Abstract
Objective
Motivation is critically important for rehabilitation, exercise, and motor performance, but its neural basis is poorly understood. Recent correlational research suggests that the dorsomedial prefrontal cortex (dmPFC) may be involved in motivation for walking activity and/or descending motor output. This study experimentally evaluated brain activity changes in periods of additional motivation during walking exercise and tested how these brain activity changes relate to self-reported exercise motivation and walking speed.
Methods
Adults without disability (N = 26; 65% women; 25 [standard deviation = 5] years old) performed a vigorous exercise experiment involving 20 trials of maximal speed overground walking. Half of the trials were randomized to include “extra-motivation” stimuli (lap timer, tracked best lap time, and verbal encouragement). Wearable near-infrared spectroscopy measured oxygenated hemoglobin responses from frontal lobe regions, including the dmPFC, primary sensorimotor, dorsolateral prefrontal, anterior prefrontal, supplementary motor, and dorsal premotor cortices.
Results
Compared with standard trials, participants walked faster during extra-motivation trials (2.43 vs 2.67 m/s; P < .0001) and had higher oxygenated hemoglobin responses in all tested brain regions, including dmPFC (+842 vs +1694 μM; P < .0001). Greater dmPFC activity was correlated with more self-determined motivation for exercise between individuals (r = 0.55; P = .004) and faster walking speed between trials (r = 0.18; P = .0002). dmPFC was the only tested brain region that showed both of these associations.
Conclusion
Simple motivational stimuli during walking exercise seem to upregulate widespread brain regions. Results suggest that dmPFC may be a key brain region linking affective signaling to motor output.
Impact
These findings provide a potential biologic basis for the benefits of motivational stimuli, elicited with clinically feasible methods during walking exercise. Future clinical studies could build on this information to develop prognostic biomarkers and test novel brain stimulation targets for enhancing exercise motivation (eg, dmPFC).
Keywords: Aerobic Exercise, Behavior Regulation, Brain, fNIRS, Gait, Locomotion
Introduction
Human motor behavior emerges from the interaction between environmental and individual constraints that are further bounded by a task goal.1–7 Motivation is a critical constraint to consider in explaining how individuals approach a movement task, are pushed to discover novel task solutions, and ultimately improve motor performance with practice.7 Self-determination theory8–10 differentiates between intrinsic and extrinsic forms of motivation, where intrinsic motivation refers to performing an action because it is inherently satisfying.8–11 Extrinsic motivation, on the other hand, refers to actions driven by external pressures or rewards.9
Many human behaviors begin with extrinsic motivation, then become more self-determined as individuals progress from strictly external regulation to higher degrees of introjection (seeking approval from self or others), then identification (conscious valuing of activity), and eventually, intrinsic motivation.9 Behaviors regulated primarily by external and introjected motivations are considered to be controlled, whereas behaviors regulated primarily by identified and intrinsic motivations are considered autonomous.10
Motivation for physical activity, especially in its more self-determined forms,12,13 can play an important positive role in an individual’s engagement, physical performance, motor skill acquisition, and outcomes in physical rehabilitation.14–19 For example, greater exercise motivation during inpatient stroke rehabilitation has been associated with increased participation in physical activity after discharge (especially intrinsic, identified, and introjected motivations).13 Daily feedback about self-selected fast gait speed during inpatient stroke rehabilitation has also been shown to significantly improve gait speed at discharge,20 potentially by augmenting identified motivation (assuming participants valued faster walking), or even intrinsic motivation (eg, if it made rehabilitation feel more like a game).
Although motivation for physical activity is generally considered to be critically important for rehabilitation, exercise, and motor performance, it has largely been studied using subjective questionnaires or interviews,13,21–24 and the neural basis remains poorly understood. We speculate that a better understanding of the neurobiology of exercise motivation could lead toward: more objective measurement (including unconscious aspects); more accurate prognostication of movement recovery and training response; and novel brain stimulation targets to enhance motivation for exercise. Broadly speaking, different aspects of motivation have been related to interactions among various brain regions, including the dorsolateral prefrontal cortex, anterior prefrontal cortex (aPFC), supplementary motor area (SMA), anterior cingulate cortex, ventral striatum, and anterior insula.25–27
However, technical challenges have made it difficult to measure motivation-related brain activity during mobility tasks like walking that involve whole-body transport and continuous head motion.28 Thus, prior studies aiming to probe the neural basis of motivation for mobility activities have primarily been limited to correlational designs. Intriguingly, these correlational mobility studies have identified a previously unconsidered brain region in the dorsomedial prefrontal cortex (dmPFC) that could play a role in motivation and/or descending motor output.28,29 Adults without disability who had greater dmPFC resting connectivity during brain magnetic resonance imaging (MRI) also walked further during a 6-Minute Walk Test,28 and persons with and without stroke who had greater dmPFC activation during imagined walking inside an MRI scanner also had better overall gait function.29 Still, the asynchronous measurement of brain activity and walking function in these correlational studies makes it impossible to draw any conclusions about the role of dmPFC or any other brain regions in motivation for walking activity.
Functional near-infrared spectroscopy (fNIRS) may help overcome this technical limitation by enabling the study of brain activity during mobility tasks like walking. fNIRS uses infrared light to measure changes in blood oxygenation, which can be used to infer cerebral cortex activity when the light sources and detectors are arranged on the scalp.30 Unlike other imaging modalities, head motion does not create signal artifact with fNIRS, as long as the positions of the light sources and detectors are maintained.30 Thus, fNIRS is well-suited to studying mobility tasks. However, no prior walking studies have capitalized on fNIRS to experimentally assess differences in brain activity underlying different motivational conditions.
The purpose of this fNIRS study was to assess brain activity changes in periods of additional motivation during walking exercise. We also tested how the magnitude of these brain activity changes relates to self-reported motivation for exercise, and to differences in walking speed between trials. In line with previous correlational studies,28,29 we hypothesized that dmPFC would be more active during periods of additional motivation and would have greater motivation-related activity than other brain regions. We also hypothesized that greater dmPFC activation would be associated with more self-determined motivation and faster walking.
Methods
This study was approved by the University of Cincinnati Institutional Review Board and performed between February 2021 and March 2022. Adult participants without disability were recruited from the University community to complete online questionnaires and perform a single visit walking experiment with fNIRS. Written informed consent was obtained prior to participation.
Participants
To be eligible, participants had to be 18 to 80 years old, able to communicate with investigators and provide informed consent, not pregnant, and without any health conditions that would require medical clearance for vigorous exercise according to the American College of Sports Medicine preparticipation screening algorithm.31
Experimental Design
Each participant performed 20 total trials of maximal speed overground walking back and forth along a straight 12-m course while wearing the fNIRS system (Fig. 1A and B). Each trial included 2 laps back and forth (48 m). Between trials, there was a mandatory 20-second standing rest break, then participants were able to start the next trial when ready. A computer was placed on a raised table at the starting and finishing end of the course, while floor tape marked the other end. PyschoPy software32 was used to present audiovisual stimuli, randomize trial conditions, time laps, and wirelessly transmit time stamps for each trial to the fNIRS data collection software, using the lab streaming layer protocol. Participants pressed a large button connected to the computer to start each trial, start the second lap, and finish the trial.
Figure 1.
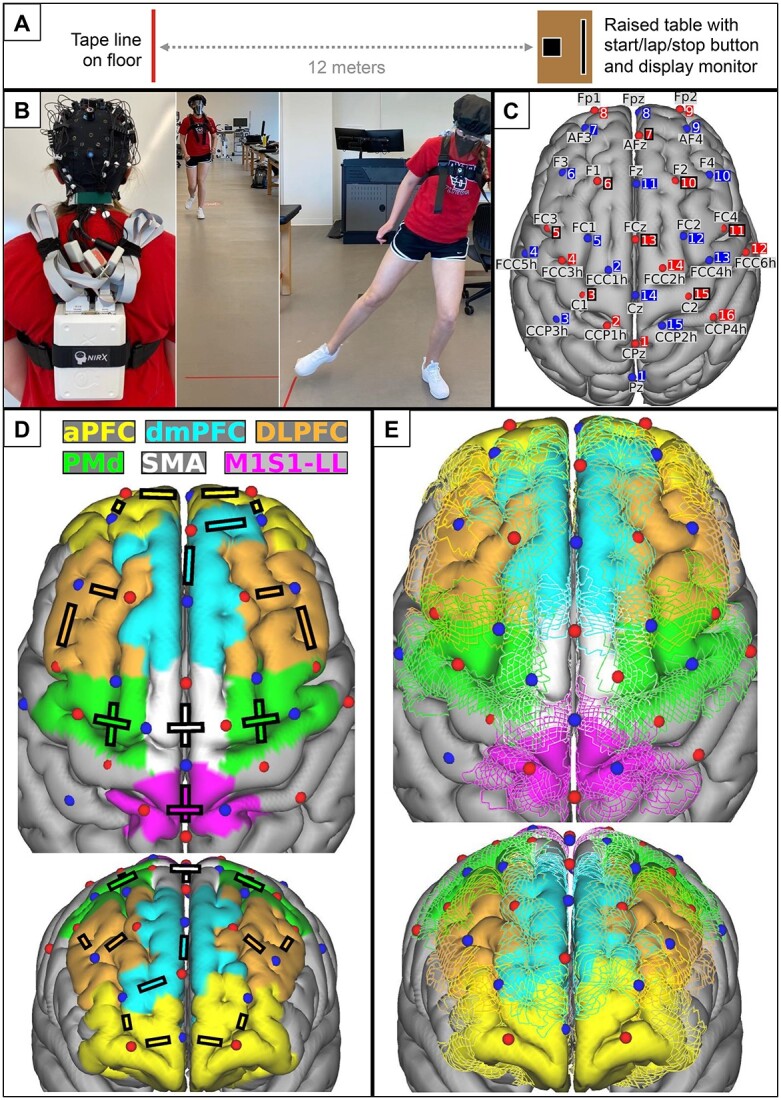
Experimental setup. (A) Walking course schematic. (B) Functional near-infrared spectroscopy (fNIRS) cap setup and wire management (left) for trial performance (right). (C) Locations of fNIRS sources (red spheres) and detectors (blue spheres) are visualized on the cortical surface of the MNI152 standard brain model. Each optode is shown with its standard 10%–5% location label and source or detector number. Black squares around source numbers 3, 5, 6, 7, 10, 11, 13, and 15 show the locations of the 8 short-channel detectors. (D) Lines between specific sources and detectors represent channels that were averaged to represent each cortical region of interest. (E) Between-participant variability in the brain regions underlying the optodes was estimated by coregistering 20 individual brain magnetic resonance images from the Human Connectome Project with the 10%–5% optode locations in standard brain space, using registration constraints to mimic cap-based optode placement at 10%–5% scalp locations. Estimated brain region boundaries from all 20 individuals are shown relative to the optode locations. aPFC = anterior prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; M1S1-LL = primary sensorimotor cortex for the lower limb; PMd = dorsal premotor cortex; SMA = supplementary motor area.
Participants were instructed to walk as fast as possible (without running) for all trials, and half of the trials were randomized to include additional audiovisual stimuli intended to augment motivation. For these “extra-motivation” trials, the computer displayed a large lap timer and the participant’s current best lap time, provided an auditory count of the lap time every 5 seconds, and said “new record!” whenever the best lap time was updated. Throughout the extra-motivation trials, at least 2 study team members also provided verbal encouragement. None of these extra stimuli were presented during standard trials.
Throughout the experiment, heart rate (HR) was also recorded at 0.33 Hz using an electrocardiographic monitor (Polar H7-H10; Polar Electro, Kempele, Finland), and participants were required to wear a face mask due to the COVID-19 pandemic.
Functional Near-Infrared Spectroscopy Data Collection
A wearable fNIRS system (NIRSport2; NIRx Medical Technologies, Berlin, Germany) was used to record brain activity during the experiment (Fig. 1B) at a sampling rate of 5.09 Hz. The 32 dual-tip optodes included 16 light sources emitting infrared wavelengths of 760 and 850 nm and 16 silicon photodiode detectors. Optodes were secured to the head at landmarks from the 10 to 5 electroencephalogram location system (10%–5% system)33 (hereafter, referred to as “10%–5%”) using an EasyCap (EasyCap GmbH, Wörthsee, Germany) and spring-loaded optode holders. Wires were managed with cable trees, ties, and holders to avoid tension on the optodes during walking, and a black shower cap was placed over the optodes to block environmental light.
Brain regions of interest included dmPFC, primary sensorimotor cortex for the lower limb (M1S1-LL), dorsolateral prefrontal cortex, aPFC, SMA, and dorsal premotor cortex (PMd). The optode configuration (Fig. 1C and D; Tab. 1) was designed by visualizing brain regions of interest on the cortical surface alongside the average locations of the 10%–5% landmarks in the same standard Montreal Neurological Institute (MNI) brain space (MNI152 6th generation).34 Cortical surface labels for the regions of interest in the MNI template brain were generated by combining the Human Motor Area Template35 with the Human Connectome Project Multimodal Parcellation36 as previously described.37
Table 1.
Functional Near-Infrared Spectroscopy Optode Configurationa
| Source | 10%–5% Label | MNI Coordinates (mm) | Detector | 10%–5% Label | MNI Coordinates (mm) |
|---|---|---|---|---|---|
| S1 | CPz | 0.83, −41.89, 75.59 | D1 | Pz | −0.98, −66.75, 62.61 |
| S2 | CCP1h | −15.04, −30.55, 77.26 | D2 | FCC1h | −14.19, 1.2, 72.89 |
| S3 | C1 | −28.43, −14.43, 71.27 | D3 | CCP3h | −43.19, −30.52, 65.78 |
| S4 | FCC3h | −40.33, 2.49, 60.71 | D4 | FCC5h | −60.8, −1.96, 37.33 |
| S5 | FC3 | −48.24, 16.31, 45.55 | D5 | FC1 | −25.88, 16.41, 62.65 |
| S6 | F1 | −20.49, 44.82, 46.59 | D6 | F3 | −39.81, 44.33, 31.74 |
| S7 | AFz | 2.90, 64.49, 25.87 | D7 | AF3 | −27.23, 64.88, 14.87 |
| S8 | Fp1 | −22.08, 67.58, −6.47 | D8 | Fpz | 3.02, 67.61, −3.04 |
| S9 | Fp2 | 25.86, 68.06, −7.13 | D9 | AF4 | 29.57, 64.55, 15.08 |
| S10 | F2 | 22.99, 44.91, 47.06 | D10 | F4 | 42.36, 43.57, 33.13 |
| S11 | FC4 | 50.05, 16.47, 46.51 | D11 | Fz | 1.46, 44.16, 49.44 |
| S12 | FCC6h | 62.88, −1.1, 37.31 | D12 | FC2 | 27.78, 17.88, 63.08 |
| S13 | FCz | 0.91, 16.38, 64.36 | D13 | FCC4h | 42.27, 2.62, 60.65 |
| S14 | FCC2h | 16.03, 2.4, 73.06 | D14 | Cz | 0.88, −13.47, 74.14 |
| S15 | C2 | 30.3, −14.79, 71.79 | D15 | CCP2h | 15.81, −30.26, 77.98 |
| S16 | CCP4h | 44.43, −29.43, 65.85 |
The channels for brain regions were as follows: dorsomedial prefrontal cortex—S7–D11 and S7–D9; primary sensorimotor cortex for the lower limb—S1–D14 and S2–D15; dorsolateral prefrontal cortex—S5–D6, S6–D6, S10–D10, and S11–D10; anterior prefrontal cortex—S8–D7, S9–D9, S8–D8, and S9–D8; supplementary motor area—S13–D14 and S14–D2; and dorsal premotor cortex—S3–D5, S4–D2, S14–D13, and S15–D12. MNI = Montreal Neurological Institute.
Between-participant variability in the brain regions underlying the optodes was estimated by coregistering 20 individual brain MRI scans from the Human Connectome Project38 with the 10%–5% optode locations in MNI space. The Human Connectome Project participants included in this analysis were the same as those included in a prior study.39 The sample included 10 women and 10 men with a mean age of 28 (SD = 3) years. Regions of interest were labeled for each individual by projecting the template cortical surface labels onto each individual cortical surface, using the MSMAll multimodal surface registrations calculated in the Human Connectome Project preprocessing pipelines.38 This registration is based on multiple structural and functional features to better account for individual differences in the location of functional cortical areas.38 Cortical region labels for each individual were coregistered with the 10%–5% optode locations in MNI space using a constrained registration procedure designed to mimic our cap-based optode placement at 10%–5% scalp locations. This registration involved skull-constrained rigid rotation and global rescaling only using the pairreg function in FSL software40 with just 7 degrees of freedom. Volumetric region-of-interest labels for each individual in MNI space were then projected onto the surface of the MNI template cortex so that individual variability in region boundaries could be visualized relative to the optode locations (Fig. 1E).
Source-detector pairs with approximately 3-cm separation formed standard channels to record cortical activity from the regions of interest. Eight short-distance detectors with 8-mm source separation also formed short-distance channels to record signal of noninterest superficial to the brain. This can be used to filter motion artifacts (from cap slippage on the head) and global physiologic noise (eg, from hemodynamic fluctuations) out of the standard channel data.41–44 The short-distance detector bundle occupied 1 of the 16 detector optodes.
Functional Near-Infrared Spectroscopy Experiment Data Processing
The NIRS Toolbox45 (version 5/25/2023) and a custom MATLAB (The MathWorks, Inc, Natick, MA, USA) script were used to process data. fNIRS data were downsampled to 5 Hz, “bad” channels with a structured noise index of <1.5 were removed,45,46 and voltages were converted to hemoglobin concentrations using the Beer–Lambert Law.45,47 The first 6 principal components from the short-channel data were regressed out of the standard channel data using autoregressive, iteratively reweighted least squares regression.44,45,48 The filtered oxygenated hemoglobin (HbO2) data were then averaged among channels representing the same cortical region (Fig. 1D).
The first 2 (practice) trials were discarded, leaving 18 for analysis (9 extra-motivation and 9 standard trials). Since walking speed and trial duration varied between trials, the time scale was normalized by interpolating HbO2 data to percentage of trial duration. For each cortical region and each trial, the HbO2 response (ΔHbO2) was calculated as the mean HbO2 value from 0% to 110% trial duration minus the mean HbO2 value just before trial onset (from −20% to 0% trial duration).
HR data were expressed as a percentage of age-predicted maximal HR, which was calculated using 206.9–0.67 × age (years).49 HR was averaged across the whole session to measure overall exercise intensity and was averaged from 100% to 150% trial duration to capture the response for each trial.
Self-Report Measures
The following questionnaires were completed online within 2 (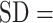 3) days (mean
3) days (mean 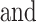 SD) of the fNIRS experiment.
SD) of the fNIRS experiment.
The International Physical Activity Questionnaire50 short form is a 9-item questionnaire that collects information on the time spent participating in vigorous-intensity, moderate-intensity, walking, and sitting activities over the last 7 days. Results were used for descriptive purposes to estimate total metabolic equivalents of energy expenditure per week and to categorize individuals as having low, moderate, or high amounts of physical activity.50
The Behavioral Regulation in Exercise Questionnaire51 includes 15 items assessing motivation for exercise and is based on self-determination theory. Each item is rated from 0 (“not true for me”) to 4 (“very true for me”). Subsets of questions were averaged to calculate scores for different dimensions of motivation (external, introjected, identified, and intrinsic),9,52 and subsets of these domains were averaged to calculate scores for autonomous (intrinsic and identified) and controlled (introjected and external) motivations.53 The primary measure of self-determined motivation for exercise was the difference between the autonomous and controlled motivation scores.53
Statistical Analysis
SAS software (SAS Institute Inc, Cary, NC, USA) version 9.4 was used for analysis, except as indicated otherwise. The effects of trial condition (extra motivation vs standard) on walking speed, HR, and brain ΔHbO2 were assessed using separate linear regression models. The models included data from each trial and assumed compound symmetry covariance between repeated trials from the same participant.
In the brain ΔHbO2 (primary) model, condition effects and (co)variances were estimated separately for each brain region, and trial HR was included as a covariate. This covariate was added to control for potential confounding effects of global hemodynamic differences (ie, higher HR) on brain ΔHbO2 differences between conditions, in case the short-channel regression did not fully remove such physiologic noise. Within this model, the effect of extra motivation on ΔHbO2 was estimated for each brain region by contrasting the 2 conditions, and the magnitude of this extra-motivation effect for dmPFC (the primary region of interest) was compared to each other region.
We also assessed how individual differences in the brain responses to extra motivation related to self-reported motivation for exercise. For this analysis, mean extra-motivation effects on ΔHbO2 for each participant and brain region were calculated by averaging across trials within conditions. Partial Pearson correlations were then calculated between these brain responses and Behavioral Regulation in Exercise Questionnaire scores, controlling for each participant’s mean HR across all trials.
To assess how brain activity related to walking speed, we used data from each trial to calculate partial repeated-measures correlations between brain ΔHbO2 and speed, controlling for trial condition (extra motivation vs standard). Repeated-measures correlations estimate common within-participant associations54 and were calculated using R software55 with the rmcorr package.54
Sample Size
Power analysis was done using the R package pwr.56 Simplified calculations based on a paired t-test indicated that 18 participants would provide 80% power at the 2-sided .05 significance level to detect a medium standardized effect (Cohen d of 0.5) between the extra-motivation and standard conditions in the primary analysis. For the correlation analyses, simplified calculations based on a bivariate Pearson correlation without repeated measures indicated that 23 participants would provide 80% power to detect a correlation as small as 0.55. Thus, we aimed to enroll at least 23 participants.
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
Among 30 persons who consented to participate, 4 were ultimately unavailable to perform the fNIRS experiment. The remaining 26 participants (Tab. 2) were enrolled and completed the study, including the full fNIRS experiment and both self-report measures.
Table 2.
Participant Characteristics (N = 26)a
| Characteristic | Mean (SD) | Range | No. (%) |
|---|---|---|---|
| Age, y | 24.8 (5.0) | 18.8 to 42.1 | |
| Women | 17 (65.4) | ||
| Body mass index, kg/m2 | 24.8 (3.9) | 19.2 to 35.3 | |
| IPAQ total physical activity, MET, min/wk | 2560 (1273) | 330 to 5346 | |
| IPAQ weekly physical activity category | |||
| High | 13 (50.0) | ||
| Moderate | 12 (46.2) | ||
| Low | 1 (3.8) | ||
| BREQ motivation for exercise scores | |||
| Overall self-determined motivation,b −4 to 4 | 0.0 (0.6) | −1.1 to 1.2 | |
| Autonomous motivation,c 0 to 4 | 2.4 (0.6) | 0.9 to 3.3 | |
| Intrinsic motivation, 0 to 4 | 2.8 (0.5) | 1.0 to 4.0 | |
| Identified motivation, 0 to 4 | 2.0 (0.6) | 0.5 to 3.0 | |
| Controlled motivation,d 0 to 4 | 2.4 (0.5) | 1.7 to 3.4 | |
| Introjected motivation, 0 to 4 | 2.1 (0.5) | 1.3 to 3.0 | |
| External motivation, 0 to 4 | 2.7 (0.6) | 1.3 to 3.8 |
BREQ = Behavioral Regulation in Exercise Questionnaire; IPAQ = International Physical Activity Questionnaire; MET = metabolic equivalents of energy expenditure.
Autonomous minus controlled.
Average of intrinsic and identified.
Average of introjected and external.
Walking Exercise Intensity
The average HR across the fNIRS experiment was 84.7% (SD = 8.1%) age-predicted maximal HR (mean 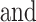 SD across participants). Compared with standard trials, participants walked faster during extra-motivation trials (2.67 [SD = 0.32] vs 2.43 [
SD across participants). Compared with standard trials, participants walked faster during extra-motivation trials (2.67 [SD = 0.32] vs 2.43 [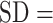 0.31] m/s; P < .0001) and had slightly higher HRs (89.1
0.31] m/s; P < .0001) and had slightly higher HRs (89.1 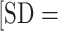 8.5] vs 87.8%
8.5] vs 87.8% 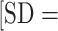 8.3%] age-predicted maximal HR; P < .0001).
8.3%] age-predicted maximal HR; P < .0001).
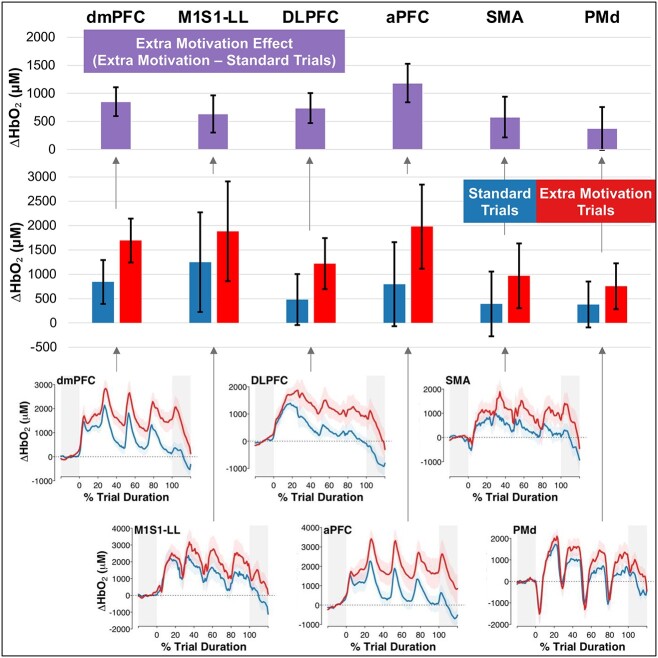
Effects of Extra Motivation on Brain Activity
Compared with standard trials, extra-motivation trials had higher ΔHbO2 in all brain regions tested (Fig. 2; Tab. 3), and the difference between trial conditions was statistically significant for every region (P 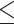 .002) except PMd (P = .053). The magnitude of this extra-motivation effect for dmPFC was significantly greater than that for PMd (P = .04) but not significantly different from that for the other regions (P
.002) except PMd (P = .053). The magnitude of this extra-motivation effect for dmPFC was significantly greater than that for PMd (P = .04) but not significantly different from that for the other regions (P 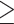 .13).
.13).
Figure 2.
Oxygenated hemoglobin responses (ΔHbO2) by task condition and brain region. Bars show mean estimates, and error bars show 95% CIs for ΔHbO2 from baseline, controlled for trial heart rate. Bottom panels show the ΔHbO2 time series averaged across trials and participants for each task condition, with a shaded region to depict the SE across participants. aPFC = anterior prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; M1S1-LL = primary sensorimotor cortex for the lower limb; PMd = dorsal premotor cortex; SMA = supplementary motor area.
Table 3.
Oxygenated Hemoglobin Responses (ΔHbO2) by Task Condition and Brain Regiona
| Brain Region | Mean Estimate (95% CI) for ΔHbO 2 From Baseline, μM, Controlled for Trial Heart Rate | ||
|---|---|---|---|
| Standard Trials | Extra-Motivation Trials | Extra-Motivation Effect (Extra − Standard) | |
| dmPFC | 842 (391 to 1293) | 1694 (1243 to 2145) | 852 (596 to 1109) |
| M1S1-LL | 1249 (225 to 2274) | 1884 (859 to 2908) | 634 (303 to 965) |
| DLPFC | 480 (−44 to 1004) | 1219 (695 to 1743) | 739 (472 to 1006) |
| aPFC | 796 (−68 to 1659) | 1979 (1116 to 2843) | 1184 (840 to 1527) |
| SMA | 390 (−275 to 1056) | 969 (304 to 1634) | 579 (216 to 941) |
| PMd | 379 (−92 to 851) | 755 (284 to 1226) | 375 (−5 to 756) |
aPFC = anterior prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; M1S1-LL = primary sensorimotor cortex for the lower limb; PMd = dorsal premotor cortex; SMA = supplementary motor area.
Brain Activity Correlations With Self-Reported Exercise Motivation Across Participants
Across participants, more self-determined motivation for exercise was correlated with greater mean brain ΔHbO2 to extra motivation (relative to standard trials) in dmPFC and aPFC (Tab. 4). None of the individual Behavioral Regulation in Exercise Questionnaire motivation domains contributing to the overall self-determination score showed significant correlations with the brain ΔHbO2, but the estimated associations for dmPFC and aPFC were all positive for autonomous forms of motivation and negative for controlled forms.
Brain Activity Correlations With Walking Speed Across Trials
Within participants and conditions (extra motivation vs standard), greater brain ΔHbO2 increases in dmPFC, M1S1-LL, SMA, and PMd were correlated with faster walking speed across trials (Tab. 4).
Discussion
This walking exercise study among adults without disability experimentally assessed brain activity changes during periods of additional motivation and examined how this brain activity relates to self-reported motivation and walking speed. The exercise protocol appeared to provide sufficient motivational challenge since it required vigorous aerobic exertion. Faster walking during trials randomized to include extra motivation also showed that the experimental manipulation successfully altered participant behavior. As hypothesized, dmPFC was more active during extra-motivation trials, and greater dmPFC activity was associated with more self-determined motivation for exercise and faster walking speeds. In addition, dmPFC was the only tested brain region that was associated with both self-reported motivation and walking speed. These results substantially strengthen prior findings from correlational MRI studies,28,29 suggesting that dmPFC plays an important role in motivation for walking activity and possibly motor output.
Conversely, our hypothesis that the dmPFC would have greater motivation-related activity than other brain regions was not supported, aside from a significant difference from PMd that could be attributable to multiple testing. Like dmPFC, most other tested regions were also more active during extra-motivation trials than during standard trials. Although this could indicate that motivation effects are not specific to dmPFC, it could also reflect extraneous differences between these conditions that were unrelated to motivation. For example, auditory stimulation was also likely higher in our extra-motivation condition and cognitive processing may also have been more demanding because of the additional stimuli. It is also possible that some brain regions not specifically involved in motivation showed greater activity during the extra-motivation trials than during the standard trials because of afferent input from brain center(s) that generate motivation signals (potentially dmPFC). Preliminary evidence from this study that supports a motivation signaling role of dmPFC includes the immediate increase in dmPFC activity at trial onset (also present for dorsolateral prefrontal cortex and aPFC), compared with the delayed activity increases for M1S1-LL, SMA, and PMd (Fig. 2, bottom); and the fact that dmPFC was the only tested brain region that was associated with both self-reported motivation and walking speed.
Across individuals, a greater dmPFC (and aPFC) response to the extra-motivation condition was specifically associated with more self-determined motivation for exercise, suggesting that these regions may specifically regulate more autonomous and less controlled behavior. More autonomous or self-determined exercise motivation is generally considered to have more positive effects on exercise behavior and experiences,12 which implies beneficial effects of higher dmPFC activity. Brain activity correlations with the different domains of self-reported motivation may also reflect the extent to which the experimental manipulation targeted each motivation domain. From this perspective, it may initially seem counterintuitive that the additional external inputs (eg, lap timer, verbal encouragement) in the extra-motivation trials appeared to be more effective at upregulating brain activity for individuals who reported more intrinsic (and less extrinsic) forms of motivation for exercise.
Table 4.
Behavioral Associations With Brain Oxygenated Hemoglobin Responses (ΔHbO2)a
| Parameter | Correlation for the Following Brain Region: | |||||
|---|---|---|---|---|---|---|
| dmPFC | M1S1-LL | DLPFC | aPFC | SMA | PMd | |
| Self-reported motivation for exercise (BREQ score) associated with mean ΔHbO2 to extra motivation (relative to standard trials)b | ||||||
| Overall self-determined motivation (autonomous − controlled) | 0.55c | 0.18 | 0.36 | 0.46c | −0.06 | 0.23 |
| Autonomous motivation (intrinsic + identified) | 0.35 | 0.24 | 0.34 | 0.32 | −0.01 | 0.38 |
| Intrinsic motivation | 0.31 | 0.23 | 0.36 | 0.23 | 0.06 | 0.38 |
| Identified motivation | 0.35 | 0.22 | 0.28 | 0.36 | −0.07 | 0.34 |
| Controlled motivation (introjected + external) | −0.23 | 0.07 | −0.03 | −0.17 | 0.06 | 0.18 |
| Introjected motivation | −0.29 | 0.02 | −0.01 | −0.21 | 0.06 | 0.03 |
| External motivation | −0.13 | 0.10 | −0.04 | −0.09 | 0.04 | 0.27 |
| Trial-by-trial ΔHbO2 associated with walking speedd | ||||||
| Walking speed | 0.18c | 0.09c | 0.07 | 0.03 | 0.16c | 0.21c |
aPFC = anterior prefrontal cortex; BREQ = Behavioral Regulation for Exercise Questionnaire; DLPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; M1S1-LL = primary sensorimotor cortex for the lower limb; PMd = dorsal premotor cortex; SMA = supplementary motor area.
Partial correlations, controlled for trial heart rate.
P < .05.
Partial repeated-measures correlations (within participants), controlled for trial type (extra motivation vs standard).
In the broader psychology literature, it was originally proposed that external rewards could only generate extrinsic motivation57 and would diminish intrinsic motivation by reducing perceived autonomy.58 However, general interest theory59 subsequently departed from this former view (cf.57) based on evidence that external rewards can actually enhance intrinsic motivation under more natural experimental conditions.60,61 For example, external rewards that are contingent on meeting a specific, challenging, and attainable performance criterion appear to increase perceived autonomy and competence, which can generate intrinsic motivation.59,62 The gain in perceived autonomy with this type of reward contingency is thought to occur because knowing what level of performance is needed to receive a reward gives the individual more control over their own reinforcement and environment.59,62 Thus, our display of the participant’s best lap time and positive feedback for each new record may have been important stimuli for generating more autonomous forms of motivation. Future studies could benefit gait rehabilitation and exercise interventions by more specifically identifying inputs and contextual conditions that optimize self-determined engagement in walking exercise.
The positive association we observed between brain responses and self-determined motivation was based on differences between individuals. Therefore, it is also possible that our external motivational inputs may have had more intrinsic salience to some participants. Individual differences in interests and experiences can influence the extent to which a particular activity is intrinsically motivating.63 Additional factors (eg, physical and social environment) may also contribute to an individual’s motivation for physical activity.64 These potential individual differences should be considered when aiming to augment motivation in future studies, exercise programs, and clinical rehabilitation.
In addition to these between-participant differences, we also analyzed within-participant differences across trials and found that greater dmPFC, M1S1-LL, SMA, and PMd activity was associated with faster walking speed while controlling for the effects of trial condition (extra motivation vs standard). Unlike M1S1-LL, SMA, and PMd, dmPFC is not typically considered a motor region, but this finding adds to a growing body of evidence that dmPFC may indeed be a novel motor output area or at least highly relevant to walking function.28,29,37,65 For example, dmPFC was recently found to be a major origin of a key descending projection for gross motor functions like walking (the corticoreticulospinal pathway).37,65
The current study cannot determine whether dmPFC exerted any direct effects on walking through the corticoreticulospinal pathway and/or indirect effects via connections with established motor regions of the cortex (M1S1-LL, SMA, PMd). Given the correlational nature of this result, it is also possible that dmPFC did not exert any effects on walking (noncausal/confounded association) or that faster walking somehow led to greater dmPFC activation (reverse causality). However, it seems unlikely that reverse causality could fully explain the observed dmPFC activity, based on its time course (Fig. 2, bottom). HbO2 concentration in dmPFC increased immediately at trial onset, and separation between the extra-motivation and standard trials began at the first peak, around 5% trial duration (~0.6–1.8 seconds into the trial based on the ranges of trial duration of 12.2–35.6 seconds). ΔHbO2 from motor actions typically has an onset latency of 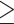 3 seconds,66,67 so afferent input from walking activity was not a likely contributor to this early dmPFC response.
3 seconds,66,67 so afferent input from walking activity was not a likely contributor to this early dmPFC response.
It is encouraging for clinical rehabilitation that most brain regions tested were more active during the extra-motivation condition than during the standard trials, including dmPFC, M1S1-LL, SMA, aPFC, and dorsolateral prefrontal cortex. These findings indicate that simple methods to augment motivation during walking exercise (eg, timing trials, providing feedback about speed and encouragement to beat previous records) can upregulate brain activity across the motor cortex and beyond. Similar motivational techniques have demonstrated feasibility among clinical populations with gait impairment,20,68 and have shown efficacy for improving gait outcomes.20 The current findings provide a possible neurobiological explanation for how this motivational input during rehabilitation might improve outcomes (via widespread upregulation of brain activity).
Limitations
This study attempted the challenging task of elucidating the neural underpinnings of a complex psychological construct (motivation), for which there is no flawless approach.69–71 Although our randomized task comparison design is widely used (eg, in the fMRI literature) and has important strengths over other methods, it still does not guarantee valid causal inference.70 This design evaluates the effects of brain activity on behavior by manipulating the dependent variable (ie, testing effects of behavioral manipulation on brain activity), which assumes a 1:1 relationship between them.70 When evaluating the brain activity underlying motivation, this strong assumption may be particularly tenuous because it is difficult to design experimental conditions that specifically alter motivation without also affecting other neural processes. Addressing the uncertain causal inference from our current results will likely require additional studies targeting the same question with complementary designs.69,70
As referenced above, the analyses correlating brain activity with self-reported motivation and walking speed may have also been confounded by other brain or behavioral variables. We attempted to mitigate this issue by statistically controlling for the most plausible confounding variable in each analysis, but false positive or negative findings are still possible. With our moderate sample size (N = 26), the large number of repeated trials provided sufficient power to compare brain activity between conditions and to detect even relatively small brain activity associations with walking speed between trials. However, we may have been underpowered to detect smaller differences in brain activity between regions or smaller brain activity associations with self-reported motivation between participants.
Mobile brain imaging technology like fNIRS is also limited to measuring activity from more superficial brain structures and has lower spatial resolution than MRI. Thus, our optode configuration was only able to measure from relatively large cortical regions that are each known to contain multiple functional subareas.36 We also did not obtain MRI data in this study to confirm that fNIRS recordings were made from the same brain locations across participants. Our analysis of individual variability in the cortical areas underlying the optodes, using MRI data from the Human Connectome Project (Fig. 1E), allowed us to select optode channels that were most likely to selectively record from each region of interest across individuals. However, we are unable to determine how specifically these regions were tested across our participants, and this individual variability may have decreased our statistical power.
Conclusions
For walking exercise among adults without disability, clinically relevant inputs designed to increase motivation (eg, timing trials, providing feedback about speed and encouragement to beat previous records) elicit widespread increases in cerebral cortex activity, including in the dmPFC, a region with recently discovered relevance to motor function. Greater activity increases in dmPFC were also related to more self-determined motivation for exercise and faster walking speed, suggesting that it could be a key region linking affective signaling to motor output.
Contributor Information
Sarah Doren, Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA.
Sarah M Schwab, Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA.
Kaitlyn Bigner, Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA.
Jenna Calvelage, Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA.
Katie Preston, Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA.
Abigail Laughlin, Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA.
Colin Drury, Department of Neurology and Rehabilitation Medicine, College of Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Brady Tincher, Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA.
Daniel Carl, Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA.
Oluwole O Awosika, Department of Neurology and Rehabilitation Medicine, College of Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Pierce Boyne, Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA.
Funding
This study was supported by grants from the National Institutes of Health (R01HD093694 and UL1TR001425) and the Oliver Family Foundation.
Ethics Approval
This study was approved by the University of Cincinnati Institutional Review Board.
Disclosures and Presentations
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest. The unreviewed version of this manuscript was posted on the bioRxiv preprint server (https://www.biorxiv.org/content/10.1101/2022.12.30.522346v1). A poster with some of the data was presented at the American Physical Therapy Association’s Combined Sections Meeting; February 2–5, 2022; San Antonio, Texas.
References
- 1. Araújo D, Davids K, Bennett SJ, Button C, Chapman G. Emergence of sport skills under constraints. In: Williams AM, Hodges NJ. (eds), Skill Acquisition in Sport. Routledge; 2004: 433–458. [Google Scholar]
- 2. Button C, Seifert L, Chow JY, Davids K, Araujo D. Dynamics of skill acquisition: an ecological dynamics approach. 2nd ed. Human Kinetics. 2020: 1–288. 10.5040/9781718214125. [DOI] [Google Scholar]
- 3. Davids K, Araújo D, Shuttleworth R, Button C. Acquiring skill in sport: a constraints-led perspective. Int J Comput Sci Sport. 2003;41:5–16. [Google Scholar]
- 4. Davids K, Button C, Bennett S. Dynamics of skill acquisition: a constraints-led approach. 1st ed. Hum Kinet. 2008: 1–264. [Google Scholar]
- 5. Guccione AA, Neville BT, George SZ. Optimization of movement: a dynamical systems approach to movement systems as emergent phenomena. Phys Ther. 2019;99:3–9. [DOI] [PubMed] [Google Scholar]
- 6. Wade MG, Whiting HTA, eds. . Motor Development in Children: Aspects of Coordination and Control . Vol 34. Norwell, MA; Dordrecht; Boston: Distributors for the U.S. and Canada, Kluwer Academic Publishers; 1986. [Google Scholar]
- 7. Renshaw I, Davids K, Newcombe D, Roberts W. The Constraints-Led Approach: Principles for Sports Coaching and Practice Design . London, UK: Routledge. 2019. [Google Scholar]
- 8. Deci EL, Ryan RM. The general causality orientations scale: self-determination in personality. J Res Pers. 1985;19:109–134. [Google Scholar]
- 9. Ryan RM, Deci EL. Intrinsic and extrinsic motivations: classic definitions and new directions. Contemp Educ Psychol. 2000;25:54–67. [DOI] [PubMed] [Google Scholar]
- 10. Ryan RM, Deci EL. Self-Determination Theory: Basic Psychological Needs in Motivation, Development, and Wellness. New York: Guilford Press; 2017: 1–756. [Google Scholar]
- 11. Frederick-Recascino CM, Schuster-Smith H. Competition and intrinsic motivation in physical activity: a comparison of two groups. J Sport Behav. 2003;26:240–254. [Google Scholar]
- 12. Thogersen-Ntoumani C, Ntoumanis N. The role of self-determined motivation in the understanding of exercise-related behaviours, cognitions and physical self-evaluations. J Sports Sci. 2006;24:393–404. [DOI] [PubMed] [Google Scholar]
- 13. Thilarajah S, Bower KJ, Pua YH et al. Modifiable factors associated with poststroke physical activity at discharge from rehabilitation: prospective cohort study. Phys Ther. 2020;100:818–828. [DOI] [PubMed] [Google Scholar]
- 14. Chow JY, Davids K, Button C, Renshaw I. Nonlinear Pedagogy in Skill Acquisition . London, UK: Routledge. 2015, 10.4324/9781315813042. [DOI] [Google Scholar]
- 15. Frederick CM, Morrison C, Manning T. Motivation to participate, exercise affect, and outcome behaviors toward physical activity. Percept Mot Skills. 1996;84:691–701. [DOI] [PubMed] [Google Scholar]
- 16. Lewthwaite R. Motivational considerations in physical activity involvement. Phys Ther. 1990;70:808–819. [DOI] [PubMed] [Google Scholar]
- 17. Peek K, Carey M, Sanson-Fisher R, Mackenzie L. Physiotherapists’ perceptions of patient adherence to prescribed self-management strategies: a cross-sectional survey of Australian physiotherapists. Disabil Rehabil. 2017;39:1932–1938. [DOI] [PubMed] [Google Scholar]
- 18. Moreno JA, González-Cutre D, Martín-Albo J, Cervelló E. Motivation and performance in physical education: an experimental test. J Sports Sci Med. 2010;9:79–85. [PMC free article] [PubMed] [Google Scholar]
- 19. Maclean N, Pound P. A critical review of the concept of patient motivation in the literature on physical rehabilitation. Soc Sci Med. 2000;50:495–506. [DOI] [PubMed] [Google Scholar]
- 20. Dobkin BH, Plummer-D'Amato P, Elashoff R et al. International randomized clinical trial, stroke inpatient rehabilitation with reinforcement of walking speed (SIRROWS), improves outcomes. Neurorehabil Neural Repair. 2010;24:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maclean N, Pound P, Wolfe C, Rudd A. Qualitative analysis of stroke patients' motivation for rehabilitation. BMJ. 2000;321:1051–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brunet J, Sabiston CM. Exploring motivation for physical activity across the adult lifespan. Psychol Sport Exerc. 2011;12:99–105. [Google Scholar]
- 23. Morris JH, Oliver T, Kroll T, Joice S, Williams B. Physical activity participation in community dwelling stroke survivors: synergy and dissonance between motivation and capability. A qualitative study. Physiotherapy. 2017;103:311–321. [DOI] [PubMed] [Google Scholar]
- 24. Swinnen E, Lefeber N, Willaert W et al. Motivation, expectations, and usability of a driven gait orthosis in stroke patients and their therapists. Top Stroke Rehabil. 2017;24:299–308. [DOI] [PubMed] [Google Scholar]
- 25. Braver TS, Krug MK, Chiew KS et al. Mechanisms of motivation-cognition interaction: challenges and opportunities. Cogn Affect Behav Neurosci. 2014;14:443–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez-Gamundi P, Yao Y, Chong TT, Heekeren HR, Mas-Herrero E, Marco-Pallarés J. The neural basis of effort valuation: a meta-analysis of functional magnetic resonance imaging studies. Neurosci Biobehav Rev. 2021;131:1275–1287. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt L, Lebreton M, Cléry-Melin M, Daunizeau J, Pessiglione M. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol. 2012;10:e1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyne P, Maloney T, DiFrancesco M et al. Resting-state functional connectivity of subcortical locomotor centers explains variance in walking capacity. Hum Brain Mapp. 2018;39:4831–4843. 10.1002/hbm.24326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boyne P, Doren S, Scholl V et al. Functional magnetic resonance brain imaging of imagined walking to study locomotor function after stroke. Clin Neurophysiol. 2021;132:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Menant JC, Maidan I, Alcock L et al. A consensus guide to using functional near-infrared spectroscopy in posture and gait research. Gait Posture. 2020;82:254–265. [DOI] [PubMed] [Google Scholar]
- 31. Riebe D, Franklin BA, Thompson PD et al. Updating ACSM's recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473–2479. 10.1249/MSS.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 32. Peirce JW. PsychoPy—psychophysics software in Python. J Neurosci Methods. 2007;162:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol. 2001;112:713–719. [DOI] [PubMed] [Google Scholar]
- 34. Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage. 2007;34:1600–1611. [DOI] [PubMed] [Google Scholar]
- 35. Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glasser MF, Coalson TS, Robinson EC et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyne P, DiFrancesco M, Awosika OO, Williamson B, Vannest J. Mapping the human corticoreticular pathway with multimodal delineation of the gigantocellular reticular nucleus and high-resolution diffusion tractography. J Neurol Sci. 2022;434:120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elam JS, Glasser MF, Harms MP et al. The human connectome project: a retrospective. Neuroimage. 2021;244:118543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang Y, Sun W, Toga AW, Ringman JM, Shi Y. A probabilistic atlas of human brainstem pathways based on connectome imaging data. Neuroimage. 2018;169:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 41. Yücel MA, Selb J, Aasted CM et al. Short separation regression improves statistical significance and better localizes the hemodynamic response obtained by near-infrared spectroscopy for tasks with differing autonomic responses. Neurophotonics. 2015;2:035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brigadoi S, Cooper RJ. How short is short? Optimum source-detector distance for short-separation channels in functional near-infrared spectroscopy. Neurophotonics. 2015;2:025005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gagnon L, Perdue K, Greve DN, Goldenholz D, Kaskhedikar G, Boas DA. Improved recovery of the hemodynamic response in diffuse optical imaging using short optode separations and state-space modeling. Neuroimage. 2011;56:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Santosa H, Zhai X, Fishburn F, Sparto PJ, Huppert TJ. Quantitative comparison of correction techniques for removing systemic physiological signal in functional near-infrared spectroscopy studies. Neurophotonics. 2020;7:035009. 10.1117/1.NPh.7.3.035009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santosa H, Zhai X, Fishburn F, Huppert T. The NIRS brain AnalyzIR toolbox. Algorithms. 2018;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhuang C, Meidenbauer KL, Kardan O et al. Scale invariance in fNIRS as a measurement of cognitive load. Cortex. 2022;154:62–76. 10.1016/j.cortex.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 47. Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58:37. 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- 48. Barker JW, Aarabi A, Huppert TJ. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed Opt Express. 2013;4:1366–1379. 10.1364/BOE.4.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK. Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc. 2007;39:822–829. [DOI] [PubMed] [Google Scholar]
- 50. Craig CL, Marshall AL, Sjöström M et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 51. Mullan E, Markland D, Ingledew DK. A graded conceptualisation of self-determination in the regulation of exercise behaviour: development of a measure using confirmatory factor analytic procedures. Personal Individ Differ. 1997;23:745–752. [Google Scholar]
- 52. Markland D, Tobin V. A modification to the behavioural regulation in exercise questionnaire to include an assessment of amotivation. J Sport Exercise Psychol. 2004;26:191–196. [Google Scholar]
- 53. Chemolli E, Gagné M. Evidence against the continuum structure underlying motivation measures derived from self-determination theory. Psychol Assess. 2014;26:575–585. [DOI] [PubMed] [Google Scholar]
- 54. Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. 2017;8:456. 10.3389/fpsyg.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2004. [Google Scholar]
- 56. Champely S, Ekstrom C, Dalgaard P et al. Basic Functions for Power Analysis (pwr). R package version 130. 2020. Comprehensive R Archive Network. Accessed December 20, 2023. https://cran.r-project.org/web/packages/pwr/.
- 57. Deci E, Ryan RM. Intrinsic Motivation and Self-Determination in Human Behavior. New York: Plenum Press; 1985. [Google Scholar]
- 58. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125:627–700. 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- 59. Eisenberger R, Pierce WD, Cameron J. Effects of reward on intrinsic motivation--negative, neutral and positive: Comment on deci, koestner, and ryan (1999). Psychol Bull. 1999;125:677–700. 10.1037/0033-2909.125.6.677. [DOI] [PubMed] [Google Scholar]
- 60. Cameron J, David PW. Reinforcement, reward, and intrinsic motivation: a meta-analysis. Rev Educ Res. 1994;64:363–423. 10.3102/00346543064003363. [DOI] [Google Scholar]
- 61. Cameron J, Pierce WD. The debate about rewards and intrinsic motivation: protests and accusations do not alter the result. Rev Educ Res. 1996;66:39–51. 10.3102/00346543066001039. [DOI] [Google Scholar]
- 62. Eisenberger R, Rhoades L, Cameron J. Does pay for performance increase or decrease perceived self-determination and intrinsic motivation. J Pers Soc Psychol. 1999;77:1026–1040. 10.1037/0022-3514.77.5.1026. [DOI] [Google Scholar]
- 63. Jovanovic D, Matejevic M. Relationship between rewards and intrinsic motivation for learning – researches revie. Procedia. 2014;149:456–460. 10.1016/J.SBSPRO.2014.08.287. [DOI] [Google Scholar]
- 64. Miller A, Pohlig RT, Wright T, Kim HE, Reisman DS. Beyond physical capacity: factors associated with real-world walking activity after stroke. Arch Phys Med Rehabil. 2021;102:1880–1887.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boyne P, Awosika OO, Luo Y. Mapping the corticoreticular pathway from cortex-wide anterograde axonal tracing in the mouse. J Neurosci Res. 2021;99:3392–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kamran MA, Jeong MY, Mannan MMN. Optimal hemodynamic response model for functional near-infrared spectroscopy. Front Behav Neurosci. 2015;9:151. 10.3389/fnbeh.2015.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yamada T, Kawaguchi H, Kato J, Matsuda K, Higo N. Functional near-infrared spectroscopy for monitoring macaque cerebral motor activity during voluntary movements without head fixation. Sci Rep. 2018;8:11941–11947. 10.1038/s41598-018-30416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Boyne P, Scholl V, Doren S et al. Locomotor training intensity after stroke: effects of interval type and mode. Top Stroke Rehabil. 2020;27:483–493. 10.1080/10749357.2020.1728953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rorden C, Karnath H. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:813–819. [DOI] [PubMed] [Google Scholar]
- 70. Sarter M, Berntson GG, Cacioppo JT. Brain imaging and cognitive neuroscience. Toward strong inference in attributing function to structure. Am Psychol. 1996;51:13–21. 10.1037//0003-066x.51.1.13. [DOI] [PubMed] [Google Scholar]
- 71. Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379:2237–2245. 10.1056/NEJMra1706158. [DOI] [PubMed] [Google Scholar]