Abstract
Sindbis virus infection of cultured cells and of neurons in mouse brains leads to programmed cell death exhibiting the classical characteristics of apoptosis. Although the mechanism by which Sindbis virus activates the cell suicide program is not known, we demonstrate here that Sindbis virus activates caspases, a family of death-inducing proteases, resulting in cleavage of several cellular substrates. To study the role of caspases in virus-induced apoptosis, we determined the effects of specific caspase inhibitors on Sindbis virus-induced cell death. CrmA (a serpin from cowpox virus) and zVAD-FMK (N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone) inhibited Sindbis virus-induced cell death, suggesting that cellular caspases facilitate apoptosis induced by Sindbis virus. Furthermore, CrmA significantly increased the rate of survival of infected mice. These inhibitors appear to protect cells by inhibiting the cellular death pathway rather than impairing virus replication or by inhibiting the nsP2 and capsid viral proteases. The specificity of CrmA indicates that the Sindbis virus-induced death pathway is similar to that induced by Fas or tumor necrosis factor alpha rather than being like the death pathway induced by DNA damage. Taken together, these data suggest a central role for caspases in Sindbis virus-induced apoptosis.
Sindbis virus is an alphavirus of the Togaviridae family which causes encephalitis in mice and thus serves as a model for closely related human encephalitic viruses. Infection of a variety of cultured cell types with Sindbis virus triggers programmed cell death (33). The induction of apoptosis in neurons of mouse brains and spinal cords correlates with the neurovirulence of the virus strain and with mortality in mice, suggesting that induction of apoptosis may be a primary cause of death of young mice (34). In support of this hypothesis, overexpressed inhibitors of apoptosis, such as Bcl-2 and IAP, can protect cultured cells from Sindbis virus-induced apoptosis, and Bcl-2 efficiently reduces mortality in mice (17, 31, 32). These findings also raise the possibility that endogenous inhibitors of apoptosis inhibit Sindbis virus-induced cell death, leading to a persistent virus infection (33, 61). Encephalitis and/or a fatal stress response may be a consequence of neuronal apoptosis (21, 59). Alternatively, there may be multiple pathways that work independently to cause fatal disease.
A crucial role for the caspase family of cysteine proteases in the execution phase of programmed cell death is supported by genetic (24, 52, 66), biochemical (29, 57), and physiological (25) evidence. A current model proposes a cascade of events by which caspases proteolytically activate other caspases (35, 39, 46). More recent evidence suggests that different death stimuli trigger the activation of a subset of upstream caspases that possess long prodomains at their N termini (3, 41, 62). These prodomains serve to target proteases to specific protein complexes, where the prodomains are removed by proteolysis to produce active proteases. These caspases proteolytically activate other downstream caspases (with shorter prodomains) that cleave key substrates to ultimately produce the characteristic apoptotic phenotype of cell shrinkage, membrane blebbing, chromatin condensation, oligonucleosomal DNA fragmentation, and cell death (42, 53). A growing list of proteolytic substrates of the caspases have been identified, including protein kinase C delta (18), the retinoblastoma tumor suppressor (56), fodrin (12, 38), lamins (30, 47), the nuclear immunophilin FKBP46 (1), Bcl-2 (7), and several autoantigens (5), and they all are cleaved after an aspartate residue (P1 position). The precise role of these cleavage events is not known, but they may either inactivate key cellular functions or produce cleavage products with pro-death activity. The cleavage product of Bcl-2 is potently proapoptotic (7), and cleavage of a novel protein designated DFF was recently shown to trigger DNA fragmentation during apoptosis (36). These proteolytic events also serve as biochemical markers of apoptosis. Furthermore, cell death can be inhibited with pseudosubstrate inhibitors of the caspases, such as the cowpox virus serpin CrmA (19, 48), and synthetic peptides such as zVAD-FMK (67). The key feature of these inhibitors is an aspartate at the P1 position, consistent with their specificity for caspases.
A role for caspases in viral infections is suggested by the finding that baculovirus infection activates an apoptotic cysteine protease in insect cells that is inhibited by the virus-encoded caspase inhibitor p35 (2). Similar work with mutant adenoviruses has suggested that the adenovirus protein E1A activates caspase 3 (CPP32), generating cleaved products of poly(ADP-ribose) polymerase (PARP) (4). In addition, PARP cleavage is detected during infection of mouse neuroblastoma cells with Sindbis virus (60). To further study the role of these proteases in Sindbis virus-induced programmed cell death, we confirmed that Sindbis virus activates cellular caspases and demonstrated the participation of a subset of caspases in the execution of the apoptotic process.
MATERIALS AND METHODS
Plasmids and viruses.
Recombinant Sindbis virus expressing CrmA (pMV19) was generating by cloning a BstEII-flanked PCR product from a plasmid (provided by David Pickup) into the Sindbis virus vector dsTE12Q as previously described (8, 31). A control virus, (pSZ20), CrmA/stop was generated by inserting a nonsense oligonucleotide linker into pMV19 after codon 55 of the CrmA open reading frame. Recombinant viruses encoding bcl-xL and bcl-xL/stop were described previously (8). Wild-type (AR339) and recombinant Sindbis viruses were quantitated by standard plaque assay on baby hamster kidney (BHK) cells.
Cell lines and cell viability.
Low-passage-number (<15) BHK and mouse neuroblastoma cells (N18) were maintained free of Mycoplasma contamination in Dulbecco’s minimal essential medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml in 5% CO2 at 37°C. Cells were seeded at 4 × 104/well in a 24-well dish and infected ∼12 h later at a multiplicity of infection (MOI) of 10. Cell viability was determined by trypan blue exclusion, with blind counting of at least 500 cells per sample, as described elsewhere (17).
Progeny virus production.
Subconfluent BHK cell monolayers were infected at an MOI of 10 in culture medium containing 1% fetal bovine serum for 1 h. Exactly 1 h before each time point, the cells were washed with 1× phosphate-buffered saline (PBS) to remove accumulated virus, and the virus-containing supernatants were harvested 1 h later and analyzed in duplicate plaque assays. Cell viabilities for the same infected wells were determined as described above.
Animal studies.
The right cerebral hemispheres of 1-day-old CD1 mice (Charles River) were inoculated with 5 × 103 PFU of CrmA and CrmA/stop recombinant viruses in 30 μl of Hanks’ balanced salt solution. For mortality experiments, four separate litters were inoculated with each virus, and mortality was determined by daily observation of the mice for 21 days after infection. For virus titration experiments, three mice per experimental group were sacrificed at days 1, 2, 3, 6, and 10 after inoculation. Brains were dissected and stored at −70°C, and freeze-thawed tissues were used to prepare 10% homogenates in Hanks’ balanced salt solution for plaque assay quantitation on BHK cells.
Detection of viral proteins.
BHK cells were seeded at 5 × 105 per well in six-well dishes and infected with recombinant Sindbis viruses (MOI, 10). After three washes with 1× PBS, cells were starved in 1 ml of 10% serum-supplemented methionine- and cysteine-free medium for 1 h and metabolically labeled with 50 μCi of 35S-Translabel (ICN) per well for 45 min. Before being harvested, the cells were washed three times with ice-cold PBS and lysed on ice with 300 μl of a buffer containing 1% Nonidet P-40, 1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 50 mM Tris (pH 7.5), 15 mM NaCl, and 25 μg of aprotinin per ml. To detect Sindbis virus structural proteins, 20 μl of lysate was resolved by SDS–15% polyacrylamide gel electrophoresis (PAGE), amplified with 1 M salicylic acid, and analyzed by autoradiography. For immunoprecipitation of Sindbis virus nonstructural proteins, labeled cell lysates were immunoprecipitated with rabbit anti-Sindbis virus antiserum raised against nsP2 (provided by Charlie Rice) at a 1:100 dilution in a total volume of 200 μl for each 60 μl of lysate as previously described (15).
Immunoblotting.
Lysates from BHK and N18 cells infected at an MOI of 10 were prepared as described above, analyzed by SDS-PAGE, and immunoblotted (ECL; Amersham) with anti-CrmA or anti-Bcl-x antibodies (provided by David Pickup and Craig Thompson, respectively). For detection of autoantigens, cells were washed three times with PBS and lysed in a buffer containing 1% Nonidet P-40, 20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, and the protease inhibitors pepstatin A, leupeptin, antipain, chymostatin, and phenylmethylsulfonyl fluoride. Proteins were analyzed by SDS–10% PAGE prior to being subjected to immunoblotting with affinity-purified human antibodies as described previously (5, 6, 20).
Protease inhibitors.
BHK or N18 cells were preincubated for 2 h with 5 to 50 μM zVAD-FMK (N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone; Enzyme Systems Products) and infected with Sindbis virus strain AR339 (MOI, 10) in the presence of zVAD-FMK. The inhibitor was replenished at ∼24 h postinfection. Cells were harvested at ∼48 h postinfection to determine their viability. zFA-FMK, a fluoromethyl ketone which is inactive against caspases, was used as a negative control.
In vitro protease assays.
A glutathione S-transferase (GST)-CrmA fusion protein expressed from a modified pGEX2T plasmid (provided by Emily Cheng) was purified from bacteria, and protein concentrations were determined by the bicinchoninic acid protein assay (Pierce). Pro-interleukin-1β (pIL-1β; provided by Jennifer Lewis and Henry George) was in vitro translated by use of the TNT System (Promega). Inhibition of caspase 1 (IL-1β-converting enzyme) protease activity by GST-CrmA was performed as described previously (48); 50 pg of caspase 1 (provided by Susan Molineaux) was preincubated for 30 min at 37°C with 1 or 5 μl of purified GST-CrmA (0.5 to 2.5 μg) or GST (2.3 to 11.5 μg) in 100 mM HEPES (pH 7.5)–10% sucrose–0.1% 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate (CHAPS)–10 mM dithiothreitol and then incubated for 90 min with 2 μl of [35S]methionine-labeled, in vitro-translated pIL-1β in a total reaction volume of 20 μl with a final concentration of GST-CrmA or GST of >1 μM. The whole reaction product was analyzed by SDS-PAGE and autoradiography.
To evaluate the effect of GST-CrmA on the Sindbis virus cysteine proteinase nsP2, unlabeled, in vitro-translated Sindbis virus polyprotein nsP123 with Gly-to-Val mutations at the cleavage sites flanking nsP2 (12V; provided by Jim and Ellen Strauss) was used as the source of active protease. A Sindbis virus construct expressing the entire nonstructural region nsP1234 with a mutation in the active site of nsP2 (Cys-to-Gly mutation at position 481; provided by Jim and Ellen Strauss) was in vitro translated in the presence of [35S]methionine and used as a substrate for active nsP2 protease. In vitro translations (TNT System; Promega) were terminated by incubating reaction mixtures with boiled RNase A and DNase I (final concentrations, 10 μg/ml and 20 U/ml, respectively) at 37°C for 10 min. To assess the effect of CrmA on nsP2 protease activity, labeled substrate was mixed with unlabeled active protease, as described by de Groot et al. (14), in the presence of a molar excess (>1 μM) of GST-CrmA or GST protein alone (26). The products were analyzed by SDS-PAGE and autoradiography.
RESULTS
Caspase activation during Sindbis virus-induced programmed cell death.
Sindbis virus induces classical apoptosis (33) by mechanisms that are still unclear. Because cellular caspases are thought to be important downstream mediators of apoptosis in a variety of cell death paradigms, we investigated the role of caspases in Sindbis virus infection. To verify that caspases are activated during infection, cells were monitored for proteolytic cleavage products of known caspase substrates. Human sera from autoimmune patients recognized the autoantigens NuMA, PARP, and U1-70kDa in uninfected BHK cells and also detected their signature cleavage products of 195, 89, and 40 kDa, respectively, at 24 h postinfection (Fig. 1). These results demonstrate that Sindbis virus infection leads to caspase activation and cleavage of intracellular target proteins in a manner that is characteristic of other cell death stimuli (5). Digestion of U1-70kDa and PARP by recombinant caspase 3 in vitro produces the same proteolytic products as those detected in apoptotic cells, suggesting that caspase 3 may be the responsible protease in dying cells (5, 50). However, caspase 3 knockout mice remain capable of cleaving PARP (27). Therefore, other, related caspases may also be involved in cleaving these substrates during apoptosis.
FIG. 1.
Cleavage of cellular proteins by caspases during Sindbis virus-induced apoptosis. BHK cells were mock infected or infected (MOI, 10) with wild-type Sindbis virus strain AR339. Lysates were collected at 24 h postinfection and immunoblotted with three different affinity-purified antibodies obtained from the sera of individuals with autoimmune disease. The positions of intact NuMA, PARP, and U1-70kDa proteins and their cleavage products are indicated. The results are representative of two independent experiments. The positions of molecular size markers (in kilodaltons) are shown on the left.
Caspase inhibitors impair Sindbis virus-induced cell death.
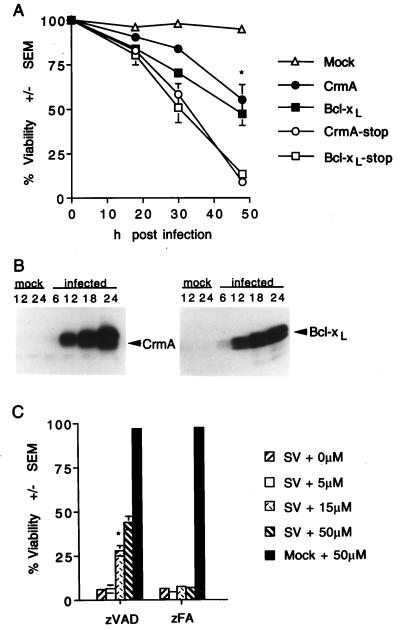
To determine if caspases have an active role in facilitating cell death during a Sindbis virus infection and are not merely markers of cell death, caspase inhibitors were tested for their effects on the outcome of a Sindbis virus infection. The ability of CrmA, a cowpox virus-encoded inhibitor of caspase 1, to impair virus-induced cell death was assessed by using the Sindbis virus vector system in which the crmA coding sequence was cloned into the Sindbis virus genome (8, 31). BHK cells infected with a recombinant virus encoding CrmA were approximately 55% viable at 48 h postinfection, while those infected with a virus in which a stop codon was inserted into the crmA open reading frame had a viability of 8% (Fig. 2A). Similar results were obtained with Bcl-xL, a Bcl-2-related protein which was previously demonstrated to protect cells from Sindbis virus-induced apoptosis (8, 33). Similar results were obtained with N18 cells (data not shown), indicating that caspase activation is a general mediator of Sindbis virus-induced cell death. Immunoblot analysis verified the occurrence of CrmA and Bcl-xL protein expression in recombinant virus-infected cells, with increasing levels of protein from 6 to 24 h postinfection (Fig. 2B). Although overexpressed CrmA is presumed to affect caspases other than caspase 1, these results suggest that CrmA-insensitive proteases may not be involved in triggering Sindbis virus-induced apoptosis (see Discussion).
FIG. 2.
CrmA and zVAD-FMK protect BHK cells from Sindbis virus-induced cell death. (A) BHK cells were mock infected or infected with recombinant Sindbis viruses encoding the indicated genes, and cell viability was determined at the indicated times by trypan blue exclusion. Data from three to nine independent experiments are shown. Error bars (indication standard deviations) are hidden by the symbol at some time points. (B) Immunoblots of N18 cells infected with recombinant viruses encoding CrmA (left) or Bcl-xL (right). Cells were harvested at the indicated times (in hours) postinfection, and equal amounts of protein were analyzed by SDS–15% PAGE. Similar results were obtained with BHK cells (data not shown). (C) The viabilities of Sindbis virus (AR339)-infected BHK cells treated with zVAD-FMK or zFA-FMK at the indicated concentrations were determined by trypan blue exclusion at 48 h postinfection. The results summarize data from three to eight independent experiments. The asterisk indicates a statistically significant difference upon comparison of the viability of each recombinant virus with that of its corresponding stop construct in panel A and upon comparison of the viability of cells treated with 15 μM zVAD-FMK with that of the other categories in panel C (P < 0.05 by Student’s t test).
The synthetic peptide inhibitor zVAD-FMK is a broad inhibitor of cysteine proteases with a specificity for Asp in the P1 position (67). BHK cells treated with zVAD-FMK were resistant to wild-type (AR339) Sindbis virus-induced cell death in a dose-dependent manner (Fig. 2C). Infected cells treated with a 50 μM concentration of a peptide inhibitor had ∼37% (sevenfold) higher viability than cells treated with a control compound, zFA-FMK, lacking an aspartate residue. Treatment with zVAD-FMK also protected N18 cells infected with Sindbis virus (data not shown).
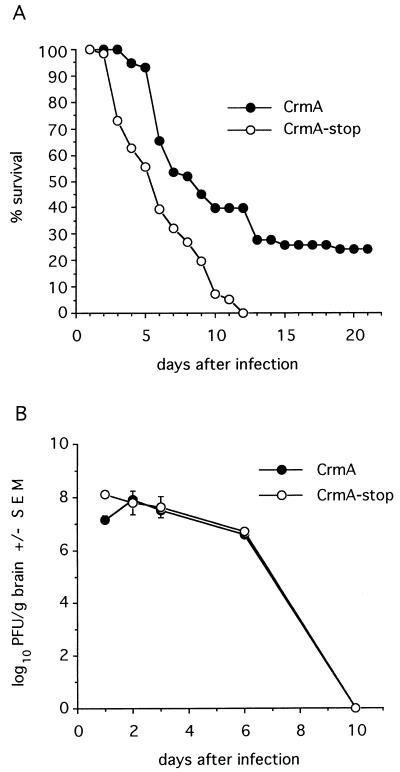
Apoptosis in mouse brains is easily detected by 24 h after intracranial inoculation with Sindbis virus (34). The mortality induced by Sindbis virus infection in mice correlates with apoptotic death of virus-infected neurons (34). To determine if caspases contribute to a fatal infection in vivo, 1-day-old mice were inoculated intracranially with recombinant viruses encoding crmA. Mice were partially protected by CrmA, as indicated by an increase in the time until death and by the survival of 25% of the animals. In contrast, recombinant viruses expressing CrmA with a premature stop codon resulted in 100% mortality by day 12 of the experiment (Fig. 3A). Taken together, the antideath effects of the serpin CrmA and the peptide inhibitor zVAD-FMK suggest that caspases have an executioner role during Sindbis virus infection both in vitro and in vivo.
FIG. 3.
CrmA enhances survival of Sindbis virus-infected mice. (A) Percent survival of mice infected with the indicated recombinant viruses was determined in four independent experiments with approximately 40 mice (total) per virus. A P value of <0.002 was obtained by life table analysis. (B) Replication of recombinant viruses in mouse brains was determined by plaque assay. Each datum point represents the geometric mean virus titer ± the standard error of the mean (SEM) of values for three mouse brains.
Effects of caspase inhibitors on Sindbis virus replication.
To determine if CrmA protected mice from a fatal Sindbis virus infection by suppressing virus replication, plaque assays of brain homogenates were performed. Although a modest reduction in the level of CrmA-expressing virus compared to that of the CrmA-stop construct was detected at 1 day postinfection, no differences in these levels were observed between days 2 and 10 postinfection, indicating that CrmA did not significantly alter progeny virus production in mouse brains (Fig. 3B).
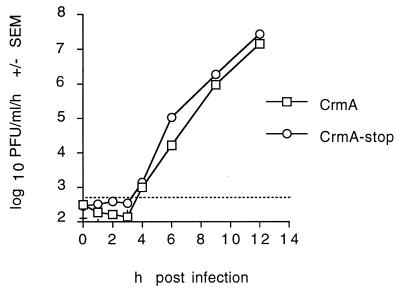
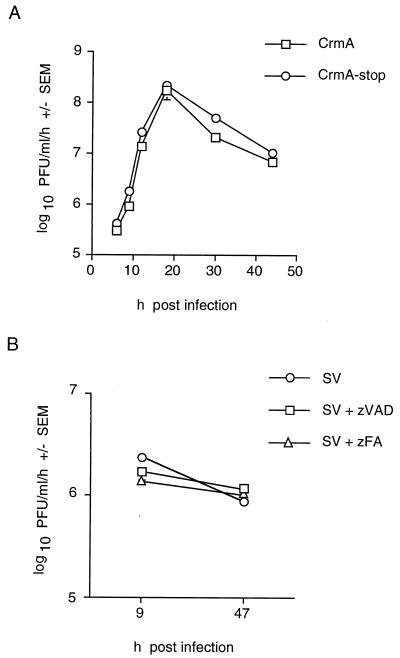
For a more detailed analysis, the effects of caspase inhibitors on Sindbis virus replication were studied in the BHK cell line. A one-step growth curve demonstrated that progeny recombinant viruses encoding either CrmA or CrmA-stop were first detectable at 4 h postinfection (Fig. 4). Therefore, CrmA did not delay the first round of virus replication.
FIG. 4.
One-step growth curves of with recombinant viruses with and without CrmA show no differences. BHK cells were infected with CrmA or CrmA-stop recombinant virus (MOI, 10), and progeny viruses produced during a 1-h period were collected from the supernatants and titered by plaque assay. The function of CrmA and CrmA-stop was confirmed by determining cell viability in control wells. The results represent the means of two independent experiments plaqued in duplicate for each time point. Bars for standard error of the mean (SEM) are hidden by the symbols, and the dashed horizontal line marks the limit of detection.
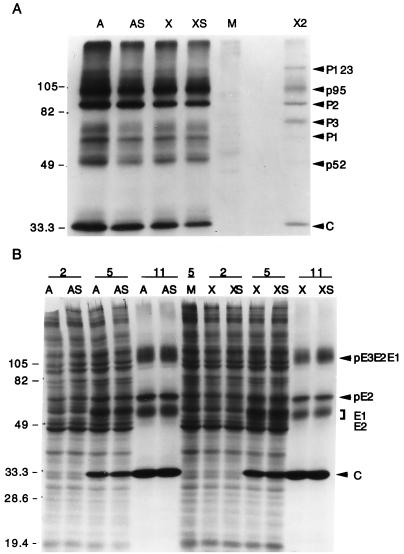
Cleavage of the nonstructural polyprotein precursor nsP1234 into the individual nsP proteins, which include the polymerase and other proteins required for plus- and minus-strand viral RNA synthesis, is accomplished by the viral cysteine protease located in nsP2. Mutations that disrupt nsP2 protease function inactivate the virus (55). Because nsP2 cleaves at sites containing a Gly at the P2 position and not at sites with an Asp at the P1 position, CrmA (or zVAD-FMK) would not be expected to inhibit the nsP2 protease. To verify that CrmA did not alter the production of Sindbis virus nonstructural proteins, the nsP protein complexes were immunoprecipitated with anti-nsP2 antibodies from lysates of pulse-labeled cells. No differences in the ratios of precursor nsP123 and individual proteins nsP1, nsP2, and nsP3 were detected in the presence of CrmA (Fig. 5A, lane A) or Bcl-xL (lane X) compared to the stop-codon controls (lanes AS and XS, respectively) as determined by densitometry (data not shown). The viral capsid protein and two cellular proteins, p95 and p52, also coprecipitated with the nonstructural proteins, as reported by others (15, 22).
FIG. 5.
CrmA does not alter production of nonstructural and structural Sindbis virus proteins. (A) Sindbis virus nonstructural proteins (P123, P1, P2, and P3) were immunoprecipitated from equal volumes of labeled lysates, prepared 11 h after infection, with recombinant viruses encoding crmA (A), crmA-stop (AS), bcl-xL (X), and bcl-xL-stop (XS) or from mock-infected cells (M) with rabbit antiserum raised against nsP2. The nonstructural proteins were identified by their molecular masses (the positions of molecular size markers [in kilodaltons] are shown on the left), by comparison to published protein patterns (15), and by comparison to nonstructural proteins immunoprecipitated with a mixture of antibodies to nsP2 and nsP3 (X2) provided by M. Gorrell and D. Griffin. Proteins were resolved by SDS–10% PAGE and processed with salicylic acid prior to autoradiography. The results are representative of three independent experiments. (B) Sindbis virus structural proteins were analyzed by SDS–15% PAGE and autoradiography of labeled whole-cell lysates harvested at the indicated times (in hours) after infection of BHK cells. These results are representative of six independent time course experiments. The arrows indicate the precursor viral glycoproteins (pE3E2E1 and pE2), the mature glycoproteins (E1 and E2), and the capsid protein (C). Molecular mass standards (in kilodaltons) are indicated.
The viral capsid protein contains a serine protease which autocatalytically cleaves itself from a polyprotein precursor, giving rise to the transmembrane proteins, which are further processed by cellular proteases to yield the glycoproteins E3, E2, and E1. To determine if CrmA or Bcl-xL altered the accumulation of virion structural proteins or impaired the virus-induced shutoff of host protein synthesis, 35S-labeled cell lysates were analyzed directly (Fig. 5B). No differences in accumulated virus structural proteins of cells infected with CrmA- or Bcl-xL-encoding viruses compared to their stop-codon controls were observed. Thus, neither CrmA nor Bcl-xL altered the time course of viral protein production (detectable by 5 h postinfection) or the inhibition of host protein synthesis (detectable at 11 h postinfection). It is unlikely that either the capsid protease or the relevant cellular proteases are inhibited (directly or indirectly) by the caspase inhibitor CrmA or the antiapoptotic Bcl-xL protein, since the capsid and glycoprotein levels were equivalent.
The effect of CrmA on progeny virus production in BHK cells was determined by measuring the titer of virus produced during a 1-h interval at various times after infection (Fig. 6A). Although the CrmA-stop virus produced approximately twofold higher titers than virus expressing CrmA, this small difference was deemed unlikely to account for the observed differences in cell viability (Fig. 2A). These results are consistent with the observation that similar levels of viral proteins (both structural and nonstructural) were detected in infected cells (Fig. 5). Likewise, treatment with zVAD-FMK had no effect on wild-type virus production in BHK cells (Fig. 6B).
FIG. 6.
Caspase inhibitors CrmA and zVAD-FMK do not significantly inhibit Sindbis virus replication. (A) Recombinant viruses produced in BHK cell supernatants (MOI, 10) during 1-h intervals were collected, and plaque assays were performed in duplicate. Each datum point is the mean of values for three independent wells harvested on the same day, and the results are representative of seven independent experiments. (B) Production of progeny Sindbis virus (AR339) per hour in BHK cells treated with zVAD-FMK or zFA-FMK was determined. The cells were pretreated with 50 μM zVAD-FMK or zFA-FMK for 2 h, and the inhibitors were replenished after 24 h. Each datum point is the mean of values for three independent wells harvested on the same day.
CrmA does not inhibit the viral nsP2 protease in vitro.
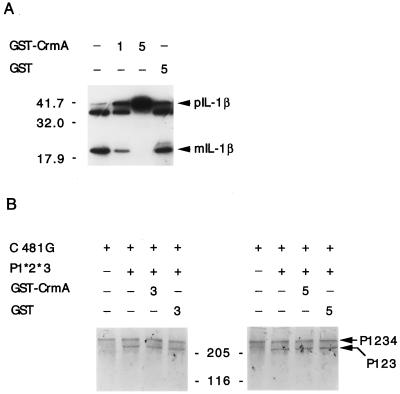
It has been pointed out that the caspase active site has greater resemblance to viral cysteine proteases than to other cellular proteases (58). Therefore, to eliminate the possibility that CrmA could impair the function of the viral nsP2 protease, purified CrmA protein was assessed for its ability to inhibit the nsP2 protease in an in vitro assay (14). 35S-labeled, in vitro-translated Sindbis virus precursor nsP1234 served as a substrate. This nsP1234 precursor contains a Cys-to-Gly mutation at position 481 of the nsP2 protease active site (Cys481Gly) to prevent it from undergoing rapid cotranslational processing. Unlabeled in vitro-translated nsP1*2*3 served as the source of active nsP2 enzyme. This construct contains mutations of the protease cleavage sites between nsP1 and nsP2 and between nsP2 and nsP3 to prevent processing of the precursor because nsP2 alone (detached from its flanking proteins) is an inefficient protease. Incubation of the protease nsP1*2*3 with the substrate nsP1234 resulted in partial cleavage of nsP4, producing nsP123 (Fig. 7B). This proteolytic event was not inhibited in the presence of 3 to 5 μl of purified GST-CrmA protein or purified GST protein alone (1 to 2 μM). In contrast, 1 μl of purified GST-CrmA fusion protein partially inhibited digestion of proIL-1β to mature IL-1β by caspase 1, and complete inhibition was obtained with 5 μl of GST-CrmA (Fig. 7A). Therefore, the proteolytic activity of viral nsP2 for its target substrate was not inhibited by CrmA in vitro.
FIG. 7.
CrmA inhibits caspase 1 but does not inhibit nsP2 protease activity in vitro. (A) In vitro-translated pIL-1β was digested with caspase 1 in the presence (1 or 5 μl) or absence (−) of purified GST-CrmA protein and then analyzed by SDS–10% PAGE. Cleavage of pIL-1β to the 17.5-kDa mature form (mIL-1β) was inhibited by GST-CrmA but not by GST protein. (B) Labeled in vitro-translated nsP1234 (C481G) was digested with unlabeled in vitro-translated nsP2 protease (P1*2*3) in the presence (3 or 5 μl) or absence (−) of purified GST-CrmA or GST protein alone. Labeled nsP123 and molecular mass standards (in kilodaltons) serve as markers.
DISCUSSION
Caspase 1 is a homolog of the Caenorhabditis elegans protease CED-3, which mediates programmed cell death in nematodes (66). Although a role for caspase 1 itself in apoptosis is less clear, 10 mammalian homologs (caspases 2 to 11) have been reported, and some of these appear to be key factors in a cell death pathway with common features in all cells (28, 37, 65). Here we have demonstrated that Sindbis virus-induced cell death is also mediated by caspases, since caspase inhibitors were found to impair virus-induced apoptosis.
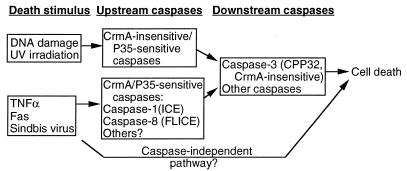
The caspases are expressed as precursors that must be cleaved to become active proteases (40). The sequence of these activating cleavage sites, together with biochemical data, indicates that caspases are proteolytically activated either autocatalytically or by other caspases, suggesting that there is a cascade of proteolytic events leading to activation of perhaps several caspases to facilitate cell death. The death signal resulting from ligation of Fas by Fas ligand or a Fas antibody, is propagated by recruiting FLICE/MACH/Mch5 (caspase 8), via its prodomain, to a protein complex bound to the cytoplasmic domain of Fas (3, 41). Caspase 8 becomes activated, perhaps autocatalytically, and subsequently cleaves and activates downstream caspases, including caspase 3 (41, 42). In vitro, CrmA specifically inhibits caspase 8 and caspase 1 over other caspases tested (44, 68), implicating the involvement of these proteases in Sindbis virus-induced apoptosis (Fig. 8). Although a role for caspase 1 in apoptosis is still controversial, virus-induced activation of caspase 1 is consistent with induction of IL-1β during a Sindbis virus infection (63).
FIG. 8.
Model of the Sindbis virus-induced apoptotic pathway. Different death stimuli appear to activate distinct upstream caspases, as determined on the basis of inhibitor profiles. Upstream caspases activate downstream caspases, leading to cell death. The presence of an alternate caspase-independent pathway cannot be ruled out.
Different upstream caspases may be activated by different death stimuli (12, 53). Ionizing-radiation-induced apoptosis of U937 cells is inhibited by the baculovirus caspase inhibitor p35 but not by CrmA (13). This was taken as evidence that CrmA-resistant proteases are involved in radiation-induced death and that this pathway is distinct from tumor necrosis factor (TNF)-induced apoptosis in U937 cells, which is inhibited by both p35 and CrmA (13). The implication from these and other studies is that CrmA is specific for a subset of intracellular caspases. CrmA inhibits cell death induced by Fas, TNF-α, nerve growth factor withdrawal, and extracellular matrix disruption but does not inhibit cell death induced by DNA-damaging agents or staurosporine. However, both the CrmA-dependent and CrmA-independent pathways lead to activation of caspase 3, a more-downstream protease in the cascade (13, 42). Because CrmA does not inhibit caspase 3 effectively, CrmA may be working to protect cells by affecting upstream proteases. However, the lack of specific inhibitors that can definitively distinguish individual caspases makes the task of delineating the cell type- or stimulus-specific death pathways difficult. Nevertheless, we found that the Sindbis virus-induced death pathway is sensitive to both p35 (43a) and CrmA. Thus, Sindbis virus-induced apoptosis may share upstream components of the death pathway mediated by Fas, TNF, and nerve growth factor withdrawal that do not involve CrmA-resistant proteases (Fig. 8). However, consistent with our study on Sindbis virus, transfected CrmA and zVAD-FMK treatment do not block cell death indefinitely, which has been assumed to mean that these inhibitors are unable to stave off all of the intracellular caspases. Nevertheless, this observation leaves open the possibility that caspase-independent pathways are also operational in Sindbis virus-infected cells (Fig. 8).
Although CrmA is a cysteine protease inhibitor, its specificity for caspases, as dictated by the presence of an Asp at the CrmA reactive site, strongly suggests that CrmA could not interfere with either the serine protease in the Sindbis virus capsid protein or the cysteine protease found in nsP2. This is an important issue because these proteases are essential for viral replication (55). Furthermore, a mutation in the protease domain of the nsP2 gene, resulting in a single amino acid change (Pro726Ser), produces a virus that fails to kill cells and establishes a persistent infection of BHK cells (16). However, because CrmA does not appear to have a direct or indirect effect on nsP2 or capsid, CrmA is not likely to impair cell killing by interfering with viral protease activity. Several pieces of information support this conclusion. (i) The viral protease cleavage sites do not contain an aspartate at the P1 position. The capsid protease of Sindbis virus cleaves after the sequence TEEW, which has no resemblance to caspase cleavage sites (e.g., DEVD or YVAD). Based on the sequences of 10 alphaviruses, the nsP2 protease cleaves following the consensus sequence XAG(A/G) (55), and substitution of the P1 glycine for valine at the nsP3-nsP4 cleavage site in Sindbis virus abolishes cleavage (14). (ii) Experimental results presented here demonstrate that CrmA did not cause an accumulation of viral nonstructural precursors, and the viral structural proteins accumulated normally between 0 and 11 h postinfection. (iii) Purified CrmA protein failed to interfere with cleavage of the nsP3nsP4 site by nsP2 in vitro.
The role of CrmA in cowpox virus infections is to reduce the host immune response by blocking production of IL-1β, and recent evidence suggests that CrmA may also prevent apoptosis of cowpox virus-infected cells (48, 49). Likewise, other antiapoptotic genes encoded by large DNA viruses appear to be required for completion of the virus replication cycle. Deletion of p35 from baculovirus or deletion of E1B 19K from adenovirus severely reduces progeny virus production because the cells die prematurely from apoptosis (11, 64). Several herpesviruses encode homologs of the cellular bcl-2 gene, a potent inhibitor of a wide variety of death stimuli (9, 23, 43). In contrast to these viruses, Sindbis virus appears to thrive in apoptotic cells, presumably in part because the replication cycle of Sindbis virus is as short as 4 h (Fig. 4), allowing abundant virus production prior to cell death. In fact, overexpressed Bcl-2 appears to suppress Sindbis virus replication both in overexpressing cell lines and in mouse brains infected with the Sindbis virus vector expressing Bcl-2 (31, 33, 61). Likewise, Bcl-2 can slow the replication of influenza virus, Semliki Forest virus, and human immunodeficiency virus (45, 51, 54). Thus, some viruses may prefer to replicate in the milieu of an apoptotic cell. In contrast to these findings with Bcl-2, we detected only slight reductions in Sindbis virus replication when caspase inhibitors were present (Fig. 2 to 4 and 6). One possible explanation for this discrepancy is that caspase inhibitors function downstream of Bcl-2 (10). (The effect of Bcl-xL overexpression on viral replication in mouse brains is currently under study.) Thus, it remains possible that viral persistence in mouse brains is facilitated not only by the presence of endogenous apoptosis inhibitors but also by a reduction in virus replication.
ACKNOWLEDGMENTS
We thank Emily Cheng for the GST-CrmA plasmid, Ellen and Jim Strauss for plasmids C481G and 12V, and Yukio Shirako for advice with the transcleavage assays. We thank Lesia Dropulic, Jennifer Lewis, and Diane Griffin for helpful suggestions and protocols and Shifa Zou for excellent technical assistance.
This work was supported by NIH grants NS34175 (J.M.H.) and AI40246 (B.L.) and by a James S. McDonnell Foundation Scholar award (B.L.). A.R. is a Pew Scholar in the Biomedical Sciences. V.E.N. is funded in part by the Consejo Nacional de Investigaciones Cientificas de Venezuela. R.J.C. is a Postdoctoral Fellow of the American Cancer Society.
REFERENCES
- 1.Ahmad M, Srinivasula S M, Wang L, Litwack G, Fernandes-Alnemri T, Alnemri E S. Spodoptera frugiperda caspase-1, a novel insect death protease that cleaves the nuclear immunophilin FKBP46, is the target of the baculovirus antiapoptotic protein p35. J Biol Chem. 1997;272:1421–1424. doi: 10.1074/jbc.272.3.1421. [DOI] [PubMed] [Google Scholar]
- 2.Bertin J, Mendrysa S M, LaCount D J, Gaur S, Krebs J F, Armstrong R C, Tomaselli K J, Friesen P D. Apoptotic suppression of baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J Virol. 1996;70:6251–6259. doi: 10.1128/jvi.70.9.6251-6259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 4.Boulakia C A, Chen G, Ng F W, Teodoro J G, Branton P E, Nicholson D W, Poirier G G, Shore G C. Bcl-2 and adenovirus E1B 19 kDa protein prevent E1A-induced processing of CPP32 and cleavage of poly(ADP-ribose) polymerase. Oncogene. 1996;12:529–535. [PubMed] [Google Scholar]
- 5.Casciola-Rosen L, Nicholson D W, Chong T, Rowan K R, Thornberry N A, Miller D K, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casciola-Rosen L A, Anhalt G J, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995;182:1625–1634. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, E. H.-Y., R. J. Clem, D. G. Kirsch, R. Ravi, M. B. Kastan, A. Bedi, K. Ueno, and J. M. Hardwick. Conversion of Bcl-2 to a Bax-like death effector by caspases. Submitted for publication. [DOI] [PubMed]
- 8.Cheng E H-Y, Levine B, Boise L H, Thompson C B, Hardwick J M. Bax-independent inhibition of apoptosis by Bcl-xL. Nature (London) 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 9.Cheng E H-Y, Nicholas J, Bellows D S, Hayward G S, Guo H-G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi’s sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinnaiyan A M, Orth K, O’Rourke K, Duan H, Poirier G G, Dixit V M. Molecular ordering of the cell death pathway. J Biol Chem. 1996;271:4573–4577. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 11.Clem R J, Miller L K. Apoptosis reduces both the in vitro replication and the in vivo infectivity of a baculovirus. J Virol. 1993;67:3730–3738. doi: 10.1128/jvi.67.7.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cryns V L, Bergeron L, Zhu H, Li H, Yuan J. Specific cleavage of a-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1β-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease. J Biol Chem. 1996;271:31277–31282. doi: 10.1074/jbc.271.49.31277. [DOI] [PubMed] [Google Scholar]
- 13.Datta R, Kojima H, Banach D, Bump N J, Talanian R V, Alnemri E S, Weichselbaum R R, Wong W W, Kufe D W. Activation of a CrmA-insensitive, p35-sensitive pathway in ionizing radiation-induced apoptosis. J Biol Chem. 1997;272:1965–1969. doi: 10.1074/jbc.272.3.1965. [DOI] [PubMed] [Google Scholar]
- 14.De Groot R J, Hardy W R, Shirako Y, Strauss J H. Cleavage-site preferences of Sindbis virus polyproteins containing the nonstructural proteinase: evidence for temporal regulation of polyprotein processing in vivo. EMBO J. 1990;9:2631–2638. doi: 10.1002/j.1460-2075.1990.tb07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desprès P, Griffin J W, Griffin D E. Effects of anti-E2 monoclonal antibody on Sindbis virus replication in AT3 cells expressing bcl-2. J Virol. 1995;69:7006–7014. doi: 10.1128/jvi.69.11.7006-7014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dryga S A, Dryga O A, Schlesinger S. Identification of mutations in a Sindbis virus variant able to establish persistent infection in BHK cells: the importance of a mutation in the nsP2 gene. Virology. 1997;228:74–83. doi: 10.1006/viro.1996.8364. [DOI] [PubMed] [Google Scholar]
- 17.Duckett C S, Nava V E, Gedrich R W, Clem R J, Van Dongen J L, Gilfillan M C, Shiels H, Hardwick J M, Thompson C B. A conserved family of apoptosis inhibitors related to the baculovirus iap gene. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 18.Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong W W, Kamen R, Weichselbaum R, et al. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagliardini V, Fernandez P-A, Lee R K K, Drexler H C A, Rotello R J, Fishman M C, Yuan Y. Prevention of vertebrate neuronal death by the crmA gene. Science. 1994;263:826–828. doi: 10.1126/science.8303301. [DOI] [PubMed] [Google Scholar]
- 20.Greidinger E L, Miller D K, Yamin T-T, Casciola-Rosen L, Rosen A. Sequential activation of three distinct ICE-like activities in Fas-ligated Jurkat cells. FEBS Lett. 1996;390:299–303. doi: 10.1016/0014-5793(96)00678-3. [DOI] [PubMed] [Google Scholar]
- 21.Griffin D E, Levine B, Tyor W R, Tucker P C, Hardwick J M. Age-dependent susceptibility to fatal encephalitis: alphavirus infection of neurons. Arch Virol. 1994;9:31–39. doi: 10.1007/978-3-7091-9326-6_4. [DOI] [PubMed] [Google Scholar]
- 22.Hardy W R, Strauss J H. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988;62:998–1007. doi: 10.1128/jvi.62.3.998-1007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson S, Hue D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hengartner M O, Ellis R E, Horvitz H R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature (London) 1992;356:494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- 25.Henkart P A. ICE family proteases: mediators of all apoptotic cell death? Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 26.Komiyama T, Ray C A, Pickup D J, Howard A D, Thornberry N A, Peterson E P, Salvesen G. Inhibition of the interleukin-1 beta converting enzyme by the cowpox virus sepin CrmA. An example of cross-class inhibition. J Biol Chem. 1994;269:19331–19337. [PubMed] [Google Scholar]
- 27.Kuida K, Zheng T S, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell R A. Decreased apoptosis in the brain and premature lethality in CPP-32 deficient mice. Nature (London) 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S. ICE-like proteases in apoptosis. Trends Biochem Sci. 1995;20:198–202. doi: 10.1016/s0968-0004(00)89007-6. [DOI] [PubMed] [Google Scholar]
- 29.Lazebnik Y A, Kaufmann S H, Desnoyers S, Poirier G G, Earnshaw W C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature (London) 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 30.Lazebnik Y A, Takahashi A, Moir R D, Goldman R D, Poirier G G, Kaufmann S H, Earnshaw W C. Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc Natl Acad Sci USA. 1995;92:9042–9046. doi: 10.1073/pnas.92.20.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine B, Hardwick J M, Griffin D E. Persistence of alphavirus in vertebrate hosts. Trends Microbiol. 1994;2:25–28. doi: 10.1016/0966-842x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 33.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature (London) 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 34.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Kim C N, Pohl J, Wang X. Purification and characterization of an interleukin-1β-converting enzyme family protease that activates cysteine protease P32 (CPP32) J Biol Chem. 1996;271:13371–13376. [PubMed] [Google Scholar]
- 36.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 37.Martin S J, Green D R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 38.Martin S J, O’Brien G A, Nishioka W K, McGahon A J, Mahboubi A, Saido T C, Green D R. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem. 1995;270:6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- 39.Miura M, Friedlander R M, Yuan J. Tumor necrosis factor-induced apoptosis is mediated by a CrmA-sensitive cell death pathway. Proc Natl Acad Sci USA. 1995;92:8318–8322. doi: 10.1073/pnas.92.18.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miura M, Yuan J. Regulation of programmed cell death by interleukin-1 beta-converting enzyme family of proteases. Adv Exp Med Biol. 1996;389:165–172. doi: 10.1007/978-1-4613-0335-0_20. [DOI] [PubMed] [Google Scholar]
- 41.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 42.Muzio M, Salvesen G S, Dixit V M. FLICE induced apoptosis in a cell-free system. J Biol Chem. 1997;272:2952–2956. doi: 10.1074/jbc.272.5.2952. [DOI] [PubMed] [Google Scholar]
- 43.Nava V E, Cheng E H-Y, Veliuona M, Zou S, Clem R J, Mayer M L, Hardwick J M. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Nava, V. E., and J. M. Hardwick. Unpublished data.
- 44.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raju S M, Smulson M E, Yamin T-T, Yu V L, Miller D K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 45.Olsen C W, Kehren J C, Dybdahl-Sissoko N R, Hinshaw V S. bcl-2 alters influenza virus yield, spread, and hemagglutinin glycosylation. J Virol. 1996;70:663–666. doi: 10.1128/jvi.70.1.663-666.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orth K, O’Rourke K, Salvesen G S, Dixit V M. Molecular ordering of apoptotic mammalian CED-3/ICE-like proteases. J Biol Chem. 1996;271:20977–20980. doi: 10.1074/jbc.271.35.20977. [DOI] [PubMed] [Google Scholar]
- 47.Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray C A, Black R A, Kronheim S R, Greenstreet T A, Sleath P R, Salvesen G S, Pickup D J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1-beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 49.Ray C A, Pickup D J. The mode of death of pig kidney cells infected with cowpox virus is governed by the expression of the crmA gene. Virology. 1996;217:384–391. doi: 10.1006/viro.1996.0128. [DOI] [PubMed] [Google Scholar]
- 50.Rosen A, Casciola-Rosen L. Macromolecular substrates for the ICE-like proteases during apoptosis. J Cell Biochem. 1997;64:50–54. doi: 10.1002/(sici)1097-4644(199701)64:1<50::aid-jcb8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 51.Scallan M F, Allsopp T E, Fazakerley J K. bcl-2 acts early to restrict Semliki Forest virus replication and delays virus-induced programmed cell death. J Virol. 1997;71:1583–1590. doi: 10.1128/jvi.71.2.1583-1590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaham S, Horvitz H R. An alternatively spliced C. elegans ced-4 RNA encodes a novel cell death inhibitor. Cell. 1996;86:201–208. doi: 10.1016/s0092-8674(00)80092-6. [DOI] [PubMed] [Google Scholar]
- 53.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri E S. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strack P R, Frey M W, Rizzo C J, Cordova B, George H J, Meade R, Ho S P, Corman J, Tritch R, Korant B D. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc Natl Acad Sci USA. 1996;93:9571–9576. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan X Q, Martin S J, Green D R, Wang J Y J. Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J Biol Chem. 1997;272:9613–9616. doi: 10.1074/jbc.272.15.9613. [DOI] [PubMed] [Google Scholar]
- 57.Tewari M, Quan L T, O’Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:1–20. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 58.Thornberry N A, Bull H G, Calaycay J R, Chapman K T, Howard A D, Kostura M J, Miller D K, Molineaux S M, Weidner J R, Aunins J, Elliston K O, Ayala J M, Casano F J, Chin J, Ding G J-F, Egger L A, Gaffney E P, Limjuco G, Palyha O C, Raju S M, Rolando A M, Salley J P, Yamin T-T, Lee T D, Shively J E, MacCross M, Mumford R A, Schmidt J A, Tocci M J. A novel heterodimeric cysteine protease is required for interleukin-1-beta processing in monocytes. Nature (London) 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 59.Trgovcich J, Ryman K, Extrom P, Eldridge C, Aronson J F, Johnston R E. Sindbis virus infection of neonatal mice results in a severe stress response. Virology. 1997;227:234–238. doi: 10.1006/viro.1996.8289. [DOI] [PubMed] [Google Scholar]
- 60.Ubol S, Park S, Budihardjo I, Desnoyers S, Montrose M H, Poirier G G, Kaufmann S H, Griffin D E. Temporal changes in chromatin, intracellular calcium, and poly(ADP-ribose) polymerase during Sindbis virus-induced apoptosis of neuroblastoma cells. J Virol. 1996;70:2215–2220. doi: 10.1128/jvi.70.4.2215-2220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ubol S, Tucker P C, Griffin D E, Hardwick J M. Neurovirulent strains of alphavirus induce apoptosis in bcl-2-expressing cells: role of a single amino acid change in the E2 glycoprotein. Proc Natl Acad Sci USA. 1994;91:5202–5206. doi: 10.1073/pnas.91.11.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincenz C, Dixit V M. Fas-associated death domain protein interleukin-1β-converting enzyme 2 (FLICE2), an ICE/Ced-3 homologue, is proximally involved in CD95- and p55-mediated death signaling. J Biol Chem. 1997;272:6578–6583. doi: 10.1074/jbc.272.10.6578. [DOI] [PubMed] [Google Scholar]
- 63.Wesselingh S L, Levine B, Fox R J, Choi S, Griffin D E. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J Immunol. 1994;152:1289–1297. [PubMed] [Google Scholar]
- 64.White E. Function of the adenovirus E1B oncogene in infected and transformed cells. Semin Virol. 1994;5:341–348. [Google Scholar]
- 65.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 66.Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1-beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 67.Zhivotovsky B, Gahm A, Ankarcrona M, Nicotera P, Orrenius S. Multiple proteases are involved in thymocyte apoptosis. Exp Cell Res. 1995;221:404–412. doi: 10.1006/excr.1995.1391. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Q, Snipas S, Orth K, Muzio M, Dixit V M, Salvesen G S. Target protease specificity of the viral serpin CrmA. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]