Abstract
In coastal waters, methane-oxidizing bacteria (MOB) can form a methane biofilter and mitigate methane emissions. The metabolism of these MOBs is versatile, and the resilience to changing oxygen concentrations is potentially high. It is still unclear how seasonal changes in oxygen availability and water column chemistry affect the functioning of the methane biofilter and MOB community composition. Here, we determined water column methane and oxygen depth profiles, the methanotrophic community structure, methane oxidation potential, and water–air methane fluxes of a eutrophic marine basin during summer stratification and in the mixed water in spring and autumn. In spring, the MOB diversity and relative abundance were low. Yet, MOB formed a methane biofilter with up to 9% relative abundance and vertical niche partitioning during summer stratification. The vertical distribution and potential methane oxidation of MOB did not follow the upward shift of the oxycline during summer, and water–air fluxes remained below 0.6 mmol m−2 d−1. Together, this suggests active methane removal by MOB in the anoxic water. Surprisingly, with a weaker stratification, and therefore potentially increased oxygen supply, methane oxidation rates decreased, and water–air methane fluxes increased. Thus, despite the potential resilience of the MOB community, seasonal water column dynamics significantly influence methane removal.
Keywords: hypoxia, methane oxidation, niche partitioning, oxygen, succession, summer stratification
Methane-oxidizing bacteria in coastal waters can mitigate diffusive methane emissions. Their metabolic versatility and resilience are potentially high. Yet, methane removal is insufficient and highly variable throughout the year.
Introduction
Coastal ecosystems are the major contributor to global ocean methane emissions, despite only covering about 15% of ocean surface area (6–12 Tg CH4 yr−1) (Borges et al. 2016, Weber et al. 2019). Due to the presence of methane-oxidizing microorganisms in the water column and in the sediment, these methane emissions are only a fraction of the methane produced in the sediment. The efficiency of this so-called methane biofilter is one of the greatest uncertainties in methane-emission predictions (Dean et al. 2018). In coastal basins, a consortium of anaerobic methane-oxidizing archaea with sulfate-reducing bacteria builds the methane biofilter in the sediment (Wallenius et al. 2021), and aerobic methane-oxidizing bacteria (MOB) dominate the methane biofilter in the water column (Reeburgh 2007, Steinle et al. 2017, Steinsdóttir et al. 2022a, Venetz et al. 2023). Deoxygenation and eutrophication can lead to increased methane production in the sediment that can exceed the methane oxidation capacity, which can result in high benthic methane fluxes into the water column (Egger et al. 2016, Zhang et al. 2020, Lenstra et al. 2023, Żygadłowska et al. 2023a). As coastal ecosystems are especially affected by eutrophication and hypoxia (Diaz and Rosenberg 2008, Breitburg et al. 2018), benthic methane fluxes are likely to increase in the future (Dean et al. 2018). A better understanding of the fate of methane in the water column is therefore crucial for better predictions of methane emissions to the atmosphere and key for adequate policymaking.
Microbial removal of methane in the water column is mostly attributed to putative aerobic methanotrophic bacteria. Although methanotrophic archaea such as anaerobic methanotrophic archaea (ANME)-related phylotypes and NC10 bacteria can be present in the water column of marine ecosystems (Schubert et al. 2006, Thamdrup et al. 2019), aerobic gamma- or alpha-proteobacterial methanotrophic bacteria (γ- and α-MOB) dominate these methanotrophic communities (Tavormina et al. 2010, Steinsdóttir et al. 2022b), even in hypoxic waters (Steinle et al. 2017). During summer stratification, such a methane biofilter can counteract the high benthic methane fluxes (Mao et al. 2022, Steinsdóttir et al. 2022b).
Coastal basins are highly influenced by seasonal water column dynamics, which affects the water column chemistry and ultimately the microbial community structure and activity (Gilbert et al. 2012, Wang et al. 2020). For instance, increasing surface water temperatures in summer can lead to a density stratification, which lowers the oxygen supply to the bottom and results in an oxycline and anoxic bottom water. Multiple potential metabolic pathways within the ambient methanotrophic community can ensure the functionality of the methane biofilter along this vertical range of oxygen (and methane) concentrations (Hernandez et al. 2015, Venetz et al. 2023). Such niche partitioning over a range of oxygen concentrations likely aids the overall resilience of the methane biofilter toward regular changes in oxygen availability in the water column (Venetz et al. 2023). For instance, thanks to the rapid succession of the MOB community, the abundance of methanotrophs and methane oxidation capacity during autumn turnover remained high and could mitigate methane emissions in a freshwater lake (Mayr et al. 2020). Another study observed increased methane oxidation accompanied by an increase of MOB abundance from spring to summer (Gründger et al. 2021). Thus, the succession of the MOB community from a mixed water column in spring to summer stratification with an oxygen gradient as well as the succession upon water column mixing is crucial for the functioning of the methane biofilter. To reduce the uncertainties in methane-emission predictions, it is thus important to understand how the methanotrophic community structure and potential removal activity at different depths respond to the seasonal changes in methane, oxygen, and nutrient availability and how this ultimately affects methane release to the atmosphere (Louca et al. 2016, Grossart et al. 2020).
Here, we studied the seasonal dynamics of the microbial methane biofilter in the water column of a marine coastal basin in the southwest Netherlands. In nine sampling campaigns between March and October 2021, we investigated the vertical distribution of methane and oxygen, the potential methane oxidation rates, the microbial community structure, and the methane fluxes to the atmosphere.
Materials and methods
Fieldwork location and sampling methodologies
Lake Grevelingen is a highly eutrophic former estuary with a total surface area of 115 km2 and an average water depth of 5.1 m. The water column of the main channel is stratified during the summer months with hypoxic or anoxic bottom waters (Wetsteijn 2011, Hagens et al. 2015). A more detailed description of the system and the study site can be found elsewhere (Egger et al. 2016, Sulu-Gambari et al. 2017). At the deepest point of Lake Grevelingen, the Scharendijke basin (51.742°N; 3.849°E, 45 mbs), the high sedimentation rates and anoxic bottom water lead to high benthic methane fluxes to the water column during summer stratification (0.6–2.7 mmol m−2 d−1) (Egger et al. 2016, Żygadłowska et al. 2023a). To monitor seasonal water column methane dynamics in the Scharendijke basin, we conducted nine research cruises with the RV Navicula between March and October 2021. During these campaigns, we measured in situ water–air methane fluxes, constructed depth profiles of water column chemistry, and took samples for microbial analysis, including incubation experiments to measure potential aerobic methane oxidation rates.
The extent of water column stratification during each sampling campaign was determined with a CTD unit (SBE 911 Plus, Sea-Bird Electronics, Bellevue, WA, USA). In addition, the oxygen distribution was simultaneously recorded by a seabird sensor (Seabird SBE43). Because of the limit of detection commonly observed in oxygen sensor data of oxygen-depleted waters, we here consider hypoxia at concentrations <63 µmol l−1 and anoxia once concentrations are <3 µmol l−1 and do not further decrease with depth.
Water samples were taken at 30 depths with a 10-l Niskin bottle. Subsequently, unfiltered water was collected in 1-l sterile plastic bottles for DNA analysis, and 0.5-l sterile Schott bottles for incubation experiments, which were stored in the dark at 4°C until further processing. Furthermore, 120-ml borosilicate serum bottles were filled for the determination of the methane concentration. To avoid air contamination, the bottles were filled from the bottom via gas-tight tubing while letting the water overflow three times, after which they were crimp-capped with an aluminum cap and a butyl stopper. To stop microbial activity, 0.25 ml of HgCl2 (sat.) was added. Samples were stored upside down at room temperature until further processing.
Methane concentration measurements
To determine methane concentrations in the water column, 5 ml of N2 gas was added to all borosilicate bottles, while simultaneously removing the same volume of the water. After equilibrating for at least 2 h, methane concentrations were measured with a Thermo Finnigan Trace™ gas chromatograph equipped with a flame ionization detector (detection limit: 0.02 µmol l−1).
DNA extraction, 16S rRNA gene sequencing, and data analysis
Water samples were filtered in the lab, within 24 h after sampling on Supor® PES 0.22-µm filters with a vacuum pump setup. After immediate freezing at −80°C, the samples were stored at −20°C until extraction. The DNA was extracted with the FastDNA™ SPIN Kit for Soil DNA Isolation Kit (MP Biomedicals) according to the protocol.
The V3-V4 region of the 16S rRNA gene was sequenced by Macrogen with an Illumina MiSeq platform (Macrogen, Amsterdam, The Netherlands) to analyze the microbial community composition. The primer pairs Bac341F (CCTACGGGNGGCWGCAG) (Herlemann et al. 2011) and Bac806R (GGACTACHVGGGTWTCTAAT) (Caporaso et al. 2012) were used for bacteria, and the primer pairs Arch349F (GYGCASCAGKCGMGAAW) (Ken et al. 2000) and Arch806R (GGACTACVSGGGTATCTAAT) (Ken et al. 2000) were used for archaeal 16S sequencing. Sequencing data were processed with RStudio. Primers were removed with cutadapt (Martin et al. 2012) with the options -g, -G, and discard-untrimmed. Low-quality reads (<Q20 forward and <Q30 reverse) were removed by truncating reads to a length of nt 270 forward and nt 240 reverse with the DADA2 pipeline (Callahan et al. 2017). Finally, amplicon sequence variants (ASVs) were inferred, forward and reverse reads were merged, and chimaeras removed. For taxonomic assignment, the 254 Silva non-redundant train set v138 (https://zenodo.org/record/3731176#.XoV8D4gzZaQ) was used. For further clustering and calculation of relative abundances, the Phyloseq package was used, and data were visualized with ggplot2, and graphs were adjusted with Adobe Illustrator. Raw reads of the 16S amplicon sequencing data can be accessed on the National Center for Biotechnology Information (NCBI) website under the accession number PRJNA1053269.
Potential methane oxidation rates
To investigate the seasonal dynamics of aerobic methane removal potential by the methanotrophic methane filter in the water column, we incubated water samples from 14 depths during each sampling campaign within 24 h after sample retrieval. For each depth, 100 ml of unfiltered, air-equilibrated sample, was put into an autoclaved 120-ml borosilicate bottle (in triplicate) and closed with bromobutyl stoppers, and crimped with an aluminum cap. To each incubation, 1 ml of 13C-CH4 (99%) was added, which resulted in a partial pressure of 5% in the headspace. All bottles were incubated under constant shaking (150 r/m) in the dark, at room temperature for the duration of the incubation.
For each time point, 3-ml liquid sample was taken from the incubation bottle and replaced with 1 ml of air. As the carbonate balance at the pH range of our sample is susceptible to small changes in pH, we acidified subsamples to a pH of <2. The liquid sample was transferred into a gas-tight air-equilibrated 3-ml vial (Labco, exetainer, UK) containing 50 µl of 0.1 M HCl. The produced 13C-CO2 was measured directly from the exetainer headspace with a gas chromatography–mass spectrometer (GC−MS) (Agilent 5975C inert MSD). Liquid 13CO2 concentrations were calculated with the Henry coefficient (Supplementary data). The linear increase in 13C-CO2 after the lag phase was used to determine methane oxidation rates.
In situ water–air flux measurements
In situ water–air fluxes of methane were measured during each sampling campaign. Fluxes were determined using a transparent, cylindrical floating chamber (ø: 390 mm, height 270 mm, TechnoCentrum, Radboud University, Nijmegen, The Netherlands) connected in a closed loop to a LICOR trace gas analyzer (LI-7810, LI-COR Environmental—UK Ltd., Cambridge, UK). The chamber frame was stabilized to withstand wave turbulence using a bespoke raft (Supplementary Fig. S1), as the surface water of the Scharendijke basin can be quite turbulent. To ensure a closed loop between the chamber and the LICOR gas analyzer, the input and output connectors of the LICOR were connected to the top of the floating chamber using gas-tight polyurethane tubes [ø: 4 mm (inside), Festo, 5 m]. The chamber was gently placed on the water surface, and the accumulation of methane was measured with the trace gas analyzer for at least 3 min in triplicate. The chamber was aerated until atmospheric methane concentrations were reached, before starting each new measurement.
Methane fluxes were then calculated with the following equation:
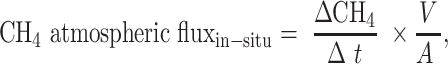 |
where ΔCH4 /Δt is the linear increase of the concentration of methane (mmol m−3) in the chamber over time (Δt), V is the volume of the chamber (m3), and A is the area of the chamber (m2). The measured partial pressures (ppb) in the chamber were converted to methane (mmol m−3) using the ideal gas law and the ambient air temperature during each deployment.
Statistical analysis
To assess the seasonal dynamics of the bacterial community, Shannon diversity and Chao1 richness were calculated to illustrate the bacterial alpha diversity of untransformed ASV counts of each sample. Before analysis, ASVs that were assigned to archaea, mitochondria, and chloroplasts were removed from the dataset.
The dissimilarity of the bacterial community between each sample was calculated as the Bray–Curtis distance of rarefied ASV counts, and distance matrices were illustrated by nonmetric multidimensional scaling (NMDS). Based on the stress factor assessment of the ordination with different dimensions, the ordination was performed on two dimensions (stress = 0.092, nonmetric fit R2 = 0.992, and linear fit R2 = 0.965). Vectors for the environmental variables O2, CH4, H2S, NO2-, NO3-, Fetot, and depth were determined with the env_fit() function of the vegan package.
Results and discussion
In this study, we monitored the seasonal dynamics of the microbial methane filter in the water column of a marine basin during nine sampling campaigns in 2021. In the following sections, we will show and discuss our results for the seasonal succession and distribution of MOB, methane oxidation potential along the depth gradient, the potential for anoxic methane removal, and the water-to-air fluxes of methane in marine Lake Grevelingen in 2021. We show that there is (i) a vertical distribution and seasonal succession of the versatile methanotrophic community, (ii) that this is related to water column dynamics and the availability of oxygen and nutrients, and (iii) that this is ultimately related to methane removal and in situ methane fluxes to the atmosphere.
Seasonal succession and vertical distribution of the methanotrophic community
The methanotrophic community was dominated by methane-oxidizing Gammaproteobacteria (γ-MOB) belonging to Methylomonadaceae. Although alphaproteobacteria (α-MOB) were present, their relative abundance never exceeded 0.07% of the bacterial 16S rRNA reads. This could be due to the eutrophic state of the basin, as α-MOB are more adapted to oligotrophic conditions and could be continuously outcompeted by Methylomonadaceae (Ho et al. 2013, Kaupper et al. 2020). The main methanotrophic genera were Milano-WF1B-03 (M1B), Marine_Methylotrophic_Group2 (MMG2), and Methyloprofundus (MP). These (γ-MOB) together built a methanotrophic community, i.e. potentially versatile in terms of oxygen metabolism, denitrification capacity, and potential for sulfur transformation (Venetz et al. 2023). Despite the low diversity of the methanotrophic community, our seasonal data demonstrated a high adaptation potential enabling both vertical niche partitioning and seasonal succession. While the relative abundance of MOB was <0.3% of the bacterial 16S rRNA reads in the mixed, oxygenated water column in March (Fig. 1A), it increased up to 9% at 39 m depth by July when the water column was stratified (Fig. 2A). This drastic increase can be attributed to the summer stratification, which resulted in the formation of chemical niches along the methane–oxygen counter gradient (Amaral and Knowles 1995, Mayr et al. 2020) and was accompanied by a shift in the MOB community structure with depth. In the oxygenated water, MOB relative abundance is low and appears to consist mostly of M1B (Fig. 2A, Supplementary Fig. S2). In the anoxic water, MOB relative abundance is up to 9% and consists of mostly MMG2 and MP (Fig. 2A). A similar niche partitioning was observed at the end of summer stratification in September 2020 (Venetz et al. 2023). There, the metabolic potential of these genera indicated metabolic adaptations to oxygen limitation with high-affinity oxidases and through potential nitrate, iron, and sulfur reduction, which could explain the shift in community composition along the oxycline. Similarly, these metabolic adaptations are also important for seasonal succession. Our seasonal data showed that despite having low diversity, the methanotrophic community could adapt to both water column mixing and summer stratification. However, eutrophication and stratification in coastal ecosystems will likely intensify in the future (Dominović et al. 2023), which may induce more pronounced shifts in the methanotrophic community during summer stratification and further decrease diversity. The diversity of the entire water column bacterial community indeed shows a drastic decrease in alpha diversity from March to October 2021 (Fig. 2C, Supplementary Fig. S3). Prolonged anoxia and warming could arguably promote even slow-growing anaerobic methanotrophs, such as anaerobic methanotrophic archaea or NC10 bacteria (Su et al. 2023). Yet, the pool of other methanotrophic microorganisms than Methylomonadaceae in marine Lake Grevelingen is very low. Thus, the low diversity of methanotrophic microorganisms can impair the resilience toward changes in oxygen availability that exceed the amplitude of the current seasonal dynamics.
Figure 1.
(A) Diffusive methane water–air fluxes measured with an in situ floating chamber. Boxes indicate the first and third quartiles, lines indicate the median, and whiskers indicate outer data points if <1.5 interquartile range from quartiles. (B) Depth profiles of oxygen (blue lines) and temperature (red lines), and (C) methane concentrations (black circles) together with potential aerobic methane oxidation rates (MOx) (orange circles) determined by incubation experiments between March and October 2021 in the Grevelingen Scharendijke basin. Error bars of MOx rates show the standard deviation between the biological replicates. The hypoxic zone (<63 µmol l−1; Breitburg et al. 2018) is shaded light and the anoxic zone (<3 µmol l−1) is shaded dark.
Figure 2.
(A) Relative abundance of methanotrophic bacteria retrieved through 16S rRNA sequencing. Light shaded areas indicate the hypoxic zone (<63 µmol l−1 h−1), and dark shaded areas indicate the anoxic zone (<3 µmol l−1). (B) Beta Diversity of all samples was calculated as Bray–Curtis distance and ordinated via two-dimensional NMDS. Short arrows indicate weak correlations and long arrows indicate strong correlations. The R2 values can be found in the Supplementary data (“NMDS_output”). (C) Alpha diversity measures Chao1 (richness) and Shannon (richness and evenness) of the total water column bacterial community of each month.
Methane removal by methanotrophic bacteria
Potential aerobic methane oxidation rate measurements (up to 0.60 µmol l−1 h−1) indicate that benthic methane is (partially) oxidized in the water column before reaching the atmosphere (Fig. 1C). These measurements confirmed that there is potential for methane removal throughout the year. Moreover, methane oxidation rates followed a seasonal pattern and varied with depth along the water column. Methane oxidation in the mixed water column in March was low (<0.002 µmol l−1 h−1) compared to May (0.008–3.6 µmol l−1 h−1) upon the onset of summer water column stratification and the development of an oxycline. During the stratification period, methane oxidation rates followed a vertical pattern. In June, a clear methane–oxygen counter gradient had formed with an oxycline between 35 and 36 m and high methane concentrations in the bottom water (up to 17 µmol l−1). The high methane oxidation rates below 9 m (0.8–2.9 µmol l−1 h−1), indicated active aerobic methane removal. However, canonical aerobic methane oxidation alone cannot explain the observed biogeochemical and microbiological pattern, which is especially pronounced in July, August, and September. Between June and July, an additional thermocline formed in between 12 and 16 m (Fig. 1B). This induced the formation of the oxycline higher up and resulted in a vertical decoupling of the methane and oxygen gradients and the formation of an oxygen- and sulfide-free (suboxic) zone (Żygadłowska et al. 2023b). Interestingly, the potential aerobic methane oxidation rates and the relative abundance of MOB were highest far below the oxycline (Figs 1C and 2A). If this de-coupling had induced a diminishing of the methane biofilter, we would have expected a decreased relative abundance of MOB, lower methane oxidation rates, and a higher methane flux to the atmosphere. However, we did not observe any of the mentioned changes. This points to a potentially active methane biofilter even under anoxic conditions. Notably, potential methane oxidation rates and relative MOB abundance were consistently highest below the oxycline, and both drastically decreased by the onset of water column mixing and reoxygenation in autumn.
MOB responsible for anaerobic methane oxidation
The high relative abundance of putatively aerobic MOB in the anoxic water column and their potential activity is an emerging paradox in a variety of aquatic ecosystems. Anaerobic methane removal is commonly attributed to slow-growing methanotrophic archaea in the sediment, where total microbial biomass is high and substrate residence time can be long. Archaea can also be involved in anaerobic methane removal in the water column. For example, in the Black Sea, methanotrophic archaea are responsible for anaerobic methane removal in the suboxic zone (Schubert et al. 2006). The relative abundance of methanotrophic archaea in the water column of marine Lake Grevelingen was highest in July with 2% (of archaeal 16S rRNA reads) at 23 m and 1.8% at 40 m and mainly belonged to the family of Methanoperedenaceae. However, their depth distribution did not follow any coherent pattern, and based on metagenomic analysis, in September 2020, the total abundance of archaea in the water column was 50–100 × lower than the abundance of bacteria (based on phyloflash analysis of the metagenome, data not shown). Moreover, the slow-growing archaea might struggle to establish a population in shallow ecosystems where the water column is fully mixed in spring and autumn and changes in water column chemistry are fast (Su et al. 2023). Therefore, we estimate the overall contribution of methanotrophic archaea to the anaerobic removal of methane in the water column to be low. It is more plausible that the dominant methanotrophic bacteria use alternative electron acceptors during oxygen limitation (Steinsdóttir et al. 2022a). The methanotrophic community in the suboxic zone in July consisted of MP and MMG2. In the euxinic zone below that, the methanotrophic community was dominated by MP alone. The shift in the MOB community toward MP and MMG2 in the anoxic zone may be explained by their versatile metabolism and adaptation potential. Methanotrophic metagenome-assembled genomes (MAGs) previously retrieved from Lake Grevelingen implied the genomic signature of low-oxygen adaptation; an MP MAG contained the high-affinity bd oxidase, which indicates the ability to scavenge oxygen at very low concentrations, and in a MAG attributed to MMG2, the potential use of nitrate or metal oxides as alternative electron acceptors was described (Venetz et al. 2023). Recent studies further indicate the high potential for anaerobic methane removal by MOB in the water column of marine ecosystems (Thamdrup et al. 2019, Steinsdóttir et al. 2022b), and laboratory incubations indicate the possibility of methane oxidation with iron oxides by methanotrophic bacteria (Zheng et al. 2020, Li et al. 2021). Suboxic zones harbor electron acceptors other than oxygen, such as metal oxides or nitrate (Murray et al. 1999). Although cryptic cycling could arguably obscure the supply of oxygen and alternative electron acceptors, nitrate is depleted in the suboxic zone (except for August/September), but iron oxides are potentially available (Żygadłowska et al. 2023b). Furthermore, recent studies suggest that external electron transfer to or with dissolved organic matter (DOM) might increase the methane oxidation capacity in wetland and brackish sediments and even in the water column of humic bog lakes (Valenzuela et al. 2019, Olmsted et al. 2023, Pelsma et al. 2023). Considering the high sedimentation in the Scharendijke basin (Egger et al. 2016) and eutrophic state of the lake, external electron transfer linked to DOM and metal oxides might be an additional mechanism supporting anaerobic methane oxidation, especially in early summer (July). Therefore, methane oxidation with alternative electron acceptors would potentially enable the removal of methane in anoxic water.
Seasonal dynamics of water–air methane fluxes
Calculations based on benthic methane flux measurements and the distribution of methane in the water column show that ebullition is a major contributor to the methane flux all year long (Żygadłowska et al. 2023b). These bubbles can bypass the microbial methane filter directly, or dissolve while traveling upward through the water and can considerably lower the methane filtering efficiency throughout the year (Żygadłowska et al. 2023b). In contrast, the in situ diffusive water–air fluxes of methane followed a clear seasonal pattern (Fig. 1A). While the fluxes increased from March to April, they were low in June, when the water column was stratified and methane concentrations had increased in the bottom waters. Together with the high relative abundance of MOB in the water and high potential methane oxidation rates (Figs 1C and 2A), this suggests that the active microbial methane filter during summer stratification mitigated most of the diffusive methane emissions to the atmosphere. However, in September, the diffusive fluxes of methane were higher despite the relative abundance of methanotrophs. While the methane water–air fluxes were lower during summer stratification (0.12–0.22 mmol m−2 d−1), the fluxes started to increase toward the end of the stratification period (Fig. 1A). Although the relative methanotrophic abundance in the anoxic water column was high at the end of August (up to 10%), methane still bypassed the MOB filter, and methane fluxes to the atmosphere increased at the end of August (0.96 mmol m−2 d−1) and in September (1.15 mmol m−2 d−1). While in the preceding months, the methane concentrations right below the oxycline never exceeded 0.5 µmol l−1, methane concentrations in the oxycline at 50% oxygen saturation were 1.6 µmol l−1 in September (Supplementary data “water_column_chemistry”). Interestingly, the methanotrophic community did not decrease significantly, which indicates that the seasonal dynamics in water column chemistry affected methane oxidation rates more than abundance or community structure (Kaupper et al. 2020). We suggest a combination of reasons that contributed to increased methane fluxes to the atmosphere from the end of August onward, despite the high relative abundance of methanotrophs: (i) the weakening of stratification increased the turbulent flux, which resulted in less time for microbial oxidation, (ii) nitrite accumulation inhibited methanotrophic activity and linked to that (iii) a shift in microbial community structure induced a change in the interactions between the methanotrophic bacteria and other members of the microbial community. The weakening of the water column methane biofilter coincided with the weakening of summer stratification. An intrusion of oxygen through a lateral influx of oxygenated water (Hagens et al. 2015, van Haren 2019) and a weakening of the stratification due to warming could increase the downward flux of oxygen and enhance the upward methane flux (Żygadłowska et al. 2023b). The high relative abundance of M1B in the anoxic water might be the result of oxygen intrusion prior to sampling at the end of August. The MAG associated with M1B, retrieved from this basin in 2020, did not reveal any genomic indication for the oxidation of methane during oxygen limitation: neither high-affinity oxidases were found, nor key genes for the utilization of alternative electron acceptors (Venetz et al. 2023). Therefore, we suggest that cryptic oxygen intrusions between July and August could have provoked a shift away from MOB adapted to oxygen limitation. This may have led to a temporary weakening of the methane oxidation filter once the introduced oxygen was depleted. In the anoxic water, MP and MMG2 were similarly abundant in August, while MP dominated in July. The MAGs associated with MMG2 and MP, retrieved from this basin in 2020, showed different adaptations to oxygen limitation. While the MAG associated with MP harbored a high-affinity bd oxidase, the MAG associated with MMG2 showed almost full potential for denitrification but only harbored the low-affinity oxidase (Venetz et al. 2023). This shift further indicates that intrusions of oxygen-rich water could have affected the community in the anoxic water as well. In September, the methane oxidation rates were much lower compared to the previous month. Notably, at the end of August, nitrite accumulated in the suboxic zone (Żygadłowska et al. 2023b), which could be caused by oxygen inhibition of nitrite oxidation (Sun et al. 2021). The NMDS plot based on the Bray–Curtis distance shows that while oxygen and methane were most predictive of the bacterial community composition (R2 = 0.86), nitrite concentrations correlated with a shift in microbial community structure (Fig. 2B; Supplementary data “NMDS_output”). It is known that nitrite can inhibit methane oxidation, especially in communities not adapted to nitrite, and in some cases, this inhibition appears to be even irreversible (King and Sylvia 1994, Dunfield and Knowles 1995). The relative abundance of nitrifiers was very low in the microbial community, and nitrite concentrations never exceeded 2 µmol l−1 in the proceeding months below the oxycline (Supplementary data “water_column_chemistry”). Therefore, the methanotrophic community was likely poorly adapted to nitrite, and nitrite accumulation might have irreversibly inhibited methanotrophic activity and resulted in low methane oxidation rates in September (Fig. 1B). This could have been accompanied by a cascading effect within the plankton community due to the above-mentioned oxygenation event. For example, it has been shown that methane oxidation can be positively or negatively influenced by the total microbial community (Gilbert et al. 2012), through nutrient competition and cross-feeding, and either the production or removal of toxic compounds by heterotrophic bacteria (Ho et al. 2014, Krause et al. 2017, Veraart et al. 2018). Therefore, factors accompanying the oxygenation event could have inhibited methane oxidation activity of MP and MMG2 in the anoxic water. All findings together suggest that a weaker stratification and increased oxygen supply together with potential nitrite accumulation and associated shifts in the microbial community might have resulted in the malfunctioning of the microbial methane filter and resulted in even higher diffusive methane fluxes to the atmosphere.
Conclusion
We conclude that the efficiency of the microbial methane filter in the water column of a shallow marine basin is strongly influenced by seasonal water column dynamics. The microbial community consists of potentially metabolic versatile Methylomonadaceae and counteracts high benthic methane fluxes during summer stratification. This is strikingly reflected in high water column methane oxidation potential and lower in situ diffusive water–air methane fluxes. Interestingly, a high relative abundance of MOB and a high potential for aerobic methane oxidation were even found in the anoxic water. We suggest that methanotrophic bacteria dominate anoxic methane removal in the water column due to the availability of iron oxides and/or external electron transfer in the suboxic zone. However, methane increasingly bypasses the biofilter as stratification weakens and the contribution of the ebullitive flux from the sediment directly to the atmosphere is high. Hence, notwithstanding the large capacity of the microbial methane filter to oxidize methane under varying redox conditions, water column mixing can decrease the methane filtering efficiency: either directly by higher vertical turbulent flux of dissolved methane or by altering other potential drivers for methane oxidation, such as nutrient limitation, changes in microbial community structure, or inhibition. This contradicts the assumption that mixing events increase methane oxidation by re-oxygenating the system. Our study demonstrates that the oxygenation state of the water column is not the ultimate factor that determines the functioning of the microbial methane filter. To accurately predict methane emissions from seasonally dynamic eutrophic coastal basins, a more complex network of drivers should be considered.
Supplementary Material
Acknowledgements
We are grateful for the excellent support of the captain and crew of the R/V Navicula during the sampling campaigns. Furthermore, we thank Koen A.J. Pelsma and Damian L. Arevalo Martinez for sharing their expertise on floating chamber gas flux measurements. For the dedicated planning and construction of the floating chamber for the flux measurements, we thank Arjan de Kleine and the team of the Techno Centrum at Radboud University, Nijmegen, The Netherlands. We also want to thank the master students Selien Op den Camp, Andreea Marin, and Sam Hoogars for their enthusiastic assistance in the lab.
Contributor Information
Jessica Venetz, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ Nijmegen, The Netherlands.
Olga M Żygadłowska, Department of Earth Sciences, Faculty of Geosciences, Utrecht University, 3508 TA Utrecht, The Netherlands.
Nicky Dotsios, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ Nijmegen, The Netherlands.
Anna J Wallenius, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ Nijmegen, The Netherlands.
Niels A G M van Helmond, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ Nijmegen, The Netherlands; Department of Earth Sciences, Faculty of Geosciences, Utrecht University, 3508 TA Utrecht, The Netherlands.
Wytze K Lenstra, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ Nijmegen, The Netherlands; Department of Earth Sciences, Faculty of Geosciences, Utrecht University, 3508 TA Utrecht, The Netherlands.
Robin Klomp, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ Nijmegen, The Netherlands; Department of Earth Sciences, Faculty of Geosciences, Utrecht University, 3508 TA Utrecht, The Netherlands.
Caroline P Slomp, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ Nijmegen, The Netherlands; Department of Earth Sciences, Faculty of Geosciences, Utrecht University, 3508 TA Utrecht, The Netherlands.
Mike S M Jetten, Department of Microbiology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ Nijmegen, The Netherlands.
Annelies J Veraart, Department of Aquatic Ecology and Environmental Biology, Radboud Institute for Biological and Environmental Sciences, Radboud University, 6525 AJ Nijmegen, The Netherlands.
Author contributions
Jessica Venetz (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing), Olga M. Żygadłowska (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing), Nicky Dotsios (Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing), Anna J. Wallenius (Data curation, Investigation, Methodology, Writing – review & editing), Niels A.G.M. van Helmond (Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing), Wytze K. Lenstra (Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing), Robin Klomp (Investigation, Methodology, Writing – review & editing), Caroline P. Slomp, Mike S.M. Jetten (Conceptualization, Data curation, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing), and Annelies J. Veraart (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing)
Conflict of interest
The authors declare that they have no competing interests.
Funding
This work was supported by the European Research Council Synergy grant MARIX [854088], the H2020 Marie Skłodowska-Curie COFUND program [847504], Netherlands Earth Systems Science Center [NESSC 024002001], and the Dutch Research Council (NWO) talent grant [VI.Veni.222.332].
Data Availability
Raw reads of the metagenome sequencing data can be accessed on the European Nucleotide Archive under the accession number PRJNA1053269 (publicly available after acceptance). Experimental data and field data are available through DANS-EASY (tbd).
References
- Amaral JA, Knowles R. Growth of methanotrophs in methane and oxygen counter gradients. FEMS Microbiol Lett. 1995;126:215–20. [Google Scholar]
- Borges AV, Champenois W, Gypens Net al. Massive marine methane emissions from near-shore shallow coastal areas. Sci Rep. 2016;6:27908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitburg D, Levin LA, Oschlies Aet al. Declining oxygen in the global ocean and coastal waters. Science. 2018;359:eaam7240. [DOI] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Holmes SP. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017;11:2639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WAet al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JF, Middelburg JJ, Röckmann Tet al. Methane feedbacks to the global climate system in a warmer world. Rev Geophy. 2018;56:207–50. [Google Scholar]
- Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–9. [DOI] [PubMed] [Google Scholar]
- Dominović I, Dutour-Sikirić M, Marguš Met al. Deoxygenation and stratification dynamics in a coastal marine lake. Estuar Coast Shelf Sci. 2023;291:108420. [Google Scholar]
- Dunfield P, Knowles R. Kinetics of inhibition of methane oxidation by nitrate, nitrite, and ammonium in a humisol. Appl Environ Microbiol. 1995;61:3129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Lenstra W, Jong Det al. Rapid sediment accumulation results in high methane effluxes from coastal sediments. PLoS One. 2016;11:e0161609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JA, Steele JA, Caporaso JGet al. Defining seasonal marine microbial community dynamics. ISME J. 2012;6:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossart H-P, Massana R, McMahon KDet al. Linking metagenomics to aquatic microbial ecology and biogeochemical cycles. Limnol Oceanogr. 2020;65:S2–S20. [Google Scholar]
- Gründger F, Probandt D, Knittel Ket al. Seasonal shifts of microbial methane oxidation in Arctic shelf waters above gas seeps. Limnol Oceanogr. 2021;66:1896–914. [Google Scholar]
- Hagens M, Slomp CP, Meysman FJRet al. Biogeochemical processes and buffering capacity concurrently affect acidification in a seasonally hypoxic coastal marine basin. Biogeosciences. 2015;12:1561–83. [Google Scholar]
- Herlemann DP, Labrenz M, Jürgens Ket al. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011;5:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez ME, Beck DA, Lidstrom MEet al. Oxygen availability is a major factor in determining the composition of microbial communities involved in methane oxidation. PeerJ. 2015;3:e801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A, de Roy K, Thas Oet al. The more, the merrier: heterotroph richness stimulates methanotrophic activity. ISME J. 2014;8:1945–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A, Kerckhof F-M, Luke Cet al. Conceptualizing functional traits and ecological characteristics of methane-oxidizing bacteria as life strategies. Environ Microbiol Rep. 2013;5:335–45. [DOI] [PubMed] [Google Scholar]
- Kaupper T, Luehrs J, Lee HJet al. Disentangling abiotic and biotic controls of aerobic methane oxidation during re-colonization. Soil Biol Biochem. 2020;142:107729. [Google Scholar]
- King GM, Sylvia S. Ammonium and nitrite inhibition of methane oxidation by Methylobacter albus BG8 and Methylosinus trichosporium OB3b at low methane concentrations. Appl Environ Microbiol. 1994;60:3508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause SMB, Johnson T, Samadhi Karunaratne Yet al. Lanthanide-dependent cross-feeding of methane-derived carbon is linked by microbial community interactions. 2017;114:358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra WK, van Helmond NAGM, Martins PDet al. Gene-based modeling of methane oxidation in coastal sediments: constraints on the efficiency of the microbial methane filter. Environ Sci Technol. 2023;57:12722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-L, Dong X, Lu Ret al. Microbial ecology of sulfur cycling near the sulfate–methane transition of deep-sea cold seep sediments. Environ Microbiol. 2021;23:6844–58. [DOI] [PubMed] [Google Scholar]
- Louca S, Hawley AK, Katsev Set al. Integrating biogeochemistry with multiomic sequence information in a model oxygen minimum zone. Proc Natl Acad Sci USA. 2016;113:E5925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S-H, Zhang H-H, Zhuang G-Cet al. Aerobic oxidation of methane significantly reduces global diffusive methane emissions from shallow marine waters. Nat Commun. 2022;13:7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Rahmann S. From cutadapt to sequencetools (sqt): a versatile toolset for sequencing projects. EMBnet J. 2012;17:35. [Google Scholar]
- Mayr MJ, Zimmermann M, Dey Jet al. Growth and rapid succession of methanotrophs effectively limit methane release during lake overturn. Commun Biol. 2020;3:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr MJ, Zimmermann M, Guggenheim Cet al. Niche partitioning of methane-oxidizing bacteria along the oxygen–methane counter gradient of stratified lakes. ISME J. 2020;14:274–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JW, Lee BS, Bullister Jet al. The Suboxic Zone of the Black Sea. In: Besiktepe ST, Ünlüata Ü, Bologa AS (eds.), Environmental Degradation of the Black Sea: Challenges and Remedies. NATO Science Series, vol 56. Dordrecht: Springer, 1999. 10.1007/978-94-011-4568-8_6. [DOI] [Google Scholar]
- Olmsted CN, Ort R, Tran PQet al. Environmental predictors of electroactive bacterioplankton in small boreal lakes. Environ Microbiol. 2023;25:705–20. [DOI] [PubMed] [Google Scholar]
- Pelsma KAJ, van Helmond NAGM, Lenstra WKet al. Anaerobic methanotrophy is stimulated by graphene oxide in a brackish urban canal sediment. Environ Microbiol. 2023;25:3104–15. [DOI] [PubMed] [Google Scholar]
- Reeburgh WS. Oceanic methane biogeochemistry. Chem Rev. 2007;107:486–513. [DOI] [PubMed] [Google Scholar]
- Schubert CJ, Coolen MJL, Neretin LNet al. Aerobic and anaerobic methanotrophs in the Black Sea water column. Environ Microbiol. 2006;8:1844–56. [DOI] [PubMed] [Google Scholar]
- Steinle L, Maltby J, Treude Tet al. Effects of low oxygen concentrations on aerobic methane oxidation in seasonally hypoxic coastal waters. Biogeosciences. 2017;14:1631–45. [Google Scholar]
- Steinsdóttir HGR, Gómez-Ramírez E, Mhatre Set al. Anaerobic methane oxidation in a coastal oxygen minimum zone: spatial and temporal dynamics. Environ Microbiol. 2022a;24:2361–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinsdóttir HGR, Schauberger C, Mhatre Set al. Aerobic and anaerobic methane oxidation in a seasonally anoxic basin. Limnol Ocean. 2022b;67:1257–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G, Lehmann MF, Tischer Jet al. Water column dynamics control nitrite-dependent anaerobic methane oxidation by Candidatus “Methylomirabilis” in stratified lake basins. ISME J. 2023;17:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulu-Gambari F, Hagens M, Behrends Tet al. Phosphorus cycling and burial in sediments of a seasonally hypoxic marine basin. Estuaries Coasts. 2017;41:921–39. [Google Scholar]
- Sun X, Frey C, Garcia-Robledo Eet al. Microbial niche differentiation explains nitrite oxidation in marine oxygen minimum zones. ISME J. 2021;15:1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K, Horikoshi K. Rapid detection and quantification of members of the Archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol. 2000;66:5066–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavormina PL, Ussler W, Joye SBet al. Distributions of putative aerobic methanotrophs in diverse pelagic marine environments. ISME J. 2010;4:700–10. [DOI] [PubMed] [Google Scholar]
- Thamdrup B, Steinsdóttir HGR, Bertagnolli ADet al. Anaerobic methane oxidation is an important sink for methane in the ocean’s largest oxygen minimum zone. Limnol Ocean. 2019;64:2569–85. [Google Scholar]
- Valenzuela EI, Avendaño KA, Balagurusamy Net al. Electron shuttling mediated by humic substances fuels anaerobic methane oxidation and carbon burial in wetland sediments. Sci Total Environ. 2019;650:2674–84. [DOI] [PubMed] [Google Scholar]
- van Haren H. Internal wave mixing in warming lake Grevelingen. Estuar Coast Shelf Sci. 2019;226:106298. [Google Scholar]
- Venetz J, Żygadłowska OM, Lenstra WKet al. Versatile methanotrophs form an active methane biofilter in the oxycline of a seasonally stratified coastal basin. Environ Microbiol. 2023;25:2277–88. [DOI] [PubMed] [Google Scholar]
- Veraart AJ, Garbeva P, van Beersum Fet al. Living apart together—bacterial volatiles influence methanotrophic growth and activity. ISME J. 2018;12:1163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenius AJ, Dalcin Martins P, Slomp CPet al. Anthropogenic and environmental constraints on the microbial methane cycle in coastal sediments. Front Microbiol. 2021;12:631621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Yan H, Peng Xet al. Community assembly of bacteria and archaea in coastal waters governed by contrasting mechanisms: a seasonal perspective. Mol Ecol. 2020;29:3762–76. [DOI] [PubMed] [Google Scholar]
- Weber T, Wiseman NA, Kock A. Global ocean methane emissions dominated by shallow coastal waters. Nat Commun. 2019;10:4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersteijn LPMJ. Grevelingenmeer: meer kwetsbaar. Een Beschrijv. van Ecol. ontwikkelingen voor periode 1000, 1999, 2008–10. [Google Scholar]
- Zhang Y, Zhang X, Wang Fet al. Exogenous nitrogen addition inhibits sulfate-mediated anaerobic oxidation of methane in estuarine coastal sediments. Ecol Eng. 2020;158:106021. [Google Scholar]
- Zheng Y, Wang H, Liu Yet al. Methane-dependent mineral reduction by aerobic methanotrophs under hypoxia. Environ Sci Technol Lett. 2020;7:606–12. [Google Scholar]
- Żygadłowska OM, Venetz J, Klomp Ret al. Pathways of methane removal in the sediment and water column of a seasonally anoxic eutrophic marine basin. Front Mar Sci. 2023a;10:1085728. [Google Scholar]
- Żygadłowska OM, Venetz J, Lenstra WKet al. High methane emissions from a eutrophic marine coastal basin driven by bubble release from the sediment. EarthArXiv, 2023b, 10.31223/X5RD6N. preprint: not peer reviewed. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw reads of the metagenome sequencing data can be accessed on the European Nucleotide Archive under the accession number PRJNA1053269 (publicly available after acceptance). Experimental data and field data are available through DANS-EASY (tbd).




