Province, Tai’an 271018, Shandong, China.
References
- 1. Wang Y, Wu WH. Potassium transport and signaling in higher plants. Annu Rev Plant Biol. 2013;64:451–76 [DOI] [PubMed] [Google Scholar]
- 2. Oosterhuis DM, Loka DA, Kawakami EM. et al. The physiology of potassium in crop production. Adv Agron. 2014;126:203–33 [Google Scholar]
- 3. de Bang TC, Husted S, Laursen KH. et al. The molecular-physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021;229:2446–69 [DOI] [PubMed] [Google Scholar]
- 4. Hu W, Jiang N, Yang J. et al. Potassium (K) supply affects K accumulation and photosynthetic physiology in two cotton (Gossypium hirsutum L.) cultivars with different K sensitivities. Field Crop Res. 2016;196:51–63 [Google Scholar]
- 5. Johnson R, Vishwakarma K, Hossen MS. et al. Potassium in plants: growth regulation, signaling, and environmental stress tolerance. Plant Physiol Biochem. 2022;172:56–69 [DOI] [PubMed] [Google Scholar]
- 6. Zorb C, Senbayram M, Peiter E. Potassium in agriculture – status and perspectives. J Plant Physiol. 2014;171:656–69 [DOI] [PubMed] [Google Scholar]
- 7. Simkin AJ, Lopez-Calcagno PE, Raines CA. Feeding the world: improving photosynthetic efficiency for sustainable crop production. J Exp Bot. 2019;70:1119–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu Z, Ren T, Pan Y. et al. Differences on photosynthetic limitations between leaf margins and leaf centers under potassium deficiency for Brassica napus L. Sci Rep. 2016;6:21725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Battie-Laclau P, Laclau JP, Beri C. et al. Photosynthetic and anatomical responses of Eucalyptus grandis leaves to potassium and sodium supply in a field experiment. Plant Cell Environ. 2014;37:70–81 [DOI] [PubMed] [Google Scholar]
- 10. Hu W, Yang J, Meng Y. et al. Potassium application affects carbohydrate metabolism in the leaf subtending the cotton (Gossypium hirsutum L.) boll and its relationship with boll biomass. Field Crop Res. 2015;179:120–31 [Google Scholar]
- 11. Hu W, Lu Z, Gu H. et al. Potassium availability influences the mesophyll structure to coordinate the conductance of CO2 and H2O during leaf expansion. Plant Cell Environ. 2022;45:2987–3000 [DOI] [PubMed] [Google Scholar]
- 12. Hu W, Ren T, Meng F. et al. Leaf photosynthetic capacity is regulated by the interaction of nitrogen and potassium through coordination of CO2 diffusion and carboxylation. Physiol Plant. 2019;167:418–32 [DOI] [PubMed] [Google Scholar]
- 13. Peng HX, Wei XY, Xiao YX. et al. Management of Valsa Canker on apple with adjustments to potassium nutrition. Plant Dis. 2016;100:884–9 [DOI] [PubMed] [Google Scholar]
- 14. Xu XX, Liu GY, Liu JQ. et al. Potassium alleviated high nitrogen-induced apple growth inhibition by regulating photosynthetic nitrogen allocation and enhancing nitrogen utilization capacity. Hortic Plant J. 2023; [Google Scholar]
- 15. Leghari SJ, Wahocho NA, Laghari GM. et al. Role of nitrogen for plant growth and development: a review. Adv Environ Biol. 2016;10:209–18 [Google Scholar]
- 16. Xuan W, Beeckman T, Xu G. Plant nitrogen nutrition: sensing and signaling. Curr Opin Plant Biol. 2017;39:57–65 [DOI] [PubMed] [Google Scholar]
- 17. Mu X, Chen Q, Chen F. et al. Within-leaf nitrogen allocation in adaptation to low nitrogen supply in maize during grain-filling stage. Front Plant Sci. 2016;7:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hou W, Trankner M, Lu J. et al. Interactive effects of nitrogen and potassium on photosynthesis and photosynthetic nitrogen allocation of rice leaves. BMC Plant Biol. 2019;19:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park M, Cho S, Park J. et al. Size-dependent variation in leaf functional traits and nitrogen allocation trade-offs in Robinia pseudoacacia and Cornus controversa. Tree Physiol. 2019;39:755–66 [DOI] [PubMed] [Google Scholar]
- 20. Sun WJ, Zhang JC, Ji XL. et al. Low nitrate alleviates iron deficiency by regulating iron homeostasis in apple. Plant Cell Environ. 2021;44:1869–84 [DOI] [PubMed] [Google Scholar]
- 21. Wen S, Liu B, Long S. et al. Low nitrogen level improves low-light tolerance in tall fescue by regulating carbon and nitrogen metabolism. Environ Exp Bot. 2022;194:104749 [Google Scholar]
- 22. Dziedek C, Hardtle W, von Oheimb G. et al. Nitrogen addition enhances drought sensitivity of young deciduous tree species. Front Plant Sci. 2016;7:1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng WT, Qi WL, Nie MM. et al. Magnesium supports nitrogen uptake through regulating NRT2.1/2.2 in soybean. Plant Soil. 2020;457:97–111 [Google Scholar]
- 24. Wang F, Ge S, Xu X. et al. Multiomics analysis reveals new insights into the apple fruit quality decline under high nitrogen conditions. J Agric Food Chem. 2021;69:5559–72 [DOI] [PubMed] [Google Scholar]
- 25. Li J, Hu W, Lu Z. et al. Imbalance between nitrogen and potassium fertilization influences potassium deficiency symptoms in winter oilseed rape (Brassica napus L.) leaves. Crop J. 2022;10:565–76 [Google Scholar]
- 26. Tsay YF, Ho CH, Chen HY. et al. Integration of nitrogen and potassium signaling. Annu Rev Plant Biol. 2011;62:207–26 [DOI] [PubMed] [Google Scholar]
- 27. Coskun D, Britto DT, Kronzucker HJ. The nitrogen-potassium intersection: membranes, metabolism, and mechanism. Plant Cell Environ. 2017;40:2029–41 [DOI] [PubMed] [Google Scholar]
- 28. Wang M, Zheng Q, Shen Q. et al. The critical role of potassium in plant stress response. Int J Mol Sci. 2013;14:7370–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu G, Fan X, Miller AJ. Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol. 2012;63:153–82 [DOI] [PubMed] [Google Scholar]
- 30. Sun T, Zhang J, Zhang Q. et al. Integrative physiological, transcriptome, and metabolome analysis reveals the effects of nitrogen sufficiency and deficiency conditions in apple leaves and roots. Environ Exp Bot. 2021;192:104633 [Google Scholar]
- 31. Perchlik M, Tegeder M. Leaf amino acid supply affects photosynthetic and plant nitrogen use efficiency under nitrogen stress. Plant Physiol. 2018;178:174–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin SH, Kuo HF, Canivenc G. et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell. 2008;20:2514–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu Z, Luo J, Han Y. et al. Low nitrogen enhances nitrogen use efficiency by triggering NO3− uptake and its long-distance translocation. J Agric Food Chem. 2019;67:6736–47 [DOI] [PubMed] [Google Scholar]
- 34. Takashima T, Hikosaka K, Hirose T. Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 2004;27:1047–54 [Google Scholar]
- 35. Zhong C, Jian SF, Huang J. et al. Trade-off of within-leaf nitrogen allocation between photosynthetic nitrogen-use efficiency and water deficit stress acclimation in rice (Oryza sativa L.). Plant Physiol Biochem. 2019;135:41–50 [DOI] [PubMed] [Google Scholar]
- 36. Lu Z, Lu J, Pan Y. et al. Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant Cell Environ. 2016;39:2428–39 [DOI] [PubMed] [Google Scholar]
- 37. Xu XX, Wang F, Xing Y. et al. Appropriate and constant potassium supply promotes the growth of M9T337 apple rootstocks by regulating endogenous hormones and carbon and nitrogen metabolism. Front Plant Sci. 2022;13:827478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wright IJ, Westoby M. Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytol. 2002;155:403–16 [DOI] [PubMed] [Google Scholar]
- 39. Xie K, Lu Z, Pan Y. et al. Leaf photosynthesis is mediated by the coordination of nitrogen and potassium: the importance of anatomical-determined mesophyll conductance to CO2 and carboxylation capacity. Plant Sci. 2020;290:110267 [DOI] [PubMed] [Google Scholar]
- 40. Tenhaken R. Cell wall remodeling under abiotic stress. Front Plant Sci. 2015;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Evans JR. Mesophyll conductance: walls, membranes and spatial complexity. New Phytol. 2021;229:1864–76 [DOI] [PubMed] [Google Scholar]
- 42. Zhu XG, Ed S, Long SP. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol. 2007;145:513–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu X, Du X, Wang F. et al. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front Plant Sci. 2020;11:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu X, Zhang X, Liu C. et al. Appropriate increasing potassium supply alleviates the inhibition of high nitrogen on root growth by regulating antioxidant system, hormone balance, carbon assimilation and transportation in apple. Sci Hortic. 2023;311:111828 [Google Scholar]
- 45. Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta Bioenerg. 1989;975:384–94 [Google Scholar]
- 46. Long SP, Bernacchi CJ. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot. 2003;54:2393–401 [DOI] [PubMed] [Google Scholar]
- 47. Harley PC, Loreto F, Di Marco G. et al. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 1992;98:1429–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grassi G, Magnani F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005;28:834–49 [Google Scholar]
- 49. Lu Z, Xie K, Pan Y. et al. Potassium mediates coordination of leaf photosynthesis and hydraulic conductance by modifications of leaf anatomy. Plant Cell Environ. 2019;42:2231–44 [DOI] [PubMed] [Google Scholar]
- 50. Liu T, Ren T, White PJ. et al. Storage nitrogen co-ordinates leaf expansion and photosynthetic capacity in winter oilseed rape. J Exp Bot. 2018;69:2995–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomas RL, Sheard RW, Moyer JR. Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron J. 1967;59:240–3 [Google Scholar]
- 52. Niinemets Ü, Tenhunen JD. A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ. 1997;20:845–66 [Google Scholar]
- 53. Lv S, Jiang P, Chen X. et al. Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol Biochem. 2012;51:47–52 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that all experimental data are available and accessible via the main text and/or the supplemental data.