We show here that wild Atlantic salmon parr and smolt from thermally distinct river systems were impacted differently by cooler and warmer thermal variability. Growth, thermal tolerance and swimming performance were all impacted by river of origin, thermal acclimation temperature and life stage, highlighting the potential need for more river specific conservation guidelines.
Keywords: Atlantic salmon, critical thermal maximum, growth rate, local adaptation, thermal acclimation, thermal performance, thermal variability
Abstract
Temperature in many natural aquatic environments follows a diel cycle, but to date, we know little on how diel thermal cycles affect fish biology. The current study investigates the growth, development and physiological performance of wild Atlantic salmon collected from the Miramichi and Restigouche rivers (NB, Canada). Fish were collected as parr and acclimated to either 16–21 or 19–24°C diel thermal cycles throughout the parr and smolt life stages. Both Miramichi and Restigouche Atlantic salmon parr grew at similar rates during 16–21 or 19–24°C acclimations. However, as smolts, the growth rates of the Miramichi (−8% body mass day−1) and Restigouche (−38% body mass day−1) fish were significantly slower at 19–24°C, and were in fact negative, indicating loss of mass in this group. Acclimation to 19–24°C also increased Atlantic salmon CTmax. Our findings suggest that both life stage and river origin impact Atlantic salmon growth and performance in the thermal range used herein. These findings provide evidence for local adaptation of Atlantic salmon, increased vulnerability to warming temperatures, and highlight the differential impacts of these ecologically relevant diel thermal cycles on the juvenile life stages in this species.
Introduction
Ectotherm physiology is strongly influenced by ambient temperature. Suboptimal temperatures can stress fishes, reduce their feed intake and consequently their growth (Jonsson et al., 2001; Handeland et al., 2008; Breau et al., 2011; Volkoff and Rønnestad, 2020). Growth rate has been a particularly important area of research because overall size of the fish can impact wild fish population age-size structure, population dynamics and inter-species relations (Thackeray et al., 2013; Carozza et al., 2019; Huang et al., 2021). The current understanding of temperature effects on growth rate in fishes is generally based on individuals acclimated to stable temperatures (Elliott and Hurley, 1997; Martell and Kieffer, 2007; Chadwick and McCormick, 2017; Scheuffele et al., 2021). However, thermal stability is an unnatural condition in temperate rivers where temperature oscillates (3–15°C amplitude) diurnally (Lynch et al., 1984; Malcolm et al., 2004). Diel thermal cycles have been shown to influence food intake (Diana, 1984; Flodmark et al., 2004), digestion efficiency (Mehner et al., 2011; Coulter et al., 2016) and routine metabolic rate (Oligny-Hébert et al., 2015; Morash et al., 2018), all factors that influence growth rate. In addition, exposure to cycling temperatures compared to stable temperature acclimation in the laboratory have been shown to alter aspects of physiology of Atlantic salmon such as their routine metabolic rate (Beauregard et al., 2013; Morash et al., 2018), fatty acid composition (Ge et al., 2021) and haematology (Tunnah et al., 2017) and have been reviewed elsewhere (Morash et al., 2021). Despite the biological significance and ecological relevance of diel thermal cycles, compared to our understanding of the effects of stable temperatures, relatively little is currently known about their impacts on overall growth and development of fishes.
The Atlantic salmon is a keystone fish species that supports the culture, economy and ecology of Nordic countries and North America (Gardner Pinfold, 2011; Myrvold et al., 2019). In the past 30 years, however, there have been significant declines in their global population and average body size, owing at least partly to climate warming (Swansburg et al., 2002; COSEWIC, 2010; Mills et al., 2013; Chaput et al., 2019; DFO, 2019; Dadswell et al. 2021). In one of the most productive river systems for Atlantic salmon, the Miramichi River (NB, Canada), water temperature can reach and even exceed 30°C in July/August, with diel thermal cycles as wide as 9°C (Caissie et al., 2013; Corey et al., 2017). The typical summer diel thermal cycle here spans ~17–22°C with mean of summer maximums across 21 sites of 29.5°C (Caissie et al., 2013). Laboratory experiments of Atlantic salmon parr (juvenile freshwater life stage) acclimated to constant temperatures have shown that water temperatures of 16–20°C elicit the fastest growth whereas temperatures between 22 and 27°C limit growth (Elliott and Hurley, 1997; Jonsson et al., 2001). Therefore, water temperatures in the Miramichi River are often outside the thermal optima for wild Atlantic salmon growth. In another highly productive Atlantic salmon river, the Restigouche River (DFO, 2022), bordering northern New Brunswick and Quebec, overall water temperature is cooler (mean of summer maximums across 21 sites 25.4°C) than the Miramichi River and has a smaller variability throughout the day (Caissie et al., 2013). To date, the effects of different thermal cycles on fish in ecologically, and importantly, thermally distinct streams are still unknown. However, recent in situ evidence from Atlantic salmon parr from rivers with different baseline thermal regimes shows variations in the temperature for the onset of behavioural thermoregulation (Corey et al., 2020). These differences in behaviour in salmon parr from thermally and geographically distinct streams suggest that the thermal nature of their natal river may impact their tolerance to temperature stress that is likely underpinned by aspects of their physiology.
The effects of water temperature are perhaps most influential during the smolt life stage of Atlantic salmon (Gillis et al., 2023). Smolts are juveniles preparing to transition from freshwater phenotype to saltwater phenotype during migration from freshwater streams to the ocean. The relationship between water temperature and initiation of smolt migration has been speculated to be related to a specific and/or cumulative temperature threshold but can also be impacted by day length and water discharge rates (McCormick et al., 1998; Whalen et al., 1999; Zydlewski et al., 2005; Teichert et al., 2020; Frechette et al., 2022). Predictive models suggest that the mean temperature in the first 90 days of the year is a significant driver of the variation in onset of smolt migration timing across streams within a watershed (Frechette et al., 2022). The survival success of the downstream migrants is also known to be dictated by environmental temperatures, and the smolts’ ability to meet certain ‘smoltification windows’ that are largely driven by temperature (McCormick et al., 1997). While Atlantic salmon smolt migration generally occurs when water temperature remains < 15°C, cases of exposure to much warmer water temperatures are beginning to emerge. For example, in hydropower regulated rivers in the southern range of the species, it is not uncommon that the water temperature rises > 20°C towards the end of smolt migration period, especially for smolts for which efficient migration is delayed by downstream passage issues at hydropower dams (e.g. Babin et al., 2020). Similar ‘high-temperature events’ during smolt migration have also recently been observed in unregulated natural rivers such as the Petitcodiac River in eastern Canada where water temperature rapidly exceeded 20°C during the peak of the smolt migration (personal communication, K. Samways, University of New Brunswick), or may occur when smolts are being trapped in downstream by-pass holding tanks with a smaller water volume while waiting for handling or transport downstream (MacLean, 2023). Exposure to rapidly changing and cycling temperatures is likely to become more common in the future due to warming climate scenarios (Borggaard et al., 2019; Thorstad et al., 2021; IPCC, 2022). Currently, in both the Atlantic salmon parr and smolt life stages, the effects of growth and metabolism under these conditions remain untested.
In this study, we investigated how wild Atlantic salmon parr and smolt growth and development differ at two ecologically relevant diel thermal cycles: 16–21 vs 19–24°C. We chose these thermal cycles as they reflect natural summer temperature conditions that are readily observed in both cool and relatively warmer rivers in Atlantic Canada (Caissie et al., 2013), while simultaneously representing a possible transition from ‘optimal’ to ‘physiologically stressful’ conditions, respectively (see e.g. Elliott and Elliott, 2010; Breau et al., 2011). We focused on comparing growth of salmon from the Miramichi River (a relatively ‘warmer’ river) to those from an on average ~4°C cooler, neighbouring Atlantic salmon-producing river system, the Restigouche River (Caissie et al., 2013). We predicted that Restigouche salmon, compared to Miramichi salmon, would develop more poorly, and grow more slowly at 19–24°C (vs 16–21°C) because this warmer thermal cycle is currently beyond the temperatures they typically experience in their natal river, but could represent a future warmer river scenario. We also predicted that Miramichi salmon would develop and grow poorly at 19–24°C (vs 16–21°C) but to a much lesser extent compared to the Restigouche fish owing to their warmer natal environment. The assessment was done primarily to obtain a better understanding of between-population differences in the parr life stage. However, we continued the assessment using the same thermal comparisons through the smolt life stage, realizing that this thermal exposure is currently rare for wild smolts, but not unforeseen during natural smolt migration period especially considering future climate change scenarios. In addition, some of the southernmost populations of Atlantic salmon smolts in Maine, USA, are already migrating in these warmer temperatures (personal communication, G. Goulette, NOAA). We assessed salmon growth rate, Fulton’s condition factor, anaerobic performance and ability to tolerate acute warm exposures.
Materials and Methods
Animal collection, care and acclimation
We collected a total of 200 wild Atlantic salmon (age 1+ and 2+; parr that had spent two or three summers in the freshwater streams, respectively) from the Rocky Brook (n = 100; an ~500-m section of third-order stream of the Miramichi River at the collection site 46°43′2.34″N; 66°38′58.17″W) in October 2018 and the Chain of Rocks Brook (n = 100; an ~500-m section of a third-order stream of the Restigouche River at the collection site; 47°57′54.9″N; 67°11′43.0″W) in September 2019 by electrofishing over 2 days (Fisheries and Oceans Canada (DFO) permits SG-RHQ-18-158 and 20 190 812-009-01-S-P, respectively). We transported the salmon parr to the Crabtree Aqualab at Mount Allison University (NB, Canada) using a 750-L insulated transport tank filled with river water (~15°C), which was kept oxygenated by periodically injecting oxygen as required to maintain a minimum oxygen saturation of 9 mg/L. Upon arrival, we transferred the salmon parr into 300-L circular fibreglass tanks (60 cm tall, 92 cm diameter). We provided tanks with recirculating freshwater, at a 12:12 light/dark photoperiod. We initially fed salmon to satiation once daily with a diet of crushed freeze-dried krill to mimic more natural food as they do not directly take commercial fish pellets. We gradually transitioned their diet to pelleted feeds (EWOS Enviroclean pigmented pellets) by mixing it in with the freeze-dried krill at increasing concentrations. After 1 month of laboratory acclimation at 16–21°C, we tagged each salmon with a unique visible implant elastomer (Northwest Marine Technology) in their dorsal fin. Subsequently, we divided salmon into different tanks based on their river origin and designated acclimation temperatures (the 16–21 or 19–24°C diel thermal cycle). We simulated diel thermal cycles with a temperature change rate of ~0.42°C h−1, a temperature maximum at 19:00 h and minimum at ~07:00 h. Mid-daylight temperatures at 13:00 and mid-nighttime temperatures at 1:00 were 18.5°C and 21.5°C, respectively. We acclimated salmon to these thermal conditions for at least 1 month after tagging before conducting further experiments. All care and subsequent experimental procedures were approved by Mount Allison Animal Care Committee following guidelines from Canadian Council on Animal Care (protocol #101929).
Experimental methods: growth, development and survivorship
Approximately once a month from 15 November 2018 to 25 September 2019 for Miramichi fish and 15 October 2019 to 23 April 2020 for Restigouche fish, we measured and recorded salmon mass, fork length and life stage, and calculated their growth rate (% body mass (BM) day−1) and Fulton’s condition factor (k; Eq. 1). Before each measurement, we fasted the salmon for 24 h then anesthetized them in a 5-L solution of 0.1 g L−1 tricaine mesylate buffered with 0.2 g L−1 sodium bicarbonate. Once anesthetized, weighed and measured, we screened individuals for the presence or absence of sexual precocity and classified their life stage (parr, transitioning to smolt or full smolt). Salmon with a distinct silvery body without parr marks were considered smolts (McCormick, 2012). Any fish mortalities were recorded daily throughout the experiment.
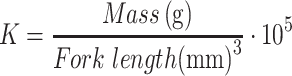 |
(1) |
Anaerobic capacity
We assessed anaerobic capacity by chasing fish (parr [n = 7–11] and smolts [n = 7–8] from each combination of temperature and river origin; see figures for exact n for each treatment) and recording the time taken until they exhaust (‘Time to Exhaustion’; Kelly et al., 2014). Unique individuals were used for each trial and fish were not repeatedly measured. Individuals were chased in a bucket (42 cm high, 48 cm diameter) filled with continuously aerated water at 18.5 or 21.5°C for salmon acclimated to 16–21 or 19–24°C cycles, respectively. We chased salmon by pinching their tail periodically until they were exhausted (unresponsive and can be emersed for 5 s without struggling). After the chase test, salmon were allowed to rest for 3–5 min before being returned to their original acclimation tank.
Acute thermal tolerance
We assessed acute thermal tolerance (CTmax) (Desforges et al., 2023) in parr (n = 6–11) and smolts (n = 8) sampled from each combination of acclimation temperature and river origin. Unique individuals were used for each trial and fish were not repeatedly measured. We first removed fish from their 16–21 or 19–24°C acclimation tank into 18.5 or 21.5°C (the mean of each thermal cycle) aerated water baths, respectively. The water bath was then warmed by 0.3°C per minute and CTmax was defined as the temperature where fish lost equilibrium (Corey et al., 2017; Morgan et al., 2018). Salmon were then transferred to a cool (16°C), aerated recovery bucket until they regained equilibrium before being returned to their original acclimation tank.
Due to a mechanical failure within our recirculation system, the Restigouche River smolts died prior to the completion of the time to exhaustion and CTmax tests. Therefore, no data are available for these two metrics for this group.
Statistical analysis
We conducted all statistical analyses in R (2021; version 3.6.2).
Growth rate
We first related salmon mass (response variable) to growth time (continuous predictor), acclimation temperature and river origin (categorical predictors) using linear mixed-effects model fitted with the function ‘lme’ (Pinheiro et al., 2016). We then tested the significance (α = 0.05) of each predictor and their interaction on growth rate (model slope) using ANOVA (Type-III). We fit separate growth models for parr and smolts due to potentially different growth behaviour between life stages. For further insights into the effect of temperature, we compared growth rates of 16–21 and 19–24°C acclimated salmon from each origin and life stage combination, using pairwise t-tests. We accounted for the multiplicity of such tests by adjusting their significance or alpha level (from α = 0.05 into αadj) using the Benjamini–Hochberg (BH) procedure with a false discovery rate of 0.05.
Survivorship
We compared survivorship of salmon from different origins and acclimation temperatures, by comparing (pairwise) their specific Kaplan–Meier survival curves using log-rank tests (Therneau, 2021). To account for the multiplicity of tests, we adjusted alpha levels based on the BH procedure described above.
Condition factor (k)
We analysed how condition factor (response variable) depended on sampling day, acclimation temperature and their interactions (all categorical factors) using a non-parametric approach due to violations of assumptions, specifically using the ‘nparLD’ model (Noguchi et al., 2012). We fit one ‘nparLD’ model for each origin that had different sampling days. At each time point, we additionally tested for the effect of temperature on condition factor of each origin group, using Mann–Whitney U tests. We adjusted alpha levels using the BH procedure, as above.
Time to exhaustion and CTmax
We first analysed how ‘Time to Exhaustion’ and CTmax (response variables) were affected by acclimation temperature (first factor), and the specific combination of salmon origin and life stage (second factor). This combination method allowed us to conduct our analysis without data from Restigouche smolts that were omitted to avoid temperature bias due to the lack of data from 16–21°C smolts. However, since temperature was found to be statistically insignificant for ‘Time to Exhaustion’, we omitted temperature as a factor in the ‘Time to Exhaustion’ model. The insignificance of temperature indicates that the available 19–24°C data for Restigouche smolts can be a fair representative for the smolts and Restigouche group. Using the 19–24°C data for Restigouche smolts, a subsequent analysis was conducted with only Life Stage and Origin as factors to ‘Time to Exhaustion’. We assessed significance (α = 0.05) of all factors to CTmax and time to exhaustion non-parametrically using permutation-based ANOVA (Type-III) with the function ‘aovp’ (Wheeler and Torchiano, 2016).
Results
Growth rate
Salmon grew exponentially over time at rates that varied depending on acclimation temperature cycle for smolts (Time: Acclimation temp; P = 0.001) and river origin for parr (Time: Origin; P = 0.009; Supplementary Table S1; Fig. 1). Parr from the Miramichi River, on average, grew 32% faster, at 1.11% BM day−1 vs 0.81% BM day−1 for Restigouche parr (Table 1). Parr from both origins grew at rates that were not significantly different between acclimate temperature cycles (Time: Acclimation temp: Origin; P = 0.905), unlike smolts (Time: Acclimation temp: Origin; P = 0.005). Smolts from the Restigouche River at 19–24°C grew ~38% slower at 0.90% BM day−1 compared to 1.31% BM day−1 at 16–21°C (P < 0.001). In contrast, smolts from the Miramichi grew only marginally slower at 19–24 (−8%) than 16–21°C (P = 0.03).
Figure 1.

Growth of Atlantic salmon (Salmo salar) collected from the Miramichi River and Restigouche River acclimated to 16–21°C (turquoise) and 19–24°C (pink) diel thermal cycles, throughout the parr and smolt life stages. Day 0 represents the beginning of the experimental acclimation of the parr. Boxplots’ central horizontal line represents the median; upper and lower hinges represent first and third quartiles; whiskers represent maximum/minimum observation within 1.5-fold of the interquartile range. Coloured bands reflect 95% confidence intervals. P values result from pairwise comparisons of growth rates (slope curve) between temperature groups. Beside each boxplot at the last time point is the n for that group.
Table 1.
Growth rates (% body mass (BM) day−1) for Atlantic salmon (Salmo salar) collected from the Miramichi River and Restigouche River, acclimated to 16–21 or 19–24°C diel thermal cycles
| Life stage | Origin | Acclimation temperature (°C) |
Growth rate (%BM day−1) |
% Diff. in growth rates (16–21 vs 19–24°C) |
|---|---|---|---|---|
| Parr | Miramichi | 16–21 | 1.07 ± 0.21 | 6.8 |
| 19–24 | 1.15 ± 0.18 | |||
| Restigouche | 16–21 | 0.78 ± 0.23 | 6.0 | |
| 19–24 | 0.83 ± 0.31 | |||
| Smolt | Miramichi | 16–21 | 0.99 ± 0.05 | 7.7* |
| 19–24 | 0.92 ± 0.05 | |||
| Restigouche | 16–21 | 1.31 ± 0.12 | 37.7* | |
| 19–24 | 0.90 ± 0.20 |
Growth rate values are presented alongside 95% confidence intervals. Asterisks (*) denote significant (pairwise t-test; P < 0.05) differences in growth rates.
Survivorship
Restigouche salmon parr and smolt life stages were less likely to survive at the warmer 19–24°C compared to 16–21°C (Fig. 2), with a statistically lower survivorship curve (P = 0.002; αadj = 0.025; Supplementary Table S2). Restigouche salmon had a shorter average survival time at 19–24°C (57 days) than at 16–21°C (89 days), with fewer surviving until the end of the experiment (~40% vs ~60%, respectively; Supplementary Table S3). In contrast, Miramichi salmon had similar odds of surviving 19–24°C (76%) and 16–21°C (71%) until the end of the experiment. For Miramichi salmon, survival probability across the life cycle did not significantly differ between the temperature cycles (P = 0.849, αadj = 0.050).
Figure 2.

Survival probabilities of wild Atlantic salmon (Salmo salar) collected from the relatively warm Miramichi River and the cooler Restigouche acclimated to 16–21°C (turquoise) and 19–24°C (pink) diel thermal cycles. Coloured numbers represent the starting number of fish for each group. Coloured bands reflect 95% confidence intervals for survival probabilities. Crosses (+) indicate when certain individuals were withdrawn from the study for reasons unrelated to acclimation temperature cycle; such individuals do not affect survival probability. P values shown result from log-rank tests comparing survival curves at 16–21 and 19–24°C. The alpha level (α = 0.05) was adjusted (into αadj) using the Benjamini–Hochberg procedure to account for multiple pairwise comparisons.
Fulton’s condition factor (k)
Regardless of river origin, salmon acclimated to 19–24 and 16–21°C generally had similar condition factors, except at specific time points for Miramichi fish (Acclimation temp: Time; P < 0.001; Supplementary Table S4). In each of the three months (days 174, 202 and 230, respectively) following smolting (median smolting Day 134), condition factor for Miramichi salmon was significantly higher in 19–24 than 16–21°C (P < αadj; Supplementary Table S5; Fig. 3). Condition factor then returned to similar values between temperature cycles, as observed for parr (Days ≤ 139). For Restigouche salmon, a similar temporal trend in condition factor was initially observed (Fig. 3). However, we did not observe a divergence in condition factor between temperature cycles after smolting occurred in Restigouche salmon, due to early termination of the project, and not necessarily the absence of such a phenomenon. We did not find significant differences between temperature cycles (Acclimation temp; P = 0.483 and Acclimation temp: Time; P = 0.229) in Restigouche salmon.
Figure 3.

Condition factor (k) of Atlantic salmon (Salmo salar) collected from the Miramichi River and Restigouche River, acclimated to 16–21°C (turquoise) and 19–24°C (pink) diel thermal cycles throughout the parr and smolt life stages. Vertical dashed lines indicate the median date for parr–smolt transformation. Points represent means; error bars represent 95% confidence interval of means; asterisks mark significant differences in k between temperature groups assessed using Mann–Whitney U tests with alpha levels adjusted using the Benjamini–Hochberg procedure to account for multiple pairwise comparisons. Numbers of fish (n) for each treatment groups are shown beside their right-most point.
Anaerobic capacity
Time to exhaustion appeared independent of acclimation temperature cycle (Acclimation Temp: P = 0.345; Fig. 4) but dependent on salmon life stage and origin (Origin & Life Stage: P < 0.001; Fig. 4). Further analysis showed that origin was a major driver of the difference in time to exhaustion (Origin; P < 0.001). On average, Restigouche parr exhausted after ~13 min of chasing, significantly longer than the ~7-min average for Miramichi parr (permutation-based test; P = 0.027) and ~3-min average for Miramichi smolts (P < 0.001).
Figure 4.

Time to exhaustion for chased Atlantic salmon (Salmo salar) collected from the Miramichi and Restigouche River, acclimated to 16–21°C (turquoise) and 19–24°C (pink) diel thermal cycles, throughout the parr and smolt life stages. Number of fish used for each group presented along the x-axis. Boxplots’ central horizontal line represents the median; upper and lower hinges represent first and third quartiles; whiskers represent the maximum/minimum observation within 1.5-fold of the interquartile range. ND denotes no data. P values shown are results from a permutation-based ANOVA (Type-III) model.
Acute thermal tolerance
Acute critical thermal tolerance, as assessed using CTmax (Fig. 5), was unaffected by salmon river origin and life stage as a combined factor (Origin & Life Stage; P = 0.238; Supplementary Table S6), but significantly increased with warmer cycling acclimation temperatures (Acclimation temp; P < 0.001). On average, individuals at 19–24°C (excluding Restigouche smolts) had a CTmax ~1°C higher than those at 16–21°C. However, the increase in CTmax in the 19–24°C groups was significantly impacted by the interaction of origin & life stage (Acclimation temp: (Origin & Life Stage); P = 0.006). Average CTmax at 19–24 (vs 16–21°C) was higher by 1.2°C for Restigouche parr, while this number was only 0.6°C and 0.5°C for Miramichi parr and smolts, respectively.
Figure 5.

CT max of Atlantic salmon (Salmo salar) collected from the Miramichi River and Restigouche River acclimated to 16–21°C (turquoise) and 19–24°C (pink) diel thermal cycles throughout the parr and smolt life stages. Number of fish used for each group presented on the x-axis. Statistical significance of salmon life stage and river origin as one factor (P = 0.238), acclimation temperature as another (P < 0.001), and their interactions (P = 0.006) were assessed using permutation-based ANOVA (Type-III); analysis excludes data from Restigouche smolts from either thermal cycle. Boxplots’ central horizontal line represents the median; upper and lower hinges represent first and third quartiles; whiskers represent the maximum/minimum observation within 1.5-fold of the interquartile range. ND denotes no data.
Discussion
Scientists’ understanding on the effects of temperature on fishes has been largely based on experiments conducted at stable temperatures, even though natural thermal environments have clear diel cycles. Here, we investigated the effects of two ecologically relevant diel thermal cycles (16–21 and 19–24°C) on growth and development of wild Atlantic salmon originating from two thermally distinct and socioeconomically important rivers in New Brunswick, Canada (Restigouche and Miramichi). We found that parr growth was similar between acclimation temperatures within each river, but Restigouche River parr grew significantly slower than Miramichi parr. In contrast, growth rate and survivorship for smolts decreased at 19–24 relative to 16–21°C in salmon from both rivers, but particularly for those from the cooler Restigouche River. Although the 19–24°C cycle appears to adversely affect salmon smolt growth, we did not observe any effect of thermal cycle on overall condition factor (k). Anaerobic performance was also not impacted by different thermal cycles, but Restigouche River salmon took longer to exhaust than those from the Miramichi. In contrast, CTmax overall was higher in salmon in the 19–24°C thermal cycle, but the differences were dependent on river of origin and life stage. Atlantic salmon parr from different river systems appear to respond differently to these relevant thermal cycles for several of the physiological metrics. The physiological impacts of these thermal cycles on fish from different river systems will provide valuable insight for predicted future warming.
There was no effect of our two natural thermal cycling acclimation conditions on the growth of salmon parr within each river of origin. However, parr from the Restigouche River grew 32% slower than those from the Miramichi on average. We initially expected an overall decrease in growth at 19–24°C based on past research using stable acclimation temperatures. For example, Norwegian Atlantic salmon parr acclimated to a range of stable temperatures grew fastest at 16 to 20°C, above which growth rate declined until it eventually ceased at 24 to 27°C (Jonsson et al., 2001). In another study on European Atlantic salmon parr, growth rate peaked at a stable 16°C, then decreased to zero at ~ 23°C (Elliott and Hurley, 1997). However, the optimal temperature for growth in juvenile salmon, determined under controlled conditions, is lower, ranging from 15 to 20°C (Elliott and Hurley, 1997; Jonsson et al., 2001; Elliott and Elliott, 2010). In another study, the thermal optimum (Topt) for Atlantic salmon parr was determined to be 15–19°C (Gillis et al., 2023). These differences between our study and others could possibly be due to feed availability and consumption. Laboratory experiments may also underestimate the effects of climate change on Topt when compared to field-based measurements where things like food availability are not controlled (Childress and Letcher, 2017). Here, we fed the fish ad libitum each day so food would not be a constraining factor. In addition, our warmest thermal cycle was within the upper thermal limit for juvenile salmon feeding in the laboratory or the wild (22–24°C; Cunjak et al., 1993; Breau et al., 2011). Smaller ration size can decrease the Topt for growth in salmonids (Brett et al., 1969; Elliott, 1976); therefore, our high rate of feeding may have increased the Topt for growth in the laboratory.
It is also possible that the salmon in this study possessed a relatively broad Topt range following acclimation to our variable thermal environments, and this may help explain the similar growth rates of parr from a given river of origin at 16–21 and 19–24°C. Diel thermal cycles tend to broaden the optimum thermal range for maximum performance as shown in green sturgeon (Acipenser medirostris; Rodgers et al., 2018) and alpine newt larvae (Triturus alpetris; Měráková and Gvoždík, 2009). However, cycles also reduce maximum performance in fruit flies (Drosophila melanogaster; Kjaersgaard et al., 2012) and a frog species (Bombina orientalis; Arrighi et al., 2013). It is possible that animals in diel thermal cycling environments benefit from a wider thermal performance curve (da Silva et al., 2019), adopting a more thermal generalist strategy vs thermal specialist (Rodgers et al., 2018). The thermal generalist strategy may be used by our salmon parr, explaining their similar growth rate at 16–21 and 19–24°C. Alternatively, if the Topt lies in the overlapping range of our thermal cycles (19–21°C), then both groups would have been exposed to their Topt for similar amounts of time each day enabling them to grow at similar rates.
Local adaptation to distinct thermal environments may also explain why our Atlantic salmon parr did not exhibit a decreased growth rate at 19–24°C as predicted by previous studies of Atlantic salmon from European origins (Elliott and Hurley, 1997; Jonsson et al., 2001). Different habitats are expected to exert different selection pressures leading to different genotypic adaptations in salmonids (Dionne et al., 2009; Weitemier et al., 2021; Zillig et al., 2021). The extent of local adaptation for salmon populations has been extensively discussed (in Garcia de Leaniz et al., 2007; Fraser et al., 2011; Primmer, 2011), and evidence for its occurrence has surfaced based on comparisons of physiology or genomics of adult (Taylor, 1991; Lee et al., 2003; Unwin et al., 2003) and juvenile (Zillig et al., 2023) salmon from different locations. For example, Sockeye salmon (Oncorhynchus nerka) from warmer environments tend to have better swim performance at warmer temperatures (Eliason et al., 2011). Atlantic salmon from warmer southern rivers possessed hearts more tolerant to acute warming (Gradil et al., 2016, North American salmon) and cardiac capacity or maximum heart rate was also increased after warm acclimation (Anttila et al., 2014, European salmon). The Atlantic salmon used in this study were a population from the warmer Miramichi River (summer Tmean 16–19°C; Caissie et al., 2013) compared to English and Norwegian rivers (summer Tmean 9–16°C; Elliott and Hurley, 1997; Jonsson et al., 2001) where most previous growth rate studies were conducted. We hypothesize that Miramichi River salmon may have developed the capability to continue to grow during the warmer summer conditions (19–24°C) compared to the Restigouche salmon. However, it is unclear whether such capability arises due to changes in genetics (local adaptation) or phenotypic plasticity (acclimation capacity) that arise from differences in developmental temperature (Zillig et al., 2021; Andrews, 2008; Jonsson and Jonsson, 2011). Historically, evidence for local thermal adaptation for growth rate of Atlantic salmon, based on individuals incubated at similar temperatures then acclimated to different stable temperatures, is lacking (Jonsson et al., 2001). The unimpaired growth of our salmon parr in laboratory conditions at 19–24 (vs 16–21°C) suggests that local thermal adaptation took place. Further study is needed to understand the facilitating mechanism and the variability of the capacity for Atlantic salmon to adapt to local watershed conditions and will be important for developing population-specific management practices (Gayeski et al., 2018; Zillig et al., 2021). This is especially important in systems such as the Miramichi where a previous long history of supplementation (i.e. stocking) may have influenced the once natural patterns of local adaptation (Wellband et al., 2019). It is also important to keep in mind that the availability and development/emergence rates of prey, mainly different macroinvertebrates, also respond to water temperature, so the effects of different temperature regimes on parr growth may manifest differently in situ vs in a laboratory study.
In a more local context, we also found that salmon parr from the cooler Restigouche River overall grew significantly slower than those from the Miramichi. Growth of Restigouche smolts decreased significantly at 19–24°C compared to smolts from the Miramichi. Restigouche salmon, which are genetically distinct from salmon populations in other river systems across Atlantic Canada (Dionne et al., 2009), inhabit a river system that is generally ~4°C cooler than the Miramichi (Caissie et al., 2013). Their cooler habitat potentially reduces the need for an ability to grow optimally at warm temperatures, explaining the lower growth rate in parr compared to the Miramichi salmon and the reduction in their growth and survival rate in smolts at 19–24°C. Notably, we only observed the warm-associated decrease in growth rate in the smolt life stage. From a physiological perspective, older and larger fishes are generally believed to have reduced thermal tolerance (Cuenco et al., 1985; Bjornsson et al., 2001; Morita et al., 2010; Audzijonyte et al., 2020; Turko et al., 2020; McKenzie et al., 2021). From an ecological perspective, smolts leave the Restigouche River from the middle of May to late June at 6–16°C (Peppar, 1982), so it is unlikely that they would typically experience these temperatures in the wild at present. Regardless, our findings revealed the greater vulnerability of Restigouche salmon to warmer and relevant thermal cycles, which will be important to consider for the predicted warming climate. Indeed, Atlantic salmon in the Northeast United States Shelf have been assessed to have extremely high biological sensitivity and climate exposure making them vulnerable to changes in population productivity (Hare et al., 2016). Our data will contribute to a knowledge base on the effects of climate warming on Atlantic salmon, which has been identified as an area of uncertainty in climate change predictions for this species (Borggaard et al., 2019; Kocik et al., 2022; Henderson et al., 2023).
Despite differences in growth, overall condition factor throughout the growth periods remained unaffected by acclimation to either thermal cycle or between rivers. Our Atlantic salmon kept high Fulton’s condition (>1) even at the warm 19–24°C across both parr and smolt developmental stages. However, we did note that Miramichi salmon at 19–24°C maintained a higher Fulton’s condition in the few months after parr–smolt transformation compared to those at 16–21°C. Fulton’s condition factor has been a popular indicator of proximate body composition and overall fish condition (Baxter, 1998; Jin et al., 2015; Naeem et al., 2016). Typically, it decreases in fish in unfavourable environments (Árnason et al., 2009; Mazumder et al., 2016; Hvas et al., 2017; Castaldo et al., 2021). A general decline in Fulton’s condition during the post-smolt period may be attributed to the parr–smolt transformation as it has also been observed previously after smoltification (Shrimpton et al., 2000; Stefansson et al., 2008; Codabaccus et al., 2011). The extent of decline in Fulton’s condition during this period, differing between anadromous vs landlocked populations (McCormick et al., 2019), has been associated with differences in gill Na+/K+-ATPase (NKA) activity and NKAα1b essential for successful sea-entry of salmon (Björnsson et al., 1989; McCormick et al., 2019; Nisembaum et al., 2020), and not necessarily their overall condition. Based on these studies, it is difficult to attribute differences in Fulton’s condition observed in the few months post-smolt as an indication of poorer condition at 19–24°C; such differences may instead suggest a temperature-sensitive smolting process for Atlantic salmon (Handeland et al., 2000; Imsland et al., 2014). It is also important to note that these fish remained in freshwater during this period. Fish with higher Fulton’s condition tend to possess greater acute thermal and hypoxia tolerance, and energy reserves (Mozsár et al., 2015; Gallant et al., 2017; Rees and Matute, 2018; Turko et al., 2020) that may lead to increased physiological performance, but this was not tested here.
Despite differences in growth and some changes to Fulton’s condition throughout development, we did not observe any differences in anaerobic performance at the warm acclimation temperature cycle. The effects of different stable acclimation temperatures on fish anaerobic performance appear to be variable/inconsistent (Kieffer, 2000). The fuel for anaerobic activity, white muscle glycogen, generally remains at similar levels regardless of stable acclimation temperature (Kieffer et al., 1994; Kieffer and Tufts, 1998; Day and Butler, 2005), although there are exceptions (Wilkie et al., 1996). Warming increased burst swimming speed in striped mullet (Muglis cephalus; Rulifson, 1977) and goldfish (Carassius auratus; Johnson and Bennett, 1995), but not consistently in threespine sticklebacks (Gasterosteus aculeatus; Guderley et al., 2001), and there was no effect on Atlantic salmon parr (Zathey, 2018). Furthermore, the time to exhaustion was not affected by acclimation temperature for four lake trout populations (Salvelinus namaycush;Kelly et al., 2014). Notably, these studies used fish acclimated to stable temperatures, which may be different physiologically compared to those acclimated to ecologically relevant diel thermal cycles (Oligny-Hébert et al., 2015; Morash et al., 2018; Rodgers et al., 2018). We showed that 16–21 and 19–24°C diel thermal cycles did not affect the time to exhaustion of Atlantic salmon, indicating unchanged anaerobic performance that could be important in overcoming velocity barriers, prey capture, and escaping predators. However, despite no effect of thermal acclimation on anaerobic performance, there would likely be differences in their aerobic capacity due to the thermal influences on aerobic metabolism (Pörtner and Knust, 2007). A warmer thermal acclimation profile may increase metabolic demands that might not be met by their capacity for oxygen uptake, which may force them to rely on anaerobic processes/substrates sooner than at cooler acclimation temperatures. Where anaerobic substrates are limiting, this could potentially limit repeat bouts of anaerobic activity at warmer temperatures.
Although time to exhaustion did not vary with acclimation temperature, it is overall higher in Restigouche salmon parr compared to those from the Miramichi. Variations in anaerobic capacity between fish populations are thought to be linked with differences in natal environment hydrology and predatory intensity (O’Steen et al., 2002; Langerhans and DeWitt, 2004) and not necessarily to temperature. For example, mosquitofish (Gambusia affinis) in environments with larger predators have been found to have faster burst swim speed (Langerhans et al., 2004). Similarly, Coho salmon (Oncorhynchus kisutch) in coastal areas, presumed to be more predator dense, have a higher burst swim speed compared to those in interior zones (Taylor and McPhail, 1985). In addition, domesticated rainbow trout (Oncorhynchus mykiss) have reduced burst swimming performance compared to their wild counterparts (Bellinger et al., 2014). The higher anaerobic performance of Restigouche salmon may be the result of differences in their natal environment that is not temperature related. This possibility awaits further investigation.
Acute thermal tolerance (as measured through CTmax) increased with warmer acclimation temperature as is typically observed in several species (Zhang and Kieffer, 2014; Comte and Olden 2016; Corey et al., 2017). The CTmax of Restigouche and Miramichi salmon increased by ~0.5 and 1.2°C, respectively, for the +3°C warmer acclimation cycle, which is about the typical expected increase in CTmax for every 3°C found in other fishes (0.9–1.2; Beitinger et al., 2000; Comte and Olden 2016; Morley et al., 2019). For some fishes under warm temperatures, CTmax increased only slightly with warm acclimation (Morrison et al., 2020; Reid and Riccairdi 2021), which could indicate that the fish is approaching its thermal ceiling and is unable to adjust CTmax. Our data indicate that our temperature cycles were within the thermal breadth of performance for Atlantic salmon in both tested populations.
In conclusion, we found that the effects of these two ecologically relevant thermal cycles on growth and physiological performance were impacted by both river of origin and life stage of the salmon. Salmon parr within each river system appear to be able to grow just as well when exposed to chronically cooler or warmer thermal cycles, which contradicts the predicted decline in growth rate from research using stable temperature acclimations. However, overall growth of salmon from the cooler Restigouche River was significantly slower despite common conditions. These differences could be suggestive of local adaptation to distinct thermal regimes between these salmon populations. In addition, smolts from the Restigouche River appear to be more vulnerable to warmer thermal cycles than those in the Miramichi. The temperatures used in this experiment are currently unlikely to be experienced by smolts in either river; however, future changes in climate and/or anthropogenic changes to river structures that inhibit or slow smolt downstream passage could make this a reality in the future. Together, our findings should inform evidence-based decision-making in support of a river-by-river conservation approach for Atlantic salmon in the Gulf region. It is unknown if local thermal adaptation occurs across all/most Canadian Atlantic salmon populations, and this could be a rewarding area for future research.
Supplementary Material
Acknowledgements
The authors wish to thank Wayne Anderson for technical help in the Harold Crabtree Aqualab, Dr Cindy Breau (DFO) for assistance with salmon tagging and Claire Pabody for technical assistance in the laboratory. Restigouche River Atlantic salmon parr collections were facilitated by the Gespe’gewa’gi Institute of Natural Understanding in close collaboration with the Listuguj Natural Resources Scientific Research team. Miramichi River Atlantic salmon parr collection was facilitated by Chris MacIntyre and Colin De Coste.
Contributor Information
Sean Andrew, Department of Biology, Mount Allison University, 62 York St., Sackville, NB E4L 1G7, Canada.
Sula Swart, Department of Biology, Mount Allison University, 62 York St., Sackville, NB E4L 1G7, Canada.
Stephanie McKenna, Department of Biology, Mount Allison University, 62 York St., Sackville, NB E4L 1G7, Canada.
Jenna Morissette, Department of Biology, Mount Allison University, 62 York St., Sackville, NB E4L 1G7, Canada.
Carole-Anne Gillis, Gespe’gewa’gi Institute of Natural Understanding, 1 Marshall Way, Listuguj, QC, G0C 2R0, Canada.
Tommi Linnansaari, Department of Biology, Faculty of Forestry and Environmental Sciences, and Canadian Rivers Institute, University of New Brunswick, 28 Dineen Drive, Fredericton, NB, E3B 5A3, Canada.
Suzanne Currie, Department of Biology, Acadia University, 33 Westwood Avenue, Wolfville, NS, B4P 2R6, Canada.
Andrea J Morash, Department of Biology, Mount Allison University, 62 York St., Sackville, NB E4L 1G7, Canada.
Author Contributions
AJM, SC, C-AG and TL conceived the study design. AJM, SA, SS, SM and JM conducted all laboratory experiments. SA conducted statistical analysis and wrote the first draft of the manuscript. All authors contributed to writing the final draft of the manuscript.
Conflict of Interest
The authors declare there are no competing interests.
Funding
This work was funded by the Atlantic Salmon Research Joint Venture (A.J.M., S.C.), NSERC Discovery Grants (A.J.M., S.C., T.L.), and the New Brunswick Innovation Foundation (S.A.) and Mount Allison University Independent Student Research Grants (S.S., S.M., J.M.).
Data availability
Data generated or analysed during this study will be available in the Borealis Data Repository upon acceptance. https://doi.org/10.5683/SP3/3P5MAY
Supplementary Material
Supplementary material is available at Conservation Physiology online.
References
- Andrews RM (2008) Effects of incubation temperature on growth and performance the veiled chameleon (Chamaeleo calyptratus). J Exp Zool A Ecol Genet Physiol 309: 435–446. 10.1002/jez.470. [DOI] [PubMed] [Google Scholar]
- Anttila K, Couturier CS, Øverli Ø, Johnsen A, Marthinsen G, Nilsson GE, Farrell AP (2014) Atlantic salmon show capability for cardiac acclimation to warm temperatures. Nat Commun 5: 4252. 10.1038/ncomms5252. [DOI] [PubMed] [Google Scholar]
- Árnason T, Bjornsson B, Steinarsson A (2009) Allometric growth and condition factor of Atlantic cod (Gadus morhua) fed to satiation: effects of temperature and body weight. J Appl Ichthyol 25: 401–406. 10.1111/j.1439-0426.2009.01259.x. [DOI] [Google Scholar]
- Arrighi J, Lencer E, Jukar A, Park D-S, Phillips P, Kaplan R (2013) Daily temperature fluctuations unpredictably influence developmental rate and morphology at a critical early larval stage in a frog. BMC Ecol 13: 18. 10.1186/1472-6785-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audzijonyte A, Richards S, Stuart-Smith R, Pecl G, Edgar G, Barrett N, Payne N, Blanchard J (2020) Fish body sizes change with temperature but not all species shrink with warming. Nat Ecol Evol 4: 809–814. 10.1038/s41559-020-1171-0. [DOI] [PubMed] [Google Scholar]
- Babin AB, Ndong M, Haralampides K, Peake S, Jones R, Curry RA, Linnansaari T (2020) Migration of Atlantic salmon (Salmo salar) smolts in a large hydropower reservoir. Can J Fish Aquat Sci 77: 1463–1476. 10.1139/cjfas-2019-0395. [DOI] [PubMed] [Google Scholar]
- Baxter . 1998. Condition Factor, K, for Salmonid Fish.pdf. State of Victoria, Department of Primary Industries. Available fromhttp://bamboorods.ca/Trout%20condition%20factor.pdf[accessed 30 January 2022].
- Beauregard D, Enders E, Boisclair D (2013) Consequences of circadian fluctuations in water temperature on the standard metabolic rate of Atlantic salmon parr (Salmo salar). Can J Fish Aquat Sci 70: 1072–1081. 10.1139/cjfas-2012-0342. [DOI] [Google Scholar]
- Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fishes 58: 237–275. 10.1023/A:1007676325825. [DOI] [Google Scholar]
- Bellinger KL, Thorgaard GH, Carter PA (2014) Domestication is associated with reduced burst swimming performance and increased body size in clonal rainbow trout lines. Annu Rev Fish Dis 420-421: 154–159. 10.1016/j.aquaculture.2013.10.028. [DOI] [Google Scholar]
- Bjornsson B, Steinarsson A, Oddgeirsson M (2001) Optimal temperature for growth and feed conversion of immature cod (Gadus morhua L.). ICES J Mar Sci 58: 29–38. 10.1006/jmsc.2000.0986. [DOI] [Google Scholar]
- Björnsson B, Thorarensen H, Hirano T, Ogasawara T, Kristinsson J (1989) Photoperiod and temperature affect plasma growth hormone levels, growth, condition factor and hypoosmoregulatory ability of juvenile Atlantic salmon (Salmo salar) during parr-smolt transformation. Aquaculture 82: 77–91. 10.1016/0044-8486(89)90397-9. [DOI] [Google Scholar]
- Borggaard, D. L., Dick, D.M., Star, J., Alexander, M., Bernier, M., Collins, M., Damon-Randall, K., Dudley, R., Griffis, R., Hayes, S., Johnson, M., Kircheis, D., Kocik, J., Letcher, B., Mantua, N., Morrison, W.Nislow, K.Saba, V., Saunders, R., Sheehan, T., Staudinger, M.D., 2019. Atlantic Salmon (Salmo salar) Climate Scenario Planning Pilot Report. Greater Atlantic Region Policy Series; 19–05. United States. National Marine Fisheries Service. Greater Atlantic Regional Fisheries Office. https://repository.library.noaa.gov/view/noaa/33877. [Google Scholar]
- Breau C, Cunjak RA, Peake SJ (2011) Behaviour during elevated water temperatures: can physiology explain movement of juvenile Atlantic salmon to cool water? J Anim Ecol 80: 844–853. 10.1111/j.1365-2656.2011.01828.x. [DOI] [PubMed] [Google Scholar]
- Brett JR, Shelbourn JE, Shoop CT (1969) Growth rate and body composition of fingerling sockeye salmon, Oncorhynchus nerka, in relation to temperature and ration size. J Fish Res Board Can 26: 2363–2394. 10.1139/f69-230. [DOI] [Google Scholar]
- Caissie D, Breau C, Hayward J, Cameron P (2013) Water temperature characteristics within the Miramichi and Restigouche rivers. DFO Canadian Science Advisory Secretariat 2012/165. vi + 31. [Google Scholar]
- Carozza DA, Bianchi D, Galbraith ED (2019) Metabolic impacts of climate change on marine ecosystems: implications for fish communities and fisheries. Glob Ecol Biogeogr 28: 158–169. 10.1111/geb.12832. [DOI] [Google Scholar]
- Castaldo G, Pillet M, Ameryckx L, Bervoets L, Town RM, Blust R, De Boeck G (2021) Temperature effects during a sublethal chronic metal mixture exposure on common carp (Cyprinus carpio). Front Physiol 12: 1–16. 10.3389/fphys.2021.651584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick JG, McCormick SD (2017) Upper thermal limits of growth in brook trout and their relationship to stress physiology. J Exp Biol 220: 3976–3987. 10.1242/jeb.161224. [DOI] [PubMed] [Google Scholar]
- Chaput G, Carr J, Daniels J, Tinker S, Jonsen I, Whoriskey F (2019) Atlantic salmon (Salmo salar) smolt and early post-smolt migration and survival inferred from multi-year and multi-stock acoustic telemetry studies in the Gulf of St. Lawrence, Northwest Atlantic. Ices. J Mar Sci 76: 1107–1121. 10.1093/icesjms/fsy156. [DOI] [Google Scholar]
- Childress E, Letcher BH (2017) Estimating thermal performance curves from repeated field observations. Ecology 98: 1377–1387. 10.1002/ecy.1801. [DOI] [PubMed] [Google Scholar]
- Codabaccus MB, Bridle AR, Nichols PD, Carter CG (2011) Effect of feeding Atlantic salmon (Salmo salar L.) a diet enriched with stearidonic acid from parr to smolt on growth and n-3 long-chain PUFA biosynthesis. Br J Nutr 105: 1772–1782. 10.1017/S0007114510005714. [DOI] [PubMed] [Google Scholar]
- Comte L, Olden J (2017) Evolutionary and environmental determinants of freshwater fish thermal tolerance and plasticity. Glob Chang Biol 23: 728–736. 10.1111/gcb.13427. [DOI] [PubMed] [Google Scholar]
- Corey E, Linnansaari T, Cunjak RA, Currie S (2017) Physiological effects of environmentally relevant, multi-day thermal stress on wild juvenile Atlantic salmon (Salmo salar). Conserv Physiol 5: cox014. 10.1093/conphys/cox014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey E, Linnansaari T, Dugdale SJ, Bergeron N, Gendron J-F, Lapointe M, Cunjak RA (2020) Comparing the behavioural thermoregulation response to heat stress by Atlantic salmon parr (Salmo salar) in two rivers. Ecol Freshw Fish 29: 50–62. 10.1111/eff.12487. [DOI] [Google Scholar]
- COSEWIC . 2010. COSEWIC assessment and status report on the Atlantic Salmon Salmo salar (Nunavik population, Labrador population, Northeast Newfoundland population, South Newfoundland population, Southwest Newfoundland population, Northwest Newfoundland population, Quebec Eastern North Shore population, Quebec Western North Shore population, Anticosti Island population, Inner St. Lawrence population, Lake Ontario population, Gaspé-Southern Gulf of St. Lawrence population, Eastern Cape Breton population, Nova Scotia Southern Upland population, Inner Bay of Fundy population, Outer Bay of Fundy population) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xlvii + 136 pp. (www.sararegistry.gc.ca/ status/status_e.cfm). [Google Scholar]
- Coulter DP, Sepúlveda MS, Troy CD, Höök TO (2016) Species-specific effects of subdaily temperature fluctuations on consumption, growth and stress responses in two physiologically similar fish species. Ecol Freshw Fish 25: 465–475. 10.1111/eff.12227. [DOI] [Google Scholar]
- Cuenco ML, Stickney RR, Grant WE (1985) Fish bioenergetics and growth in aquaculture ponds: I. Individual fish model development. Ecol Model 27: 169–190. 10.1016/0304-3800(85)90001-8. [DOI] [Google Scholar]
- Cunjak RA, Caissie D, Eljabi N, Hardie P, Conlon JH, Pollock TL, Giberson DJ, Komadina-Douthwright S (1993) The Catamaran Brook (New Brunswick) Habitat Research Project: biological, physical and chemical conditions (1990-1992). Can Tech Rep Fish Aquat Sci 1914: 81. [Google Scholar]
- Dadswell M, Spares A, Reader J, McLean M, McDermott T, Samways K, Lilly J (2022) The decline and impending collapse of the Atlantic salmon (Salmo salar) population in the North Atlantic Ocean: a review of possible causes. Rev Fish Sci Aquac 30: 215–258. 10.1080/23308249.2021.1937044. [DOI] [Google Scholar]
- Day N, Butler PJ (2005) The effects of acclimation to reversed seasonal temperatures on the swimming performance of adult brown trout Salmo trutta. J Exp Biol 208: 2683–2692. 10.1242/jeb.01669. [DOI] [PubMed] [Google Scholar]
- DFO (2019) Update of indicators to 2018 of adult Atlantic Salmon for the Miramichi River (NB), Salmon fishing area 16, DFO Gulf Region. (n.d.). Atlantic Salmon 11. [Google Scholar]
- DFO (2022) Update of indicators of Atlantic Salmon (Salmo salar) in DFO Gulf Region Salmon fishing areas 15 - 18 for 2020 and 2021. DFO Can Sci Advis Sec Sci Resp 2022/021. [Google Scholar]
- Diana JS (1984) The growth of largemouth bass, Micropterus salmoides (Lacepede), under constant and fluctuating temperatures. J Fish Biol 24: 165–172. 10.1111/j.1095-8649.1984.tb04787.x. [DOI] [Google Scholar]
- Dionne M, Caron F, Dodson J, Bernatchez L (2009) Comparative survey of within-river genetic structure in Atlantic salmon; relevance for management and conservation. Conserv Genet 10: 869–879. 10.1007/s10592-008-9647-5. [DOI] [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112American Association for the Advancement of Science. 10.1126/science.1199158. [DOI] [PubMed] [Google Scholar]
- Elliott JM (1976) The energetics of feeding, metabolism and growth of brown trout (Salmo trutta L.) in relation to body weight, water temperature and ration size. J Anim Ecol 45: 923–948. 10.2307/3590. [DOI] [Google Scholar]
- Elliott JM, Elliott JA (2010) Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: predicting the effects of climate change. J Fish Biol 77: 1793–1817. 10.1111/j.1095-8649.2010.02762.x. [DOI] [PubMed] [Google Scholar]
- Elliott JM, Hurley MA (1997) A functional model for maximum growth of Atlantic Salmon parr, Salmo salar, from two populations in Northwest England. Funct Ecol 11: 592–603. 10.1046/j.1365-2435.1997.00130.x. [DOI] [Google Scholar]
- Finstad AG, Jonsson B (2012) Effect of incubation temperature on growth performance in Atlantic salmon. Mar Ecol Prog Ser 454: 75–82. 10.3354/meps09643. [DOI] [Google Scholar]
- Flodmark L, Vøllestad L, Forseth T (2004) Performance of juvenile brown trout exposed to fluctuating water level and temperature. J Fish Biol 65: 460–470. 10.1111/j.0022-1112.2004.00463.x. [DOI] [Google Scholar]
- Fraser DJ, Weir LK, Bernatchez L, Hansen MM, Taylor EB (2011) Extent and scale of local adaptation in salmonid fishes: review and meta-analysis. Heredity 106: 404–420. 10.1038/hdy.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechette DM, Hawkes JP, Kocik JF (2022) Managing for Atlantic salmon smolt run timing variability in a changing climate. N Am J Fish Manag 43: 517–538. 10.1002/nafm.10868. [DOI] [Google Scholar]
- Gallant M, Leblanc S, Maccormack T, Currie S (2017) Physiological responses to a short-term, environmentally realistic, acute heat stress in Atlantic salmon, Salmo salar. FACETS 2: 330–341. 10.1139/facets-2016-0053. [DOI] [Google Scholar]
- Garcia de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, Aubin-Horth N, Lajus D, Letcher BH, Youngson AFet al. (2007) A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biol Rev 82: 173–211. 10.1111/j.1469-185X.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Gayeski NJ, Stanford JA, Montgomery DR, Lichatowich J, Peterman RMWilliams RN (2018) The failure of wild salmon management: Need for a place-based conceptual foundation. Fisheries 43: 303–309. 10.1002/fsh.10062. [DOI] [Google Scholar]
- Ge J, Zhou Y, Huang M, Gao Q, Dong Y, Dong S (2021) Effects of constant and diel cyclic temperatures on the liver and intestinal phospholipid fatty acid composition in rainbow trout Oncorhynchus mykiss during seawater acclimation. BMC Zool 6: 21. 10.1186/s40850-021-00086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis C-A, Ouellet V, Breau C, Frechette D, Bergeron N (2023) Assessing climate change impacts on north American freshwater habitat of wild Atlantic salmon - urgent needs for collaborative research. Can Water Resour J 48: 222–246. 10.1080/07011784.2022.2163190. [DOI] [Google Scholar]
- Gradil K, Garner S, Wilson C, Farrell A, Neff B (2016) Relationship between cardiac performance and environment across populations of Atlantic salmon (Salmo salar): a common garden experiment implicates local adaptation. Evol Ecol 30: 877–886. 10.1007/s10682-016-9847-2. [DOI] [Google Scholar]
- Guderley H, Leroy PH, Gagné A (2001) Thermal acclimation, growth, and burst swimming of threespine stickleback: enzymatic correlates and influence of photoperiod. Physiol Biochem Zool 74: 66–74. 10.1086/319313. [DOI] [PubMed] [Google Scholar]
- Handeland S, Imsland A, Stefansson S (2008) The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283: 36–42. 10.1016/j.aquaculture.2008.06.042. [DOI] [Google Scholar]
- Handeland SO, Berge Å, Björnsson BT, Lie Ø, Stefansson SO (2000) Seawater adaptation by out-of-season Atlantic salmon (Salmo salar L.) smolts at different temperatures. Aquaculture 181: 377–396. 10.1016/S0044-8486(99)00241-0. [DOI] [Google Scholar]
- Hare JA, Morrison WE, Nelson MW, Stachura MM, Teeters EJ, Griffis RB, Alexander MA, Scott JD, Alade L, Bell RJet al. (2016) A vulnerability assessment of fish and invertebrates to climate change on the northeast U.S. continental shelf. PloS One 11: e0146756. 10.1371/journal.pone.0146756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ME, Mills KE, Alexander MA, Barajas M, Collins MJ, Dzaugis M, Kircheis D, Sheehan TF (2023) A synthesis of US Atlantic salmon habitat requirements and implications for future suitability under a changing climate. ICES J Mar Sci 80: 2051–2073. 10.1093/icesjms/fsad127. [DOI] [Google Scholar]
- Huang M, Ding L, Wang J, Ding C, Tao J (2021) The impacts of climate change on fish growth: a summary of conducted studies and current knowledge. Ecol Indic 121: 106976. 10.1016/j.ecolind.2020.106976. [DOI] [Google Scholar]
- Hvas M, Folkedal O, Imsland A, Oppedal F (2017) The effect of thermal acclimation on aerobic scope and critical swimming speed in Atlantic salmon, Salmo salar. J Exp Biol 220: 2757–2764. 10.1242/jeb.154021. [DOI] [PubMed] [Google Scholar]
- Imsland AK, Handeland SO, Stefansson SO (2014) Photoperiod and temperature effects on growth and maturation of pre- and post-smolt Atlantic salmon. Aquac Int 22: 1331–1345. 10.1007/s10499-014-9750-1. [DOI] [Google Scholar]
- IPCC (2022) Climate Change 2022: Impacts, Adaptation, and Vulnerability. In Pörtner H-O, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller Vet al., eds, Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. Cambridge University Press, Cambridge, UK and New York, NY, USA, p. 3056 [Google Scholar]
- Jin S, Yan X, Zhang H, Fan W (2015) Weight–length relationships and Fulton’s condition factors of skipjack tuna (Katsuwonus pelamis) in the Western and Central Pacific Ocean. PeerJ 3: e758. 10.7717/peerj.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T, Bennett A (1995) The thermal acclimation of burst escape performance in fish: an integrated study of molecular and cellular physiology and organismal performance. J Exp Biol 198: 2165–2175. 10.1242/jeb.198.10.2165. [DOI] [PubMed] [Google Scholar]
- Jonsson B, Forseth T, Jensen AJ, Næsje TF (2001) Thermal performance of juvenile Atlantic salmon, Salmo salar L. Funct Ecol 15: 701–711. 10.1046/j.0269-8463.2001.00572.x. [DOI] [Google Scholar]
- Jonsson B, Jonsson N (2011) Climatic effects on Atlantic salmon and brown trout. Ecology of Atlantic Salmon and Brown Trout 33: 473–515. 10.1007/978-94-007-1189-1_9. [DOI] [Google Scholar]
- Kelly N, Burness G, McDermid J, Wilson C (2014) Ice age fish in a warming world: minimal variation in thermal acclimation capacity among lake trout (Salvelinus namaycush) populations. Conserv Physiol 2: cou025–cou025. 10.1093/conphys/cou025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer J (2000) Limits to exhaustive exercise. Comp Biochem Physiol A Mol Integr Physiol 126: 161–179. 10.1016/S1095-6433(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Kieffer J, Currie S, Tufts B (1994) Effects of environmental temperature on the metabolic and acid-base responses of rainbow trout to exhaustive exercise. J Exp Biol 194: 299–317. 10.1242/jeb.194.1.299. [DOI] [PubMed] [Google Scholar]
- Kieffer JD, Tufts BL (1998) Effects of food deprivation on the white muscle energy reserves in rainbow trout (Oncorhynchus mykiss): the relations between body size and temperature. Fish Physiol Biochem 19: 239–245. 10.1023/A:1007759407275. [DOI] [Google Scholar]
- Kjaersgaard A, Nguyêt L, Demontis D, Kurbalija Novicic Z, Loeschcke V, Pertoldi C (2012) The effect of developmental temperature fluctuation on wing traits and stressed locomotor performance in Drosophila melanogaster, and its dependence on heterozygosity. Evol Ecol Res 14: 803–819. [Google Scholar]
- Kocik JF, Hayes SA, Carlson SM, Cluer B (2022) A resist-accept-direct (RAD) future for Salmon in Maine and California: salmon at the southern edge. Fish Manag Ecol 29: 456–474. 10.1111/fme.12575. [DOI] [Google Scholar]
- Langerhans RB, DeWitt TJ (2004) Shared and unique features of evolutionary diversification. Am Nat 164: 335–349. 10.1086/422857. [DOI] [PubMed] [Google Scholar]
- Langerhans RB, Layman CA, Shokrollahi AM, DeWitt TJ (2004) Predator-driven phenotypic diversification in Gambusia affinis. Evolution 58: 2305–2318. 10.1111/j.0014-3820.2004.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Lee CG, Farrell AP, Lotto A, MacNutt MJ, Hinch SG, Healey MC (2003) The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J Exp Biol 206: 3239–3251. 10.1242/jeb.00547. [DOI] [PubMed] [Google Scholar]
- Lynch JA, Rishel GB, Corbett ES (1984) Thermal alteration of streams draining clear cut watersheds quantification and biological implications. Hydrobiologia 111: 161–169. 10.1007/BF00007195. [DOI] [Google Scholar]
- MacLean H (2023) Atlantic Salmon (Salmo salar) Smolt Downstream Passage in a Tobique Narrows Generating Station. MSc Thesis, University of New Brunswick Department of Biology, Tobique River.
- Malcolm IA, Hannah DM, Donaghy MJ, Soulsby C, Youngson AF (2004) The influence of riparian woodland on the spatial and temporal variability of stream water temperatures in an upland salmon stream. Hydrol Earth Syst Sci 8: 449–459. 10.5194/hess-8-449-2004. [DOI] [Google Scholar]
- Martell DJ, Kieffer JD (2007) Persistent effects of incubation temperature on muscle development in larval haddock (Melanogrammus aeglefinus L.). J Exp Biol 210: 1170–1182. 10.1242/jeb.002188. [DOI] [PubMed] [Google Scholar]
- Mazumder SK, Das SK, Bakar Y, Ghaffar MA (2016) Effects of temperature and diet on length-weight relationship and condition factor of the juvenile Malabar blood snapper (Lutjanus malabaricus Bloch & Schneider, 1801). J Zhejiang Univ Sci B 17: 580–590. 10.1631/jzus.B1500251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S (2012) Smolt physiology and endocrinology. Fish Physiol 32: 199–251. 10.1016/B978-0-12-396951-4.00005-0. [DOI] [Google Scholar]
- McCormick SD, Hansen LP, Quinn TP, Saunders RL (1998) Movement, migration, and smolting of Atlantic Salmon (Salmo salar). Can J Fish Aquat Sci 55: 77–92. 10.1139/d98-011. [DOI] [Google Scholar]
- McCormick SD, Regish AM, Ardren WR, Björnsson BT, Bernier NJ (2019) The evolutionary consequences for seawater performance and its hormonal control when anadromous Atlantic salmon become landlocked. Sci Rep 9: 968. 10.1038/s41598-018-37608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick SD, Shrimpton JM, Zydlewski JD (1997) Temperature effects on osmoregulatory physiology of juvenile anadromous fish. In Wood CM, McDonald DG, eds, Global Warming: Implications for Freshwater and Marine Fish. Cambridge University Press, Cambridge, pp. 279–302 [Google Scholar]
- McKenzie DJ, Zhang Y, Eliason EJ, Schulte PM, Claireaux G, Blasco FR, Nati JJH, Farrell AP (2021) Intraspecific variation in tolerance of warming in fishes. J Fish Biol 98: 1536–1555. 10.1111/jfb.14620. [DOI] [PubMed] [Google Scholar]
- Mehner T, Schiller S, Staaks G, Ohlberger J (2011) Cyclic temperatures influence growth efficiency and biochemical body composition of vertically migrating fish. Freshw Biol 56: 1554–1566. 10.1111/j.1365-2427.2011.02594.x. [DOI] [Google Scholar]
- Měráková E, Gvoždík L (2009) Thermal acclimation of swimming performance in newt larvae: the influence of diel temperature fluctuations during embryogenesis. Funct Ecol 23: 989–995. 10.1111/j.1365-2435.2009.01588.x. [DOI] [Google Scholar]
- Mills KE, Pershing AJ, Sheehan TF, Mountain D (2013) Climate and ecosystem linkages explain widespread declines in north American Atlantic salmon populations. Glob Chang Biol 19: 3046–3061. 10.1111/gcb.12298. [DOI] [PubMed] [Google Scholar]
- Morash A, Neufeld C, MacCormack T, Currie S (2018) The importance of incorporating natural thermal variation when evaluating physiological performance in wild species. J Exp Biol 221: jeb164673. 10.1242/jeb.164673. [DOI] [PubMed] [Google Scholar]
- Morash AJ, Speers-Roesch B, Andrew S, Currie S (2021) The physiological ups and downs of thermal variability in temperate freshwater ecosystems. J Fish Biol 98: 1524–1535. 10.1111/jfb.14655. [DOI] [PubMed] [Google Scholar]
- Morgan R, Finnøen MH, Jutfelt F (2018) CTmax is repeatable and doesn’t reduce growth in zebrafish. Sci Rep 8: 7099. 10.1038/s41598-018-25593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Fukuwaka M, Tanimata N, Yamamura O (2010) Size-dependent thermal preferences in a pelagic fish. Oikos 119: 1265–1272. 10.1111/j.1600-0706.2009.18125.x. [DOI] [Google Scholar]
- Morley SA, Peck LS, Sunday JM, Heiser S, Bates AE (2019) Physiological acclimation and persistence of ectothermic species under extreme heat events. Glob Ecol Biogeogr 28: 1018–1037. 10.1111/geb.12911. [DOI] [Google Scholar]
- Morrison SM, Mackey TE, Durhack T, Jeffrey JD, Wiens LM, Mochnacz NJ, Hasler CT, Enders EC, Treberg JR, Jeffries KM (2020) Sub-lethal temperature thresholds indicate acclimation and physiological limits in brook trout Salvelinus fontinalis. J Fish Biol 97: 583–587. 10.1111/jfb.14411. [DOI] [PubMed] [Google Scholar]
- Mozsár A, Boros G, Sály P, Antal L, Nagy S (2015) Relationship between Fulton’s condition factor and proximate body composition in three freshwater fish species. J Appl Ichthyol 31: 315–320. 10.1111/jai.12658. [DOI] [Google Scholar]
- Myrvold KM, Mawle GW, Andersen O, Aas Ø (2019) The social, economic and cultural values of wild Atlantic salmon. A review of the literature for the period 2009–2019 and an assessment of changes in values 89. NINA Report 1668.Norwegian Institute for Nature Research [Google Scholar]
- Naeem M, Salam A, Zuberi A (2016) Proximate composition of freshwater rainbow trout (Oncorhynchus mykiss) in relation to body size and condition factor from Pakistan. Pakistan J Agri Res 53: 468–472. 10.21162/PAKJAS/16.2653. [DOI] [Google Scholar]
- Nisembaum L, Martin P, Fuentes M, Besseau L, Magnanou E, McCormick S, Falcón J (2020) Effects of a temperature rise on melatonin and thyroid hormones during smoltification of Atlantic salmon, Salmo salar. J Comp Physiol B 190: 731–748. 10.1007/s00360-020-01304-2. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Gel YR, Brunner E, Konietschke F (2012) nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw 50: 1–23. 10.18637/jss.v050.i12.25317082 [DOI] [Google Scholar]
- O’Steen S, Cullum AJ, Bennett AF (2002) Rapid evolution of escape ability in Trinidadian guppies (Poecilia reticulata). Evolution 56: 776–784. 10.1111/j.0014-3820.2002.tb01388.x. [DOI] [PubMed] [Google Scholar]
- Oligny-Hébert H, Senay C, Enders EC, Boisclair D (2015) Effects of diel temperature fluctuation on the standard metabolic rate of juvenile Atlantic salmon (Salmo salar): influence of acclimation temperature and provenience. Can J Fish Aquat Sci 72: 1306–1315. 10.1139/cjfas-2014-0345. [DOI] [Google Scholar]
- Peppar JL (1982) Atlantic salmon smolt investigations, Restigouche river system, New Brunswick. In Canadian Manuscript Report of Fisheries and Aquatic Sciences No. 1641. vii + 15
- Gardner Pinfold . 2011. Available fromhttps://0104.nccdn.net/1_5/13f/2a0/0fe/value-wild-salmon-final.pdf [accessed 29 January 2022].
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2016) Nlme: linear and nonlinear mixed effect models R Package Version 3. 1–126.
- Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315: 95–97. 10.1126/science.1135471. [DOI] [PubMed] [Google Scholar]
- Primmer CR (2011) Genetics of local adaptation in salmonid fishes. Heredity 106: 401–403. 10.1038/hdy.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- Rees BB, Matute LA (2018) Repeatable interindividual variation in hypoxia tolerance in the gulf killifish, Fundulus grandis. Physiol Biochem Zool 91: 1046–1056. 10.1086/699596. [DOI] [PubMed] [Google Scholar]
- Reid HB, Ricciardi A (2022) Ecological responses to elevated water temperatures across invasive populations of the round goby (Neogobius melanostomus) in the Great Lakes basin. Can J Fish Aquat Sci 79: 277–288. 10.1139/cjfas-2021-0141. [DOI] [Google Scholar]
- Rodgers E, Cocherell D, Nguyen T, Todgham A, Fangue N (2018) Plastic responses to diel thermal variation in juvenile green sturgeon, Acipenser medirostris. J Therm Biol 76: 147–155. 10.1016/j.jtherbio.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Rulifson RA (1977) Temperature and water velocity effects on the swimming performances of young-of-the-year striped mullet (Mugil cephalus), spot (Leiostomus xanthurus), and pinfish (Lagodon rhomboides). J Fish Res Bd Can 34: 2316–2322. 10.1139/f77-310. [DOI] [Google Scholar]
- Scheuffele H, Rubio-Gracia F, Clark TD (2021) Thermal performance curves for aerobic scope in a tropical fish (Lates calcarifer): flexible in amplitude but not breadth. J Exp Biol 224: jeb243504. 10.1242/jeb.243504. [DOI] [PubMed] [Google Scholar]
- Shrimpton J, Björnsson BT, McCormick S (2000) Can Atlantic salmon smolt twice? Endocrine and biochemical changes during smolting. Can J Fish Aquat Sci 57: 1969–1976. 10.1139/cjfas-57-10-1969. [DOI] [Google Scholar]
- Silva C, Riginos C, Wilson R (2019) An intertidal fish shows thermal acclimation despite living in a rapidly fluctuating environment. J Comp Physiol B 189: 385–398. 10.1007/s00360-019-01212-0. [DOI] [PubMed] [Google Scholar]
- Stefansson S, BTh B, Ebbesson L, McCormick S (2008) Smoltification. In Finn, RN (Ed.), Fish Larval Physiology, 639–681. 10.1201/9780429061608-27. [DOI] [Google Scholar]
- Swansburg E, Chaput G, Moore D, Caissie D, El-Jabi N (2002) Size variability of juvenile Atlantic salmon: links to environmental conditions. J Fish Biol 61: 661–683. 10.1111/j.1095-8649.2002.tb00903.x. [DOI] [Google Scholar]
- Taylor E (1991) A review of local adaptation in Salmonidae, with particular reference to Pacific and Atlantic salmon. Aquaculture 98: 185–207. 10.1016/0044-8486(91)90383-I. [DOI] [Google Scholar]
- Taylor EB, McPhail JD (1985) Variation in burst and prolonged swimming performance among British Columbia populations of coho salmon, Oncorhynchus kisutch. Can J Fish Aquat Sci 42: 2029–2033. 10.1139/f85-250. [DOI] [Google Scholar]
- Teichert N, Benitez J-P, Dierckx A, Tétard S, Oliveira E, Trancart T, Feunteun E, Ovidio M (2020) Development of an accurate model to predict the phenology of Atlantic salmon smolt spring migration. Aquatic Conserv: Mar Freshw Ecosyst 30: 1552–1565. 10.1002/aqc.3382. [DOI] [Google Scholar]
- Thackeray SJ, Henrys PA, Feuchtmayr H, Jones ID, Maberly SC, Winfield IJ (2013) Food web de-synchronization in England’s largest lake: an assessment based on multiple phenological metrics. Glob Chang Biol 19: 3568–3580. 10.1111/gcb.12326. [DOI] [PubMed] [Google Scholar]
- Therneau, T. 2021. A package for survival analysis in R. R package version 3. 2–13, https://CRAN.R-project.org/package=survival.
- Thorstad EB, Bliss D, Breau C, Damon-Randall K, Sundt-Hansen LE, Hatfield EMC, Horsburgh G, Hansen H, Maoiléidigh NÓ, Sheehan Tet al. (2021) Atlantic salmon in a rapidly changing environment—facing the challenges of reduced marine survival and climate change. Aquatic Conserv: Mar Freshw Ecosyst 31: 2654–2665. 10.1002/aqc.3624. [DOI] [Google Scholar]
- Tunnah L, Currie S, MacCormack TJ (2017) Do prior diel thermal cycles influence the physiological response of Atlantic salmon (Salmo salar) to subsequent heat stress? Can J Fish Aquat Sci 74: 127–139. 10.1139/cjfas-2016-0157. [DOI] [Google Scholar]
- Turko AJ, Nolan CB, Balshine S, Scott GR, Pitcher TE (2020) Thermal tolerance depends on season, age and body condition in imperilled redside dace Clinostomus elongatus. Conserv Physiol 8: coaa062. 10.1093/conphys/coaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin M, Kinnison M, Boustead N, Quinn T (2003) Genetic control over survival in Pacific salmon (Oncorhynchus spp.): experimental evidence between and within populations of New Zealand Chinook salmon (O. tshawytscha). Can J Fish Aquat Sci 60: 1–11. 10.1139/f02-167. [DOI] [Google Scholar]
- Volkoff H, Rønnestad I (2020) Effects of temperature on feeding and digestive processes in fish. Temperature (Austin) 7: 307–320. 10.1080/23328940.2020.1765950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier K, Penaluna BE, Hauck LL, Longway LJ, Garcia T, Cronn R (2021) Estimating the genetic diversity of Pacific salmon and trout using multigene eDNA metabarcoding. Mol Ecol 30: 4970–4990. 10.1111/mec.15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellband K, Mérot C, Linnansaari T, Elliott JAK, Curry RA, Bernatchez L (2019) Chromosomal fusion and life history-associated genomic variation contribute to within-river local adaptation of Atlantic salmon. Mol Ecol 28: 1439–1459. 10.1111/mec.14965. [DOI] [PubMed] [Google Scholar]
- Whalen KG, Parrish DL, McCormick SD (1999) Migration timing of Atlantic salmon smolts relative to environmental and physiological factors. Trans Am Fish Soc 128: 289–301. . [DOI] [Google Scholar]
- Wheeler B, Torchiano M (2016) lmPerm: permutation tests for linear models. R package version 2.1.0.
- Wilkie MP, Davidson K, Brobbel MA, Kieffer JD, Booth RK, Bielak AT, Tufts BL (1996) Physiology and survival of wild Atlantic salmon following angling in warm summer waters. Trans Am Fish Soc 125: 572–580. . [DOI] [Google Scholar]
- Zathey, N. 2018. Effects of water temperature, rearing temperature and population on swimming performance and temperature preference in Atlantic salmon (Salmo salar). Electronic Thesis and Dissertation RepositoryAvailable fromhttps://ir.lib.uwo.ca/etd/5804.
- Zhang Y, Kieffer JD (2014) Critical thermal maximum (CTmax) and hematology of shortnose sturgeons (Acipenser brevirostrum) acclimated to three temperatures. Can J Zool 92: 215–221. 10.1139/cjz-2013-0223. [DOI] [Google Scholar]
- Zillig KW, FitzGerald AM, Lusardi RA, Cocherell DE, Fangue NA (2023) Intraspecific variation among Chinook salmon populations indicates physiological adaptation to local environmental conditions. Conserv Physiol 11: coad044. 10.1093/conphys/coad044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig KW, Lusardi RA, Moyle PB, Fangue NA (2021) One size does not fit all: variation in thermal eco-physiology among Pacific salmonids. Rev Fish Biol Fish 31: 95–114. 10.1007/s11160-020-09632-w. [DOI] [Google Scholar]
- Zydlewski GB, Haro A, McCormick SD (2005) Evidence for cumulative temperature as an initiating and terminating factor in downstream migratory behavior of Atlantic salmon (Salmo salar) smolts. Can J Fish Aquat Sci 62: 68–78. 10.1139/f04-179. [DOI] [Google Scholar]
- Desforges JE, Birnie-Gauvin K, Jutfelt F, Gilmour KM, Eliason EJ, Dressler TL, McKenzie DJ, Bates AE, Lawrence MJ, Fangue N, Cooke SJ. (2023). The ecological relevance of critical thermal maxima methodology for fishes. Journal of Fish Biology 102: 1000–1016. 10.1111/jfb.15368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated or analysed during this study will be available in the Borealis Data Repository upon acceptance. https://doi.org/10.5683/SP3/3P5MAY


